Abstract
Acellular pertussis vaccines typically consist of antigens isolated from Bordetella pertussis, and pertussis toxin (PT) and filamentous hemagglutinin (FHA) are two prominent components. One of the disadvantages of a multiple-component vaccine is the cost associated with the production of the individual components. In this study, we constructed an in-frame fusion protein consisting of PT fragments (179 amino acids of PT subunit S1 and 180 amino acids of PT subunit S3) and a 456-amino-acid type I domain of FHA. The fusion protein was expressed by the commensal oral bacterium Streptococcus gordonii. The fusion protein was secreted into the culture medium as an expected 155-kDa protein, which was recognized by a polyclonal anti-PT antibody, a monoclonal anti-S1 antibody, and a monoclonal anti-FHA antibody. The fusion protein was purified from the culture supernatant by affinity and gel permeation chromatography. The immunogenicity of the purified fusion protein was assessed in BALB/c mice by performing parenteral and mucosal immunization experiments. When given parenterally, the fusion protein elicited a very strong antibody titer against the FHA type I domain, a moderate titer against native FHA, and a weak titer against PT. When given mucosally, it elicited a systemic response and a mucosal response to FHA and PT. In Western blots, the immune sera recognized the S1, S3, and S2 subunits of PT. These data collectively indicate that fragments of the pertussis vaccine components can be expressed in a single fusion protein by S. gordonii and that the fusion protein is immunogenic. This multivalent fusion protein approach may be used in designing a new generation of acellular pertussis vaccines.
Pertussis is a disease of the respiratory tract that affects humans of all ages but has the greatest morbidity and mortality in young children. This disease is due to infection by Bordetella pertussis, which elaborates a number of virulence factors, including pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin, and fimbriae. Prevention of pertussis is achieved by administration of an acellular vaccine included in a trivalent diphtheria-tetanus-pertussis vaccine during the childhood immunization regimen. Acellular pertussis vaccines typically consist of antigens isolated from B. pertussis, and PT and FHA are two prominent components. One of the disadvantages of a multiple-component vaccine is the cost associated with the production of the individual components.
PT is an AB toxin; the A protomer (S1 subunit) is the toxic subunit, and the B oligomer is the pentamer that binds to cell receptors (30). The S1 subunit is immunodominant (5). Antibodies against the S1 subunit have been shown to neutralize PT in vitro and to protect mice from aerosol and intracerebral B. pertussis challenges (10, 28, 29). The B oligomer is composed of one subunit each of S2, S3, and S5 and two subunits of S4. The S2 and S3 amino acid sequences exhibit >80% identity. Antibodies against the B oligomer or the S2 and S3 subunits confer protection against B. pertussis infection in animal models but are less effective than antibodies against S1 (10). FHA is a 220-kDa protein with multiple domains for interactions with sulfated glycoconjugates on cells of the respiratory tract (11). The type I domain of FHA is a 456-amino-acid region located at the C terminus of the protein. Sera from patients with pertussis and from vaccinated infants specifically recognize the type I domain, as well as a type II domain located at the N terminus of FHA, indicating that the type I domain is one of the immunodominant regions of FHA (20).
Cloning and expression of the S1 subunit have been described for gram-negative bacteria, such as Escherichia coli (1, 2, 32) and vaccine strains of Salmonella enterica serovar Typhimurium (4, 32). These reports demonstrated that recombinant S1 is immunogenic, but the levels of protective antibodies present in the anti-recombinant S1 antisera varied from zero to low. Expression of S1 in gram-positive bacteria has been described for Bacillus subtilis (26, 27), for Streptomyces lividans (25), and recently for Mycobacterium bovis (24). In both Bacillus and Streptocmyces, the S1 subunit was expressed as a soluble extracellular protein. In B. subtilis, recombinant S1 was found to be immunogenic in animals, but whether recombinant S1 can induce protective antibodies has not been determined (26). In S. lividans, recombinant S1 was extensively degraded by proteases. In M. bovis, recombinant S1 induced protective immunity against an intracerebral B. pertussis challenge in mice (24). In recent work, we described surface expression of the N-terminal 179-amino-acid sequence of S1 in the oral commensal bacterium Streptococcus gordonii (19). Parenteral immunization with recombinant S. gordonii conferred protection against the toxic effect of PT, as shown by the leukocytosis-promoting and histamine sensitization assays (19). Oral colonization of mice with S1-expressing S. gordonii elicited a mucosal immune response (18). To date, cloning and expression of FHA have been limited to attenuated strains of Salmonella and E. coli (7, 8, 23). Immune responses to FHA were reported following oral immunization of mice with recombinant Salmonella strains (7, 23).
All the work reported above described expression of a single pertussis antigen. In the present study, we investigated expression of a multivalent pertussis antigen consisting of the S1 and S3 fragments genetically fused to the FHA type I domain by using S. gordonii as a host. The approach used was different from the approaches used in previous studies (19, 21, 22) since the fusion protein was a soluble protein that could be recovered easily from the culture supernatant.
MATERIALS AND METHODS
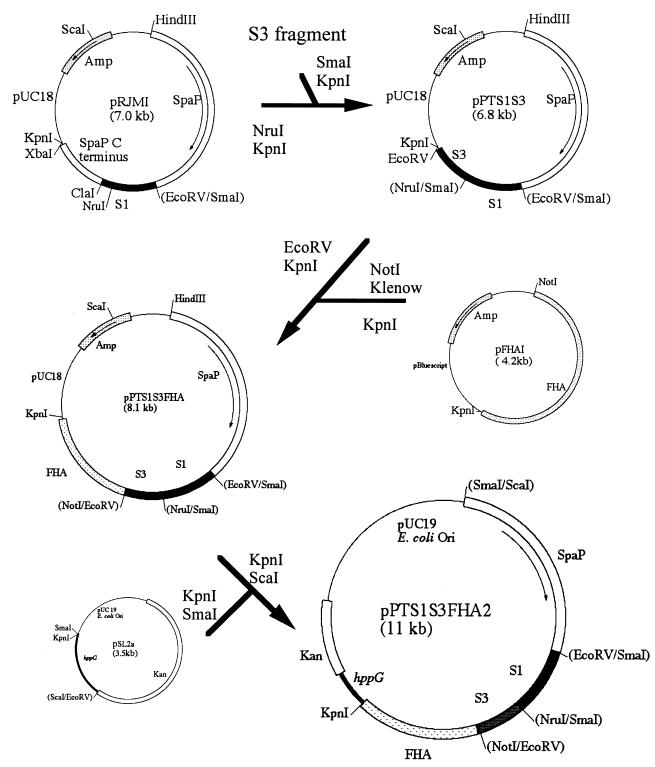
Construction of the S1S3FHA fusion protein.
The DNA coding for the 180-amino-acid sequence of the S3 subunit of PT was amplified by using Taq DNA polymerase and primers SL128 (TTACCCGGGACCCAACAGGGCGGCGC [SmaI site underlined]) and SL127 (CTCGGTACCGCGATATCGAGGGAAATGCCGGTGA [KpnI and EcoRV sites underlined]) under conditions described previously (13). The PCR product was restricted with SmaI and KpnI and cloned into the NruI and KpnI sites in pRJMI (19), creating pPTS1S3 (Fig. 1). This created an in-frame fusion of the 180 amino acids of S3 to the C-terminal 179 amino acids of S1. The DNA coding for the 456-amino-acid type I domain of FHA was initially subcloned from pMAL85 (20) into the BamHI-PstI sites of pBluescript. The resulting plasmid, pFHAI, was restricted with NotI, blunt ended with the Klenow fragment, and restricted with KpnI. The 1.3-kb DNA fragment was then ligated into the EcoRV and KpnI sites of pPTS1S3, creating pPTS1S3FHA. The 6.5-kb KpnI-ScaI fragment was subcloned into the streptococcal integration vector pSL2a (Lee and Halperin, unpublished data), which carried a 400-bp DNA fragment of the oligopeptide transport gene hppG (15) for homologous recombination. The resulting plasmid, pPTS1S3FHA2, was transformed into S. gordonii DL-1 as described previously (13).
FIG. 1.
Diagram depicting construction of the S1S3FHA fusion protein.
Purification of the S1S3FHA fusion protein.
S. gordonii #8 was grown in 10 liters of TYG (1% K2HPO4, 1% Trypticase peptone, 0.5% yeast extract, 1% [wt/vol] glucose) until late exponential growth was obtained. The culture supernatant was obtained by centrifugation (10,000 × g, 15 min, 4°C), and the proteins were precipitated with 60% saturated (NH4)2SO4. The precipitate collected by centrifugation was dialyzed against four changes of 3 liters of phosphate-buffered saline (PBS) over a 24-h period. The resulting solution was clarified by centrifugation and applied to a monoclonal anti-FHA immunoglobulin G (IgG) column, which was prepared by cross-linking the anti-FHA monoclonal 9F IgG to Affi-Gel protein A agarose (Bio-Rad Laboratories, Mississauga, Ontario, Canada) by using methods described previously (12). Briefly, 7 ml of Affi-Gel protein A agarose was washed twice with 0.15 M sodium borate buffer (pH 8.5) and incubated with 4.7 ml of anti-FHA monoclonal 9F ascites fluid in the same buffer at room temperature for 1 h. The agarose beads were washed five times with the borate buffer. The IgG was cross-linked to the protein A by treatment with 20 mM dimethylpimelimidate at room temperature for 30 min. The cross-linking reaction was stopped by washing with 0.2 M ethanolamine for 2 h. The beads were packed into a 10-ml syringe. Proteins were loaded onto this column, washed with 42 ml of PBS, and eluted with 18 ml of 0.1 M glycine (pH 2.75). The eluate was neutralized immediately by collection in 1.8 ml of 1 M Tris (pH 8.0). To maximize the recovery of the S1S3FHA fusion protein, each sample was chromatographed four times.
The eluted proteins were concentrated by ultrafiltration and then chromatographed onto a Sephacryl S-200-HR column (2.5 by 96 cm). The proteins were eluted with PBS at a flow rate of 15.6 ml/h. Fractions (5.5 ml) were collected, and duplicate 30-μl samples from every third fraction were assayed by an enzyme-linked immunosorbent assay by using anti-FHA monoclonal antibody 5E (1/2,000) and a rabbit anti-PT antibody (1/200) (19). Fractions showing reactivity with both antibodies were pooled, concentrated by ultrafiltration, and stored at −70°C.
Immunization and measurement of antibody responses.
Five 3-week-old female BALB/c mice were immunized intraperitoneally with 10 μg of the fusion protein in Freund's complete adjuvant and boosted with the same amount of antigen in Freund's incomplete adjuvant on days 8, 15, 25, and 42. The mice were euthanized on day 53, and blood was obtained by heart puncture. A second group of mice (n = 5) was immunized with 0.1 ml of a commercial vaccine containing 4 μg of the pertussis toxoid and 4 μg of FHA (Quadracel; Connaught Laboratories Ltd., Toronto, Ontario, Canada) by using the schedule described above. A third group of mice (n = 5) was immunized intranasally by pipetting 20 μl (10 μg) of the fusion protein in PBS slowly into the nostrils. The animals were boosted on days 8, 15, 29, and 41. A fourth group of mice (n = 5) was similarly immunized with the commercial vaccine, which had been concentrated by freeze-drying so that it contained 10 μg of the pertussis toxoid and 10 μg of FHA in 20 μl. On day 46, saliva was obtained from the intranasally immunized mice following carbachol stimulation (9). On day 47, blood, vaginal washes, and bronchoaveolar lavage fluids were also collected from this group of mice. Vaginal washes were obtained by pipetting two 50-μl aliquots of PBS containing 0.02% NaN3 into the vagina. The samples were subsequently clarified by centrifugation. Bronchoaveolar lavage fluids were collected as described previously (10).
Specific antibodies in samples were measured by an enzyme-linked immunosorbent assay in end point dilutions as described previously (18). Briefly, twofold-diluted samples were assayed in triplicate in 96-well microtiter plates that had been primed with 200 ng of PT (Chiron Corporation, Emeryville, Calif.) per ml, a maltose binding protein-FHA type I domain fusion protein (rFHAI), or native FHA (List Biological Laboratories, Campbell, Calif.). rFHAI was purified from a 1-liter culture of E. coli harboring pMal85 (20) by using an amylose column and methods similar to methods described previously (14). The purified rFHAI occurred as a single 85-kDa protein on a Coomassie blue-stained sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel. The specific IgG antibodies were detected with a goat anti-mouse IgG alkaline phosphatase conjugate (1/20,000; Fab specific; Sigma Chemical Co., Oakville, Ontario, Canada). The specific IgA antibodies were detected with a biotinylated goat anti-mouse IgA (1/20,000; alpha-chain specific; Sigma), followed by an avidin-alkaline phosphatase conjugate (1/10,000; Sigma). Titers were defined as the reciprocals of the dilutions that gave A405 readings which were 0.05 greater than those of the preimmune samples.
SDS-PAGE and immunoblotting.
Purified S1S3FHA (0.2 μg) was electrophoresed on an SDS-10% PAGE gel (17) and stained with a silver staining kit (Sigma). PT (1 μg; List Biologicals) was separated on an SDS-15% PAGE gel. For Western immunoblotting, the proteins were transferred to nitrocellulose membranes as described previously (31). The S1S3FHA fusion protein was reacted with rabbit anti-PT (1/200), anti-S1 monoclonal antibody A4 (1/2,000 [10]), anti-S3 monoclonal antibody B9 (1/2,000 [10]), or anti-FHA monoclonal antibody 5E (1/2,000). PT subunits were reacted with preimmune and immune sera (1/75). The antibody-protein complex was detected with either a goat anti-rabbit IgG or a goat anti-mouse IgG alkaline phosphatase conjugate (1/20,000; Sigma).
Protein content estimation.
Protein content was estimated by the Bradford method (3) by using bovine serum albumin as the standard.
RESULTS
Construction and expression of S1S3FHA fusion protein in S. gordonii.
Construction of the S1S3FHA fusion gene is depicted in Fig. 1. The fusion gene was fused to the 5′ end of the Streptococcus mutans spaP gene (16) at the convenient EcoRV site to facilitate expression and secretion of the fusion protein in S. gordonii. Previous studies showed that the fusion created at this site within the spaP gene functioned well in S. gordonii (19). Hence, the fusion protein consisted of a 523-amino-acid sequence of SpaP and a 715-amino-acid sequence of S1S3FHA. The final construct, pPTSS3FHA2, was verified by restriction analysis (data not shown). E. coli harboring pPTS1S3FHA2 was shown to express a 155-kDa protein that was recognized by a rabbit anti-PT antibody and an anti-FHA monoclonal antibody (data not shown). pPTS1S3FHA2 was introduced into S. gordonii by natural transformation. Several hundred kanamycin-resistant transformants were obtained. Ten transformants were randomly selected and screened for S1S3FHA expression by Western blotting, and all were found to produce in the culture supernatant a 155-kDa protein recognized by the rabbit anti-PT antibody. One of the transformants (transformant #8) was selected for further study. In cell fractionation studies performed by using previously described methods (19), the fusion protein was detected by Western blotting mainly in the culture supernatant (95%), and only a small amount (ca. 5%) was associated with the cell lysate (data not shown).
Purification of the S1S3FHA fusion protein.
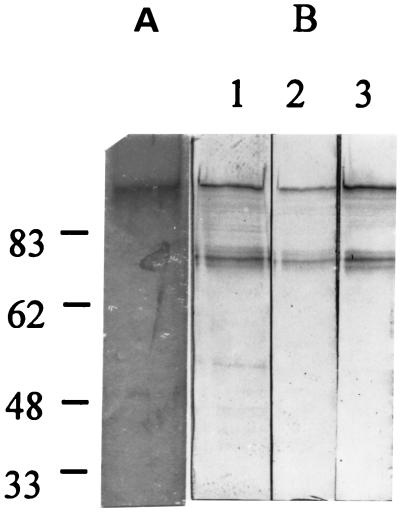
The S1S3FHA fusion protein produced by S. gordonii #8 was isolated from the culture supernatant fluid by affinity chromatography followed by gel permeation chromatography. The average yield of S1S3FHA was 15 μg per liter of culture. The isolated protein produced a single band at 155 kDa on a silver-stained SDS-PAGE gel (Fig. 2A). In Western immunoblotting analysis, the 155-kDa protein was recognized by the rabbit polyclonal anti-PT antibody, an anti-S1 monoclonal antibody, and an anti-FHA monoclonal antibody (Fig. 2B) but was only weakly recognized by an anti-S3 monoclonal antibody (data not shown). These results indicate that the 155-kDa protein was the S1S3FHA fusion protein. In addition to the 155-kDa protein, the antibodies also recognized a lower-molecular-weight band.
FIG. 2.
Silver-stained SDS-PAGE gel (A) and Western immunoblots (B) of the S1S3FHA fusion protein isolated from S. gordonii #8. In panel B, the protein was detected with a rabbit polyclonal anti-PT antibody (lane 1), an anti-S1 monoclonal antibody (lane 2), and an anti-FHA monoclonal antibody (lane 3).
Immunogenicity of S1S3FHA fusion protein.
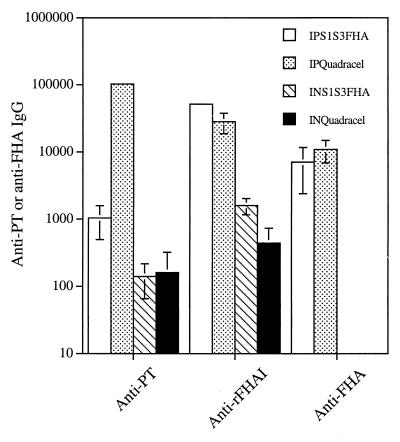
To assess the immunogenicity of the S1S3FHA fusion protein, BALB/c mice were immunized intraperitoneally with the purified protein. As shown in Fig. 3, the purified fusion protein elicited a weak serum anti-PT IgG titer (1,040) but a strong anti- rFHAI titer (51,200). For comparison, mice immunized with the commercial vaccine Quadracel gave anti-PT and anti-rFHAI titers of 102,400 and 28,160, respectively. The antisera from the S1S3FHA-immunized mice had a titer of 7,040 against the native FHA. A similar titer (10,880) against the native FHA was observed for the sera from Quatracel-immunized mice.
FIG. 3.
Immune responses in sera following intraperitoneal (IP) and intranasal (IN) immunizations with the S1S3FHA fusion protein or the commercial vaccine Quadracel. The values are mean ± standard error titers for individual animal samples. The absence of error bars means that the titers for the individual samples were identical.
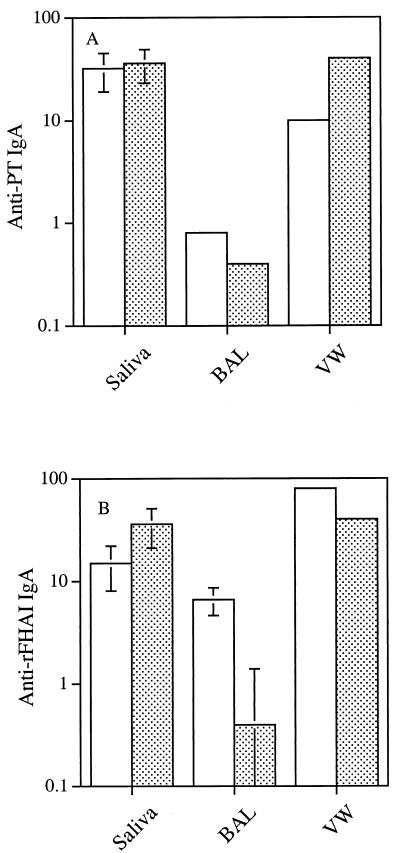
The purified fusion protein was also used to immunize mice intranasally. Immune responses to PT and FHA were observed in sera and mucosal samples. In sera, the anti-rFHAI and anti-PT titers were 1,600 and 140, respectively (Fig. 3). Interestingly, mice immunized intranasally with Quadracel gave similar anti-PT and anti-rFHAI titers. The S1S3FHA fusion protein also elicited a mucosal immune response. Anti-PT and anti-rFHAI IgA antibodies were detected in saliva, bronchoaveolar lavage fluid, and vaginal washes from the immunized animals (Fig. 4). Mice immunized intranasally with Quadracel showed similar levels of anti-PT and anti-rFHAI IgA in mucosal samples.
FIG. 4.
Immune responses in mucosal samples following intranasal immunization with the S1S3FHA fusion protein (open bars) or Quadracel (stippled bars). (A) Anti-PT IgA titers; (B) anti-rFHAI IgA titers. For saliva and bronchoaveolar lavage fluid (BAL), the values are mean ± standard error titers for individual animal samples; for vaginal washes (VW), the values are titers for pooled samples. In panel A, the titers for the bronchoaveolar lavage fluid samples are identical; therefore, there are no error bars.
In Western immunoblots the immune sera raised with the fusion protein, but not the preimmune sera, recognized the S1, S2, and S3 subunits of PT (Fig. 5).
FIG. 5.
Western immunoblots showing recognition of PT subunits S1, S2, and S3 by sera from mice immunized intraperitoneally (lane 2) and intranasally (lane 3) with the S1S3FHA fusion protein. Lane 1 contained pooled preimmune sera.
DISCUSSION
In this study, fragments of PT and FHA, two of the prominent components of the acellular pertussis vaccine, were used to genetically construct a fusion protein. The fusion protein consisted of the N-terminal 179 amino acids of S1, the 180 amino acids of S3, and the 456 amino acids of the type I domain of FHA. In-frame fusion was demonstrated by reactions with specific anti-PT and anti-FHA antibodies (Fig. 2). The S1S3FHA fusion protein had the expected molecular mass (155 kDa) and was secreted into the culture supernatant. The fusion protein was purified by affinity and gel permeation chromatography. The protein obtained was relatively pure, as demonstrated by the single band produced on an SDS-PAGE gel. In Western immunoblots, a smaller immunoreactive band was also observed. This immunoreactive band was likely a degraded product of the full-length fusion protein. The reactivity of this band with the three antibodies indicates that the proteolytic cleavage occurs at the SpaP sequence N terminal to the S1S3FHA sequences. The yield of the fusion protein was ca. 15 μg per liter of culture, which is relatively low. The low yield is most likely attributable to the presence of a single copy of the fusion gene on the chromosome and the fact that the spaP promoter is not highly active in S. gordonii. The introduction of the fusion gene into the chromosome should allow the gene to be maintained by the bacterium without antibiotic selection. The recombinant S. gordonii can then be used in oral colonization studies in an animal model (18). In future expression studies, the yield may be improved by placing the fusion gene on a multicopy plasmid, such as pDL276 (6), and by using an inducible promoter in place of the spaP promoter.
Our results clearly showed that multiple pertussis antigens can be expressed by S. gordonii in a single fusion protein. The ability to express multiple pertussis antigens in one immunogenic protein has potential for lowering the production cost of acellular pertussis vaccines. The use of a heterologous host for expression has potential for producing an antigen in a background free of other pertussis virulence factors that may contribute to the side effects of some vaccine preparations.
When injected intraperitoneally into mice, the purified fusion protein elicited a very strong antibody response to rFHAI and a weaker response to the native FHA. Similar levels of responses to rFHAI and native FHA were observed for sera obtained with the commercial vaccine. The discrepancy may be explained by the differences in the immunogens and the antibodies elicited. Antibodies against the fusion protein are directed to the type I domain only, while the antibodies raised with the commercial vaccine are directed against the entire FHA molecule. The much lower titer against the native FHA than against rFHAI for anti-S1S3FHA sera may be explained by the many hidden epitopes in the native FHA. This may also be true for the sera raised against the commercial vaccine.
The anti-PT response generated by parenteral immunization with S1S3FHA was very low compared to that generated by parenteral immunization with the commercial vaccine. In fact, the titer in the anti-S1S3FHA sera was much lower than that (12,800) in the sera generated by the surface-localized SpaP-S1 recombinant S. gordonii whole cells (19). The reason for this difference is not clear. Despite the low titer, specific antibodies do recognize the S1 and S3 subunits, as demonstrated by Western immunoblotting (Fig. 5). Recognition of the S2 subunit by the sera may be explained by the high level of homology between S2 and S3. Cross-reactivity between S2 and S3 was previously observed with an anti-S3 monoclonal antibody (10).
The fusion protein is also immunogenic when it is administered intranasally. Anti-rFHAI and anti-PT IgA were detected in mucosal samples. The titers of the specific IgA antibodies, however, were low. The mucosal immune response may be improved by using adjuvants in the future. Intranasal immunization also elicited a serum IgG response, but the antibody levels were much lower than those elicited by parenteral immunization. The anti-PT IgG from the sera of mucosally immunized mice also recognized S1, S2, and S3 in Western blots. Interestingly, similar levels of anti-PT and anti-FHA IgG were observed for the commercial vaccine.
In conclusion, our results provided proof that fragments of the pertussis vaccine components can be expressed in a single fusion protein by S. gordonii and that the fusion protein is immunogenic when it is given via systemic and mucosal routes. The multivalent fusion protein approach may be used in designing the new generation of acellular pertussis vaccines.
Acknowledgments
We thank Yi-Jing Li, Maxine Langman, and Ann MacMillian for their technical assistance. We thank Camille Locht for providing pMal85 and Michael Brennan for providing the anti-FHA 9F and 5E hybridomas.
This study was supported by an MRC Regional Partnership Program grant to the IWK Health Centre. J. B. Knight was a recipient of an IWK Summer Studentship award.
REFERENCES
- 1.Barbieri, J. T., D. Armellini, J. Molkentin, and R. Rappuoli. 1992. Construction of a diphtheria toxin A fragment-C180 peptide fusion protein which elicits a neutralizing antibody response against diphtheria toxin and pertussis toxin. Infect. Immun. 60:5071-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher, P., H. Sato, Y. Sato, and C. Locht. 1994. Neutralizing antibodies and immunoprotection against pertussis and tetanus obtained by use of a recombinant pertussis toxin-tetanus toxin fusion protein. Infect. Immun. 62:449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Dalla-Pozza, T. H. Yan, D. Meek, C. A. Guzman, and M. J. Walker. 1998. Construction and characterization of Salmonella typhimurium aroA simultaneously expressing the five pertussis toxin subunits. Vaccine 16:522-529. [DOI] [PubMed] [Google Scholar]
- 5.De Magistris, M. T., M. Romano, A. Bartoloni, R. Rappuoli, and A. Tagliabue. 1989. Human T cell clones define S1 subunit as the most immunogenic moiety of pertussis toxin and determine its epitope map. J. Exp. Med. 169:1519-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunny, G. M., L. N. Lee, and D. J. LeBlanc. 1991. Improved electroporation and cloning vector system for gram-positive bacteria. Appl. Environ. Microbiol. 57:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman, C. A., R. M. Brownlie, J. Kadurugamuwa, M. J. Walker, and K. N. Timmis. 1991. Antibody responses in the lungs of mice following oral immunization with Salmonella typhimurium aroA and invasive Escherichia coli strains expressing the filamentous hemagglutinin of Bordetella pertussis. Infect. Immun. 59:4391-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman, C. A., M. J. Walker, M. Rohde, and K. N. Timmis. 1991. Direct expression of Bordetella pertussis filamentous hemagglutinin in Escherichia coli and Salmonella typhimurium aroA. Infect. Immun. 59:3787-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 10.Halperin, S. A., T. B. Issekutz, and A. Kasina. 1991. Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J. Infect. Dis. 163:355-361. [DOI] [PubMed] [Google Scholar]
- 11.Hannah, J. H., F. D. Menozzi, G. Renauld, C. Locht, and M. J. Brennan. 1994. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect. Immun. 62:5010-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 13.Homonylo-McGavin, M. K., and S. F. Lee. 1996. Role of the C terminus in antigen P1 surface localization in Streptococcus mutans and two related cocci. J. Bacteriol. 178:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homonylo-McGavin, M. K., S. F. Lee, and G. H. Bowden. 1999. Subcellular localization of the Streptococcus mutans P1 protein C terminus. Can. J. Microbiol. 45:536-539. [PubMed] [Google Scholar]
- 15.Jenkinson, H. F., R. A. Baker, and G. W. Tannock. 1996. A binding-lipoprotein-dependent oligopeptide transport system in Streptococcus gordonii essential for uptake of hexa- and heptapeptides. J. Bacteriol. 178:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, C., P. Evans, L. A. Bergmeier, S. F. Lee, A. Progulske-Fox, A. S. Bleiweis, and T. Lehner. 1989. Sequence analysis of the cloned streptococcal cell surface antigen I/II (P1). FEBS Lett. 258:127-132. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. F., S. A. Halperin, H. Wang, and A. MacArthur. 2002. Oral colonization and immune responses to Streptococcus gordonii expressing a pertussis toxin S1 fragment in mice. FEMS Microbiol. Lett. 208:175-178. [DOI] [PubMed]
- 19.Lee, S. F., R. March, S. A. Halperin, G. Faulkner, and L. Q. Gao. 1999. Surface expression of a protective recombinant pertussis toxin S1 subunit fragment in Streptococcus gordonii. Infect. Immun. 67:1511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leininger, E., S. Bowen, G. Renauld-Mongenie, J. H. Rouse, F. D. Menozzi, C. Locht, I. Heron, and M. J. Brennan. 1997. Immunodominant domains present on the Bordetella pertussis vaccine component filamentous hemagglutinin. J. Infect. Dis. 175:1423-1431. [DOI] [PubMed] [Google Scholar]
- 21.Medaglini, D., G. Pozzi, T. P. King, and V. Fischetti. 1995. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. USA 92:6868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 23.Molina, N. C., and C. D. Parker. 1990. Murine antibody response to oral infection with live aroA recombinant Salmonella dublin vaccine strains expressing filamentous hemagglutinin antigen from Bordetella pertussis. Infect. Immun. 58:2523-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nascimento, I. P., W. O. Dias, R. P. Mazzantini, E. N. Miyaji, M. Gamberini, W. Quintilio, V. C. Gebara, D. F. Cardoso, P. L. Ho, I. Raw, N. Winter, B. Gicquel, R. Rappuoli, and L. C. Leite. 2000. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect. Immun. 68:4877-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paradis, F. W., F. Shareck, C. Dupont, D. Kluepfel, and R. Morosoli. 1996. Expression and secrection of beta-glucuronidase and pertussis toxin S1 by Streptomyces lividans. Appl. Microbiol. Biotechnol. 45:646-651. [DOI] [PubMed] [Google Scholar]
- 26.Runeberg-Nyman, K., O. Engstrom, S. Lofdahl, S. Ylostalo, and M. Sarvas. 1987. Expression and secretion of pertussis toxin S1 in Bacillus subtilis. Microb. Pathog. 3:461-468. [DOI] [PubMed] [Google Scholar]
- 27.Saris, P., S. Taira, U. Airaksinen, A. Palva, M. Sarvas, I. Palva, and K. Runeberg-Nyman. 1990. Production and secretion of pertussis toxin subunits in Bacillus subtilis. FEMS Microbiol. Lett. 56:143-148. [DOI] [PubMed] [Google Scholar]
- 28.Sato, H., A. Ito, J. Chiba, and Y. Sato. 1984. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect. Immun. 46:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato, H., Y. Sato, and I. Ohishi. 1991. Comparison of pertussis toxin (PT)-neutralizing activities and mouse-protective activities of anti-PT mouse monoclonal antibodies. Infect. Immun. 59:3832-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura, M., K. Nogimori, S. Murai, M. Yajima, K. Ito, T. Katada, M. Ui, and S. Ishii. 1982. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516-5522. [DOI] [PubMed] [Google Scholar]
- 31.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker, M. J., M. Rohde, K. N. Timmis, and C. A. Guzman. 1992. Specific lung mucosal and systemic immune responses after oral immunization of mice with Salmonella typhimurium aroA, Salmonella typhi Ty21a, and invasive Escherichia coli expressing recombinant pertussis toxin S1 subunit. Infect. Immun. 60:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]