Abstract
Epigenetic modulation enables precise gene regulation without altering DNA sequences. While histone acetylation has been widely utilized for gene activation, the therapeutic potential of histone methylation remains underexplored. In this study, we developed a new epigenetic activator by fusing the histone methyltransferase SETD7 to deactivated Cas9 (dCas9). The optimized SETD7-dCas9 fusion protein successfully induced H3K4 mono-methylation and activated transcription at multiple target loci. We further established a prediction model using promoter CpG methylation status to identify genes most responsive to SETD7-dCas9-mediated activation. To evaluate therapeutic relevance, we targeted the medium-wavelength-sensitive opsin gene (OPN1MW), which is crucial for cone photoreceptor function as a strategy for treating retinitis pigmentosa. SETD7-dCas9-mediated activation of OPN1MW restored light absorption properties comparable with rhodopsin, effectively compensating for rhodopsin deficiency in an in vitro disease model. These findings demonstrate the potential of histone methylation-based gene activation as a mutation-independent therapeutic strategy. The SETD7-dCas9 system represents a promising epigenome editing platform for precision gene regulation in diverse diseases.
Keywords: MT: RNA/DNA Editing, epigenetic regulation, SET domain-containing 7, retinitis pigmentosa, opsin, CpG methylation, CpG epigenome engineering
Graphical abstract

SETD7-dCas9 enables targeted histone methylation to activate gene expression. By restoring medium-wavelength opsin in retinal cells, this epigenetic approach offers a mutation-independent strategy for treating retinitis pigmentosa, highlighting histone methylation-based gene activation as a versatile therapeutic platform.
Introduction
Epigenetic modulation is a critical mechanism for regulating gene expression without altering the underlying DNA sequences.1 This modulation occurs through processes such as DNA methylation, histone modification, and non-coding RNA regulation, which can either activate or repress gene expression.2 Among these mechanisms, post-translational modifications (PTMs) to histone tails, such as acetylation, methylation, phosphorylation, and ubiquitination, play critical roles in regulating chromatin structure and gene expression. Histone PTMs modulate gene transcription as they regulate the accessibility of transcription factors and chromatin remodelers by inducing euchromatin or a heterochromatic conformational state. Histone acetylation has been shown to correlate often with gene activation by relaxing chromatin structure, whereas histone methylation either activates or represses gene expression depending on the specific lysine or arginine residues modified and the extent of methylation (mono-, di-, or tri-methylation).
The methylation of histone H3 at lysine 4 (H3K4), has been demonstrated to enhance the transcriptional activity. SETD7 (also known as SET7/9), the histone-lysine N-methyltransferase, was shown to catalyze the addition of methyl groups to H3K4, promoting a more accessible chromatin structure that facilitates gene activation.3 SETD7 contains a SET domain that is responsible for catalysis of the cofactor S-adenosylmethionine and the subsequent transfer of a methyl group to a lysine residue. SETD7 is mainly composed of the N-terminal domain and the C-terminal domain (or SET domain). The N-terminal domain is a non-conserved domain, whereas the SET domain is highly conserved among almost all methyltransferases and is responsible for their catalytic function. By limiting chromatin condensation, SETD7 methylates H3 at lysine 4, which improves transcriptional activity.3 H3K4me1 is frequently located around enhancer binding sites and the transcription start sites of actively transcribed genes; H3K4me1 is identified by the “reader” proteins, which are subsequently recruited to local chromatin to drive the transcription of certain genes.3 Thus, H3K4me1 could be a potential target site for transcriptional activation utilizing histone methylation.
Recent advancements in CRISPR-Cas9 technology have provided powerful tools for precise genome targeting and modification. By converting Cas9 into a catalytically inactive form (dCas9), it can be repurposed to deliver various functional domains to specific genomic loci without inducing double-strand breaks.4 This has led to the development of epigenetic editing tools, where dCas9 is fused with epigenetic effector domains to modify the epigenetic landscape at targeted sites, thereby regulating gene expression.5
A wide range of dCas9-based epigenetic editing systems have been developed to modulate chromatin structure and regulate gene activity, primarily through histone acetylation, DNA methylation, or the recruitment of transcriptional activators and repressors.6 While histone methylation has been extensively studied in the context of transcriptional regulation, its targeted application for gene activation using dCas9-based tools has received comparatively less attention. Emerging studies have demonstrated that dCas9 fused to histone methyltransferases can deposit H3K4 methylation at specific loci, expanding the repertoire of epigenetic editing strategies beyond traditional approaches. For example, Kim et al. developed a dCas9-SMYD3 (SET and MYND domain-containing protein 3) fusion protein capable of inducing H3K4 trimethylation (H3K4me3), thereby promoting the expression of target genes implicated in cancer cell proliferation and invasion.7 Similarly, Cano-Rodriguez et al. introduced the dCas9-PRDM9 fusion system, which facilitates chromatin remodeling through site-specific H3K4me3 deposition.8 While previous studies have predominantly focused on H3K4me3, which is associated with active transcription, this study investigates the potential of SETD7-mediated H3K4 mono-methylation (H3K4me1), a hallmark of enhancer activation and poised transcriptional states. This distinction is significant, as H3K4me1 plays a pivotal role in transcriptional priming and enhancer-mediated gene regulation, potentially enabling a more precise and therapeutically relevant approach to epigenetic activation.
Retinitis pigmentosa (RP) stands as the most prevalent subtype among inherited retinal diseases, characterized by significant genetic heterogeneity and leading to progressive vision loss and potential blindness for millions worldwide.9,10 One of RP’s leading causes is mutations in the RHO gene, which codes the major photoreceptor for scotopic vision.11,12 Despite its prevalence, effective therapies and clinical trials for RP remain limited, posing a substantial challenge in managing this condition. In human eyes, photoreceptor cells comprise cones and rods, each playing distinct roles in visual perception.13 Cones, located mainly in the central retina (fovea), facilitate color vision and operate under bright light conditions. They express three types of opsins: long-wavelength sensitive, medium-wavelength-sensitive (MW), and short-wavelength-sensitive opsins. In contrast, rods are more abundant in the peripheral retina and are specialized for vision under low-light conditions, primarily expressing RHO.3 Recent studies in the mouse model have shown functional similarities between RHO and cone opsins.14 If RHO is defective, OPN1MW has been shown to potentially compensate for the loss of RHO function.15,16,17,18 This compensatory mechanism explains the importance of understanding the interplay between different photoreceptor pigments in maintaining visual function and provides insights into potential therapeutic strategies for RP.19,20
Our study focuses on optimizing the novel dCas9-based histone methyltransferase system designed to precisely target gene expression. Initially, we developed a synthetic epigenetic modulation enzyme, SETD7-dCas9, to activate gene regulation via H3K4me1 at transcription start sites (TSSs) in HEK293 cells. Subsequently, we validated the efficacy of SETD7-dCas9 in activating OPN1MW expression and confirmed that OPN1MW exhibited light absorption properties comparable with RHO in NIH3T3 cells. Importantly, our findings demonstrated that enhancing OPN1MW expression could effectively compensate for RHO deficiency. This research contributes to expanding our understanding of novel methods for activating specific gene expression by histone methylation, particularly in the context of photoreceptor pigments.
Results
Development of CRISPR-dCas9-based histone methyltransferase, SETD7-dCas9
To develop a new CRISPR-dCas9-based epigenetic activator system that specifically targets histone methylation, we utilized dCas9 for conferring the target specificity and SETD7, a histone methyltransferase known for promoting H3K4me1 methylation (Figure 1A). We engineered this system by fusing SETD7 to the N terminus of dCas9. In our design, dCas9, guided by specific guide RNA (gRNA), binds to the TSS of target genes. This positioning allows SETD7 to methylate the H3 histone at these sites, potentially activating gene expression. In this study, the function of SETD7-dCas9 fusion protein was validated by enhancing opsin expression for alternating RHO to respond against light in the cells. To understand the structural dynamics of the SETD7-dCas9 fusion protein, we performed simulation modeling. Figure 1B illustrates that the inclusion of a flexible linker between SETD7 and dCas9 provides SETD7 with sufficient flexibility to interact effectively with its substrate. The flexibility was conferred via a 15-amino acid (GGS)5 linker between dCas9 and SETD7. This flexibility is crucial for the proper positioning of SETD7 to methylate histone H3 when targeted to specific gene loci by dCas9. In Figure 1C, the simulation model shows the binding interaction between the SETD7 domain of our fusion protein and the histone H3. This model demonstrates that the SETD7 component of the fusion protein can access and bind to its histone target, positioning it appropriately for methylation at the H3K4 site. This alignment supports the functional hypothesis of our design.
Figure 1.
Schematic and structural modeling of the SETD7-dCas9 fusion protein
(A) Design of the SETD7-dCas9 system targeting histone H3K4 methylation. dCas9, guided by gRNAs, binds to the TSS of target genes, allowing SETD7 to methylate histone H3. (B) Illustration of the SETD7-dCas9 fusion protein showing the flexibility provided by the linker, which is crucial for the interaction between SETD7 and its substrate. (C) Simulation model depicting the binding interaction between the SETD7 domain and histone H3, demonstrating the proper alignment for H3K4 methylation (left) and the surface electrostatic potential map of the SETD7-dCas9 depicted colored from −10 kT/e (red) to +10 kT/e (blue) (right).
Validation of enzymatic activity and function of SETD7-dCas9
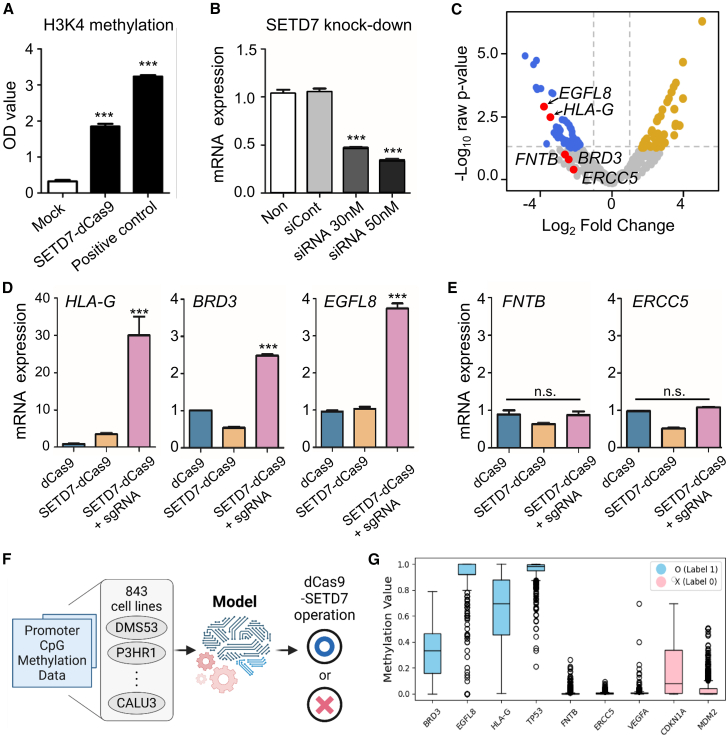
Following the successful construction of the SETD7-dCas9 system, we focused on validating its H3K4 methylation activity in vitro. To confirm the methylation activity of the SETD7-dCas9 fusion protein, we performed an in vitro histone methylation assay using H3K4 as the substrate. As shown in Figure 2A, the assay results verified that the SETD7-dCas9 protein retains its enzymatic activity, successfully catalyzing the methylation of H3K4. This experimental evidence supports the capability of the SETD7-dCas9 system to mediate H3K4 methylation, a crucial mark associated with gene activation. These analyses collectively demonstrate that the SETD7-dCas9 fusion protein not only retains structural flexibility and binding capability, but also effectively performs H3K4 methylation.
Figure 2.
Validation of SETD7-dCas9 enzymatic activity and development of a predictive model
(A) Results of the in vitro histone methylation assay confirming that the SETD7-dCas9 fusion protein catalyzes H3K4 methylation, validating its functional activity. (B) Efficiency of knockdown of SETD7 using siRNAs in HEK293 cells. (C) RNA-seq analysis comparing transcriptome-wide changes between WT and SETD7 knockdown HEK293 cells. (D) Enhanced mRNA expression of the target genes, HLA-G, BRD3, and EGFL8 by SETD7-dCas9. (E) Non-responding genes, FNTB, and ERCC5, by SETD7-dCas9. (F) Development of the prediction model for the SETD7-dCas9 operation. (G) Boxplot showing the median, quartiles, and range of DNA methylation value of promoter CpGs for each cell line of nine genes (BRD3, EGFL8, HLA-G, TP53, FNTB, ERCC5, VEGFA, CDKN1A, and MDM2) based on DNA methylation profiles from the CCLE RRBS dataset (DepMap, 2018 release). Error bars represent SD over biological replicates. p values were obtained using One-way ANOVA. ns; no statistical significance, ∗∗∗p < 0.001.
To evaluate whether the SETD7-dCas9 fusion proteins can modulate endogenous histone methylation or alter gene expression, we first identified the natural targets of SETD7 through comparative RNA sequencing (RNA-seq). Using small interfering RNA (siRNA), we generated SETD7 knockdown in HEK293 cells and compared the transcriptome-wide changes with wild-type (WT) HEK293 cells (Figures 2B and 2C). The RNA-seq analysis revealed significant differences in gene expression: 45 genes were downregulated and 46 genes were upregulated in SETD7 knockdown cells compared with WT cells (Figure 2C). To test the functionality of the SETD7-dCas9 fusion protein, we selected the top five most significantly downregulated genes, including human leukocyte antigen G (HLA-G), bromodomain-containing protein 3 (BRD3), epidermal growth factor-like domain 8 (EGFL8), protein farnesyltransferase subunit β (FNTB), and DNA repair protein complementing XP-G cells (ERCC5) for further analysis. We designed gRNAs to target the TSS of these genes and recruited the SETD7-dCas9 fusion proteins to these sites. Interestingly, co-transfection of SETD7-dCas9 with gRNA specifically targeting HLA-G, BRD3, and EGFL8 led to significant activation of these genes (Figure 2D; Tables 1 and 2). However, FNTB and ERCC5 did not show increased expression, suggesting that they might not be direct targets of SETD7 and, therefore, are not responsive to the SETD7-dCas9 system (Figure 2E; Tables 1 and 2).
Table 1.
gRNA sequence
| sgRNA | Position | Sequence (20 bp) |
|---|---|---|
| OPN1MW | −510 TSS | GCTCCCATGGAAAAGCGGGT |
| −343 TSS | TTGTGGACCAGAGTGTGAGT | |
| −104 TSS | CTGATCTCTTAATTGGGCCC | |
| RORA | −559 TSS | CGAAGCCACTGGGACCGTCC |
| −435 TSS | CGATAAATGCGCCAGCTCGT | |
| −390 TSS | TCTCTATAAGCTTATACTCC | |
| −282 TSS | CTCGCCTTCGGGATCACCTG | |
|
POU5F121 |
OCT4_PP_Region2_A | ACTCCACTGCACTCCAGTCT |
| OCT4_PP_Region2_B | TCTGTGGGGGACCTGCACTG | |
| OCT4_PP_Region2_C | GGGGCGCCAGTTGTGTCTCC | |
| OCT4_PP_Region2_D | ACACCATTGCCACCACCATT |
Table 2.
Primer sequence
| Gene | Sequence (5′–3′) | Size (bp) | |
|---|---|---|---|
| HLA-G | forward | CTGGTTGTCCTTGCAGCTGTAG | 80 |
| reverse | CCTTTTCAATCTGAGCTCTTCTTTCT | ||
| GAPDH | forward | GTCAGTGGTGGACCTGACCT | 166 |
| reverse | AAAGGTGGAGGAGTGGGTGT | ||
| BRD3 | forward | AGATGGATAGCCGAGAGTACC | 147 |
| reverse | GCAAACCTCATCTCAAACACATC | ||
| EGFL8 | forward | CCCGCTCCACTACAACGAGT | 106 |
| reverse | AACGCGGTACATGGTCCTGT | ||
| FNTB | forward | GTTCAACTGTGCCAGAGGAAAC | 146 |
| reverse | CTGGTGGAAGAAAGATAGAAACCC | ||
| ERCC5 | forward | GCTCCCATTAGTGCCGTC | 77 |
| reverse | CCCTGCTCCTACACAACAA | ||
Development of prediction model for SETD7-dCas9 operation
Epigenetic regulation of gene expression is a complex interplay between DNA methylation and histone modifications. SETD7 catalyzes the mono-methylation of histone H3 at lysine 4 (H3K4me1), a mark associated with enhancers and transcriptionally poised promoters.22,23 While DNA methylation at promoter CpG islands is typically repressive,24,25 emerging evidence suggests that methylated promoters can remain accessible and be reactivated under appropriate chromatin remodeling cues.26,27 Based on this view, we hypothesized that promoters with dense CpG methylation but preserved regulatory potential may be particularly responsive to targeted activation by SETD7-dCas9-mediated H3K4me1 deposition.26 Therefore, we used DNA methylation profiling to identify candidate promoter regions likely to benefit from epigenetic activation.
To predict which genes are most responsive to dCas9-SETD7-mediated activation, we assessed DNA methylation profiles of CpG clusters at transcription start sites from the Cancer Cell Line Encyclopedia (CCLE) RRBS dataset (Table 3). Specifically, we evaluated CpG methylation across 17 promoter clusters derived from 9 genes of interest (BRD3, EGFL8, HLA-G, TP53, FNTB, ERCC5, VEGFA, CDKN1A, and MDM2) (Figures 2E and 2F; Tables 3 and S1). These promoter clusters were defined based on hierarchical clustering of CpG sites located within ±3 kb of the TSS in each gene. This clustering approach allowed us to capture local epigenetic variation in promoter regions, enabling identification of subgroups with distinct methylation patterns that may differentially influence transcriptional responsiveness. By focusing on these methylation-defined promoter clusters, we aimed to enhance the resolution of our prediction model and increase its sensitivity to subtle yet functionally relevant epigenetic configurations.
Table 3.
Representative statistical information of the dataset (DepMap, CCLE_RRBS_tss_CpG_clusters_20181022)
| Average DNA methylation value for 843 cell lines | |||||
|---|---|---|---|---|---|
| Gene symbol | Mean ± SD | Median | Maximum | Minimum | Applicability |
| BRD3 | 0.311 ± 0.196 | 0.331 | 0.930 | −0.310 | O |
| EGFL8 | 0.919 ± 0.200 | 1.000 | 1.000 | 1.000 | O |
| HLA-G | 0.643 ± 0.273 | 0.697 | 1.528 | −0.197 | O |
| TP53 | 0.955 ± 0.079 | 0.983 | 1.074 | 0.870 | O |
| FNTB | 0.008 ± 0.026 | 0.000 | 0.000 | 0.000 | X |
| ERCC5 | 0.005 ± 0.009 | 0.002 | 0.015 | −0.009 | X |
| VEGFA | 0.009 ± 0.032 | 0.004 | 0.018 | −0.008 | X |
| CDKN1A | 0.174 ± 0.195 | 0.079 | 0.839 | −0.499 | X |
| MDM2 | 0.040 ± 0.081 | 0.003 | 0.104 | −0.062 | X |
Maximum (Q3 + 1.5 IQR), minimum (Q1 – 1.5 IQR).
A logistic regression algorithm was employed to construct a binary classification model of dCas9-SETD7 operation (Figure 2G). Given the limited number of training instances, we conducted leave-one-out cross-validation (LOOCV) to evaluate the predictive performance of the model. In detail, the LOOCV process involved training the model on all instances except one and then testing the model on the excluded instance. This process was repeated for every instance in turn. To evaluate the generalization capability for the model in cross-validation, receiver operating characteristic (ROC) curve and precision-recall (PR) curve were generated, and the area under the curve (AUC) was computed. Following the cross-validation, we also evaluated the final model by generating a confusion matrix, ROC curves, and PR curves (Figures S1A–S1C). These results confirmed that the final model outperformed the cross-validation results. Specifically, the average accuracy of LOOCV was 0.882 (95% CI, 0.706–1.000), with six true negatives and nine true positives (Figure S1A). The accuracy of the final model was 0.941 (95% CI, 0.824–1.000), indicating an improvement in specificity. The ROC AUC for LOOCV was 0.886 (95% CI, 0.652–1.000), which improved to 0.986 (95% CI, 0.917–1.000) for the final model (Figure S1B). Similarly, the PR AUC for LOOCV was 0.947 (95% CI, 0.806–1.000), while the final model exhibited a significant increase to 0.990 (95% CI, 0.942–1.000) (Figure S1C). Additionally, to determine the optimal probability threshold for operational prediction, the best thresholds based on the F1 score were evaluated. During LOOCV, the best threshold was computed for each iteration, and the average was obtained. After training the final model, another best threshold was calculated based on the F1 score. The best threshold for the final model (0.177, F1 = 0.952) provided a higher F1 score compared with LOOCV (0.571, F1 = 0.900), suggesting that the threshold from the final model was more optimal. Since the final model was trained on the entire dataset, this improved threshold is likely to represent a more accurate decision boundary.
Among these, EGFL8 and TP53 exhibited consistently high DNA methylation levels, which likely contributed to the robust predictive performance of our classification model by representing promoter regions with a repressed yet responsive epigenetic state. These findings are consistent with the functional role of SETD7 as a histone methyltransferase that catalyzes H3K4me1, a mark enriched at poised or repressed regulatory elements. Our analysis suggests that SETD7-dCas9 activity may be particularly effective at epigenetically silenced, hypermethylated promoters, supporting the use of DNA methylation as a predictive biomarker for targeted gene activation via this system.
Confirmation of SETD7-dCas9 operation
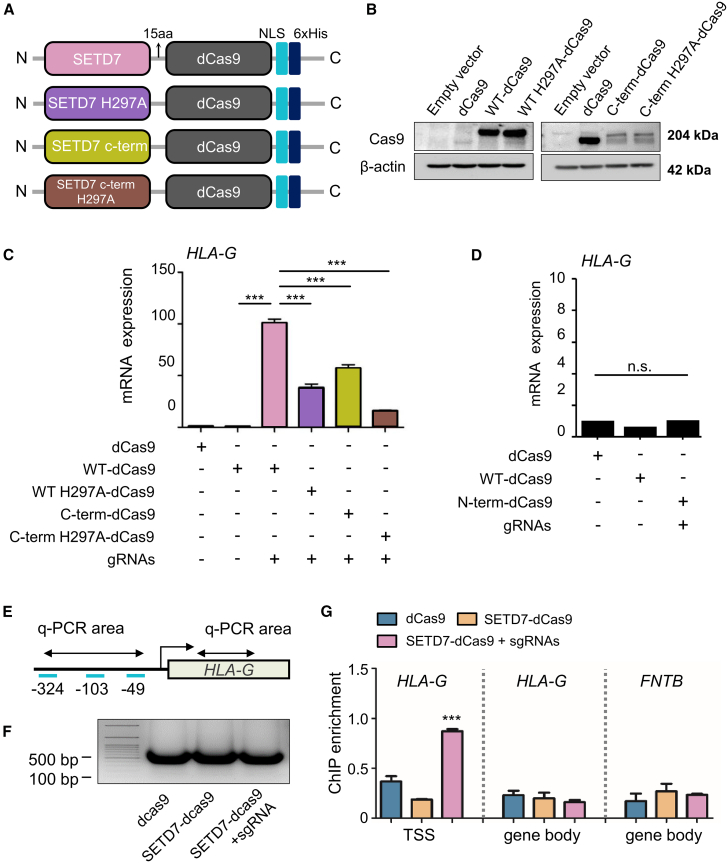
Since the ability of SETD7-dCas9 in activation of target genes was demonstrated, we tried to further confirm that the function of SETD7-dCas9 comes from enzymatic activity of SETD7 by using the constructs with a mutation in the C-terminal domain of SETD7, since this domain has a catalytic function. SETD7 is composed of the N-terminal domain and the C-terminal domain (or SET domain). The function of the SET domain has been shown for their catalytic function. Limiting chromatin condensation by SETD7 methylating H3 at lysine 4 is expected to improve transcriptional activity. To confirm the enzymatic function of the SET domain in the SETD7-dCas9 in the activation of the target genes, we constructed SETD7-dCas9 with a SETD7 H297A mutation, eliminating its methylation activity, and also constructed SETD7 C-terminal domain-dCas9 and its H297A mutation version (Figure 3A). First, following the transfection of the constructs into HEK293 cells, we verified the expression of each fusion protein using western blot analysis (Figure 3B). The expected size of the full-length SETD7-dCas9 fusion protein was approximately 204 kDa, and our results confirmed its successful expression in the cells. Next, we assessed the gene activation potential of different SETD7-dCas9 constructs, focusing specifically on the HLA-G gene. We transfected HEK293 cells with the four different SETD7-dCas9 fusion constructs and corresponding gRNAs for HLA-G, then measured mRNA expression levels by RT-PCR (Figure 3C). WT SETD7-dCas9 exhibited the highest efficacy in activating HLA-G expression compared with the other constructs. SETD7 C terminus-dCas9, containing only the regulatory C-terminal domain of SETD7, was less effective than the full-length WT SETD7-dCas9. SETD7 H297A-dCas9, which abolishes the methyltransferase activity, resulted in a significant reduction in HLA-G mRNA expression, emphasizing the importance of SETD7’s catalytic activity for gene activation. Similarly, SETD7 C-terminal domain H297A-dCas9 also showed reduced efficacy in gene activation. To further validate these findings, we compared the WT SETD7-dCas9 with an SETD7 N-terminal domain-dCas9 construct targeting HLA-G. The results confirmed that the N-terminal domain fusion construct could not activate HLA-G expression, reinforcing that the function SETD7-dCas9 in gene activation relies on enzymatic activity of SETD7 (Figure 3D). To assess the specificity of SETD7-dCas9-mediated gene activation, we performed ChIP-qPCR analysis targeting the TSS of the HLA-G gene, as well as a distal gene body region and the TSS of an unrelated gene, FNTB (Figures 3E, 3F, and 3G). Our results demonstrated a marked enrichment of H3K4me1 at the HLA-G TSS, with no significant enrichment observed at the HLA-G gene body or FNTB promoter (Figure 3G). These findings indicate that SETD7-mediated histone methylation occurs locally at the targeted promoter, suggesting minimal off-target chromatin remodeling. However, we acknowledge that our current analysis does not capture genome-wide dCas9 binding activity. Future studies employing unbiased techniques such as dCas9 ChIP-seq will be critical to comprehensively assess off-target binding and further validate the specificity of our epigenetic editing platform.
Figure 3.
Functional validation of SETD7-dCas9 fusion constructs and target specificity
(A) Design of the SETD7-dCas9 constructs containing the WT SETD7, SETD7 H297A, C terminus SETD7, and C terminus SETD7 H297A. (B) Western blot analysis confirming the expression of the SETD7-dCas9 fusion proteins and its mutant constructs in HEK293 cells. The expected size for the full-length fusion protein is approximately 204 kDa. (C) Comparison of HLA-G mRNA expression levels in HEK293 cells transfected with different SETD7-dCas9 constructs. (D) Analysis of HLA-G activation by N-terminal SETD7-dCas9 fusion. (E) Design of three gRNAs targeting the TSS of HLA-G for ChIP-qPCR analysis. (F) Chromatin fragmentation results showing fragment sizes after sonication. (G) ChIP-qPCR results indicating H3K4 methylation at the TSS of HLA-G but not at its gene body or the TSS of FNTB. Error bars represent SD over biological replicates. P values were obtained using One-way ANOVA. ns; no statistical significance, ∗∗∗p < 0.001.
Validation of target gene upregulation by SETD7-dCas9
To evaluate the broader applicability of the SETD7-dCas9 fusion system beyond its natural targets, we investigated its ability to activate non-natural target genes. This analysis aimed to understand how the system functions when directed to genes not typically associated with SETD7 activity. We selected POU5F1 as a control target gene for our system. POU5F1 is a well-studied pluripotency marker and is commonly used in the evaluation of various dCas9-based gene activation systems; and we also selected the OPN1MW and RORA genes, which are responsible for maintaining vision, to explore the potential of SETD7-dCas9 for therapeutic purposes. To validate applicability of the targets in the SETD7-dCas9 system, we first applied the constructed prediction model of SETD7-dCas9 operation to new data, including three genes (POU5F1, OPN1LW, and RORA). The model predicted that dCas9-SETD7 would act on all three genes shown in the heatmap, visualizing differentially methylated promoter CpG sites on eight selected genes (HLA-G, BRD3, EGFL8, POU5F1, OPN1LW, RORA, FNTB, and ERCC5) with the threshold of 0.06 (Figure 4A). To obtain the optimal midpoint for DNA methylation values, the optimal split point was identified by investigating the decision tree algorithm using the average DNA methylation values of 12 genes.
Figure 4.
Prediction and validation of gene activation by SETD7-dCas9
(A) Heatmap visualization of differentially methylated promoter CpG sites on 8 selected genes (HLA-G, BRD3, EGFL8, POU5F1, OPN1LW, RORA, FNTB, and ERCC5) in 748 different cell lines. These datasets are from the Broad Institute’s Cancer Cell Line Encyclopedia (CCLE), which includes a diverse array of 1,036 human cancer cell lines representing 36 different tumor types. Various cell lines are arranged in columns clustered using Pearson correlation, and the corresponding gene name of the CpG site is arranged in row. A scale is shown on the top, in which red and blue correspond to a higher and a lower methylation status, respectively. Gene expression analysis demonstrating the activation of POU5F1 (B), MW opsin, and RORA (C) by the SETD7-dCas9 system. Information of gRNA sequences in Table 1. Error bars represent SD over biological replicates. p values were obtained using One-way ANOVA. ∗∗∗p < 0.001.
To confirm the prediction, we co-transfected SETD7-dCas9 with gRNAs targeting the POU5F1 gene into cells and assessed gene activation. The results showed successful activation of POU5F1, demonstrating that the SETD7-dCas9 fusion protein can effectively upregulate this critical gene (Figure 4B). Next, we designed specific gRNAs to target these genes and transfected them along with SETD7-dCas9 (Figure 4C). Three gRNAs targeting the transcription start site of the OPN1MW gene were co-transfected with SETD7-dCas9 into HEK293 cells. This gene is crucial in photoreceptor function and light absorption, making it an important target for therapeutic applications. Similarly, we co-transfected gRNAs targeting the RORA gene along with SETD7-dCas9 into HEK293 cells. RORA is involved in various cellular processes, including inflammation in the eyes. The data indicated that the SETD7-dCas9 system successfully upregulated both OPN1MW and RORA gene expression in the cell types (Figure 4C). These findings expand the utility of SETD7-dCas9 beyond its natural targets, exhibiting its potential as a versatile tool for gene activation in diverse contexts. This setup facilitates gene activation by directing the SETD7-dCas9 system to these specific genomic loci. The data provide insights into the activation potential of the SETD7-dCas9 system compared with baseline expression. These findings also demonstrate that DNA methylation values effectively contribute to binary classification tasks. Further validation and application to a broader range of genes will be necessary to enhance the robustness of the model.
Therapeutic potential of OPN1MW activation for compensating RHO deficiency in retinal disorders
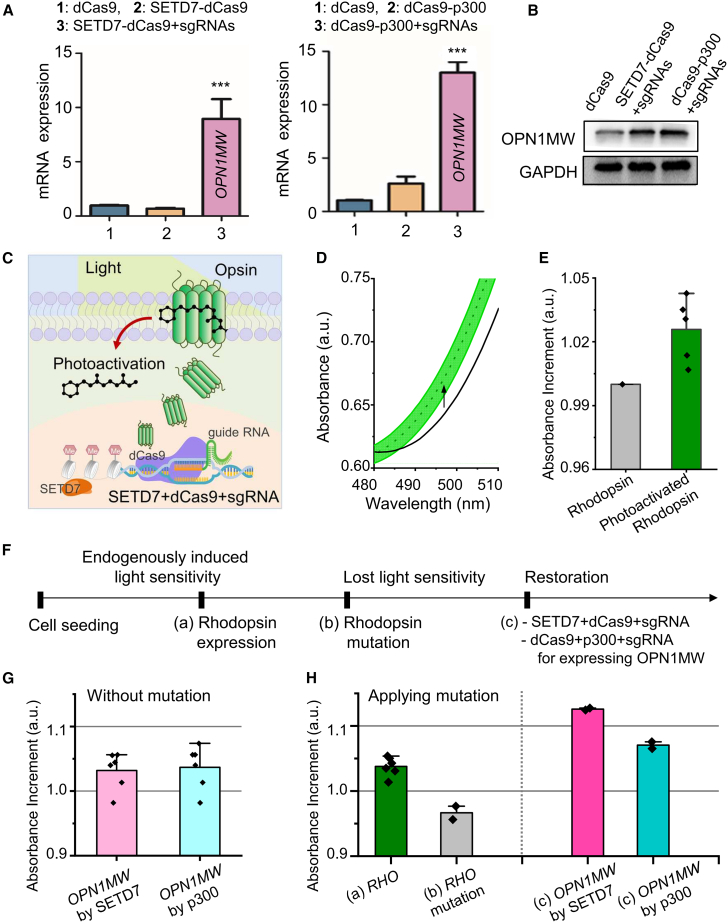
Pigmentosa, a progressive retinal degenerative disorder characterized by rod photoreceptor dysfunction and apoptosis, followed by secondary cone photoreceptor degeneration. To evaluate whether targeted activation of OPN1MW by the SETD7-dCas9 could serve as a compensatory strategy for RHO deficiency, we conducted a series of experiments using NIH3T3 cells, which exhibit low basal expression of OPN1MW. We transfected these cells with SETD7-dCas9 and three gRNAs targeting the TSS region of the OPN1MW gene. The goal was to enhance OPN1MW opsin expression. As a positive control, we used the dCas9-p300 system, a well-established activator that increases gene expression through histone acetylation. Our results showed that co-transfection of SETD7-dCas9 with the OPN1MW-specific gRNAs significantly increased OPN1MW expression at both the mRNA (Figure 5A) and protein levels (Figure 5B). Similar results were observed in cells transfected with dCas9-p300, validating the effectiveness of our approach in upregulating OPN1MW expression.
Figure 5.
Therapeutic activation of OPN1MW to compensate for RHO deficiency
Analysis of OPN1MW expression levels in NIH3T3 cells following transfection with SETD7-dCas9 and OPN1MW-specific gRNAs. Both mRNA (A) and protein levels (B) were significantly increased, similar to the results obtained with the positive control, dCas9-p300. (C) Light absorption properties of NIH3T3 cells expressing OPN1MW, activated by SETD7-dCas9 and treated with 11-cis-retinal. The changes in absorption spectra confirm the functional activation of OPN1MW. (D) Absorbance spectra of NIH3T3 cells expressing OPN1MW, activated by SETD7-dCas9, shows increased light absorption. (E) NIH3T3 cells expressing WT RHO shows increased light absorption at 495 nm. (F) Schematics of experimental procedures for comparative light absorption analysis demonstrating the potential of OPN1MW to compensate for RHO deficiencies. (G) The light absorption in NIH3T3 cells with OPN1MW expression by SETD7-dCas9 and dCas9-p300 systems. (H) Comparative light absorption analysis among cells expressing WT RHO, cells with RHO mutations, and cells engineered to produce MW opsin, demonstrating the potential of OPN1MW to compensate for RHO deficiencies. Error bars represent SD over biological replicates. p values were obtained using One-way ANOVA. ∗∗∗p < 0.001.
To determine whether the upregulated OPN1MW was functionally active and capable of mimicking RHO,28,29 we assessed light absorption profiles following 11-cis-retinal treatment. Cells expressing OPN1MW exhibited a marked shift in light absorption spectra consistent with functional opsin activation (Figures 5C and 5D). NIH3T3 cells expressing WT RHO served as positive controls and demonstrated a similar absorption response (Figure 5E), confirming the optical functionality of the induced OPN1MW.
To simulate pathological conditions, we engineered an RP-like environment using RHO-deficient HEK293 cells expressing an E-F loop deletion mutant of RHO (Figure 5F). In this model, co-expression of OPN1MW via either SETD7-dCas9 or dCas9-p300 restored light absorption profiles in a manner comparable with WT RHO expression (Figure 5G). Spectral analyses showed that OPN1MW expression compensated for the reduced absorbance associated with RHO dysfunction, thereby rescuing phototransduction-related responses (Figure 5H). Collectively, these findings highlight the therapeutic potential of the SETD7-dCas9 system to epigenetically activate alternative opsin genes and functionally compensate for RHO deficiency. This approach may offer a mutation-independent strategy for treating retinal degenerative diseases such as RP.
Discussion
In this study, we successfully developed and optimized a novel CRISPR-dCas9-based histone methyltransferase system designed to specifically activate gene expression. Our approach leverages the fusion of SETD7 to dCas9 directing H3K4me1 to targeted genomic loci, which is a modification associated with poised and active promoters. Validation in HEK293 cells demonstrated that SETD7-dCas9 effectively deposited H3K4me1 marks at the TSS and induced transcription of endogenous genes, establishing its functionality as a programmable epigenetic activator.
Importantly, we observed that the DNA methylation status of promoter CpG regions strongly influenced the responsiveness to dCas9-SETD7-mediated activation. Genes with densely methylated promoters such as HLA-G, BRD3, and EGFL8 showed robust transcriptional upregulation, consistent with the notion that these hypermethylated promoters may represent “locked but primed” chromatin states. In contrast, genes such as FNTB and ERCC5, which displayed low promoter methylation, were unresponsive to SETD7-dCas9. These findings support the model that hypermethylated yet accessible promoters retain latent regulatory potential that can be unlocked through targeted histone methylation, while unmethylated, transcriptionally inert loci may require additional factors such as enhancers or coactivators for activation. This pattern reinforces promoter DNA methylation as a predictive biomarker for gene responsiveness to SETD7-mediated epigenetic activation.
To explore therapeutic relevance, we demonstrated that SETD7-dCas9 can robustly induce the expression of OPN1MW in NIH3T3 cells. OPN1MW is a cone photoreceptor-specific gene essential for color and daylight vision. By co-delivering SETD7-dCas9 with guide RNAs targeting the OPN1MW promoter, we observed strong induction at both mRNA and protein levels, comparable with the well-established dCas9-p300 activator system. This result has translational implications for retinal degenerative diseases such as RP, in which rod photoreceptor loss often due to mutations in the RHO gene leads to progressive vision loss. As OPN1MW activation in residual retinal cells may compensate for the lack of rod input, our approach suggests a gene-independent strategy for restoring phototransduction in RP.
We further propose that this platform can be applied to bipolar cells, neurons preserved during early RP, to express opsins and preserve retinal circuitry. Unlike ganglion cell-targeted optogenetics, targeting ON/OFF bipolar cells could maintain more naturalistic visual perception. Although our work is currently limited to in vitro studies, these findings establish a strong foundation for in vivo testing in RP models and the development of bipolar cell-specific promoters for targeted delivery.
A major translational hurdle is the delivery of large fusion proteins like SETD7-dCas9. Although the AAV2 serotype has shown safety and efficacy in retinal gene therapy such as Luxturna for RPE65 mutations, its limited packaging capacity necessitates dual-vector systems, split-intein strategies, or alternative non-viral delivery systems such as lipid nanoparticles.30,31 Future work should integrate these delivery strategies to enable in vivo application of SETD7-dCas9 systems.
Unlike permanent DNA editing technologies, our SETD7-dCas9 platform does not alter the underlying genomic sequence, reducing the risk of off-target mutations or genomic instability. This epigenetic modality enables reversible, tunable gene regulation, which is particularly appealing for diseases involving dynamic or tissue-specific gene expression. As such, our findings not only provide mechanistic insights into chromatin state-dependent gene activation but also lay the groundwork for a broader, safer epigenetic therapy platform.
Altogether, our study establishes SETD7-dCas9 as a programmable, chromatin-modifying activator capable of targeting disease-relevant genes. Compared with conventional transcriptional activators, this system harnesses endogenous chromatin dynamics to unlock silenced loci, offering a precision-based, mutation-independent, and potentially safer approach to therapeutic gene regulation.
Materials and methods
Plasmid construction
To clone SETD7-dCas9, we first generated dCas9 from pET-Cas9-NLS-6xHis vector (Addgene) by mutagenesis kit. We connected SETD7 with dCas9 with a small linker, which can enhance the binding affinity of dCas9 to target genes. SETD7 was merged to dCas9 via NEBuilder HiFi DNA Assembly (NEB, E2621) and cloned into pCDNA3 and pET backbone plasmid. pCDNA3-SETD7 H279A-dCas9, pCDNA3-SETD7 Cterm-dCas9, pCDNA3 SETD7 H279A Cterm-dCas9, and pCDNA3 SETD7 Nterm-dCas9 were cloned via NEBuilder HiFi DNA Assembly (NEB, E2621). All enzymes were obtained from New England Biolabs.
Purification of the Cas9 fusion protein
pET-SETD7-dCas9-NLS-6xHis plasmids were transformed into Escherichia coli BL21 (DE3) competent cells and incubated overnight at 37°C using Luria-Bertani agar plates containing ampicillin (100 μg/mL). The transfected BL21 cells were selected and cultured overnight at 120 rpm at 20°C in 3 L of LB-ampicillin broth with 1 mM isopropyl-β-D-thiogalactopyranoside. Cells were collected by ultra-centrifugation and lysed with lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM imidazole [pH 8.0]). After ultra-centrifugation at 18,000 rpm for 40 min at 4°C, the soluble lysate was incubated at Ni-NTA resin (ThermoFisher Scientific) for 2 h at 4°C. After washing with washing buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole [pH 8.0]), it was eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 100 mM imidazole [pH 8.0], 50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole [pH 8.0]). After concentration, the buffer was changed with PBS. The purity of the SETD7-Cas9 protein was confirmed by Coomassie blue SDS-PAGE gel staining.
Complex structural modeling
The 3D structure of Cas9 was modeled with the Galaxy Loop method based on the template structure (PDB: 6IFO). The single-stranded structures of mRNA1X and mRNA3X were modeled using the Rosetta software suite. Based on the predicted secondary structures of RNA strands by the RNAFold server, the 3D structures were generated using the Rna_de_novo program of the Rosetta suite. All figures of protein structures were illustrated by ChimeraX.
mRNA transcriptome sequencing
HEK293 cells were knocked down by 50 ng of SETD7 siRNA. After that, total mRNA was extracted from the knockdown cells using the RNeasy Mini Kit (QIAGEN, cat. no. 74104). Samples were analyzed by TruSeq Stranded mRNA LT Sample Prep Kit using the Illumina platform for next-generation sequencing (Macrogen). The experiment flow was based on Nat. Rev. Genet. 2011 Sep 7;12(10):671–82. The data were analyzed by Macrogen. Genes with a mean q value smaller than 0.05 were considered a target of differential expression between WT and SETD7 siRNA knockdown cells. The candidate genes were selected based on a fold change of more than 4 and a read count value of more than 100.
gRNA design, synthesis, and transfection
gRNA sequencing was designed by the crispr-era.stanford.edu website to target the gene’s promoter region at various locations upstream of the TSS (Table 1). The 20 bp binding site sequences of single-guide (sgRNA)-targeting specific genes were cloned into pRG2 using BsaI restriction enzyme ligation. pRG2 was kindly provided from Jin-Soo Kim (Addgene, plasmid no. 104174). For targeted gene activation, multiple single gRNAs were used to bind distinct positions within the promoter region of each target gene, typically within 200–400 bp upstream of the TSS. All gRNAs targeting a specific promoter were co-transfected simultaneously to enhance the recruitment of the SETD7-dCas9 fusion protein and increase activation efficiency. gRNAs were cloned into individual U6-driven expression vectors and combined at equimolar ratios prior to transfection. Lipofectamine 3000 was used for transfection according to the manufacturer’s protocol. Transfections were performed in HEK293 or NIH3T3 cells seeded at ∼70% confluency, and downstream analyses were conducted 48–72 h post-transfection.
ChIP-qPCR enrichment assay
Totals of 8.4 μg of pCDNA3-dCas9-6His or pCDNA3-SETD7-dCas9-6His and 2.4 μg of sgRNA were co-transfected with 15 μL Lipofectamine 2000 into 7 × 106 HEK293 cells in 10 cm plates. Harvested cells were crosslinked with 11% fixation buffer and the reaction was stopped by glycine at a 1:10 ratio. Chromatin was sheared on ice for 25 min with 15 s sonication at 30 s intervals with a Bioruptor KR (CosmoBio, Tokyo, Japan) to obtain DNA fragments of an average length of 100–500 bp. The ChIP-qPCR enrichment assay was processed following ChIP Kit Magnetic qPCR (ab270816). Sheared chromatin was incubated overnight with the following antibodies previously bound to magnetic bead antibody to H3K4me1 (EMD Millipore, 07-473), antibody to H3K27ac (EMD Millipore, 07-360), IgG. After reverse crosslinking and DNA isolation, primers were designed to amplify regions −324 to –49 bp spanning the HLA-G-binding motif within gene promoter regions.
RT-PCR
Total RNA in macrophage cells was extracted using the RNeasy Mini Kit (QIAGEN, cat. no. 74104). Total cellular RNA (2.5 μg) was converted to cDNA by SuperScript VILO Master Mix Kit for cDNA synthesis (Invitrogen, cat. no. 11755250). All the primers used for real-time PCR are listed in Table 2. GAPDH was used as an internal control. Primers were purchased from Bioneer (Daejeon). Real-time PCR was performed using diluted cDNA by the Applied Biosystems models 7500 real-time cyclers, and relative levels of mRNA gene expression were analyzed using the 2−ΔΔCT method.
Histone H3 (K4) methyltransferase in vitro assay
H3K4 methyl transferase activity of SETD7-dCas9 protein was measured by histone H3 (K4) methyl transferase activity quantification assay kit (ab 113452).
Human cell lines
Human cancer cell lines HEK293 and NIH3T3 cells were purchased from Korean Cell Line Bank. The cell lines HEK293 and NIH3T3 were maintained in DMEM medium supplemented with 10% FBS from Gibco (Carlsbad, CA) and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin).
Modeling RHO dysfunction and functional compensation by OPN1MW
To mimic RHO dysfunction in vitro, a mutant RHO gene lacking the E-F loop (amino acids 231–252) was generated via Gibson assembly. This region is essential for transducin activation, and its deletion results in impaired RHO function.32 HEK293 cells were first transfected with plasmids encoding either WT RHO or the E-F loop deletion mutant fused to GFP using Lipofectamine 3000 for 24 h. After initial expression of mutant RHO, cells were subsequently transfected with either dCas9-SETD7 or dCas9-p300 in combination with sgRNAs targeting the OPN1MW promoter.
After 48 h, light absorption assays were also performed to assess the functional restoration of opsin activity. HEK293 cells transfected with WT RHO serving as positive controls. This experimental model enabled the evaluation of whether SETD7-dCas9-mediated epigenetic activation of OPN1MW could compensate for defective RHO signaling.
Measurement of light absorption
The light absorption was measured using a UV-vis spectrometer (Lambda 365+, PerkinElmer) in visible light. Specifically, we monitored the variation in light absorption at a wavelength of 495 nm, which is characteristic of RHO. This feature shows the function of RHO in the cells produced by the genetic activation of our technique. Without adding 11-cis-retinal to the cells, the intrinsic light absorption of the samples was measured, and the photo-activated samples with 11-cis-retinal were checked several times.
Statistical analysis
All experiments conducted in the present study were performed in triplicate. All statistical values are presented as mean ± standard deviation (SD) or ± standard error. In addition, Student’s t test was used to determine the statistical significance of the results obtained in this study. A p value of less than 0.05 was considered significant (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Data and code availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
This work was supported by National Research Foundation (NRF) grant funded by the Korea government (RS-2023-00208795 and RS-2023-00260529) and a grant from Kyung Hee University in 2022 (KHU- 20222227) (to S.J.O.). This work was also supported by UST Young Scientist Research Program 2020 through the University of Science and Technology (grant no. 2G11250), KIST Institutional Program (nos. 2E33231 and 2E32451), and NRF grant funded by the Korean government (MSIT) (NRF-2021R1C1C2013750) (to B.P.) The artwork in this study was created using Biorender.com.
Author contributions
Conceptualization, N.L.T., S.J.O., and S.-H.K.; data acquisition, N.L.T., Y.E.K., H.J., Y.K., S.C.S., and B.P.; data analysis, N.L.T., Y.E.K., H.J., Y.K., and B.P.; writing, all authors; funding acquisition, S.J.O.
Declaration of interests
N.L.T. and S.J.O. have a patent application related to this work.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2025.102677.
Contributor Information
Byeongho Park, Email: t12509@kist.re.kr.
Seung Ja Oh, Email: seungja.oh@khu.ac.kr.
Supplemental information
References
- 1.Waterland R.A. Epigenetic mechanisms and gastrointestinal development. J. Pediatr. 2006;149:S137–S142. doi: 10.1016/j.jpeds.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y., Wang Y. Role of epigenetic regulation in plasticity of tumor immune microenvironment. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.640369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batista I.D.A., Helguero L.A. Biological processes and signal transduction pathways regulated by the protein methyltransferase SETD7 and their significance in cancer. Signal Transduct. Tar. 2018;3 doi: 10.1038/s41392-018-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Geen H., Bates S.L., Carter S.S., Nisson K.A., Halmai J., Fink K.D., Rhie S.K., Farnham P.J., Segal D.J. Ezh2-dCas9 and KRAB-dCas9 enable engineering of epigenetic memory in a context-dependent manner. Epigenetics Chromatin. 2019;12 doi: 10.1186/s13072-019-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakore P.I., Black J.B., Hilton I.B., Gersbach C.A. Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat. Methods. 2016;13:127–137. doi: 10.1038/nmeth.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.-M., Kim K., Schmidt T., Punj V., Tucker H., Rice J.C., Ulmer T.S., An W. Cooperation between SMYD3 and PC4 drives a distinct transcriptional program in cancer cells. Nucleic Acids Res. 2015;43:8868–8883. doi: 10.1093/nar/gkv874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano-Rodriguez D., Gjaltema R.A.F., Jilderda L.J., Jellema P., Dokter-Fokkens J., Ruiters M.H.J., Rots M.G. Writing of H3K4Me3 overcomes epigenetic silencing in a sustained but context-dependent manner. Nat. Commun. 2016;7 doi: 10.1038/ncomms12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daiger S.P., Sullivan L.S., Bowne S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013;84:132–141. doi: 10.1111/cge.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 11.Beryozkin A., Levy G., Blumenfeld A., Meyer S., Namburi P., Morad Y., Gradstein L., Swaroop A., Banin E., Sharon D. Genetic Analysis of the Rhodopsin Gene Identifies a Mosaic Dominant Retinitis Pigmentosa Mutation in a Healthy Individual. Investig. Ophthalmol. Vis. Sci. 2016;57:940–947. doi: 10.1167/iovs.15-18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrar G.J., Kenna P.F., Humphries P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002;21:857–864. doi: 10.1093/emboj/21.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- 14.Sakami S., Maeda T., Bereta G., Okano K., Golczak M., Sumaroka A., Roman A.J., Cideciyan A.V., Jacobson S.G., Palczewski K. Probing Mechanisms of Photoreceptor Degeneration in a New Mouse Model of the Common Form of Autosomal Dominant Retinitis Pigmentosa due to P23H Opsin Mutations. J. Biol. Chem. 2011;286:10551–10567. doi: 10.1074/jbc.M110.209759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y., Kefalov V., Luo D.G., Xue T., Yau K.W. Quantal noise from human red cone pigment. Nat. Neurosci. 2008;11:565–571. doi: 10.1038/nn.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kefalov V.J. Rod and cone visual pigments and phototransduction through pharmacological, genetic, and physiological approaches. J. Biol. Chem. 2012;287:1635–1641. doi: 10.1074/jbc.R111.303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai K., Onishi A., Imai H., Chisaka O., Ueda Y., Usukura J., Nakatani K., Shichida Y. Physiological properties of rod photoreceptor cells in green-sensitive cone pigment knock-in mice. J. Gen. Physiol. 2007;130:21–40. doi: 10.1085/jgp.200609729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi G., Yau K.W., Chen J., Kefalov V.J. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J. Neurosci. 2007;27:10084–10093. doi: 10.1523/JNEUROSCI.2211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cideciyan A.V., Aleman T.S., Swider M., Schwartz S.B., Steinberg J.D., Brucker A.J., Maguire A.M., Bennett J., Stone E.M., Jacobson S.G. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum. Mol. Genet. 2004;13:525–534. doi: 10.1093/hmg/ddh048. [DOI] [PubMed] [Google Scholar]
- 20.Berson E.L., Rosner B., Sandberg M.A., Hayes K.C., Nicholson B.W., Weigel-DiFranco C., Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch. Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Mahata B., Escobar M., Goell J., Wang K., Khemka P., Hilton I.B. Programmable human histone phosphorylation and gene activation using a CRISPR/Cas9-based chromatin kinase. Nat Commun. 2021;12:896. doi: 10.1038/s41467-021-21188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barski A., Cuddapah S., Cui K., Roh T.-Y., Schones D.E., Wang Z., Wei G., Chepelev I., Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Zang C., Rosenfeld J.A., Schones D.E., Barski A., Cuddapah S., Cui K., Roh T.-Y., Peng W., Zhang M.Q. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 25.Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 26.Rishi V., Bhattacharya P., Chatterjee R., Rozenberg J., Zhao J., Glass K., Fitzgerald P., Vinson C. CpG methylation of half-CRE sequences creates C/EBPα binding sites that activate some tissue-specific genes. Proc. Natl. Acad. Sci. USA. 2010;107:20311–20316. doi: 10.1073/pnas.1008688107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blattler A., Farnham P.J. Cross-talk between site-specific transcription factors and DNA methylation states. J. Biol. Chem. 2013;288:34287–34294. doi: 10.1074/jbc.R113.512517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park B., Yang H., Ha T.H., Park H.S., Oh S.J., Ryu Y.S., Cho Y., Kim H.S., Oh J., Lee D.K. Artificial Rod and Cone Photoreceptors with Human-Like Spectral Sensitivities. Adv. Mater. 2018;30 doi: 10.1002/adma.201706764. [DOI] [PubMed] [Google Scholar]
- 29.Shichida Y., Matsuyama T. Evolution of opsins and phototransduction. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2881–2895. doi: 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.-F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire A.M., Russell S., Chung D.C., Yu Z.-F., Tillman A., Drack A.V., Simonelli F., Leroy B.P., Reape K.Z., High K.A. Durability of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease: phase 3 results at 3 and 4 years. Ophthalmology. 2021;128:1460–1468. doi: 10.1016/j.ophtha.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Franke R., Sakmar T., Graham R., Khorana H. Structure and function in rhodopsin. Studies of the interaction between the rhodopsin cytoplasmic domain and transducin. J. Biol. Chem. 1992;267:14767–14774. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.