Abstract
Meiotic crossovers promote correct chromosome segregation and the shuffling of genetic diversity. However, the measurement of crossovers remains challenging, impeding our ability to decipher the molecular mechanisms that are necessary for their formation and regulation. Here we demonstrate a novel repurposing of the single-nucleus Assay for Transposase Accessible Chromatin with sequencing (snATAC-seq) as a simple and high-throughput method to identify and characterize meiotic crossovers from haploid testis nuclei. We first validate the feasibility of obtaining genome-wide coverage from snATAC-seq by using ATAC-seq on bulk haploid mouse testis nuclei, ensuring adequate variant detection for haplotyping. Subsequently, we adapt droplet-based snATAC-seq for crossover detection, revealing >25 000 crossovers in F1 hybrid mice. Comparison between the wild type and a hyper-recombinogenic Fancm-deficient mutant mouse model confirmed an increase in crossover rates in this genotype, however with a distribution which was unchanged. We also find that regions with the highest rate of crossover formation are enriched for PRDM9. Our findings demonstrate the utility of snATAC-seq as a robust and scalable tool for high-throughput crossover detection, offering insights into meiotic crossover dynamics and elucidating the underlying molecular mechanisms. It is possible that the research presented here with snATAC-seq of haploid post-meiotic nuclei could be extended into fertility-related diagnostics.
Introduction
Meiosis is a specialized cell division which is necessary for the formation of haploid gametes in sexually reproducing species. Meiosis halves ploidy through one round of DNA replication, being followed by two consecutive rounds of chromosome division. The first round of chromosome segregation (meiosis I) is a reductional division, where homologues are pulled to opposite poles. The second round of chromosome segregation (meiosis II) is an equational division where sister chromatids are pulled to opposite poles [1]. At the onset of meiosis, hundreds of DNA double-strand breaks (DSBs) are formed [2]. Homologous recombination is used to repair the DSBs as either crossovers or non-crossovers [1–4]. Meiotic crossovers are large reciprocal exchanges between homologous chromosomes. For chromosomes to segregate correctly at the first meiotic division, in most species, all homologue pairs must have at least one ‘obligate’ crossover. Loss of the obligate crossover leads to aneuploid gametes and a reduction in fertility [1]. Aneuploid gametes used in fertilization can lead to aneuploid karyotypes, such as trisomy 21 in humans [5–7]. The obligate crossover is linked to a phenomenon known as crossover interference, where the occurrence of one crossover reduces the probability of another crossover on the same chromosome in the same meiosis in a distance-dependent manner [1, 8].
Assaying for genome-wide crossovers remains challenging, despite more than a century since the discovery of meiotic crossing over in Drosophila and how it informs on physical chromosome structure and enables genetic map construction [9, 10]. These detection challenges mostly relate to the need for generating and genotyping pedigrees that require at least three generations and many offspring, and this is assuming that crosses can be controlled in the species of interest. The challenges for studying crossover formation mechanisms still exist, even for well-studied species such as mice. However, with the growing flexibility in the species that can be studied with genomic tools, due to the reduction in cost for de novo genome sequencing and assembly, researchers who wish to use these species for studies of meiosis would benefit from new tools that can assess genome-wide crossover distributions with greater ease.
Reporter assays continue to play an essential role in deciphering the mechanisms of DSB repair and crossover formation. Some examples include assays using linked phenotypic markers, targeted recombination reporters, and tetrad analysis across an array of species [9, 11–16]. These assays can allow quantitative analysis of a variety of parameters at a single locus or adjacent loci, e.g. formation of DSBs, crossovers, and non-crossovers, and repair on sister chromatids. A strength of these methods is their reproducibility once established and potential for comparisons across experimental conditions. On the other hand, cytological analysis of DSB and crossover formation informs on chromosome-scale and genome-wide events, assuming a species is conducive to cytology. However, these methods, while providing rich and important data, tend to be more labour intense, particularly for quantification of events being scored.
Whole-genome single-gamete sequencing provides the opportunity to combine the advantages of different styles of assays for crossover detection and quantification. Single-gamete sequencing for genome-wide crossover analysis can reduce the number of generations required to access the recombined products of meiosis. Interest in understanding crossover regulation has driven the adaptation of various technologies to sequence individual gametes [17–23] and the development of methods for analysis of the data [24, 25]. Notably, droplet-based gamete sequencing methods are a powerful tool for investigating genome-wide crossover formation, because despite the low level of coverage typically obtained per cell, the coverage is sufficient to determine haplotypes [21, 24, 26, 27]. However, despite advances in single-gamete sequencing approaches, some of the specialized kits used in previous studies have been discontinued, which results in established methods becoming unavailable. We therefore sought to establish a method that we consider should remain accessible for longer or could be re-established with in-house reagents in the case of product discontinuation. The Assay for Transposase Accessible Chromatin using sequencing (ATAC-seq) was developed for profiling chromatin accessibility at a genome-wide scale [28]. This method of DNA library preparation could be adapted for single-nucleus gamete sequencing for crossover detection applications, because it generates reads that map genome-wide —in some cell lines and tissues—and has already been used for single-cell sequencing assays [29]. Further, open-source methods for Tn5 expression and purification are well established which can facilitate in-house development of ATAC-based methods. However, the extent to which gametes can be sequenced with ATAC-based methods is less clear given the different chromatin structure—which is protamine rich—in mammalian sperm compared with somatic tissue.
We demonstrate here that single-nuclues ATAC-seq (snATAC-seq) libraries can be used to sequence genomic DNA for crossover quantification and analyses. Using hybrid mice (C57BL/6J × FVB/N), we isolated haploid testis nuclei, henceforth referred to as haploid nuclei, by fluorescence-activated cell sorting (FACS) based on DNA content and prepared libraries using modified snATAC-seq methods. We obtained high-quality libraries which had ample coverage for variant calling, phasing, and crossover detection. Further, the number of high-quality cells recovered was far greater than other methods that we have used (Tsui et al. [26]). Using an established hyper-crossover mouse model, we could also demonstrate that this robust method can detect changes in crossover frequencies, opening up the scope for other applications in research and diagnostics.
Materials and methods
Animals
All animal experiments were approved by the Animal Ethics Committees at St Vincent's Hospital Melbourne and conducted in accordance with Australian NHMRC Guidelines on Ethics in Animal Experimentation. All mice were housed at the BioResources Centre, St. Vincent's Hospital, in a controlled environment with a 12 h light/dark cycle, and with food and water provided ad libitum. The FancmΔ2/Δ2 mice used in this study were previously described [26].
Isolation of haploid nuclei
Haploid nuclei were isolated from testicular single-nucleus suspensions and enriched for haploids using FACS as previously described [30]. Testes were dissected from C57BL/6J × FVB/N hybrid males, aged between 10 and 14 weeks of age at the time of tissue collection. Tissues were processed immediately for isolation of nuclei.
Briefly, testes were dissected out from adult mice and placed in 1 ml of chilled Nuclei EZ Lysis Buffer (Sigma). The testes were gently squeezed with pointed tweezers to releases seminiferous tubules into the solution. Simultaneously, the spleen was also dissected and homogenized with 2 ml of Dulbecco's phosphate-buffered saline (DBPS) before being passed through a 40 μm strainer; this sample served as a diploid control for flow cytometry. A 300 μl aliquot of splenic homogenate was added to 1 ml of Nuclei EZ Lysis Buffer and both the testis and splenic samples were incubated for 5 min on ice, with mixing by gentle inversion after 3 min. To avoid somatic contaminant, 600 μl of the testis suspension was transferred to a fresh 1.5 ml Lo-Bind microcentrifuge tube. Suspensions were centrifuged at 500 g for 5 min at 4°C. The supernatant was removed, and the pellet was resuspended with 1 ml of Nuclei EZ Lysis Buffer. After a 5 min incubation on ice, the samples were again centrifuged at 500 g for 5 min at 4°C. Without disrupting the pellet, 1 ml of Nuclei Wash and Resuspension Buffer [NWRB; DPBS with 1% (v/v) bovine serum albumin (BSA)] was slowly added and allowed to buffer exchange for 5 min on ice. The pellet was resuspended by gentle pipetting up and down 10 times using a 1 ml wide-bore pipette tip. Samples were centrifuged at 500 g for 5 min at 4°C, and most of the supernatant was removed, leaving behind just enough to cover the pellet. Each sample was resuspended with 300 μl of NWRB supplemented with 4′,6-diamidino-2-phenylindole (DAPI; 10 μg/μl), and then filtered using a 40 μm Flowmi Cell Strainer (Bel-Art) into a 5 ml polypropylene tube. Samples were FACS sorted, and haploid nuclei (peaks with DAPI content half that of diploid control peaks) were collected.
Bulk ATAC-sequencing of haploid sperm nuclei
Bulk tagmentation was performed using either commercial pre-assembled Tn5 transposase (Diagenode) or home-made Tn5 transposases produced and assembled using previously published methods [31] (Supplementary Fig. S1).
Libraries were prepared using previously published methods [32] with minor modifications. Approximately 50 000 haploid nuclei were FACS sorted into a 5 ml polypropylene tube containing 300 μl of NWRB with 0.1% (w/v) BSA. Nuclei were centrifuged at 500 g for 5 min at 4°C and the supernatant was aspirated. Without disrupting the pellet, 1 ml of OMNI-ATAC RSB buffer (10 mM Tris–HCl pH 8.0, 10 mM NaCl, 3 mM MgCl2) was slowly added and the sample was incubated for 5 min, then resuspended gently using a wide-bore tip. Nuclei were centrifuged at 500 g for 5 min at 4°C and the supernatant was aspirated. Nuclei were resuspended in 100 μl of chilled OMNI-ATAC RSB buffer containing 0.1% (v/v) Tween-20, 0.1% (v/v) IGEPAL, and 0.01% (v/v) digitonin, and incubated on ice for 2 min. Nuclei were centrifuged again at 500 g for 5 min at 4°C and the supernatant was aspirated. Nuclei were resuspended in 50 μl of freshly prepared transposition mix [33 mM Tris–HCl pH 8.0, 66 mM potassium acetate, 10 mM magnesium acetate, 15% (v/v) N,N-dimethylformamide, 0.01% (v/v) digitonin, 2.5 μl of Tn5-assembled enzyme] and mixed by pipetting up and down 10 times. Transposition reactions were incubated at 37°C for 45 min in a thermocycler with gentle mixing by tapping the tube at 5 min intervals. The transposition reaction was stopped with 50 μl of 2× ATAC-STOP buffer (10 mM Tris–HCl pH 8.0, 20 mM EDTA). Afterwards, the samples were purified using a Monarch Nuclei Acid Purification Kit (NEB) as per the manufacturer's instructions and eluted in 20 μl of nuclease-free water. For the Tn5-based library preparation, oligonucleotides containing unique barcodes were used for sample indexing, as described previously [33]. The following sequences (5′–3′) were used (barcodes in bold):
CAAGCAGAAGACGGCATACGAGATTCGCCTTAGTCTCGTGGGCTCGGAGATGT,
CAAGCAGAAGACGGCATACGAGATCTAGTACGGTCTCGTGGGCTCGGAGATGT,
CAAGCAGAAGACGGCATACGAGATTTCTGCCTGTCTCGTGGGCTCGGAGATGT,
CAAGCAGAAGACGGCATACGAGATGCTCAGGAGTCTCGTGGGCTCGGAGATGT.
Single-haploid nuclei ATAC-seq library preparation and sequencing
Preparation of single-haploid nuclei ATAC-seq libraries was performed with modifications to our previously published protocol [30]. Briefly, for each snATAC-seq library, haploid nuclei were FACS sorted into a 5 ml polypropylene tube containing 300 μl of NWRB with 0.1% (w/v) BSA. Nuclei were centrifuged at 500 g for 5 min at 4°C and the supernatant was aspirated. Nuclei were resuspended in NWRB with 0.1% (w/v) BSA supplemented with 0.01% (v/v) digitonin and incubated on ice for 5 min. Nuclei were centrifuged again at 500 g for 5 min at 4°C and the supernatant was aspirated. A 500 μl aliquot of diluted nuclei buffer (10x Genomics) was slowly added to the nuclei without disrupting the pellet and allowed to buffer exchange for 5 min on ice. The nuclei were resuspended in 10 μl of diluted nuclei buffer (10x Genomics) and quantified using a haemocytometer. The concentration of nuclei was adjusted to attain a capture number of ∼1000 nuclei, accounting for loss during subsequent steps. Prepared nuclei were added to the Transposition Mix (10x Genomics), and transposition, library preparation, and clean-up for snATAC-seq were performed according to the manufacturer’s protocol using the Chromium Single-Cell ATAC Kit v1.1 (10x Genomics). Each library was sequenced on the NovaSeq 6000 (Illumina) using a 50/8/16/50 bp configuration (Read 1/Index 1/Index 2/Read2).
Data processing
Bulk ATAC-sequencing fastq files were trimmed using cutadapt (v3.4) [34] and mapped to the mm39 reference genome using bwa-mem with default settings [35]. Duplicate reads were removed using MarkDuplicates (picard tools).
For snATAC-seq, fastq files were processed using Cell Ranger ATAC (v2.1.0; 10x Genomics) to align reads to the mm39 reference genome. Outputs from Cell Ranger were used in downstream analysis.
Sequencing coverage analysis
Coverage files for bulk and snATAC experiments were generated from BAM files using samtools depth [36]. Data were summarized in 10 kb bins based on chromosome lengths from mm39. Genomic tracks were visualized with Gviz, with gene annotations added from the UCSC genome browser [37].
Single-nucleus ATAC-sequencing analysis with Signac and Seurat
The outputs from Cell Ranger containing peak and fragment information were used to generate coverage estimates of the snATAC-seq data by identifying a set of common peaks across all samples, indicative of open chromatin regions. These peaks were called by aggregating accessibility signals across all filtered nuclei, and were primarily used for quality control metrics such as percentage of reads in peaks and transcription start site (TSS) enrichment scores, rather than for biological interpretation.
Barcodes with <500 fragments per cell were filtered out. Fragment counts were then normalized using term-frequency inverse-document-frequency (TF-IDF) to result in the logarithmic ratio of the total number of reads per cell, relative to total cells.
The normalized data were used to assess coverage in specified genetic regions, as well as to assess various quality control (QC) metrics, including: TSS enrichment scores, fragment counts (nCounts), nucleosome signal, and percentage of reads in peaks. Dimensionality reduction was performed using singular value decomposition (SVD), and uniform manifold approximation and projection (UMAP) plots were generated to visualize clusters, with latent semantic indexing (LSI) applied for data reduction. Spare clusters were filtered, and UMAP plots were separated into sequencing batches. Gene activities were annotated using the Ensembl database and the mm39 reference genome. Cluster-specific markers with positive fold changes between clusters were identified, and Enrichr was used to identify putative cell types based on the top differentially accessible genes.
Single-nucleus ATAC-sequencing crossover calling with sgcocaller and comapr
Crossover calling was conducted on the Cell Ranger BAM output file using sgcocaller [24], with the following parameters: –cmPmb 0.1 –maxDP 20 –maxTotalDP 450 –minTotalDP 0 –minDP 0 –thetaREF 0.2 –thetaALT 0.8. FVB/N-specific variants differing from the mm10 reference genome were obtained from the Mouse Genome Project (FVB_NJ.mgp.v5.snps.dbSNP142.vcf) and lifted over for compatibility with the mm39 reference genome.
The outputs from Cell Ranger were filtered to generate a list of barcodes from intact cells with >10 000 reads. To refine crossover calls, the filtered barcode file and output of sgcocaller were processed utilizing comapr [24], generating genetic distance maps with the Kosambi mapping function, and identifying inter-crossover distances. The following thresholds were set in comapr: minSNP = 10, minCellSNP = 100, maxRawCO = 5, minLogllRatio = 30, bpDist = 1e5. Artefacts with double crossovers occurring within 3 bp were removed from the analysis.
Marker segregation for single-nucleus ATAC-sequencing
Segregation states of single nucleotide polymorphisms (SNPs) were analysed in 10 Mb bins, based on chromosome lengths from the mm39 reference genome, using output files generated by sgcocaller. SNP positions and their underlying segregation states were used to calculate the ratio of C57BL/6J and FVB/N genetics, where 1 indicated FVB/N and 0 indicated C57BL/6J. Haplotype ratios were computed, and binomial distribution tests were performed to assess bias in marker segregation. Telomeric regions were removed due to low coverage, which resulted in inaccurate state imputation.
Permutation tests and generation of null distributions
Permutation tests using label swapping and null distribution generation were conducted following previously described methods [26]. Briefly, to generate the null distribution of inter-crossover distances, the positions of the second crossovers were randomly shuffled within chromosomes, preserving their original widths. The positions of the first crossovers remained fixed to obtain the null distribution. This permutation was conducted 1000 times for each chromosome, using parallel processing. The null distribution was plotted using absolute values. The observed and permuted inter-distances between crossovers one and two were compared. Comparative analysis was between snATAC, Tsui et al. [26], and Hinch et al.[19] single-cell datasets.
The fastq files from both datasets [available from NCBI’s Sequence Read Archive (SRA)] were aligned to the mouse reference genome mm10. The data from Hinch et al. [19] were aligned using minimap2 v2.7 with options –ax sr. GATK v4.0 was then used to mark duplicates and add readgroups, before sorting with samtools. Prior to merging reads from each sperm with samtools, the cell barcode tags were appended to each DNA read using appendCB [24]. Informative SNP markers, differing between BL6 and CAST, were identified as described previously [19] and downloaded from the Mouse Genome Project. Informative SNPs were used to call crossovers with sgcocaller, which was run on a final bam file containing tagged and merged DNA reads from each sperm. The single-cell dataset of Tsui et al. was demultiplexed and aligned using cellranger-dna cnv (v1.1.0) [26]. Informative SNPs differing between C57BL/6J and FVB/N were downloaded from the Mouse Genome Project. The informative SNPs were used for crossover calling, using sgcocaller. Crossover refining was conducted as described above, using comapr, with total crossover counts and genetic distance calculated using the Kosambi mapping function. Nuclei counts and crossover proportions were calculated using individual cell barcodes and the associated number of crossovers.
Comparisons of crossover genomic locations between datasets were conducted first by lifting over the mm10 variant positions to the mm39 genome positions, obtained from UCSC. Overlapping positions were determined using GRanges in R, and the signficance of the relationship between overlapping positions for each dataset was calculated by shuffling the positions of one dataset across the genome and comparing the relationship of the other dataset. A two-tailed P-value was calculated from the permutation test. To compare the overall degree of overlapping positions, a Jaccard index was calculated by comparing the genomic positions with a randomly generated dataset, based on the mm39 genome size.
Chromatin immunoprecipitation-sequencing compared with crossover analysis
C57BL/6 PRDM9 chromatin immunoprecipitation-sequencing (ChIP-seq) data [38] were downloaded from NCBI’s SRA, using the SRA toolkit. ChIP-seq reads were aligned to the mm39 reference genome using bowtie2 [39], and filtered to remove low-quality reads and duplicates using samtools (fixmate, sort, and markdup [36]). PRDM9 knockout samples were excluded from wild-type analysis, and ChIP-seq peaks were called using MACS2 [40].
Crossover positions were derived from the snATAC-seq data, processed using sgcocaller and comapr. Overlapping and ChIP-seq and crossover positions were identified using the findOverlaps function from GenomicRanges [41]. Statistical comparisons were conducted using one-sided Wilcoxon signed-rank tests, and as permutation tests, comparing observed ChIP-seq signals with the null distribution.
Results
Bulk ATAC-sequencing libraries using haploid nuclei produce genome-wide coverage
ATAC-seq assays use the enzymatic activity of Tn5 to generate DNA libraries for high-throughput sequencing. Tn5 simultaneously cuts accessible regions of double-stranded DNA while ligating sequencing adapters compatible with high-throughput whole-genome sequencing platforms [28, 42, 43]. However, chromatin in post-meiotic haploid nuclei is particularly condensed and protamine rich [44, 45], and it was unclear if libraries could generate read coverage suitable for haplotyping and crossover measurement. Therefore, we first sought to test if sufficient coverage of DNA from haploid nuclei could be obtained in bulk ATAC-seq samples to justify using ATAC-seq with single haploid nuclei.
We first performed ATAC-seq by isolating 50 000 bulk-sorted haploid nuclei extracted from F1 (C57BL/6J × FVB/N) hybrid mice, using our SSNIP-seq protocol [30] for isolation of high-quality nuclei. We expressed and purified Tn5 (Supplementary Fig. S1) using a plasmid previously described [31] and then generated tagmented sequencing libraries using our home-made and commercially available hyperactive Tn5 enzymes. Our sequencing libraries served as a proof-of-concept, demonstrating that genome-wide sequencing coverage derived from haploid nuclei is attainable via ATAC-sequencing.
Our bulk ATAC-sequencing libraries were used to generate ∼152 × 106 to 189 × 106 paired-end reads, with an average coverage of ∼6–7× across the mouse genome (Table 1). Our libraries exhibited minimal GC bias and covered 42–50% of the informative SNPs between C57BL/6J and FVB/N backgrounds (Table 1), which we used for haplotyping and crossover detection previously [26]. While our ATAC-sequencing data do not map homogenously throughout the genome at nucleotide resolution (Table 1; Fig. 1A–C)—as is expected with ATAC-seq—with the high number of SNPs in the genome we predicted it would be sufficient for haplotyping and crossover calling. Our prediction was based on the observation that when considering ‘bins’ throughout the genome (Fig. 1A–C), reads map relatively homogenously to broader regions, even if at the resolution of a gene there is skewing of reads towards regulatory elements and exons (Fig. 1C). We therefore proceeded to test and repurpose single-gamete ATAC-sequencing methods for meiotic crossover detection.
Table 1.
Bulk sequencing results from haploid nuclear DNA prepared with Tn5
| Tn5 source | Genotype | Total mapped read pairs | Read length (bp) | Average genome-wide coverage | %GC | Proportion of informative SNPs with at least one mapped read |
|---|---|---|---|---|---|---|
| Home-made | Fancm +/+ | 188 998 455 | 150 | 6.7 | 49 | 49.7% |
| Commercial | Fancm +/+ | 151 991 673 | 150 | 5.5 | 50 | 41.5% |
| Home-made | Fancm Δ2/Δ2 | 179 633 072 | 150 | 6.1 | 51 | 45.4% |
| Commercial | Fancm Δ2/Δ2 | 162 885 127 | 150 | 5.8 | 51 | 41.5% |
Sequence output metrics are provided for bulk ATAC-seq libraries produced from haploid sperm DNA.
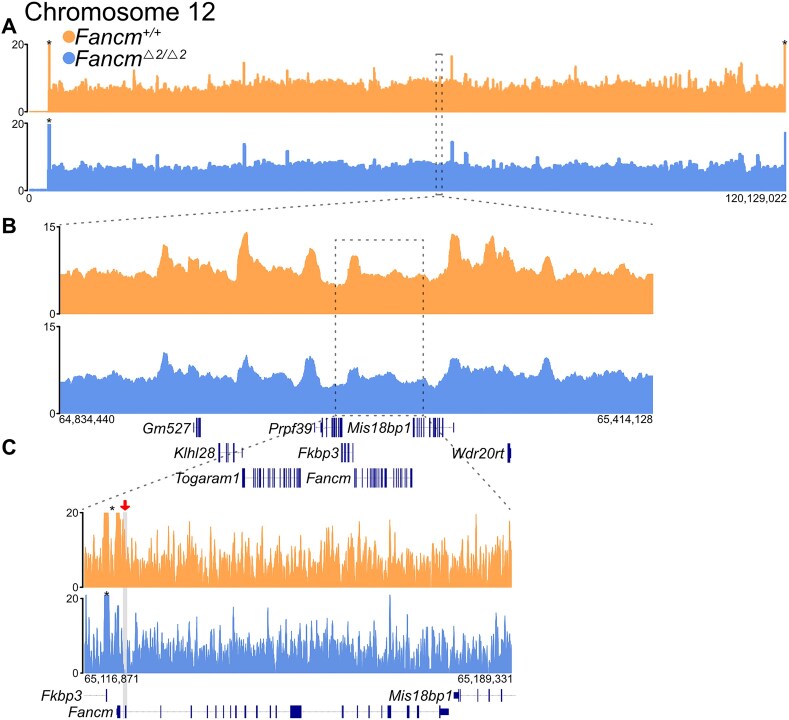
Figure 1.
Bulk sequencing of tagmented haploid nuclei generates fragments that map broadly with genome-wide coverage. Libraries were generated by Tn5 fragmentation (home-made Tn5, shown above) of haploid nuclei from wild-type and mutant F1 hybrid mouse testis, with reads aligned to the mouse reference genome (mm39). Tracks show reads from wild-type (orange) and mutant (blue) samples, aligned to (A) the length of chromosome 12 (64 kb bins). (B) A zoomed-in ∼580 kb region (chr12: 64 834 440–65 414 128; 10 kb bins). (C) A zoomed-in ∼72 kb region (chr12: 65 116 871–65 189 331; 100 bp bins). The grey box highlights the locus of exon 2 of Fancm; indicated by a red arrow for clarity, showing the absence of aligned reads in the mutant dataset at this locus. *Maximum displayed coverage threshold set to 20.
Single-nucleus ATAC-sequencing library generation, pre-processing, and filtering
To assay meiotic crossovers with high throughput, we generated snATAC-seq libraries (10x Genomics) using FACS-sorted haploid nuclei isolated from six F1 hybrid male mice; three F1.Fancm+/+ and three F1.FancmΔ2/Δ2. Coverage estimates of the snATAC data were performed through assessing the number of fragments at common regions of open chromatin across samples. Read coverage showed that the snATAC-seq data from haploid nuclei had relatively homogeneous coverage across bins of the genome (Fig. 2A), replicating the genome-wide coverage observed in the bulk ATAC-seq data (Fig. 1; Table 1).
Figure 2.
Quality control and validation of the haploid sperm snATAC-seq libraries. (A) Comparison of genome coverage across an ∼60 kb window of chromosome 12 (chr12: 65 120 000– 65 180 000) from each mouse single-gamete library. The locus contains Fancm, with no reads mapping to exon 2 of the F1.FancmΔ2/Δ2, which carry biallelic deletions (grey) of exon 2. (B) Scatter plot of snATAC-seq data displaying QC metrics (unique fragments per cell and TSS enrichment). Low TSS enrichment highlights the condensed nature of haploid post-meiotic chromatin. (C) Violin plot showing the percentage of reads that cluster into peaks for each wild-type and mutant replicate. Three replicates (wild-type/mutant pairs) were sequenced across two sequencing runs (batch 1 and 2). (D) UMAP visualizations of snATAC-seq of haploid nuclei, from sequencing batches 1 and 2, showing cells grouped in nine clusters. (E) UMAP of snATAC data for wild-type and mutant samples, post-filtering.
Given that chromatin in haploid nuclei is more condensed than in other cell types, bioinformatic tools Signac (v1.13.0) [46] and Seurat (v5.0.3) [47] were employed to assess chromatin accessibility and evaluate the quality of the dataset. We calculated the number of fragments per cell, which provided a measure of chromatin accessibility for each cell in the sample group. Additionally, TSS enrichment scores were computed to indicate the accessibility of chromatin near TSS regions. These metrics were visualized using a density scatter plot (Fig. 2B). The relative number of counts per cell aligns with the known inaccessibility of sperm chromatin [45]. snATAC-seq libraries were produced from three biological replicates of each genotype (F1.Fancm+/+ and F1.FancmΔ2/Δ2) in two batches and sequenced in multiple runs (Fig. 2C–E). When assessing the percentage of reads in peaks (Fig. 2C), the data segregated into two groups, indicating possible batch effects; however, this does not affect the ability to detect crossovers (below). When exploring the overall structure of the data in a UMAP plot, the samples from the first sequencing batch appeared to have an increased number of artefacts, as indicated by the large number of smaller clusters consisting of a few cells (Fig. 2D). Artefactual cells were removed, and the top differentially accessible genes were identified from each cluster, for each genotype. When comparing wild-type and mutant samples from the filtered dataset, gene set enrichment analysis (see the Materials and methods) indicated that cluster 0 corresponds to late-stage spermatocytes, while cluster 1 represents early-stage spermatocytes (Fig. 2E). These annotations are based on gene set enrichment for chromatin accessibility and should be interpreted with caution and as a relative guide of spermatocyte differentiation, particularly because snATAC-seq data may not be representative of cell type to the extent of snRNA-seq.
Cluster 2 could not be confidently annotated, indicating that it may consist of contamination, unfiltered artefacts, or an uncharacterized cell state. However, without single-cell RNA-sequencing or cytological analysis, confirming the exact identity of this cluster remains challenging. Despite this ambiguity, the results confirm the correct cell types were sequenced with minimal contamination, making the data suitable for further processing and crossover calling analysis (Supplementary Fig. S2). Together, these filtering steps allow us to focus on the analysis of confidently identified haploid nuclei, improving data quality for downstream crossover detection.
The single haploid sequencing library was filtered for barcodes which were present with >10 000 high-quality fragments, and we excluded likely doublets as well as cells in which one or more chromosomes could not be confidently detected. The final dataset included >3800 haploid genomes from Fancm+/+ and FancmΔ2/Δ2 samples (Table 2; Supplementary Table S1). Each cell was sequenced to an average depth of 0.01× of the haploid genome, covering ∼30 × 106 bp per cell on average. About 0.65% of the heterozygous SNPs between the two mouse strains had at least one mapped read, which is sufficient for haplotyping (Table 2). To assess alignment bias and sequencing bias, we analysed allelic segregation ratios and found that throughout the genome most loci segregated very close to a ratio of 1:1 in the three F1.Fancm+/+ and three F1.FancmΔ2/Δ2 samples (Fig. 3). Some telomeric skewing remains on a limited number of chromosomes after filtering repetitive regions, typically representing a mapping bias towards the reference genome.
Table 2.
Summary of crossovers detected from ATAC-sequencing of single haploid nuclei
| Genotype | Cellsa | Single crossovers | Double crossovers | >2 crossovers | Total crossovers |
|---|---|---|---|---|---|
| Fancm +/+ | 2251 | 14 778 | 1402 | 63 | 16 243 |
| Fancm Δ2/Δ2 | 1581 | 10 496 | 1617 | 104 | 12 217 |
Data were summarized from ATAC-sequencing results. Total numbers of crossoverss from Fancm+/+ and FancmΔ2/Δ2 samples were broken down into chromosomes with single, double, and >2 crossovers.
aIndividual gametes were identified and filtered for a unique barcode which was represented by >10 000 high-quality fragments (see the Materials and methods).
Figure 3.
Marker segregation for the 19 autosomal chromosomes in Fancm wild-type and mutant snATAC-seq data. Genome-wide patterns of marker segregation from gametes produced by an F1(C57BL/6J × FVB/N) mouse with two haplotypes were calculated in chromosome bins (of size 10 Mb) and found to match Mendelian segregation expectations, except for subtelomeric regions (excluded from analysis due to mapping biases in repetitive regions). Hypothesis testing using a binomial test was performed to evaluate if marker segregation ratios differ from 0.5; no significant differences were observed in any chromosomes. The y-axis units of the haplotype state ratio represent FVB/N or C57BL/6J ratios for given genomic bins, ‘1’ represents the alternative allele, which is FVB/N, and ‘0’ represents the reference allele C57BL/6J. Most genomic regions have a haplotype state ratio close to 0.5, which is consistent with chromosome segregation showing expected Mendelian ratios.
Meiotic crossover detection with single-nucleus ATAC-sequencing libraries
Using sgcocaller and comapr [24], we identified > 25 000 meiotic crossovers within our combined snATAC-seq samples. We found that crossover detection was robust and was not affected by coverage within the ranges of read depth in our samples (Fig. 4).
Figure 4.
Analysis of coverage as a function of genetic distance [centiMorgans (cM)] reveals a robust assay for crossover detection. Coverage is defined as reads per million (RPM) mapped reads per sample. We find no significant correlation between genetic distance and coverage for both wild-type and mutant F1 mouse samples using snATAC-seq, in 10 kb bins.
Notably, the average number of crossovers inferred per haploid nucleus was higher in F1.FancmΔ2/Δ2 samples compared with F1.Fancm+/+ controls, with a median of 12 and 10 crossovers identified per haploid nucleus, respectively (two-sided Wilcoxon signed rank test with continuity correction, P< 2.2 × 10−16; Fig. 5A), and the genetic map lengths were significantly longer in mutant (1172.4 cM) than wild-type (1015.2 cM) samples (two-sided Wilcoxon signed rank test with continuity correction, P< 2.2 × 10−16; Fig. 5B). These findings align with our previous work indicating that Fancm acts as a meiotic crossover suppressor in mammals [26]. Most individual chromosomes had genetic lengths of at least 50 cM, indicating good variant detection along the length of each chromosome (Fig. 5C). The differences in genetic distance found in Fancm mice were observed along the length of individual chromosomes in bins of 10 Mb (Supplementary Table S2). Crossover numbers were elevated in mutant mice at a chromosome level, with adjusted P-values >0.01 in all chromosomes except 4, 5, 9, 15, and 19, as shown by both Kruskal–Wallis and permutation testing with false discovery rate (FDR) correction (Supplementary Table S2). Although each chromosome had increased crossovers in the mutant mice, the general crossover distribution—at any given locus—remained comparable between both genotypes, with no significant difference between the 10 kb bins (permutation testing with FDR correction, P> 0.05; Fig. 5D). These results are consistent with our previous findings using single-nucleus, bulk sequencing, and PCR-based methods for crossover detection [26], suggesting that ATAC-seq provides a robust tool for high-throughput crossover detection.
Figure 5.
snATAC-seq profiling of meiotic crossovers. Crossovers were assayed using snATAC-seq of isolated haploid mouse nuclei. (A) Distribution of crossover frequency assayed per haploid nucleus (n = 3 animals per genotype). The boxplot represents the median, upper, and lower quartile; whiskers represent the lowest and highest values within the 1.5 interquartile range. (B) Recombination rates measured for F1.Fancm+/+ and F1.FancmΔ2/Δ2 samples. Observed crossover fractions were converted into genetic distances (cM) via the Kosambi mapping function and presented as cumulative cM across the genome. (C) Average recombination frequency (cM) per chromosome in mutant and wild-type samples. Individual replicate values are provided in Supplementary Table S2. (D) Recombination rates (in cM) measured per 10 kb window along each chromosome position (M, megabases) for Fancm+/+ (top) and FancmΔ2/Δ2 (bottom, flipped) autosomes reveal the increased crossover rate in mutant mice.
Comparative analysis of single-nucleus ATAC-sequencing data with published methods
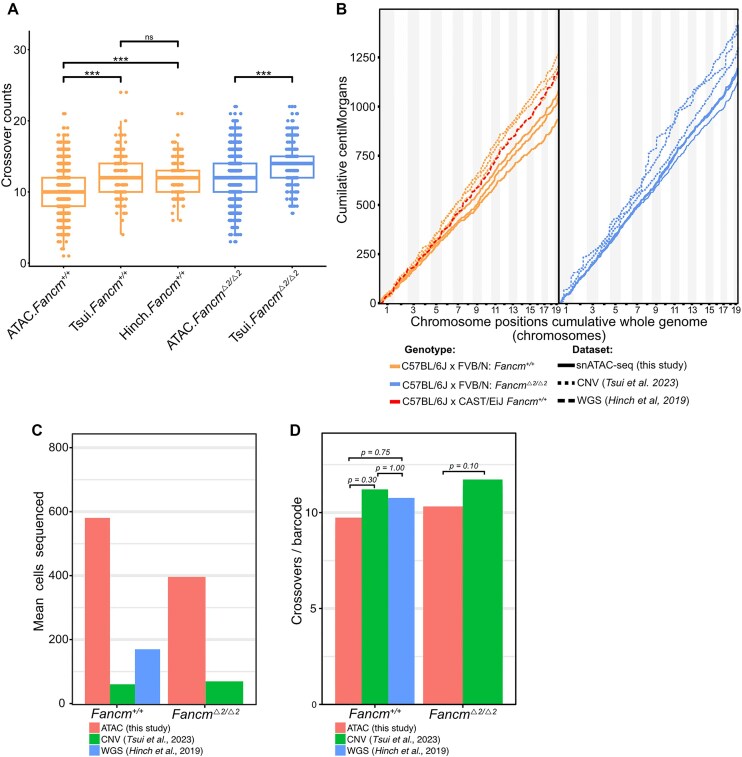
To evaluate how our data align with previous findings, we compared the snATAC-seq crossover data with previously published single-cell sperm datasets from Tsui et al. [26] and Hinch et al. [19]. Tsui et al. sequenced sperm from F1 mice of C57BL/6J × FVB/N origin using single-cell copy number variation (CNV) sequencing technology from 10x Genomics, while Hinch et al. [19] used single-cell whole-genome sequencing of sperm from F1 mice carrying wild-type or humanized PRDM9 alleles, derived from a cross between C57BL/6J and CAST/EiJ strains.
We re-analysed both datasets using the same sgcocaller and comapr pipeline applied to snATAC data, enabling direct comparison across datasets. Total crossover counts and genetic distance were assessed in each dataset. The snATAC-seq dataset had 28 458 crossovers, compared with 1862 in Hinch et al. and 4502 in Tsui et al. (Fig. 6A). The total genetic distance from sequenced crossovers was significantly lower in the snATAC-seq data compared with both the reference datasets [pairwise Wilcoxon test with Benjamini–Hochberg (BH) correction, P < 2 × 10−7; Fig. 6B]. This probably reflects the higher number of sequenced haploid nuclei that passed filtering in the snATAC-seq dataset (2949 cells), compared with 173 in Hinch et al. and 408 in Tsui et al. (Fig. 6C; Supplementary Table S3). Given similar per-cell sequencing coverage, each haploid cell in the snATAC-seq dataset would have had fewer SNPs sequenced, leading to lower genetic distance recovered per cell.
Figure 6.
Comparative analysis of crossover calling from different single-cell sequencing methodologies. Crossovers called from the snATAC-seq data were compared with single-cell sequencing datasets from Tsui et al. [26] and Hinch et al. [19]. The snATAC and Tsui et al. datasets both contained F1.FancmΔ2/Δ2 and F1.Fancm+/+ (n = 3) of C57BL/6J × FVB/N origin. The Hinch et al. dataset contains an F1.Fancm+/+ mouse of C57BL/6J and CAST/EiJ strains (n = 1). (A) The total crossovers called from each dataset, per single cell. The boxplot represents the median, upper, and lower quartile; whiskers represent the lowest and highest values within the 1.5 interquartile range. (B) The cumulative genetic distances (cM) calculated via the Kosambi mapping function, representing recombination rates for crossovers from all datasets. (C) The mean number of cells sequenced per sample is shown for each respective study. (D) The number of crossovers detected per barcode is shown for the respective genotypes and studies.
When assessing the number of crossovers as a proportion of haploid cells sequenced for each dataset, no significant difference was detected across the datasets (pairwise Wilcoxon test with BH correction, P > 0.05; Fig. 6D). To assess whether crossover locations overlapped across datasets, we performed permutation testing. We compared overlaps between snATAC-seq and CNV data from Tsui et al., as well as between the snATAC-seq and data from Hinch et al. To test if the overlapping regions exceeded what would be expected by chance, genomic coordinates from one dataset were randomly shuffled 1000 times while preserving crossover distance, and the number of overlapping regions was calculated. Two-tailed P-values from these tests indicated significant overlaps from both comparisons: P = 0.002 for snATAC versus Tsui et al., and P = 0.0286 for snATAC versus Hinch et al.
These results indicate that the crossovers which overlap do so significantly more than would be expected by random chance. However, this result does not test for the overall number of crossovers which overlap. To assess the overall degree of overlapping genomic positions, the Jaccard index was used, which showed non-significant crossover overlap between the snATAC-seq and the Hinch et al. dataset (P = 1), but significant overlap between snATAC and Tsui et al. (P = 0.01). These results suggest that the genomic location of crossovers in the snATAC-seq and CNV datasets are highly similar. This is expected, as both studies utilized F1 mice from a C57BL/6J × FVB/N background. In contrast, lower overlap with the Hinch dataset is likely to reflect genetic background differences, as those F1 mice were generated from a C57BL/6 × CAST cross. While fewer overlapping crossovers were observed in this comparison, those that did overlap occurred more frequently than expected by chance, suggesting conserved hotspot usage across backgrounds.
Genome-wide crossover distributions detected with single-nucleus ATAC-sequencing
Additionally, we investigated whether crossover interference could be detected with the snATAC-seq method of crossover detection. Permutation testing via label swapping was used to generate random null distributions of inter-crossover distances to simulate the absence of crossover interference (null hypothesis), as we conducted previously [26]. We next filtered our dataset to chromosomes with only two crossovers (1402 wild-type and 1617 mutant chromosomes; Table 2) and calculated the inter-crossover distance for each pair of crossovers. Analysis of the spacing between two crossover events on the same chromosome revealed that inter-crossover distance did not fit a random distribution in both wild-type and mutant samples, indicative of a functioning crossover interference mechanism (Fig. 7A–C). The observed median inter-crossover distance was 88.8 Mb in F1.Fancm+/+, and reduced to 82.6 Mb in F1.FancmΔ2/Δ2 (pairwise comparisons using Wilcoxon rank sum test with continuity correction, P< 2 ×10−7; Fig. 7A–C). The increase in crossovers in the absence of Fancm is consistent with previous findings [26, 48–56], suggesting the additional crossovers generated in gametes lacking Fancm are likely to arise from the class II (non-interfering) crossover pathway. These findings suggest that snATAC-seq libraries generated with methods here and using SSNIP-seq [30] can be used for high-throughput and genome-wide measurements of crossover interference.
Figure 7.
Analysis of crossover interference in haploids using snATAC-seq data. The distance of observed crossovers was compared with the null hypothesis generated from permutation via label swapping to simulate the absence of crossover interference. (A) The median distance for observed double crossover chromosomes from wild-type samples was ∼88.8 Mb, compared with 44.0 Mb in the null hypothesis. Pairwise comparisons using Wilcoxon rank sum test with continuity correction, P= 2 × 10−16. (B) The median distance for observed double crossover chromosomes from mutant samples was ∼82.6 Mb, compared with 46.1 Mb in the null hypothesis. Pairwise comparisons using Wilcoxon rank sum test with continuity correction, P< 2 × 10−16. (C). Median intercrossover distances were reduced by 6.2 Mb in mutant crossovers, with an interquartile range of 5.9 Mb. These findings suggest that the additional crossovers in Fancm-deficient mice are likely to be derived from the non-interfering (type II) crossover pathway. Pairwise comparisons using Wilcoxon rank sum test with continuity correction, P< 2 × 10−7. Non-parametric statistical testing was used due to non-normal data distribution.
Crossover hotspots are associated with PRDM9 recombination hotspots
Crossovers identified in the snATAC-seq dataset here correlate with published PRDM9 binding locations, identified through ChIP-seq analysis from C57BL/6J mice [38]. Permutation testing was conducted by comparing the mean signal at overlapping regions with the null distribution, generated by shuffling crossovers. P-values were calculated by comparing the observed mean signal with the null distribution, testing if the association differs from a random distribution. The results indicated that there is a significant association between the crossovers, pooled from both genotypes, and PRDM9 binding locations (P< 1 × 10−4; Fig. 8A), indicating a broad requirement for PRDM9 for crossover localization, which is also consistent with the shared PRDM9 allele in C57BL/6J × FVB/N (see the Discussion). Crossovers from both genotypes were pooled as no differences in distributions were detected (Fig. 5D), even if there are generally more crossovers in Fancm-deficient mice.
Figure 8.
Crossover sites in F1 haploid nuclei have a strong association with PRDM9-binding sites. (A) Permutation test comparing the observed mean distance between crossovers (COs) and PRDM9-binding sites (red line) with a null distribution generated by randomizing the PRDM9 ChIP-seq signal (signal fold change, compared with background) locations. Analysis includes all COs identified in snATAC-seq samples, P< 1 × 10−4. (B) One-sided Wilcoxon signed rank test for the PRDM9 ChIP-seq signal (signal fold change) compared with the top 10% of COs identified in snATAC-seq; P= 0.02. (C) Visualization of the PRDM9 ChIP-seq signal (fold change, compared with background) and crossover rate (cM/Mb, scaled by ×10 for plotting) along chromosome 12 (Mbp) for the top 10% of COs. The top track displays the PRDM9 ChIP-seq signal (signal fold change, compared with background); the bottom mirrored track shows the CO signal from snATAC-seq data. (D) One-sided Wilcoxon signed rank test for the PRDM9 ChIP-seq signals compared with the additional COs 'Higher CO loci' identified in F1.FancmΔ2/Δ2 samples, compared with all other COs 'All regions'; P= 0.14. (E) Permutation test to compare the observed mean (red line) with null distribution of PRDM9 ChIP-seq with additional F1.FancmΔ2/Δ2 COs; P< 1 × 10−4.
Crossover hotspots were identified by sub-setting the data for the top 10% of crossovers in both F1.FancmΔ2/Δ2 and F1.Fancm+/+ genotypes. Hotspots were enriched for PRDM9 ChIP-seq signals; one-sided Wilcoxon signed rank test with continuity correction, P= 0.02 (Fig. 8B, C). There were no significant differences in PRDM9 peak association with CO sites when considering genotype, i.e. F1.FancmΔ2/Δ2 and F1.Fancm+/+, as a variable.
The F1.FancmΔ2/Δ2 mutants have an increased number of crossovers, which are consistent with type II crossover distributions (Fig. 7A–C). When assessing the ChIP-seq signal in the loci with increased crossover frequencies in F1.FancmΔ2/Δ2 compared with all F1.Fancm+/+ (assessing only the ‘extra’ crossovers), there was no significant association with PRDM9 and crossovers (Fig. 8D, E), but permutation testing indicated a significant association between PRDM9 and the extra crossovers, compared with the null distribution. This is likely to be due to the strong association with PRDM9 and all crossovers, observed in Fig. 8A.
Discussion
Tn5-based single-gamete library preparation can be used for sequencing library production
The detection of meiotic crossover events has historically relied on pedigree data, large population cohorts, or cytogenetics. However, single-gamete library sequencing methods allow one individual to serve as the exclusive source of data for high-throughput and accurate crossover detection. These single-gamete approaches are scalable, with several studies having developed methods for library preparation and crossover calling software [17–21, 24–26]. Despite these advantages, there are some practical risks that can limit the uptake of single-gamete sequencing for investigating crossover regulation, which we have aimed to partially mitigate in this study: some published methods employ reagents that are no longer commercially available (Bell et al. [20]; Tsui et al.[26]). Therefore, we sought to demonstrate proof-of-concept for single-gamete library preparation with a view to then use these data for crossover detection. While this study uses the 10x Genomics platform for single-nucleus library preparation, the overall strategy of using ATAC-seq for crossover detection from haploid nuclei could be implemented using alternative approaches. We suggest that it is important to be able to adapt methods with home-made reagents to protect against flux of commercial kits, by developing robust methods for crossover detection such as the method which described isolation of individual sperm and sequencing to a median depth of 6.3× [19]. There is precedent for such an approach with a Tn5-based library generation method, e.g. plate-based methods have been established for library generation with somatic cells where bulk-tagmented nuclei are sorted into individual wells of a 384-well plate for unique barcoding [57]. We anticipate that single-gamete sequencing with ATAC-seq is amenable to plate-based approaches, using home-made Tn5 [31], allowing for a cheaper alternative to commercial approaches. Similarly, we also anticipate that this approach could be adapted to other species where gametes, particularly sperm, can be readily obtained, and may open up access in new areas where it has not been technically feasible to study crossover formation due to biology and anatomy, or perhaps ethical constraints around invasive access to testicular biopsies. To enable crossover detection, key requirements would include access to a reference genome and sufficient genetic polymorphism between parental strains or individuals. In cases where no reference genome exists, de novo assembly or the use of long-read sequencing to scaffold a draft reference may be necessary, although this would increase the technical burden. In systems where gametes are limited, such as oocytes or species with low fecundity, adaptation to plate-based barcoding protocols would offer a practical low-throughput solution. By demonstrating that crossover detection is compatible with open reagents and flexible workflows, we aim to support broader adoption of this approach in non-model systems and across evolutionary, ecological, and agricultural research contexts.
Single-nucleus ATAC-sequencing adaption for crossover calling from haploid nuclei
Here, we repurposed an snATAC-seq library preparation and sequencing method as a straightforward, reproducible, and highly scalable approach for genome-scale haplotyping of haploid sperm genomes. For sample preparation, we used our previously published SSNIP-seq nuclei isolation protocol [30], which allows for isolation of high-quality nuclei suitable as input for ATAC-seq. The library preparation assay itself can be completed in a single day with the collection of the sample, preparation, to library generation, and amplification and QC. We sequenced the genomes of 3842 haploid mouse sperm, achieving uniform coverage across the genome at the kilobase scale. Using our established bioinformatic approaches [24], we analysed the sequencing data to detect >30 000 crossovers. We validated the utility of this novel approach by using our established mouse model, F1.FancmΔ2/Δ2, which has increased crossover rates (Tsui et al., [26]), whereby we showed that the snATAC-seq method detects this altered crossover behaviour. We also showed that this assay is highly robust for crossover detection, as there was no observed correlation between coverage and crossover rates, within the range of coverage that we obtained. Further, with the high number of nuclei that were sequenced, this study offers the best resolution to date of the effect of loss of function of Fancm in mammals, compared with our previous work. We show that there is a genome-wide increase in crossover rates in the absence of Fancm, these crossovers occur in the same loci as in the wild type, and the effect in the mutant appears to be an increase in amplitude of what occurs in the wild type. This suggests that the same DSB formation mechanisms are used, but the fate of some DSB repair intermediates is to progress down a crossover formation rather than non-crossover pathway. We note that crossover numbers in subtelomeric regions may be underestimated, as suggested by some chromosomes showing haplotype skewing at chromosome ends. This could stem from a lack of FVB/N variants in these regions, and hence a reference mapping bias. In turn this may be the reason for some chromosomes not quite reaching a length of 50 cM in the wild-type snATAC-seq data.
The association between crossover hotspots and PRDM9 binding has previously been established [19, 58–61]. It is therefore not unexpected that the crossovers observed in this study show a similar association; however, it is important to note the degree of shared crossover hotspots in our data (Fig. 8C) with the binding sites of pro-crossover factor PRDM9 from independent work [38]. Of note, C57BL/6J × FVB/N share a common PRDM9Dom2 allele, which should permit assessing for associations between PRDM9-binding sites and crossover distributions in these different strains, and their crosses. SNPs from FVB/N may change some PRDM9 target sequences; however, this should not change the broader results from genome-wide crossover studies.
Conclusion and future perspectives
Our development of snATAC-seq for F1 sperm, coupled with bioinformatic analysis, establishes this as a reliable method for studying crossover events and the mechanisms required for their formation. This approach has revealed that Fancm plays a key role in limiting the formation of class II crossovers mediated by intrinsic levels of DMC1. We anticipate that these findings and tools will have broader applications in fertility research and diagnostics, potentially aiding in the quantification of sperm aneuploidy linked to chromosome segregation errors (Templado et al. [62]). Future studies could further examine the interaction of Fancm with other crossover pathways, advancing our understanding of recombination and reproductive health.
Supplementary Material
Acknowledgements
We are grateful to all members of the Crismani laboratory for helpful comments on the manuscript. Thank you to Tim Semple for helpful discussions about ATAC-seq methods, and to Adam Thomas for assistance with Tn5 production. Thanks also to Ruqian Lyu for helpful discussions and guidance on the implementation of sgcocaller.).
Author contributions: All authors wrote, reviewed, and discussed the manuscript. S.N. and C.H. prepared the figures. All authors read and approved the final manuscript.
Contributor Information
Stevan Novakovic, DNA Repair and Recombination Laboratory, St Vincent's Institute of Medical Research, Fitzroy VIC 3065, Australia.
Caitlin Harris, DNA Repair and Recombination Laboratory, St Vincent's Institute of Medical Research, Fitzroy VIC 3065, Australia.
Ruijie Liu, Bioinformatics and Cellular Genomics, St Vincent's Institute of Medical Research, Fitzroy VIC 3065, Australia.
Davis J McCarthy, Bioinformatics and Cellular Genomics, St Vincent's Institute of Medical Research, Fitzroy VIC 3065, Australia; The Faculty of Medicine, Dentistry and Health Science, The University of Melbourne, Carlton VIC 3010, Australia.
Wayne Crismani, DNA Repair and Recombination Laboratory, St Vincent's Institute of Medical Research, Fitzroy VIC 3065, Australia; The Faculty of Medicine, Dentistry and Health Science, The University of Melbourne, Carlton VIC 3010, Australia.
Supplementary data
Supplementary data is available at NAR Genomics & Bioinformatics online.
Conflict of interest
The authors declare that they have no competing interests.
Funding
The Australian National Health and Medical Research Council [GNT1112681, GNT1129757, GNT1185387, and GNT2011299 to W.C. and D.J.M.].
Data availability
The raw data from this study are available from the NCBI SRA under the BioProject PRJNA1221601. Code for analytical workflows and the figures used in this article are available at https://drr-public.svi.edu.au/snATAC_public/
References
- 1. Hunter N Meiotic recombination: the essence of heredity. Cold Spring Harb Perspect Biol. 2015; 7:a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam I, Keeney S Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb Perspect Biol. 2015; 7:a016634. 10.1101/cshperspect.a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gray S, Cohen PE Control of meiotic crossovers: from double-strand break formation to designation. Annu Rev Genet. 2016; 50:175–210. 10.1146/annurev-genet-120215-035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szostak JW, Orr-Weaver TL, Rothstein RJ et al. The double-strand-break repair model for recombination. Cell. 1983; 33:25–35. 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 5. Warren AC, Chakravarti A, Wong C et al. Evidence for reduced recombination on the nondisjoined chromosomes 21 in Down syndrome. Science. 1987; 237:652–4. [DOI] [PubMed] [Google Scholar]
- 6. Sherman SL, Takaesu N, Freeman SB et al. Trisomy 21: association between reduced recombination and nondisjunction. Am J Hum Genet. 1991; 49:608–20. [PMC free article] [PubMed] [Google Scholar]
- 7. Hassold T, Sherman S Down syndrome: genetic recombination and the origin of the extra chromosome 21. Clin Genet. 2000; 57:95–100. 10.1034/j.1399-0004.2000.570201.x. [DOI] [PubMed] [Google Scholar]
- 8. Berchowitz LE, Copenhaver GP Genetic interference: don’t stand so close to me. Curr Genomics. 2010; 11:91–102. 10.2174/138920210790886835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgan TH Sex limited inheritance in Drosophila. Science. 1910; 32:120–2. [DOI] [PubMed] [Google Scholar]
- 10. Sturtevant AH The linear arrangement of six sex-linked factors in Drosophila, as shown by their mode of association. J Exp Zool. 1913; 14:43–59. 10.1002/jez.1400140104. [DOI] [Google Scholar]
- 11. Schwacha A, Kleckner N Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994; 76:51–63. 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 12. Francis KE, Lam SY, Harrison BD et al. Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proc Natl Acad Sci USA. 2007; 104:3913–8. 10.1073/pnas.0608936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole F, Baudat F, Grey C et al. Mouse tetrad analysis provides insights into recombination mechanisms and hotspot evolutionary dynamics. Nat Genet. 2014; 46:1072–80. 10.1038/ng.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fogel S, Mortimer RK Recombination in yeast. Annu Rev Genet. 1971; 5:219–36. 10.1146/annurev.ge.05.120171.001251. [DOI] [PubMed] [Google Scholar]
- 15. McClintock B The order of the genes c, sh and wx in Zea mays with reference to a cytologically known point in the chromosome. Proc Natl Acad Sci USA. 1931; 17:485–91. 10.1073/pnas.17.8.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yelina NE, Ziolkowski PA, Miller N et al. High-throughput analysis of meiotic crossover frequency and interference via flow cytometry of fluorescent pollen in Arabidopsis thaliana. Nat Protoc. 2013; 8:2119–34. 10.1038/nprot.2013.131. [DOI] [PubMed] [Google Scholar]
- 17. Lu S, Zong C, Fan W et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science. 2012; 338:1627–30. 10.1126/science.1229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun H, Rowan BA, Flood PJ et al. Linked-read sequencing of gametes allows efficient genome-wide analysis of meiotic recombination. Nat Commun. 2019; 10:4310. 10.1038/s41467-019-12209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hinch AG, Zhang G, Becker PW et al. Factors influencing meiotic recombination revealed by whole-genome sequencing of single sperm. Science. 2019; 363:eaau8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bell AD, Mello CJ, Nemesh J et al. Insights about variation in meiosis from 31,228 human sperm genomes. Nature. 2019; 2019:625202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campoy J, Sun H, Goel M et al. Gamete binning: chromosome-level and haplotype-resolved genome assembly enabled by high-throughput single-cell sequencing of gamete genomes. Genome Biol. 2020; 21:36. 10.1186/s13059-020-02235-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo C, Li X, Zhang Q et al. Single gametophyte sequencing reveals that crossover events differ between sexes in maize. Nat Commun. 2019; 10:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie H, Li W, Guo Y et al. Long-read-based single sperm genome sequencing for chromosome-wide haplotype phasing of both SNPs and SVs. Nucleic Acids Res. 2023; 51:8020–34. 10.1093/nar/gkad532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lyu R, Tsui V, Crismani W et al. sgcocaller and comapr: personalised haplotype assembly and comparative crossover map analysis using single-gamete sequencing data. Nucleic Acids Res. 2022; 50:e118. 10.1093/nar/gkac764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carioscia SA, Weaver KJ, Bortvin AN et al. A method for low-coverage single-gamete sequence analysis demonstrates adherence to Mendel's first law across a large sample of human sperm. eLife. 2022; 11:e76383. 10.7554/eLife.76383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsui V, Lyu R, Novakovic S et al. Fancm has dual roles in the limiting of meiotic crossovers and germ cell maintenance in mammals. Cell Genomics. 2023; 3:100349. 10.1016/j.xgen.2023.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leung AQ, Bell AD, Mello CJ et al. Single cell analysis of DNA in more than 10,000 individual sperm from men with abnormal reproductive outcomes. J Assist Reprod Genet. 2021; 38:2975–83. 10.1007/s10815-021-02300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buenrostro JD, Giresi PG, Zaba LC et al. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013; 10:1213–8. 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cusanovich DA, Daza R, Adey A et al. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015; 348:910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novakovic S, Tsui V, Semple T et al. SSNIP-seq: a simple and rapid method for isolation of single-sperm nucleic acid for high-throughput sequencing. PLoS One. 2022; 17:e0275168. 10.1371/journal.pone.0275168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Picelli S, Björklund ÅK, Reinius B et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 2014; 24:2033–40. 10.1101/gr.177881.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corces MR, Trevino AE, Hamilton EG et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods. 2017; 14:959–62. 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buenrostro JD, Wu B, Chang HY et al. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr Protoc Mol Biol. 2015; 109:21.29.1–9. 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j. 2011; 17:10. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 35. Li H Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv26 May 2013, preprint: not peer reviewedhttps://arxiv.org/abs/1303.3997.
- 36. Li H, Handsaker B, Wysoker A et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009; 25:2078–9. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hahne F, Ivanek R Visualizing genomic data using Gviz and bioconductor. Methods Mol Biol. 2016; 1418:335–51. [DOI] [PubMed] [Google Scholar]
- 38. Biot M, Toth A, Brun C et al. Principles of chromosome organization for meiotic recombination. Mol Cell. 2024; 84:1826–1841. 10.1016/j.molcel.2024.04.001. [DOI] [PubMed] [Google Scholar]
- 39. Langmead B, Salzberg SL Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012; 9:357–9. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Liu T, Meyer CA et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008; 9:R137. 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lawrence M, Huber W, Pagès H et al. Software for computing and annotating genomic ranges. PLoS Comput Biol. 2013; 9:e1003118. 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adey A, Morrison HG, Asan et al. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 2010; 11:R119. 10.1186/gb-2010-11-12-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goryshin IY, Reznikoff WS Tn5 in vitro transposition. J Biol Chem. 1998; 273:7367–74. 10.1074/jbc.273.13.7367. [DOI] [PubMed] [Google Scholar]
- 44. Hammoud SS, Nix DA, Zhang H et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009; 460:473–8. 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oliva R Protamines and male infertility. Hum Reprod Update. 2006; 12:417–35. 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 46. Stuart T, Srivastava A, Madad S et al. Single-cell chromatin state analysis with Signac. Nat Methods. 2021; 18:1333–41. 10.1038/s41592-021-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stuart T, Butler A, Hoffman P et al. Comprehensive integration of single-cell data. Cell. 2019; 177:1888–1902. 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crismani W, Girard C, Froger N et al. FANCM limits meiotic crossovers. Science. 2012; 336:1588–90. [DOI] [PubMed] [Google Scholar]
- 49. Blary A, Gonzalo A, Eber F et al. FANCM limits meiotic crossovers in Brassica crops. Front Plant Sci. 2018; 9:368. 10.3389/fpls.2018.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lorenz A, Osman F, Sun W et al. The fission yeast FANCM ortholog directs non-crossover recombination during meiosis. Science. 2012; 336:1585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Girard C, Chelysheva L, Choinard S et al. AAA-ATPase FIDGETIN-LIKE 1 and helicase FANCM antagonize meiotic crossovers by distinct mechanisms. PLoS Genet. 2015; 11:e1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Girard C, Crismani W, Froger N et al. FANCM-associated proteins MHF1 and MHF2, but not the other Fanconi anemia factors, limit meiotic crossovers. Nucleic Acids Res. 2014; 42:9087–95. 10.1093/nar/gku614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Séguéla-Arnaud M, Choinard S, Larchevêque C et al. RMI1 and TOP3α limit meiotic CO formation through their C-terminal domains. Nucleic Acids Res. 2016; 45:1860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Séguéla-Arnaud M, Crismani W, Mazel J et al. Multiple mechanisms limit meiotic crossovers: TOP3 α and two BLM homologs antagonize crossovers in parallel to FANCM. Proc Natl Acad Sci USA. 2015; 112:4713–8. 10.1073/pnas.1423107112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mieulet D, Aubert G, Bres C et al. Unleashing meiotic crossovers in crops. Nat Plants. 2018; 4:1010–6. 10.1038/s41477-018-0311-x. [DOI] [PubMed] [Google Scholar]
- 56. Fernandes JB, Séguéla-Arnaud M, Larchevêque C et al. Unleashing meiotic crossovers in hybrid plants. Proc Natl Acad Sci USA. 2018; 115:2431–6. 10.1073/pnas.1713078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu W, Wen Y, Liang Y et al. A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility. Nat Protoc. 2021; 16:4084–107. 10.1038/s41596-021-00583-5. [DOI] [PubMed] [Google Scholar]
- 58. Baudat F, Buard J, Grey C et al. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science. 2010; 327:836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Myers S, Bowden R, Tumian A et al. Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science. 2010; 327:876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parvanov ED, Petkov PM, Paigen K Prdm9 controls activation of mammalian recombination hotspots. Science. 2010; 327:835. 10.1126/science.1181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li R, Bitoun E, Altemose N et al. A high-resolution map of non-crossover events reveals impacts of genetic diversity on mammalian meiotic recombination. Nat Commun. 2019; 10:3900. 10.1038/s41467-019-11675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Templado C, Uroz L, Estop A et al. New insights on the origin and relevance of aneuploidy in human spermatozoa. Mol Hum Reprod. 2013; 19:634–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data from this study are available from the NCBI SRA under the BioProject PRJNA1221601. Code for analytical workflows and the figures used in this article are available at https://drr-public.svi.edu.au/snATAC_public/