Abstract
Background
Co-occurrence of type 2 diabetes mellitus (T2DM) and heart failure (HF) elevates the risk of morbidity and mortality. Recent research emphasizes treatment strategies that go beyond glycemic control to enhance heart function.
Aim
To assess the effectiveness and safety of the fixed-drug combination of dapagliflozin and sitagliptin (FDC D/S) in T2DM patients with HF.
Methods
This was a retrospective, multicenter, observational study that included data from 168 T2DM patients with HF receiving treatment with FDC D/S. Outcome parameters included glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), postprandial plasma glucose (PPG), hypertension, N-terminal pro-B-type natriuretic peptide (NT-proBNP), estimated glomerular filtration rate (eGFR), and adverse events.
Results
The mean age of the patients was 55.5 ± 10.5 years. Most patients had comorbidities such as hypertension (86.3%) and dyslipidemia (75%), with more than 53% being obese. A significant (P < 0.0001) reduction was observed in HbA1c, FPG, PPG, and NT-proBNP levels, and in systolic blood pressure (SBP) and diastolic blood pressure (DBP) after 3 months of treatment with FDC D/S, while a significant (P < 0.0001) increase was observed in ejection fraction and eGFR, indicating improved glycemic control and heart function. Urinary tract infections (29.8%), dehydration (17.9%), hypoglycemia (14.9%), and genital mycotic infection (6.6%) were the common adverse events encountered with FDC D/S.
Conclusion
FDC D/S enhances glycemic control in T2DM patients with HF, leading to reductions in HbA1c, FPG, PPG, and cardiovascular risk factors such as NT-proBNP, SBP, and DBP, while also improving eGFR. The FDC D/S was generally well-tolerated, making it an effective and convenient treatment option.
Keywords: adverse effects, dapagliflozin, drug combinations, heart failure, sitagliptin, treatment outcome, type 2 diabetes mellitus
Introduction
The prevalence of diabetes in India is increasing at an alarming rate. A 2023 report by the Indian Council of Medical Research–India Diabetes estimated that approximately 101 million people in India live with diabetes [1]. The 10th edition of the International Diabetes Federation Diabetes Atlas projects a rising trend in diabetes prevalence, with the number of affected individuals expected to reach 124.9 million by 2045 [2]. The weighted prevalence of diabetes in 2023 was estimated to be 11.4% [1]. In parallel, a global analysis from 1995 to 2025 highlighted a similar alarming trend for type 2 diabetes mellitus (T2DM), positioning India as the country with the second-highest prevalence of T2DM in the world, after China [3]. The condition is more common among males (12.1%) than females (10.7%) [1,4].
T2DM has been closely associated with cardiovascular diseases (CVDs), the most prevalent cause of morbidity and mortality in patients with T2DM [5]. The risk of heart failure (HF) has been observed to be 2–4-fold higher in patients with T2DM, with a prevalence ranging between 9 and 22% compared to people without T2DM. The prevalence is even higher in elderly patients (aged ≥60 years) with T2DM [6]. Moreover, T2DM is strongly associated with higher hospitalization due to heart failure (HHF) and all-cause mortality, especially in Asians compared with the Western population [7]. While HF remains the most common cause of cardiac-related hospitalizations globally, the mortality rate for first-time heart attacks is notably higher in India, ranging from 15 to 20%, compared with the 4 to 5% seen in developed nations [8].
The management of T2DM has been demonstrated to influence clinical outcomes in patients with concurrent HF. Evidence indicates that specific classes of diabetes medications, such as sodium–glucose cotransporter-2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP4) inhibitors, not only control glycemia but also improve cardiac function and reduce the incidence and severity of HF events [9–11]. The 2023 American Association of Clinical Endocrinology Clinical Practice Guideline update on a comprehensive T2DM care plan recommends that if there is an established or high risk of HF, clinicians should prescribe an SGLT2 inhibitor or a glucagon-like peptide-1 (GLP-1) receptor agonist with proven efficacy, independent of glycemic control [12]. The European Society of Cardiology 2023 guidelines recommend SGLT2 inhibitors (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin, or sotagliflozin) for patients with T2DM and multiple atherosclerotic cardiovascular disease (ASCVD) risk factors or established ASCVD to reduce the risk of hospitalization for HF. Further, SGLT2 inhibitors such as sotagliflozin, empagliflozin, and dapagliflozin are also recommended in patients with T2DM having HF with reduced ejection fraction (HFrEF) for lowering the risk of hospitalization or cardiovascular-related mortality [13]. Nevertheless, pioglitazone and the DPP4 inhibitor saxagliptin are associated with an increased risk of HF in patients with T2DM and are therefore not recommended for lowering glucose levels [13]. The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, conducted among patients with T2DM, demonstrated that dapagliflozin significantly outperformed placebo in reducing the worsening of HF and cardiovascular-related mortality [14]. The DELIVER trial also demonstrated significantly reduced cardiovascular mortality; high-risk events, including first event and subsequent events; and urgent HF visits compared with placebo (receiving usual care) [15]. The TECOS trial found that sitagliptin, while improving glycated hemoglobin levels, was noninferior to placebo in terms of cardiovascular death, nonfatal stroke, hospitalization for cardiac events, and nonfatal myocardial infarction and did not increase the risk of these adverse events [16]. Despite the proven effectiveness of SGLT2 inhibitors, an observational study reported that metformin was the most frequently prescribed drug (59.8%), while SGLT2 inhibitors were under-prescribed (2.1%) among patients with T2DM having HF [17].
Combination therapy with SGLT2 inhibitors and DPP4 inhibitors offers a comprehensive approach to managing T2DM by targeting multiple pathways to enhance glucose control. SGLT2 inhibitors promote glucose excretion through the kidneys, leading to reduced blood glucose levels, weight loss, and lower blood pressure. DPP4 inhibitors increase incretin hormones, boosting insulin secretion, and lowering glucagon levels. The combination of these medications provides additional benefits, resulting in improved glycemic management, reduced cardiovascular risk, and better overall patient outcomes compared to using either drug alone (Fig. 1) [18].
Fig. 1.
Enhanced effects of combining SGLT2 inhibitors and DPP4 inhibitors in patients with T2DM. DPP4, dipeptidyl peptidase-4; SGLT2, sodium–glucose cotransporter-2; T2DM, type 2 diabetes mellitus.
SGLT2 inhibitors and DPP4 inhibitors have individually shown significant benefits in reducing the risk of cardiovascular events; however, despite these demonstrated benefits, there remains some residual risk of cardiovascular death that warrants attention. In addition, the Asian-Indian phenotype of T2DM is uniquely marked by cardiometabolic risk [19]. This phenotype necessitates comprehensive management strategies that not only focus on hyperglycemia but also target the broader spectrum of metabolic abnormalities to mitigate the risk of cardiovascular events. Combination therapy of SGLT2 inhibitors and DPP4 inhibitors can be particularly advantageous in the Indian population [18]. An expert opinion on the evidence of the effectiveness of the dapagliflozin and sitagliptin combination reported that this dual therapy is more effective than monotherapy with either drug alone or with metformin combined with diet and lifestyle modifications. The combination therapy offers a safe option, with no increased risk of inducing hypoglycemia [20]. The experts also opined that the sequence of addition of gliflozin to gliptin was more efficacious in lowering glucose levels than the addition of gliptin to gliflozin [20]. Dapagliflozin and sitagliptin lower glucose levels via different and complementary mechanisms, which adds an advantage for patients with HF [20]. Further, the combination therapy also targets six components of the ominous octet of T2DM [18,21]. Considering HF as a critical outcome in patients with T2DM, it is important to understand the prescribing pattern of antihyperglycemic agents and individualize the choice of various combinations in T2DM patients with HF. This study aimed to assess the clinical characteristics, treatment patterns, clinical effectiveness, and safety of the fixed-drug combination of dapagliflozin and sitagliptin (FDC D/S) in T2DM patients with HF.
Methods
Study design
This was a retrospective, multicenter, observational study to collect data anonymously from clinicians from various locations across India. The data were collected about T2DM patients with HF from multiple hospitals and diabetes clinics, both before and after 3 months of treatment with FDC D/S using case report forms (CRFs).
Study population
Inclusion criteria
The study included patients with T2DM with HF who were aged over 18 and receiving treatment with FDC D/S (dapagliflozin 10 mg/sitagliptin 100 mg), regardless of gender. HF was defined as the presence of symptoms or signs of HF, together with objective evidence of cardiac dysfunction. The severity of HF was classified using the New York Heart Association (NYHA) classification: class I (no symptoms with ordinary activity), class II (mild symptoms with ordinary activity), class III (marked limitation with less-than-ordinary activity), and class IV (symptoms at rest). In addition, HF was categorized based on left ventricular ejection fraction: HFrEF (≤40%), HF with mildly reduced ejection fraction (41–49%), or HF with preserved ejection fraction (≥50%) in the presence of elevated natriuretic peptides, structural heart disease, or both.
Exclusion criteria
Patients with T2DM free from HF or any other associated CVDs, and not receiving FDC D/S were excluded from the study. In addition, pregnant women with T2DM and individuals intolerant to either dapagliflozin or sitagliptin were excluded.
Data collection
Data from 168 patients were collected from 24 cardiologists at multiple centers over a period of 7 months, from September 2023 to April 2024, using digital CRFs. All the identifiers were removed before data collation. The collected data included information on BMI, relevant medical history, the reasons for adding FDC D/S, measuring efficacy through lab investigations using the same standardized testing methods for HbA1c, fasting plasma glucose (FPG), postprandial plasma glucose (PPG), hypertension, N-terminal pro-B-type natriuretic peptide (NT-proBNP), estimated glomerular filtration rate (eGFR), and adverse events experienced. NT-proBNP levels were obtained retrospectively from patient records, where they had already been measured in clinical practice. The measurements were performed on blood samples collected in EDTA tubes, which were centrifuged and frozen for storage. These measurements were performed using an immunoassay kit following the manufacturer’s instructions.
Outcome parameters
The primary outcome focused on evaluating the effectiveness and safety of FDC D/S. Effectiveness outcomes included HbA1c, FPG, PPG, hypertension, NT-proBNP, and eGFR, while the safety profile was assessed based on the reported adverse events. Secondary outcomes included the pattern of prescribing FDC D/S in T2DM patients with HF; percentage of patients with risk factors such as hypertension, dyslipidemia, or renal problems; eGFR variability in patients treated with FDC D/S; and percentage of patients achieving target FPG, PPG, HbA1c less than 7%, and reduction in NT-proBNP levels after FDC D/S prescription. Both pretreatment and posttreatment values (at 3 months) were recorded for each parameter.
Data analysis and interpretation
Statistical analysis was conducted using SPSS Version 23.0 software, and mean and standard deviations were obtained. The improvement in HbA1c, FPG, PPG, NT-proBNP, hypertension, and eGFR was determined based on the difference between post- and pretreatment values. Fisher’s exact test was used for categorical variables with two categories and χ² test for categorical variables with more than two categories. Statistical significance was considered at P value less than 0.05.
Ethical consideration
The study adhered to the requirements set by the institutional review boards of the participating clinical sites, following the principles of the International Conference on Harmonization of Good Clinical Practice and the Declaration of Helsinki. Approval was provided by the institutional review board at the primary study site (Indira IVF Hospital Institutional Ethics Committee, Chandigarh). Data were collected anonymously, coded for confidentiality, and securely stored with access control. As the data were collected retrospectively, informed consent was not required.
Results
Patient demographics
A total of 168 patients (69.6% males, 29.8% females, and 0.6% others) with a mean age of 55.5 ± 10.5 (range: 18–85) years were included in the study. The baseline characteristics are presented in Table 1. The medical history of patients included dyslipidemia, hypertension, obesity, smoking, and physical inactivity. The majority of the patients were overweight (38.69%) or obese (53.0%) (Table 1).
Table 1.
Baseline characteristics of the study population (N = 168)
| Characteristics | N (%) |
|---|---|
| Medical history | |
| Dyslipidemia | 126 (75) |
| Hypertension | 145 (86.3) |
| Obesity | 110 (65.5) |
| Smoking | 66 (39.3) |
| Physical inactivity | 64 (38.1) |
| BMI | |
| ≤18.5 kg/m2 (underweight) | 4 (2.4) |
| 18.5–22.9 kg/m2 (normal weight) | 10 (5.9) |
| 23–24.9 kg/m2 (overweight) | 65 (38.7) |
| ≥25 kg/m2 (obese) | 89 (53.0) |
The distribution of patients based on the NYHA classification of HF and as per ejection fraction–based HF classification is detailed in Table 2. Around 42.3% of the patients presented with some symptoms of HF as per the NYHA classification and around 43.4% of the patients had HF with mildly reduced or reduced ejection fraction according to the ejection fraction–based HF classification (Table 2).
Table 2.
Distribution of patients based on New York Heart Association and ejection fraction–based classification of heart failure
| HF class | N (%) |
|---|---|
| NYHA classification | |
| NYHA class I | 97 (57.7) |
| NYHA class II | 51 (30.4) |
| NYHA class III | 18 (10.7) |
| NYHA class IV | 2 (1.2) |
| EF-based classification | |
| HFmEF | 50 (29.8) |
| HFpEF | 95 (56.5) |
| HFrEF | 23 (13.7) |
EF, ejection fraction; HF, heart failure; HFmEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
The decision to add FDC D/S to the treatment regimen was driven by several factors, with the primary reasons being managing uncontrolled glycemia (89.9%) and addressing glycemic variability (75.6%) (Table 3).
Table 3.
Reasons for combining dapagliflozin and sitagliptin
| Reason | Number of patients (%) |
|---|---|
| Uncontrolled glycemia | 151 (89.9) |
| Weight gain | 56 (33.3) |
| Hypoglycemia due to other GLP-1 analog/insulin/other OADs | 40 (23.8) |
| Glycemic variability | 127 (75.6) |
| Hospitalization because of cardiovascular disease | 83 (49.4) |
GLP-1, glucagon-like peptide 1; OAD, oral antidiabetic drug.
Effectiveness of fixed-drug combination of dapagliflozin and sitaglipt after 3 months of treatment
Treatment with FDC D/S led to reduced HHF, improved eGFR variability, and a reduction in BMI levels in a maximum number of patients (Table 4).
Table 4.
Improvement in outcome parameters after 3 months of treatment with a fixed-dose combination of dapagliflozin and sitagliptin
| Outcome parameter | N (%) |
|---|---|
| Reduced HHF | 158 (94.0) |
| Improved eGFR variability | 162 (96.4) |
| Reduced BMI levels | 167 (99.4) |
eGFR, estimated glomerular filtration rate; HHF, heart failure hospitalization.
After 3 months of treatment with FDC D/S, a significant (P < 0.0001) reduction (16.8%) in HbA1c, from 8.9 to 7.4%, was observed in the patients (Fig. 2a). Consistently, both FPG and PPG also showed a significant reduction (P < 0.0001) (Figs. 2b and c). NT-proBNP dipped from 306.7 to 184 pg/ml (P < 0.0001) (Fig. 2d), while the ejection fraction in 2D echocardiography [showed an improvement (6.7%) from 43.1 to 45.99% (P < 0.0001); Fig. 2e]. The reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP) was significant (P < 0.0001) after 3 months of treatment with FDC D/S, from 151.6 to 133.6 mmHg, demonstrating an 11.9% reduction, and from 98.8 to 87.4 mmHg, demonstrating an 11.5% reduction, respectively (Figs. 2f and g). In addition, eGFR improved by 17.2%, from 63.08 to 73.96 ml/min/1.73 m2, after 3 months of treatment (P < 0.0001) (Fig. 2h).
Fig. 2.
Results (mean ± SD) obtained before and after treatment for (a) HbA1c, (b) FPG, (c) PPG, (d) NT-proBNP, (e) 2D ECHO, (f) SBP, (g) DBP, and (h) eGFR. A significant difference (P < 0.0001) was observed for all parameters. DBP, diastolic blood pressure; ECHO, echocardiography; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PPG, postprandial plasma glucose; SBP, systolic blood pressure.
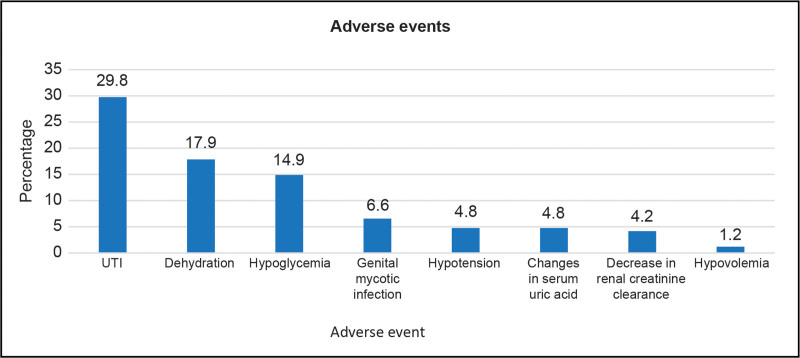
Adverse events
The most common adverse events reported after treatment with FDC D/S were urinary tract infections (UTIs) (29.8%), dehydration (17.9%), hypoglycemia (14.9%), and genital mycotic infection (6.55%). Other adverse events included hypotension, changes in serum uric acid, decreased renal creatinine clearance, and hypovolemia (Fig. 3).
Fig. 3.
Adverse events reported with the use of FDC D/S. FDC D/S, fixed-drug combination of dapagliflozin and sitagliptin; UTI, urinary tract infection.
Discussion
The management of T2DM is rapidly transitioning from concentrating solely on lowering glucose to a cardiometabolic approach [22]. Dapagliflozin and sitagliptin are antidiabetic agents that lower blood glucose through distinct mechanisms, each having proven effectiveness and safety in patients with T2DM. A meta-analysis of randomized controlled trials (RCTs) supports the efficacy and safety of dapagliflozin as monotherapy in lowering HbA1c, body weight, and FPG in patients with T2DM, without increasing the risk of hypoglycemia [23]. Similarly, a 10-year follow-up study on sitagliptin has demonstrated improvement in glycemic control among patients with T2DM with no prior history of CVD and has shown to have long-lasting islet-protective effects [24]. Regarding cardiovascular effects, a meta-analysis of dapagliflozin monotherapy demonstrated a significant reduction in HHF (risk ratio = 0.72) and cardiovascular death (risk ratio = 0.83) [25] among T2DM patients with HF. Similarly, a meta-analysis by Zeng et al. [26] provided evidence supporting the use of sitagliptin, as the drug was associated with no increased risk of cardiovascular events in patients with T2DM. Given the effectiveness of dapagliflozin and sitagliptin as monotherapies, it is evident from the literature that their combination yields even more pronounced improvements in glycemic control, while simultaneously addressing the complications associated with T2DM [21,27,28]. The present real-world study provided evidence about the effectiveness of FDC D/S in reducing HbA1c, FPG, PPG, NT-proBNP, ejection fraction, SBP, and DBP, and improving eGFR among T2DM patients with HF.
In the present study, FDC D/S was effective in reducing HbA1c, which is consistent with existing research [21,28]. Dapagliflozin and sitagliptin, a real-world retrospective study among Indian patients with T2DM, reported a significant reduction in HbA1c from 8.9 to 7.2% at the follow-up of 12 weeks with the use of FDC D/S [21]; however, another real-world study among Indian patients assessed improvement in glycemic control using the time-in-target (TIT) metric, as HbA1c alone may not provide accurate evidence for T2DM management. The combination therapy in the study conducted by Bhattacharyya et al. [28] in 2023 demonstrated significant improvement in average daily glucose (19.41% reduction), time-above-target (31.1% reduction), and TIT (34.5% increase) at the end of 15 days of the treatment.
The FDC D/S in the present study was also effective in decreasing FPG. In line with the findings of the present study, FPG decreased from 178.8 to 124.0 mg/dl and PPG from 273.9 to 176.0 mg/dl, demonstrating significant glucose level improvement in patients using FDC D/S therapy [21]. Correspondingly, a multicenter, randomized, placebo-controlled trial showed a reduction in FPG from 157.3 to 135.3 mg/dl at 24 weeks after adding dapagliflozin to sitagliptin, substantiating the effectiveness of an FDC of D/S [27]. A significant reduction in NT-proBNP was observed with FDC D/S therapy, from baseline to 3 months posttreatment, indicating improved cardiac function and a reduced risk of HF in the patients of the present study. In accordance with this, a post hoc subgroup analysis of the CANDLE trial reported a decrease in NT-proBNP levels from 213.9 at baseline to 207.7 at 24 weeks in patients with T2DM managed by the addition of SGLT2 inhibitor in those using DPP4 inhibitors, indicating a potential improvement in cardiac function associated with this treatment approach [29].
The FDC D/S has also demonstrated its positive effect on renal function by improving eGFR among patients with T2DM included in the present study. This improved eGFR is likely to prevent the progression of diabetic nephropathy, reduce the risk of chronic kidney disease, and lower the likelihood of associated complications such as cardiovascular events and end-stage renal disease (ESRD). These findings align with the post hoc analysis of the VERTIS CV study, which showed that combination therapy with an SGLT2 inhibitor and a DPP4 inhibitor notably decreased the risk of kidney composite outcome (defined as a sustained decrease of 40% or more in eGFR, dropping to less than 60 ml/min/1.73 m2). Furthermore, the combination therapy also reported a reduced risk of new ESRD and death because of renal complications compared with placebo [30]. In addition, post hoc analysis of the DECLARE-TIMI 58 study demonstrated comparable renal outcomes (1.5%), that is, new ESRD, death from renal complications, and a sustained decrease of 40% or more in eGFR (dropping to less than 60 ml/min/1.73 m2) among patients with T2DM managed by a combination of dapagliflozin and a DPP4 inhibitor and those managed by dapagliflozin without the DPP4 inhibitor; however, the renal-specific outcomes were relatively less in combination therapy (1.5%) compared with placebo (2.4%) [31]. These findings support the use of this combination therapy as a viable option for improving renal outcomes and managing diabetic kidney disease.
The FDC D/S demonstrated a reduction in HHF in 94% of the patients in the present study, indicating its effectiveness. According to the DAPA-HF trial, the event of HHF with a combination therapy of dapagliflozin and a DPP4 inhibitor was 11.2%, while it was 15.5% in patients with T2DM managed by dapagliflozin alone [32]. Similarly, the percentage of patients with HHF or CV death was 4.7% in those with T2DM managed by the combination of the SGLT2 inhibitor and DPP4 inhibitor, while the rate was 8.5% in patients with T2DM managed by SGLT2 inhibitor alone [30]. The results of the present study are also in line with the CANVAS study, wherein T2DM patients managed with a combination of SGLT2 inhibitor and DPP4 inhibitor reported HHF or cardiac death of 0.98%, while the rate was 1.7% in T2DM patients managed by SGLT2 inhibitor without DPP4 inhibitor [33]. The results are supported by the post hoc analysis of the DECLARE-TIMI 58 study, wherein HHF was reported in 2.1% of patients on combination therapy of dapagliflozin and DPP4 inhibitor while it was slightly more (i.e. 2.5%) in patients managed by dapagliflozin without DPP4 inhibitor [31]. The findings of the present study, supported by existing literature, reinforce the role of FDC D/S not only in glycemic control but extending beyond to reduce HHF.
It is important to note that the improved cardiovascular health in T2DM patients with HF is attributable to the additional effects of combining SGLT2 and DPP4 inhibitors, each contributing through their distinct mechanisms. For SGLT2 inhibitors, the drug’s multiple mechanisms of action include systemic effects, myocardial effects, and combined effects. SGLT2 inhibitors enhance vascular function, volume regulation, and cardiorenal effects, thereby reducing cardiac workload and blood pressure. Further, SGLT2 inhibitors also improve ketone utilization, reduce hypertrophy and fibrosis, and decrease inflammation and oxidative stress. These drugs also promote better ion homeostasis and myocardial contractility, effectively protecting against HF and other cardiovascular issues [34]. The DPP4 inhibitors inhibit HF by increasing GLP-1 levels, which leads to improved myocardial glucose uptake, reducing oxidative stress and exerting anti-inflammatory effects. Also, it increases the levels of stromal cell-derived factor 1, which is associated with the repair and regeneration of cardiac tissue by promoting stem cell homing, hematopoiesis, and angiogenesis. Furthermore, it causes downregulation of collagen type III production, thereby improving myocardial fibrosis. It also modulates the renin–angiotensin–aldosterone system, thereby controlling myocardial hypertrophy and fibrosis and reducing sodium and water retention [35].
It is noteworthy that comorbidities, particularly hypertension, dyslipidemia, and obesity, were prevalent in the majority of the study patients. In addition, 39.3% of the patients were smokers and 38.1% were physically inactive. This underscores the burden of comorbidities and risk factors in these patients and the importance of incorporating these parameters into the management practices of T2DM for a comprehensive approach. The FDC D/S in the present study not only aimed at achieving glycemic control but also demonstrated its effectiveness in reducing T2DM-associated complications, particularly HHF, variability in eGFR, and BMI levels. Expert opinions by cardiologists in India have also opined that the use of FDC D/S in T2DM patients with HF is useful as it offers the advantage of lower cardiovascular risk with a once-daily dosing [22].
Adverse events, particularly UTI, genital tract infection (GTI), and renal diseases have been documented in the literature for both monotherapies and combination therapies of FDC D/S. A meta-analysis of RCTs demonstrated a significant increase in the risk of UTI [odds ratio (OR) = 1.36], gastrointestinal tract infections (OR = 3.65), and renal diseases (OR = 1.57) with the use of dapagliflozin compared with placebo [36]. The safety outcomes were similar in another meta-analysis with increased risk of UTI [relative risk (RR) = 1.74] and GTI (RR = 3.52) associated with dapagliflozin use [23]. In the present study, UTI (29.8%), dehydration (17.9%), hypoglycemia (14.9%), and genital mycotic infection (6.55%) were the most common adverse events reported for FDC D/S use. The adverse events reported were in accordance with the adverse events reported by Bhattacharjee et al.,[21] comprising hypoglycemia (22.6%) and UTI or genital mycotic infection (12%), with no cases of serious adverse events with the use of FDC D/S. In contrast, a real-world study among Indian patients revealed negligible adverse events, such as nocturnal and daytime hypoglycemia, with FDC D/S use [28]. Further, although FDC D/S is known for its low risk of hypoglycemia, the rates of hypoglycemia in the present study were high. This may be attributed to the inclusion of patients having a higher susceptibility to hypoglycemia due to factors such as advanced age. Around 37.5% of patients in the present study were aged greater than or equal to 60 years. It is important to note that older adults with T2DM are more susceptible to hypoglycemia because of their impaired adaptive physiologic responses to low glucose levels. The reduced hormone responses and compromised autonomic processes hinder proper glucose secretion. This affects brain function, slowing recovery after hypoglycemia. In addition, aging affects the pharmacokinetics of oral medications and insulin, altering drug absorption, distribution, and renal elimination [37]. Another reason for a higher rate of hypoglycemia in this study may be because of 86.3% of the patients being hypertensive. Beta-blockers used for managing hypertension are reported to be associated with hypoglycemia in patients with T2DM [38]. Thus, concomitant use of beta-blockers for managing hypertension may have contributed to the higher rate of hypoglycemia in the present study.
Strengths and limitations
This study had several strengths. The study population included a diverse patient pool with comorbidities, which allowed a better understanding of the impact of FDC D/S on glycemic control and heart function. Large-scale digital data collection enabled robust analysis, and the transparent reporting of adverse events highlighted the need for careful monitoring. Nevertheless, certain limitations cannot be overlooked. Being a retrospective design, the study may have an inherent issue of selection bias resulting in a limited generalizability of the results. Furthermore, controlling the confounding factors in the retrospective design is constrained, which might have affected the validity of the results. In addition, the follow-up duration of the patients receiving FDC D/S was short (3 months), restricting the ability to assess the long-term effectiveness and safety of the drug in T2DM patients with HF.
Conclusion
On the basis of the real-world evidence from this study, it can be concluded that FDC D/S positively impacts T2DM patients with HF, leading to a reduction in HbA1c, FPG, PPG, and cardiovascular risk factors such as NT-proBNP, SBP, and DBP, while also improving eGFR. The advantages of this combination therapy, including its convenient single-pill formulation and high patient compliance, make FDC D/S a well-established and effective treatment option for managing T2DM in patients with HF; however, there is a need for well-designed RCTs to validate these results and provide data on the long-term effectiveness and safety of FDC D/S in T2DM patients with HF. Furthermore, future studies can aim at incorporating patient-reported outcomes in addition to clinical outcomes to obtain a more holistic understanding of the impact of combination therapy on the quality of life of the patients.
Acknowledgements
The authors extend special thanks to Mr. Animesh Sahoo, Mr. Sumit Kakirde, and Ms. Pallavi Vanmali. The authors also thank BioQuest Solutions for medical writing support.
This research was funded by USV Private Limited.
All the authors of the manuscript have contributed equally to the conception, design, drafting, review, and finalization of the manuscript.
The manuscript has been read and approved by all the authors. The requirements for authorship, as stated earlier in this document, have been met, and each author believes that the manuscript represents honest work.
Conflicts of interest
The present study was initiated and supported by USV Private Limited. The authors R.N.K., A.P., and A.P. are employees of USV and for the remaining authors, there are no conflicts of interest.
References
- 1.Anjana RM, Unnikrishnan R, Deepa M, Pradeepa R, Tandon N, Das AK, et al. ; ICMR-INDIAB Collaborative Study Group. Metabolic non-communicable disease health report of India: The ICMR-INDIAB National Cross-sectional Study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol 2023; 11:474–489. [DOI] [PubMed] [Google Scholar]
- 2.Magliano DJ, Boyko EJ. IDF Atlas 2021. Edition 10. https://www.ncbi.nlm.nih.gov/books/NBK581934/. [Accessed 7 June 2024]. [Google Scholar]
- 3.Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 2020; 10:14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol 2021; 69:2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einarson TR, Acs A, Ludwig C, Panton U. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018; 17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. ; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and the Heart Failure Society of America. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019; 140:e294–e324. [DOI] [PubMed] [Google Scholar]
- 7.Bank IEM, Gijsberts CM, Teng TK, Benson L, Sim D, Yeo PSD, et al. Prevalence and clinical significance of diabetes in Asian versus white patients with heart failure. JACC Heart Fail 2017; 5:14–24. [DOI] [PubMed] [Google Scholar]
- 8.ICC – National Heart Failure Registry. The current situation. 2024. https://www.iccnhfr.org/the-current-situation. [Accessed 7 June 2024]. [Google Scholar]
- 9.Shen J, Greenberg BH. Diabetes management in patients with heart failure. Diabetes Metab J 2021; 45:158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muscoli S, Barillà F, Tajmir R, Meloni M, Morte DD, Bellia A, et al. The new role of SGLT2 inhibitors in the management of heart failure: current evidence and future perspective. Pharmaceutics 2022; 14:1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini K, Sharma S, Khan Y. DPP-4 inhibitors for treating T2DM – hype or hope? An analysis based on the current literature. Front Mol Biosci 2023; 10:1130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, et al. American Association of Clinical Endocrinology Consensus statement: Comprehensive type 2 diabetes management algorithm – 2023 update. Endocr Pract 2023; 29:305–340. [DOI] [PubMed] [Google Scholar]
- 13.Marx N, Federici M, Schütt K, Muller-Wieland D, Ajjan RA, Antunes MJ, et al. ; ESC Scientific Document Group. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J 2023; 44:4043–4140. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 15.Jhund PS, Claggett BL, Talebi A, Butt JH, Gasparyan SB, Wei LJ, et al. Effect of dapagliflozin on total heart failure events in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER Trial. JAMA Cardiol 2023; 8:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. ; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242. Erratum in: N Engl J Med. 2015; 373:586 [DOI] [PubMed] [Google Scholar]
- 17.Lin SN, Phang KK, Toh SH, Chee KH, Huri HZ. Heart failure with type 2 diabetes mellitus: association between antihyperglycemic agents, glycemic control, and ejection fraction. Front Endocrinol (Lausanne) 2020; 11:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadha M, Das AK, Deb P, Gangopadhyay KK, Joshi S, Kesavadev J, et al. Expert opinion: optimum clinical approach to combination-use of SGLT2I + DPP4I in the Indian diabetes setting. Diabetes Ther 2022; 13:1097–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravikumar L, Kiwalkar RS, Ravindra HS, Lokesh B, Dabhade D, et al. Dapagliflozin and sitagliptin combination therapy: an overview of clinical utility in type 2 diabetes mellitus with multiple cardiovascular risk factors. Cardiol Cardiovasc Med 2023; 7:141–144. [Google Scholar]
- 20.Scheen AJ. DPP-4 inhibitor plus SGLT-2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol 2016; 12:1407–1417. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharjee R, Rai M, Joshi P, Prasad A, Birla A. The real DAPSI: a real-world retrospective study on assessing the efficacy and safety of a fixed-dose combination of dapagliflozin and sitagliptin in the Indian population. Cureus 2023; 15:e46767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soumitra R, Ezhilan J, Karnik R, Prasad A, Dhar R. Expert opinion on fixed dose combination of dapagliflozin plus sitagliptin for unmet cardiovascular benefits in type 2 diabetes mellitus. J Diabetol 2024; 15:131–141. [Google Scholar]
- 23.Feng M, Lv H, Xu X, Wang J, Lyu W, Fu S. Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Medicine (Baltim) 2019; 98:e16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattori S. Ten-year follow-up of sitagliptin treatment in patients with type 2 diabetes mellitus. Diabetol Metab Syndr 2021; 13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng XD, Qu Q, Jiang XY, Wang ZY, Tang C, Sun JY. Effects of dapagliflozin on cardiovascular events, death, and safety outcomes in patients with heart failure: a meta-analysis. Am J Cardiovasc Drugs 2021; 21:321–330. [DOI] [PubMed] [Google Scholar]
- 26.Zeng DK, Xiao Q, Li FQ, Tang YZ, Jia CL, Tang XW. Cardiovascular risk of sitagliptin in treating patients with type 2 diabetes mellitus. Biosci Rep 2019; 39:BSR20190980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabbour SA, Hardy E, Sugg J, Parikh S; Study 10 Group. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care 2014; 37:740–750. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharyya S, Muchhala S, Jhaveri K. Efficacy of fixed-dose combination of dapagliflozin and sitagliptin in type 2 diabetes mellitus using continuous glucose monitoring: a real-world study in India. J Adv Med Pharm Sci 2023; 25:1–9. [Google Scholar]
- 29.Tanaka A, Toyoda S, Imai T, Shiina K, Tomiyama H, Matsuzawa Y, et al. ; CANDLE Trial Investigators. Effect of canagliflozin on N-terminal pro-brain natriuretic peptide in patients with type 2 diabetes and chronic heart failure according to baseline use of glucose-lowering agents. Cardiovasc Diabetol 2021; 20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagogo-Jack S, Cannon CP, Cherney DZI, Cosentino F, Liu J, Pong A, et al. Cardiorenal outcomes with ertugliflozin assessed according to baseline glucose-lowering agent: an analysis from VERTIS CV. Diabetes Obes Metab 2022; 24:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahn A, Wiviott SD, Mosenzon O, Murphy SA, Goodrich EL, Yanuv I, et al. Cardiorenal outcomes with dapagliflozin by baseline glucose-lowering agents: post hoc analyses from DECLARE-TIMI 58. Diabetes Obes Metab 2021; 23:29–38. [DOI] [PubMed] [Google Scholar]
- 32.Docherty KF, Jhund PS, Bengtsson O, DeMets DL, Inzucchi SE, Kober L, et al. ; DAPA-HF Investigators and Committees. Effect of dapagliflozin in DAPA-HF according to background glucose-lowering therapy. Diabetes Care 2020; 43:2878–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation 2018; 138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 inhibitors and their mode of action in heart failure-has the mystery been unravelled? Curr Heart Fail Rep 2021; 18:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SY, Kong XQ, Zhang KF, Luo S, Wang F, Zhang J-J. DPP4 as a potential candidate in cardiovascular disease. J Inflamm Res 2022; 15:5457–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.She L, Wu H. Meta-analysis on safety of dapagliflozin in patients with type 2 diabetes mellitus. Yangtze Med 2018; 02:129–145. [Google Scholar]
- 37.Sircar M, Bhatia A, Munshi M. Review of hypoglycemia in the older adult: clinical implications and management. Can J Diabetes 2016; 40:66–72. [DOI] [PubMed] [Google Scholar]
- 38.Tsujimoto T, Sugiyama T, Shapiro MF, Noda M, Kajio H. Risk of cardiovascular events in patients with diabetes mellitus on β-blockers. Hypertension 2017; 70:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]