Abstract
A seasonal study of the distribution of lysogenic bacteria in Tampa Bay, Florida, was conducted over a 13-month period. Biweekly water samples were collected and either were left unaltered or had the viral population reduced by filtration (pore size, 0.2 μm) and resuspension in filtered (pore size, 0.2 μm) water. Virus-reduced and unaltered samples were then treated by adding mitomycin C (0.5 μg ml−1) to induce prophage or were left untreated. In order to test the hypothesis that prophage induction was phosphate limited, additional induction experiments were performed in the presence and absence of phosphate. Induction was assessed as an increase in viral direct counts, relative to those obtained in controls, as detected by epifluorescence microscopy. Induction of prophage was observed in 5 of 25 (20%) unaltered samples which were obtained during or after the month of February, paralleling the results from a previous seasonal study. Induction of prophage was observed in 9 of 25 (36%) of the virus-reduced samples, primarily those obtained in the winter months, which was not observed in a prior seasonal study (P. K. Cochran and J. H. Paul, Appl. Environ. Microbiol. 64:2308-2312, 1998). Induction was noted in the months of lowest bacterial and primary production, suggesting that lysogeny was favored under conditions of poor host growth. Phosphate addition enabled prophage induction in two of nine (22%) experiments. These results indicate that prophage induction may occasionally be phosphate limited or respond to increases in phosphate concentration, suggesting that phosphate concentration may modulate the lysogenic response of natural populations.
Lysogeny, the process whereby phage genomes establish silent infections in their host, is a common occurrence among cultivated bacteria of terrestrial (1) and marine (16) origin. Lysogeny can benefit the host by the process of conversion or the expression of phage genes, including genes coding for toxin production, antibiotic resistance, expanded metabolic capabilities, and homoimmunity (resistance to superinfection by the same or closely related phages) (19).
The occurrence of lysogenic bacteria and temperate phages in the environment may be due to a variety of environmental and biological factors. Stewart and Levin (22) hypothesized that lysogenic interactions between viruses and host cells may provide a means of survival for viral populations that are threatened by poor host cell abundance and therefore cannot sustain population numbers through lytic infection alone.
The importance of lysogeny as an alternative to lytic infection in natural populations of marine bacteria is poorly understood. Prior research in the Gulf of Mexico indicated that two-thirds of the marine environments sampled contained inducible prophage (6). Additionally, Jiang and Paul (17) found that 43% of marine heterotrophic bacterial isolates contained inducible prophage, indicating that lysogens comprised a significant portion of the heterotrophic microbial population. Conversely, other studies have demonstrated that lysogeny is not an important virus-host interaction in the marine environment. Both Weinbauer and Suttle (30) and Tapper and Hicks (24) found that only a small fraction of bacteria contained inducible prophage in both the Gulf of Mexico and Lake Superior, respectively. Another study conducted by Weinbauer and Suttle (29) found that the production of phage by lysogenic induction was not an important mechanism of phage production or bacterial mortality in the marine environment. Additional studies have shown that lysogeny is more prevalent in oligotrophic offshore waters than in nutrient-rich coastal environments (30, 33). Thus, the factors regulating the occurrence of lysogeny (or its capability of being detected) are not known.
The abundance of nutrients may be a factor regulating lysogeny in the environment. Nutrient concentrations often determine host cell abundance. Wilson and Mann (35) found that lysogeny was favored when nutrient concentrations were low and the virus-to-host cell ratio was high. Inorganic nutrient limitation, particularly PO43− limitation, has been suggested as a possible constraint on viral replication and/or prophage induction (35). Bratbak et al. (2) provided evidence that phosphate limitation resulted in inhibition of successful viral replication in the marine coccolithophorid Emiliania huxleyi and suggested that these results may have been caused by the viruses' high nucleic acid-to-protein ratio. Wilson et al. (34) investigated the effect of phosphate limitation on the Synechococcus sp. strain WH7803 and found that 100% of cyanobacterial cells were lysed by viruses under phosphate-replete conditions compared to 9.3% of cells lysed under phosphate-depleted conditions. Tuomi et al. (25) observed a significant decrease in bacterial abundance upon addition of inorganic phosphate to phosphorus-depleted seawater samples taken from Raunefjorden, Norway. In contrast, F. Rohwer (personal communication) has shown that Roseophage entered into a pseudolysogenic relationship with its host under phosphate-replete conditions but displayed a lytic relationship under conditions of phosphate limitation.
A prior study of the seasonal occurrence of lysogens as determined by prophage induction of ambient populations indicated that about half of the seasonal samples contained lysogens, and these were obtained between the months of February and October when water temperature was above 19°C (5). This study suggested that the occurrence (or detection) of lysogeny might be linked to microbial activity, yet no activity measurements were performed. To investigate the environmental factors that might influence the seasonal variation of lysogeny, a 13-month study of lysogeny in Tampa Bay, Fla., was conducted. In this study, we compared the direct method of prophage induction of Cochran and Paul (5) to the viral reduction method of Weinbauer and Suttle (29) for the detection of lysogeny. Additionally, microbial activity (heterotrophic and autotrophic production) as well as inorganic nutrient concentrations were measured in the present study. We report here a clear seasonal variation in lysogeny and relate this to times of diminished primary and bacterial production, low nutrient input, and minimal water temperature.
MATERIALS AND METHODS
Sampling site.
Water samples were collected biweekly from Tampa Bay, Fla., at the St. Petersburg City Pier for a period of 13 months, starting on 21 October 1999 and concluding on 23 October 2000. The sampling site is located on the western shore of Tampa Bay and is influenced by urban runoff from the city of St. Petersburg, Fla. Water was collected from the far east end of the St. Petersburg City Pier in a plastic bucket and transferred to four sterile, acid-washed 2-liter polycarbonate flasks. Water samples were processed within 1 h of collection.
Viral reduction of sampled seawater.
Sixty milliliters of the sample water was filtered through a Poretics filter (pore size, 0.22 μm) using a 47-mm polycarbonate filtration apparatus to reduce the volume to approximately 5 ml. Fifty milliliters of filtered (pore size, 0.02 μm) sample water was added back to the remaining 5 ml of the filtered (pore size, 0.22 μm) sample, and the volume was once again reduced to approximately 5 ml through filtration. The bacteria retained on the filter, along with the filter, were resuspended in 40 ml of filtered (pore size, 0.02 μm) virus-free seawater in a 125-ml flask. The filter was removed, and filtered (pore size, 0.02 μm) virus-free sample water was added to bring the final volume to 60 ml (29). Ten milliliters of the virus-reduced sample was fixed with filtered (pore size, 0.02 μm) formalin for viral and bacterial direct counts. The remainder of the virus-reduced sample was equally divided (25 ml) and placed in sterile 50-ml Sarstedt test tubes (Corning Inc., Corning, N.Y.) for determination of lysogeny.
Enumeration of viruses and bacteria.
Water samples were stained according to the Noble and Fuhrman (20) method, except that SYBR Gold nucleic acid stain was used and staining time was reduced to 12 min.
Induction of virus-reduced and unaltered samples with mitomycin C.
Replicate virus-reduced and unaltered samples were either treated with the mutagen mitomycin C (1.0 μg ml−1; Sigma Chemical Co., St. Louis, Mo.) or left untreated as controls. All samples were incubated statically at room temperature in the dark for 24 h. Samples were fixed with filtered (pore size, 0.02 μm) formalin following incubation.
Induction of virus-reduced and unaltered samples in the presence of phosphate.
Experiments designed to determine the effect of phosphate stimulation on induction began 26 June 2000. In treatment and control samples, the ambient viral population was reduced by the same procedure mentioned above. Replicate virus-reduced samples (25 ml) were either treated with a combination of mitomycin C (1.0 μg ml−1) and phosphate (KH2PO4 [final concentration, 10 μM]) or treated with phosphate alone as controls. All samples were incubated and fixed as mentioned above.
Bacterial production by [3H]leucine incorporation.
Rates of bacterial production were measured by the [3H]leucine incorporation technique as described by Kirchman et al. (18) and Chin-Leo and Kirchman (4). [3H]leucine (44.0 Ci mmol−1; NEN, Boston, Mass.) was added to sterile acid-washed treatment and trichloroacetic acid (TCA)-killed control flasks containing sample water. Flasks were wrapped in foil and incubated on a dock in an incubator kept at the sample temperature by recirculating ambient bay water. Triplicate subsamples were immediately removed and added to 15-ml Sarstedt test tubes containing 50% TCA, and this was repeated again at 30 and 60 min. Tubes were placed on ice for 1 min, incubated at 85°C for 15 min in order to degrade the DNA and RNA fraction of precipitated macromolecules, and then placed on ice again for 8 min in order to precipitate the bacterial protein fraction. Samples were then filtered through Millipore GS filters. Filters were washed two times with ice-cold TCA, placed in liquid scintillation vials, and dissolved with ethyl acetate. Liquid scintillation counting fluid was added to the vials, which were gently shaken for 30 min, and radioactivity was determined by liquid scintillation counting.
NaH14CO3 carbon fixation.
Carbon fixation was determined using the technique of Strickland and Parsons (23) as modified by Carpenter and Lively (3). The counting efficiency was calculated by liquid scintillation counting of a 14C standard (28). Total carbon dioxide and carbonate alkalinity were measured through titration with 0.01 N HCl (23).
Estimation of autotrophic biomass through chlorophyll a measurements.
Triplicate 50-ml water samples were filtered through Whatman GF/F filters. Filters were wrapped in aluminum foil and stored at −20°C until processing. The filters were extracted in the dark in methanol, and the chlorophyll a content was fluorometrically determined (14).
Nutrient analyses.
Sampling for nutrient analysis began on 9 February 2000. NO3 plus NO2 concentrations and PO4 concentrations were determined for unfiltered and filtered water samples. For the filtered samples, a Swinex filtration device containing a Poretics filter (pore size, 0.4 μm) was rinsed first with 10 ml of 10% HCl in order to clean the filter and then rinsed again with ultrapure water (Millipore Milli-Q). All water samples were stored in 30-ml polycarbonate bottles (Nalgene) at −20°C until processing. One portion of the nutrient samples (February 1999 to August 2000) was analyzed by an Astoria Pacific segmented flow analyzer (9), while the remaining nutrient samples (August 2000 to October 2000) were analyzed by an Alpkem RFA II segmented flow analyzer (10, 11, 12, 26, 27).
Calculations of the induced prophage, lysogenic fraction of the bacterial population, and induced burst size.
The amount of induced prophage was calculated as VI − VC, where VI is the number of viruses enumerated in the induced sample at 24 h and VC is the number of viruses enumerated in the control sample. The lysogenic fraction (LF) of the bacterial population was determined by two methods. The first, termed the burst size method, was calculated using the following equation: LF = [(VI − VC)/BZ]/BC·100, where BC is the number of bacteria enumerated in the control sample at 24 h and BZ is the burst size. Two values were used for BZ, one derived from an average of literature values (BZ = 50 [37]) and a second derived from transmission electron microscopy observation of Tampa Bay samples (BZ = 30 [15]). The second method for calculation of the lysogenic fraction used the following equation: LF = (BC − BI)/BC·100, where BC and BI are the number of bacteria enumerated in the control and induced samples at 24 h, respectively. This was corrected by subtracting the average mortality caused by mitomycin C in uninduced samples. Induced burst size (BZI) was calculated using the following equation: BZI = (VI − VC)/(BC − BI).
RESULTS
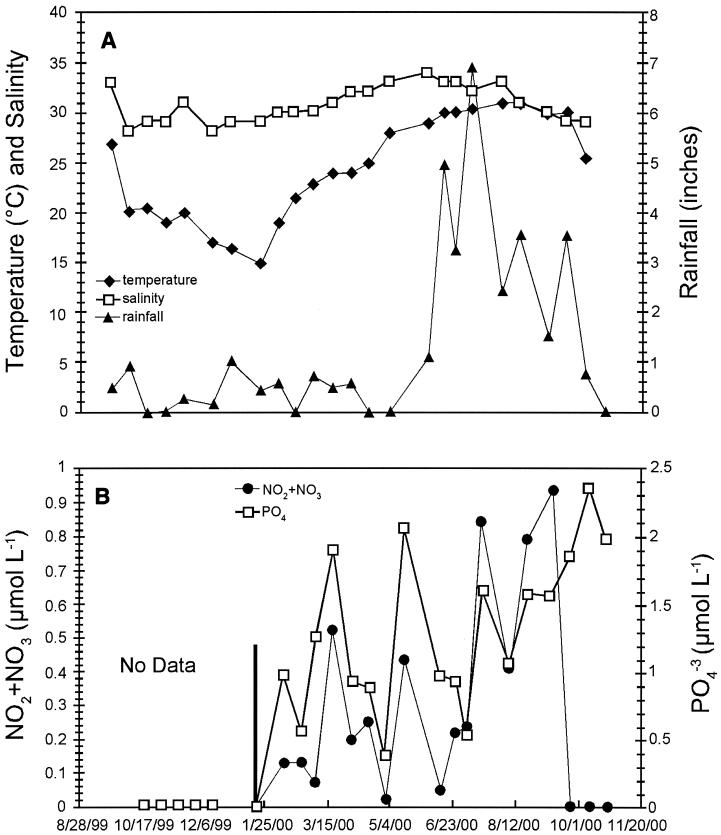
Figure 1 depicts the seasonal trends observed in temperature, salinity, and rainfall (Fig. 1A) and the fluctuations observed in the filtered NO2 plus NO3 and PO43− concentrations measured at the sampling site (Fig. 1B). Drought conditions were reflected in below-average annual rainfall. The least amount of precipitation (9.12 in.) occurred during the first half of the study (October 1999 to March 2000), while the second half (April 2000 to October 2000) experienced the highest amount of rainfall (40.72 in.). The increased rainfall had minimal effect on the salinity, which ranged from 29 to 34 ppt.
FIG. 1.
Seasonal variations in water temperature, salinity, and rainfall (A) and fluctuations observed in the filtered NO2 plus NO3 and PO43− concentrations measured at the St. Petersburg City Pier (B).
Inorganic nutrient levels (NO3 plus NO2, PO43−) were measured only from February 2000 to October 2000 at the St. Petersburg City Pier. The NO3 plus NO2 levels were greatest during times of the greatest rainfall (July to October) (r = 0.464; P = 0.053), suggesting that runoff was the major source of these nitrogen compounds. Patterns of phosphate concentrations mirrored nitrogen concentration until late in the study, when a sharp decrease in NO2 plus NO3 concentration corresponded to an increase in PO43− concentration.
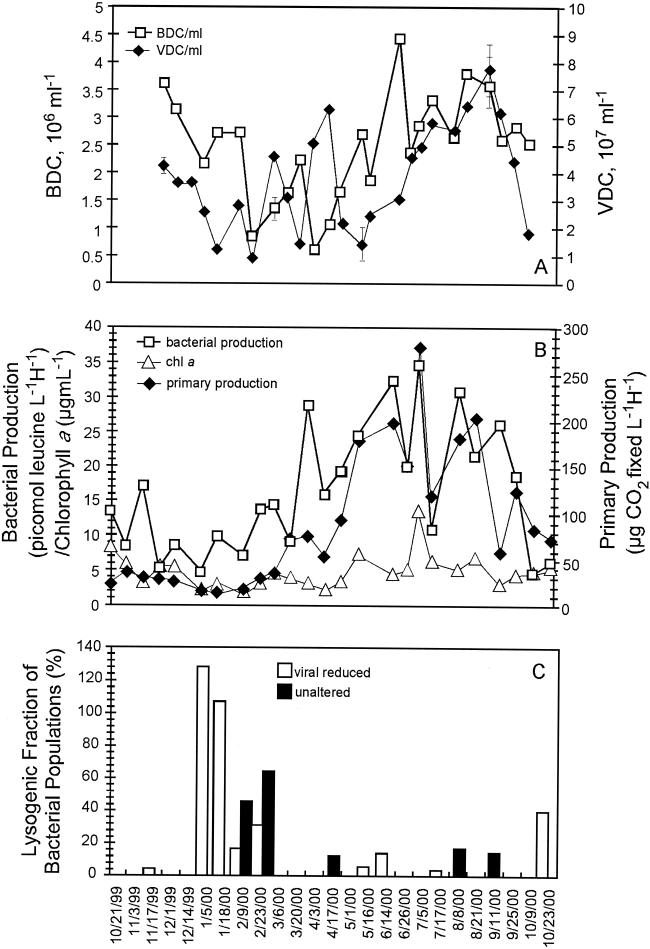
The seasonal variations in viral and bacterial abundance are shown in Fig. 2A. The lowest bacterial abundances (8.51 × 105 and 6.16 × 105 cells ml−1) were observed in January and March, respectively, while bacterial abundance increased throughout the spring, reaching a maximum of 4.42 × 106 cells ml−1 in June. Viral abundance decreased throughout the fall and winter, with the lowest viral direct counts occurring in January (9.45 × 106 viruses ml−1). Although viral abundance fluctuated throughout the winter and early spring, viral direct counts began to steadily increase throughout the summer months, reaching a maximum in September (7.78 × 107 viruses ml−1). Large peaks in viral abundance either coincided with a large increase in bacterial production (2 April 2000) or followed closely behind a large increase in bacterial production (11 September 2000) (Fig. 2B). The virus-to-bacterium ratio for this study had an average value of 19.4, and ranged from 4.8 to 82.6. The large peak in viral abundance that occurred on 11 September 2000 also coincided with the maximum NO2 plus NO3 concentration measured, and a positive correlation between viral abundance and NO2 plus NO3 concentration was observed (r = 0.604; P = 0.008).
FIG. 2.
Seasonal variations in viral and bacterial abundance (A) as well as seasonal variations in bacterial production, chlorophyll a, and primary production (B) are presented. (C) Seasonal occurrence of lysogeny for unaltered and virus-reduced water samples.
The lysogenic fraction of bacterial populations was calculated (using a standard burst size of 50) for both unaltered and virus-reduced water samples (Fig. 2C) that displayed a statistically significant (90 to 95% confidence interval) increase in viral direct counts upon treatment with mitomycin C. Five of 25 (20%) unaltered water samples were positive for induction of prophage. The occurrence of lysogeny in the unaltered water samples displayed a seasonal trend, with all induction events occurring in or after the month of February. These results are very similar to the results of a previous seasonal study conducted by Cochran and Paul (5), in which they observed induction of prophage in or after the month of February when water temperatures were above 19°C. Additionally, positive correlations were noted between the number of induced prophage per milliliter in unaltered and virus-reduced water samples and the calculated lysogenic fraction of the bacterial population determined by the mortality method (for the unaltered sample, r = 0.711 and P < 0.001; for the virus-reduced sample, r = 0.663 and P < 0.001).
Lysogeny was detected in virus-reduced samples mostly in the winter months between November 1999 and February 2000 (Fig. 2C) and therefore displayed a slightly different seasonal trend than the trend observed in unaltered water samples. Filtration of water samples to reduce background levels of viruses resulted in 9 of 25 (36%) samples being positive for the induction of prophage. The majority of the induction events (78%) occurred in or prior to the month of February, which has not been previously observed in any other seasonal study.
The biological parameters of chlorophyll a, primary production, and bacterial production all displayed a clear seasonal trend (Fig. 2B). Seasonal variation in primary productivity was reflected in chlorophyll a values, and the two parameters were positively correlated (r = 0.642; P = 0.001). Primary production and bacterial production also showed a strong positive correlation (r = 0.740; P < 0.001).
Seventy-three percent of the induction events that occurred in both the unaltered and virus-reduced water samples were detected during periods of low bacterial production, as determined by [3H]leucine incorporation. Additionally, the specific growth rate (μ) of the bacterial populations during periods of low bacterial production (average ± standard deviation, 0.272 ± 0.205) was approximately half of the specific growth rate during times of high bacterial production (average ± standard deviation, 0.500 ± 0.290) (P = 0.036). Bacterial production values were lowest during periods of decreased water temperature, and the two parameters were correlated (r = 0.669; P < 0.001). Water temperature was positively correlated to several of the environmental and biological parameters measured, including salinity (r = 0.67; P < 0.001), viral abundance (r = 0.539; P = 0.005), bacterial abundance (r = 0.499; P = 0.011), primary production (r = 0.791; P < 0.000), chlorophyll a (r= 0.489; P = 0.013), and bacterial production (r = 0.669; P < 0.000). The number of induced prophage per milliliter (r = −0.468; P = 0.018), the calculated lysogenic fraction determined by the burst size (r = −0.505; P = 0.010) and mortality (r = −0.435; P = 0.030) methods, and the calculated burst size for virus-reduced samples (r = −0.470; P = 0.018) were all negatively correlated with water temperature. In addition, the number of induced prophage per milliliter and bacterial abundance were negatively correlated in virus-reduced water samples (r = −0.432; P = 0.031), indicating that a portion of the bacteria in these samples were lysogenized. These results support the theory that viruses enter into lysogenic interactions with their hosts during periods of low host density and growth rate.
The effect of phosphate addition on prophage induction appears in Table 1. Two of nine samples (22%) displayed a statistically significant increase in viral direct counts upon treatment with mitomycin C and phosphate. The induction event that was observed on 23 October 2000 in the phosphate-amended treatment also occurred in the non-phosphate-amended, mitomycin C-treated sample, suggesting that phosphate did not stimulate prophage induction. Although we did not initiate phosphate amendment studies until June 2000, all significant increases in viruses occurred in August or September.
TABLE 1.
Induction of natural populations of bacteria by addition of mitomycin C and PO43−a
| Date (mo/day/yr) | VDC ml−1
|
% Change | |
|---|---|---|---|
| Treatmentb | Controlc | ||
| 6/26/00 | 1.07 × 107 ± 1.68 × 106 | 1.06 × 107 ± 4.04 × 105 | 0.94 ± 16.4 |
| 7/5/00 | 1.06 × 107 ± 7.60 × 105 | 1.07 × 107 ± 6.03 × 105 | −0.93 ± 10.0 |
| 7/17/00 | 6.24 × 106 ± 1.48 × 106 | 6.46 × 106 ± 1.06 × 105 | −3.4 ± 23.6 |
| 8/8/00 | 2.70 × 106 ± 1.02 × 105 | 9.81 × 106 ± 2.25 × 106 | −72.5 ± 30.0 |
| 8/21/00d | 1.36 × 107 ± 1.78 × 106 | 8.80 × 106 ± 1.81 × 106 | 54.5 ± 18.2 |
| 9/11/00 | 1.05 × 107 ± 3.33 × 106 | 8.46 × 106 ± 8.49 × 105 | 24.1 ± 27.2 |
| 9/25/00 | 1.81 × 107 ± 4.59 × 106 | 2.57 × 106 ± 4.08 × 105 | 604.3 ± 288.9 |
| 10/9/00 | 1.34 × 107 ± 1.17 × 107 | 6.64 × 106 ± 1.45 × 106 | 101.8 ± 125.4 |
| 10/23/00 | 9.50 × 106 ± 6.37 × 105 | 7.19 × 106 ± 8.23 × 105 | 32.1 ± 11.7 |
Results are presented as means ± SD.
Treatment with mitomycin C plus PO43−.
Mitomycin C alone.
Boldface type indicates a date on which significant induction was observed.
Table 2 summarizes the lysogenic fractions, induced prophage per milliliter, and burst sizes for the unaltered, virus-reduced, and phosphate-treated water samples that were positive for induction of prophage. The number of induced prophage per milliliter calculated for all samples was 4.00 × 107 ± 1.34 × 107 and ranged from 1.67 × 106 to 2.32 × 108 (unless otherwise noted, results are presented as averages ± standard deviations). The calculated lysogenic fractions determined by the burst size method (BZ = 50 and BZ = 30) were 27.6% ± 24.7% and 45.9% ± 41.2%, respectively, ranging from 0.92 to 126% (BZ = 50) and 1.5 to 210.0% (BZ = 30). The lysogenic fraction was also calculated by the mortality method, based on reduction in bacterial direct counts after mitomycin C treatment. Because a portion of the bacterial mortality was caused by the toxicity of mitomycin C, and not solely by prophage-induced cell lysis, we examined the mortality in samples that displayed no prophage induction. The mortality average in such samples was 27.6% ± 36.2%. When this “toxicity factor” was subtracted from the lysogenic fraction calculated by the mortality method, the average lysogenic fraction was 21.6% ± 58.4% and ranged from 0 to 51.2%. Lastly, the calculated induced burst size for all induced samples was 27.6 ± 61.3 viral particles per host cell, ranging from 1.4 to 102.8 viral particles liberated per host cell.
TABLE 2.
Calculation of lysogenic fractions of bacterial populations in Tampa Bay, Fla.a
| Samples and date (day/mo/yr) | No. of induced prophage ml−1 | Lysogenic fraction (%) determined by:
|
Induced burst size (ΔVDC/ΔBDC) | ||
|---|---|---|---|---|---|
| Assuming BZ = 50% | Assuming BZ = 30% | Mortality methodb | |||
| Unfiltered | |||||
| 2/9/00 | 4.60 × 107 ± 9.50 × 106 | 43.6 ± 30.7 | 72.6 ± 51.2 | 24.0 ± 52.2 | 42 ± 218.7 |
| 2/23/00 | 6.16 × 107 ± 1.34 × 107 | 60.4 ± 52.3 | 101.0 ± 87.2 | 12.6 ± 53.9 | 75.1 ± 252.3 |
| 4/17/00 | 9.22 × 106 ± 5.69 × 105 | 7.7 ± 2.3 | 12.8 ± 3.8 | 51.2 ± 36.9 | 4.8 ± 5.6 |
| 8/7/00 | 2.53 × 107 ± 3.18 × 106 | 12.8 ± 2.8 | 21.4 ± 4.6 | 23.3 ± 36.5 | 12.6 ± 3.7 |
| 9/11/00 | 2.26 × 107 ± 1.22 × 107 | 10.8 ± 5.9 | 18.1 ± 9.8 | 13.2 ± 36.7 | 13.3 ± 19.8 |
| Virus reduced | |||||
| 11/17/99 | 16.7 × 106 ± 4.10 × 105 | 1.4 ± 1.0 | 2.3 ± 1.6 | 6.4 ± 44.0 | 2.0 ± 5.3 |
| 1/5/00 | 1.47 × 108 ± 6.43 × 107 | 126.0 ± 113.1 | 210.0 ± 188.4 | 33.4 ± 137.9 | 102.8 ± 132.5 |
| 1/18/00 | 2.32 × 108 ± 6.05 × 107 | 103.8 ± 119.0 | 172.9 ± 198.8 | 47.6 ± 114.4 | 68.8 ± 165.8 |
| 2/9/00 | 1.50 × 107 ± 7.82 × 106 | 12.6 ± 13.8 | 21.0 ± 23.2 | 38.8 ± 113.9 | 9.4 ± 44.0 |
| 2/23/00 | 4.10 × 107 ± 2.89 × 107 | 27.4 ± 22.3 | 45.7 ± 37.2 | 34.6 ± 36.5 | 22 ± 19.6 |
| 5/16/00 | 4.80 × 106 ± 2.08 × 106 | 2.6 ± 1.1 | 4.3 ± 1.9 | 26.5 ± 38.3 | 2.4 ± 1.7 |
| 6/14/00 | 4.41 × 107 ± 3.44 × 106 | 10.8 ± 0.6 | 18.1 ± 1.0 | 35.1 ± 38.0 | 8.6 ± 2.0 |
| 7/17/00 | 1.87 × 106 ± 2.72 × 106 | .92 ± 0.7 | 1.5 ± 1.1 | 5.7 ± 45.6 | 1.4 ± 8.6 |
| 10/23/00 | 5.82 × 106 ± 6.62 × 106 | 36.4 ± 26.0 | 60.8 ± 43.3 | 4.1 ± 58.1 | 57.6 ± 80.1 |
| Virus reduced (10 μM phosphate) | |||||
| 8/21/00 | 4.80 × 106 ± 9.80 × 105 | 2.0 ± 0.7 | 3.4 ± 1.2 | 6.8 ± 39.0 | 2.9 ± 3.3 |
| 9/25/00 | 1.55 × 107 ± 4.91 × 106 | 7.9 ± 2.9 | 13.2 ± 4.8 | ≤0 ± 53.2 | 40.8 ± 18.0 |
| Avg | 4.00 × 107 ± 1.34 × 107 | 27.6 ± 24.7 | 45.9 ± 41.2 | 21.6 ± 58.4 | 27.6 ± 61.3 |
The lysogenic fraction of bacterial populations, the number of induced prophage per milliter, and the induced burst size were calculated for all unaltered, virus- reduced, and phosphate-treated samples that displayed statistically significant (90 to 95% confidence interval) increases in viral direct counts relative to control samples. Results are means ± standard deviations.
Mortality caused by mitomycin C in uninduced samples = 27.6% ± 36.2%.
DISCUSSION
The factors that determine the occurrence of lysogeny in the marine environment are poorly understood. In this study, we determined that the occurrence of lysogeny in Tampa Bay, Fla., displayed a seasonal trend. The viral reduction method for lysogenic induction revealed a slightly different seasonal pattern than that observed in unaltered water samples. The dramatic increase in the calculated lysogenic fraction of the bacterial population in unaltered water samples occurred in the month of February, marking the beginning of the seasonal trend. The seasonality of lysogeny observed in unaltered water samples is similar to the seasonal trend observed by Cochran and Paul (5), in which all induction events occurred in or after the month of February when water temperatures were greater than 19°C. In contrast, the occurrence of lysogeny in virus-reduced water samples was first detected in November, thereby shifting the beginning of the seasonal trend to earlier in the winter season. Induction of prophage was only detected on a few occasions during the summer months. This suggests that either the production of viruses in Tampa Bay at this time was predominantly through the lytic pathway or prophage were already induced by other mechanisms in the environment, such as elevated water temperature or levels of UV radiation.
Viral reduction of seawater by filtration (pore size, 0.22 μm) to reduce background levels of viruses and enumeration of viruses by epifluorescent microscopy may have allowed for a more sensitive means of detection of induced prophage in natural marine samples. Alternately, the increased detection of prophage may have resulted from the manipulation of the water sample by filtration. The viral reduction method enables detection of lysogeny in more seasonal samples, perhaps by metabolic stimulation caused by filtration. However, these lysogens were in the sample prior to filtration, which enabled their detection, and should not be viewed as an artifact.
The majority of the induction events in unaltered and virus-reduced water samples were detected during periods of low water temperature, rainfall, nutrient concentrations, primary productivity, and bacterial productivity. This suggests that viruses entered into lysogenic interactions with their bacterial hosts under conditions that were not favorable to bacterial growth and production. These results do not agree with previous studies for culturable bacteria, which have shown that induction of prophage occurs mainly in cultures that are in exponential phase (1).
The lowest bacterial production rates observed during the winter and early spring appeared to be influenced by several factors. The positive correlations between bacterial abundance or production and water temperature suggested that cooler water temperatures were a contributing factor to repressed bacterial growth and production. In addition, bacterial production and abundance in both freshwater and marine habitats have been shown to fluctuate with water temperature (13, 32).
The seasonal trend observed in viral abundance also appeared to be related to water temperature, with the lowest viral concentrations occurring in the winter and the highest viral concentrations occurring in the summer. Similar seasonal trends in viral abundance were observed in previous studies conducted on the occurrence of lysogeny in Tampa Bay by Jiang and Paul (16) and Cochran and Paul (5). The peaks in viral abundance that occurred either concomitantly with (2 April 2000) or closely behind (11 September 2000) peaks in bacterial production may have been the result of lytic production of viruses in response to increased bacterial production and host density. Successful virulent phage production requires the host-phage encounter rate to exceed the rate of destruction and inactivation of the viruses produced (37). The large increases observed in bacterial production may have resulted in a sufficiently high host density to support virulent infection.
Another biological factor that was positively correlated with bacterial production in the seasonal study was primary production. It is well known that primary production in marine systems fuels secondary production, including bacterial production. Efficient transfer of primary production to secondary producers can result in a depletion of excess organic matter that can be used as a food source due to the rapid cycling of this material during the summer months (38). Primary productivity and bacterial productivity were closely coupled throughout the seasonal study, and it was not surprising to see positive correlations between primary and bacterial production, as well as positive correlations between chlorophyll a (an estimate of autotrophic biomass) and bacterial production. The increasing trend in bacterial production that occurred throughout the spring and summer months lagged slightly behind the almost-identical trends observed in primary production and chlorophyll a.
The concentration of nutrients has been proposed as one of the environmental factors that influence the lysogenic decision (19) by modulating the metabolic activity of the host population. Although inorganic nutrient concentrations are well known to modulate autotrophic production, heterotrophic bacteria have demonstrated the potential to compete successfully with algae for dissolved inorganic nutrients due to the high affinity of bacterial transport systems (7). Several studies have indicated that inorganic nutrients act as limiting factors for heterotrophic bacterial growth. Horrigan et al. (15) demonstrated that supplementation of seawater off of Scripps pier (San Diego, Calif.) with inorganic nitrogen increased bacterial biomass. Additionally, Wheeler and Kirchman (31) demonstrated that the growth rate of heterotrophic bacteria was significantly higher following the addition of a mixture of organic (glucose) and inorganic (ammonium) nutrients to seawater samples from the Gulf Stream compared to the addition of organic nutrients alone (amino acid mixture).
In this study, induction of prophage was generally not observed during periods of elevated NO2 plus NO3 concentrations. The exception to this observation was the induction event that occurred on 11 September 2000 in an unaltered water sample. This suggests that elevated inorganic nitrogen concentrations may favor lytic rather than lysogenic production of viruses in the marine environment.
Since inorganic-nutrient data were not collected during the first 4 months of the seasonal study, we compared nutrient data that were collected during this time period by the Hillsborough County Environmental Protection Commission in order to gain a better understanding of nutrient concentrations during the early months of the seasonal study (references 10, 11, and 12 and 1999 and 2000 surface water quality raw data summaries, Hillsborough County Water Department, Hillsborough County, Fla.). The Hillsborough County Environmental Protection Commission (EPC) monitors 52 stations in Tampa Bay on a monthly basis for a variety of environmental parameters, including nutrient concentrations. Station 82 is located just offshore of the St. Petersburg City Pier. Although the PO43− concentrations reported are from two distinct sites that are within close proximity of one another, the concentrations from both EPC station 82 and our sampling site displayed a similar trend when sampled approximately at the same time (EPC data not shown). According to the EPC data, a significant decrease in phosphate concentration occurred between the months of November 1999 and January 2000, which coincided with a significant increase in the calculated lysogenic fraction of bacterial populations (Fig. 2). The decrease in PO43− concentration may have been a contributing factor to the occurrence of lysogeny at this time. Since phosphate amendment studies were not conducted during this time, we cannot conclude that prophage induction was a result of phosphate limitation during this period of the seasonal study. Many studies have been conducted on the marine environment in which PO43− concentrations have been manipulated in order to determine the effect on viral activity and production (2, 8, 25, 34, 36). The results of these studies suggest that PO43− does behave as a determinative factor in the lysogenic decision (21).
Treatment of virus-reduced water samples with a combination of mitomycin C and 10 μM PO43− resulted in the detection of lysogeny in 22% of the samples. Although all induction events occurred in or after the month of August, we could not determine if induction of prophage in response to PO43− addition occurred on a seasonal basis, since treatment of virus-reduced water samples in this manner did not begin until June 2000. It is not clear why the addition of PO43− to water samples stimulated the induction of prophage on two occasions when phosphate did not appear to be a limiting nutrient on the dates that induction was observed. Although the NO2 plus NO3 concentration did exceed the PO43− concentration on 21 August 2000 when induction in phosphate-amended samples was first observed, the PO43− concentration greatly exceeded the NO2 plus NO3 concentration on 25 September 2000, when the second induction event in phosphate-amended samples was observed (Fig. 1B). The low percentage of samples induced by phosphate stimulation may have been due to the elevated phosphate levels present at that time and the apparent high incidence of lytic infection.
The calculations of the lysogenic fraction of bacterial populations by the burst size method (BZ = 50% [27.6% ± 24.7%]; BZ = 30% [45.9% ± 41.2%]), the mortality method (47.7% ± 39.7%), and the mortality method after correction (21.6% ± 58.4%) for unaltered, virus-reduced, and phosphate-treated samples indicate that a significant portion of the bacteria in Tampa Bay are lysogenic during certain times of the year. Water samples that were not positive for induction of prophage upon treatment with mitomycin C still underwent a decrease in bacterial abundance, probably due to mitomycin C toxicity. We therefore calculated the mortality caused by mitomycin C in these samples and deducted this from the prophage-induced mortality calculations. This correction resulted in a more realistic estimate of the lysogenic fraction of bacterial populations. The burst size method should also be employed when calculating lysogenic fractions since it utilizes increases in viral direct counts which occurs upon treatment with mitomycin C, thereby confirming the presence of lysogenic bacteria in the sample.
Although our calculations of the lysogenic fraction of bacterial populations indicate that a significant portion of bacteria in Tampa Bay are lysogenic, there are still additional factors to be addressed that have a direct influence on the detection and subsequent calculation of lysogenic bacteria in the marine environment. For example, we cannot assume that all lysogenic bacteria present in the Tampa Bay estuarine system are inducible by mitomycin C. Although mitomycin C has been proven to be an effective mechanism for inducing prophage in both marine and nonmarine lysogenic systems, other chemical inducing agents such as a polychlorobiphenyl mixture and Aroclor 1248 were more efficient at inducing prophage in the marine environment in a previous study (6). Another problem with the mitomycin C induction method is that it will not detect lysogens that are already induced by natural mechanisms. Finally, if a certain portion of lysogens present in this study were metabolically inactive, we would not have been able to detect induction of prophage by mitomycin C, since induction by this method requires actively growing cells (at least for culturable cells). All of these factors taken together suggest that our estimates of the lysogenic fraction of bacterial populations in Tampa Bay are inherently conservative.
According to our methods of detection, lysogenic bacteria appear to comprise a significant portion of the bacterial populations in Tampa Bay during certain times of the year. Furthermore, the occurrence of lysogeny appears to be under the direct or indirect influence of a variety of environmental and biological parameters such as water temperature, nutrient concentration, primary productivity, and bacterial productivity. Environmental factors such as inorganic nutrient concentration may act as a direct influence on viral replication and/or prophage induction (as in the case of phosphate), or indirectly on the nutritional status of the host cell population, thereby influencing the lysogenic decision.
Acknowledgments
This research was supported by a grant from the National Science Foundation OCE 9811319 and Florida Sea Grant R/LR-MB-3.
REFERENCES
- 1.Ackermann, H. W., and M. S. Dubow. 1987. Viruses of prokaryotes, vol. I. General properties of bacteriophages. CRC Press, Boca Raton, Fla.
- 2.Bratbak, G., J. K. Egge, and M. Heldal. 1993. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar. Ecol. Prog. Ser. 93:39-48. [Google Scholar]
- 3.Carpenter, D. J., and J. S. Lively. 1980. Review of estimates of algal growth using 14C-tracer techniques, p. 161-178. In P. G. Falkowski (ed.), Primary productivity in the sea. Plenum Press, New York, N.Y.
- 4.Chin-Leo, G., and D. L. Kirchman. 1988. Estimating bacterial production in marine waters from the simultaneous incorporation of thymidine and leucine. Appl. Environ. Microbiol. 54:1934-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochran, P. K., and J. H. Paul. 1998. Seasonal abundance of lysogenic bacteria in a subtropical estuary. Appl. Environ. Microbiol. 64:2308-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochran, P. K., C. A. Kellog, and J. H. Paul. 1998. Prophage induction of indigenous marine lysogenic bacteria by environmental pollutants. Mar. Ecol. Prog. Ser. 164:125-133. [Google Scholar]
- 7.Currie, D. J., and J. Kalff. 1984. Can bacteria outcompete phytoplankton for phosphorous? A chemostat test. Microb. Ecol. 10:205-221. [DOI] [PubMed] [Google Scholar]
- 8.Egge, J. K., and D. L. Aksnes. 1992. Silicate as regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 83:281-289. [Google Scholar]
- 9.Gordon, L. I., J. R. Jennings, Jr., A. A. Ross, and J. M. Krest. 1993. A suggested protocol for continuous flow automated analysis of seawater nutrients, p. 1-52. In WOCE Operation Manual, WHP Office Report 90-1, WOCE Report 77 No. 68/91.
- 10.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. Standard method 4500-NH3. American Public Health Association, Washington, D.C.
- 11.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. Standard method 4500-P. American Public Health Association, Washington, D.C.
- 12.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. Standard method 4500-SiF. American Public Health Association, Washington, D.C.
- 13.Hoch, M., and D. L. Kirchman. 1993. Seasonal and inter-annual variability in bacterial production and biomass in a temperate estuary. Mar. Ecol. Prog. Ser. 98:283-295. [Google Scholar]
- 14.Holm-Hansen, O., and B. Riemann. 1978. Chlorophyll a determination: improvements in methodology. Oikos 30:438-448. [Google Scholar]
- 15.Horrigan, S. G., A. Hagstrom, I. Koike, and F. Azam. 1988. Inorganic nitrogen utilization by assemblages of marine bacteria in sea water culture. Mar. Ecol. Prog. Ser. 50:147-150. [Google Scholar]
- 16.Jiang, S. C., and J. H. Paul. 1994. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriogenicity in the marine environment. Mar. Ecol. Prog. Ser. 104:163-172. [Google Scholar]
- 17.Jiang, S. C., and J. H. Paul. 1996. Occurrence of lysogenic bacteria in marine microbial communities as determined by prophage induction. Mar. Ecol. Prog. Ser. 142:27-38. [Google Scholar]
- 18.Kirchman, D. L., E. K'ness, and R. E. Hodson. 1985. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl. Environ. Microbiol. 49:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin, B. R., and R. E. Lenski. 1983. Coevolution in bacteria and their viruses and plasmids. In D. J. Futuyma and M. Slatkin (ed.), Coevolution. Sinauer, Sunderland, Mass.
- 20.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 21.Scanlan, D. J., and W. H. Wilson. 1999. Application of molecular techniques to addressing the role of P as a key effector in marine ecosystems. Hydrobiologia 401:149-175. [Google Scholar]
- 22.Stewart, F. M., and B. R. Levin. 1984. The population biology of bacterial viruses: why be temperate? Theor. Popul. Biol. 26:93-117. [DOI] [PubMed] [Google Scholar]
- 23.Strickland, J. D. H., and T. R. Parsons. 1968. A manual of seawater analysis, 2nd ed. Bull. Fish. Res. Bd. Can. 125:1-311. [Google Scholar]
- 24.Tapper, M. A., and R. E. Hicks. 1998. Temperate viruses and lysogeny in Lake Superior bacterioplankton. Limnol. Oceanogr. 43:95-103. [Google Scholar]
- 25.Tuomi, P., K. M. Fagerbakke, G. Bratbak, and M. Heldal. 1995. Nutritional enrichment of a microbial community: the effects on activity, elemental composition, community structure, and virus production. FEMS Microbiol. Ecol. 16:123-134. [Google Scholar]
- 26.U.S. Environmental Protection Agency. 1993. EPA standard method 353.2. Nitrate-nitrite by automated colorimetry. In U.S. Environmental Protection Agency (ed.), Method for the determination of inorganic substances in environmental samples (EPA/600/R-93/100). U.S. Environmental Protection Agency, Washington, D.C.
- 27.U.S. Environmental Protection Agency. 1993. EPA standard method 365.1. Phosphorous by automated colorimetry. In U.S. Environmental Protection Agency (ed.), Method for the determination of inorganic substances in environmental samples (EPA/600/R-93/100). U.S. Environmental Protection Agency, Washington, D.C.
- 28.Wang, D. H., D. L. Willis, and W. D. Loveland. 1975. Radiotracer methodology in the biological, environmental, and physical sciences. Prentice-Hall, Englewood Cliffs, N.J.
- 29.Weinbauer, M. G., and C. A. Suttle. 1996. Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico. Appl. Environ. Microbiol. 62:4374-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinbauer, M. G., and C. A. Suttle. 1999. Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquat. Microb. Ecol. 18:217-225. [Google Scholar]
- 31.Wheeler, P. A., and D. L. Kirchman. 1986. Utilization of inorganic and organic nitrogen by bacteria in marine systems. Limnol. Oceanogr. 31:998-1009. [Google Scholar]
- 32.White, P. A., J. Kalff, J. B. Rasmussen, and J. M. Gasol. 1991. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb. Ecol. 21:99-118. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox, R. M., and J. A. Fuhrman. 1994. Bacterial viruses in coastal seawater: lytic rather than lysogenic production. Mar. Ecol. Prog. Ser. 114:35-45. [Google Scholar]
- 34.Wilson, W. H., N. G. Carr, and N. H. Mann. 1996. The effect of phosphate status on the kinetics of infection in the oceanic cyanobacterium Synechococcus sp. WH7803. J. Phycol. 32:506-516. [Google Scholar]
- 35.Wilson, W. H., and N. H. Mann. 1997. Lysogenic and lytic production in marine microbial communities. Aquat. Microb. Ecol. 13:95-100. [Google Scholar]
- 36.Wilson, W. H., S. Turner, and N. H. Mann. 1998. Population dynamics of phytoplankton and viruses in a phosphate-limited mesocosm and their effect on DMSP and DMS production. Estuar. Coast. Shelf Sci. 46(Suppl. A):49-59. [Google Scholar]
- 37.Wommack, K. E., and R. R. Colwell. 2000. Viroplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zweifel, U. L., B. Norrman, and A. Hagstrom. 1993. Consumption of dissolved organic carbon by marine bacteria and demand for inorganic nutrients. Mar. Ecol. Prog. Ser. 101:23-32. [Google Scholar]