Abstract
Although anthropogenic land use has major impacts on the exchange of soil and atmosphere gas in general, relatively little is known about its impacts on carbon monoxide. We compared soil-atmosphere CO exchanges as a function of land use, crop type, and tillage treatment on an experimental farm in Parãna, Brazil, that is representative of regionally important agricultural ecosystems. Our results showed that cultivated soils consumed CO at rates between 3 and 6 mg of CO m−2 day−1, with no statistically significant effect of tillage method or crop. However, CO exchange for a pasture soil was near zero, and an unmanaged woodlot emitted CO at a rate of 9 mg of CO m−2 day−1. Neither nitrite, aluminum sulfate, nor methyl fluoride additions affected CO consumption by tilled or untilled soils from soybean plots, indicating that CO oxidation did not depend on ammonia oxidizers and that CO oxidation patterns differed in part from patterns reported for forest soils. The apparent Km for CO uptake, 5 to 11 ppm, was similar to values reported for temperate forest soils; Vmax values, approximately 1 μg of CO g (dry weight)−1 h−1, were comparable for woodlot and cultivated soils in spite of the fact that the latter consumed CO under ambient conditions. Short-term (24-h) exposure to elevated levels of CO (10% CO) partially inhibited uptake at lower concentrations (i.e., 100 ppm), suggesting that the sensitivity to CO of microbial populations that are active in situ differs from that of known carboxydotrophs. Soil-free soybean and corn roots consumed CO when they were incubated with 100-ppm concentrations and produced CO when they were incubated with ambient concentrations. These results document for the first time a role for cultivated plant roots in the dynamics of CO in an agricultural ecosystem.
Anthropogenic activity disturbs numerous elemental cycles and gas exchanges between the atmosphere and biosphere (42). Agriculture in particular impacts carbon, nitrogen, and phosphorous cycles, altering patterns of microbial activity and elemental distribution relative to what occurs in natural systems (see, e.g., references 16, 42, and 43). In addition, agriculture disturbs fluxes of methane, nitrous oxide, and nitric oxide (5, 26, 32, 38, 40, 42, 44). Each of these gases plays important roles in atmospheric radiative forcing and contributes significantly to greenhouse warming through various direct and indirect effects (15, 37, 45). Agriculture also affects carbon monoxide dynamics, though there remains some uncertainty about the nature and sign (positive or negative) of impacts (see, e.g., references 29 and 33).
Changes in CO dynamics merit attention because CO largely determines the oxidative capacity of the troposphere by reacting rapidly with hydroxyl radicals (13, 37). Current best estimates suggest that global natural and anthropogenic sources contribute 2,600 Tg of CO year−1 to the atmosphere, with soils removing about 300 Tg year−1 (24). Therefore, soils play a role in atmospheric CO consumption similar to the role that they play in methane consumption (8, 25). However, much less is known about the biogeochemistry of soil CO and the factors that control consumption and emission (8).
A number of studies have documented the impacts of soil organic matter, water content, and temperature (9-11, 20, 28, 33, 34, 36, 46), but responses to many aspects of soil structure and chemistry have been explored to only a limited extent (e.g., references 8 and 27). In addition, relatively few studies have addressed the impact of agricultural practices on soil-atmosphere CO exchanges (29, 33, 39-41, 46), in spite of the fact that agricultural ecosystems have well-known profound effects on nitrogen gases and methane. Of those studies, two have reported short-term stimulatory effects of tilling on CO consumption, presumably caused by enhancing gas exchange (39, 40). Whether or not these effects persist is unknown. King (29) has suggested that enhanced CO consumption in temperate soils may accompany decreases in soil organic matter due to conventional tillage. However, Moxley and Smith (33) suggest that agricultural land use inhibits CO consumption and Bender and Conrad (4) indicate that patterns of CO consumption may be sensitive to ammonium fertilization, which affects populations of methanotrophs and ammonia oxidizers, both of which oxidize CO fortuitously (2).
In this study, we compared soil-atmosphere CO exchanges for regionally important tropical land uses on an experimental farm in Parãna, Brazil. We estimated kinetic parameters for CO consumption in selected soils, determined responses to soil moisture and elevated CO, and used a selective inhibitor to evaluate contributions of ammonia- or methane-oxidizing bacteria to CO consumption. In addition, we estimated the rates of CO production and potential CO consumption by washed, excised roots of soybean and corn plants. Our results showed that CO was consumed rapidly by most soils independently of the type of land use. CO consumption was most likely due to carboxydotrophs or other heterotrophic CO oxidizers that were not inhibited by nitrite or aluminum. Incubations with elevated CO (10% CO) inhibited uptake at atmospheric levels. CO consumption was also observed for soil-free soybean and corn roots when they were incubated with 100-ppm concentrations. However, when they were incubated with ambient CO, soil-free roots rapidly produced CO, with soybean plants showing substantial rates. Roots may contribute to belowground CO pools and may affect soil CO dynamics.
MATERIALS AND METHODS
Field description.
All assays were conducted with soil or plants collected from field sites at or adjacent to the EMBRAPA-Soja research station, Londrina, Parãna, Brazil, during February 2001. At this time, cultivated plots contained either soybeans (Glycine max) or corn (Zea mays L.). The cultivated soils have been previously classified within the Haplorthox group (6). A pasture site and unmanaged wooded site adjacent to the cultivated sites have not been specifically characterized but are similar to the cultivated soils in origin. Various aspects of the field sites, including soil characteristics, have been reported by Cattelan et al. (6).
Soil-atmosphere CO exchange.
Intact cores (7.5 cm [inner diameter] by 15 cm [length]) were collected during February 2001 from sites adjacent to soybean or corn plant stems or from an unmanaged wooded area and an adjacent pasture dominated by the grass Panicum maximum. Plots containing soybeans included conventionally tilled and nontilled areas of various ages; corn plots were conventionally tilled. Triplicate cores from each treatment were returned to the laboratory within 1 h and sealed with polypropylene end caps fitted with a septum to create headspaces of ambient air that were sampled with a needle and syringe for CO analysis. CO fluxes were estimated from headspace CO concentrations obtained at 3- to 4-min intervals over a period of 12 to 20 min by using a model RGD-2 gas chromatograph (Trace Analytical, Inc.) equipped with a mercury vapor detector. Over this time interval, CO levels did not change appreciably in empty core tubes used as blanks. The detector response was standardized with zero-grade air (Scott Specialty Gases, Inc.) containing 294 ppb CO as determined by comparison with a certified standard (103 ppb; National Oceanic and Atmospheric Administration-Climate Modeling and Diagnostics Laboratory, Boulder, Colo.). Net CO flux and production rates were estimated by using a model involving simultaneous CO production and consumption (9) and a nonlinear-curve-fitting algorithm from Kaleidagraph software (Synergy, Inc.). For this study, net CO uptake is taken as a positive value and emission is taken as a negative value.
Soil assays.
Surface soil (from depths of 0 to 2 cm) was collected with a hand trowel from a soybean plot that had been managed under a no-tillage regimen for 20 years. Five-gram (fresh weight) (gfw) subsamples were transferred to 110-cm3 glass jars that were subsequently sealed with neoprene stoppers as described above. One set of triplicates was incubated with ambient air for 24 h; a second set of triplicates was incubated with 10% CO for an equivalent period. A 10% headspace concentration was chosen to reflect values typically used in carboxydotroph enrichment. After the soils were reequilibrated with ambient air, the jars were sealed and rates of CO consumption were determined as before.
One-half-milliliter volumes of deionized water, 20 mM potassium nitrite, or 10 mM aluminum sulfate were added to 10- or 5-gfw soil samples (for nitrite or aluminum treatments, respectively) in jars allocated to three treatments in triplicate experiments. Final nitrite and aluminum concentrations were 1 and 2 μmol gfw of soil−1, respectively. Jars with additions of deionized water served as a control. Jars were sealed with neoprene stoppers, and consumption of ambient levels of CO was monitored as described above. Similar assays were conducted with 5-gfw soil samples incubated with or without the addition of 1% methyl fluoride; methyl fluoride at these levels inhibits both methane oxidizers and ammonia oxidizers (35).
Ammonia oxidation rates and the efficacy of methyl fluoride as an ammonia oxidation inhibitor were determined by transferring 1-gfw soil samples from conventionally tilled and 20-year-old nontilled soybean plots to 50-cm3 disposable centrifuge tubes. Each tube contained 10 ml of deionized water with 1 mM ammonium chloride and 10 mM sodium chlorate, an effective inhibitor of nitrite oxidation (3). For each soil type, a set of triplicate tubes sealed with neoprene stoppers was incubated at ambient temperature with shaking and with or without 1% methyl fluoride in the headspace. At various intervals over a 24-h period, 0.5-cm3 volumes of the soil slurries were subsampled with a needle and syringe for nitrite analysis by a spectrophotometric method (22). Nitrite accumulation in the presence of chlorate provided an estimate of ammonia oxidation rates (3).
The responses of CO exchange to increasing water content were assayed by using 5-gfw soil subsamples from a 20-year-old nontilled soybean plot. Subsamples (in 110-cm3 jars) were allocated to two treatments, one with an ambient water content (about 23%). A second was subjected to stepwise additions of 0.5-ml volumes of deionized water to yield water contents of 36, 48, 60, and 72%. Soils were equilibrated for about 30 min after each water addition prior to the monitoring of headspace CO and estimation of rates as before. Approximately 2 h elapsed between additions.
Water contents were determined for soil samples at all sites by drying the samples at 105°C. Ambient and soil temperatures were determined with a National Institute of Standards and Technology-traceable digital thermometer (Fisher Scientific, Inc.). Organic contents were measured for select samples by using the modified Walkley-Black perchlorate digestion procedure (18); pH was assayed for selected samples by using a 1:1 dilution (by weight) of fresh soil with deionized water and a Beckman model 71 pH meter.
CO uptake kinetics.
Previous assays with a variety of soils have confirmed that CO consumption rates respond to increasing CO concentrations up to modest levels (e.g., 1,000 ppm) according to a simple Michaelis-Menten kinetic model (23, 28). This model predicts that apparent one-half-saturation constants (appKm) can be approximated by Vmax/k, where Vmax is the maximum uptake velocity determined at a saturating CO concentration and k is a first-order rate constant determined at low (i.e., ambient) CO concentrations. Since appKm values of 10 to 20 ppm have been observed consistently for other soils, headspace CO concentrations of 100 to 200 ppm were chosen to determine Vmax values for surface soils (sampled at 0 to 2 cm) from an unmanaged woodlot and a 6-year-old nontilled soybean plot. CO uptake rate constants for these soils were determined initially as previously described. Subsequently, CO was added to a final concentration of 100 ppm, and uptake was measured as before, with the exception that a 15-μl sample loop was used on the gas chromatograph injection valve and rates were estimated from linear fits to time course data.
Root CO production and consumption.
Intact plants (stems and roots) were excavated from soybean and corn plots and returned to the laboratory within 1 h. Soil was gently but thoroughly washed from the roots with nonchlorinated tap water. After excision, roots were submerged and agitated to remove loosely adhering or nonadhering soil. Live roots were also separated from nonliving roots and particulate organic matter. Wash water was exchanged several times until no additional soil was freed. Washed roots were blotted to remove excess water, and approximately 1 gfw of fine roots (including nodules for soybeans) was transferred to 110-cm3 aluminum foil-covered glass jars fitted with neoprene rubber stoppers. Jar headspaces were subsampled at 3- to 4-min intervals with a needle and syringe during incubations at room temperature for analysis of headspace CO as previously described. Subsequent to CO production assays, the jar headspaces were equilibrated with room air and CO was added to final concentrations of 100 ppm. CO consumption by excised roots was assayed as described above. Dry weights of plant roots and nodules were determined after incubation at 110°C for about 24 h. All assays were conducted in triplicate.
RESULTS
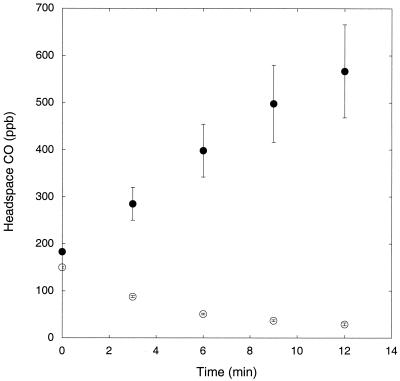
CO consumption was typically rapid (Fig. 1) for all cultivated soils, with substantial CO depletion occurring within 9 to 15 min. Consumption did not vary significantly among plots based on crop type or tillage treatment (Table 1; P > 0.1). Among the cultivated soils, the lowest and highest CO consumption rates were observed for a 20-year-old nontilled soybean plot (3.2 ± 0.5 mg of CO m−2 day−1) and a conventionally tilled soybean plot (5.5 ± 0.8 mg of CO m−2 day−1), respectively. In contrast, CO consumption was essentially zero (0.2 ± 0.7 mg of CO m−2 day−1) for pasture soils while CO was emitted from an unmanaged woodlot (Fig. 1) (−8.8 ± 2.2 mg of CO m−2 day−1).
FIG. 1.
Time course of headspace CO concentrations for samples of soils from a nontilled soybean plot (○) and an unmanaged woodlot (•). Data represent means ± 1 standard error for triplicate (soybean) or quadruplicate (woodlot) assays.
TABLE 1.
Rates of soil-atmosphere CO exchange for various crop and tillage treatmentsa
| Plot type | Rate (mg of CO m−2 day−1)b |
|---|---|
| Zea mays L. crop land | 5.5 ± 0.9 |
| Glycine max crop land | 5.3 ± 1.1 |
| Land not tilled for 7 yr | 5.6 ± 0.6 |
| Land not tilled for 20 yr | 3.2 ± 0.5 |
| Pasture | 0.2 ± 0.6 |
| Woodlot | −8.6 ± 2.3 |
All data are means of quadruplicate determinations ± 1 standard error of the mean.
Positive and negative values indicate inputs to and losses from the soils, respectively.
CO consumption did not vary consistently with bulk soil chemical parameters, such as pH, organic content, and major nutrients, for which values were similar among sites (see Table 2 for selected values). The unmanaged woodlot was exceptional in that organic contents of surface soils were about fourfold higher than those of all other soils (1.7 versus 0.4%). The relatively high organic contents at this site were associated with net CO emission, in contrast to consumption at other sites.
TABLE 2.
Selected results for chemical analyses of soils from EMBRAPA, Parãna, Brazila
| Site | pHb | Water contentc | Organic content (%)d |
|---|---|---|---|
| Soybean plot, tilled | 5.2 | 34.0 | 0.52 |
| Soybean plot, not tilled | 5.2 | 38.6 | 0.79 |
| Pasture | 5.1 | 30.5 | 0.34 |
| Unmanaged woodlot | 5.3 | 74.7 | 1.70 |
Values are means of duplicate determinations.
Determined from 1:1 slurries of deionized water and fresh soil.
Grams of water per gram (dry weight) of soil.
Grams of organic matter per gram (dry weight) of soil times 100%.
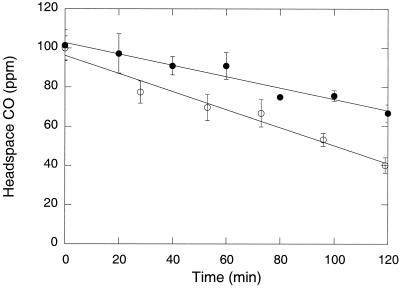
When CO was added at initial concentrations of 100 ppm, surface soil from a 20-year-old nontilled soybean plot readily consumed it (Fig. 2). Uptake was linear over a 2-h period, indicating that 100 ppm CO saturated activity. However, preincubation with a headspace containing 10% CO for about 24 h reduced uptake rates by 37% (analysis of variance; F = 8.86, P = 0.04) relative to those of controls maintained with ambient air only (Fig. 2). In contrast, additions of sodium nitrite, aluminum sulfate, or methyl fluoride had no significant effect on CO uptake (P > 0.05; data not shown). However, methyl fluoride completely inhibited ammonium oxidation in the same soils while controls incubated without methyl fluoride rapidly oxidized exogenous ammonium (data not shown). Rates of nitrite accumulation were significantly greater (about fivefold, P < 0.05) for nontilled soils (2.1 nmol gfw−1 h−1) than for tilled soils (0.4 nmol gfw−1 h−1).
FIG. 2.
Headspace CO concentrations during uptake assays of surface soils from a 20-year-old nontilled soybean plot. Samples were preincubated with ambient air (○) or 10% CO (•). All data are means ± 1 standard error for triplicate assays.
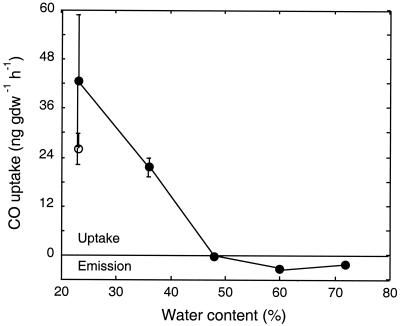
Changes in water content markedly affected CO exchange (Fig. 3). Increases to 48% or more changed activity distinctly from consumption to net emission. Though not statistically significant (P > 0.05), emission rates appeared to drop somewhat at a water content of 72%. At this level, the soil was saturated and slurry-like. CO uptake by controls maintained at ambient water levels (23%) did not change consistently over time, and these soils consumed CO throughout the incubation.
FIG. 3.
Atmospheric CO uptake rates for surface soils from a 20-year-old nontilled soybean plot. Samples were preincubated with ambient air and amended with deionized water sequentially to vary water content. Data for soils with varied water contents (•) are means ± 1 standard error for triplicate assays; the value for controls (○) is the mean of rates determined in triplicate at five intervals, during which control water contents were varied.
Based on uptake rates at a starting CO concentration of approximately 200 ppm and on first-order uptake rate constants determined from incubations with ambient CO, appKm values for the 0- to 2-cm depth interval of soil from a 6-year-old nontilled plot were 5.0 ± 2.4 ppm (about 5 nM dissolved CO). Maximum uptake rates for the same soils were 0.9 ± 0.4 μg of CO g (dry weight) (gdw)−1 h−1. For the 0- to 2-cm depth interval of unmanaged woodlot soils, maximum uptake and appKm values were 0.7 ± 0.04 μg of CO gdw−1 h−1 and 11.4 ± 1.7 ppm (about 12 nM), respectively.
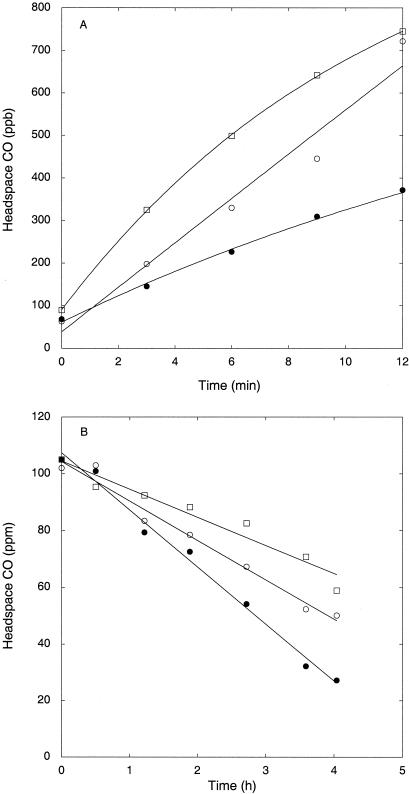
When incubated with ambient CO levels, soybean and corn roots produced CO with net average rates of 18 ± 8 and 2 ± 0.4 ng of CO gdw−1 min−1, respectively; rates were significantly higher for soybean than corn roots (P < 0.05; Fig. 4A [soybean only]). Gross CO production rates, corrected for a small simultaneous consumption term, were slightly higher than net rates, since CO consumption at near-ambient concentrations accounted for 7 and 16% of the respective production rates. However, when incubated with headspace concentrations of 100 ppm, all roots consumed CO rapidly, with activity for corn being about fourfold lower than that for soybean (66 ± 2 and 18 ± 9 ng of CO gdw−1 min−1, respectively; P = 0.02) (Fig. 4B [soybean only]). CO consumption rates at 100-ppm concentrations greatly exceeded net production and consumption rates (1.4 ± 0.8 and 0.4 ± 0.1 ng of CO gdw−1 min−1, respectively) estimated at near-ambient CO levels.
FIG. 4.
Headspace CO concentrations during assays of freshly collected, excised soil-free soybean roots (in parts per billion) (A) and after the addition of CO to initial values of approximately 100 ppm (in parts per million) (B). Different symbols indicate individual replicates.
DISCUSSION
Previously, King (29) reported that agricultural land use enhances atmospheric CO consumption relative to that of nonagricultural land in temperate systems. Differences in soil organic matter contents were proposed as a primary factor accounting for differences in CO uptake, with lower organic levels contributing to smaller amounts of abiological CO production. Decreased abiological CO production presumably promoted greater net CO consumption.
In this study, cultivated soils consumed atmospheric CO at higher rates than those of pasture or woodlot soils (Table 1). However, except with the woodlot, soil organic contents were very low (<1%) and did not vary consistently with crop type or agricultural practice (Table 2). Moreover, uptake rates for cultivated sites were comparable to data for temperate soils with higher organic levels (29). Further, woodlot CO emission was associated with much lower organic concentrations than those found in temperate soils that routinely consumed CO. Thus, organic matter plays a well-documented role in abiological CO production (10, 20, 34), but concentrations per se only partially explain CO flux patterns among varied land uses. Organic-matter turnover may play an additional role. In tropical systems, relatively rapid organic turnover (42) may be accompanied by greater CO production per unit of organic carbon and by a transition from CO consumption to emission at organic levels lower than those associated with emission in temperate soils.
Analysis of tillage treatments offers possibilities for exploring relationships among soil organic matter, organic turnover, and CO fluxes, since no-tillage practices generally accumulate organic matter relative to that of conventional tillage (16, 19, 21, 43). However, in this study, which is the first to compare tillage impacts, neither organic matter nor CO fluxes varied significantly among treatments, even though some plots were untilled for approximately 20 years (Fig. 2b). In part, this may reflect the lability of legume biomass as well as the relatively high rates of organic matter mineralization characteristic of many tropical systems.
The similarity of CO fluxes among treatments suggests that previously described plowing impacts (39, 40) are short-lived in tropical soils. The results also suggest that tropical soil management for carbon sequestration may have minimal near-term (20-year) effects. However, due to the increased application of no-tillage methods and their potential to sequester carbon (carbon farming) as a means to improve anthropogenic CO2 emissions, additional comparisons of CO fluxes of nontilled soils are warranted, especially in temperate systems, for which no data are currently available.
Similarities between kinetic parameters for nontilled and woodlot soils provided additional insights about the importance of organic matter as a determinant for net soil-atmosphere CO exchanges. Although appKm and Vmax values were equivalent at both sites, woodlot soils emitted CO while no-till soils consumed it (Fig. 1). However, net emission did not indicate an absence of CO consumption, since time course data were consistent with a model of simultaneous production and consumption at all sites (9, 23, 28). Emission for woodlot soils simply reflected a relatively high (1.1 ± 0.2 ppm) compensation point (the concentration where CO production and consumption levels are equal) compared to that for nontilled soils and to estimated woodlot appKm values (11.4 ± 1.7 ppm). Accordingly, CO production, which is related to organic matter content, exceeded CO consumption under ambient conditions. In contrast, at elevated concentrations (100 ppm), CO uptake greatly exceeded CO production and was comparable to values for cultivated soils.
Results from short-term incubations with high CO concentrations illustrate an additional aspect of the physiological ecology of CO utilization. Preincubation with 10% CO for as little as 24 h inhibits uptake at lower concentrations (e.g., 100 ppm) (Fig. 2). This response is likely due to CO toxicity (14). Long-term (20-day) incubations also indicate that elevated CO (20%) inhibits activity in temperate forest soils (23). Collectively, these observations suggest that CO oxidizers active in situ differ physiologically from typical carboxydotrophs and that attempts to isolate or otherwise characterize them must consider CO sensitivity in the design of enrichment protocols.
The identities of active CO-oxidizing microbes in situ remain largely unknown (1, 12, 23). However, insensitivity to methyl fluoride, complete inhibition of ammonia oxidation by methyl fluoride, and significant variation among sites in ammonia levels but not in CO oxidation effectively eliminate methanotrophs and ammonia-oxidizing bacteria as important contributors. Similar results have been reported for temperate forest soils (28) but are notable here, since Bender and Conrad (4) have shown that ammonia oxidizers consume CO in ammonium-fertilized temperate soils. The discrepancy between this study and that of Bender and Conrad (4) may be because the Brazilian soils chosen for analysis were not heavily fertilized. Accordingly, results reported here and by others (39) imply that CO utilization is dominated by heterotrophs, not lithotrophs.
CO consumption by nontilled soils varies sensitively with water content (Fig. 3). Such sensitivity typifies most soil-gas exchange processes (see, e.g., reference 17). In this case, the optimum water content must be less than approximately 20%, since activity decreased substantially at higher values, a pattern similar to that for a temperate forest (28). These data indicate that CO consumption by Brazilian soils may shift dramatically from the dry to rainy season and with irrigation. Sanhueza et al. (39) have described a similar phenomenon for tilled Venezuelan soils.
Data from root incubations indicate that plants affect soil CO dynamics (Fig. 4A), with the greatest impacts occurring during the growing season. Both corn and soybean roots produce CO, although activity is much greater for the latter. Similarly, King and Crosby (30) have documented substantial CO production by a wide range of temperate and tropical plants, with the highest values for legumes. Their results suggest that roots represent a belowground direct source of CO that is comparable in magnitude to the amount of CO which is removed globally from the atmosphere. They have also reported an inverse correlation between soybean root CO production and atmospheric CO consumption at sites described in this study (30), thus documenting impacts of root activity at a local level.
In addition, data presented here and by King and Crosby (30) show that roots consume CO actively (Fig. 4B) due to a rhizoplane microbiota that reduces fluxes from plants to soil by about 10 to 15%. Rhizoplane CO oxidizers are as yet largely uncharacterized, but enrichment cultures have yielded a number of new strains, including representatives of Mesorhizobium, Bradyrhizobium, Burkholderia, Stenotrophomonas, and Xanthobacter (G. M. King and H. Crosby, Abstr. Gen. Meet. Am. Soc. Microbiol., abstr. I-4, p. 247, 2002). Thus, plants appear to support diverse CO oxidizers and may account for the maintenance in soil of low-affinity populations, for which an ecological role has been uncertain (see, e.g., references 7 and 31).
Acknowledgments
G. M. King thanks the staff of EMBRAPA-Soja for a pleasant and productive visit.
G. M. King also acknowledges support from the Charles and Anne Morrow Lindbergh Foundation, the USDA, and the National Science Foundation. M. Hungria acknowledges support from EMBRAPA-Soja.
Footnotes
Contribution 372 from the Darling Marine Center.
REFERENCES
- 1.Bartholomew, G. W., and M. Alexander. 1982. Microorganisms responsible for the oxidation of carbon monoxide in soil. Environ. Sci. Technol. 16:300-301. [DOI] [PubMed] [Google Scholar]
- 2.Bedard, C., and R. Knowles. 1989. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 53:68-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belser, L. W., and E. L. Mays. 1980. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl. Environ. Microbiol. 39:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, M., and R. Conrad. 1994. Microbial oxidation of methane, ammonium and carbon monoxide, and turnover of nitrous oxide and nitric oxide in soils. Biogeochemistry 27:97-112. [Google Scholar]
- 5.Castro, M. S., W. T. Peterjohn, J. M. Mellilo, P. A. Steudler, H. L. Gholz, and D. Lewis. 1994. Effects of nitrogen fertilization on the fluxes of N2O, CH4, and CO2 from soils in a Florida slash pine plantation. Can. J. For. Res. 24:9-13. [Google Scholar]
- 6.Cattelan, A. J., C. A. Gaudencio, and T. A. Silva. 1997. Sistemas de rotacao de culturas em plantio direto e os microorganismos do solo, na cultura da soja, em Londrina. Rev. Brasil Ciencias Solo Campinas 21: 293-301. [Google Scholar]
- 7.Conrad, R. 1988. Biogeochemistry and ecophysiology of atmospheric CO and H2. Adv. Microb. Ecol. 10:231-283. [Google Scholar]
- 8.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad, R., and W. Seiler. 1980. Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl. Environ. Microbiol. 40:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad, R., and W. Seiler. 1985. Characteristics of abiological carbon monoxide formation from soil organic matter, humic acids, and phenolic compounds. Environ. Sci. Technol. 19:1165-1169. [DOI] [PubMed] [Google Scholar]
- 11.Conrad, R., and W. Seiler. 1985. Influence of temperature, moisture, and organic carbon on the flux of H2 and CO between soil and atmosphere: field studies in subtropical regions. J. Geophys. Res. 90(D):5699-5709. [Google Scholar]
- 12.Conrad, R., O. Meyer, and W. Seiler. 1981. Role of carboxydobacteria in consumption of atmospheric carbon monoxide by soil. Appl. Environ. Microbiol. 42:211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crutzen, P. J., and L. T. Gidel. 1983. A two-dimensional photochemical model of the atmosphere. II. The tropospheric budgets of the anthropogenic chlorocarbons, CO, CH4, CH3Cl and the effect of various NOx sources on tropospheric ozone. J. Geophys. Res. 88(C):6641-6661. [Google Scholar]
- 14.Cypionka, H., and O. Meyer. 1982. Influence of carbon monoxide on growth and respiration of carboxydobacteria and other aerobic organisms. FEMS Microbiol. Lett. 15:209-214. [Google Scholar]
- 15.Daniel, J. S., and S. Solomon. 1998. On the climate forcing of carbon monoxide. J. Geophys. Res. 103:13249-13260. [Google Scholar]
- 16.Davidson, E. A., and I. L. Ackerman. 1993. Changes in soil carbon inventories following cultivation in previously untilled soils. Biogeochemistry 20:161. [Google Scholar]
- 17.Fenchel, T., G. M. King, and T. H. Blackburn. 1999. Bacterial biogeochemistry: an ecophysiological analysis of mineral cycles. Academic Press, London, United Kingdom.
- 18.Forster, J. C. 1995. Organic carbon, p. 59-65. In K. Alef and P. Nannipieri (ed.), Methods in applied soil microbiology and biochemistry. Academic Press, New York, N.Y.
- 19.Franzluebbers, A. J., and M. A. Arshad. 1997. Particulate organic carbon content and potential mineralization as affected by tillage and texture. Soil Sci. Soc. Am. J. 61:1382-1386. [Google Scholar]
- 20.Göode, M., K. Meuser, and R. Conrad. 2000. Hydrogen consumption and carbon monoxide production in soils with different properties. Biol. Fertil. Soils 32:129-134. [Google Scholar]
- 21.Grant, R. F. 1997. Changes in soil organic matter under different tillage and rotation: mathematical modeling in ecosys. Soil Sci. Soc. Am. J. 61:1159-1175. [Google Scholar]
- 22.Grasshoff, K. 1976. Methods of seawater analysis, p. 134-137. Verlag Chemie, New York, N.Y.
- 23.Hardy, K., and G. M. King. Enrichment of high-affinity CO oxidizer in Maine forest soil. Appl. Environ. Microbiol. 67:3671-3676. [DOI] [PMC free article] [PubMed]
- 24.Khalil, M. A. K., J. P. Pinto, and M. J. Shearer. 1999. Atmospheric carbon monoxide. Chemosph. Glob. Change Sci. 1:ix-xi. [Google Scholar]
- 25.King, G. M. 1992. Ecological aspects of methane oxidation, a key determinant of global methane dynamics. Adv. Microbial. Ecol. 12:431-468. [Google Scholar]
- 26.King, G. M. 1997. Responses of atmospheric methane consumption by soils to global climate change. Glob. Change Biol. 3:351-362. [Google Scholar]
- 27.King, G. M. 1999. Characteristics and significance of atmospheric carbon monoxide consumption by soils. Chemosph. Glob. Change Sci. 1:53-63. [Google Scholar]
- 28.King, G. M. 1999. Attributes of atmospheric carbon monoxide oxidation by Maine forest soils. Appl. Environ. Microbiol. 65:5257-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King, G. M. 2000. Impacts of land use on atmospheric carbon monoxide consumption by soils. Global Biogeochem. Cycles 14:1161-1172. [Google Scholar]
- 30.King, G. M., and H. Crosby. Impact of plant roots on soil CO cycling and soil-atmosphere CO exchange. Glob. Change Biol., in press.
- 31.Morsdorf, G., K. Frunzke, D. Gadkari, and O. Meyer. 1992. Microbial growth on carbon monoxide. Biodegredation 3:61-82. [Google Scholar]
- 32.Mosier, A. R., J. A. Delgado, and M. Keller. 1998. Methane and nitrous oxide fluxes in an acid Oxisol in western Puerto Rico: effects of tillage, liming and fertilization. Soil Biol. Biochem. 30:2087-2093. [Google Scholar]
- 33.Moxley, J. M., and K. A. Smith. 1998. Factors affecting utilisation of atmospheric CO by soils. Soil Biol. Biochem. 30:65-79. [Google Scholar]
- 34.Moxley, J. M., and K. A. Smith. 1998. Carbon monoxide production and emission by some Scottish soils. Tellus 50(B):151-162. [Google Scholar]
- 35.Oremland, R. S., and C. W. Culbertson. 1992. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl. Environ. Microbiol. 58:2983-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potter, C. S., S. A. Losster, and R. B. Chatfield. 1996. Consumption and production of carbon monoxide in soils: a global model analysis of spatial and seasonal variation. Chemosphere 6:1175-1193. [Google Scholar]
- 37.Prather, M. J., R. Derwent, D. Ehhalt, P. Fraser, E. Sanhueza, and X. Zhou. 1995. Other trace gases and atmospheric chemistry, p. 73-126. In J. T. Houghton, L. G. Meira Filho, J. Bruce, H. Lee, B. A. Callender, E. Haites, N. Harris, and K. Maskell (ed.), Climate change 1994. Cambridge University Press, Cambridge, United Kingdom.
- 38.Robertson, G. P., E. A. Paul, and R. R. Hardwood. 2000. Greenhouse gases in intensive agriculture: contributions of individual gases to the radiative forcing of the atmosphere. Science 289:1922-1925. [DOI] [PubMed] [Google Scholar]
- 39.Sanhueza, E., L. Donoso, D. Scharffe, and P. Crutzen. 1994. Carbon monoxide fluxes from natural, managed, or cultivated savanna grasslands. J. Geophys. Res. 99(D):16421-16427. [Google Scholar]
- 40.Sanhueza, E., L. Cardenas, L. Donoso, and M. Santana. 1994. Effect of plowing on CO2, CO, CH4, N2O and NO fluxes from tropical savanna soils. J. Geophys. Res. 99(D):16429-16434. [Google Scholar]
- 41.Scharffe, D., W. M. Hao, L. Donoso, P. J. Crutzen, and E. Sanhueza. 1990. Soil fluxes and atmospheric concentrations of CO and CH4 in the northern part of the Guyana Shield, Venezuela. J. Geophys. Res. 95(D):22475-22480. [Google Scholar]
- 42.Schlesinger, W. H. 1997. Biogeochemistry: an analysis of global change. Academic Press, New York, N.Y.
- 43.Scholes, R. L., and M. C. Scholes. 1995. The effect of land use on non-living organic matter in soils, p. 209-225. In R. G. Zepp and C. Sonntag (ed.), The role of non-living organic matter in the Earth's carbon cycle. Wiley and Sons, Chichester, United Kingdom.
- 44.Steudler, P. A., J. M. Mellilo, B. J. Feigl, C. Neill, M. C. Piccolo, and C. C. Cerri. 1996. Consequence of forest-to-pasture conversion on CH4 fluxes in the Brazilian Amazon basin. J. Geophys. Res. 101:18547-18554. [Google Scholar]
- 45.Watson, R. T., H. Rodhe, H. Oeschger, and U. Siegenthaler. 1990. Greenhouse gases and aerosols, p. 1-40. In J. T. Houghton, G. J. Jenkins, and J. J. Ephraums (ed.), Climate change: the IPCC scientific assessment. Cambridge University Press, Cambridge, United Kingdom.
- 46.Yonemura, S., S. Kawashima, and H. Tsuruta. 2000. Carbon monoxide, hydrogen and methane uptake in soils in a temperate arable field and a forest. J. Geophys. Res. 105(D):14347-14362. [Google Scholar]