Abstract
Electron microscopy, sodium dodecyl sulfate-polyacrylamide gel electrophoresis with silver staining and 1H, 13C, and 31P-nuclear magnetic resonance (NMR) were used to detect and characterize the lipopolysaccharides (LPSs) of several Shewanella species. Many expressed only rough LPS; however, approximately one-half produced smooth LPS (and/or capsular polysaccharides). Some LPSs were affected by growth temperature with increased chain length observed below 25°C. Maximum LPS heterogeneity was found at 15 to 20°C. Thin sections of freeze-substituted cells revealed that Shewanella oneidensis, S. algae, S. frigidimarina, and Shewanella sp. strain MR-4 possessed either O-side chains or capsular fringes ranging from 20 to 130 nm in thickness depending on the species. NMR detected unusual sugars in S. putrefaciens CN32 and S. algae BrYDL. It is possible that the ability of Shewanella to adhere to solid mineral phases (such as iron oxides) could be affected by the composition and length of surface polysaccharide polymers. These same polymers in S. algae may also contribute to this opportunistic pathogen's ability to promote infection.
Shewanella organisms are generally associated with aquatic habitats and play important roles in the cycling of particulate iron and organic matter, but they can also be opportunistic pathogens (33, 45). Because of their environmental significance, they are presently under vigorous investigation and many new species were recently included in the genus (45). Most Shewanella organisms are capable of dissimilatory reduction of a wide range of electron acceptors, including metal oxides [e.g., those of Fe(III) and Mn(IV)]. Several reductive mechanisms are possible. Electron flux from the organism to the solid oxide may occur via (i) direct contact of bacterial outer membrane and oxide surface (2, 9, 21, 31), (ii) organic shuttles (such as humic acids or quinones to mediate electron flow (22, 32), or (iii) a combination of both processes. For the direct contact model, metal reductases (such as c-type cytochromes) seem to be embedded in the outer membrane of dissimilatory metal-reducing bacteria to facilitate electron flow (13, 29, 30). Lipopolysaccharide (LPS) and outer membrane proteins could play principal roles in establishing and maintaining contact with oxide minerals so that electron transport to the terminal acceptor occurs. The junction between cell and mineral must be tight so as to ensure that the reductase functions effectively (23). However, the cell surface structure and LPS of Shewanella, which could affect the cell-mineral connection, are poorly understood (28, 39, 40, 48). In this present article we characterize those structural elements that can extend beyond the outer face of the outer membrane, i.e., the LPS O-side chains and capsular polymers.
Bacterial strains and growth conditions.
The strains used in this study are shown in Table 1. Most of these strains were kindly provided by Doug Lies (Jet Propulsion Laboratory, Pasadena, Calif.). S. algae BrY was supplied by both D. Lies and F. Caccavo, Jr. (while he was at the Department of Microbiology, University of New Hampshire, Durham); the former is designated S. algae BrYDL and the latter BrYFC. All were cultured on either tryptic soy broth or tryptic soy broth supplemented with 2% (wt/vol) NaCl (i.e., for S. pealeana and S. woodyi). Cultures were grown aerobically on a rotary shaker (150 rpm) at temperatures from 5 to 37°C and were harvested at a mid-exponential growth phase (optical density at 470 nm [OD470] = ∼0.8).
TABLE 1.
Shewanella strains used in this study
| Strain | Collection no. | Characteristics |
|---|---|---|
| S. putrefaciens CN32 | ATCC BAA-453 | Commonly used Fe oxide-reducing strain |
| S. putrefaciens | NCTC 10695 | Reference strain for Owen's homology group I |
| S. oneidensis MR-1 | ATCC 700550 | Type strain |
| S. oneidensis DLM-7 | ||
| S. algae OK-1 | ATCC 51192 | Type strain |
| S. algae 136-2 | NCTC 10738 | Reference strain for Owen's homology group IV |
| S. algae BrY | ATCC 51181 | Commonly used Fe oxide-reducing strain |
| S. algae BCM-8 | ||
| Shewanella sp. strain MR-4 | ||
| Shewanella sp. strain CL 256/73 | NCTC 12093 | Reference strain for Owen's homology group III |
| S. baltica 63 | NCTC 10735 | Type strain |
| S. amazonensis SB2B | ATCC 700329 | Type strain |
| S. frigidimarina | ACAM 591 | Type strain |
| S. pealeana ANG-SQ1 | ATCC 700345 | Type strain |
| S. woodyi MS32 | ATCC 51908 | Type strain |
SDS-PAGE and nuclear magnetic resonance (NMR) LPS analyses.
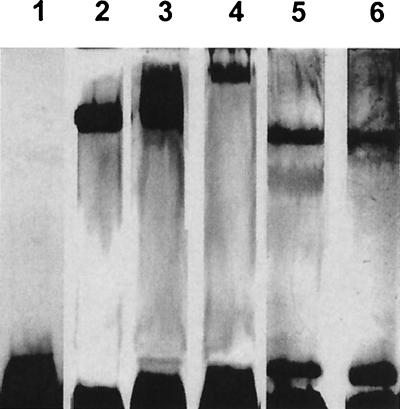
Proteinase K/sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to analyze LPS (16, 24), and the gels were silver stained (41). Gels of S. putrefaciens, S. oneidensis, S. baltica, and Shewanella sp. strains MR-4 and CL 256/73 displayed only low-Mr bands when grown at 26°C. Gels did not display high-Mr bands or “laddering” (indicative of smooth LPS [S-LPS]), suggesting that these contained rough LPS (R-LPS) with core oligosaccharide and no O-side chains (Fig. 1, lane 1, is representative). S. amazonensis, S. frigidimarina, S. pealeana, S. woodyi, and most S. algae organisms produced S-LPS (or possibly capsular polysaccharides) as evidenced by bands located in the upper regions of the gel (Fig. 1, lanes 2 to 6, is representative). These bands sometimes were smeared (Fig. 1, lane 3) suggesting that each band possessed similar but not identical polymer lengths. At this point in our study, it was impossible to distinguish these S-LPSs from capsular material. By definition capsular macromolecules contain long-branched or unbranched homo- or heteropolysaccharides linked to a lipid substituent, which anchors them to the outer membrane. Capsular polymers can have either lipid A or entirely different lipid anchors, such as diacylglycerolphosphate, but the major differentiating property is polymer length (46). Capsular polysaccharides are longer than those of LPS and, as such, should not enter the gels. Strangely, S. algae BrYFC lacked bands in the top region of the gel (Fig. 1, lane 1), being different from all other tested strains of S. algae, including BrYDL.
FIG. 1.
Silver-stained SDS-PAGE gel of LPS from S. algae BrYFC (lane 1), S. algae 136-2 (lane 2), S. algae BCM-8 (lane 3), S. amazonensis SB2B (lane 4), S. pealeana (lane 5), and S. woodyi (lane 6) cultured at 26°C.
1H-, 13C-, and 31P-NMR was performed on selected strains and spectra analyzed according to the method described by Vinogradov et al. using a Varian Inova spectrometer (44). Most cores that have been studied possess high concentrations of carboxyl and phosphoryl sites, making them highly polar so as to interact with metal ions (10, 20) and inanimate surfaces (26). S. putrefaciens CN32 (and related strains [28, 47]) and S. oneidensis MR-1 have R-LPS (Table 2), which may aid their adhesion and close fit to iron oxide minerals. 1H-, 13C-, and 31P-NMR analyses on CN32 indicated that its oligosaccharide backbone consisted of β-Galf-(1-3)-β-Gal-(1-4)-β-Glc-α-DDHep2PEtN-(1-5)-α-Kdo4P-(1-6)-β-GlcN4P-(1-6)-α-GlcN1P, which possesses phosphate and carboxylate groups (i.e., 3-deoxy-d-manno-2-octulosonic acid [Kdo]) suggesting that this region is polar and can be ionized. Phosphorylated Kdo is rare among extensively studied bacteria, such as enterobacteria, and difficult to detect by conventional colorimetric means (17). At this point, since so few Shewanella LPSs have been studied in detail (39, 40), it is impossible to say if phosphorylated Kdo could be a common trait of this genus or species.
TABLE 2.
Surface properties of used Shewanella strains
| Strain | Presence of S-LPS/PSa | Presence of fringe (freeze substitution) |
|---|---|---|
| S. putrefaciens CN32 | − | − |
| S. putrefaciens NCTC 10695 | − | − |
| S. baltica 63 | − | − |
| Shewanella sp. strain CL 256/73 | − | NDb |
| Shewanella sp. strain MR-4 | − | + |
| S. oneidensis MR-1 | − | + |
| S. oneidensis DLM-7 | − | + |
| S. amazonensis SB2B | + | − |
| S. algae OK-1 | + | + |
| S. algae BrYDL | + | ±c |
| S. algae BrYFC | − | ± |
| S. algae 136-2 | + | ND |
| S. algae BCM-8 | + | ND |
| S. frigidimarina ACAM 591 | + | + |
| S. pealeana ANG-SQ1 | + | ND |
| S. woodyi MS32 | + | ND |
PS, capsular polysaccharide. Noted as absence (−) or presence (+) of upper bands on SDS-PAGE gels.
ND, not determined.
±, indicates that only a certain population of cells had the fringe.
From a dissimilatory medal-reducing bacterium point of view, it may be advantageous for a bacterium to have its length of LPS constrained so that the microbe can fit closely to the oxide, thereby more efficiently using the mineral as a terminal electron acceptor. Indeed, for a close fit to any inert surface, as long as the hydophobicity and hydrophilicity properties are appropriate, short LPSs should be more beneficial. Dissimilatory reduction of metal oxides requires anaerobiosis, and our Shewanella was grown aerobically, but even under these conditions, close oxide-bacterium union is seen (14). Almost one-half of our strains possessed R-LPS (Table 2), suggesting that core oligosaccharide was the LPS terminus. Because of the high potential charge and relatively short terminus (core oligosaccharide) on CN32 LPS, it is attractive to suggest that these two characteristics allow a tight union between bacterium and iron oxide, allowing good contact of the putative outer membrane iron reductase so that electron flow is ensured (23, 29).
Many enterobacteria and pseudomonads are noted for the strong antigenicity that is expressed by their LPSs. Although endotoxicity is more a function of lipid A, O-side chains differentiate these bacteria into specific immunodominant groupings. For example, Pseudomonas aeruginosa PAO1 belongs to serogroup 05 and expresses two separate LPSs under normal growth conditions, A-band LPS (“common antigen”) and B-band LPS (“serotype-specific antigen”) (37). Here there are not only distinct differences in chemistry of the O-polysaccharides but also in their polymer lengths. LPSs frequently form distinctive ladderlike patterns on silver-stained gels. Many of our strains showed an S-LPS phenotype, often without a ladderlike appearance, suggesting that the O-polymers are of a relatively constant length. It was interesting that most S. algae strains contained S-LPS, since this species, unlike others, has been implicated in certain infections (33, 45). Here, the O-side chain could be a contributing virulence factor. NMR analysis of the polysaccharide chain of BrYDL LPS revealed -3)-α-d-BacNAc4Nbu-(1-3)-α-l-Rha-(1-2)-α-l-Rha-(1,2)-l-malyl-(4-2)-α-l-FucN-(1- to be the basic backbone structure with a malic acid linkage unit (44), which is distinctive for a gram-negative pathogen and different from S. algae strain 48055 (40). Interestingly, the distinctive malic acid linkage unit should provide increased flexibility of the O-polysaccharide chain, thereby allowing easier bending and compression than side chains of enteric bacteria, a feature that could also aid tighter bonding to mineral surfaces under environmental settings.
Influence of growth temperature.
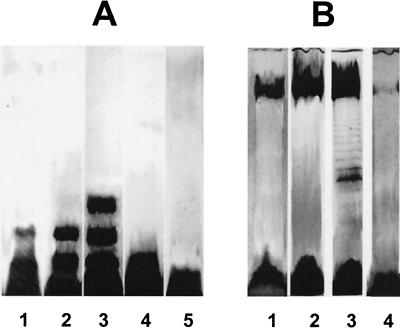
Growth temperature can markedly influence the polymeric organization of LPS (1, 18, 25, 27, 35, 42), and this was true with some Shewanella strains. Temperatures below 25°C resulted in S- to semirough LPS in S. oneidensis MR-1, which corresponded to the appearance of one to three bands above the putative core (Fig. 2A). Maximum size heterogeneity of LPS was observed at 15 to 20°C. Most apparent was S. frigidimarina, where a characteristic ladderlike banding pattern appeared at 15°C (Fig. 2B). A further decrease of growth temperature caused a decrease in this LPS size heterogeneity; however, the LPS profiles of most strains were not affected by temperature. The low temperature effect on some strains was not surprising, since many Shewanella organisms are either psychrotolerant or psychrophilic (7, 43).
FIG. 2.
(A) Silver-stained SDS-PAGE gel of LPS from S. oneidensis MR-1 cells cultured at 5oC (lane 1), 15oC (lane 2), 20oC (lane 3), 26oC (lane 4), and 30°C (lane 5); (B) gel of LPS from S. frigidimarina cells cultured at 5oC (lane 1), 15oC (lane 2), 22oC (lane 3), and 26°C (lane 4).
Ultrastructural analyses.
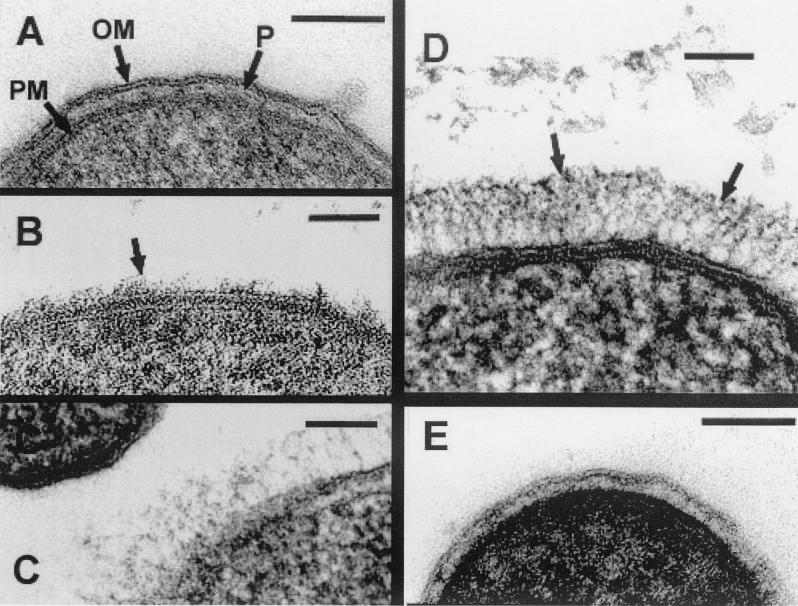
For conventional embeddings for thin sections the glutaraldehyde-osmium tetroxide protocol of Beveridge et al. (5) was followed and cells were embedded in LR White. Ruthenium red was also used as suggested by Beveridge et al. (5). Freeze substitution was according to the method described by Graham et al. (15). Sections were imaged with a Philips EM300 under standard operating conditions. All conventionally processed strains possessed envelope profiles typical of gram-negative cells having an outer membrane, periplasmic space, peptidoglycan layer, and plasma membrane (Fig. 3A is representative). No specific surface structures, such as capsules, S-layers, exopolymeric substances, sheaths, spinae, pili (fimbriae), or flagella (see references 4 and 6), could be discerned.
FIG. 3.
All are thin sections and the bar in each image is 100 nm. (A) Conventionally embedded S. oneidensis MR-1; OM, outer membrane; P, periplasm; PM, plasma membrane. Note the absence of fibrous material on the outer membrane of this cell. (B) Freeze-substitution of S. oneidensis MR-1 (a patch of fringe on the outer membrane is shown by the arrow, whereas adjacent regions do not have the fringe). (C) Freeze-substitution of S. algae BrYFC (only one of the two cells has a fringe). (D) Freeze-substitution of Shewanella sp. strain MR-4 (arrows indicate the ordered fibrous material external to the outer membrane). (E) Freeze-substitution of S. putrefaciens CN32 (no fringe is seen).
Since freeze substitution has been shown to preserve finely detailed surface structures such as capsules and LPS O-side chains (3, 15, 19), it was also used to examine the surfaces of these Shewanella organisms. All strains contained a periplasmic gel (3, 6). S. oneidensis strains revealed a fibrous fringe extending about 20 to 30 nm and 60 to 80 nm from the cell surfaces of MR-1 and DLM 7, respectively (Fig. 3B is representative). A more extensive fringe was seen on S. algae BrYDL and BrYFC reaching up to 60 to 90 nm. However, cell populations in the samples were very heterogeneous, since in each population, some cells possessed different fringe heights, some possessed only patches of fringe, and some had no fringe at all (Fig. 3C is representative), suggesting that there was unequal expression of fringe within a single culture. Shewanella sp. strain MR-4 possessed the most extensive fringe (70 to 130 nm) of all (Fig. 3D). Taken together, the variance of fringe thickness and patchiness and the heterogeneity of fringe expression corroborated the (sometimes) smeared appearance of bands within SDS-PAGE gels, since a variation of polymer lengths would be expected.
Some variations in fiber arrangement of the fringes were also observed. The fibers of MR-4 extended directly away from the cell envelope in quite an organized manner (Fig. 3D), while those of S. oneidensis and S. algae (Fig. 3B and C) were more randomly arranged, forming a netlike mesh; these could be a softer, less ordered matrix than that of MR-4. Taking into account the structure and substantial thickness of these three fibrous layers, it would be reasonable to designate them as comprising capsules based solely on polymer length. In contrast, the cell surfaces of S. putrefaciens (CN32 and NCTC 10695), S. baltica, and S. amazonensis were devoid of any fibrous material and were not capsulated (Fig. 3E).
Ruthenium red is a stain that is frequently used to contrast acidic surface polymeric substances, such as capsules (4, 5). Strangely, when this stain was used with all our strains, no fringes were seen, even on those strains shown to have capsules by freeze substitution (data not shown). It is possible that the capsules of Shewanella are too delicate to withstand conventional fixation-ruthenium red processing or that the capsules' overall electronegative charge density is too low to bind the stain.
Because the electron microscopy and LPS analyses have produced many data points in our study, we have tabulated results in Table 2. Here it is revealed that ∼50% of the strains have R-LPS and that about one-half of these possess a fringe (freeze substitution) that is presumably a capsule. The other strains possess S-LPS or low-Mr polysaccharides (since they enter the SDS-PAGE gel) that can only infrequently be seen by freeze substitution.
Possible implications.
Surface polysaccharides, whether they are capsular or LPS, strongly affect the physicochemistry and adhesion qualities of gram-negative bacteria (8, 10, 11, 12, 34, 38, 48). Often it has been assumed that strains possessing R-LPS are more hydrophobic than their S-LPS counterparts, but this is not necessarily true (19, 25). Rough strains often have more exposed ionizable groups, and O-side chains can be so rapidly in motion that long-lived interactions between ions or surfaces can be rare (3, 49). Capsules, which are typically considered to aid adhesion, can be either adhesive (via charge-charge interaction) or nonadhesive (34, 46). Chemistry and length of both capsular and O-polysaccharides and core oligosaccharides all come to bear as they help to produce the net physicochemistry of a bacterial surface. In some instances, strong polarity would be required for adhesion, whereas in others, strong hydrophobicity would be required. Phenotypic plasticity is important (36), and the most successful bacteria are those that can modulate their surface properties according to the environment and the chosen attachment surface. For this reason, we propose that certain Shewanella organisms, such as CN32, have developed short R-LPS of high charge character to aid close adherence to inanimate surfaces, such as iron oxides. Under anaerobic conditions this would allow the Fe(III)-Fe(II) couple to aid electron capture during respiration. MR-1, on the other hand, retains the R-LPS phenotype but also requires the aid of a capsule of low charge density to aid its adhesion. BrYDL has adapted itself to live under natural environmental conditions but can also be an opportunistic pathogen. Possibly, the O-side chains aid in pathogenicity, but its flexible malic acid hinge-like linkage could also allow close contact to hard materials. There is no doubt that the surfaces of the other Shewanella organisms in our study will utilize their structural combinations in similar self-serving ways.
Acknowledgments
We acknowledge the excellent technical assistance of Bob Harris, Dianne Moyles, and Sean Langley of our laboratory, and we thank Chris Whitfield of our Department for his helpful comments on capsular material.
This work was funded by NABIR-DOE grants to T.J.B. and Y.G. The electron microscopy was done in the NSERC Guelph Regional STEM Facility (GRSF), which is partially funded by an NSERC-Major Facilities Access grant to T.J.B.
REFERENCES
- 1.Al-Hendy, A., P. Toivanen, and M. Skurnik. 1991. The effect of growth temperature on the biosynthesis of Yersinia enterocolitica O:3 lipopolysaccharide: temperature regulates the transcription of the rfb but not the rfa region. Microb. Pathog. 10:81-86. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, R. G., T. J. DiChristina, and M. R. Hoffmann. 1988. Reductive dissolution of Fe(III) oxides by Pseudomonas sp. 200. Biotechnol. Bioeng. 32:1081-1096. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beveridge, T. J. 1981. Ultrastructure, chemistry, and function of the bacterial cell wall. Int. Rev. Cytol. 72:229-317. [DOI] [PubMed] [Google Scholar]
- 5.Beveridge, T. J., D. Moyles, and R. Harris. Electron microscopy. In C. A. Reddy, M. Bagdasarian, T. J. Beveridge, J. A. Breznak, G. A. Murzluf, and T. M. Schmidt (ed.), Methods for general and molecular microbiology, in press. ASM Press, Washington, D.C.
- 6.Beveridge, T. J., and L. L. Graham. 1991. Surface layers of bacteria. Microbiol. Rev. 55:684-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman, J. P., S. A. McCammon, D. S. Nichols, J. H. Skerratt, S. M. Rea, P. D. Nichols, and T. A. McMeekin. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 47:1040-1047. [DOI] [PubMed] [Google Scholar]
- 8.Busscher, H. J., J. Sjollema, and H. C. van der Mei. 1990. Relative importance of free energy as a measure of hydrophobicity in bacterial adhesion to solid surfaces, p. 335-359. In R. J. Doyle and M. Rosenberg (ed.), Microbial cell surface hydrophobicity. American Society for Microbiology, Washington, D.C.
- 9.Caccavo, F., Jr., B. Frolund, F. Van Ommen Kloeke, and P. H. Nielsen. 1996. Deflocculation of activated sludge by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Appl. Environ. Microbiol. 62:1487-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris, F. G., and T. J. Beveridge. 1986. Site specifity of metallic ion binding in Escherichia coli K-12 lipopolysaccharide. Can. J. Microbiol. 32:52-55. [DOI] [PubMed] [Google Scholar]
- 11.Flemming, C. A., R. J. Palmer, Jr., A. A. Arrage, H. C. Van Der Mei, and D. C. White. 1998. Cell surface physicochemistry alters biofilm development of Pseudomonas aeruginosa lipopolysaccharide mutants. Biofouling 13:213-231. [Google Scholar]
- 12.Fletcher, M. 1980. Adherence of microorganisms to smooth surfaces, p. 345-374. In E. H. Beachey (ed.), Bacterial adherence. Chapman & Hall, New York, N.Y.
- 13.Gaspard, S., F. Vazquez, and H. Holliger. 1998. Localization and solubilization of the iron(III) reductase of Geobacter sulfurreducens. Appl. Environ. Microbiol. 64:3188-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasauer, S., S. Langley, and T. J. Beveridge. 2001. The sorption of Fe (hydr)oxides to the surface of Shewanella putrifaciens: cell-bound fine-grained minerals are not always formed de novo. Appl. Environ. Microbiol. 67:5544-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, L. L., R. Harris, W. Villiger, and T. J. Beveridge. 1991. Freeze-substitution of gram-negative eubacteria: general cell morphology and envelope profiles. J. Bacteriol. 173:1623-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo, S., Y. Haishima, and K. Hisatsune. 1992. Taxonomic implication of the apparent undetectability of 3-deoxy-D-manno-2-octulosonate (Kdo) in lipopolysaccaharides of representatives of the family Vibrionaceae and the occurrence of Kdo 4-phosphate in their inner core regions. Carbohydr. Res. 231:55-64. [DOI] [PubMed] [Google Scholar]
- 18.Kropinski, A. M., V. Lewis, and D. Berry. 1987. Effect of growth temperature on the lipids, outer membrane proteins, and lipopolysaccharides of Pseudomonas aeruginosa PAO1. J. Bacteriol. 169:1960-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam, J. S., L. L. Graham, J. Lightfoot, T. Dasgupta, and T. J. Beveridge. 1992. Ultrastructural examination of the lipopolysaccharides of Pseudomonas aeruginosa strains and their isogenic rough mutants by freeze-substitution. J. Bacteriol. 174:7159-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langley, S., and T. J. Beveridge. 1999. Effect of O-side-chain-lipopolysaccharide chemistry on metal binding. Appl. Environ. Microbiol. 65:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley, D. R., J. L. Fraga, J. D. Coates, and E. L. Blunt-Harris. 1999. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1:89-98. [DOI] [PubMed] [Google Scholar]
- 23.Lower, S. K., M. F. Hochella, Jr., and, T. J. Beveridge. 2001. Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and α-FeOOH. Science 292:1360-1362. [DOI] [PubMed] [Google Scholar]
- 24.Lugtenberg, B., J. Meijers, R. Peters, P. van der Hock, and L van Alhen. 1975. Electrophoretic resolution of the ′major outer membrane protein' of Escherichia coli K-12 into four bands. FEBS Lett. 58:254-258. [DOI] [PubMed] [Google Scholar]
- 25.Makin, S. A., and T. J. Beveridge. 1996. Pseudomonas aeruginosa PAO1 ceases to express serotype-specific lipopolysaccharide at 45oC. J. Bacteriol. 178:3350-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makin, S. A., and T. J. Beveridge. 1996. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology 142:299-307. [DOI] [PubMed] [Google Scholar]
- 27.McConnell, M., and A. Wright. 1979. Variation in the structure and bacteriophage-inactivating capacity of Salmonella anatum lipopolysaccharide as a function of growth temperature. J. Bacteriol. 137:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moule, A. L., and S. G. Wilkinson. 1989. Composition of lipopolysaccharides from Alteromonas putrefaciens (Shewanella putrefaciens). J. Gen. Microbiol. 135:163-173. [Google Scholar]
- 29.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers, C. R., and J. M. Myers. 1997. Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochim. Biophys. Acta 1326:307-318. [DOI] [PubMed] [Google Scholar]
- 31.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 32.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transport. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 33.Nozue, H., T. Hayashi, Y. Hashimoto, T. Ezaki, K. Hamasaki, K. Ohwada, and Y. Terawaki. 1992. Isolation and characterization of Shewanella alga from human clinical specimens and emendation of the description of S. alga Simudu et al., 1990, 335. Int. J. Syst. Bacteriol. 42:628-634. [DOI] [PubMed] [Google Scholar]
- 34.Ong, Y. L., A. Razatos, G. Georgiou, and M. M. Sharma. 1999. Adhesion forces between E. coli bacteria and biomaterial surfaces. Langmuir 15:2719-2725. [Google Scholar]
- 35.Poole, K., and V. Braun. 1988. Influence of growth temperature and lipopolysaccharide on hemolytic activity of Serratia marcescens. J. Bacteriol. 170:5146-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley, M. S., V. S. Cooper, R. E. Lenski, L. J. Forney, and, T. L. Marsh. 2001. Rapid change and diversification of a soil bacterium during 1000 generations of experimental condition. Microbiology 147:995-1006. [DOI] [PubMed] [Google Scholar]
- 37.Rivera, M., and E. J. McGroarty. 1989. Analysis of common-antigen lipopolysaccharide from Pseudomonas aeruginosa. J. Bacteriol. 171:2244-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg, M., and S. Kjellenberg. 1986. Hydrophobic interactions: role in bacterial adhesion. Adv. Microb. Ecol. 9:353-393. [Google Scholar]
- 39.Shashkov, A. S., S. N. Senchenkova, E. V. Nazarenko, V. A. Zubkov, N. M. Gorshkova, Y. A. Knirel, and R. P. Gorshkova. 1997. Structure of phosphorylated polysaccharide from Shewanella putrefaciens strain S29. Carbohydr. Res. 303:333-338. [DOI] [PubMed] [Google Scholar]
- 40.Shashkov, A. S., S. N. Senchenkova, E. V. Nazarenko, V. A. Zubkov, N. M. Gorshkova, Y. A. Knirel, and R. P. Gorshkova. 1998. Structure of the acidic polysaccharide chain of the lipopolysaccharide of Shewanella alga 48055. Carbohydr. Res. 309:103-108. [DOI] [PubMed] [Google Scholar]
- 41.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in the polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 42.Van der Mei, H. C., M. M. Cowan, M. J. Genet, P. G. Rouxhet, and H. J. Busscher. 1992. Structural and physicochemical properties of Serratia marcescens strains. Can. J. Microbiol. 38:1033-1041. [Google Scholar]
- 43.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 44.Vinogradov, E., A. Korenevsky, and T. J. Beveridge. The structure of the O-specific polysaccharide chain of the Shewanella algae BrY lipopolysaccharide. Carbohydr. Res., in press. [DOI] [PubMed]
- 45.Vogel, B. F., H. M. Holt, P. Gerner-Smidt, A. Bundvad, P. Søgaard, and L. Gram. 2000. Homogeneity of Danish environmental and clinical isolates of Shewanella algae. Appl. Environ. Microbiol. 66:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitfield, C., and M. A. Valvano. 1993. Biosynthesis and expression of cell-surface polysaccharides in Gram-negative bacteria. Adv. Microb. Physiol. 35:135-246. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson, S. G., L. Galbraith, and G. A. Lightfoot. 1973. Cell walls, lipids, and lipopolysaccharides of Pseudomonas species. Eur. J. Biochem. 33:158-174. [DOI] [PubMed] [Google Scholar]
- 48.Williams, V., and M. Fletcher. 1996. Pseudomonas fluorescens adhesion and transport through porous media are affected by lipopolysaccharide composition. Appl. Environ. Microbiol. 62:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao, X., J. Walter, S. Burke, S. Stewart, M. H. Jeriho, D. A. Pink, R. Hunter, and T. J. Beveridge. 2002. Atomic force microscopy, computer simulations and theoretical considerations of the surface properties of bacteria. Colloids Surf. B: Biointerfaces 23:213-230. [Google Scholar]