Abstract
During aerobic degradation of naphthalene-2-sulfonate (2NS), Sphingomonas xenophaga strain BN6 produces redox mediators which significantly increase the ability of the strain to reduce azo dyes under anaerobic conditions. It was previously suggested that 1,2-dihydroxynaphthalene (1,2-DHN), which is an intermediate in the degradative pathway of 2NS, is the precursor of these redox mediators. In order to analyze the importance of the formation of 1,2-DHN, the dihydroxynaphthalene dioxygenase gene (nsaC) was disrupted by gene replacement. The resulting strain, strain AKE1, did not degrade 2NS to salicylate. After aerobic preincubation with 2NS, strain AKE1 exhibited much higher reduction capacities for azo dyes under anaerobic conditions than the wild-type strain exhibited. Several compounds were present in the culture supernatants which enhanced the ability of S. xenophaga BN6 to reduce azo dyes under anaerobic conditions. Two major redox mediators were purified from the culture supernatants, and they were identified by high-performance liquid chromatography-mass spectrometry and comparison with chemically synthesized standards as 4-amino-1,2-naphthoquinone and 4-ethanolamino-1,2-naphthoquinone.
Wastewaters from textile industries are often highly colored due to residual dyestuff from the dyeing processes. It has been estimated that up to 60% of the total dyestuff used in the dyeing processes may be found in the wastewaters (3). Only small amounts of these dyes are removed by conventional aerobic biological wastewater treatment systems (37, 46). On the other hand, it is well known that azo dyes are reduced anaerobically by different microorganisms, which usually results in the generation of colorless aromatic amines (2, 5, 7, 12, 14, 51, 56). These amines are in most cases recalcitrant under anaerobic conditions (6). Therefore, it has been repeatedly suggested that two-stage anaerobic-aerobic treatment systems should be used to reduce the azo dyes anaerobically and to mineralize the amines formed in a subsequent aerobic stage (1, 36, 38, 45, 51, 54). The first successful example of mineralization of a sulfonated azo dye by an anaerobic-aerobic treatment process involved a 6-aminonaphthalene-2-sulfonate-degrading mixed bacterial culture. This culture consisted of the naphthalenesulfonate-degrading strain Sphingomonas xenophaga BN6 in a mutualistic coculture with other bacterial strains (21, 34, 50). Recently, it was found that strain BN6 uses a new mechanism for conversion of azo dyes. During aerobic degradation of naphthalene-2-sulfonate (2NS), redox mediators are released, which mediate the reduction of azo dyes under anaerobic conditions (27). It was proposed that these redox mediators shuttle electrons from the cells to the azo dyes, which results in purely chemical, extremely nonspecific, extracellular reductive cleavage of the azo bond. Aerobic preincubation of strain BN6 with 2NS increased subsequent anaerobic reduction of the azo dyes almost 20-fold. It was suggested that 1,2-dihydroxynaphthalene (1,2-DHN), which is the first detectable intermediate formed from 2NS, or its autoxidation product(s) acts as a quinone-hydroquinone redox couple during the anaerobic reduction of the azo dyes (27). The results of the previous study suggested that the presumed redox mediators were present only at very low concentrations in the culture supernatants of strain BN6. Therefore, in the present study we attempted to increase the concentration of the redox mediators formed by genetic techniques and to analyze the products by highly sensitive liquid chromatography (LC)-mass spectrometry (MS) techniques in order to identify the naturally formed redox mediator(s).
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. Strain BN6 was grown at 30°C on minimal medium (34) containing 0.5% (wt/vol) glucose (MMG) as the sole source of carbon and energy. Mutants of BN6 were grown on MMG supplemented with the appropriate antibiotic. Escherichia coli strains were cultivated at 37°C in 2×YT medium. The antibiotics ampicillin, kanamycin, and tetracycline were used at final concentrations of 100, 50, and 20 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| S. xenophaga BN6 strains | ||
| Wild type (DSM 6383) | NSA+ | 34 |
| AKE1 | ΔnsaC::neo (ΔDHNDO), Kmr | This study |
| E. coli strains | ||
| JM109 | endA1 recA1 gyrA96 thi hsdR17 relA1 supE44 Δ(lac-proAB) F′[traD36 proAB lacIqlacZΔM15] | 57 |
| SI7-1 | recA pro hsdR hsdM+ RP4(Tc::Mu, Km::Tn7, Tpr Smr) | 47 |
| Plasmids | ||
| pAKE35.1 | Conjugative plasmid for gene replacement containing the sacB gene; Tcr | 26 |
| pAKE14 | pAKE35.1 carrying a fragment of the 3′ and 5′ flanking region of nsaC and neo cloned into XbaI/NdeI restriction sites; Tcr Kmr | This study |
| pUT/Km | Kmr | 22 |
NSA+, conversion of naphthalenesulfonate; ΔDHNDO, insertion mutant of the gene coding for the dihydroxynaphthalene dioxygenase.
Turnover of naphthalenesulfonates by resting cells of strain BN6.
Cells of strain BN6 were grown aerobically in MMG to an optical density at 600 nm (OD600) of approximately 0.6. Then 0.5 mM salicylate was added to the exponentially growing cells to induce the degradative pathway for 2NS (34), and the cells were grown for an additional 3 h. Cells were harvested by centrifugation, washed once, and resuspended in 50 mM Na/K phosphate buffer (pH 7.3) to an OD600 of about 5. The cell suspensions were incubated with 1 mM naphthalenesulfonate at 30°C on a rotary shaker (200 rpm). Samples (1 ml) were centrifuged (10 min, 20,800 × g) and the supernatants were stored at −20°C until they were analyzed.
Conversion of amaranth with whole cells.
Bacterial cultures (100 to 250 ml) were grown aerobically under the conditions described above until they reached the late exponential growth phase. Cells were harvested by centrifugation (8,000 × g), washed, and resuspended in Na/K phosphate buffer (50 mM, pH 7.7) to an OD600 of about 5. The cell suspensions were transferred to serum bottles, and oxygen was removed by repeated evacuation and flushing with nitrogen gas. The serum bottles were transferred to an anaerobic incubation chamber (Toepfer Lab System, Göppingen, Germany), and aliquots (usually 20 μl) were transferred under strictly anaerobic conditions to the wells of 96-well microtiter plates. The wells of the microtiter plates contained (in a total volume of 200 μl) 50 mM Na/K phosphate buffer (pH 7.7), 10 mM glucose, 0.1 mM amaranth, the appropriate quinone at a concentration of 2 to 200 μM (or, as indicated below, cell supernatants or fractions of supernatants containing the unknown redox mediators), and cells (OD600, ∼0.5). The microtiter plates were transferred to a microtiter plate reader (Power Wave 340; Biotek Kontron, Neufahrn, Germany) which was located inside the anaerobic chamber, and the decrease in absorbance at 520 nm was determined for 30 min (by using 1-min measuring intervals). Reaction rates were calculated by using a molar extinction coefficient (ɛ520) of 27 mM−1 cm−1 (29).
DNA manipulation, DNA preparation, and cell transformation.
Small-scale plasmid preparation was performed by the method of Kieser (28). Genomic DNA was isolated by using a DNA extraction kit (NucleoSpin C+T) purchased from Machery-Nagel (Düren, Germany), and all DNA manipulations were carried out as described by Sambrook et al. (42). All enzymes were purchased from Roche Diagnostics GmbH (Mannheim, Germany) and were used according to the manufacturer's suggestions. E. coli was transformed with plasmid DNA by the method of Chung et al. (11).
Conjugation.
Conjugation with E. coli as the donor and S. xenophaga BN6 as the recipient was performed as described previously (27).
PCR analysis.
The PCR mixtures (total volume, 40 μl) contained 10 to 100 ng of DNA, forward and reverse primers (MWG Biotech GmbH, Ebersberg, Germany) at a concentration of 0.5 μM each, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, each deoxynucleoside triphosphate (Pharmacia Biotech, Uppsala, Sweden) at a concentration of 0.2 mM, and 2.5 U of Taq polymerase (Pharmacia Biotech). The mixtures were placed in a thermal cycler (PTC-200; MJ Research, Watertown, Mass.). The first step consisted of denaturation for 1 min at 94°C and was followed by 30 cycles of denaturation for 1 min at 92°C, annealing of primers for 1 min at 35 to 50°C, and extension for 2 min at 72°C, with extension for 5 min during the last cycle. The PCR fragments were separated by electrophoresis through 1% agarose gels at 10 V/cm and were stained with ethidium bromide.
nsaC gene disruption.
Suicide vector pAKE14 carrying a fusion between the 5′-3′ flanking region of the nsaC gene and the kanamycin resistance (neo) gene was constructed by inserting the neo gene into the coding region of the nsaC gene of wild-type strain BN6 (BN6wt) via gene splicing by overlap extension (SOE) (23). Primers S1690 (5′-TATATCTAGAGCACAGTGCTGACGTGGTAT-3′) and S1691 (5′-CTTGCTGTTTACTGCTCTCCCAAA-3′) were used to amplify a 1-kb fragment of the 5′ flanking region of the nsaC gene by PCR, and primers S1694 (5′-TCTTCTGATAAGCCGCCTCCAGA-C-3′) and S1695 (5′-AATAATTCATATGAATCGGCTTATATTGGACGT-3′) were used to amplify a 1-kb fragment of the 3′ flanking region of the nsaC gene with genomic DNA of strain BN6wt as the template. The neo gene was amplified with its own promoter region by using primers S1692 (5′-AGCAGTAA-ACA-GCA-AGC-GAA-CCG-G-3′) and S1693 (5′-GCGGCTTA-TCA-GAA-GAA-CTC-GTC-A-3′) and pUT/Km (22) as the template DNA to obtain a 944-bp fragment. Primers S1691, S1692, S1693, and S1694 were constructed to obtain hybrid genes of the 5′ and 3′ flanking regions of nsaC and the neo gene by use of SOE (25). To facilitate cloning of the SOE product, XbaI and NdeI restriction sites were added to primers S1690 and S1695, respectively (the relevant sequences are underlined in the oligonucleotide sequences shown above). The SOE product was cut with XbaI/NdeI and inserted into XbaI/NdeI-cleaved pAKE35.1 (26) to obtain pAKE14. Southern blotting was performed by using a digoxigenin-labeled (Roche Diagnostics) nsaC gene fragment, a neo gene fragment, and 5′ and 3′ flanking sequence fragments of nsaC as probes. The sizes of the detected fragments were determined by comparison to HindIII-digested λ DNA, which was used as a marker.
Verification of the introduced mutation.
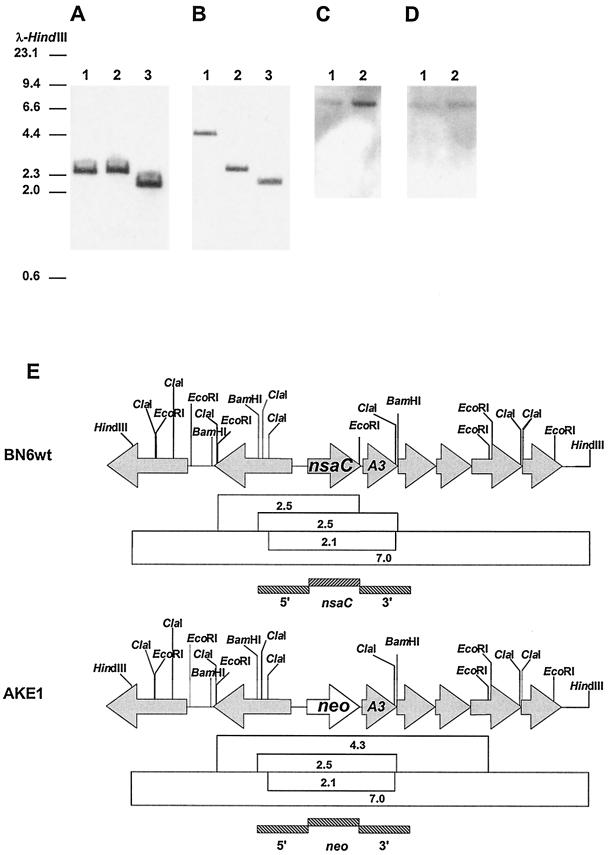
In order to verify integration of the neo gene into the nsaC gene locus, chromosomal DNA from strain AKE1 and the wild-type strain were digested with different restriction enzymes and hybridized with the nsaC gene, with the neo gene, and with 1-kb fragments of the flanking sequences of the nsaC gene (Fig. 1). As expected, the nsaC probe did not hybridize with the chromosomal DNA of strain AKE1 and the neo probe did not hybridize with the chromosomal DNA of the wild-type strain. The hybridization signals of the chromosomal DNA of strains BN6wt and AKE1 with the nsaC and neo genes (Fig. 1A and B) corresponded to the expected DNA fragments according to the restriction maps in Fig. 1E. This was also true for the 5′ and 3′ flanking regions (Fig. 1C and D). The expected 7-kb fragments were detected in the chromosomal DNA of both strains. The molecular analysis revealed that the neo gene was inserted into the nsaC locus by homologous recombination via double crossover, creating mutant strain AKE1.
FIG. 1.
Southern blot analysis of chromosomal DNA of strains AKE1 and BN6wt. DNA was digested, electrophoresed on a 0.7% agarose gel, transferred to a nylon membrane, and hybridized. HindIII-digested λ DNA was used as a marker. (A) Southern hybridization of chromosomal DNA of BN6wt digested with different restriction enzymes using the nsaC gene fragment as the probe. Lane 1, EcoRI; lane 2, BamHI; lane 3, ClaI. (B) Southern hybridization of chromosomal DNA of strain AKE1 digested with different restriction enzymes using the neo gene fragment as the probe. Lane 1, EcoRI; lane 2, BamHI; lane 3, ClaI. (C) Hybridization of HindIII-digested chromosomal DNA using the 1-kb 5′ flanking sequence of nsaC as the probe. Lane 1, strain AKE1; lane 2, strain BN6wt. (D) Hybridization of HindIII-digested chromosomal DNA using the 1-kb 3′ flanking sequence of nsaC as the probe. Lane 1, strain AKE1; lane 2, strain BN6wt. (E) Restriction map of the genomic DNA of these strains. The position of the nsaA3 gene encoding the ferredoxin subunit of the NSDO which was identified adjacent to nsaC (26) is shown. The sizes (in kilobases) of restriction fragments detected by Southern hybridization are indicated in open boxes. DNA fragments representing the probes used for hybridization are indicated by shaded boxes.
Preparation of cell extracts and determination of protein content.
The method used to prepare cell extracts of strain BN6 was described by Nörtemann et al. (34). The protein content was determined by the method of Bradford (4) with bovine serum albumin as the standard.
The protein content of whole cells was determined by a modification of the biuret assay (43) with bovine serum albumin as the standard.
Enzyme assays.
One unit of enzyme activity was defined as the amount of enzyme that converted 1 μmol of substrate per min. 1,2-Dihydroxynaphthalene dioxygenase (DHNDO), 2′-hydroxybenzalpyruvate aldolase, and salicylaldehyde dehydrogenase activities were determined as described previously (30, 31).
The activity of napthalenesulfonate dioxygenase (NSDO) was determined by using whole cells and naphthalene-2-carboxylate as the substrate. NSDO converts naphthalene-2-carboxylate to 1,2-dihydroxy-1,2-dihydronaphthalene-2-carboxylate as a dead-end product (35). Therefore, production of 1,2-dihydroxy-1,2-dihydronaphthalene-2-carboxylate was thought to represent specifically the presence of an NSDO activity in the wild-type and mutant strains.
Analytical methods.
The concentration of the azo dye amaranth was determined spectrophotometrically (Ultraspec 3000; Pharmacia Biotech, Freiburg, Germany) at 520 nm (ɛ520, 27 mM−1 cm−1) (29).
Metabolites resulting from the conversion of 2NS and naphthalene-2-carboxylate were analyzed by high-pressure liquid chromatography (HPLC). An S1121 solvent delivery system equipped with an S3205 UV-visible light (UV-Vis) detector from Sykam GmbH (Gilching, Germany) was used. A reverse-phase column (250 mm by 4 mm [inside diameter]; GROM, Herrenberg, Germany) filled with 5-μm-diameter particles of Lichrospher RP18 (Merck, Darmstadt, Germany) was used as the stationary phase. The mobile phase (flow rate, 0.7 ml min−1) consisted of 50% (vol/vol) methanol, 49.7% (vol/vol) water, and 0.3% (vol/vol) H3PO4. The separated compounds were detected spectrophotometrically at 210 nm and were identified by standard addition of reference compounds.
Initial detection of the quinones which accumulated in the cell supernatants of strain BN6 and its mutants was performed by reverse-phase HPLC. The apparatus (Waters Associates, Milford, Mass.) consisted of two pumps (type 510), an autosampler (type 717), and a photodioide array detector (type 996), which were controlled by the Millenium software. A reverse-phase column (250 by 4.6 mm [inside diameter]) with a precolumn (10 by 4.6 mm) packed with 5-μm particles of Nucleosil 100 was used as the stationary phase (GROM). The separated compounds were detected at 210 nm, at 265 nm, or at the wavelengths of maximal absorbance of the compounds. The solvent system consisted of a sodium formate buffer (20 mM, pH 3.15) and methanol. A solvent gradient with increasing concentrations of methanol (0 to 80%, vol/vol) and a flow rate of 0.4 ml min−1 was used.
For identification of metabolites by LC-tandem MS, a Quattro LC triple quadrupole mass spectrometer (Micromass, Manchester, United Kingdom) equipped with the Z-spray interface and an electrospray probe was used. An HP1100 LC system (Hewlett-Packard) consisting of a membrane degasser, a binary high-pressure gradient pump, an autosampler, and a column thermostat (operated at 40°C) was coupled to the mass spectrometer. A Nucleosil 100-5 C18AB column (125 by 3 mm; Macherey & Nagel) with a precolumn (8 by 3 mm) was used for separation by using a flow rate of 0.5 ml/min and the following binary gradient: at zero time, 20% eluent B; at 2 min, 20% eluent B; at 10 min, 60% eluent B; at 11 min, 60% eluent B; at 12 min, 20% eluent B; and at 17 min, 20% eluent B. Eluent A contained 240 ml of H2O, 12.5 ml of methanol, and 100 μl of acetic acid, and eluent B contained 25 ml of H2O, 225 ml of methanol, and 100 μl of acetic acid.
The mass spectrometer was operated in the positive mode with the probe capillary voltage set at 3.2 kV and the probe tip kept at a temperature of 250°C. The cone voltage was set at 29 V, and the source temperature was 120°C. Daughter ion spectra were recorded from m/z 40 to m/z 250 in 2 s with the collision energies varied between 15 and 30 eV.
Nuclear magnetic resonance (NMR) spectra were recorded with a Bruker ARX 500 spectrometer in deuterated dimethyl sulfoxide at 500.153 MHz.
Quantitation of 4-amino-1,2-naphthoquinone (4-A-1,2-NQ) and 4-ethanolamino-1,2-naphthoquinone (4-EA-1,2-NQ) in culture supernatants of strains BN6 and AKE1.
Bacterial cultures were grown with glucose, induced by addition of salicylate, and finally incubated with 2NS as described above. The culture supernatants (10 ml) were applied to a solid-phase extraction column (500 mg of Butyl C4 [Applied Separations, Allentown, Pa.]), which was preequilibrated with Na/K phosphate buffer (50 mM, pH 7.3). The fractions were eluted with steps consisting of Na/K phosphate buffer (2 ml), water (2 ml), 20% (vol/vol) methanol (0.8 ml), 50% (vol/vol) methanol (0.6 ml), 75% (vol/vol) methanol (0.6 ml), and 100% (vol/vol) methanol (1 ml). The fractions that were obtained with methanol concentrations higher than 50% (vol/vol) were collected (1.5 ml), and the solvent was completely removed under a constant flow of N2. The dry residue was redissolved in 200 μl of methanol-water (1:1, vol/vol) and analyzed by HPLC as described above.
Chemicals.
4-EA-1,2-NQ was synthesized from 1,2-naphthoquinone (1,2-NQ) via 4-ethoxy-1,2-naphthoquinone. The 4-ethoxy-1,2-naphthoquinone was synthesized as described by Takuwa et al. (53). 1,2-NQ (250 mg, 1.6 mmol) was dissolved in 50 ml of ethanol at room temperature, 600 mg (1.6 mmol) of CeCl3 · 7 H2O and 316 mg (1.6 mmol) of sodium iodate were added, and the reaction mixture was stirred for 30 min at room temperature. The mixture was then poured into a 10% aqueous solution of ammonium chloride and extracted repeatedly with chloroform. The combined chloroform phases were washed with water and dried with Na2SO4. The product was purified by column chromatography (Silica Gel 60; Merck) with chloroform as the eluent. The dark red fraction containing the product was collected, and the solvent was evaporated. The precipitated product was recrystallized from 15 ml of a mixture of benzene and hexane (1:2, vol/vol), filtered, and finally dried under a constant stream of nitrogen gas. In this way, 73.5 mg of orange needles was obtained, with the following parameters: mp, 122.5 to 123°C; value given in the literature, 123°C; UV-Vis spectrum (pH 7.7) λmax, 252 nm (ɛ = 44.3 mM−1 cm−1), 282 nm (ɛ = 19.8 mM−1 cm−1), 342 nm (ɛ = 4.3 mM−1 cm−1), and 415 nm (ɛ = 3.9 mM−1 cm−1).
4-EA-1,2-NQ was synthesized from 4-ethoxy-1,2-naphthoquinone and ethanolamine by using a general strategy described by Fieser and Fieser (17) for the synthesis of different 4-alkylamino-1,2-naphthoquinones from 4-ethoxy-1,2-naphthoquinone. 4-Ethoxy-1,2-naphthoquinone (39.4 mg, 0.2 mmol) was dissolved in 1.2 ml of ethanol at 45°C, and ethanolamine (24 μl, 0.4 mmol) was added. This resulted in an immediate change of the color from orange to red and subsequent formation of a red precipitate. After 10 min, the precipitate was collected by centrifugation, washed with 20 μl of cold ethanol, and dried under a stream of nitrogen gas. In this way, 12.4 mg of red platelets was obtained, with the following parameters: mp, 240 to 242°C under decomposition; absorbance maxima at pH 7.7, 238 nm (ɛ = 10.5 mM−1 cm−1), 270 nm (ɛ = 11.5 mM−1 cm−1), 305 nm (ɛ = 7.2 mM−1 cm−1), and 465 nm (ɛ = 3.1 mM−1 cm−1); 1H-NMR (d-DMSO), 8.38 (N-H), 8.16 to 7.62 (m, 5-, 6-, 7-, 8-H), 5.75 (s, 3-H), 3.67 (2H, t, CH2OH), 3.46 (2H, CH2NH); 13C-NMR (d-DMSO), 182.3 (C-1), 175.1 (C-2), 155.5 (C-4), 134.5 to 123.8 (6 C-Ar), 98.6 (C-3), 58.8 (CH2OH), 46.3 (CH2NH); LC-MS retention time, 3.3 min; daughter ions of m/z 218 (M+H)+, m/z 190 (M+H-CO)+, m/z 172 (M+H-CO-H2O)+, and m/z 146 (M+H-CO-CH2CHOH)+.
The LC-MS data for 4-A-1,2-NQ are as follows: retention time, 3.6 min; daughter ions of m/z 174 (M+H)+ and m/z 146 (M+H-CO)+.
Naphthalene-1-sulfonate and 2NS were obtained from Bayer AG, Leverkusen, Germany. trans-2-Hydroxybenzalpyruvate and 2-hydroxychromene-2-carboxylic acid were prepared as described by Kuhm et al. (30, 31). All other chemicals were obtained from Sigma-Aldrich Chemie (Deisenhofen, Germany) or Merck. Biochemicals were obtained from Roche Diagnostics.
Nucleotide sequence accession number.
The sequence of the nahC gene has been deposited in the GenBank nucleotide sequence database under accession number U65001.
RESULTS
Construction of a mutant of S. xenophaga BN6 inactivated in the nsaC gene.
It was suggested previously that the formation of 1,2-DHN during degradation of 2NS was essential for the formation of the extracellular redox mediator(s) (27). The sequence of the gene (nsaC) encoding DHNDO has recently been determined (26). Therefore, we attempted to increase formation of this redox mediator by inactivation of nsaC by gene replacement (10). The suicide vector pAKE14 carrying the levan sucrase gene (sacB) and a fusion between the 5′-3′ flanking region of the nsaC gene and the neo gene was constructed (see Materials and Methods). Plasmid pAKE14 was transformed into E. coli S17-1 and conjugatively transferred to S. xenophaga BN6wt. Transconjugants were selected on MMG agar plates supplemented with kanamycin and tetracycline. Since plasmid pAKE14 (neo tetA) does not replicate in strain BN6, kanamycin- and tetracycline-resistant colonies should be integration mutants (cointegrates) in which the integration cassette has been inserted into the BN6 genome by homologous recombination between the nsaC flanking sequences in plasmid pAKE14 and the BN6 genome. To isolate integration mutants which had lost the vector fragment by homologous recombination, mutants were selected on MMG supplemented with 4% sucrose and kanamycin. The resulting strain, AKE1, was not able to grow on tetracycline. Formation of a double-crossover mutant was verified by different hybridization experiments performed with labeled probes for nsaC and neo (see Materials and Methods).
Biochemical characterization of strain AKE1.
A cell suspension of AKE1 was grown to the early exponential growth phase (OD600 = 0.5) in MMG containing kanamycin. The culture was incubated for an additional 3 h with salicylate (0.5 mM) to induce the 2NS degradation pathway (34). Cell extracts of strains AKE1 and BN6wt grown in the presence or absence of salicylate were prepared, and the DHNDO, 2′-hydroxybenzalpyruvate aldolase, and salicylaldehyde dehydrogenase activities were determined. It was found that strain AKE1 had lost DHNDO activity but retained 2′-hydroxybenzalpyruvate aldolase and salicylaldehyde dehydrogenase activities (Table 2). The presence of an NSDO in the mutant strain was demonstrated by its ability to convert naphthalene-2-carboxylate to 1,2-dihydroxy-1,2-dihydronaphthalene-2-carboxylate. Thus, the biochemical analysis of insertion mutant AKE1 showed that with the exception of the DHNDO activity, the enzyme activities which are involved in the 2NS degradation pathway were unaffected.
TABLE 2.
Biochemical characterization of strain AKE1
| Strain | Growth conditionsa | Sp act (U/g)b
|
|||
|---|---|---|---|---|---|
| NSDO | DHNDO | HBPA | SalDH | ||
| AKE1 | Glucose | 3.2 | 0 | 25 | 82 |
| Glucose + salicyclate | 7.7 | 0 | 158 | 142 | |
| BN6wt | Glucose | 1.4 | 0 | 31 | 84 |
| Glucose + salicyclate | 7.3 | 430 | 156 | 111 | |
Cell suspensions of strain AKE1 (ΔnsaC::neo) and BN6wt were grown exponentially in MMG or in MMG supplemented with 0.5 mM salicylate for 3 h.
Specific enzyme activities for the catabolism of 2NS in strain BN6wt were determined as described in Materials and Methods. Specific activity of the ring-hydroxylating dioxygenase NSDO was measured with resting cells by using naphthalene-2-carboxylic acid as the substrate (35). HBPA, 2′-hydroxybenzalpyruvate aldolase; SalDH, salicylaldehyde dehydrogenase.
Conversion of 2NS by mutant strain AKE1.
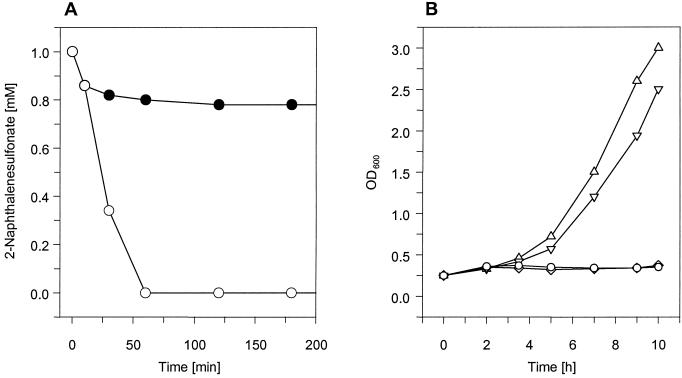
Resting cells of strain AKE1 were incubated with 2NS in order to assay the NSDO activity and to analyze the formation of putative products. The resting cells converted 2NS (initial concentration, 1 mM), but turnover of 2NS was completely inhibited after conversion of about 0.2 mM 2NS (Fig. 2A). In contrast, 2NS was completely converted by the wild-type strain under the same conditions. This suggested that intermediates which were produced during the incomplete degradation of 2NS by AKE1 inhibited the turnover of 2NS and/or were toxic for the mutant strain. To investigate the toxicity of the intermediate(s), different concentrations of 2NS were added to exponentially growing cells of AKE1 (OD600 = 0.3) in MMG. 2NS concentrations higher than 0.1 mM completely inhibited the growth of strain AKE1 (Fig. 2B). This demonstrated that degradation of 2NS by insertion mutant AKE1 was prevented by the accumulation of a toxic intermediate(s). Surprisingly, no accumulation of 1,2-DHN or the autoxidation product 1,2-NQ (or any other metabolite of the 2NS degradative pathway) could be found by HPLC analysis of the culture supernatants.
FIG. 2.
(A) Degradation of 2NS by wild-type strain S. xenophaga BN6 or mutant strain AKE1. Resting cells of strain BN6wt (○) or AKE1 (•) (OD600 = 5) were incubated aerobically with 2NS (1 mM). (B) Growth behavior of strain AKE1 in MMG supplemented with 2NS. No 2NS (▵), 0.01 mM 2NS (▿), 0.1 mM 2NS (○), or 1 mM 2NS (⋄) was added to exponentially growing cells of strain AKE1 (OD600 = 0.3) in MMG at 30°C under aerobic conditions.
Anaerobic reduction of the azo dye amaranth with strain AKE1.
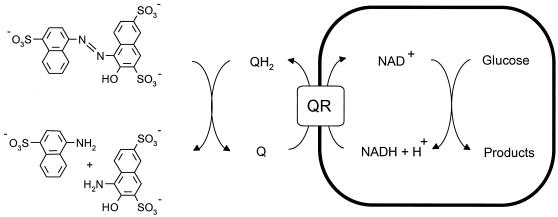
Previously, it was suggested that 1,2-DHN or its autoxidation product(s) acts under anaerobic conditions as a redox mediator(s) which increases the rate of reduction of azo dyes (27).Therefore, we examined whether strain AKE1 has an enhanced azo dye reduction capacity compared to that of BN6wt. The strains were grown with glucose and salicylate, incubated aerobically with 2NS (0.5 mM), and then incubated anaerobically with amaranth (0.5 mM) (the structural formula of amaranth is shown in Fig. 3) and glucose (10 mM). Strain AKE1 showed a much higher reduction rate for amaranth (4.1 μmol of amaranth min−1 g of soluble cell protein−1) than strain BN6wt (1.5 μmol of amaranth min−1 g of soluble cell protein−1).
FIG.3.
Proposed mechanism for the reduction of amaranth by S. xenophaga BN6 in the presence of a quinoid redox mediator (Q). QR, quinone reductase.
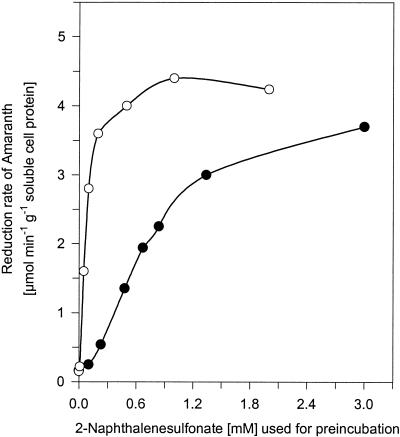
Cell suspensions of strains AKE1 and BN6wt were preincubated aerobically with different concentrations of 2NS and then incubated anaerobically with amaranth. With all initial concentrations of 2NS, strain AKE1 exhibited higher anaerobic reduction rates for amaranth than BN6wt exhibited (Fig. 4). Saturation kinetics were observed with both strains at higher initial concentrations of 2NS. These results revealed the expected increased accumulation in the redox mediator(s) during aerobic preincubation of strain AKE1 with 2NS. Thus, the substrate of DHNDO or its reaction product(s) seemed to act as a redox mediator(s).
FIG. 4.
Anaerobic reduction of amaranth by strains AKE1 and BN6wt preincubated with 2NS. Cell suspensions of strains AKE1 (○) and BN6wt (•) were aerobically preincubated with different amounts of 2NS and then incubated anaerobically with 0.5 mM amaranth and 10 mM glucose. The amaranth reduction activity was measured spectrophotometrically at 520 nm.
Isolation of redox mediators from culture supernatants of strain AKE1.
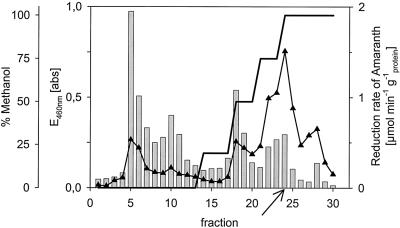
Supernatants of strain AKE1 cultures after incubation with 2NS (1 mM) showed an intense orange-brown color with an absorbance maximum (λmax) at 465 nm. This absorbance maximum reversibly disappeared under anaerobic conditions after incubation with molecular hydrogen and a hydrogenation catalyst. These observations indicated that the color was caused by a quinoide compound(s) which could also be responsible for the redox mediator activity of the culture supernatants. The culture supernatants were extracted with a solid-phase extraction cartridge, and the colored products were eluted with a gradient containing increasing amounts of methanol. Five major colored fractions were detected and assayed in the microtiter plate test for azoreductase activity under anaerobic conditions by using cells of strain BN6 and amaranth. Four of the colored fractions (which all showed a typical quinone absorbance maximum between 450 and 500 nm) showed redox mediator activity (Fig. 5). A comparison of the mediator activities present in these fractions suggested that fraction 24 (Fig. 5), which eluted at a rather high methanol concentration, represented the largest portion of the mediator activity present in the single fractions and accounted for approximately 15% of the total mediator activity which was present in the culture supernatant.
FIG. 5.
Separation of different redox mediators from a culture supernatant of S. xenophaga AKE1. The culture supernatant (10 ml) was applied to a solid-phase extraction column (500 mg of Butyl C4 [Applied Separations]), which was preequilibrated with Na/K phosphate buffer (50 mM, pH 7.3). The fractions were eluted with steps consisting of Na/K phosphate buffer (2 ml), water (2 ml), 20% (vol/vol) methanol (0.8 ml), 50% (vol/vol) methanol (0.6 ml), 75% (vol/vol) methanol (0.6 ml), and 100% (vol/vol) methanol (1 ml). Fractions (150 μl) were collected, and the absorbance at 460 nm was determined (bars) by using a microtiter plate spectrophotometer. Aliquots (20 μl) of each fraction were tested with resting cells of strain BN6 in the standard microtiter plate assay under anaerobic conditions for the decolorization of amaranth (▴). The fraction (fraction 24) which was subsequently analyzed is indicated by an arrow.
Identification of 4-A-1,2-NQ and 4-EA-1,2-NQ in the culture supernatants.
Fraction 24 was analyzed further by HPLC, and two major compounds were identified, which had almost identical (typical quinoide) in situ UV-Vis spectra (λmax, 270, 300 [shoulder], and 465 nm). The UV-Vis spectra of these compounds at pH 3.2 were clearly different from those of 1,2-NQ (λmax, 251, 345, and 408 nm) and 2-hydroxy-1,4-naphthoquinone (lawsone) (λmax, 250, 277, and 335 nm) but strongly resembled the UV-Vis spectrum of 4-A-1,2-NQ (λmax, 271 and 464 nm). The metabolites were isolated from a larger volume of culture supernatant of strain AKE1 (120 ml) by solid-phase extraction and were analyzed by HPLC-MS. One of the compounds (retention time, 3.6 min) was identified by its retention time, its in situ recorded UV-Vis spectrum, and its daughter ion mass spectrum as 4-A-1,2-NQ based on comparisons with the commercial reference material. The second compound (retention time, 3.3 min), which had a molecular cation (M+H)+ of m/z 218 (compared to m/z 174 for 4-A-1,2-NQ), showed a fragmentation pattern similar to that of 4-A-1,2-NQ. This compound was therefore identified as 4-EA-1,2-NQ. Because 4-EA-1,2-NQ has not been described previously, the compound was chemically synthesized and its structure was confirmed by NMR and LC-MS analyses (see Materials and Methods). The chemically synthesized reference compound was identical to the unknown compound from the culture supernatants according to the UV-Vis spectra and the LC-MS analyses.
The synthesized 4-EA-1,2-NQ and the commercial 4-A-1,2-NQ were used to quantify the concentrations of the compounds in the culture supernatants of strain AKE1 by HPLC. The culture supernatants contained 3.8 μM 4-A-1,2-NQ and 1.4 μM 4-EA-1,2-NQ. After they were identified, both quinones could also be detected in culture supernatants of wild-type strain BN6 by HPLC-UV and HPLC-MS analyses (daughter ion spectra), but the concentrations were significantly lower (0.5 μM 4-A-1,2-NQ and 0.3 μM 4-EA-1,2-NQ, respectively, after incubation with 3 mM 2NS).
4-A-1,2-NQ and 4-EA-1,2-NQ are active as redox mediators for anaerobic decolorization of azo dyes.
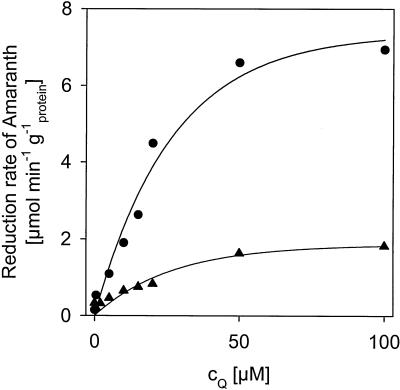
In order to verify the ability of 4-A-1,2-NQ and 4-EA-1,2-NQ to act as redox mediators, the chemically synthesized standards were incubated under anaerobic conditions with strain BN6 cells and amaranth. Both quinones significantly enhanced the anaerobic reduction of amaranth in the relevant concentration range (Fig. 6).
FIG. 6.
Effects of different concentrations of 4-EA-1,2-NQ (•) or 4-A-1,2-NQ (▴) on the reduction of amaranth by whole cells of S. xenophaga BN6 under anaerobic conditions in the standard microtiter plate test. The decrease in absorbance at 570 nm was measured spectrophotometrically. cQ, concentration of the respective quinone tested.
DISCUSSION
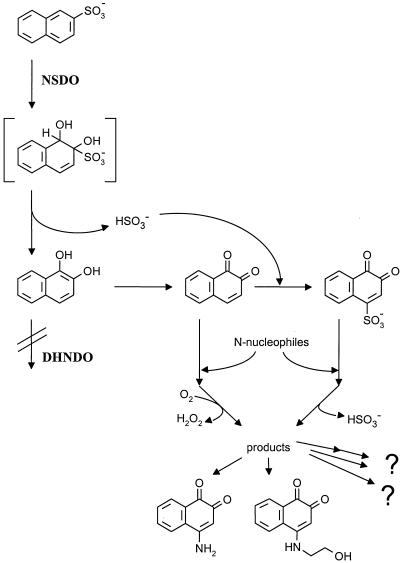
The mutant strain obtained during the present study clearly demonstrated that formation of the redox mediators which are active in the anaerobic reduction of azo dyes is dependent on formation of 1,2-DHN during the degradation of 2NS. It was originally thought that the redox couple 1,2-DHN-1,2-NQ is responsible for the enhanced reduction of azo dyes under anaerobic conditions, but it was shown that addition of 1,2-DHN or 1,2-NQ did not lead to enhancement of the reduction rates similar to the enhancement observed for the naturally formed redox mediator (27). Furthermore, a comparison of the redox potentials of 1,2-DHN (E0′ = 140 mV) (18) and amaranth (E0′ = ca. −250 mV) (15) suggested that 1,2-DHN was not responsible for the observed effects. These observations together suggested that 1,2-DHN formed during the degradation of 2NS was further converted (biologically or chemically) to products which are more active as redox mediators. From the experiments performed with wild-type strain BN6, it was evident that this strain accumulated only very small amounts of the putative mediator compounds. Construction of the strain carrying a mutation in the DHNDO gene, therefore, offered the opportunity to accumulate larger quantities of the compounds of interest. It was rather surprising that 4-amino-substituted 1,2-NQs constitute a quantitatively important part of the mediator effect (a schematic representation of the reactions discussed below is shown in Fig. 7). Nevertheless, the formation of these compounds can be readily explained by the known chemical behavior of 1,2-NQ, as many nucleophiles form (usually 1,4) addition products with o-quinones. The action of electron-rich nucleophiles (like amines or thiols) usually results in the formation of substituted quinones, which show redox potentials lower than that of the parent compound (20). The reduction potentials of 4-A-1,2-NQ and 4-EA-1,2-NQ were estimated by polarography to be −96 and −112 mV, respectively (39, 40). The formation of amino adducts of 1,2-NQ under physiological conditions has been observed previously. Rees and Pirie (41) studied the reactions of amino acids with 1,2-NQs by UV-Vis spectroscopy and suggested that most amino acids (but not cysteine and tryptophan) react via the amino group. The formation of amino acid adducts has been studied recently in some detail by Sridhar et al. (48), who demonstrated the formation of 1,4-Michael addition products from 1,2-NQ and protected derivatives of the amino acids lysine and histidine in a potassium phosphate buffer at pH 7.0. Similar inter- and intramolecular addition reactions have also been proposed for ortho-benzoquinone derivatives; these reactions include the addition of serine to 4-methyl-o-benzoquinone and the formation of cyclization products from o-dopaminequinone [4-(2-aminoethyl)-1,2-benzoquinone] (9, 19).
FIG. 7.
Schematic representation of the putative reactions leading to the formation of the mediator compounds detected.
A putative source of the 4-ethanolamino moiety of 4-EA-1,2-NQ could be phosphatidylethanolamine, which is a quantitatively important component of the cell membrane of S. xenophaga BN6 and other sphingomonads (52). It can be speculated that the rather unpolar compound 1,2-NQ may accumulate in the cell membranes of strain BN6 and there comes in close contact with phosphatidylethanolamine, which may allow reaction of the free amino group of the ethanolamine moiety with 1,2-NQ, followed by hydrolysis of the phosphorester group and formation of 4-EA-1,2-NQ. A second possibility for the formation of 4-EA-1,2-NQ may be a reaction of serine with the quinone nucleus, followed by decarboxylation. Serine may be present at higher concentrations in sphingomonads than in other bacteria because the members of this genus contain high concentrations of sphingolipids in their membranes, which are synthesized via a decarboxylative condensation of serine with palmitoyl coenzyme A or other coenzyme A esters (8, 24).
Apart from a purely chemical reaction of 1,2-NQ with amines as suggested above, we cannot eliminate the possibility that some unknown enzymatic activities are involved in the conversion of 1,2-NQ to the observed amino-substituted derivatives or that 1,2-NQ is first chemically activated to a more reactive species. One possible precursor is 1,2-naphthoquinone-4-sulfonate, which is formed in a spontaneous reaction of 1,2-NQ and sulfite (17). Both reaction constituents are generated from 2NS by the NSDO reaction. This proposal is supported by the detection (via HPLC-MS) of 1,2-naphthoquinone-4-sulfonate in supernatants from strain BN6 and AKE1 cultures. Furthermore, it was shown that 1,2-naphthoquinone-4-sulfonate reacted with ethanolamine to form 4-EA-1,2-NQ (data not shown).
The present study demonstrated that formation of very low concentrations (<10 μM) of metabolites, which in most cases cannot be detected by standard analytical techniques, may significantly change the fate of important environmental pollutants. Because the reaction of 1,2-NQ with amines or other nucleophiles is rather unspecific, it may be expected that several other compounds with mediator activity are also formed in the system studied here. Similar reactions may also be relevant for other biological systems, because aromatic 1,2-dihydroxy compounds are formed as intermediates during the bacterial degradation of almost all aromatic compounds and hydroquinones have been shown to be able to reduce a wide range of natural and man-made substances, such as nitroaromatic compounds and ferric iron (13, 16, 32, 33, 44, 49, 55).
Acknowledgments
This work was supported by the German Minister for Education, Science, Research and Technology (BMB+F, Bonn, Germany) (grant A3.3U, ZSP Stuttgart) and by the German Research Council (DFG, Bonn, Germany) through SFB 193 “Biological treatment of industrial wastewater” project A14 at Technische Universität Berlin.
REFERENCES
- 1.An, H., Y. Qian, X. Gu, and W. Z. Tang. 1996. Biological treatment of dye wastewaters using an anerobic-oxic system. Chemosphere 33:2533-2542. [DOI] [PubMed] [Google Scholar]
- 2.Banat, I. M., P. Nigam, D. Singh, and R. Marchant. 1996. Microbial decolorization of textile-dye-containing effluents: a review. Biores. Technol. 58:217-227. [Google Scholar]
- 3.Beckmann, W., and U. Sewekow. 1991. Farbige Abwässer aus der Reaktivfärberei: Probleme und Wege zur Lösung. Text. Prax. Int. 46:346-348. [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-252. [DOI] [PubMed] [Google Scholar]
- 5.Bromley-Challenor, K. C. A., J. S. Knapp, Z. Zhang, N. C. C. Gray, M. J. Hetheridge, and M. R. Evans. 2000. Decolorization of an azo dye by unacclimated activated sludge under anaerobic conditions. Water Res. 34:4410-4418. [Google Scholar]
- 6.Brown, D., and B. Hamburger. 1987. The degradation of dyestuffs: part III. Investigations of their ultimate degradability. Chemosphere 16:1539-1553. [Google Scholar]
- 7.Brown, D., and P. Laboureur. 1983. The degradation of dyestuffs: part I. Primary biodegradation under anaerobic conditions. Chemosphere 12:397-404. [Google Scholar]
- 8.Busse, H.-J., P. Kämpfer, and E. B. M. Denner. 1999. Chemotaxonomic characterisation of Sphingomonas. J. Ind. Microbiol. Biotechnol. 23:242-251. [DOI] [PubMed] [Google Scholar]
- 9.Cabanes, J., F. Garcia-Cánovas, and F. Garcia-Carmona. 1987. Chemical and enzymatic oxidation of 4-methylcatechol in the presence and absence of l-serine. Spectrophotometric determination of intermediates. Biochim. Biophys. Acta 914:190-197. [DOI] [PubMed] [Google Scholar]
- 10.Chanama, S., and R. L. Crawford. 1997. Mutational analysis of pcpA and its role in pentachlorophenol degradation by Sphingomonas (Flavobacterium) chlorophenolica ATCC 39723. Appl. Environ. Microbiol. 63:4833-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung, C. T., S. L. Niemela, and R. H. Miller. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 86:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, K.-T., G. E. Fulk, and M. Egan. 1978. Reduction of azo dyes by intestinal anaerobes. Appl. Environ. Microbiol. 35:558-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates, J. D., D. J. Ellis, E. L. Blunt-Harris, C. V. Gaw, E. E. Roden, and D. R. Lovley. 1998. Recovery of humic-reducing bacteria from a diversity of environments. Appl. Environ. Microbiol. 64:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delée, W., C. O'Neill, F. R. Hawkes, and H. M. Pinheiro. 1998. Anaerobic treatment of textile effluents: a review. J. Chem. Technol. Biotechnol. 73:323-335. [Google Scholar]
- 15.Dubin, P., and K. L. Wright. 1975. Reduction of azo food dyes in cultures of Proteus vulgaris. Xenobiotica 5:563-571. [DOI] [PubMed] [Google Scholar]
- 16.Field, J. A., F. J. Cervantes, F. P. van der Zee, and G. Lettinga. 2000. Role of quinones in the biodegradation of priority pollutants: a review. Water Sci. Technol. 42:215-222. [Google Scholar]
- 17.Fieser, L. F., and M. Fieser. 1935. The reduction potentials of various naphthoquinones. J. Am. Chem. Soc. 57:491-494. [Google Scholar]
- 18.Fultz, M. L., and R. A. Durst. 1982. Mediator compounds for the electrochemical study of biological redox systems: a compilation. Anal. Chim. Acta 140:1-18. [Google Scholar]
- 19.Garcia-Moreno, M., J. N. Rodriguez-López, F. Martinez-Ortiz, J. Tudela, R. Varón, and F. Garcia-Cánovas. 1991. Effect of pH on the oxidation pathway of dopamine catalysed by tyrosinase. Arch. Biochim. Biophys. 288:427-434. [DOI] [PubMed] [Google Scholar]
- 20.Grundmann, C. 1979. Ortho-Chinone, p. 144. In Methoden der organischen Chemie (Houben-Weyl), 4th ed., vol. VII/3b. Georg Thieme, Stuttgart, Germany.
- 21.Haug, W., A. Schmid, B. Nörtemann, D. C. Hempel, A. Stolz, and H.-J. Knackmuss. 1991. Mineralization of the sulfonated azo dye Mortant Yellow 3 by a 6-aminonaphthalene-2-sulfonate-degrading bacterial consortium. Appl. Environ. Microbiol. 57:3144-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 24.Ikushiro, H., H. Hayashi, and H. Kagamiyama. 2001. A water-soluble homodimeric serine palmitoyltransferase from Sphingomonas paucimobilis EY2395T strain. J. Biol. Chem. 276:18249-18256. [DOI] [PubMed] [Google Scholar]
- 25.Kaluz, S., M. Kaluzova, and A. P. F. Flint. 1995. Improvement of the gene splicing overlap (SOE) method. BioTechniques. 19:186-188. [PubMed] [Google Scholar]
- 26.Keck, A. 2000. Conversion of azo dyes by a redox mediator dependent mechanism which is linked to the naphthalenesulfonate degradation of Sphingomonas sp. BN6. Ph.D. thesis. University of Stuttgart, Stuttgart, Germany.
- 27.Keck, A., J. Klein, M. Kudlich, A. Stolz, H. J. Knackmuss, and R. Mattes. 1997. Reduction of azo dyes by redox mediators originating in the naphthalenesulfonic acid degradation pathway of Sphingomonas sp. strain BN6. Appl. Environ. Microbiol. 63:3684-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieser, T. 1984. Factors affecting the isolation of ccc DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 29.Kudlich, M., A. Keck, J. Klein, and A. Stolz. 1997. Localization of the enzyme system involved in anaerobic reduction of azo dyes by Sphingomonas sp. strain BN6 and effect of artificial redox mediators on the rate of azo dye reduction. Appl. Environ. Microbiol. 63:3691-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhm, A. E., A. Stolz, K. L. Ngai, and H.-J. Knackmuss. 1991. Purification and characterization of a 1,2-dihydroxynaphthalene dioxygenase from a bacterium that degrades naphthalenesulfonic acids. J. Bacteriol. 173:3795-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhm, A. E., H. J. Knackmuss, and A. Stolz. 1993. Purification and properties of 2′-hydroxybenzalpyruvate aldolase from a bacterium that degrades naphthalenesulfonates. J. Biol. Chem. 268:9484-9489. [PubMed] [Google Scholar]
- 32.Lovley, D. R., J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 33.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 34.Nörtemann, B., J. Baumgarten, H. G. Rast, and H.-J. Knackmuss. 1986. Bacterial communities degrading amino- and hydroxynaphthalene-2-sulfonates. Appl. Environ. Microbiol. 52:1195-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nörtemann, B., A. E. Kuhm, H.-J. Knackmuss, and A. Stolz. 1994. Conversion of substituted naphthalenesulfonates by Pseudomonas sp. BN6. Arch. Microbiol. 161:320-327. [Google Scholar]
- 36.O'Neill, C., A. Lopez, S. Esteves, F. R. Hawkes, D. L. Hawkes, and S. J. Wilcox. 2000. Azo-dye degradation in an anaerobic-aerobic treatment system operating on simulated textile effluent. Appl. Microbiol. Biotechnol. 53:249-254. [DOI] [PubMed] [Google Scholar]
- 37.Pagga, U., and D. Brown. 1986. The degradation of dyestuffs. Part II. Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 15:479-491. [Google Scholar]
- 38.Rajaguru, P., K. Kalaiselvi, M. Palanivel, and V. Subburam. 2000. Biodegradation of azo dyes in a sequential anaerobic-aerobic system. Appl. Microbiol. Biotechnol. 54:268-273. [DOI] [PubMed] [Google Scholar]
- 39.Rau, J. 2002. Die anaerobe Reduktion von Azofarbstoffen durch Bakterien in Gegenwart von Redoxmediatoren. Ph.D. thesis. Universität Stuttgart, Stuttgart, Germany.
- 40.Rau, J., H.-J. Knackmuss, and A. Stolz. 2002. Effect of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ. Sci. Technol. 36:1497-1504. [DOI] [PubMed] [Google Scholar]
- 41.Rees, J. R., and A. Pirie. 1967. Possible reactions of 1,2-naphthoquinone in the eye. Biochem. J. 102:853-863. [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Schmidt, K., S. L. Jensen, and H. G. Schlegel. 1963. Die Carotinoide der Thiorhodaceae. Arch. Mikrobiol. 46:117-126. [PubMed] [Google Scholar]
- 44.Schwarzenbach, R. P., R. Stierli, K. Lanz, and J. Zeyer. 1990. Quinone and iron porphyrin mediated reduction of nitroaromatic compounds in homogeneous aqueous solution. Environ. Sci. Technol. 24:1566-1574. [Google Scholar]
- 45.Seshadri, S., P. L. Bishop, and A. M. Agha. 1994. Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag. 15:127-137. [Google Scholar]
- 46.Shaul, G. M., T. J. Holdsworth, C. R. Dempsey, and K. A. Dostal. 1991. Fate of water soluble azo dyes in the activated sludge process. Chemosphere 22:107-119. [Google Scholar]
- 47.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host-range mobilization system for in vivo genetic engineering and transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 48.Sridhar, G. R., V. S. Murty, S. H. Lee, I. A. Blair, and T. M. Penning. 2001. Amino acid adducts of PAH o-quinones: model studies with naphthalene-1,2-dione. Tetrahedron 57:407-412. [Google Scholar]
- 49.Stahl, J. D., S. J. Rasmussen, and S. D. Aust. 1995. Reduction of quinones and radicals by a plasma membrane redox system of Phanerochaete chrysosporium. Arch. Biochem. Biophys. 322:221-227. [DOI] [PubMed] [Google Scholar]
- 50.Stolz, A. 1999. Degradation of substituted naphthalenesulfonic acids by Sphingomonas xenophaga BN6. J. Ind. Microbiol. Biotechnol. 23:391-399. [DOI] [PubMed] [Google Scholar]
- 51.Stolz, A. 2001. Basic and applied aspects in the microbial degradation of azo dyes. Appl. Microbiol. Biotechnol. 56:69-80. [DOI] [PubMed] [Google Scholar]
- 52.Stolz, A., C. Schmidt, E. B. M. Denner, H.-J. Busse, T. Egli, and P. Kämpfer. 2000. Description of Sphingomonas xenophaga for strains BN6 and N,N which degrade xenobiotic aromatic compounds. Int. J. Syst. E vol. Bacteriol. 50:35-41. [DOI] [PubMed] [Google Scholar]
- 53.Takuwa, A., O. Soga, H. Iwamoto, and K. Maruyama. 1986. The addition of alcohol to 1,2-naphthoquinone promoted by metal ions. A facile synthesis of 4-alkoxy-1,2-naphthoquinones. Bull. Chem. Soc. Jpn. 59:2959-2961. [Google Scholar]
- 54.Tan, N., F. X. Prenafeta-Boldú, J. L. Opsteeg, G. Lettinga, and J. A. Field. 1999. Biodegradation of azo dyes in cocultures of anaerobic granular sludge with aerobic aromatic amine degrading enrichment cultures. Appl. Microbiol. Biotechnol. 51:865-871. [DOI] [PubMed] [Google Scholar]
- 55.Tratnyek, P. G., and D. L. Macalady. 1989. Abiotic reduction of nitro aromatic pesticides in anaerobic laboratory systems. J. Agric. Food. Chem. 37:248-254. [Google Scholar]
- 56.Walker, R. 1970. The metabolism of azo compounds: a review of the literature. Food Cosmet. Toxicol. 8:659-676. [DOI] [PubMed] [Google Scholar]
- 57.Yanish-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]