Abstract
Anhydrobiotic engineering aims to increase the level of desiccation tolerance in sensitive organisms to that observed in true anhydrobiotes. In addition to a suitable extracellular drying excipient, a key factor for anhydrobiotic engineering of gram-negative enterobacteria seems to be the generation of high intracellular concentrations of the nonreducing disaccharide trehalose, which can be achieved by osmotic induction. In the soil bacterium Pseudomonas putida KT2440, however, only limited amounts of trehalose are naturally accumulated in defined high-osmolarity medium, correlating with relatively poor survival of desiccated cultures. Based on the enterobacterial model, it was proposed that increasing intracellular trehalose concentration in P. putida KT2440 should improve survival. Using genetic engineering techniques, intracellular trehalose concentrations were obtained which were similar to or greater than those in enterobacteria, but this did not translate into improved desiccation tolerance. Therefore, at least for some populations of microorganisms, trehalose does not appear to provide full protection against desiccation damage, even when present at high concentrations both inside and outside the cell. For P. putida KT2440, it was shown that this was not due to a natural limit in desiccation tolerance since successful anhydrobiotic engineering was achieved by use of a different drying excipient, hydroxyectoine, with osmotically preconditioned bacteria for which 40 to 60% viability was maintained over extended periods (up to 42 days) in the dry state. Hydroxyectoine therefore has considerable potential for the improvement of desiccation tolerance in sensitive microorganisms, particularly for those recalcitrant to trehalose.
Disaccharides and other polyols have been shown to be highly effective stabilizers of dried biological molecules and membranes in vitro, and the protection conferred by trehalose, in particular, has attracted considerable attention (7, 9, 10). Trehalose is also thought to be crucial for the survival of many anhydrobiotic organisms, which are able to maintain viability throughout long periods in a dried state (reviewed in reference 8). It is therefore reasonable to suppose that trehalose (or related molecules, such as sucrose) could be used to improve the desiccation tolerance of otherwise sensitive organisms, and several groups have demonstrated this for microorganisms (2, 17, 21, 23, 37).
However, the stability of the dried bacteria in these experiments falls far short of that observed in anhydrobiotic organisms. For example, Louis et al. (23) showed that Escherichia coli loses between 1 and 4 logs of viability 6 weeks after being dried in 0.5 M trehalose and stored at 4°C. Welsh and Herbert (37) reported maximal survival rates of 4.2 and 6.5% of initial CFU after 50 days at ambient temperature when E. coli was dried in the presence of 0.25 M extracellular trehalose or with a similar concentration of intracellular trehalose, respectively. Billi et al. (2) genetically engineered E. coli to produce up to 25 mM sucrose, and after freeze drying and immediate rehydration, 2.5% of the bacteria were viable. While these survival rates are clearly higher than those of controls, they do not match those of anhydrobiotic microorganisms such as Deinococcus radiodurans, for which in excess of 60% survival is observed after a 6-week storage period at ambient temperature (27).
Anhydrobiotic engineering aims to achieve levels of desiccation tolerance in sensitive organisms which are comparable to those of anhydrobiotes (15, 16). This has been demonstrated for the gram-negative enterobacteria E. coli and Salmonella enterica serovar Typhimurium, for which between 50 and 80% survival after vacuum drying has been maintained over storage periods of up to 6 weeks at above-ambient temperatures (4, 15; A. G. Tunnacliffe, D. T. Welsh, B. J. Roser, K. S. Dhaliwal, and C. Colaço, 1996, PCT patent application WO9824882A1). A crucial element of this success is thought to be the presence of high concentrations of trehalose both inside and outside the cell prior to drying (35). Growth in defined medium of high osmolarity can result in intracellular concentrations of trehalose between 190 and 400 mM in E. coli and S. enterica serovar Typhimurium (4, 13, 16, 20, 36), in which the disaccharide is used as a compatible solute (reviewed in reference 33). This has been exploited for the anhydrobiotic engineering of these enterobacterial species, which are first osmotically preconditioned by growth in an appropriate medium and then dried in a trehalose solution.
A similar approach with the gram-negative soil bacterium Pseudomonas putida KT2440 was, however, less successful, since long-term viability after drying was ≤20% that of nondried controls (16). This was attributed to the fact that although trehalose is synthesized by P. putida during growth in high-osmolarity defined medium, the intracellular concentration never naturally exceeds 50 mM. In E. coli, this level of trehalose provides suboptimal protection against desiccation (37; our unpublished results). One way to improve desiccation tolerance in P. putida KT2440, therefore, may be simply to increase the intracellular trehalose concentration. In this report, we describe the genetic engineering of P. putida KT2440 with trehalose synthase genes from E. coli and the effect of the resultant elevated intracellular trehalose concentration on desiccation tolerance. In addition, we compare this effect with that of extracellular hydroxyectoine, a common compatible solute in halophilic microorganisms (31) which has been reported previously to offer some protection to desiccating E. coli (23). Our results suggest that, at least for P. putida, hydroxyectoine is superior to trehalose as a desiccation protectant and offers significant potential as a drying excipient for live microorganisms recalcitrant to trehalose.
MATERIALS AND METHODS
Reagents.
Chemicals were obtained from Sigma or Fisher, unless otherwise stated. High-grade trehalose was obtained from Alchemy International Ltd., Hambrook, United Kingdom; hydroxyectoine [(S)-2-methyl-5 hydroxy-1,4,5,6-tetra-hydropyrimidine-4-carboxylic acid] was purchased from Bitop GmbH (Witten, Germany).
Bacterial strains and culture conditions.
The strains and plasmids used in this study are shown in Table 1. Bacteria were grown in Luria Broth (LB) or in M9 minimal medium with glucose (20 mM) as the sole carbon source at 30°C for P. putida and at 37°C for E. coli as previously described (16). To generate hypersaline minimal medium (HMM), NaCl was added to M9 at a final concentration of 0.4 and 0.6 M for P. putida and E. coli, respectively. When necessary for maintaining plasmid stability, gentamicin was added to a final concentration of 10 and 66 μg/ml for E. coli and P. putida, respectively; kanamycin was used at 25 and 50 μg/ml, respectively. When trehalose, maltose, or hydroxyectoine was used as a carbon source, it was used at a final concentration of 20 mM.
TABLE 1.
Strains and plasmids
Harvesting, drying, and storage of cells.
Aliquot volumes (15 ml) of cultures were harvested by centrifugation. Cell pellets were rinsed and then resuspended at 107 to 109 cells/ml in 100 μl of a solution of drying excipient (usually trehalose or hydroxyectoine) plus 1.5% (wt/vol) polyvinylpyrrolidone (PVP) as a viscosity enhancer; all manipulations were done at ambient temperature. Drying was performed in serum vials under vacuum without freezing in a modified freeze dryer (Dura-Stop μP; FTS Systems, Stone Ridge, N.Y.) at 30°C shelf temperature and 15 mtorr (2 Pa; 2 × 10−5 atm) for 20 h followed by temperature ramping of 2.5°C/min with a 15-min pause after every increase of 2°C, up to a maximum temperature of 40°C. Samples were sealed under vacuum and stored for variable periods at 30°C after which they were resuspended in LB to a total volume of 1 ml. For assessment of viability, serial dilutions (in LB) were plated on LB agar plates, incubated at 37°C for 24 h, and counted to determine CFU.
Constructs.
The otsBA operon from plasmid pFF13 (19) was amplified by PCR using the following set of primers which introduced a BspHI site (underlined below) at the 5′ end and an EcoRI site at the 3′ end: forward primer 5′-GAGAACCTCATGACAGAACCGTTAACCG-3′ and reverse primer 5′-CTTACGGGGAATTCACCGCTCCTACG-3′. The digested PCR product was ligated into the expression vector pJB861 (3) digested with BspHI and EcoRI to create pHA1. The otsBA operon was amplified from a colony of E. coli MC4100 by PCR using the primers otsBPst (5′-CATGTCTGCAGAGCGCGTTC-3′) and otsAEco (5′-CGAATTCTACGCAAGCTTTGG-3′) and after digestion was cloned into pUCP22NotI (28) at the EcoRI and PstI sites. The insert was correctly oriented by excision with NotI and religation, creating pSTO22.
Determination of intracellular trehalose concentration.
Samples of cultures (1 to 6 ml) were centrifuged and washed in growth medium lacking the C source followed by a second centrifugation. Bacterial pellets were extracted with 70% (wt/vol) ethanol at 80°C using 0.05 mg of sucrose as internal standard, cell debris was removed by centrifugation, and supernatant was extracted with chloroform-water. The water-soluble fraction was dried by evaporation and samples were derivatized using a 1:1 mix of pyridine and N,O-bis(trimethylsilyl)trifluoroacetimide (Aldrich, Poole, United Kingdom, and Pierce [Perbio Science, Tattenhall, United Kingdom], respectively) for 1 h at 50°C. Gas chromatography was carried out on a Perkin-Elmer 8410 machine fitted with a DB-5 30-m by 0.25-mm column (J&W Scientific Inc.) and a split injector. Helium was used as the carrier gas at a rate of 50 ml/min. Injector and detector temperatures were set at 320°C. The column temperature was set at 250°C upon injection and for the first 3 min and then increased at 10°C/min up to 300°C, where it was allowed to remain for a further 5 min. Trehalose concentrations were calculated against a sucrose standard with reference to viable cell counts.
RESULTS
Genetic engineering of P. putida with the E. coli otsBA operon.
The maximum intracellular concentration of trehalose in P. putida KT2440 grown in high-osmolarity medium is low in comparison with E. coli MC4100 (16; A. R. Strøm, A. Tøndervik, and H. Bredholt, Abstr. Pap. Am. Chem. Soc. 219:4-BTEC, 2000). To increase the intracellular trehalose in P. putida, two expression constructs were made with the E. coli otsBA operon, which encodes the enzymes trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase (19). In plasmid pHA1, the operon was cloned without its promoter into the medium-copy-number vector pJB861 (3) to generate pHA1. This placed the otsBA genes under the control of the inducible Pm promoter and the regulatory xylS gene from the P. putida KT2440 TOL plasmid pWW0 (14); transcription occurs in the presence of 3-methyl-benzoate (3mBz). In plasmid pSTO22, the same promoterless otsBA fragment was cloned into the low-copy-number vector pUCP22NotI (28) under the control of the lac promoter; expression is constitutive in P. putida, which lacks lacI.
Figure 1 shows intracellular trehalose concentration in P. putida carrying pSTO22 or pUCP22NotI when cultured under osmotic stress in HMM containing glucose for 21 h. A general increase in trehalose accumulation was observed in both strains, reaching a maximum ∼17 h after inoculation and decreasing slightly during stationary phase. However, the level of trehalose in P. putida (pSTO22) was severalfold higher than in the cells with the empty vector, reaching values over 200 fg/cell during late exponential and stationary phases, suggesting that intracellular concentrations on the order of 400 mM were attained. Even higher concentrations were obtained with P. putida (pHA1) after induction with 3mBz (data not shown). Perhaps surprisingly, this increase in intracellular trehalose concentration did not improve the normally slow growth rate of P. putida in hyperosmotic medium. Chemical analyses indicated that this may be due to the levels of other compatible solutes being decreased as that of trehalose increases (H. Bredholt, A. Tøndervik, A. Hagen, and A. R. Strøm, unpublished results).
FIG. 1.
Accumulation of trehalose in P. putida KT2440 containing the otsBA genes expressed from the lac promoter in pSTO22 (squares) or containing the control plasmid pUCP22NotI (triangles).
High intracellular trehalose concentration does not improve desiccation tolerance in P. putida.
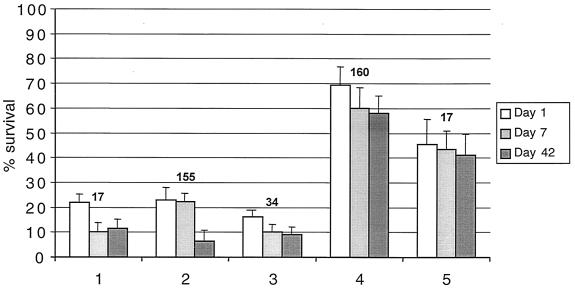
To determine whether the increased intracellular trehalose concentration conferred by the otsBA genes could improve the desiccation tolerance of P. putida, cells carrying pSTO22, pUCP22NotI, or no plasmids were grown in HMM; osmotically preconditioned E. coli MC4100 was included as a control. Figure 2 presents results for cells harvested at mid-exponential phase (12 h after inoculation). The bacteria were dried under vacuum (without freezing) in 1 M trehalose plus 1.5% PVP and survival was determined immediately after drying (day 1) and after storage at 30°C for 7 or 42 days. For all samples of P. putida cells dried in trehalose (samples 1 to 3), the survival on day 1 was in the range of 16 to 22% that of nondried controls. The survival profile was similar over the 42-day storage period, with bacteria containing high trehalose concentrations showing little difference to controls. In contrast, E. coli cells dried under the same conditions showed markedly higher survival levels, in agreement with previous results (16). Experiments in which the above procedure was repeated for cells harvested in late exponential or stationary growth phase or in which 3mBz-induced P. putida carrying pHA1 or pJB861 was dried gave similar results. It was concluded, therefore, that intracellular trehalose concentrations above those naturally obtained by osmotic induction did not have a beneficial effect on the survival of P. putida KT2440 dried with trehalose.
FIG. 2.
The effect of high intracellular trehalose concentration on desiccation tolerance of P. putida KT2440 compared with E. coli MC4100 and with hydroxyectoine as drying excipient. Samples 1 to 4 were vacuum dried in 1 M trehalose-1.5% PVP, while sample 5 was dried in 1 M hydroxyectoine-1.5% PVP. Samples 1, 2, 3, and 5 are P. putida carrying pSTO22 (2), plasmid pUCP22NotI (3), or no plasmid (1 and 5); sample 4 is unmodified E. coli. The intracellular concentration of trehalose (in femtograms per cell) in each sample is indicated above the bars. Error bars indicate standard deviations.
Drying and storage of osmotically preconditioned P. putida in hydroxyectoine.
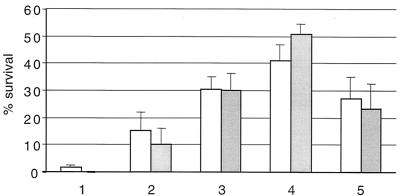
One interpretation of the above result is that a natural limit of desiccation tolerance in P. putida has been reached, perhaps due to the vulnerability of sensitive cellular structures which cannot be completely protected by trehalose. To test this hypothesis, the tetrahydropyrimidine hydroxyectoine, which has been described previously as a drying excipient for E. coli (23), was used. For this experiment, P. putida cells harvested from exponential-phase cultures in HMM were vacuum dried in 0, 0.25, 0.5, 1, or 2 M hydroxyectoine plus 1.5% PVP. Depending on the hydroxyectoine concentration, up to 50% of the P. putida cells survived drying and remained viable over the storage period of 7 days at 30°C. The observed viability increased up to 1 M hydroxyectoine, while viability with 2 M hydroxyectoine declined, possibly due to osmotic stress or a degree of toxicity at this high concentration; 1 M hydroxyectoine was used in subsequent experiments (Fig. 3). These data confirm previous findings that in the absence of external protectant, survival is poor.
FIG. 3.
Viability of P. putida after vacuum drying in different concentrations of hydroxyectoine. Samples were dried in 1.5% PVP without hydroxyectoine (1) or with 0.25 M (2), 0.5 M (3), 1 M (4), or 2 M (5) hydroxyectoine. After drying, samples were vacuum sealed and stored at 30°C for 7 days. Viability was measured at days 1 and 7 after drying (white and gray bars, respectively). Error bars indicate standard deviations.
The highest survival rate obtained with hydroxyectoine was significantly higher than the best results obtained with trehalose. In order to make a more direct comparison of the protective capacity of these compounds, P. putida cells dried in 1 M hydroxyectoine were included in the experiment described for Fig. 2. The result showed that survival with hydroxyectoine was maintained above 40% for up to 42 days (Fig. 2, bar set 5). Thus, anhydrobiotic engineering of P. putida is possible, but manipulation of trehalose concentrations alone is not sufficient. Instead, other protective components, a role played here by hydroxyectoine, are required to maintain high viability levels.
Hydroxyectoine is metabolized by P. putida KT2440.
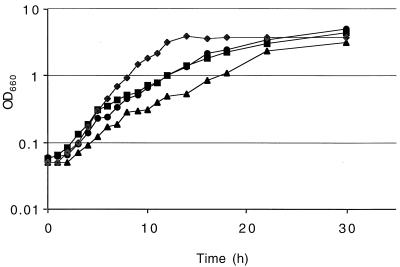
Hydroxyectoine and the related tetrahydropyrimidine ectoine are endogenously synthesized compatible solutes in many halotolerant bacteria (31). E. coli cannot synthesize these compounds, but ectoine is taken up by the nonspecific transporters ProU and ProP and confers osmoprotection to a similar degree as glycine betaine (18). The results presented in Fig. 4 show that hydroxyectoine (20 mM) stimulated growth of E. coli, but not P. putida, in glucose-HMM. Thus, P. putida may lack appropriate transport systems that can produce the high-level accumulation of hydroxyectoine needed for osmoprotection, although glycine betaine stimulated growth of P. putida in HMM (data not shown).
FIG. 4.
Effect of the addition of hydroxyectoine to cultures of P. putida or E. coli grown in high-osmolarity medium. Growth was spectrophotometrically measured at 600 nm over a 30-h period. Cells were grown in HMM with or without 20 mM hydroxyectoine. P. putida cultures are represented by squares (with hydroxyectoine) or circles (without hydroxyectoine); E. coli cultures are represented by diamonds (with hydroxyectoine) or triangles (without hydroxyectoine).
In order to compare the ability of E. coli and P. putida to metabolize hydroxyectoine, the bacteria were subcultured twice sequentially in HMM with a 20 mM concentration of this compound as the sole carbon source. Control cultures with medium without a carbon source were included. Under these conditions, only P. putida grew. The growth rate with hydroxyectoine was lower than that normally observed with glucose but was apparent nevertheless. This suggests that P. putida is able to internalize and degrade hydroxyectoine (or a derivative); the existence of a catabolic pathway may explain why hydroxyectoine cannot also be utilized as an osmolyte. Alternatively, the hydroxyectoine transporter may operate like the PutP proline permease of S. enterica serovar Typhimurium, which is required when proline serves as the sole carbon and nitrogen source but does not facilitate the osmoprotective effect of proline (11). Separate experiments revealed that P. putida did not grow in M9 or HMM medium with trehalose or maltose as the carbon source; it is well known that E. coli can utilize these substrates.
DISCUSSION
P. putida KT2440 displays relatively poor desiccation tolerance when trehalose is used as a drying excipient: over a storage period of 40 days the survival of dried bacteria is 10 to 20%, compared to 50 to 70% for E. coli (Fig. 2) (16). In this paper, the hypothesis was tested that low intracellular trehalose concentrations in P. putida were responsible for this poor desiccation tolerance. High intracellular concentrations of trehalose were generated using two different plasmid systems expressing the E. coli otsBA operon either constitutively or inducibly. However, in contrast to results with E. coli (37; A. Tunnacliffe, unpublished data), increased intracellular trehalose concentrations did not produce any improvement in desiccation tolerance. This was not due to a natural limit to desiccation tolerance in P. putida since using hydroxyectoine as a drying excipient resulted in 40 to 50% viability which was maintained through drying and storage phases. This is directly comparable to survival rates in true microbial anhydrobiotes such as D. radiodurans (27) and therefore demonstrates successful anhydrobiotic engineering of P. putida.
The crucial factor leading to improved desiccation tolerance of osmotically preconditioned P. putida is the use of extracellular hydroxyectoine rather than trehalose (cf. data sets 1 and 5 in Fig. 2). It is not clear why hydroxyectoine outperforms trehalose in this context, but one explanation might be that certain sensitive extracytoplasmic structures in P. putida, either in the periplasm or at the outer face of the cytoplasmic membrane, are preferentially protected by hydroxyectoine. Access of trehalose to the periplasm might be blocked by the selectivity of outer membrane porins towards mono- and disaccharides: it is known that OprB, the carbohydrate-specific porin in Pseudomonas spp., facilitates diffusion of glucose and maltose but restricts uptake of galactose, sucrose, and lactose (30).
The results reported indicate a limited role for intracellular trehalose in anhydrobiotic engineering of P. putida. Previously it was shown that survival of dried stationary-phase P. putida cells was higher over time than that of equivalent growth-phase bacteria (16). This was attributed to the modest concentration (∼50 mM) of trehalose in osmotically induced stationary-phase cells; trehalose levels are very low in these bacteria during growth phase, when mannitol is used as a compatible solute. However, other responses to the combined stresses of stationary phase and high osmolarity, perhaps part of the regulons governed by sigma factors σS and σH, may be responsible for improved desiccation tolerance in stationary-phase cells; such a stationary-phase effect is well documented (29). Whether intracellular trehalose is required at all for desiccation tolerance in P. putida could be tested using mutants of the trehalose biosynthetic genes. In P. putida, trehalose biosynthesis apparently proceeds through isomerization of maltose (treS) (34) or by isomerization and hydrolysis of maltodextrins (treYZ) (26). Both these gene systems, but not otsBA, have been identified in the P. putida genome (García de Castro et al., unpublished data) and therefore could be inactivated by insertional mutagenesis.
Although the role of intracellular trehalose in the desiccation tolerance of P. putida might be questioned, extracellular trehalose clearly does have a beneficial effect (16). However, this is not sufficient to give the survival rates required for anhydrobiotic engineering. The observation that extracellular hydroxyectoine gave excellent results was surprising but is not restricted to P. putida: osmotically preconditioned E. coli, vacuum dried from a solution of hydroxyectoine, exhibits stability similar to that observed when dried in trehalose; when neither excipient is used, relatively poor survival is seen (Manzanera et al., unpublished data). Hydroxyectoine has previously been reported to modestly improve survival of freeze-dried E. coli (23). It can also protect susceptible enzymes against desiccation and other stresses in vitro (1, 22), although other authors have shown that hydroxyectoine inhibits certain enzyme activities and DNA-protein complex formation (25), implying a potentially negative impact on metabolism.
While the genes governing synthesis of the related tetrahydropyrimidine ectoine have been defined (6, 24), those for biosynthesis of hydroxyectoine have not. Since both the work of Louis et al. (23) and our unpublished results show that hydroxyectoine is more effective than ectoine at stabilizing dried bacteria, it will be of great interest to identify the genes responsible for hydroxyectoine biosynthesis, which may proceed by two alternative pathways in halophiles (5). Genetic modification of P. putida KT2440 with these genes, particularly if they were positively regulated by dehydration, could increase its drought tolerance and improve its potential for survival in the field, where it has been proposed as a vector for biopesticide and bioremediation use (reviewed in reference 12).
Acknowledgments
This work was supported by EC grant BIO4-CT98-0283, by BBSRC ROPA grant 8/9912302, and by Merck Chemicals Ltd. A.T. is the AWG Senior Research Fellow of Pembroke College, Cambridge, United Kingdom.
REFERENCES
- 1.Andersson, M. M., J. D. Breccia, and R. Hatti-Kaul. 2000. Stabilizing effect of chemical additives against oxidation of lactate dehydrogenase. Biotechnol. Appl. Biochem. 32:145-153. [DOI] [PubMed] [Google Scholar]
- 2.Billi, D., D. J. Wright, R. F. Helm, T. Prickett, M. Potts, and J. H. Crowe. 2000. Engineering desiccation tolerance in Escherichia coli. Appl. Environ. Microbiol. 66:1680-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatny, J. M., T. Brautaset, H. C. Winther Larsen, P. Karunakaran, and S. Valla. 1997. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid 38:35-51. [DOI] [PubMed] [Google Scholar]
- 4.Bullifent, H. L., K. Dhaliwal, A. M. Howells, K. Goan, K. Griffin, C. D. Lindsay, A. Tunnacliffe, and R. W. Titball. 2001. Stabilisation of Salmonella vaccine vectors by the induction of trehalose biosynthesis. Vaccine 19:1239-1245. [DOI] [PubMed] [Google Scholar]
- 5.Canovas, D., N. Borges, C. Vargas, A. Ventosa, J. J. Nieto, and H. Santos. 1999. Role of N-gamma-acetyldiaminobutyrate as an enzyme stabilizer and an intermediate in the biosynthesis of hydroxyectoine. Appl. Environ. Microbiol. 65:3774-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canovas, D., C. Vargas, M. I. Calderon, A. Ventosa, and J. J. Nieto. 1998. Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst. Appl. Microbiol. 21:487-497. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter, J. F., B. Martin, L. M. Crowe, and J. H. Crowe. 1987. Stabilization of phosphofructokinase during air-drying with sugars and sugar/transition metal mixtures. Cryobiology 24:455-464. [DOI] [PubMed] [Google Scholar]
- 8.Clegg, J. S. 2001. Cryptobiosis—a peculiar state of biological organization. Comp. Biochem. Physiol. B 128:613-624. [DOI] [PubMed] [Google Scholar]
- 9.Colaço, C., S. Sen, M. Thangavelu, S. Pinder, and B. Roser. 1992. Extraordinary stability of enzymes dried in trehalose: simplified molecular biology. Bio/Technology 10:1007-1011. [DOI] [PubMed] [Google Scholar]
- 10.Crowe, J. H., L. M. Crowe, and R. Mouradian. 1983. Stabilization of biological membranes at low water activities. Cryobiology 20:346-356. [DOI] [PubMed] [Google Scholar]
- 11.Csonka, L. N. 1982. A third l-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J. Bacteriol. 151:1433-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lorenzo, V. 2000. Pseudomonas entering the postgenomic era. Environ. Microbiol. 2:349-354. [DOI] [PubMed] [Google Scholar]
- 13.Dinnbier, U., E. Limpinsel, R. Schmid, and E. P. Bakker. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 150:348-357. [DOI] [PubMed] [Google Scholar]
- 14.Franklin, F. C. H., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta-cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García de Castro, A., J. Lapinski, and A. Tunnacliffe. 2000. Anhydrobiotic engineering. Nat. Biotechnol. 18:473. [DOI] [PubMed] [Google Scholar]
- 16.García de Castro, A., H. Bredholt, A. R. Strom, and A. Tunnacliffe. 2000. Anhydrobiotic engineering of gram-negative bacteria. Appl. Environ. Microbiol. 66:4142-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israeli, E., B. T. Shaffer, and B. Lighthart. 1993. Protection of freeze-dried Escherichia coli by trehalose upon exposure to environmental conditions. Cryobiology 30:519-523. [DOI] [PubMed] [Google Scholar]
- 18.Jebbar, M., R. Talibert, K. Gloux, T. Bernard, and C. Blanco. 1992. Osmoprotection of Escherichia coli by ectoine—uptake and accumulation characteristics. J. Bacteriol. 174:5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaasen, I., J. McDougall, and A. R. Strøm. 1994. Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli and homology of the OtsA and OtsB proteins to the yeast trehalose-6-phosphate synthase phosphatase complex. Gene 145:9-15. [DOI] [PubMed] [Google Scholar]
- 20.Larsen, P. I., L. K. Sydnes, B. Landfald, and A. R. Strøm. 1987. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid and trehalose. Arch. Microbiol. 147:1-7. [DOI] [PubMed] [Google Scholar]
- 21.Leslie, S. B., S. A. Teter, L. M. Crowe, and J. H. Crowe. 1994. Trehalose lowers membrane phase transitions in dry yeast cells. Biochim. Biophys. Acta 1192:7-13. [DOI] [PubMed] [Google Scholar]
- 22.Lippert, K., and E. A. Galinski. 1992. Enzyme stabilisation by ectoine-type compatible solutes—protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61-65. [Google Scholar]
- 23.Louis, P., H. G. Truper, and E. A. Galinski. 1994. Survival of Escherichia coli during drying and storage in presence of compatible solutes. Appl. Microbiol. Biotechnol. 41:684-688. [Google Scholar]
- 24.Louis, P., and E. A. Galinski. 1997. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143:1141-1149. [DOI] [PubMed] [Google Scholar]
- 25.Malin, G., R. Iakobashvili, and A. Lapidot. 1999. Effect of tetrahydropyrimidine derivatives on protein nucleic acids interaction—type II restriction endonucleases as a model system. J. Biol. Chem. 274:6920-6929. [DOI] [PubMed] [Google Scholar]
- 26.Maruta, K., K. Hattori, T. Nakada, M. Kubota, T. Sugimoto, and M. Kurimoto. 1996. Cloning and sequencing of trehalose biosynthetic genes from Arthrobacter sp. Q36. Biochim. Biophys. Acta 1289:10-13. [DOI] [PubMed] [Google Scholar]
- 27.Mattimore, V., and J. R. Battista. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen, R. H., G. DeBusscher, and R. R. McCombie. 1982. Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J. Bacteriol. 150:60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potts, M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saravolac, E. G., N. F. Taylor, R. Benz, and R. E. W. Hancock. 1992. Purification of glucose-inducible outer-membrane protein OprB of Pseudomonas putida and reconstitution of glucose-specific pores. J. Bacteriol. 173:4970-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severin, J., A. Wohlfarth, and E. A. Galinski. 1992. The predominant role of recently discovered tetrahydropyrimidines for the osmoadaptation of halophilic eubacteria. J. Gen. Microbiol. 138:1629-1638. [Google Scholar]
- 32.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Strøm, A. R. 1998. Osmoregulation in the model organism Escherichia coli: genes governing the synthesis of glycine betaine and trehalose and their use in metabolic engineering of stress tolerance. J. Biosci. 23:437-445. [Google Scholar]
- 34.Tsusaki, K., T. Nishimoto, T. Nakada, M. Kubota, H. Chaen, T. Sugimoto, and M. Kurimoto. 1996. Cloning and sequencing of trehalose synthase gene from Pimelobacter sp. R48. Biochim. Biophys. Acta 1290:1-3. [DOI] [PubMed] [Google Scholar]
- 35.Tunnacliffe, A., A. García de Castro, and M. Manzanera. 2001. Anhydrobiotic engineering of bacterial and mammalian cells: is intracellular trehalose sufficient? Cryobiology 43:124-132. [DOI] [PubMed] [Google Scholar]
- 36.Welsh, D. T., R. H. Reed, and R. A. Herbert. 1991. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J. Gen. Microbiol. 137:745-750. [DOI] [PubMed] [Google Scholar]
- 37.Welsh, D. T., and R. A. Herbert. 1999. Osmotically induced intracellular trehalose, but not glycine betaine accumulation, promotes desiccation tolerance in Escherichia coli. FEMS Microbiol. Lett. 174:57-63. [DOI] [PubMed] [Google Scholar]
- 38.Williams, P. A., and K. Murray. 1974. Metabolism of benzoates and methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of new TOL plasmids. J. Bacteriol. 125:818-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worsey, M. J., F. C. H. Franklin, and P. A. Williams. 1978. Regulation of the degradative pathway enzymes coded for by the TOL plasmid (pWW0) from Pseudomonas putida mt-2. J. Bacteriol. 134:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]