Abstract
Coconut flesh, the solid endosperm, of coconut, which is rich in fat, protein and polyphenols. To investigate the impact of ultrasound treatment on the biosynthesis of polyphenols in tender coconut flesh during storage, the targeted metabolomic and transcriptomic analyses were employed. A total of 36 phenolic compounds were identified, of which catechin, epicatechin, gossypol and vanillic acid were the most abundant ones in ‘Hainan Tall’ coconut flesh. Ultrasound treatment maintained the levels of syringic acid, catechin and epicatechin, while suppressing the expression of most associated genes. Correlation analysis revealed that, downregulation expressions of FLS, 4CL2, F3′5′H, CHS2 and CHS3 decreased kaempferol, isoliquiritigenin and luteoloside content but increased catechin content. Furthermore, downregulation of CSE, DFR and CHI reduced contents of luteolin, whereas downregulation of LAR and ANS elevated the contents of catechin and epicatechin. Unraveling the effect of ultrasound on phenolic biosynthesis in the tender coconut flesh at metabolite and transcript levels provides technical and theoretical support for the high-value development and utilization of Hainan coconut resources.
Keywords: Cocos nucifera L., RNA-Seq, Differentially expressed metabolites, Polyphenolic compounds, Pathway analysis
Highlights
-
•
A total of 36 phenolic compounds were identified in tender coconut flesh.
-
•
Ultrasonic treatment maintained the content of polyphenols.
-
•
Ultrasonic treatment inhibited the expression of most genes.
-
•
Genes of CSE, DFR, LAR and CHI were positively correlated with luteolin.
-
•
LAR and ANS were negatively correlated with catechin and epicatechin.
1. Introduction
Polyphenolic compounds encompass diverse and complex structures, primarily comprising phenolic acids, flavonoids, coumarins, and lignans (Jiang et al., 2021). The content and proportion of different types of polyphenols in plants vary significantly, depending on plant genotype, growth environment, soil type, and harvesting and storage conditions (Dias et al., 2021). Evidence indicates that polyphenols are widely distributed in agricultural products, including vegetables, fruits, grains, and nuts (Rodríguez-García et al., 2019), where they regulate plant growth, development, flower color, fruit flavor, and resistance to biotic or abiotic stress (Havsteen, 2002). Furthermore, polyphenols demonstrate well-documented health benefits, including antioxidant, anti-inflammatory, and anti-cancer activities (Akifumi et al., 2012; Arivalagan et al., 2018; Catalkaya et al., 2020).
The coconut palm (Cocos nucifera L.) is extensively utilized, with nearly all components possessing significant economic value (Lima et al., 2015). Tender coconut (6–9 months old), containing both water and flesh (endosperm), is globally sought after on the global market because of their high nutritional value and some potential therapeutic properties (Debmandal & Mandal, 2011). Tender coconut water is sweet and refreshing, it contains minerals, sugar and vitamins and has isotonic properties, so it can be used as fresh water or sports drink products (Hidalgo, 2017). Tender coconut flesh is nutritious and delicious, which can be eaten directly or used to make jams or coconut milk (Rina, & Novelina & Perdana, P. D., 2023; Zhang et al., 2020). Research confirms that polyphenol extracts from coconut endosperm can scavenge free radicals, protect DNA, and regulate the production of apoptotic genes and reactive oxygen species (Geetha et al., 2016; Radhakrishnan et al., 2016). However, fresh coconuts are highly perishable post-harvest, resulting in a short shelf life. Storage leads to perianth blackening, increases in amino acid, nucleotide and sugar metabolism, and a decline in total polyphenols within the water (Haseena et al., 2010; Shen et al., 2022). Given the direct connection between the liquid endosperm (water) and solid endosperm (flesh), quality deterioration in the flesh can directly impact the flavor of the water (Meethaworn et al., 2019; Shen et al., 2024).
Several pretreatment methods are employed to maintain the quality of post-harvest coconut. Among them, as an advanced non-thermal technology, ultrasound treatment has been widely applied owing to its safety, low cost, and environmental friendliness (Ashokkumar, 2015; Jiang et al., 2020). The primary mechanism of ultrasound is the cavitation effect, resulting from the sudden energy release during the generation, growth, and collapse of microscopic bubbles (Iqbal et al., 2019). During the cavitation process, instantaneous high pressure and temperature (1.01 × 108 Pa and 5000 K) are generated, inducing changes in protein structure and enzyme activity. In addition, ultrasound generates free radicals that disrupt enzyme-substrate interactions between the enzyme and the substrate. Research demonstrates that ultrasound damages the molecular structure and protein interaction of acid invertase, supporting its application for tender coconut preservation (Wu et al., 2021).
Recently, transcriptomic and metabolomic techniques have become essential for revealing quality changes in plants during growth and storage (Guo et al., 2023; Li, Jiang, Chen, & Jackson, 2021; Wang et al., 2024). For instance, in peach, ultraviolet-C irradiation promoted polyphenol accumulation by regulating genes such as CYP98A, COMT, CHS, CHI, and DFR (Han et al., 2023). Shen et al. (2022) reported that coconut shelf life at 25 °C is approximately 28 days, noting that down-regulation of sugars and up-regulation of organic acids likely contribute to taste deterioration of coconut water. In the present study, the regulatory mechanism of ultrasound on polyphenols in tender coconut flesh during post-harvest storage were comprehensively analyzed using integrated targeted metabolomics and transcriptomics. Unraveling the phenolics biosynthesis in the tender coconut flesh at the metabolite and molecular levels will provide technical and theoretical support for the enhanced utilization of Hainan coconut flesh resources.
2. Material and methods
2.1. Samples preparation
Mature (approximately 8-month-old) ‘Hainan Tall’ coconuts exhibiting uniform appearance and no surface damage or pests were selected for the experiment. Coconuts assigned to the ultrasound group (UG) were treated at 20 kHz and 2400 W for 20 min by an ultrasound processor (XC-3000, Jining Xinxin ultrasonic electronic equipment Co., Ltd., Jining, China) according to Wu et al. (2021), with slight modifications. Untreated coconuts served as the control group (CG). All samples were stored at room temperature (26 ± 1 °C, 70–75 % relative humidity) for 15 days. To elucidate the regulatory mechanism of ultrasound treatment on phenolic compound biosynthesis in tender coconut flesh during storage, targeted metabolomic and transcriptomic analyses determined metabolite levels and transcript expression on days 0, 6 and 15. At each time point, flesh from three coconuts was blended to form a pooled sample, with three biological replicates collected per storage time. After collection, tender coconut flesh was frozen in liquid nitrogen and stored at −80 °C.
2.2. Polyphenols extraction
Frozen tender coconut flesh was pulverized using a mixer mill (MM 400, Retsch, Haan, Germany). Subsequently, 100 mg of powder was weighed, and 0.5 mL of 80 % methanol solution (Thermo Fisher Scientific, Waltham, USA) (containing 0.2 % vitamin C (Yuanye Bio-Tech, Shanghai, China)) was added for ultrasonic extraction (40 kHz and 600 W) 30 min. The supernatant was obtained by centrifugation (16,099 ×g, 4 °C, 10 min). The extraction and centrifugation steps were repeated once. Combined supernatants were filtered through a 0.22-μm membrane prior to analysis.
2.3. Metabolomic analysis
LC conditions: Phenolic compounds in tender coconut flesh were performed on the Ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) system. Detailed standard information is provided in Supplemental Table S1. Separation was achieved on a Waters UPLC HSS T3 column (50 × 2.1 mm, 1.8 μm; Waters, Milford, USA), and maintained at 40 °C. mobile phase A was 0.1 % acetic acid water solution (Thermo Fisher Scientific, Waltham, USA), and mobile phase B was 0.1 % acetic acid acetonitrile solution (Thermo Fisher Scientific, Waltham, USA). The gradient elution stared with 90 % solvent A within 0–2 min, decreased linearly to 40 % over 6 min and hold for 3 min, and continued to increase to 90 % solvent A over 9.1 min, and hold for 2.9 min. The flow rate was 0.3 mL/min, and the injection volume was 2 μL.
MS conditions: Full MS/dd-MS2 mode was employed and with a primary scan range of 100–900 m/z. Key parameters were: sheath gas flow, 40 arb; auxiliary gas flow, 10 arb; ion spray voltage, −2800 V; capillary temperature, 320 °C; auxiliary gas heater temperature, 350 °C.
2.4. Transcriptome sequencing
2.4.1. RNA extraction, cDNA library construction and illumina sequencing
According to the manufacturer's procedure, total RNA was extracted from five pooled samples (CG0, CG6, UG6, CG15, UG15) of tender coconut flesh in triplicate by Trizol reagent (Thermo Fisher Scientific, Waltham, USA). The purity and integrity of total RNA were assessed using NanoDrop ND-1000 (NanoDrop, Wilmington, USA) and Bioanalyzer 2100 (Agilent, Santa Clara, USA). Samples with RNA Integrity Number (RIN) > 7.0 were selected for library construction. Then, Polyadenylated mRNA was isolated and purified using Dynabeads Oligo (dT) (Thermo Fisher Scientific, Waltham, USA). Subsequently, the mRNA fragments were performed with Magnesium RNA Fragmentation Module (NEBNext, Ipswich, USA) under high temperature conditions, and then followed by first-strand cDNA synthesis using SuperScript™ II Reverse Transcriptase (Invitrogen, Waltham, USA). E.coli DNA polymerase I (NEB, Ipswich, USA) and RNase H (NEB, Ipswich, USA) were used to synthesize Second-stranded DNA. Finally, paired-end sequencing (PE150) was conducted on the Illumina Novaseq™ 6000.
2.4.2. Transcriptome data analysis
Raw reads were processed by Cutadapt (https://cutadapt.readthedocs.io/en/stable/, version: cutadapt-1.9) and verified using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, 0.11.9) to obtain high-quality clean reads. Then, HISAT2 (https://daehwankimlab.github.io/hisat2/,version:hisat2-2.2.1) package was used to maps the clean reads to the Cocos nucifera L. reference genome, and transcript assembly from mapped reads was performed by StringTie (http://ccb.jhu.edu/software/stringtie/,version:stringtie-2.1.6). The expression abundance of transcripts and mRNAs was expressed by FPKM (fragments per kilogram of transcripts per million aligned reads) value.
Genes identified using thresholds of |log2FC| ≥ 1 and padj (an adjusted p value) < 0.05 were considered to differentially expressed genes (DEGs). Functional annotations of DEGs were assigned using Gene Ontology (GO) (https://geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations (https://www.kegg.jp/kegg/).
2.5. Correlation analysis between metabolome and transcriptome
To assess regulatory relationships of gene expression level on polyphenols synthesis, Pearson ‘s correlation coefficients (PCC) were calculated. Associations with |PCC| > 0.8 and p value less than 0.05 were utilized for the construction of transcript-metabolite network.
2.6. Statistical analysis
Data are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) was performed using SPSS Statistics 26 (IBM, Chicago, USA). Figures were created using Origin 2022 (OriginLab, Northampton, MA). Principal component analysis (PCA) was performed using SIMCA18.0.1 software (Sartorius Stedim Data Analytics AB, Umea, Sweden). The volcano plot and heat map analysis were performed by R software (https://www.r-project.org/).
3. Results
3.1. Correlation analysis of samples
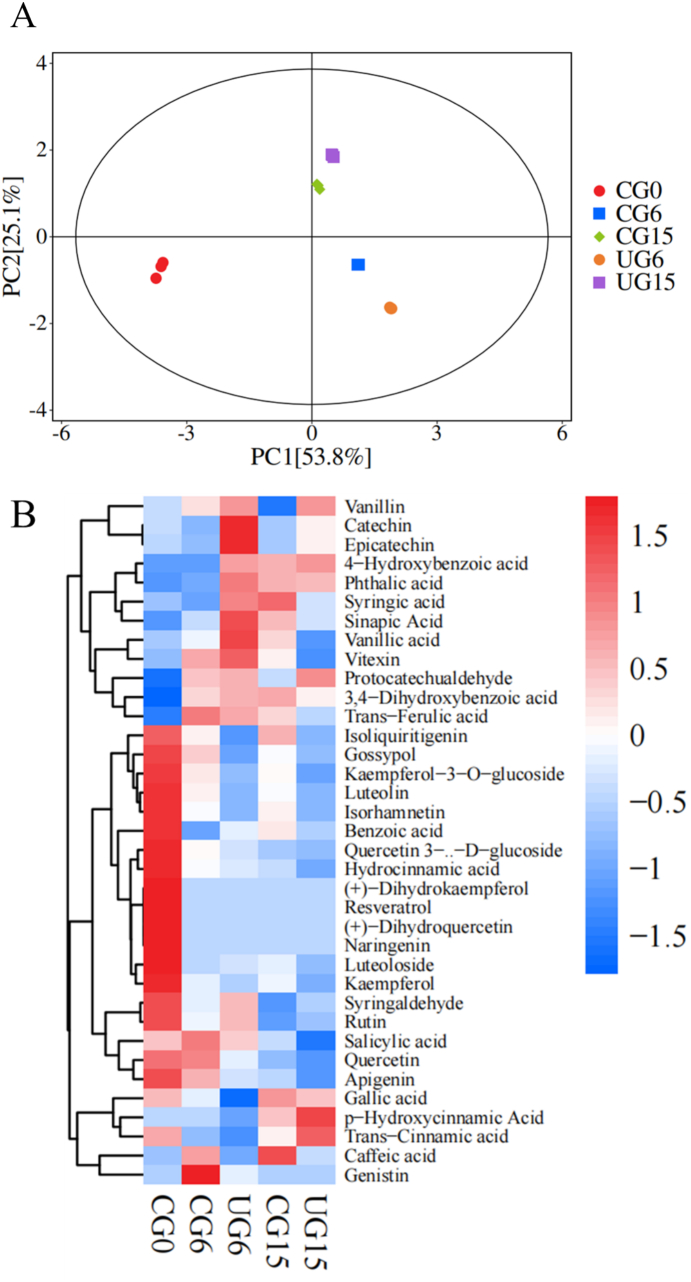
PCA, a dimensionality-reduction statistical method, transforms possible correlated variables into linear uncorrelated variables, thus revealing the internal structure and retaining the intrinsic data structure of the data. As shown in Fig. 1A, the five experimental sample groups could be well distinguished, and the high intragroup repeatability. The first principal component accounted for 53.8 %, and the second principal component occupied 25.1 %. All samples determined were within the 95 % confidence interval. This indicates that the sample meets the experimental requirements, and the experimental results were highly reliable.
Fig. 1.
The PCA score plot (A) and heatmap (B) of tender coconut meat during storage.
3.2. The composition and content of polyphenols
The UPLC-MS / MS analysis method was carried out to detect and quantify polyphenols in tender coconut flesh. A total of 36 polyphenols were detected and quantified during the storage of tender coconut flesh, and their contents and changes were shown in Table. 1. The content of catechin ranged from 435.10 to 2379.56 ng/g, which was the highest among 36 polyphenols, followed by epicatechin (279.57–1088.92 ng/g). The content of gossypol ranged between 187.81 ng/g and 530.90 ng/g. In addition, the contents of vanillic acid, caffeic acid, benzoic acid and syringic acid ranked 4th to 7th. However, the contents of isoliquiritigenin, vitexin, luteoloside and resveratrol were relatively low, with an average content of less than 1 ng/g.
Table 1.
Changes in the content of polyphenols in tender coconut meat during storage.
| Metabolites | CG0 | CG6 | UG6 | CG15 | UG15 |
|---|---|---|---|---|---|
| Gallic acid | 9.15 ± 0.22b | 6.37 ± 0.32c | 0.03 ± 0d | 10.43 ± 0.49a | 8.91 ± 0.97b |
| 3,4-Dihydroxybenzoic acid | 8.76 ± 0.43c | 12.21 ± 0.33ab | 12.72 ± 0.27a | 12.81 ± 0.21a | 11.78 ± 0.55b |
| Protocatechualdehyde | 5.63 ± 0.35c | 8.79 ± 0.48a | 9.11 ± 0.23a | 7.54 ± 0.96b | 9.47 ± 0.26a |
| 4-Hydroxybenzoic acid | 17.82 ± 1.34b | 17.68 ± 0.08b | 33.88 ± 1.14a | 32.49 ± 1.36a | 34.31 ± 3.55a |
| Phthalic acid | 16.63 ± 0.4b | 17.32 ± 0.28b | 26.49 ± 0.47a | 24.55 ± 3.79a | 24.24 ± 2.86a |
| Catechin | 733.13 ± 21.25c | 435.1 ± 2.36e | 2379.56 ± 32.36a | 595.49 ± 35.48d | 1135.58 ± 33.52b |
| Vanillic acid | 132.9 ± 3.44c | 165.82 ± 20.73b | 261.13 ± 32.09a | 192.3 ± 5.86b | 99.92 ± 10.67d |
| Caffeic acid | 112.98 ± 0.79d | 266.53 ± 3.54b | 82.68 ± 5.35e | 336.51 ± 28.73a | 141.79 ± 5.26c |
| Syringic acid | 40.16 ± 0.99b | 17.62 ± 0.29c | 136.12 ± 21.74a | 149.86 ± 13.11a | 59.84 ± 0.85b |
| Epicatechin | 386.48 ± 22.1c | 279.57 ± 3.69d | 1088.92 ± 90.87a | 332.57 ± 10.99 cd | 578.05 ± 33.5b |
| Vanillin | 9.07 ± 0.19b | 10.07 ± 1.15ab | 10.92 ± 0.74a | 7.19 ± 0.76c | 10.94 ± 1.31a |
| p-Hydroxycinnamic Acid | 5.57 ± 1.31a | 5.53 ± 0.79a | 5.01 ± 2.25a | 6.4 ± 1.51a | 7.3 ± 1.45a |
| Syringaldehyde | 5.51 ± 0.43a | 2.9 ± 0.23c | 4.17 ± 0.17b | 1.23 ± 0.08e | 2.27 ± 0.09d |
| Rutin | 4.32 ± 0.42a | 2.35 ± 0.05c | 3.19 ± 0.08b | 1.12 ± 0.17e | 1.64 ± 0.13d |
| Vitexin | 0.14 ± 0.02c | 0.42 ± 0.03b | 0.54 ± 0.05a | 0.3 ± 0.16b | 0.03 ± 0d |
| Trans-Ferulic acid | 50.21 ± 5.04d | 114.24 ± 3.49a | 104.88 ± 7.43b | 96.36 ± 3.27b | 75.8 ± 5.14c |
| Sinapic Acid | 25.62 ± 1.92d | 58.84 ± 2.64c | 133.47 ± 4.41a | 97.61 ± 3.44b | 62.28 ± 1.16c |
| Salicylic acid | 3.98 ± 0.14b | 4.43 ± 0.12a | 3.95 ± 0.4b | 3.37 ± 0.23c | 2.51 ± 0.07d |
| Luteoloside | 0.95 ± 0.21a | 0.42 ± 0.03c | 0.63 ± 0.04b | 0.81 ± 0.07ab | 0.03 ± 0d |
| Quercetin 3-β-D-glucoside | 18.96 ± 0.71a | 11.22 ± 0.34b | 9.52 ± 1.47c | 8.03 ± 0.35d | 7.33 ± 0.31d |
| Genistin | 0.03 ± 0c | 12.12 ± 0.31a | 1.96 ± 0.31b | 0.03 ± 0c | 0.03 ± 0c |
| (+)-Dihydroquercetin | 12.86 ± 0.2a | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b |
| Benzoic acid | 206.91 ± 19.11a | 124.25 ± 3.41d | 150.49 ± 5.78bc | 160.9 ± 6.19b | 139.21 ± 4.23 cd |
| Kaempferol-3-O-glucoside | 7.38 ± 0.36a | 5.67 ± 0.24b | 4.45 ± 0.42c | 5.46 ± 0.31b | 4.11 ± 0.23c |
| (+)-Dihydrokaempferol | 3.72 ± 0.16a | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b |
| Resveratrol | 0.53 ± 0.01a | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b |
| Luteolin | 6.24 ± 1.5a | 3.39 ± 0.17b | 1.73 ± 0.06c | 3.16 ± 0.46b | 1.73 ± 0.17c |
| Quercetin | 38.31 ± 4.48a | 37.73 ± 1.92a | 32.53 ± 5.12ab | 29.79 ± 0.98b | 27.91 ± 1.23b |
| Hydrocinnamic acid | 5.72 ± 0.37a | 2.96 ± 0.22b | 2.64 ± 0.11bc | 2.4 ± 0.11c | 1.53 ± 0.19d |
| Trans-Cinnamic acid | 6.59 ± 0.38b | 3.83 ± 0.13d | 2.82 ± 0.97d | 5.43 ± 0.16c | 7.72 ± 0.86a |
| Naringenin | 1.3 ± 0.08a | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b | 0.03 ± 0b |
| Apigenin | 2.13 ± 0.03a | 1.64 ± 0.18b | 1.01 ± 0.19c | 0.91 ± 0.06c | 0.46 ± 0.04d |
| Kaempferol | 6.68 ± 0.44a | 3.21 ± 0.3b | 2.53 ± 0.26c | 3.3 ± 0.28b | 1.77 ± 0.2d |
| Isorhamnetin | 6.6 ± 0.47a | 3.2 ± 0.21b | 1.54 ± 0.18c | 3.5 ± 0.01b | 1.61 ± 0.2c |
| Isoliquiritigenin | 0.46 ± 0.02a | 0.25 ± 0.02c | 0.03 ± 0e | 0.34 ± 0.04b | 0.08 ± 0d |
| Gossypol | 530.9 ± 23.44a | 387.08 ± 1.45b | 187.81 ± 2.05e | 324.83 ± 21.74c | 229.57 ± 22.2d |
Data were expressed as the mean ± standard deviations, and different letters represent significant differences (p < 0.05).
Through the heatmap analysis, 36 polyphenols can be divided into three groups according to the changing trend (Fig. 1B). There were 12 polyphenols in group I, which were up-regulated during storage. Group II contained 19 compounds, of which content showed a downward trend. The left 5 compounds were listed in Group III, which had fluctuating trend and showed no obvious change rules.
The differentially expressed metabolites (DEMs, p < 0.05) were screened for volcano plots and heatmaps analysis, and 22 and 24 DEMs were screened on the 6th and 15th days, respectively (Fig. 2). On the 6th day, the contents of syringic acid, benzoic acid, 4-hydroxybenzoic acid, phthalic acid, sinapic acid, luteoloside, vanillic acid and vitexin significantly higher in UG versus CG. Moreover, ultrasound significantly up-regulated the contents of vanillin, trans-cinnamic acid and protocatechualdehyde on day 15. Catechin, syringaldehyde, epicatechin, and rutin were consistently upregulated on day 6 and 15. Among them, the content of syringic acid changed the most, 7.7-fold versus CG. Followed by catechin and epicatechin, which were 5.5 times and 4 times than CG, respectively.
Fig. 2.
The volcano plots and heatmaps of DEMs between UG and CG during storage.
3.3. Transcriptome analysis
To further understand the metabolic changes of tender coconut flesh, the cDNA libraries of CG0, CG6, UG6, CG15 and UG15 were constructed and sequenced, yielded 95.57 Gb clean data, and the GC content ranged from 46.5 % to 48.5 % in 15 samples (Supplemental Table S2). The Phred-like quality score of Q20 (a valid ratio of 99 %) ranged from 99.69 % to 99.79 %, and the Q30 (a valid ratio of 99.9 %) ranged from 97.9 % to 98.4 %. This indicates that the constructed transcriptome library has good sequencing quality and could meet the requirements of subsequent analysis.
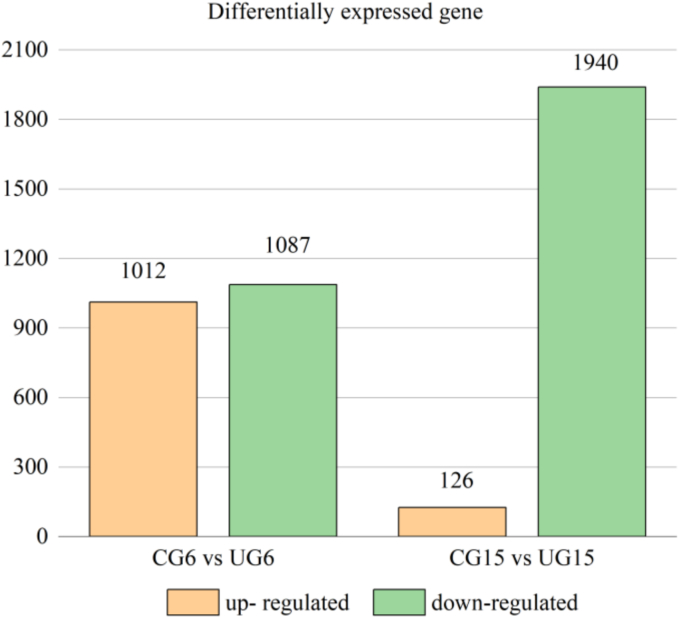
On the 6th day, a total of 2099 DEGs were screened from tender coconut flesh. Among them, the up-regulated and down-regulated DEGs were 1012 and 1087, respectively (Fig. 3). Interestingly, most genes in the UG showed a downward trend on the 15th day (93.9 %). This suggesting ultrasound-induced suppression of physiological processes by ultrasound treatment at the end of storage.
Fig. 3.
The up- regulated and down-regulated DEGs of tender coconut meat at day 6 and 15.
GO enrichment analysis was performed on functional annotation of DEGs, and the significantly enriched GO items (Q value) of DEGs were shown in Fig. 4. On the 6th day, terms such as cell wall organization (GO:0071555, 31 upregulated, 9 downregulated), xyloglucan metabolic process (GO:0010411, 16 upregulated, 1 downregulated) and cellulose biosynthetic process (GO:0030244, 14 upregulated, 2 downregulated) were significantly enriched in biological process. In the cellular component category, the most abundant subcategory was plasma membrane (GO:0005886, 166 upregulated, 155 downregulated), followed by extracellular region (GO:0005576, 82 upregulated, 34 downregulated). In addition, kinase activity (GO:0016301, 47 upregulated, 38 downregulated) and hydrolase activity, hydrolyzing O-glycosyl compounds (GO:0004553, 19 upregulated, 14 downregulated) were significantly enriched in molecular function. Different from day 6, almost all DEGs in significantly enriched terms were down-regulated on day 15, indicating that ultrasound treatment inhibited the expression of most genes.
Fig. 4.
GO enrichment of DEGs between UG and CG of tender coconut meat at day 6 (A) and 15 (B).
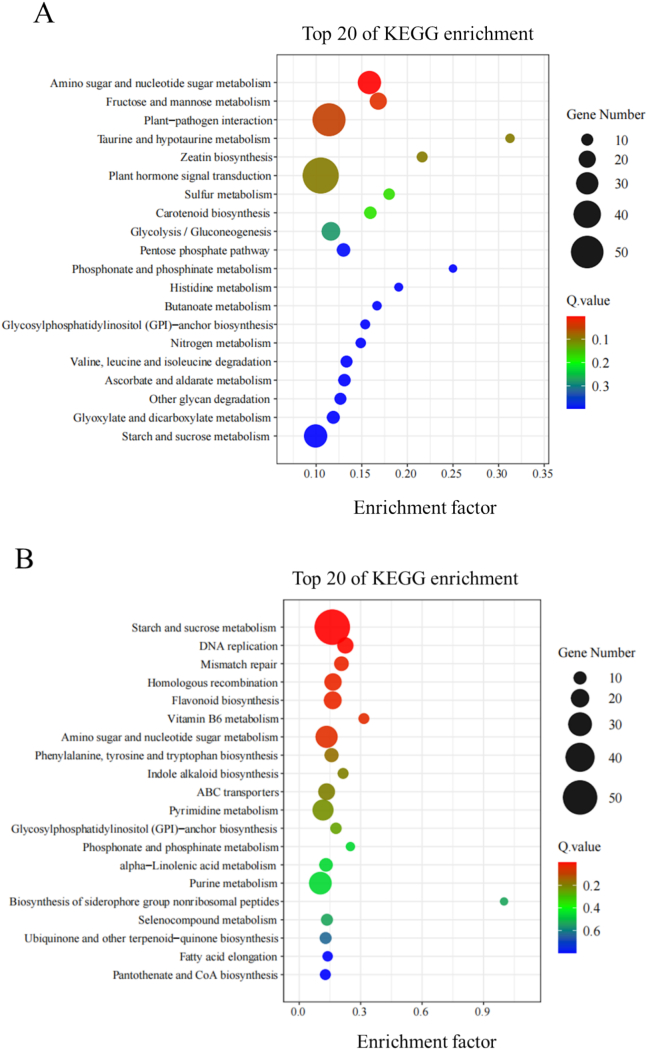
Pathway annotation of DEGs was performed by KEGG enrichment pathway analysis, and the significantly enriched pathways are shown in Fig. 5. It can be seen that amino sugar and nucleotide sugar metabolism, fructose and mannose metabolism, starch and sucrose metabolism and flavonoid biosynthesis pathways were significantly enriched. Fourteen phenylpropanoid/flavonoid biosynthesis-related genes were identified, including F3H, two 4CLs, F3′5′H, CYP73A, two CHSs, CHI, FLS, LAR, CSE, DFR, ANS and COMT.
Fig. 5.
KEGG pathway classification of DEGs between UG and CG of tender coconut meat at day 6 (A) and 15 (B).
3.4. Correlation analysis of metabolome and transcriptome
By integrating multi-omics analysis, the metabolite-gene interaction network and polyphenol biosynthetic pathway were constructed (Fig. 6, Fig. 7). The down-regulation of FLS, 4CL2, F3′5′H, CHS2, CHS3 and ANS resulted the decrease of kaempferol, isoliquiritigenin and luteoloside and the increase of catechin. CSE, DFR, LAR and CHI were exhibited positive correlations with luteolin but negative correlations with epicatechin. 4CL1 positively regulated sinapic acid, while F3H was negatively correlated with sinapic acid (Fig. 7). Down-regulation of COMT decreased the contents of luteolin, while the down-regulated expressions of CYP73A increased the contents of trans-cinnamic acid (Fig. 6). On day 6, ultrasound treatment enhanced the expression of genes 4CL1, CHS2 and ANS. However, almost all genes associated with phenylpropanoid and flavonoid biosynthesis were repressed on day 15.
Fig. 6.
The pathway associated with polyphenols biosynthesis in tender coconut meat.
Fig. 7.
Connection network between DEGs and DEMs.
4. Discussion
Polyphenols have garnered significant research interest because of their potent antioxidant activity, which underpins their anti-inflammatory, anti-tumor, anti-allergic and other beneficial health effects (Afnan Saleem et al., 2022). At the same time, coconut flesh is rich in polyphenols, which may indirectly regulate the quality of coconut water. Previous studies identified gallic acid, p-coumaric acid, salicylic acid and caffeic acid are the predominant polyphenols of Thai aromatic coconut cv. Nam Hom (Mahayothee et al., 2016). In this study, 36 polyphenols were found in the flesh of ‘Hainan Tall’ coconuts. Among them, catechin, epicatechin, gossypol and vanillic acid were the most crucial ones. These compositional differences likely stem from variations in cultivar, maturity, and cultivation conditions.
Ultrasound treatment is a non-thermal physical sterilization technology, which can protect the nutrients in fruits and vegetables and prolong the shelf life. Lagnika et al. (2013) showed that ultrasound treated white mushrooms had lower polyphenol oxidase activity, better preserving the content of phenolics. Moreover, the combination of ultrasound and ultraviolet-C radiation was useful to improve the bioactive substances and antioxidant capacity of tomatoes during storage (Esua et al., 2019). Wu et al. (2021) found that during the storage of tender coconut, ultrasound could inhibit acid invertase and did not affect the content of volatile substances in coconut water, which suggesting its utility for tender coconut preservation. In this study, ultrasound treatment effectively maintained the contents of syringic acid, catechin and epicatechin, which may be due to the denaturation of polyphenol oxidase and peroxidase by ultrasound (Rajashri et al., 2020).
Integrated multi-omics analysis elucidated the phenolics biosynthesis pathway and interaction network (Fig. 6, Fig. 7). Phenylalanine could synthesize phenolic acids through shikimic acid pathway, and it could be converted into trans-cinnamic acid by PAL and PTAL (Wang et al., 2018). CYP73A hydroxylates trans-cinnamic acid to p-coumaric acid, which CSE then converts to caffeic acid. Subsequently, COMT catalyzes the conversion of caffeic acid to ferulic and sinapic acids. Down-regulation of CSE and COMT reduced ferulic acid, caffeic acid and sinapic acid (Fig. 6, Fig. 7).
In addition, 4CL catalyzes p-coumaric acid to p-coumaroyl-CoA, a key intermediate linking phenylpropanoid metabolism pathway and lignin biosynthesis pathway in plants. In the present study, 4CL1 positively correlated with sinapic acid, vitexin, luteolin and luteoloside, while 4CL2 negatively regulated catechin, epicatechin, rutin and trans-cinnamic acid. Chalcone synthase (CHS) utilizes p-coumaroyl-CoA to form isoliquiritigenin and naringenin chalcone (Zhang et al., 2017). CHS2 and CHS3 were positively correlated with kaempferol and luteoloside, while negatively regulated catechin. Through CHI or spontaneous isomerization, naringenin chalcone is isomerized to naringenin. In arabidopsis, CHI promotes the accumulation of flavonoids (Jiang et al., 2015). Flavone synthase I (FNSI) converts naringenin and eriodictyol to apigenin and luteolin (Zuk et al., 2019).
Flavanone 3-hydroxylase (F3H) converts naringenin and eriodictyol to dihydrokaempferol and dihydroquercetin, which were further form kaempferol and quercetin by FLS (Park et al., 2020; Wang et al., 2020). F3′5′H catalyzes the conversion of kaempferol to quercetin. DFR can catalyze dihydroquercetin to generate leucocyanin, is the key enzyme of anthocyanin and proanthocyanidin synthesis (Kenjiro et al., 2017). Leucocyanidin is converted to catechin by leucoanthocyanidin reductase (LAR) or to anthocyanins by anthocyanidin synthase (ANS); anthocyanins are subsequently reduced to epicatechin by anthocyanidin reductase (ANR) (Yan et al., 2021). Interestingly, the expressions of LAR and ANS were down-regulated, while catechin and epicatechin were accumulated. This maybe the reduction of their conversion to other substances or the existence of another synthetic pathways. The synthesis of polyphenols involves complex regulatory networks, and our findings delineate this network of polyphenols in tender coconut flesh, providing a significant foundation for subsequent scientific research and application.
5. Conclusion
A total of 36 polyphenols were identified in tender coconut flesh in our research, with catechin, epicatechin, gossypol, and vanillic acid being the predominant compounds. Ultrasound treatment maintained polyphenol content and suppressed the expression of most genes by the end of storage. Correlation analysis revealed that down-regulation of FLS, 4CL2, F3′5′H, CHS2 and CHS3 correlated with reduced levels of kaempferol, isoliquiritigenin and luteoloside, alongside elevated catechin. CSE, DFR and CHI exhibited positive associations with luteolin, whereas LAR and ANS showed negative correlations with catechin and epicatechin. In conclusion, this study revealed that ultrasound treatment could regulate the biosynthesis of phenolic compounds at metabolite and molecular levels in tender coconut flesh, which will broaden our understanding of coconut polyphenols and establishing a foundation for subsequent research.
CRediT authorship contribution statement
Cheng Fang: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Xie Huang: Methodology, Investigation, Data curation. Yanpei Huang: Methodology, Investigation, Data curation. Ming Zhang: Writing – review & editing, Supervision, Project administration, Funding acquisition. Haiming Chen: Investigation, Formal analysis, Data curation. Weijun Chen: Investigation, Formal analysis, Data curation. Qiuping Zhong: Supervision. Jianfei Pei: Software, Resources. Ying Lv: Supervision. Rongrong He: Software, Resources. Wenxue Chen: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (32201977) and Key Laboratory of Tropical Fruits and Vegetables Quality and Safety for State Market Regulation (TDYJ-2024001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2025.100289.
Contributor Information
Ming Zhang, Email: m.zhang@hainanu.edu.cn.
Wenxue Chen, Email: chwx@hainanu.edu.cn.
Appendix A. Supplementary data
Supplementary material
Supplementary material
Data availability
Data will be made available on request.
References
- Afnan Saleem A., Akhtar M.F., Sharif A., Akhtar B., Siddique R., Ashraf G.M.…Alharthy S.A. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules. 2022;27(21):7286. doi: 10.3390/molecules27217286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akifumi A., Hiroshi Y., Yoshiko K., Shozo K. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta. 2012;236(4):1067–1080. doi: 10.1007/S00425-012-1650-X. [DOI] [PubMed] [Google Scholar]
- Arivalagan M., Roy T.K., Yasmeen A.M., Pavithra K.C., Jwala P.N., Shivasankara K.S.…Kanade S.R. Extraction of phenolic compounds with antioxidant potential from coconut (Cocos nucifera l.) testa and identification of phenolic acids and flavonoids using uplc coupled with tqd-ms/ms. Lwt. 2018;92:116–126. doi: 10.1016/j.lwt.2018.02.024. [DOI] [Google Scholar]
- Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrasonics - Sonochemistry. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Catalkaya G., Venema K., Lucini L., Rocchetti G., Delmas D., Daglia M.…Capanoglu E. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Frontiers. 2020;1(2):109–133. doi: 10.1002/fft2.25. [DOI] [Google Scholar]
- Debmandal M., Mandal S. Coconut (Cocos nucifera l.: Arecaceae): In health promotion and disease prevention. Asian Pacific Journal of Tropical Medicine. 2011;4(3):241–247. doi: 10.1016/S1995-7645(11)60078-3. [DOI] [PubMed] [Google Scholar]
- Dias M.C., Pinto D.C.G.A., Silva A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules. 2021;26(17):5377. doi: 10.3390/molecules26175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esua O.J., Chin N.L., Yusof Y.A., Sukor R. Effects of simultaneous uv-c radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chemistry. 2019;270:113–122. doi: 10.1016/j.foodchem.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Geetha V., Bhavana K.P., Chetana R., Gopala K.A., Suresh K.G. Studies on the composition and in-vitro antioxidant activities of concentrates from coconut testa and tender coconut water. Journal of Food Processing & Technology. 2016;07(05) doi: 10.4172/2157-7110.1000588. [DOI] [Google Scholar]
- Guo H., Li C., Lai J., Tong H., Cao Z., Wang C., Zhao W., He L., Wang S., Yang J., Long T. Comprehensive analysis of metabolome and transcriptome reveals the regulatory network of coconut nutrients. Metabolites. 2023;13(6):683. doi: 10.3390/metabo13060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Cai H., Yu H., Yu Z., Wu Y. Targeted metabolomic and transcriptomic reveal the regulation of uv–c on phenolics biosynthesis of peach fruit during storage. Lwt. 2023;190 doi: 10.1016/j.lwt.2023.115573. [DOI] [Google Scholar]
- Haseena M., Kasturi Bai K.V., Padmanabhan S. Post-harvest quality and shelf-life of tender coconut. Journal of Food Science and Technology. 2010;47(6):686–689. doi: 10.1007/s13197-010-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen B.H. The biochemistry and medical significance of the flavonoids. Pharmacology and Therapeutics. 2002;96(2):67–202. doi: 10.1016/S0163-7258(02)00298-X. [DOI] [PubMed] [Google Scholar]
- Hidalgo H.A. Market potential of pasteurized coconut water in the philippine beverage industry. International Journal on Advanced Science, Engineering and Information Technology. 2017;7(3):898–903. doi: 10.18517/ijaseit.7.3.1897. [DOI] [Google Scholar]
- Iqbal A., Murtaza A., Hu W., Ahmad I., Ahmed A., Xu X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food and Bioproducts Processing. 2019;117:170–182. doi: 10.1016/j.fbp.2019.07.006. [DOI] [Google Scholar]
- Jiang N., Zhu H., Liu W., Fan C., Jin F., Xiang X. Metabolite differences of polyphenols in different litchi cultivars (litchi chinensis sonn.) based on extensive targeted metabonomics. Molecules. 2021;26(4):1181. doi: 10.3390/molecules26041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Zhang M., Xu B. Application of ultrasonic technology in postharvested fruits and vegetables storage: A review. Ultrasonics Sonochemistry. 2020;69 doi: 10.1016/j.ultsonch.2020.105261. [DOI] [PubMed] [Google Scholar]
- Jiang W., Yin Q., Wu R., Zheng G., Liu J., Dixon R.A., Pang Y. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in arabidopsis thaliana. Journal of Experimental Botany. 2015;66(22):7165–7179. doi: 10.1093/jxb/erv413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenjiro K., Rintaro S., Wataru T., Noritoshi I., Toshimasa Y., Tomomi H.…R, W. A., Kiyoshi, F., & Katsuhiro, M. A new buckwheat dihydroflavonol 4-reductase (dfr), with a unique substrate binding structure, has altered substrate specificity. BMC Plant Biology. 2017;17(1):239. doi: 10.1186/s12870-017-1200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnika C., Zhang M., Mothibe K.J. Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biology and Technology. 2013;82:87–94. doi: 10.1016/j.postharvbio.2013.03.006. [DOI] [Google Scholar]
- Li X., Jiang J., Chen Z., Jackson A. Transcriptomic, proteomic and metabolomic analysis of flavonoid biosynthesis during fruit maturation in rubus chingii hu. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.706667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima E.B.C., Sousa C.N.S., Meneses L.N., Ximenes N.C., Júnior M.A.S., Vasconcelos G.S.…Vasconcelos S.M.M. Cocos nucifera (l.) (Arecaceae): A phytochemical and pharmacological review. Brazilian Journal of Medical and Biological Research. 2015;48(11) doi: 10.1590/1414-431X20154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahayothee B., Koomyart I., Khuwijitjaru P., Siriwongwilaichat P., Nagle M., Müller J. Phenolic compounds, antioxidant activity, and medium chain fatty acids profiles of coconut water and flesh at different maturity stages. International Journal of Food Properties. 2016;19(9):2041–2051. doi: 10.1080/10942912.2015.1099042. [DOI] [Google Scholar]
- Meethaworn K., Luckanatinwong V., Zhang B., Chen K., Siriphanich J. Off-flavor caused by cold storage is related to induced activity of lox and hpl in young coconut fruit. Lwt. 2019;114 doi: 10.1016/j.lwt.2019.108329. [DOI] [Google Scholar]
- Park S., Kim D.-H., Yang J.-H., Lee J.-Y., Lim S.-H. Increased flavonol levels in tobacco expressing acfls affect flower color and root growth. International Journal of Molecular Sciences. 2020;21(3):1011. doi: 10.3390/ijms21031011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan D., Chacko S., Bhaskara P., Sandya S., Govindan N. Polyphenolic extract from coconut kernel modulates apoptotic genes, reactive oxygen species production, and prevents proliferation of human colon cancer cell line. International Journal of Clinical and Experimental Physiology. 2016;3(3):113–121. doi: 10.4103/2348-8832.191585. [DOI] [Google Scholar]
- Rajashri K., Roopa B.S., Negi P.S., Rastogi N.K. Effect of ozone and ultrasound treatments on polyphenol content, browning enzyme activities, and shelf life of tender coconut water. Journal of Food Processing and Preservation. 2020;44(3) doi: 10.1111/jfpp.14363. [DOI] [Google Scholar]
- Rina Y., Novelina, & Perdana, P. D. The effect of citric acid addition on physicochemical and organoleptic characteristics of young coconut flesh (Cocos nucifera, l.) and butterfly pea (clitoria ternatea) sheet jam. IOP Conference Series: Earth and Environmental Science. 2023;1177(1) doi: 10.1088/1755-1315/1177/1/012033. [DOI] [Google Scholar]
- Rodríguez-García C., Sánchez-Quesada C., Gaforio J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants. 2019;8(5):137. doi: 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Wang Y., Ran L., Liu R., Sun X., Hu L., Chen F. Flavor deterioration of liquid endosperm in postharvest tender coconut revealed by lc-ms-based metabolomics, gc-ims and e-tongue. Postharvest Biology and Technology. 2022;187, Article 111866 doi: 10.1016/j.postharvbio.2022.111866. [DOI] [Google Scholar]
- Shen X., Xiong F., Niu X., Gong S., Sun X., Xiao Y., Yang Y., Chen F. Molecular mechanism of quality changes in solid endosperm of tender coconut during room temperature storage based on transcriptome and metabolome. Food Chemistry. 2024;436 doi: 10.1016/j.foodchem.2023.137615. [DOI] [PubMed] [Google Scholar]
- Wang H., Gang H., Chen J., Liu J., Zhang X., Fu C., Shao K., Wang X., Qin D., Huo J. Transcriptomic and metabolomic analyses reveal molecular and metabolic regulation of anthocyanin biosynthesis in three varieties of currant. Food Research International. 2024;196 doi: 10.1016/j.foodres.2024.115056. [DOI] [PubMed] [Google Scholar]
- Wang L., Lui A.C.W., Lam P.Y., Liu G., Godwin I.D., Lo C. Transgenic expression of flavanone 3-hydroxylase redirects flavonoid biosynthesis and alleviates anthracnose susceptibility in sorghum. Plant Biotechnology Journal. 2020;18(11):2170–2172. doi: 10.1111/pbi.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharmaceutical Biology. 2018;56(1):465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen H., Chen W., et al. Effect of ultrasonic treatment on the activity of sugar metabolism relative enzymes and quality of coconut water. Ultrasonics Sonochemistry. 2021:79105780. doi: 10.1016/j.ultsonch.2021.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Pei X., Zhang H., Li X., Zhang X., Zhao M.…Zhao X. MYB-mediated regulation of anthocyanin biosynthesis. International Journal of Molecular Sciences. 2021;22(6):3103. doi: 10.3390/ijms22063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen H., Wang W., Jiao W., Chen W., Zhong Q.…Chen W. Characterization of volatile profiles and marker substances by hs-spme/gc-ms during the concentration of coconut jam. Foods. 2020;9(3):347. doi: 10.3390/foods9030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Carolina A., Colquhoun T.A., Liu C.J. A proteolytic regulator controlling chalcone synthase stability and flavonoid biosynthesis in arabidopsis. The Plant Cell. 2017;29(5):1157–1174. doi: 10.1105/tpc.16.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M., Szperlik J., Hnitecka A., Szopa J. Temporal biosynthesis of flavone constituents in flax growth stages. Plant Physiology and Biochemistry. 2019;142:234–245. doi: 10.1016/j.plaphy.2019.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Data Availability Statement
Data will be made available on request.