Abstract
This study was carried out in order to investigate human enteric virus contaminants in mussels from three sites on the west coast of Sweden, representing a gradient of anthropogenic influence. Mussels were sampled monthly during the period from February 2000 to July 2001 and analyzed for adeno-, entero-, Norwalk-like, and hepatitis A viruses as well as the potential viral indicator organisms somatic coliphages, F-specific RNA bacteriophages, bacteriophages infecting Bacteroides fragilis, and Escherichia coli. The influence of environmental factors such as water temperature, salinity, and land runoff on the occurrence of these microbes was also included in this study. Enteric viruses were found in 50 to 60% of the mussel samples, and there were no pronounced differences between the samples from the three sites. E. coli counts exceeded the limit for category A for shellfish sanitary safety in 40% of the samples from the sites situated in fjords. However, at the site in the outer archipelago, this limit was exceeded only once, in March 2001, when extremely high levels of atypical indole-negative strains of E. coli were registered at all three sites. The environmental factors influenced the occurrence of viruses and phages differently, and therefore, it was hard to find a coexistence between them. This study shows that, for risk assessment, separate modeling should be done for every specific area, with special emphasis on environmental factors such as temperature and land runoff. The present standard for human fecal contamination, E. coli, seems to be an acceptable indicator of only local sanitary contamination; it is not a reliable indicator of viral contaminants in mussels. To protect consumers and get verification of “clean” mussels, it seems necessary to analyze for viruses as well. The use of a molecular index of the human contamination of Swedish shellfish underscores the need for reference laboratories with high-technology facilities.
The commercial shellfish industry is well developed in many countries in Europe, but in Sweden, production is low (24). However, there are plans for the expansion of production, with the focus on the cultivation of blue mussels, Mytilus edulis. There are two reasons for an expansion. (i) M. edulis specimens can have a great impact on local marine ecosystems since they are filter-feeding organisms with a high filtering capacity and, as such, are able to significantly reduce the phytoplankton biomass (10, 11, 40, 41, 54), thereby preventing oxygen depletion in eutrophicated areas. (ii) Cultivation of M. edulis can be of economic importance as a contribution to the commercial food industry. However, the most clearly identified health risk associated with coastal pollution by urban wastewater is the transmission of diseases by the consumption of shellfish harvested in contaminated areas (53). In particular, the filter-feeding bivalves constitute a human health risk as carriers of pathogenic organisms (38, 48, 50, 52). The European Community (EC; directive 91/492) has pronounced the gram-negative bacterium Escherichia coli an indicator organism for fecal contamination in bivalves. On the other hand, many authors (32, 35, 36, 46) have reported enteric viruses as the major infectious agents causing diseases when bivalves are consumed. Compared to E. coli, the viruses seem to survive longer both in the marine environment and in the digestive tracts of bivalves (44). Thus, a more reliable indicator than E. coli has been sought. The current methods for analyses of human viral pathogens that can potentially be present in bivalve tissue are so far considered too expensive and complicated for routine monitoring. Bacteriophages infecting human fecal bacteria have shown slow elimination kinetics, which appears to be representative of human enteric viruses (26, 47). Phages such as somatic coliphages, F-specific RNA (F-RNA) bacteriophages, and phages infecting Bacteroides fragilis are easily enumerated and may be possible to use as indicators of viral contaminants. However, environmental conditions such as seawater temperature and salinity and land runoff may influence the occurrence of both the viral contaminants and the potential indicator organisms in seawater and therefore also their accessibility to the mussels. In addition, physiological functions in bivalves and, consequently, also the accumulation and elimination of microbes are affected by, e.g., temperature and salinity (8). Such phenomena may partly explain seasonal (9) and geographical differences in the indicator content in bivalves. For example, strains of bacteriophages infecting B. fragilis have been shown to be useful as enteric viral indicators in some geographical areas, such as South Africa and Southern Europe (4, 5, 21) but, e.g., not in the United States (31).
In this work, we focused on the sanitary aspect of mussel consumption, with the aim of giving recommendations on how to control the health risks for consumers. This study was performed in order to investigate the presence of some human viruses that are excreted in feces. We investigated the influence of environmental factors such as temperature, salinity, and land runoff on the variability of these enteric viruses and their potential indicator organisms in mussels from a coastal area in eastern Skagerrak. A comprehensive study of this kind, by the inclusion of human viruses, has never before been performed in Scandinavia.
MATERIALS AND METHODS
Site selection, sampling, and environmental parameters.
The Skagerrak coast of Sweden has a large archipelago and many fjords. The inner parts of the fjords are considered eutrophicated and are of interest for developing mussel production (25). For the study of pathogenic contaminants in mussels, three sites in the coastal area were investigated. So far, this area has been poorly investigated in terms of human pathogens. The sites, referred to herein as A, B, and C (Fig. 1), were assumed to represent different degrees of anthropogenic influence and land runoff. Site A is situated in the outer part of the archipelago, which is characterized by a dynamic exchange of surface water and a minor influence from local sources of pathogenic contaminants. Site B is positioned in the inner part of a short fjord, with a direct connection to the Skagerrak area, but the area is affected by local agricultural activities and sewage treatment plants. Site C is situated in the inner part of an open-ended fjord system with a predominating counterclockwise surface current. The water at this site is transported through narrow fjords and is supposed to be affected by outlets from agricultural activities and sewage treatment plants more than the other sites.
FIG. 1.
Map showing the coastal area of Skagerrak. The sampling sites, referred to as A, B, and C, are situated along the southwestern coast of Sweden.
Mussels from an independent site in the outer archipelago were collected and transferred to the three sampling sites, where they were kept in sets of two baskets each from 6 to 8 weeks at a 2-m depth (tidal amplitude, 0.2 m) prior to monthly samplings. Samplings were repeated 18 times during the period from February 2000 to July 2001. On the sampling day, mussels were collected from the different sites and brought by car to the Kristineberg Marine Research Station within 45 min. The length of the mussels was ca. 6 to 7 cm, and the weight was 33.5 ± 7 g. Fifteen thoroughly cleaned mussels were used to analyze for E. coli, and 2 × 15 mussels were used for assaying somatic coliphages, phages infecting B. fragilis, and F-RNA phages. For analyses of adenoviruses (Ads), enteroviruses, Norwalk-like viruses (NLVs), and hepatitis A viruses (HAVs), 20 mussels from each area were sent by air cargo in a chilled thermos to the Department of Virology, University of Umeå, Umeå, Sweden, and were received by the laboratory within 24 h of dispatch.
Water temperature and salinity were measured at each sampling site in connection with the mussel sampling. Water was collected at a 2-m depth with a modified Ruthner sampler. Temperature (Celsius) was immediately measured with Thermapen thermometer (Electronic Temperature Instruments Ltd., West Sussex, United Kingdom) calibrated against a high-accuracy thermometer (Tempmaster; Pentronic, Gunnebo, Sweden). Salinity (in practical salinity units [PSU]) was measured with a laboratory salinometer (Tsurumi-Seiki E2; Swedaq HB, Malmö, Sweden) calibrated with certified standard seawater (Ocean Scientific International Limited, Hampshire, United Kingdom). Daily measurements of the water flow (cubic meters per second) in the river Göta Älv were acquired from the Swedish Meteorological and Hydrological Institute and were assumed to represent the regional land runoff since the river drains a large area of southwestern Sweden.
Isolation of E. coli, bacteriophages, and human viruses.
Mussels were aseptically opened in a laminar airflow cabinet. The wet-flesh weight of the mussels was determined. For analyses of E. coli and F-RNA phages, the meat and intravalvular fluid were homogenized in peptone buffer (pH 7.2) according to the method of Doré and Lees (14). For somatic coliphages and phages infecting B. fragilis, 0.25 M glycine buffer (pH 10) was used (43). The homogenates used for F-RNA analyses were centrifuged for 5 min at room temperature at 2,170 × g (Beckman centrifuge J2-21 M/E). The homogenates used for assaying somatic coliphages and phages infecting B. fragilis were centrifuged at the same speed but for 15 min at 4°C, and the pH of the supernatants was adjusted to 7.2 ± 0.2. Opening and homogenization of the mussels used for the detection of human viruses followed the same procedures as for the detection of somatic coliphages and phages infecting B. fragilis viruses except that only the digestive glands of the mussels were used. Previous data suggest that viruses are concentrated mainly in the digestive gland of the animal and that a specific dissection of this organ will reduce the final volume and also reduce the presence of inhibitors of the enzymatic reactions used for the final detection of the viruses (20). After the pH adjustment, the samples for virus isolation were centrifuged for 45 min at 4°C at 39,800 × g (Beckman centrifuge J2-21 M/E). The supernatant was then ultracentrifuged for 1 h at 4°C at 100,000 × g (Beckman ultracentrifuge L5-65B) to pellet virus particles as well as suspended organic material. The pellets were finally dissolved in 50 μl of sterile phosphate-buffered saline × g of digestive gland used−1.
Detection and analysis of E. coli.
The most probable number (MPN × 100 g of sample−1) of E. coli was determined by the standard multiple tube method (13) with five tubes and three dilutions in mineral-modified glutamate broth (CM607; Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). The tubes were incubated for 24 ± 2 h at 37°C and thereafter examined for the presence of acid, denoted by a yellow coloration of the medium. Thermotolerant E. coli was confirmed by inoculation of an acid-positive tube on tryptone bile glucuronide agar (Lab 162; Lab M) plates, followed by incubation for 22 ± 2 h at 44°C and examination for the presence of blue-green colonies. E. coli NCTC 9001E (β-glucuronidase positive; Culture Centre, University of Göteborg) was used as a positive control, and Klebsiella aeruginosa (β-glucuronidase negative; Centre for Environment, Fisheries & Aquaculture Science) was used as a negative control. Ten isolated strains of E. coli from one sampling occasion (March 2001) were biochemically verified by the Culture Centre, University of Göteborg, with the API 32E strip system (bioMérieux Vitek, Inc.). All of these strains were further tested for enterohemorrhagic toxin (verotoxins 1 and 2) by specific PCR according to the protocol of Welinder-Olsson et al. (51). Briefly, amplification of the 16S rRNA gene between the universal regions U1 and U4 (767-bp fragment) was performed by using the primers SSU1 (CGGCAGGCCTAACACATGCAAGTCG) and 806R (GGACTACCAGGGTATCTAAT). The reaction mixture was UV irradiated for 10 min before the addition of 10 μl of a suspension of lysed bacteria in order to avoid the presence of any unidentified DNA in the reaction mixture before amplification. DNA was denaturated at 94°C for 15 s, annealed at 55°C for 30 s, and extended at 72°C for 1 min. A temperature delay step of 10 min at 72°C was done after 35 cycles to complete elongation. After gel electrophoresis, the amplification products of the expected size were extracted from the gel matrix (gel extraction kit; Qiagen, Hilden Germany) and subjected to sequencing with an ABI Prism BigDye Terminator cycle sequencing kit (version 1; Applied Biosystems, Foster City, Calif.) strategy on an automated ABI 310 DNA sequencer (Applied Biosystems).
Detection and analysis of phages.
The bacteriophages were analyzed by standard double-overlay agar methods (3a) for detection and enumeration (PFU × 100 g of sample−1) by incubating the sample with the appropriate host bacteria. All stock cultures used for phage analysis were cultured, stored, and checked in accordance with the instructions given in the respective ISO guidelines. The host bacteria used for assaying the mussels for somatic coliphages (3b) were E. coli WG5 cells (ATCC 13706) cultured in modified Scholten's (MS) media (MS broth, MS agar), and φX174 phage (ATCC 13706-B1) was used as reference. For assaying F-RNA phages (adjusted standard ISO 10705, version 1, December 1996), Salmonella enterica serovar Typhimurium WG49 cells (NCTC 12484) cultured in tryptone-yeast extract-glucose (TYG) medium (TYG broth, TYG agar) were used as host bacteria and MS-2 phage (ATCC 15597-B1) was used as reference. To confirm the bacteriophages detected to be RNA bacteriophages, the samples were assayed in parallel with RNase (from bovine pancreas; ICN Biomedicals, Costa Mesa, Calif.). Phages infecting B. fragilis (3c) were detected by using strain RYC2056 (ATCC 700786) anaerobically cultured in bacteroides phage recovery medium (BPRM broth, BPRM agar), and B56-3 (ATCC 700786-B1) phage was used as reference. Briefly, 15 ml of the supernatants from the samples was assayed for the phages by using 1-ml portions mixed with the appropriate host bacteria and molten agar. The solution was poured on top of a previously prepared semisolid agar layer, which contained half of the mass of the top agar layer, in 90-mm-diameter petri dishes and incubated for 18 ± 2 h at 36 ± 2°C. Incubation for the detection of bacteriophages infecting B. fragilis was performed in an anaerobic jar at the same temperature but for 22 ± 2 h.
Detection and analysis of human viruses.
Ad genomic DNA and enterovirus, HAV, and NLV genomic RNA were extracted by the silica method of Boom et al. (6) with minor modifications (45). The extract was used in cDNA synthesis combined with PCR amplification for the detection of RNA viruses or directly for PCR amplification of Ad DNA. Specific primers used for the nucleic acid amplifications of enterovirus and HAV (43), Ad (3), and NLV (22, 37) have been described previously. cDNA synthesis was performed with 0.5 or 5 μl of eluted RNA in a final 10-μl mixture of 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3; 25°C), 50 mM KCl, 1 mM (each) deoxynucleoside triphosphates, and 25 M first-step PCR reverse primer. The reaction mixture was incubated at 95°C for 5 min before the addition of 5 U of Moloney murine leukemia virus reverse transcriptase (Applied Biosystems) and 10 U of RNase inhibitor (Applied Biosystems). Reverse transcription (RT) was performed at 42°C for 30 min, followed by 5 min of reaction termination at 95°C. The tubes were then chilled on ice, and 10 μl of the RT mixture was added to 40 μl of PCR mixture in a final concentration of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphates, 0.4 μM (each) reverse and forward primers, and 2 U of Taq polymerase (Applied Biosystems). Ten or one microliters of silica-extracted nucleic acids was directly used for PCR amplification of Ad DNA under the conditions already described for PCR. In the PCR assays for Ads, HAVs, and enteroviruses, the first cycle of denaturation was carried out for 2 min at 95°C, followed by 30 amplification cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 1 min with a final extension of 72°C for 5 min. In the NLV PCR assay, amplification was performed with the Taq polymerase AmpliTaq Gold (Applied Biosystems), which needs an initial cycle of 7 to 8 min at 95°C to be activated. This denaturation and activation step was followed by 35 amplification cycles of 94°C for 1 min, 37°C for 1 min, and 72°C for 1 min with a final extension at 72°C for 10 min. Second-round (nested) amplifications were carried out with 1 μl of the first-round amplicon and 49 μl of an identical PCR mixture but with 0.18 μM (each) nested primers for enterovirus or HAV or, alternatively, 0.4 μM NLV- or Ad-nested primers. The cycling parameters were unchanged. Amplicons were analyzed by gel electrophoresis of 15 μl of the reaction mixture in agarose gels (2% NuSieve GTG, 1% SeaKem ME; FMC Bioproducts, Rockland, Maine). Molecular weights were compared with those of HaeIII-digested X174 DNA (Promega Corporation). For quality control, all the samples were analyzed twice in independent experiments with a negative and a positive control for the nucleic acid extraction step and additional controls for every enzymatic step. Undiluted samples and 1:10 dilutions of the nucleic acid extracts were analyzed on all samples in order to reduce the likelihood of false-negative results caused by inhibition of the reactions. The number of positive controls included echovirus type 11 of enterovirus, Ad type 5 (Ad5), and the vaccine strain of HAV. A dilution series from 10−1 to 10−8 was used for the theoretical calculation of enterovirus and HAV particles obtained by end-titration experiments. For Ad, the calculations were made by Taqman real-time PCR. Aliquots (60 μl each) of dilutions for all viruses corresponding to ca. 500 virus particles were used as positive controls during the preparation procedure of viral nucleic acids isolated from shellfish material. For NLV (nonculturable), a fecal sample associated with an outbreak of gastroenteritis was used as a positive control and sequencing analysis confirmed the identity of the strain present as Oberschleissheim virus. Due to the high titer of virus in this particular clinical sample, a 10−3 dilution was used as the NLV control.
Taqman quantitative real-time PCR, Ad primer, and probe design.
A pair of degenerated PCR primers was selected from the conserved region of the first part of the Ad hexon gene. The upstream and downstream primer sequences were 5′-C(AT)T ACA TGC ACA TC(GT) C(CG)G G-3′ and 5′-C(AG)C GGG C(GA)A A(CT)T GCA CCA G-3′, respectively. Two fluorogenic Ad probes, Ad:ACDEF [5′-(6FAM) CCG GGC TCA GGT ACT CCG AGG CGT CCT (TAMRA)-3′] and Ad:B [5′-(6FAM) CCG GAC TCA GGT ACT CCG AAG CAT CCT (TAMRA)-3′], with sequences located between the PCR primers were synthesized by Applied Biosystems. 6FAM stands for 6-carboxyfluorescein and TAMRA is 6-carboxytetramethyl rhodamine. Two different probes were needed to ensure the amplification and detection of all Ad types. When testing the general primer-probe system against all 51 Ad serotypes and 42 additional genome variants, it was necessary to use a specific probe for the detection of the Ad subgenus B members, i.e., Ad3, -7, -11, -14, -16, -21, -34, -35, and -51. The other Ad probe, Ad:ACDEF, was homologous to all the Ad sequences of the remaining types. The origins of the serotypes and the different genome variants of the Ads tested here have been described elsewhere (3).
Quantification of Ad DNA by Taqman PCR.
Amplification was performed in a 25-μl reaction mixture with a PCR core reagent (Applied Biosystems). The reaction mixture contained 5 μl of silica-purified DNA or 10 μl of titrated standard DNA purified by the QIAamp blood mini kit protocol (Qiagen); 1× Taqman core buffer; 5 mM MgCl2; a 200 μM concentration (each) of dATP, dCTP, and dGTP; 400 μM dUTP; a 900 nM concentration of each primer; 225 nM probe; 0.25 U of AmpErase uracil N-glycosylase; and 1 U of Taq Gold polymerase. Following activation of the uracil N-glycosylase (2 min, 50°C) and activation of the AmpliTaq Gold for 10 min at 95°C, 40 cycles (15 s at 95°C and 1 min at 60°C) were performed with an ABI 7700 sequence detector system (Applied Biosystems). The principle of real-time PCR has been described elsewhere (28).
Standard curves were generated by using serial dilutions (range, 10 to 106) of known amounts of linearized plasmids containing the entire hexon region of Ad11p or Ad41. Known amounts of full-length Ad DNA of serotypes 1, 5, 7b, 11a, 19a, and 35 were compared and optimized to the plasmid standard curves. Several serotypes were tested as standard curves for accurate calibration and standardization of the quantification system. Full-length viral DNA from Ad5 was chosen as template for a standard curve since plasmids are high risk factors for contamination. Ad5 DNA was diluted such that 10 μl of the sample contained 5 × 105, 5 × 104, 5 × 103, 5 × 102, 5 × 101, or 5 copies of DNA. All samples were run in triplicate, and positive and negative controls were included. The amount of DNA was defined as the median of the triplicate data obtained.
Typing of viral DNA and cDNA by sequencing.
Ten microliters of each positive nested PCR product was purified by using the QIAquick (Qiagen) PCR purification protocol according to the manufacturer's instructions. Both strands of the purified DNA were sequenced with the ABI Prism BigDye Terminator cycle sequencing kit (version 1.0; Applied Biosystems), according to the manufacturer's instructions. The results were checked with the ABI Prism 3700 DNA analyzer (Applied Biosystems). For suggestion of Ad type, the obtained sequences were compared with all sequences in the GenBank and EMBL databases by using the PubMed National Center for Biotechnology Information BLAST program. So far, hexon sequences are available for 27 out of the 51 different Ad serotypes, which have to be taken under consideration when typing is performed by this method.
In accordance with the protocol described by Henshilwood et al. (29), the method for sequencing NLVs was modified by cloning NLV-positive amplicons prior to sequencing to overcome the problem with mixed infections commonly identified in bivalve mollusks. A minimum of five clones from each specific nested RT-PCR was sequenced to facilitate the identification of more than one target sequence. All clones were sequenced in both orientations. The primers were identified and removed, and ambiguities in base calling were resolved by comparison of ABI 310 chromatograms (Applied Biosystems).
Statistical analyses.
Comparisons of the mean numbers of potential indicator organisms with the mean values of environmental factors were analyzed by using repeated measures analysis of variance followed by Tukey's test to allow pairwise multiple comparison. Analysis of the relationship between the environmental factors and the potential indicator organisms was performed by using multiple linear regression analysis (y = b0 + b1x1 + b2x2 + b3x3 +… + bkxk, where y is the dependent variable, x1 through xk are the k-independent variables, and b0 through bk are the k regression coefficients). Analyses of the relationship between environmental factors, indicator organisms, and binary data of viruses were performed by using multiple logistic regression analyses:
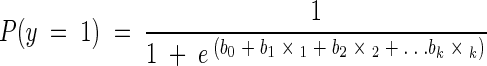 |
where y is the dependent variable, P(y = 1) is the predicted probability that the dependent variable is a positive response or has a value of 1, b0 through bk are the k regression coefficients, and x1 through xk are the independent variables. A homogeneity test of variances was carried out before the analysis, and whenever heterogeneity was proved, a log transformation of data was performed. All of the statistical analyses were performed by using Sigma Stat (version 2; Jandel Scientific Software, San Rafael, Calif.). The level of significance was set at P < 0.05.
RESULTS
Environmental parameters.
During the winter, the water temperature was ca. 1 to 3°C, and during the summer, it was ca. 18 to 20°C (Fig. 2). The temperatures were similar between sites (site A, 9.4 ± 5.9°C; site B, 9.5 ± 6.0°C; site C, 9.9 ± 6.4°C). The salinity was significantly lower at site C (mean salinity = 19.1 ± 2.6 PSU) compared to that at sites A (23.7 ± 3.6 PSU) and B (23.3 ± 3.2 PSU) (one-way repeated measures analysis of variance, P < 0.001). No seasonal fluctuations in salinity were observed. During the first 9 months of the investigational period (referred to as period I), from February 2000 until October 2000, the water flow in the river Göta Älv was slightly higher than the mean value calculated from 34 years (1967 to 2000) of daily measurements (data from the Swedish Meteorological and Hydrological Institute). Thereafter, the flow increased and during the last 9 months (referred to as period II), from November 2000 until July 2001, it was ca. 50% above the mean. The change in the water flow in the river did not seem to influence the salinity at the sampling sites (mean salinities: site A, period I, 22.9 ± 4.6 PSU, and period II, 24.4 ± 2.3 PSU; site B, period I, 22.8 ± 4.1 PSU, and period II, 23.8 ± 2.0 PSU; site C, period I, 19.3 ± 1.8 PSU, and period II, 19.0 ± 3.3 PSU). However, a temporary decrease in salinity was shown at site C during the first 3 months of the flooding period, from December 2000 to January 2001.
FIG. 2.
Graphs from sites A, B, and C showing the occurrence of Ad, enterovirus, and NLV in relation to the presence of E. coli (indicated by columns). The water flow (cubic meters per second) in the river Göta Älv indicating regional land runoff is illustrated with an area diagram, and water temperature (Celsius) and salinity (PSU) are shown with line diagrams (right y axis). The left y axis that indicates the number of E. coli cells (MPN × 100 g−1) is truncated in all three panels to better visualize the differences and fluctuations in the columns. The x axis indicates the time period from February 2000 to July 2001, with each month designated by the first letter of the name of the month. The correct number of E. coli microbes for the following sampling months are as follows: A, March 2001, MPN = 1.6 × 106 × 100 g−1; B, February and March 2001, MPN = 17,500 and 1.6 × 106 × 100 g−1, respectively; C, March 2001, MPN = 1.6 × 106 × 100 g−1.
E. coli.
At both sites B and C, ca. 40% of the E. coli samples exceeded the limit for the A category (MPN, 230 × 100 g−1, according to EC directive 91/492) (Fig. 2). At site A, this limit was exceeded on only one occasion. This occurred in March 2001, when high numbers were found at all sites (MPN, approximately 1.6 × 106 × 100 g−1). Ten of these March E. coli isolates, four from site A, five from site B, and one from site C, were further tested in order to exclude the occurrence of potentially pathogenic strains. In addition, two E. coli isolates that were obtained from mussels at site C in August 2001 were tested. The results from the API 32E test system showed that there were three different groups of E. coli strains in the mussels that March. Group 1 had negative ornithine decarboxylase, lysin decarboxylase, and sorbitol reactions. Group 2 differed from this pattern only by being positive for sorbitol. Group 3 showed positive reactions in the ornithine decarboxylase and lysin decarboxylase tests and was sorbitol negative. An uncommon characteristic for all three groups was a negative indole reaction. However, the two E. coli isolates from site C obtained in August 2001 were indole positive. One isolate was also 5-ketogluconate positive, while the other could ferment d-arabitol. All isolates fit a nonenterohemorrhagic E. coli profile. This was especially clear for the rhamnose, trehalose, β-glucuronidase, and β-glucosidase reactions (34). This was also further confirmed by PCR analyses of the potential verotoxin 1 and 2 genes and by 16S rRNA gene sequencing.
When the extremely high values found in March were excluded, the mean number of E. coli cells was significantly higher at sites B and C compared to that at site A (Table 1). Multiple linear regression analysis showed that the water flow of the river Göta Älv significantly influenced the prediction of the occurrence of E. coli. The numbers of this bacterium increased with water flow (Table 2).
TABLE 1.
Means counts of E. coli cells and bacteriophages and comparisons of sampling sites by Tukey's test
| Site(s) | E. coli | Phage
|
||
|---|---|---|---|---|
| Somatic coliphage | FRNA phage | Phage infecting B. fragilis | ||
| Means ± SEa | ||||
| A | 55 ± 13 | 1,334 ± 169 | 476 ± 97 | 616 ± 208 |
| B | 1,332 ± 1,015 | 2,705 ± 493 | 703 ± 139 | 711 ± 261 |
| C | 723 ± 274 | 4,187 ± 1,605 | 443 ± 102 | 778 ± 287 |
| Comparisons of meansb | ||||
| B vs A | 0.574*c | 0.241* | 0.304 | 0.171 |
| B vs C | 0.003 | 0.033 | 0.540* | 0.474 |
| C vs A | 0.571* | 0.209 | −0.235 | −0.298 |
For E. coli, data are given in MPN × 100 g of sample−1, for phages, data are given in PFU × 100 g of sample−1.
Differences of means of log10-transformed data.
*, P < 0.05.
TABLE 2.
Multiple linear regression analysis describing the relationship between potential indicator organisms for viral contaminants in mussel tissue and the environmental factors water temperature, salinity, and land runoff measured during an investigational period of 18 months
| Log10b (SE b)a | ||||
|---|---|---|---|---|
| Parameter (units) | E. coli | Phage
|
||
| Somatic coliphage | F-RNA bacteriophage | Bacteriophage-infecting B. fragilis | ||
| Intercept | 1.59 (1.31) | 3.66 (0.44) | 4.02 (0.82) | 6.99 (1.03) |
| Temperature (Degrees Celsius) | −0.04 (0.04) | −0.19 (0.01) | −0.03 (0.03) | −0.13 (0.03)*** |
| Salinity (PSU) | −0.01 (0.04) | −0.03 (0.01) | 0.01 (0.03) | 0.02 (0.03) |
| Water flow (m3 × s−1) | 1.4 × 10−3 (0.8 × 10−3)* | 0.49 × 10−4 (2.7 × 10−4) | −1.8 × 10−3 (0.5 × 10−3)*** | −4.6 × 10−3 (0.5 × 10−3)*** |
| Time (months) | 0.01 (0.07) | 0.04 (0.02) | −0.01 (0.04) | 0.07 (0.04) |
b, mean value of the regression coefficient; SE b, standard error of the regression coefficient for 18 measurements. *, P < 0.05; ***, P < 0.001.
Bacteriophages.
The mean number (log10; n = 18) of somatic coliphages was significantly higher at site B compared to that at sites A and C (Table 1). At site C, high numbers were found during the winter (Fig. 3). Multiple linear regression analysis showed that the occurrence of this phage was not predicted by the environmental variables (Table 2). The number of F-RNA bacteriophages was significantly higher at site B than that at site C but not higher than that at site A (Table 1). The water flow of the river Göta Älv had a significantly negative correlation to this phage (Table 2). The mean number of phages infecting B. fragilis was similar at all sites (Table 1). This phage could be predicted from the temperature and water flow of the river Göta Älv. The number of phages increased at a low temperature and at a low water flow (Table 2).
FIG. 3.
The potential indicator organisms somatic coliphages, F-RNA phages, and phages infecting B. fragilis (PFU × 100 g−1) measured in mussel tissue from sites A, B, and C during the period from February 2000 to July 2001, with each month designated by the first letter of the name of the month.
Viruses.
HAV was not found in any sample during the investigational period. Ads, enteroviruses, and NLVs were found at all sites (Fig. 2). At site A, viruses were found in 50% of the samples (Fig. 4), and at sites B and C, viruses were found in ca. 60% of the samples. Ads were the most frequently occurring viruses at all sites (site A, 17%; site B, 20%; and site C, 37%). Enteroviruses were found in ca. 15% at all three sites. NLVs were found in ca. 20% of the samples at both sites A and B. At site C, 8% of the samples were NLV positive. Multiple logistic regression analysis showed that the environmental variables influenced the viruses differently (Table 3). Ads were positively influenced by the water flow in the river Göta Älv as well as by the temperature. The environmental variables did not appear to predict the occurrence of enteroviruses or NLVs. Enteroviruses coexisted with the F-RNA phages, but there was no significant relationship between the other viruses and the phages or E. coli.
FIG. 4.
Graphs showing the human viruses detected in mussels from sites A, B, and C. For each site, the relationship between the percentages of virus-free samples and positive findings of enterovirus, Ad, and NLV, respectively, is presented for the investigational period from February 2000 to July 2001.
TABLE 3.
Multiple logistic regression analysis describing the relationship between the NLV and enterovirus and the potential indicator organisms, as well as the environmental parameters during the investigational period of February 2000 to July 2001a
| Parameter |
b (SE Log10b)a
|
||
|---|---|---|---|
| Ad | NLV b | Enterovirus | |
| Intercept | −11.2 (5.2)* | 2.88 (4.96) | −7.0 (6.2) |
| E. coli | 0.3 × 10−6 (1.0 × 10−6) | −0.4 × 10−6 (1.0 × 10−5) | −1.1 × 10−3 (1.3 × 10−3) |
| Somatic coliphages | 1.2 × 10−4 (1.0 × 10−4) | 1.1 × 10−4 (1.0 × 10−4) | 1.6 × 10−4 (2.1 × 10−4) |
| F-specific phages | −3.0 × 10−4 (0.9 × 10−4) | 6.4 × 10−4) (9.1 × 10−4) | 3.7 × 10−3 (1.8 × 10−3)* |
| Phages infecting B. fragilis | 6.2 × 10−4 (0.6 × 10−4) | −0.4 × 10−4 (6.0 × 10−4) | −0.5 × 10−3 (0.6 × 10−3) |
| Temperature | 0.37 (0.14)** | −0.23 (0.18) | −0.33 (0.19) |
| Salinity | 0.09 (0.12) | 0.03 (0.11) | 0.17 (0.14) |
| Water flow | 6.1 × 10−3 (3.2 × 10−3)* | −1.3 × 10−3 (3 × 10−3) | 1.0 × 10−3 (3.5 × 10−3) |
| Sampling site | 0.47 (0.54) | −0.98 (0.61) | 0.73 (0.70) |
| Time | −0.35 (0.17)* | −0.06 (0.17) | 0.27 (0.19) |
For units of measurement, see Table 2.
b, mean value of the regression coefficient; SE b, standard error of the regression coefficient for 18 measurements. *, P < 0.05; **, P < 0.01.
Typing and identification of human viruses isolated from the mussels.
From 17 out of the 19 positive Ad samples, 6 different serotypes could be identified, altogether representing Ad species groups A, B, C, and F (Table 4). The positive homology of strain identification was 95 to 100% in 11 cases, whereas the remaining six samples had positive strain identifications between 90 and 95%. In three cases, DNA was prepared twice from the same batch of mussels. In two of the cases, divergent results were obtained, i.e., two different types from the same area. This can be explained by multicontamination in one single mussel or different types contaminating neighbor mussels in the same batch.
TABLE 4.
Typing and quantification of human Ads isolated from mussels from the three sitesa
| Date | Site | No. of digestive glands analyzed | No. of Ad particles g of digestive gland−1 | Ad type |
|---|---|---|---|---|
| March 2000 | B | 13 | 116 | Ad2 |
| March 2000 | A | 12 | ND | Ad5 |
| May 2000 | A | 14 | 103 | Ad2 |
| May 2000 | C | 14 | 153 | ND |
| September 2000 | B | 20 | 78 | Ad5 |
| September 2000 | C | 20 | 30 | ND |
| November 2000 | C | 20 | ND | Ad41 |
| January 2001 | C | 20 | 14 | Ad41/7 |
| February 2001 | A | 19 | 27 | Ad2 |
| February 2001 | C | 20 | 11 | Ad41 |
| March 2001 | C | 20 | 33 | Ad31/2 |
| May 2001 | B | 20 | 263 | Ad41 |
| May 2001 | A | 20 | 62 | Ad41 |
| May 2001 | C | 20 | 1,994 | Ad7 |
| June 2001 | B | 21 | 1,866 | Ad7 |
| June 2001 | C | 21 | 590 | Ad1 |
| July 2001 | B | 20 | 33 | Ad2 |
| July 2001 | A | 20 | 735 | Ad7 |
| July 2001 | C | 20 | 15 | Ad2 |
Results from the quantification analysis are expressed as the number of adenovirus particles found per gram of mixed digestive glands; the weight of each digestive gland differed from 0.5 to 1.5 g. In samples from January 2001 and March 2001, both representing site C, typing was done twice by two separate DNA extractions, with results indicating mixed infections. ND, not detected.
NLVs can be divided into two different genogroups based on sequence diversity. Genogroup-specific primers were used in the PCR assay for group identification, but the results in the tables and figures present the total number of positive NLV samples regardless of group identity. Cloning and sequencing among 14 positive NLV samples could identify five different strains; two of the strains were from the same batch of mussels. In all of the strains found, the results from the sequence divergence-similarity plot showed a positive strain identification of ≥95%. Three strains were of genogroup I origin, two were strain Valetta (sites A and B, January 2001), and one was strain Haytread (site B, October 2000). Two samples were identified as genogroup II strains, one was identified as strain Grimsby (site C, February 2000), and the other was identified as strain Hawaii (site B, October 2000).
Quantification of Ad particles.
Seventeen out of the 19 positive Ad samples could also be quantified for the number of virus particles. In 10 samples, the number of virus particles × g of digestive gland−1 was low (<100); in 6 samples, the number of particles was between 100 and 700; and in 2 samples, the number of virus particles was ca. 2,000 × g of digestive gland−1 (Table 4). The high levels were detected in May and June 2001 in batches from sites B and C, and both represented Ad7.
DISCUSSION
The high frequency of human viruses that we found in Swedish mussels was not expected. Sweden has strict legislation concerning the treatment of wastewater and sludge, and untreated water is not discharged into the environment. However, the inactivation and removal of potent human pathogens throughout the sewage treatment processes have not been explored. Pathogens entering the marine environment may be recycled to the community through, e.g., filter-feeding bivalves. Hence, the content of enteric viruses in bivalves should occur sporadically, depending on the viral circulation in the community. Today, there are no situations where HAV is endemic in Sweden, and this virus was not found in any samples. Occasionally, limited outbreaks have been caused by the consumption of mainly imported contaminated food. Another potent human pathogenic virus in shellfish, NLV, was, however, present at all sampling sites, and it is suggested that only a few particles are needed to cause an acute episode of gastroenteritis (16, 33, 36). Of the five strains that were possible to identify, two were homologous to the strain Valetta, a newly emergent strain that has now become the most widely circulating strain in Europe. The community-circulating theory was supported as the Valetta strain, found in our study at sites A and B in January 2001, was also isolated and identified in human fecal material collected in the regional community during the same period (N. Nenonen [Academy of Sahlgrenska, University of Göteborg, Göteborg, Sweden], personal communication). Epidemiological studies for enteroviruses and Ads are difficult to perform, since these viruses very often act endemically and those infected by the viruses may act as carriers without showing any symptoms. Most of the Ads identified represented respiratory types (Ad1, -2, -5, and -7) that are normally circulating in society and can be excreted in human fecal material a long time after infection (2, 17). Ad31 can, e.g., give gastrointestinal problems, and Ad41 is a well-known cause of severe gastrointestinal infection in children, both types, then, also expected in sewage water. The quantified levels of Ad particles found in the mussels were quite low, and we do not know if these quantities are sufficient for infecting humans. However, in May and June 2001, we were able to detect higher levels of Ad in two different batches, both representing Ad7. Since Ad7 causes a respiratory infection, we suggest that this type is not responsible for disease because it passes through the gastrointestinal tract. Further studies are needed to define the relationship between the level of enterovirus and Ad contamination detected by PCR in shellfish and the potential effect of these shellfish after consumption.
The amount of fecal contaminants in seawater is supposed to be highly linked to domestic drainage. Besides the disposal from wastewater treatment plants, there are small river outlets and diffuse land runoff which can be affected by agricultural activities and septic tank leakages, etc. In this study, the water flow of the river Göta Älv was used to estimate the variability of land runoff. The river enters the Swedish west coast approximately 50 nautical miles upstream of the sampling area (Fig. 1) and does not directly affect the sampling sites. Thus, it was used as an independent variable reflecting the more general amount of land runoff at the Skagerrak part of the coast. During November and December 2000, there was an extraordinary situation, with heavy rains and flooded areas in the western part of Sweden. From the winter until the thawing of the ground in March 2001, much of the floodwater was retained, frozen, in the ground. Based on 34 years of daily measurements in the river Göta Älv, the water flow was 50% higher during the period of November 2000 to July 2001 than the long-term mean. This high flow of the river indicated a large input of domestic drainage to the seawater during the latter half of the investigational period.
Multiple regression analysis showed that this land runoff could not be used to predict the presence of all the different viruses or the potential indicator organisms. The occurrences of Ads were more frequent when the land runoff was high, and so was the number of E. coli bacteria. On the other hand, in such situations, the number of phages infecting B. fragilis and F-RNA phages decreased. These contradictory results may indicate that the microbes are tracers of different water masses. The origin of surface water is known to be highly variable in the sampling area (12, 23). Briefly, the Jutland coastal current brings water to the Swedish west coast from the German Bight and the Baltic current brings water from the Baltic Sea. High-salinity North Sea water can enter from Skagerrak, and the land runoff can also affect the coastal water. The exchange and/or mixing of these water masses can be very dynamic, and their temporal and spatial variability is not possible to forecast for more than a few days. At the two sampling sites, situated in the fjords, ca. 40% of the samples were contaminated by E. coli to a level which exceeded the limit for category A for shellfish sanitary safety (EC directive 91/492). In the outer archipelago, this limit was exceeded just once. However, we did not find any relationship between high numbers of E. coli bacteria and viral contaminants. Enteric viruses were found in 50 to 60% of the mussel samples, and there were no pronounced differences among the three sites. Thus, E. coli might be useful for tracing local sources of contamination but not as an indicator of the more-resistant enteric viruses in mussels.
The extremely high numbers of E. coli bacteria that were found in March 2001 (MPN, ca. 1.6 × 106 × 100 g−1) cooccurred with the thawing of the frozen ground. Whether these released E. coli bacteria were of animal or human origin could not be determined from our data. E. coli is a common commensal or parasitic inhabitant of the intestine in all mammals and most birds, and it can survive in fertilized soil for several months (49). Analyses of the biochemical pattern obtained through the API 32E system showed that all the tested isolated strains resembled nonenterohemorrhagic E. coli strains. This was also confirmed by negative PCRs for the verotoxins and by analyses of the partial 16S rRNA gene sequences. However, all the March strains were indole negative. This is a very uncommon characteristic for E. coli. A positive indole reaction is normally used as a basic criterion for the proper identification of E. coli in both medical and food samples (7, 30, 42). Thus, this basic identification characteristic has to be reconsidered, at least in dealing with the analysis of mussels. Since the few isolates obtained from mussels in August showed a positive indole phenotype, we can only speculate that environmental factors favored the negative phenotype during wintertime.
Besides the influence of the water flow, the microbes were also different in their appearance in relation to water temperature. There was a significant increase in the occurrence of Ads with increased temperature, but the other viruses were not influenced by temperature according to the overall statistical results. Seasonal differences describing large amounts of NLVs in shellfish during the winter period, from October to March, have been reported (29). However, we could find just the slightest indication of an increased level of NLVs during the cold months of the year at sites A and B (Fig. 2). Among the potential indicators, the phages infecting B. fragilis were the only ones that were significantly affected by temperature, but in contrast to the Ad, this type of phage decreased in number with temperature. Elimination of bacteria in contaminated bivalves is known to be less effective at low temperatures (18, 27). Likewise, it has been shown that the ability of bivalve hemocytes to kill bacteria is inhibited at low temperatures (19). The extremely large quantity of land runoff that dominated half of the investigational period was recorded over seasons and may have overshadowed the influence of temperature. This means that the influence of temperature on the occurrence of the different microbes may have been underestimated.
This study showed that the sampling sites represented a gradient in the amount of domestic drainage in relation to local origin. In comparison to the outer archipelago, sites B and C seemed to have a larger local supply of pathogens in connection with a high degree of land runoff. However, the water masses that dominate the outer archipelago seemed to bring long-distance-transported viruses to this area. Therefore, the viral contaminants were at a level in the outer archipelago similar to that in the areas more influenced by land runoff. This could not be traced with the indicator organism E. coli. It should be pointed out that we chose to examine mussels which were feeding close to the shore. Mussels from farms, which are placed more distant from the shore, most likely contain fewer E. coli cells. The underestimation of viral fecal contamination may therefore be even worse for those mussels. In addition, when assaying viruses in the mussels, only the digestive gland was used due to its analytical advantages over using the whole animal. This might give an underestimation of the viruses since these can also be absorbed through the gills (1). Thus, the mismatch between E. coli and viruses can in reality be even larger than we had observed. Among the potential indicator organisms, the F-RNA phages could be regarded as a reliable indicator with respect to enteroviruses but certainly not for Ads. Doré et al. (15) showed a satisfactory correlation between F-RNA bacteriophages and NLVs in oysters from the United Kingdom. Their evaluation was based on a comparison of the correlations between the geometric means of the phages and the frequency of the viruses between sites. Our three sites were too few for such a site level evaluation. This kind of evaluation can be used to classify areas for sanitary safety, but it will not tell if a certain batch of mussels is virus contaminated.
With respect to the influence of the environmental factors observed in our study, none of the phages can be regarded as a substantially better indicator organism than E. coli was. Although the F-RNA phage could be useful for the prediction of enteroviruses, it is uncertain if this indicator will increase the safety. NLV is considered the most potent infectious agent of the viruses used in this investigation, and the NLV in mussels seemed to reflect its circulation in the community. Additionally, the types of Ads found in our study were shown to be human specific. Although Ad found in mussels may not be infectious, this virus was, in agreement with other studies, the most prevalent (20, 39, 43) and thus probably a representative indicator of fecal contaminants. Moreover, Ad is a DNA virus and thus, the detection procedure is somewhat simple compared to the procedure for RNA viruses. In addition, it has been found in previous studies on the viral contamination of seawater and shellfish (39, 43) that human Ads were generally detected when enterovirus and HAV were found to be positive. However, a molecular approach may have benefits for the future, but still this can only be performed in specialized laboratories with high-technology facilities. However, to improve the safety of mussel consumption, the overall goal must be to improve the methodology so that it can be used for routine analyses. E. coli used together with, e.g., NLV or Ad for the verification of “clean” areas would be a better guarantee of sanitary safety. Prior to the implementation of the methodology, a risk assessment should be done for every specific area of interest and special emphasis should be placed on environmental factors such as temperature and land runoff. Mussel sanitary control programs may be designed differently due to the variability of these factors. An approach not tested in this study is to investigate the importance of and possibilities for pretreatments, like the proper preparation of bivalves before consumption, to reduce the content of pathogens.
Acknowledgments
This research was supported by FAIR-CT98-4039 from the European Union and the Swedish Foundation for Strategic Environmental Research (MISTRA; SuCoZoMa).
We thank K. Henshilwood, CEFAS, Weymouth, Dorset, United Kingdom, for help with identifying NLV strains and Kjell Pettersson, School of Economics and Commercial Law, Göteborg University, Göteborg, Sweden, for statistical advice.
REFERENCES
- 1.Abad, F. X., R. M. Pinto, R. Gajardo, and A. Bosch. 1997. Viruses in mussels: public health implications and depuration. J. Food Prot. 60:677-681. [DOI] [PubMed] [Google Scholar]
- 2.Allard, A., B. Albinsson, and G. Wadell. 1992. Detection of adenoviruses in stools from healthy persons and patients with diarrhea by two-step polymerase chain reaction. J. Med. Virol. 37:149-157. [DOI] [PubMed] [Google Scholar]
- 3.Allard, A., B. Albinsson, and G. Wadell. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 39:498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Anonymous. 1996. ISO 10705-1 (amended version): Water quality. Detection and enumeration of bacteriophages-part 1: enumeration of F-specific RNA bacteriophages. International Organization for Standardization, Geneva, Switzerland.
- 3b.Anonymous. 1999. ISO/FDIS 10705-2: Water quality. Detection and enumeration of bacteriophages-part2: enumeration of somatic coliphages. International Organization for Standardization, Geneva, Switzerland.
- 3c.Anonymous. 1999. ISO/FDIS 10705-2: Water quality. Detection and enumeration of bacteriophages-part 4: enumeration of bacteriophages infecting Bacteroides fragilis. International Organization for Standardization, Geneva, Switzerland.
- 4.Araujo, R., A. Puig, J. Lasobras, F. Lucena, and J. Jofre. 1997. Phages of enteric bacteria in fresh water different levels of fecal pollution. J. Appl. Microbiol. 82:281-286. [DOI] [PubMed] [Google Scholar]
- 5.Armon, R., and Y. Kott. 1995. Distribution comparison between coliphages and phages of anaerobic bacteria (Bactericides fragilis) in water sources and their reliability as faecal pollution indicator in drinking water. Water Sci. Technol. 31:215-222. [Google Scholar]
- 6.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Norordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulte, M., and G. Reuter. 1989. Glucuronidase detection and indol capillary test as reliable rapid identification procedures for the detection of E. coli in foods—toxinogenic strains included. Zentbl. Hyg. Umweltmed. 188:284-293. [PubMed] [Google Scholar]
- 8.Cabelli, V. J., and W. P. Heffernan. 1970. Accumulation of Escherichia coli by the northern quahaug. Appl. Microbiol. 19:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabelli, V. J., and W. P. Heffernan. 1971. The elimination of bacteria by the northern quahaug: variability of the response of individual animals and development of criteria. Proc. Natl. Shellfish Assoc. 61:102-108. [Google Scholar]
- 10.Carlson, D. J., D. W. Townsend, A. L. Hilyard, and J. F. Eaton. 1984. Effect on an intertidal mudflat on plankton of overlying water column. Can. J. Fish. Aquat. Sci. 41:1523-1528. [Google Scholar]
- 11.Cohen, R. D. H., P.V. Dresler, E. J. P. Phillips, and R. L. Cory. 1984. The effect of the Asiatic clam, Corbicula fluminea, on phytoplankton of the Potomac River, Maryland. Limnol. Oceanogr. 29:170-180. [Google Scholar]
- 12.Danielssen, D. S., L. Edler, S. Fonselius, L. Hernroth, M. Ostrowski, E. Svendsen, and L. Talpsepp. 1997. Oceanographic variability in the Skagerrak and Northern Kattegat, May-June, 1990. ICES (Int. Counc. Explor. Sea) J. Mar. Sci. 54:753-773. [Google Scholar]
- 13.Donovan, T. J., S. Gallacher, N. J. Andrews, M. H. Greenwood, J. Graham, J. E. Russell, D. Roberts, and R. Lee. 1998. Modification of the standard method used in the United Kingdom for counting Escherichia coli in live bivalve molluscs. Commun. Dis. Public Health 1:188-196. [PubMed] [Google Scholar]
- 14.Doré, W. J., and D. N. Lees. 1995. Behavior of Escherichia coli and male-specific bacteriophage in environmentally contaminated bivalve molluscs before and after depuration. Appl. Environ. Microbiol. 61:2830-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doré, W., K. Henshilwood, and D. N. Lees. 2000. Evaluation of F-specific RNA bacteriophage as a candidate human enteric virus for bivalve molluscan shellfish. Appl. Environ. Microbiol. 66:1280-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 17.Fox, J. P., C. E. Hall, and M. K. Cooney. 1977. The Seattle virus watch. VII. Observations of adenovirus infections. Am. J. Epidemiol. 105:362-386. [DOI] [PubMed] [Google Scholar]
- 18.Fuks, D., and Z. Filic. 1977. Microbiological control of shellfish growing waters and bacteria elimination by mussel Mytilus galloprovincialis. Acta Biol. Iugosl. Ser. E Ichthyol. 9:101-106. [Google Scholar]
- 19.Genthner, F. J., A. K. Volety, L. M. Oliver, and W. S. Fisher. 1999. Factors influencing in vitro killing of bacteria by hemocytes of the eastern oyster (Crassostrea virginica). Appl. Environ. Microbiol. 65:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girones, R. 2002. Development of techniques for monitoring and control of human viral contamination in shellfish, EC project FAIR CT 98 4039. Department of Microbiology, Biology School, University of Barcelona, Barcelona, Spain.
- 21.Grabow, W. E. K., R. Kfir, and B. Genthe. 1993. Advantage and disadvantage of the use of immunodetection techniques for the enumeration of microorganisms and toxins in water. Water Sci. Technol. 27:243-252. [Google Scholar]
- 22.Green, J., K. Henshilwood, C. I. Gallimore, D. W. G. Brown, and D. N. Lees. 1998. A nested reverse transcriptase PCR assay for detection of small round-structured viruses in environmentally contaminated molluscan shellfish. Appl. Environ. Microbiol. 64:858-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson, B. 1999. High frequency variability of the surface layers in the Skagerrak during SKAGEX. Continental Shelf Res. 19:1021-1047. [Google Scholar]
- 24.Haamer, J., et al. 1997. The mussel industry of Sweden. NOAA (Natl. Ocean Atmos. Adm.) Tech. Rep. NMFS (Natl. Mar. Fish. Serv.) 129:1-6. [Google Scholar]
- 25.Haamer, J., A.-S.Holm, L. Edebo, O. Lindahl, F. Norén, and B. Hernroth. 1999. Strategisk Musselodling för att Skapa Kretslopp och Balans i Ekosystemet-Kunskapsöversikt och Förslag till Åtgärder. Fiskeriverket Rapp. 6:5-29. [Google Scholar]
- 26.Havelaar, A. H. 1987. Bacteriophages as model organisms in water treatment. Microbiol. Sci. 4:362-364. [PubMed] [Google Scholar]
- 27.Haven, D. S., F. O. Perkins, R. Morales-Alamo, and M. W. Rhodes. 1977. Coliform depuration of Chesapeake Bay oysters, p. 49-59. In D. S. Wilt (ed.), Proceedings of the 10th National Shellfish Sanitation Workshop, U.S. Department of Health, Education, and Welfare, Shellfish Sanitation Branch, Washington, D.C.
- 28.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 29.Henshilwood, K., J. Green, and D. N. Lees. 1998. Monitoring the marine environment for small round structured viruses (SRSVS): a new approach to combine the transmission of these viruses by molluscan shellfish. Water Sci. Technol. 12:51-56. [Google Scholar]
- 30.Iritani, B., and T. J. Inzana. 1988. Evaluation of a rapid tube assay for presumptive identification of Escherichia coli from veterinary specimens. J. Clin. Microbiol. 26:564-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kator, H., and M. Rodes. 1993. Evaluation of male-specific coliphage as indicators of fecal contamination in point and nonpoint source impacted shellfish growing areas. Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, Va.
- 32.Kohn, M. R., T. A. Farley, T. Ando, M. Curtis, S. A. Wilson, Q. Jin, S. S. Monroe, R. C. Baron, L. M. Mcfarland, and R. I. Glass. 1995. An outbreak of Norwalk virus gastroenteritis associated with eating raw oysters—implications for maintaining safe oyster beds. JAMA 273:466-471. [DOI] [PubMed] [Google Scholar]
- 33.Kukkula, M., L. Maunula, E. Silvennoinen, and C.-H. von Bonsdorff. 1999. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk-like viruses. J. Infect. Dis. 180:1771-1776. [DOI] [PubMed] [Google Scholar]
- 34.Leclercq, A., B. Lambert, D. Pierard, and J. Mahillon. 2001. Particular biochemical profiles for enterohemorrhagic Escherichia coli O157:H7 isolates on the ID 32E system. J. Clin. Microbiol. 39:1161-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lees, D. 2000. Viruses in bivalve shellfish. Int. J. Food Microbiol. 59:81-116. [DOI] [PubMed] [Google Scholar]
- 36.Le Guyader, F., F. H. Neill, M. K. Estes, S. S. Monroe, T. Ando, and R. T. Atmar. 1996. Detection and analysis of a small round-structured virus strain in oysters implicated in an outbreak of acute gastroenteritis. Appl. Environ. Microbiol. 62:4268-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maguire, A. J., J. Green, D. W. G. Brown, U. Desselberger, and J. J. Gray. 1999. Molecular epidemiology of outbreaks of gastroenteritis associated with small round-structured viruses in East Anglia, United Kingdom, during the 1996-1997 season. J. Clin. Microbiol. 37:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matches, J. R., and C. Abeyta. 1983. Indicator organisms in fish and shellfish. Food Technol. 37:114-117. [Google Scholar]
- 39.Muniain-Mujika, I., R. Girones, and F. Lucena. 2000. Viral contamination of shellfish: evaluation of methods and analysis of bacteriophages and human viruses. J. Virol. Methods 89:109-118. [DOI] [PubMed] [Google Scholar]
- 40.Nichols, F. H. 1985. Increased benthic grazing: an alternative explanation for low phytoplankton biomass in Northern San Francisco Bay during the 1976-1977 drought. Estuar. Coast. Shelf Sci. 21:379-388. [Google Scholar]
- 41.Norén, F., Haamer, J., and O. Lidahl. 1999. Changes in the plankton community passing a Mytilus edulis mussel bed. Mar. Ecol. Prog. Ser. 191:187-194. [Google Scholar]
- 42.Perez, J. L., C. I. Berrocal, and L. Berrocal. 1986. Evaluation of a common beta-glucuronidase test for the rapid and economical identification of Escherichia coli. J. Appl. Bacteriol. 61:541-545. [DOI] [PubMed] [Google Scholar]
- 43.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Power, U. F., and J. K. Collins. 1990. Tissue distribution of a coliphage and Esherichia coli in mussels after contamination and depuration. Appl. Environ. Microbiol. 56:803-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puig, M., J. Jofre, F. Lucena, A. Allard, G. Wadell, and R. Girones. 1994. Detection of adenovirus and enterovirus in polluted waters by nested PCR amplification. Appl. Environ. Microbiol. 60:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards, G. P. 1985. Outbreaks of shellfish-associated enteric virus illness in the United States: requisite for development of viral guidelines. J. Food Prot. 48:815-823. [DOI] [PubMed] [Google Scholar]
- 47.Richards, G. P. 1988. Microbial purification of shellfish: a review of depuration and relaying. J. Food Prot. 51:218-251. [DOI] [PubMed] [Google Scholar]
- 48.Rippey, S. R. 1994. Infectious diseases associated with molluscan shellfish consumption. Clin. Microbiol. Rev. 7:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selander, R. K., D. A. Caugant, and T. S. Whittam. 1983. Genetic structure and variation in natural populations of Escherichia coli. Am. Nat. 122:732-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sockett, P. N., P. A. West, and M. Jacob. 1985. Shellfish and public health. PHLS Microbiol. Digest 2:29-35. [Google Scholar]
- 51.Welinder-Olsson, C., E. Kjellin, M. Badenfors, and B. Kaijser. 2000. Improved microbiological techniques using the polymerase chain reaction and pulsed-field gel electrophoresis for diagnosis and follow-up of enterohaemorrhagic Escherichia coli infection. Eur. J. Clin. Microbiol. Infect. Dis. 19:843-851. [DOI] [PubMed] [Google Scholar]
- 52.Wilson, I. G., and J. E. Moore. 1996. Presence of Salmonella spp. and Campylobacter spp. in shellfish. Epidemiol. Infect. 116:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization and United Nations Environmental Programme (UNEP). 1987. Environmental quality criteria for shellfish-growing waters and shellfish in the Mediterranean. WHO/EURO document EUR/ICP/CEH 051. World Health Organization—Europe, Copenhagen, Denmark.
- 54.Wright, R. T., R. B. Collin, C. Persing, and D. Pearson. 1982. Field and laboratory measurements of bivalve filtration of natural marine bacterioplankton. Limnol. Oceanogr. 27:91-98. [Google Scholar]






