Abstract
One of the major hurdles in solid organ transplantation is graft rejection, which must be prevented with lifelong general immunosuppression. However, modern maintenance immunosuppression is accompanied by serious side effects, such as an increased risk of infection and malignancies. The search for alternative therapies specifically controlling allogeneic responses is fueling renewed interest in extracorporeal photopheresis (ECP). Despite guideline indications for ECP in cardiothoracic transplantation, potential applications in liver and kidney transplantation have not been adequately investigated. Presently, limited understanding of the pharmacodynamic effects of ECP and lack of consensus biomarkers are hindering the development of standardized multiparametric assays to assess patient responses. This review explores current knowledge about immune responses after ECP in transplant recipients and collates a set of biomarkers associated with favorable treatment responses.
Extracorporeal photopheresis (ECP) is an ex vivo immunomodulatory therapy in which leukocytes are treated with a photosensitizing agent and then exposed to UV A (UVA) irradiation to induce apoptosis before reinfusion into patients.1 ECP was first approved by the US States Food and Drug Administration in 1988 for treating Sezary syndrome, an advanced type of cutaneous T-cell lymphoma.2 Originally, ECP was introduced as a procedure to debulk circulating malignant T cells, thereby reducing cutaneous lymphoid infiltrates. This concept was naturally extended to increasing turnover of pathogenic T cells and B cells responsible for allo- and autoimmune disease.
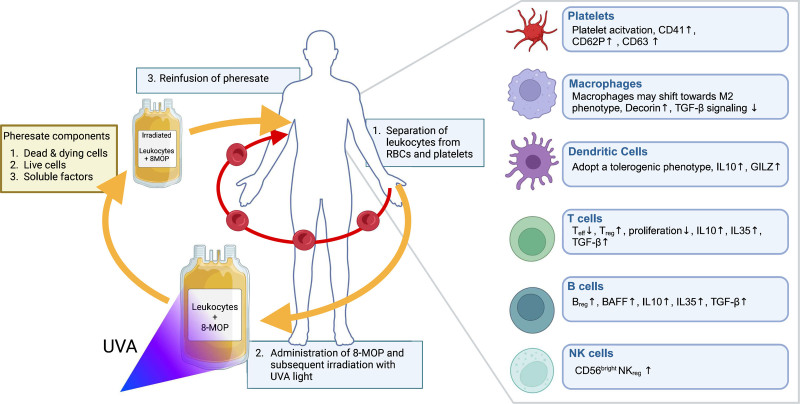
During ECP treatment, whole blood is collected from the patient through a peripheral venous catheter. White blood cells (WBCs) are then separated from red blood cells and platelets by centrifugation. Red blood cells, platelets, and plasma are immediately returned to the patient, and a concentrated WBC fraction is retained (Figure 1). To these WBCs, 8-methoxypsoralen (8-MOP) is then added, which binds intercalates into DNA, sensitizing cells to UVA light. On UVA irradiation, 8-MOP forms covalent DNA adducts that result in double-stranded DNA breaks, which trigger apoptosis in treated cells. The resulting mixture of living and dying leukocytes, as well as the conditioned plasma in which they are suspended, is called a “photopheresate.” This immunologically complex product is then reinfused into patients.3 ECP regimens vary widely depending upon clinical indications, local practice, and the particular devices being used. However, historical precedent means that most protocols consist of cycles of 2 consecutive days of ECP treatment, which are repeated with intervals of several weeks.1
FIGURE 1.
A standard extracorporeal photopheresis procedure. Blood is drawn from the patient continuously and leukocytes are separated from RBCs and platelets. The concentrated leukocyte fraction is exposed to 8-MOP, which binds covalently to the DNA. Subsequent irradiation with UVA light causes activation of 8-MOP resulting in extensive DNA damage. The treated leukocyte fraction called pheresate consists of soluble factors, dead and dying cells, and live cells. Subsequent intravenous administration of the pheresate leads to immunomodulation. Created in BioRender. Veltman, H. (2025; https://BioRender.com/1qaq2hv). 8-MOP, 8-methoxypsoralen; RBC, red blood cell; UVA light, UV A light.
Major advances have been made in treating both acute and chronic graft-versus-host disease (GvHD) and rejection of heart and lung transplants.4 Over many decades, ECP has proven to be safe, with only limited procedurally related side effects, the most common being mild hypotension.5,6 This lies in stark contrast with the severe side effects associated with immunosuppressant (IS) regimens used to prevent transplant rejection. These treatments heighten the risk of infections and malignancies and, in the case of calcineurin inhibitors, are nephrotoxic.6,7
ECP is only sparsely applied and currently not approved for clinical use in solid organ transplantation (SOT), despite some trials showing promising results in preventing and reducing rejection events, increasing graft lifespan, and allowing for IS sparing.8 This possibly reflects our current lack of mechanistic knowledge, absence of consensus guidelines about ECP in abdominal organ transplant recipients, and inability to measure the therapeutic effects of ECP in patients. To address these deficiencies, our group is participating in exTra, an EU-funded collaborative network investigating applications of ECP and standardized clinical analysis in SOT.9-12 This review aims to discuss potential biomarkers of therapeutic responses to ECP in SOT recipients.
COMPOSITION OF PHERESATES AND ITS SYSTEMIC EFFECTS
The composition of pheresates can vary significantly depending on the modality used. The 2 primary systems are inline and offline ECP. The inline method is a closed system in which leukocyte separation and treatment occur simultaneously, whereas the offline method separates these procedures. A third approach, mini-ECP, is designed for patients with contraindications to conventional ECP, such as low body weight or limited venous access. In a single procedure, the inline, offline, and mini-ECP methods collect approximately ~5%–10%, ~12%–20%, and <10% of total circulating leukocytes, respectively.13–15 Significant differences in platelet counts between modalities are reported too, which could affect monocyte activation.14,16 Although differences in pheresate composition have been described, its effects on treatment efficacy have not been fully explored.3,14,17
While the immediate effects of ECP remain unclear, several events are known to occur. First, platelet interaction with plastic surfaces activates monocytes via P-selectin glycoprotein ligand-1 ligation. 13,18 Second, 8-MOP and UVA further activate monocytes, inducing differentiation into monocyte-derived dendritic cells (DCs). Third, injured leukocytes release damage-associated molecular patterns recognized by myeloid cells.19 Fourth, DNA damage initiates apoptosis, especially in proliferating lymphocytes. In vitro ECP studies show most lymphocytes undergo apoptosis within 48 h in 2 waves: immediate apoptosis through mitochondrial dysfunction and caspase activation after 24 h.20,21 Stepien et al described ECP-related cell death in further detail.21
Clinically, photopheresates are infused continuously or shortly after treatment, receiving a complex suspension of secondarily necrotic, apoptotic, preapoptotic, and living cells, as well as plasma fraction containing soluble mediators, metabolites, subcellular debris, and altered plasma proteins.22,23 Altered cytokine profiles in both monocytes and lymphocytes have been reported.24-29 Notably, interleukin (IL)-1β levels increase in both pheresate and peripheral blood after ECP therapy.24-29 The systemic effects may extend beyond cytokines. MicroRNAs such as miR-23 b-3p and miR-155 are upregulated in lung transplant patients.29,30 Additionally, ECP significantly increases neutrophil extracellular traps formation immediately after treatment in chronic GvHD, further highlighting its systemic effects.24–31
The effectiveness of ECP in treating a wide range of inflammatory and T cell–mediated diseases suggests its benefits stem from different photopheresate components. Some studies link increased apoptosis to clinical success, whereas others propose that surviving leukocytes mediate therapeutic effects. Standardized proliferation and cell death assays are routinely used to characterize and validate the pheresate.32 The tetrazolium salt assay, combined with 7-aminoactinomycin D (7AAD) and carboxyfluorescein succinimidyl ester, quantifies metabolically active cells and indicates clinical potency of pheresates.33 However, the effect of ECP may not exclusively be mediated by leukocytes. ECP strongly induces platelet activation, as evidenced by increased expression of CD41, CD62P, and CD63.34 Activated platelets are known contributors to monocyte activation and modulate lymphocyte function.13,35 A major limitation is the poor documentation of photopheresate composition in clinical studies. Our research aims to identify key markers for photopheresates applicable in clinical and research settings. We hypothesize that standardized analysis will improve clinical response predictions and highlight the most immunologically relevant components.
IMMUNE MODULATORY EFFECTS OF ECP IN SOT
Dendritic Cells
Under homeostatic conditions, tissue resident immature DCs take up apoptotic cells and present self-antigens in the peripheral lymph nodes to naive T cells, inducing peripheral tolerance. It is hypothesized that ECP uses this mechanism to induce immune tolerance.35 ECP has shown to modulate the immune landscape in both animal and human studies by increasing the frequency of regulatory T cells (Treg) in circulation, both CD4+ FoxP3+ Treg and IL-10 producing CD4+ Tr1 cells; a process mediated by tolerogenic DCs (TolDCs).36-39 ECP-induced preapoptotic leukocytes, administered intravenously, migrate to the spleen and secondary lymphoid tissues, where they release apoptotic-associated molecular patterns, which in turn lead to phagocytosis by resident DCs.40 Removal of apoptotic leukocytes in the absence of inflammatory signals induces peripheral immune tolerance.41,42 TolDCs show reduced CD80/86 and increased programmed death-ligand 1 expression, along with higher IL-10 and transforming growth factor beta (TGF-β) secretion.43 Glucocorticoid-induced leucine zipper, a key regulator of this phenotype, is elevated in monocytes after ECP and is proposed as a marker for TolDCs44
T Cells
T cells play a central role in cell-mediated rejection by recognizing alloantigens and orchestrating the subsequent immune response. ECP increases Treg frequency and reduces T-cell proliferation and effector function through cell contact–dependent interactions and inhibitory cytokines, most importantly IL-10, IL-35, and TGF-β.45-47 Quality control testing after ECP using a simple CD71 staining in the peripheral blood of healthy volunteers showed strong inhibition of T-cell proliferation, which may be used to assess response.48 Interactions between FoxP3+ Treg and DCs through these same mechanisms inhibit DC maturation and antigen presentation, further reducing T-cell activation.45,49 This is a crucial first step in the response to ECP treatment, as T cells play a central role in cell-mediated rejection.
The effect of ECP in Treg subsets is extensively studied in GvHD. Increased and decreased CD4+ and CD8+ T cells, respectively, and increased CD4+CD25+CD127− Treg populations are associated with response in steroid-resistant patients.50 This Treg subset can be further divided into HLA-DR– and HLA-DR+ populations, with higher levels of the latter associated with a reduced risk of developing GvHD.51 Elevated expression levels of CCR7 in central memory CXCR3+ T cells are also associated with response, suggesting that lymph node homing is essential.52 In heart transplant recipients, a highly suppressive FoxP3 Treg subset called FoxP3-E2 (exon 2) with elevated CD39 expression levels was found to be increased after ECP treatment.53 Dieterlen et al54 also reported increased levels of CD39+ Treg, and increased and decreased expressions of CD62L and CD120b, respectively, were ascertained.55
Macrophages
After transplantation, recipient macrophages play a key role in tissue regeneration by removing cellular debris and promoting revascularization.56 However, during rejection, macrophages shift phenotypically into a proinflammatory state, secreting tumor necrosis factor alpha, IL-6, and IL-1β. In addition, excessive expression of TGF-β contributes to tissue fibrosis, which is an important cause of graft failure.57,58 ECP may counteract this proinflammatory role by promoting an increase in M2 macrophages exhibiting a tolerogenic and tissue-restorative phenotype. Regulatory macrophages have previously been shown to modulate T-cell immunity and induce a TIGIT+ FoxP3+ Treg phenotype.59 Moreover, enhanced IL-10 production and reduced alloreactivity inhibit effector T (Teff) cells and promote Treg expansion.58 In a lung transplant mouse model, ECP was shown to decrease fibrosis by reducing alveolar macrophage-TGF-β signaling through expression of its antagonist, decorin, which may serve as a potential biomarker in bronchiolitis obliterans syndrome.60 Research on the effects of ECP on macrophage function is limited and is currently being investigated by our collaborative partners.61,62
Natural Killer Cells
Natural killer (NK) cells are innate lymphocytes that recognize and kill allogeneic cells due to lack of self-major histocompatibility complex or through antibody-mediated cytotoxicity. During acute rejection, NK cells infiltrate the graft, releasing cytotoxic enzymes and expressing interferon-gamma and tumor necrosis factor alpha, which recruit T lymphocytes and myeloid cells.63 Several studies in patients with GvHD show that ECP modulates NK cells by reducing their immune activity 24 h posttreatment,64-66 promoting a regulatory phenotype while preserving their antiviral and antitumor functions.65,66 Regulatory NK cells can differentiate from a CD56bright to a CD56dim population and subsequently maturate, gaining the capacity to kill alloreactive T cells.66 Moreover, CD56bright NK cells have been associated with a positive response to ECP in patients with GvHD.67 These data reveal a clear effect of ECP on NK cell function, which may contribute to increased graft acceptance. Thus, the CD56bright NK cell subset may be a suitable candidate to measure the response to ECP in transplant patients.
B Cells
Antibody-mediated rejection is another major contributor to transplant rejection. B lymphocytes play a key role in the humoral response by producing donor-specific antibodies, which activate the complement system, cause inflammation, and eventually cytolysis.68 However, B cells can also suppress T-cell activity and promote graft tolerance through expression of IL-10, IL-35, and TGF-β. These cytokines together with cell contact–dependent mechanisms reduce antigen presentation in DCs and macrophages. Furthermore, regulatory B cells (Breg) increase programmed death-ligand 1 and FasL expression, inducing T-cell apoptosis.69 B-cell depletion in transplantation models can either mitigate or exacerbate rejection, depending on the extent of rejection and timing of B-cell depletion, highlighting their dual role in graft rejection and acceptance.70,71 Chandra et al72 found increased levels of IL-10-producing Breg and Wang et al73 observed increased Breg, defined as CD24+CD38high, in GvHD patients after ECP therapy. Furthermore, reduced levels of immature CD21low B cells and decreased serum B-cell activating factor have also been associated with positive response in GvHD,74,75 which were previously proposed as predictive biomarkers in Mankarious et al.76 In lung transplant recipients(LTRs), ECP was found to reduce circulating donor-specific antibodies, which was associated with reduced lung function decline.77 Additionally, quantifying antibody-secreting cells using standardized enzyme-linked immunospot assays may provide a reliable method to assess reduced antibody-mediated rejection after ECP therapy, as discussed in detail by Nogueira et al.78
The effect of ECP on B-cell responses in SOT remains unclear and the definition of Breg is still under debate. Rather than a distinct cell type, multiple B-cell subsets can adopt a regulatory phenotype depending on the microenvironment. Different B-cell subsets can initiate and subsequently terminate IL-10 expression, demonstrating the transient characteristics of Breg.79 Despite these dynamics, multiple Breg subsets have been identified including B10, regulatory B1 (Br1), GZMB+ Breg, and TIM1+ Breg.80 Further studies regarding the effect of ECP on Breg populations in SOT will be realized by our consortium partners.78
Myeloid-derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are a heterogenous group of myeloid cells that suppress T cells by creating a microenvironment rich in free radicals and depleted of l-arginine and cysteine, essential amino acids for T-cell expansion and function.81-83 Although their precise role in SOT remains unclear, increased MDSC levels have been associated with graft tolerance. Meng et al84 found that MDSCs frequency correlated with prolonged graft survival and promoted expansion of CD4+FoxP3+ Treg while reducing IL-17 expression in vitro. Iglesias-Escudero et al85,86 observed increased monocytic MDSCs in kidney transplant recipients up to 12 mo posttransplantation and elevated polymorphonuclear (PMN)-MDSCs in LTRs, with the latter linked to long-term outcome. Heigl et al87 also found increased PMN-MDSCs in stable LTRs compared with patients with chronic lung allograft dysfunction.
Despite promising results, limited data are available on the effect of ECP on MDSCs. Rieber et al88 observed a sustained increase in PMN-MDSCs with strong T-cell inhibitory potential in GvHD patients post-ECP, correlating with remission. Increased monocytic MDSCs in acute GvHD after ECP therapy have been described too.73 However, no data exist on the impact of ECP on MDSCs in SOT, and a lack of specific markers complicates standardized analysis. Nevertheless, their strong T-cell inhibitory capacity should not be overlooked when assessing transplant tolerance. Table 1 summarizes the role of each immune cell type in graft rejection and how ECP modulates their function.
TABLE 1.
The effect of ECP on immune cells and their role in graft rejection
| Cell type | Role in organ rejection | Effect of ECP on cell fuction |
|---|---|---|
| DCs | Direct alloantigen-specific rejection through activation of naive T cells, induces expansion of Teff cells and expression of IL-6, IL-12, and TNF-α89 | Secrete IL-10, inhibit Teff cells and induce expansion of Tregs38,90 |
| Macrophages | Increase tissue fibrosis due to excessive expression of TGF-β and expression of TNF-α, IL-6, and IL-1β57,58 | Promotes M2 phenotype, induces IL-10 expression, inhibits Teff cells, and induces expansion of Treg60,61 |
| Teff cells | CD4+ T cells orchestrate immune response through expression of cytokines/CD8+ T cells express cytolytic enzymes targeting graft cells91 | Dampenes Teff function and inhibits expansion38 |
| Treg | Reduced activity in acute and chronic rejection92 | Contributes to graft tolerance, increases Treg expansion, induces expression of IL-10, and reduces Teff function50,53,55,93 |
| NK cells | Recognize and kill allogeneic cells, antibody-mediated cytotoxicity, release cytotoxic enzymes, express proinflammatory cytokines (INF-γ, TNF-α)63 | Reduces cytotoxic activity, kills alloreactive T cells, and maintains antiviral and antitumoral activity66,67 |
| B cells | Present antigens to T cells, produce DSAs, expression of proinflammatory cytokines (IL-6, TNF-α)68 | Increases regulatory phenotype, inhibits alloreactive T cells through IL-10 and IL-35 expression, and reduces antigen presentation in DCs and macrophages68,72,73 |
| MDSCs | Immunosuppressive regimens affect MDSC numbers and potentially reduce their immunoregulatory capacity85,86 | Strong suppressive effect on Teff cells81-83 |
DC, dendritic cell; DSA, donor-specific antibody; ECP, extracorporeal photopheresis; IFN, interferon; IL, interleukin; MDSC, myeloid-derived suppressor cell; NK, natural killer; Teff cell, effector T cell; TGF, transforming growth factor; TNF, tumor necrosis factor; Treg, regulatory T cell.
ADVANCING PREDICTIVE TOOLS FOR ECP RESPONSE IN SOT
ECP effectively prevents rejection and prolongs graft survival in multiple trials,4,94-97 but predicting patient response remains challenging, highlighting the need for biomarkers. Its effects vary by organ: heart transplant patients often experience prolonged rejection-free periods, whereas LTRs show only reduced functional decline.4,94,95 No studies have analyzed biomarkers related to outcome in specific organ transplants, despite immune cell subset relevance varying by transplant type. Thus, organ-specific studies should identify markers associated with treatment response.
Currently, outcome assessment relies solely on clinical data (ie, rejection events, organ performance), requiring months of therapy. Standardized multiparametric immune assays with key biomarkers could help clinicians monitor response and predict outcome, potentially enabling the timely tapering of immunosuppressants. Achieving this requires comprehensive immune profiling to quantify and phenotype regulatory/effector cells and analyze the cytokine milieu. Furthermore, markers of allograft injury, such as donor cell-free DNA, can help detect injury at an early stage,98 and advanced predictive tools like iBOX may enable clinicians to predict and assess superiority of ECP therapy.99
Spectral flow cytometry and multiparametric immunoassays are promising tools for standardized immune profiling. Spectral flow offers high resolution and is already used for immune monitoring,100-102 but consensus on relevant markers and assay standardization is needed and interassay variability must be addressed. Standardized immune phenotyping strategies exist, which could aid in establishing immune phenotype profiles for ECP.103,104 Similarly, multiplex immunoassays, already effective in cytokine profiling in disease, may be crucial for understanding cytokine dynamics in ECP-treated transplant patients.104
CONCLUSIONS
ECP is a valuable addition to the clinician’s toolbox in SOT, increasing organ lifespan and enabling IS sparing. Animal and human studies across various fields, including SOT, have partly elucidated the mechanism(s) of action of ECP. The availability of affordable multiparametric tools enhances our understanding of the immunomodulatory effects of ECP and helps identify key parameters in SOT. However, more randomized controlled trials are needed, particularly in the context of liver, kidney, and lung transplantation. These trials should include detailed immune monitoring to help establish standardized comprehensive immune profiling assays.
Footnotes
This work was funded by the European Union through the exTra doctoral network (grant 101119855).
The authors declare no conflicts of interest.
H.V. participated in writing the original draft. M.I.-E. and E.M.-C. participated in writing, reviewing, and editing.
Contributor Information
Hendrik Veltman, Email: hendrikveltman96@gmail.com.
Eva Martinez-Caceres, Email: Emmartinez.germanstrias@gencat.cat.
REFERENCES
- 1.Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. N Engl J Med. 1987;316:297–303. [DOI] [PubMed] [Google Scholar]
- 2.Parsonidis P, Wekerle T. Extracorporeal photopheresis: does it have a potential place among cell-based therapies? Transplant Direct. 2025;11:e1808. [Google Scholar]
- 3.Bennett D, Fossi A, Ferrari S, et al. Extracorporeal photopheresis in lung transplantation. J Heart Lung Transplant. 2020;39:S307. [Google Scholar]
- 4.Barten MJ, Sax B, Schopka S, et al. European multicenter study on the real-world use and clinical impact of extracorporeal photopheresis after heart transplantation. J Heart Lung Transplant. 2023;42:1131–1139. [DOI] [PubMed] [Google Scholar]
- 5.Mörtzell Henriksson M, Newman E, Witt V, et al. Adverse events in apheresis: an update of the WAA registry data. Transfus Apher Sci. 2016;54:2–15. [DOI] [PubMed] [Google Scholar]
- 6.Barten MJ, Fisher AJ, Hertig A. The use of extracorporeal photopheresis in solid organ transplantation—current status and future directions. Am J Transplant. 2024;24:1731–1741. [DOI] [PubMed] [Google Scholar]
- 7.Tönshoff B. Immunosuppressants in organ transplantation. Handb Exp Pharmacol. 2020;261:441–469. [DOI] [PubMed] [Google Scholar]
- 8.Alemanno S, Jaksch P, Benazzo A. Extracorporeal photopheresis in lung transplantation: present applications and emerging research. Transplant Direct. 2025;11:e1831. [Google Scholar]
- 9.Nicoli M, Rovira J, Diekmann F. Exploring the role of extracorporeal photopheresis in kidney transplant management. Transplant Direct. 2025;11:e1809. [Google Scholar]
- 10.extra-horizon.eu/.
- 11.Morgado I, Vinnakota JM, Zeiser R. Extracorporeal photopheresis: from animal models to clinical practice. Transplant Direct. 2025;11:e1824. [Google Scholar]
- 12.Knobler R, Barr ML, Couriel DR, et al. Extracorporeal photopheresis: past, present, and future. J Am Acad Dermatol. 2009;61:652–665. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo N, Putzulu R, Massini G, et al. Inline and offline extracorporeal photopheresis: device performance, cell yields and clinical response. J Clin Apher. 2021;36:118–126. [DOI] [PubMed] [Google Scholar]
- 14.Várkonyi A, Kállay K, Dobos K, et al. A novel, closed-line mini extracorporeal photopheresis (ECP) technique using the therakos TM CellEx TM photopheresis system, developed for patients with contraindications against conventional ECP therapy. Blood. 2023;142(Suppl 1):6945–6945. [Google Scholar]
- 15.Brosig A, Hähnel V, Orsó E, et al. Technical comparison of four different extracorporeal photopheresis systems. Transfusion. 2016;56:2510–2519. [DOI] [PubMed] [Google Scholar]
- 16.Helmberg W, Sipurzynski S, Groselje-Strehle A, et al. Does offline beat inline treatment: investigation into extracorporeal photopheresis. Transfus Med Hemother. 2020;47:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatsuno K, Yamazaki T, Hanlon D, et al. Extracorporeal photochemotherapy induces bona fide immunogenic cell death. Cell Death Dis. 2019;10:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durazzo TS, Tigelaar RE, Filler R, et al. Induction of monocyte-to-dendritic cell maturation by extracorporeal photochemotherapy: initiation via direct platelet signaling. Transfus Apher Sci. 2014;50:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bladon J, Taylor PC. Extracorporeal photopheresis in cutaneous T-cell lymphoma and graft-versus-host disease induces both immediate and progressive apoptotic processes. Br J Dermatol. 2002;146:59–68. [DOI] [PubMed] [Google Scholar]
- 20.Budde H, Berntsch U, Riggert J, et al. In vitro effects of different 8-methoxypsoralen treatment protocols for extracorporeal photopheresis on mononuclear cells. Cent Eur J Immunol. 2017;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stępień J, Eggenhofer E. ECP-induced apoptosis in leucocytes: how cell death promotes tissue repair. Transplant Direct. 2025;11:e1816. [Google Scholar]
- 22.Garcia-Almeida H, Heger L, Hackstein H. Extracorporeal Photopheresis: Soluble Factors that Promote Immunomodulation. Transplant Direct. 2025;11:e1840. [Google Scholar]
- 23.Yakut E, Jakobs C, Peric A, et al. Extracorporeal photopheresis promotes IL-1β production. J Immunol. 2015;194:2569–2577. [DOI] [PubMed] [Google Scholar]
- 24.Vowels BR, Cassin M, Boufal MH, et al. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-α by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Investig Dermatol. 1992;98:686–692. [DOI] [PubMed] [Google Scholar]
- 25.Di Renzo M, Rubegni P, De Aloe G, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T‐cell lymphoma. Immunology. 1997;92:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo N, Tokura Y, Matsumoto K, et al. Tumour-specific cytotoxic T lymphocyte activity in Th2-type Sézary syndrome: its enhancement by interferon-gamma (IFN-γ) and IL-12 and fluctuations in association with disease activity. Clin Exp Immunol. 2001;112:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet a radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459–462. [DOI] [PubMed] [Google Scholar]
- 28.Bladon J, Taylor P. Extracorporeal photopheresis reduces the number of mononuclear cells that produce pro‐inflammatory cytokines, when tested ex‐vivo. J Clin Apher. 2002;17:177–182. [DOI] [PubMed] [Google Scholar]
- 29.Bozzini S, Del Fante C, Morosini M, et al. Mechanisms of action of extracorporeal photopheresis in the control of bronchiolitis obliterans syndrome (BOS): involvement of circulating miRNAs. Cells. 2022;11:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg I, Granot G, Telerman A, et al. Extracorporeal photopheresis induces NETosis in neutrophils derived from patients with chronic graft‐vs‐host disease. J Clin Apher. 2023;38:615–621. [DOI] [PubMed] [Google Scholar]
- 31.Faivre L, Lecouflet L, Liu WQ, et al. Quality control of extracorporeal photochemotherapy: proliferation assay using CFSE validated according to ISO 15189:2007 standards. Cytometry B Clin Cytom. 2015;88:30–39. [DOI] [PubMed] [Google Scholar]
- 32.Chieregato K, Alghisi A, Borghero C, et al. Evaluation of lymphocytes inactivation by extracorporeal photopheresis using tetrazolium salt based-assay. Transfus Apher Sci. 2015;53:242–245. [DOI] [PubMed] [Google Scholar]
- 33.Budde H, Mohr L, Bogeski I, et al. Extracorporeal photopheresis and the cellular mechanisms: effects of 8‐methoxypsoralen and UVA treatment on red blood cells, platelets and reactive oxygen species. Vox Sang. 2023;118:775–782. [DOI] [PubMed] [Google Scholar]
- 34.Li N. Platelet–lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. [DOI] [PubMed] [Google Scholar]
- 35.Steinman RM, Hawiger D, Liu K, et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. [DOI] [PubMed] [Google Scholar]
- 36.Lamioni A, Parisi F, Isacchi G, et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–850. [DOI] [PubMed] [Google Scholar]
- 37.Vogiatzis R, Krüger W, Jünger M, et al. Effects of extracorporeal photopheresis on quality of life and the course of diseases in patients with mycosis fungoides and graft-versus-host disease: a single-center analysis. Cureus. 2023;15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda A, Schwarz A, Bullinger A, et al. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181:5956–5962. [DOI] [PubMed] [Google Scholar]
- 39.Roncarolo MG, Gregori S, Bacchetta R, et al. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity. 2018;49:1004–1019. [DOI] [PubMed] [Google Scholar]
- 40.Savill J, Dransfield I, Gregory C, et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. [DOI] [PubMed] [Google Scholar]
- 41.Kleinclauss F, Perruche S, Cahn JY, et al. Administration of donor apoptotic cells: an alternative cell-based therapy to induce tolerance? Transplantation. 2003;75(Suppl):43S–45S. [DOI] [PubMed] [Google Scholar]
- 42.Saas P, Tiberghien P, de Carvalho Bittencourt M. Cell-based therapy approaches using dying cells: from tumour immunotherapy to transplantation tolerance induction. Expert Opin Biol Ther. 2002;2:249–263. [DOI] [PubMed] [Google Scholar]
- 43.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. [DOI] [PubMed] [Google Scholar]
- 44.Futterleib JS, Feng H, Tigelaar RE, et al. Activation of GILZ gene by photoactivated 8-methoxypsoralen: potential role of immunoregulatory dendritic cells in extracorporeal photochemotherapy. Transfus Apher Sci. 2014;50:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knobler R, Arenberger P, Arun A, et al. European dermatology forum—updated guidelines on the use of extracorporeal photopheresis 2020—part 1. J Eur Acad Dermatol Venereol. 2020;34:2693–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of Treg-mediated T cell suppression. Front Immunol. 2012;3:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi S, Mikami N, Wing JB, et al. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–566. [DOI] [PubMed] [Google Scholar]
- 48.Schwab L, Michel G, Bein G, et al. CD71 surface analysis of T cells: a simple alternative for extracorporeal photopheresis quality control. Vox Sang. 2020;115:81–93. [DOI] [PubMed] [Google Scholar]
- 49.Misra N, Bayry J, Lacroix-Desmazes S, et al. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. [DOI] [PubMed] [Google Scholar]
- 50.Amat P, López‐Corral L, Goterris R, et al. Biomarker profile predicts clinical efficacy of extracorporeal photopheresis in steroid‐resistant acute and chronic graft‐vs‐host disease after allogenic hematopoietic stem cell transplant. J Clin Apher. 2021;36:697–710. [DOI] [PubMed] [Google Scholar]
- 51.Crocchiolo R, Cesana C, Girgenti D, et al. Correction to: Tregs and GvHD prevention by extracorporeal photopheresis: observations from a clinical trial. Exp Hematol Oncol. 2021;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aoki M, Marin‐Esteban V, Weigl R, et al. Decreased pro‐inflammatory cytokines and increased CCR7 expression on T‐lymphocyte subsets are predictive of response to extracorporeal photopheresis in patients with GvHD. Br J Haematol. 2011;154:409–413. [DOI] [PubMed] [Google Scholar]
- 53.Mottola M, Bruzzaniti S, Piemonte E, et al. Extracorporeal photopheresis enhances the frequency and function of highly suppressive FoxP3+ treg subsets in heart transplanted individuals. Transplantation. 2024;109:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dieterlen MT, Bittner HB, Pierzchalski A, et al. Immunological monitoring of extracorporeal photopheresis after heart transplantation. CLIN Exp Immunol. 2014;176:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dieterlen MT, Klaeske K, Bernhardt AA, et al. Immune monitoring assay for extracorporeal photopheresis treatment optimization after heart transplantation. Front Immunol. 2021;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang YY, Jiang H, Pan J, et al. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28:2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Li C, Zhuang Q, et al. The evolving roles of macrophages in organ transplantation. J Immunol Res. 2019;2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riquelme P, Haarer J, Kammler A, et al. TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity. Nat Commun. 2018;9:2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Z, Liao F, Zhu J, et al. Reprogramming alveolar macrophage responses to TGF-β reveals CCR2+ monocyte activity that promotes bronchiolitis obliterans syndrome. J Clin Investig. 2022;132. doi:10.1172/JCI159229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arella F, Schlitt HJ, Riquelme P. The potential use of ECP to promote tissue reparative macrophages. Transplant Direct. 2025;11:e1812. [Google Scholar]
- 62.Tocco C, Ochando J. Potential impact of extracorporeal photopheresis on trained immunity and organ transplant acceptance. Transplant Direct. 2025;11:e1835. [Google Scholar]
- 63.Pontrelli P, Rascio F, Castellano G, et al. The role of natural killer cells in the immune response in kidney transplantation. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apostolova P, Unger S, von Bubnoff D, et al. Extracorporeal photopheresis for colitis induced by checkpoint-inhibitor therapy. N Engl J Med. 2020;382:294–296. [DOI] [PubMed] [Google Scholar]
- 65.Becherucci V, Allegro E, Brugnolo F, et al. Extracorporeal photopheresis as an immunomodulatory agent: haematocrit‐dependent effects on natural killer cells. J Clin Apher. 2017;32:257–265. [DOI] [PubMed] [Google Scholar]
- 66.Ni M, Wang L, Yang M, et al. Shaping of CD56bri natural killer cells in patients with steroid-refractory/resistant acute graft-vs.-host disease via extracorporeal photopheresis. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iniesta P, Revilla N, Chen‐Liang TH, et al. An early increase of CD56 bright natural killer subset as dominant effect and predictor of response to extracorporeal photopheresis for graft‐versus‐host disease. Transfusion (Paris). 2018;58:2924–2932. [DOI] [PubMed] [Google Scholar]
- 68.Yi SG, Gaber AO, Chen W. B-cell response in solid organ transplantation. Front Immunol. 2022;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng B, Ming Y, Yang C. Regulatory B cells: the cutting edge of immune tolerance in kidney transplantation. Cell Death Dis. 2018;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiLillo DJ, Griffiths R, Seshan SV, et al. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol. 2011;186:2643–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clatworthy MR. Targeting B cells and antibody in transplantation. Am J Transplant. 2011;11:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandra DJ, Hosoba S, Giver C, et al. Response to extracorporeal photopheresis in chronic graft versus host disease correlates with levels of IL-10 producing regulatory B cells. J Immunol. 2016;196(1_Suppl):140.18–140.18. [Google Scholar]
- 73.Wang L, Ni M, Hückelhoven-Krauss A, et al. Modulation of B cells and homing marker on NK cells through extracorporeal photopheresis in patients with steroid-refractory/resistant graft-vs-host disease without hampering anti-viral/anti-leukemic effects. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuzmina Z, Greinix HT, Knobler R, et al. Proportions of immature CD19+CD21− B lymphocytes predict the response to extracorporeal photopheresis in patients with chronic graft-versus-host disease. Blood. 2009;114:744–746. [DOI] [PubMed] [Google Scholar]
- 75.Whittle R, Taylor PC. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood. 2011;118:6446–6449. [DOI] [PubMed] [Google Scholar]
- 76.Mankarious M, Matthews NC, Snowden JA, et al. Extracorporeal photopheresis (ECP) and the potential of novel biomarkers in optimizing management of acute and chronic graft vs. host disease (GvHD). Front Immunol. 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baskaran G, Tiriveedhi V, Ramachandran S, et al. Efficacy of extracorporeal photopheresis in clearance of antibodies to donor-specific and lung-specific antigens in lung transplant recipients. J Heart Lung Transplant. 2014;33:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nogueira F, van Ham SM, ten Brinke A. Extracorporeal photopheresis in solid organ transplantation: modulating B cell responses to improve graft survival. Transplant Direct. 2025;11:e1833. [Google Scholar]
- 79.Maseda D, Smith SH, DiLillo DJ, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188:1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baba Y, Matsumoto M, Kurosaki T. Signals controlling the development and activity of regulatory B-lineage cells. Int Immunol. 2015;27:487–493. [DOI] [PubMed] [Google Scholar]
- 81.Raber PL, Thevenot P, Sierra R, et al. Subpopulations of myeloid‐derived suppressor cells impair T cell responses through independent nitric oxide‐related pathways. Int J Cancer. 2014;134:2853–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I–producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srivastava MK, Sinha P, Clements VK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng F, Chen S, Guo X, et al. Clinical significance of myeloid-derived suppressor cells in human renal transplantation with acute T cell-mediated rejection. Inflammation. 2014;37:1799–1805. [DOI] [PubMed] [Google Scholar]
- 85.Iglesias-Escudero M, Sansegundo-Arribas D, Riquelme P, et al. Myeloid-derived suppressor cells in kidney transplant recipients and the effect of maintenance immunotherapy. Front Immunol. 2020;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iglesias-Escudero M, Segundo DS, Merino-Fernandez D, et al. Myeloid-derived suppressor cells are increased in lung transplant recipients and regulated by immunosuppressive therapy. Front Immunol. 2022;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heigl T, Singh A, Saez-Gimenez B, et al. Myeloid-derived suppressor cells in lung transplantation. Front Immunol. 2019;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rieber N, Wecker I, Neri D, et al. Extracorporeal photopheresis increases neutrophilic myeloid-derived suppressor cells in patients with GvHD. Bone Marrow Transplant. 2014;49:545–552. [DOI] [PubMed] [Google Scholar]
- 89.Ochando J, Ordikhani F, Jordan S, et al. Tolerogenic dendritic cells in organ transplantation. Transplant Int. 2020;33:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Capitini CM, Davis JPE, Larabee SM, et al. Extracorporeal photopheresis attenuates murine graft-versus-host disease via bone marrow–derived interleukin-10 and preserves responses to dendritic cell vaccination. Biol Blood Marrow Transplant. 2011;17:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Short S, Lewik G, Issa F. An immune atlas of T cells in transplant rejection: pathways and therapeutic opportunities. Transplantation. 2023;107:2341–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim JYV, Assadian S, Hollander Z, et al. Regulatory T cell biomarkers identify patients at risk of developing acute cellular rejection in the first year following heart transplantation. Transplantation. 2023;107:1810–1819. [DOI] [PubMed] [Google Scholar]
- 93.George JF, Gooden CW, Guo WH, et al. Role for CD4+CD25+ T cells in inhibition of graft rejection by extracorporeal photopheresis. J Heart Lung Transplant. 2008;27:616–622. [DOI] [PubMed] [Google Scholar]
- 94.Benazzo A, Bagnera C, Ius F, et al. A European multi-center analysis of extracorporeal photopheresis as therapy for chronic lung allograft dysfunction. Transpl Int. 2024;36:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leroux J, Hirschi S, Essaydi A, et al. Initiation of extracorporeal photopheresis in lung transplant patients with mild to moderate refractory BOS: a single-center real-life experience. Respir Med Res. 2022;81:100913. [DOI] [PubMed] [Google Scholar]
- 96.Tamain M, Sayegh J, Lionet A, et al. Extracorporeal photopheresis for the treatment of graft rejection in 33 adult kidney transplant recipients. Transfus Apher Sci. 2019;58:515–524. [DOI] [PubMed] [Google Scholar]
- 97.Urbani L, Mazzoni A, De Simone P, et al. Avoiding calcineurin inhibitors in the early post‐operative course in high‐risk liver transplant recipients: the role of extracorporeal photopheresis. J Clin Apher. 2007;22:187–194. [DOI] [PubMed] [Google Scholar]
- 98.Kobashigawa JA, Patel J, Kransdorf E, et al. Does cell-free DNA detect the development of de novo donor specific antibodies. J Heart Lung Transplant. 2019;38:S288. [Google Scholar]
- 99.Klein A, Loupy A, Stegall M, et al. Qualifying a novel clinical trial endpoint (iBOX) predictive of long-term kidney transplant outcomes. Transpl Int. 2023;36:1496–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma S, Boyer J, Teyton L. A practitioner’s view of spectral flow cytometry. Nat Methods. 2024;21:740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bauman JE, Ohr J, Gooding WE, et al. Phase I study of ficlatuzumab and cetuximab in cetuximab-resistant, recurrent/metastatic head and neck cancer. Cancers (Basel). 2020;12:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ng HHM, Lee RY, Goh S, et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J ImmunoTher Cancer. 2020;8:e000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Streitz M, Miloud T, Kapinsky M, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplant Res. 2013;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vera EJ, Chew YV, Burns H, et al. Standardized whole-blood immunophenotyping panels on flow cytometry for transplant recipients and clinical trials. Transplantation. 2018;102(Suppl 7):S103. [Google Scholar]