Abstract
Deep-ocean hydrothermal-vent environments are rich in heavy metals and metalloids and present excellent sites for the isolation of metal-resistant microorganisms. Both metalloid-oxide-resistant and metalloid-oxide-reducing bacteria were found. Tellurite- and selenite-reducing strains were isolated in high numbers from ocean water near hydrothermal vents, bacterial films, and sulfide-rich rocks. Growth of these isolates in media containing K2TeO3 or Na2SeO3 resulted in the accumulation of metallic tellurium or selenium. The MIC of K2TeO3 ranged from 1,500 to greater than 2,500 μg/ml, and the MIC of Na2SeO3 ranged from 6,000 to greater than 7,000 μg/ml for 10 strains. Phylogenetic analysis of 4 of these 10 strains revealed that they form a branch closely related to members of the genus Pseudoalteromonas, within the γ-3 subclass of the Proteobacteria. All 10 strains were found to be salt tolerant, pH tolerant, and thermotolerant. The metalloid resistance and morphological, physiological, and phylogenetic characteristics of newly isolated strains are described.
Selenium (Se), a naturally occurring element, is essential for biological systems at low concentrations and toxic at higher levels. Under aerobic conditions Se is present in several redox forms, including the elemental form Se; however, it exists predominantly in the high-valence toxic and soluble forms selenate (SeVIO42−) and selenite (SeIVO32−) (4). The roles of Se in the biosphere, both beneficial and detrimental, are gradually being determined (5), and it is becoming evident that microorganisms play a major role in the global Se cycle. It has been suggested that various species of bacteria reduce selenite to elemental selenium (insoluble and nontoxic) to eliminate the toxic character of this compound (31, 48). Selenite is also involved in anaerobic respiration of several species (Thauera selenatis, Sulfospirillum barnesii, Bacillus arsicoselenatis, and Bacillus selenitireducens) (27, 44).
About a quarter of a century ago, tellurium (Te) was considered almost an exotic element and was treated with certain diffidence by the most serious chemists. The impressive number of publications on Te compounds during the last few years shows that Te is now widely used in applied chemical reactions (37). The natural Te cycle, however, has not been investigated, and the role of microbes in this process has not yet been elucidated. Te has not been shown to be a biologically essential element thus far, and potassium tellurite in concentrations as low as 1 μg/ml is toxic to most microorganisms (42). In fact, tellurite has been employed as an antimicrobial agent in growth media (42) and, prior to the development of modern antibiotics, as a therapeutic agent for the treatment of leprosy, tuberculosis, dermatitis, cystitis, and eye infections (45).
Intrinsic low-level resistance to tellurite has been reported for some gram-positive bacteria; however, the mechanism of this resistance is still poorly understood (42). Resistance to tellurite in some gram-negative bacteria has been shown to be plasmid mediated (42, 52). In Escherichia coli the low-level basal resistance to tellurite is a result of a secondary function of nitrate reductase (1). Resistance to extremely high levels of tellurite has been reported to occur in members of the phototrophic purple non-sulfur bacterial family Rhodospirilliaceae (31, 32, 33) and in some members of the aerobic phototrophic bacteria, which have been shown to resist and reduce concentrations of tellurite as high as 2,700 μg/ml (55). Recently, a haloalkaliphilic archaeon, Natronococcus occultus, was also shown to be resistant to and to be able to reduce high levels of tellurite (36).
Although the exact mechanism of toxicity of selenite and tellurite is not understood, the toxicity of these oxyanions has been attributed to their strong oxidizing activity, which may interfere with basic cellular process (42). Reduction of tellurite by bacteria results in black colonies on agar plates and a black culture in liquid media due to the accumulation of intracellular crystals of elemental Te (31, 46). When grown in the presence of high levels of sodium selenite, cells accumulate intracellular deposits of red, amorphous, elemental Se, giving cultures an orange to dark-red color (31, 40).
There is a narrow concentration range between Se as an essential nutrient and as a toxic substance for animals and humans. While in some regions of the world the daily food intake is artificially supplemented with Se for health reasons, other regions are polluted with Se (22). In the San Joaquin Valley of California, bird malformations due to excess Se have been reported (34). Chemical detoxification of metal- and metalloid-polluted sites is very expensive and often results in secondary effects on the environment. In contrast, biological solutions can be developed. Te is relatively rare in the environment, but it can be found at high concentrations near waste discharge sites. Native elemental Te is uncommon (an abundance in the lithosphere of 2 × 10−7%), usually occurring in conjunction with elemental sulfur or as tellurides of lead, copper, silver, gold, and antimony (2). The extraction of Te is difficult because of its low content in natural ores, and Te compounds are usually obtained as by-products of metal-refining processes (20). Today, microbial bioremediation of toxic compounds and microbial concentration of metals from natural ores and from mining tailings with metal levels too low for smelting are becoming more popular (28). However, it is currently estimated that only about 1% of microbial species have been described and obtained in laboratory culture. Thus, a continuous search for new microbes with biotechnological and industrial potential is of high importance.
Volcanic and hydrothermal processes associated with the global mid-ocean ridge system support a potentially vast, complex ecosystem on and beneath the deep-ocean floor. Deep ocean hydrothermal vent fluids possess a wide range of chemical compositions (3). These fluids have been found to be enriched in metal sulfides, which include iron, copper, calcium, silicon, and zinc, as well as metalloids (3, 8). Whereas the average concentrations of Te (550 fmol/kg) and Se (1.9 nmol/kg) in the ocean are relatively low (24, 30), their concentrations around deep-ocean vents are significantly higher. The measured values for Se in the particle fraction from the vents at Guaymas Basin and from 21°N, East Pacific Rise, range from 15 to 103 nmol/kg (50, 51). The concentrations of Te and Se in sulfide rocks of the Galapagos Rift were measured as 111 and 569 μmol/kg, respectively (21). The high concentrations of metals and metalloids in fluids and mineral deposits surrounding the vents (3) may make them an ideal location for the isolation of metalloid-resistant microorganisms. In this study, we report on the isolation, enumeration, and the characterization of major physiological properties of several strains that resist and reduce high concentrations of tellurite and selenite. These bacteria were recovered from samples taken in the vicinity of deep-ocean hydrothermal vents located in the Main Endeavor Segment of the Juan de Fuca Ridge in the Pacific Ocean.
MATERIALS AND METHODS
Collection of samples.
Samples were obtained in June, 1998, on the research vessel Atlantis by using the deep-ocean-submersible vessel ALVIN at the Juan de Fuca Ridge's main Endeavor Field in the Pacific Ocean (47.57′N, 129.05′W), from depths of 2,000 to 2,200 m. The vent plume water collected in Niskin bottles, bacterial-film-like formations collected with a slurp gun, and pieces of sulfide rocks were obtained to isolate metalloid-reducing bacteria (Table 1).
TABLE 1.
General characteristics of isolated strains
| Strain | Color and type of colonies without metal addition | Color of colonies isolated on medium containing:
|
Cell morphology (size [μm]) | Site (depth of isolation) | |
|---|---|---|---|---|---|
| K2TeO3 | Na2SeO3 | ||||
| Te-1-1 | Opaque, creamy | Black | NAa | Short ovoid rod (0.7 × 1.0) or rod (0.8 × 2.8) | Bacterial-mat-like community, Melarie Summit, Main Endeavor Field (≈2,200 m) |
| Te-1-2 | Transparent, colorless | Black | NA | Curved rod (0.9 × 2.1), vibrio (0.7 × 2.0), or short spirillum (0.7 × 3.5) | Same as for strain Te-1-1 |
| Se-1-1-or | Opaque, creamy | NA | Orange | Short ovoid (0.9 × 1.4) or elongated (1.1 × 2.4) rods | Same as for strain Te-1-1 |
| Se-1-2-or | Opaque, creamy | NA | Red-orange | Short ovoid (0.7 × 1.0) or elongated (1.0 × 2.4) rods | Same as for strain Te-1-1 |
| Se-1-2-red | Opaque, creamy | NA | Bright red | Short (0.9 × 1.4) or slightly curved long (0.8 × 2.5) rods | Same as for strain Te-1-1 |
| Se-1-3-red | Transparent, creamy | NA | Bright red | Short ovoid (1.2 × 1.8) or elongated (1.0 × 2.7) rods | Same as for strain Te-1-1 |
| Te-2-1 | Transparent, colorless | Black | NA | Vibrio (0.8 × 2.4) or short spirillum (0.6 × 5.3) | Water beneath Lobo Flange, Main Endeavor Field (≈2,194 m) |
| Te-2-2 | Transparent, colorless | Black | NA | Vibrio (0.6 × 1.9) or short spirillum (0.7 × 3.2) | Same as for strain Te-2-1 |
| Se-6-1-or | Transparent, colorless | NA | Bright red | Short ovoid (0.7 × 1.2) or elongated (0.8 × 2.6) rods | Wash from sulfide rocks, Site Dante, Main Endeavor Field (≈2,186 m) |
| Se-6-2-red | Transparent, creamy | NA | Dark red | Short ovoid (1.0 × 1.5) or elongated (0.7 × 2.3) rods | Same as for strain Se-6-1-or |
NA, not applicable.
Isolation and enumeration of metalloid-resistant bacteria.
Isolation and enumeration of strains was performed using a solid (2% agar) medium (designated HM medium) that contained (in grams per liter) NaCl, 20; KH2PO4, 0.3; NH4Cl, 0.3; KCl, 0.3; CaCl2·2H2O, 0.05; Na-acetate, 1; Na-malate, 0.3; and yeast extract, 0.1. HM medium was supplemented with a mixture of vitamins ([per liter]: 20 μg of vitamin B12, 200 μg of nicotinic acid, 80 μg of biotin, and 400 μg of thiamine) and 1.0 ml of a trace element solution per liter (7). Filter-sterilized cysteine and methionine (both at 0.3 mM; 5 ml of each per liter of medium) were added after autoclaving of the basal medium. For selection of selenite- and tellurite-resistant bacteria, 100 μg of either sodium selenite or sodium tellurite (from filter-sterilized 10% solutions) per ml of medium was added. The pH was adjusted to 7.8.
Media used for subsequent cultivation.
Isolates were grown in D/O medium, which contains the following (grams per liter): KH2PO4, 0.3; MgSO4, 0.5; NH4Cl, 0.3; KCl, 0.3; CaCl2, 0.05; Na acetate, 1.0; yeast extract, 0.5; Casamino Acids, 0.5; and NaCl, 15.0. Media were adjusted to pH 7.8 to 8.0 and supplemented with a mixture of vitamins ([per liter]: 40 μg of vitamin B12, 400 μg of nicotinic acid, 160 μg of biotin, and 800 μg of thiamine) and 2.0 ml of a trace element solution per liter (7).
Plates (supplemented with 2% agar) and liquid cultures were incubated aerobically in the dark at 28°C. Physiological and biochemical tests were performed using D/O medium unless otherwise specified.
Morphology and cytology.
The Gram test was done by the method of Gregersen (14). The sizes and shapes of cells were determined by phase-contrast and electron microscopy of cells from cultures grown in D/O and HM media (100 μg of metal oxide per ml). The cytology of cells was investigated from thin sections examined in an electron microscope. For thin sections, the bacteria were embedded in Epon after fixation with 1% glutaraldehyde and 1% osmium tetroxide as described previously (18).
Resistance to and reduction of metalloid oxides.
Strains were grown on D/O medium plates overnight, and cells were transferred into HM medium to prepare a dense cell suspension. A loopful of cell suspension was deposited on plates of HM medium (supplemented with an appropriate volume of either 10% K2TeO3 or 10% Na2SeO3) and incubated for up to 6 days at 30°C in the dark. Resistance was determined by the appearance of growth and the reduction of oxyanions to their respective elemental forms, which was indicated by the color of the growth: grey to dark black in the case of tellurite reduction and orange to dark red in the case of selenite reduction. The MIC was defined as the lowest concentration of either K2TeO3 or Na2SeO3 that inhibited growth.
The viability of cells following exposure to high levels of either tellurite or selenite was determined after 5 days of incubation on plates containing 2,000 μg of K2TeO3 per ml or 2,000 μg of Na2SeO3 per ml by removing cells with a loop and streaking them on D/O medium plates.
The ability to grow anaerobically using either K2TeO3 or Na2SeO3 as a terminal electron acceptor for anaerobic respiration was tested in screw-cap tubes completely filled with HM medium containing appropriate concentrations of K2TeO3 or Na2SeO3. Optical density was measured using a Klett-Summerson photoelectric colorimeter before and after incubation at 30°C for 10 days. Anaerobic growth was also tested on HM medium plates incubated in an anaerobic jar containing a CO2-H2 headspace (BBL GasPak) at 30°C for 14 days, as well as on modified HM medium plates on which Na-acetate and malic acid were replaced by the addition of 1.0 g of glucose per liter.
Biochemical and physiological properties.
Physiological and biochemical tests were performed as previously described (56, 57). The ability to reduce nitrate was examined in tubes that were partially filled with D/O medium containing 0.1% KNO3 and that were incubated without shaking for 10 days. Following incubation, α-naphthylamine and sulfanilic acid were added to determine the presence of nitrite. If no nitrite was present, zinc dust was added to evaluate the presence of nitrate remaining in the medium. Tubes that contained inverted Durham vials were used to detect gas production (38).
To test for sugar fermentation, 0.1% of either glucose or fructose was added to D/O medium free of other organic carbon sources. Stationary tubes were incubated for 7 days, pH was measured to determine acid production, and inverted Durham vials were used to detect gas production.
Phylogenetic analysis.
Extraction of genomic DNA, PCR-mediated amplification of the 16S rRNA gene (rDNA), and direct sequencing of the purified PCR product were carried out as described previously (39). The 16S rDNA sequences were aligned with published sequences obtained from the EMBL Nucleotide Sequence Database (Cambridge, United Kingdom) and the Ribosomal Database Project, using the ae2 editor (29). Evolutionary distances were calculated and phylogenetic dendrograms were constructed as described previously (16, 6). Bootstrap analysis was used to evaluate the tree topology of the neighbor-joining data by performance of 500 resamplings (9).
Nucleotide sequence accession numbers.
The 16S rDNA sequences determined in this study were deposited in the EMBL database under the following accession numbers: AJ314843 for strain Te-1-1, AJ314842 for strain Te-2-2, AJ314844 for strain Se-1-2-or, and AJ314845 for strain Se-1-2-red.
RESULTS AND DISCUSSION
Isolation and enumeration.
Samples of hydrothermal-vent fluids were directly inoculated onto selective HM medium. Small pieces of sulfide rock samples were washed with sterile liquid base of HM medium in tubes using a vortex mixer, and the resultant suspensions were used to inoculate selective (metalloid-containing) plates. Plates were incubated at 30°C in the dark for a week. Tellurite-resistant (black-colored due to the accumulation of elemental Te) and selenite-resistant (orange- to red-colored due to the accumulation of elemental Se) colonies were subsequently streaked on metalloid-free HM medium plates to complete the purification of the isolates. Pure strains were used for further investigation. The tellurite-resistant (Te-2-1 and Te-2-2) and selenite-resistant (Se-6-1-or and Se-6-2-red) strains were obtained from these samples (Table 1).
Bacterial-film-like formations with surrounding vent fluid obtained at the Melarie Summit site were used to enumerate metalloid-resistant or -reducing bacteria present in this community (Table 1). Bacterial aggregates were initially homogenized and later inoculated as decimal dilutions onto selective HM agar plates and onto HM agar plates without metal addition (controls). After incubation, the total number of cells that grew on metalloid-free medium was compared with the number of metalloid-resistant cells that grew on HM-selenite and HM-tellurite media. The numbers of CFU obtained from control plates showed up to 60,000 CFU/ml of sample as white, creamy, and colorless bacteria. The medium selective for tellurite-resistant strains yielded black colonies, and plates selective for selenite-resistant strains gave rise to a variety of orange- and red-colored colonies (Fig. 1). The results of this enumeration indicated that the number of tellurite-resistant strains (1,200 CFU/ml) in the samples was much smaller than the number of selenite-resistant strains (the total of the numbers of CFU of all types of selenite-reducing strains was 14,240 per ml). We estimate that about 24% of strains able to grow on HM medium were selenite resistant and that only about 2% of the population were tellurite resistant. However, we have two reservations. On the one hand, as an alternative to genuine dissimilatory metal reduction (an anaerobic process), some bacteria are thought to use metal oxide reduction for the disposal of electrons (e.g., reoxidation of NADH, NADPH, FADH2, reduced cytochromes, and quinones), thereby maintaining an appropriate redox poise in vivo (31, 32). Therefore, it is possible that some metalloid-resistant strains grew on selective media only because growth was stimulated by the presence of the metalloid oxide. On the other hand, the newly isolated strains are phylogenetically related to described species of Pseudoalteromonas (see “Phylogenetic analysis” above). Some species of Pseudoalteromonas have been found to exhibit strong bacteriolytic activity against both gram-positive and gram-negative bacteria (41), which is a significant trait for bacteria living in oligotrophic environments. Because of this possible bacteriolytic activity in Pseudoalteromonas strains, it is possible that some gram-positive as well as gram-negative metalloid-resistant bacteria did not form colonies on the selective plates. Tellurite-resistant (Te-1-1 and Te-1-2) and selenite-resistant (Se-1-1-or, Se-1-2-or, Se-1-2-red, and Se-1-3-red) strains were isolated from the bacterial mat sample (Table 1).
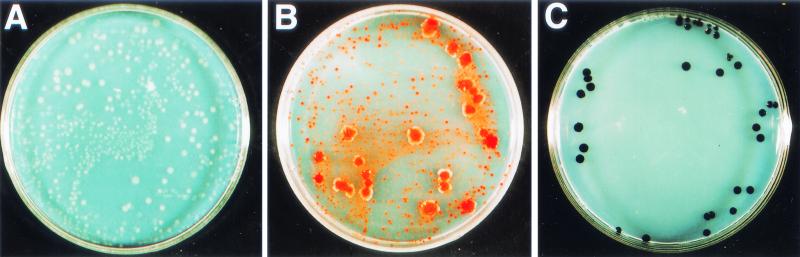
FIG. 1.
Plates used for the enumeration of metalloid-resistant or -reducing bacteria in bacterial-film-like formations taken from the Melarie Summit site, Main Endeavor Field. (A) Metalloid-free control plate. (B and C) Plates containing 100 μg of Na2SeO3 per ml and 100 μg of K2TeO3 per ml, respectively. The red-orange color of colonies (B) is due to the accumulation of elemental Se, and the black color (C) is due to the accumulation of elemental Te.
Morphology and cytology.
Four strains (Te-1-1, Te-1-2, Te-2-1, and Te-2-2) isolated on tellurite-containing selective HM agar plates produced black, circular, and lustrous colonies. Some of the strains isolated from Se-containing selective HM agar plates formed small, orange to red-orange colonies (Se-1-1-or, Se-1-2-or, and Se-6-1-or), whereas others formed bright-red to dark-red colonies (strains Se-1-2-red, Se-1-3-red, and Se-6-2-red). Growth of strains Te-1-1, Se-1-1-or, Se-1-2-or, and Se-1-2-red on D/O agar plates without metalloid addition produced opaque, creamy colonies. The colonies of strains Te-1-2, Te-2-1, Te-2-2, and Se-6-1-or were transparent and colorless, whereas the colonies of Se-1-3-red and Se-6-2-red were transparent and creamy (Table 1).
Colonies of two strains, Te-1-1 and Se-1-2-red, softened the agar and produced halos of clearing after 24 to 48 h of incubation at 30°C, indicating the production of an extracellular agarase. Extracellular agarase activity has been previously found in several bacterial strains from marine environments, including members of the genera Alteromonas and Pseudoalteromonas (15). It appears that the bacterial degradation of agar occurs by two mechanisms based on the specificities of the enzymes β- and α-agarase. Most of the reported agarolytic Pseudoalteromonas strains have been found to produce extracellular β-agarase, whereas a Pseudoalteromonas agarolyticus strain was reported to produce both α- and β-agarases (15). Because strains Te-1-1 and Se-1-2-red are phylogenetically related to Pseudoalteromonas, it would be of interest to determine which mechanism of agar hydrolysis is used by these strains.
Two distinct morphological types were identified when the isolated strains were grown in D/O medium without metalloid addition. One cluster (strains Te-1-1, Se-1-1-or, Se-1-2-or, Se-1-2-red, Se-1-3-red, Se-6-1-or, and Se-6-2-red) included bacteria which have rod-shaped cells. The sizes of rods in culture were different and seemed to reflect the age of the cell. The cells divided by binary fission. The mature mother cell, which was an elongated rod 1.0 × 2.4 to 0.8 × 2.8 μm in size, depending on the strain, produced after division two short ovoid rods (sometimes almost coccoid cells) 0.9 × 1.4 to 0.7 × 1.9 μm in size. Motility was found in two strains (Te-1-1 and Se-1-2-red), whereas motility was not observed in the other five strains of this cluster (Table 1). Another cluster (strains Te-1-2, Te-2-1, and Te-2-2) was represented by bacteria with vibrio (0.6 × 1.9 to 0.8 × 2.4 μm) or short spirillum (0.7 × 3.2 to 0.6 × 5.3 μm) morphology. As is characteristic of vibrios and spirilla, all strains of this cluster are motile.
The Gram test and ultrathin electron microscope sections indicated that all strains have a gram-negative cell wall. Phase-contrast and electron microscopy showed that most of the strains, excluding three vibrio-spirillum-type strains, Te-1-2, Te-2-1, and Te-2-2, produced capsule- or matrix-like extracellular compounds. Exopolysaccharide (EPS)-producing strains are common within the genera Pseudoalteromonas and Alteromonas. Interestingly, Alteromonas sp. strain HYD-1545, isolated from tube worms, produces an EPS containing acidic sugars, which were demonstrated to have heavy metal binding properties (49). This bacterium was suggested to be important for the survival of its host organism, which lives in an environment where the exposure to chemicals (e.g., toxic metalloid compounds) is high. It is generally thought that bacterial EPS may serve as protection against antibacterial substances, control bacterial attachment, protect against predation by protozoa, function as enhancers for nutrient uptake, and reduce the diffusion of substances into and out of cells (10, 12, 49, 53). However, the functional role as well as the chemical and structural compositions of the extracellular excretions in our deep ocean strains awaits future studies.
Interesting changes in morphology were observed in strains that make a rod-shaped cluster depending on growth conditions and the presence of metalloid oxides (Fig. 2; strain Se-1-2-red as an example). In liquid medium these strains produced separate, short, ovoid, and elongated rods, whereas on agar plates they tended to form chains (of up to 8 cells). The addition of selenite or tellurite to the HM medium provoked changes in cell morphology, the reduction of these oxides, accumulation of elemental Se or Te, and the development of red-orange or black color in colonies, respectively.
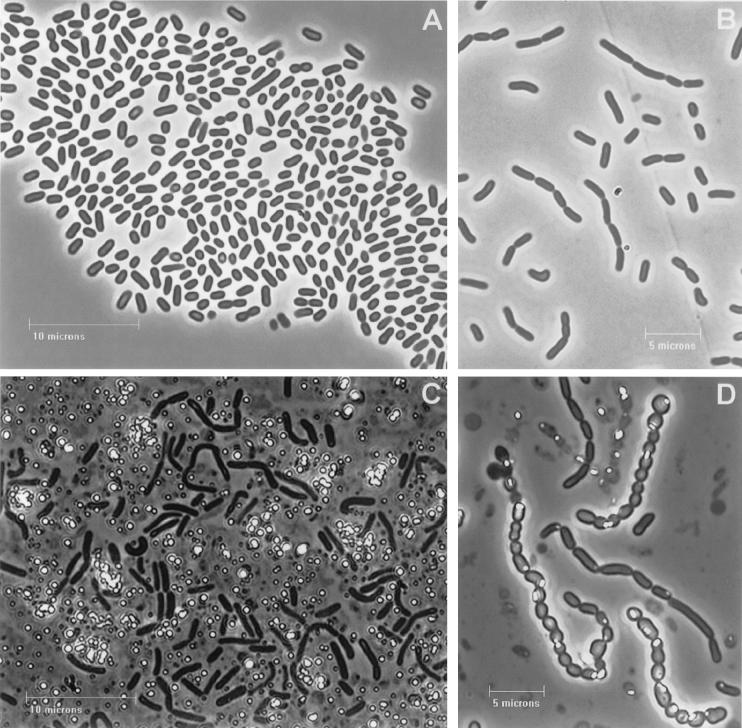
FIG. 2.
Strain Se-1-2-red under phase-contrast microscopy. (A) Cells grown in the absence of metalloids; (B) 48-h-old culture grown in the presence of tellurite; (C and D) 72-h-old cultures grown in the presence of selenite and tellurite, respectively. Most of the Se globules were excreted from cells and present as cell-free light-refractile particles (C), whereas most of the Te globules were located inside the cells (D).
During the first 48 h of growth in the presence of metalloids, light-orange (with Na2SeO3 added) or greyish (with K2TeO3 added) colors developed. The cells in chains became more elongated, and a few light-refractile globules of Te or Se were seen inside as well as outside the cells. After 72 h of growth, the colonies turned to bright orange or red (with K2SeO3 added) or lustrous black (with Na2TeO3 added). At this time, most of the Se was observed as cell-free particles (Fig. 2C). However, after 72 h in tellurite-reducing cultures, the cells in chains were much shorter and some were almost rounded, with most of the Te located inside the cells (Fig. 2D). The initial intracellular accumulation of Se and Te and the apparent later release of these particles by cells was also indicated in ultrathin electron microscope sections (Fig. 3). The three strains in the vibrio-spirillum cluster also reduced metalloid oxides and accumulated metalloid globules in the cytoplasm of the cell, with subsequent release but without noticeable changes in the morphology of cells.
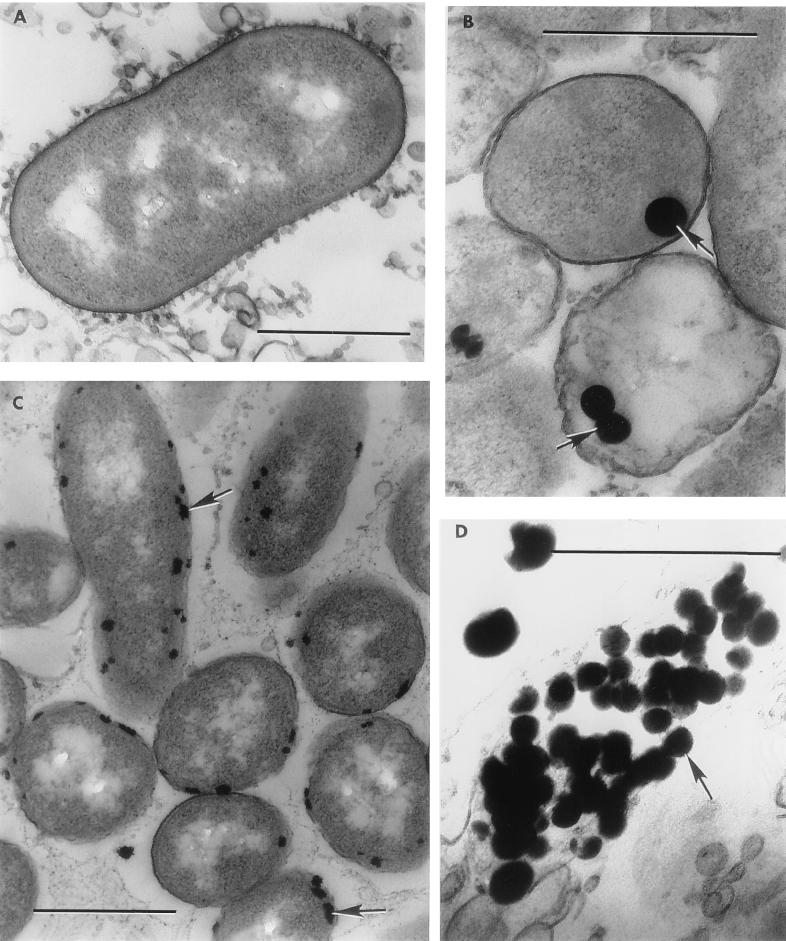
FIG. 3.
Electron microscopy of ultrathin sections. (A) Strain Se-1-2-red grown in metalloid-free medium; (B and C) intracellular localizations of Se (B) and Te (C), as reduction products of selenite and tellurite, in strains Se-1-2-red (48-h-old culture) and Te-1-1 (72-h-old culture), respectively; (D) granules of Se released from cells of Se-1-2-red (48-h-old culture). Bars: 0.5 μm.
Multiple detoxification processes may occur during selenite and tellurite reduction by microorganisms because elemental Se and Te have been described as deposits in the cytoplasm, in the periplasm, and outside the cell (13, 19, 25, 26, 43, 45, 46, 47, 54, 55, 58). According to Tomei et al. (47), particles containing elemental Se found outside cells are released by cell lysis, whereas Losi and Frankenberger (26) suggested that the reduction reaction occurs close to the membrane, possibly as a result of a membrane-associated reductase, and that the particles are rapidly expelled by a membrane efflux pump. Kessi et al. (19) speculated that a vesicular mechanism of Se excretion occurs in Rhodospirillum rubrum. Therefore, it will be a challenge to elucidate the mechanism of metal excretion in the metalloid reducers described above.
Metalloid oxide resistance and reduction.
Although the strains described in this paper were isolated using a selective medium that contained either sodium tellurite or sodium selenite, all isolates revealed high resistance to both metalloid oxides. All strains, when grown on plates containing moderate levels of K2TeO3 (100 μg/ml) grew well and reduced TeO32− to elemental Te, appearing as black colonies (Fig. 1C), and as electron-dense formations inside of cells in electron micrographs (Fig. 3C) (25, 31).
The ability to resist high levels of K2TeO3 as well as the ability to reduce tellurite to elemental Te varied among strains, as shown in Table 2. All strains except Te-1-1, Te-1-2, Se-1-2-or, and Se-6-1-or were resistant to concentrations of K2TeO3 as high as 2,500 μg/ml, although in all cases growth at concentrations above 2,000 μg/ml was suboptimal and the ability of cells to reduce large amounts of TeO32− appeared to be hindered. Attempts to increase the concentration of K2TeO3 above 2,500 μg/ml in the medium employed (pH 7.5 to 8.0) resulted in the formation of a white precipitate, and so higher concentrations were not tested.
TABLE 2.
Comparative physiological characteristics of the strains isolated from the Juan de Fuca Ridge
| Characteristic | Result for straina:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Te-1-1 | Te-1-2 | Te-2-1 | Te-2-2 | Se-1-1-or | Se-1-2-or | Se-6-1-or | Se-1-2-red | Se-1-3-red | Se-6-2-red | |
| Utilization of: | ||||||||||
| Acetate | + | + | + | + | W | − | W | − | W | − |
| Pyruvate | + | + | W | − | W | − | W | + | + | − |
| Glutamate | + | ++ | + | ++ | + | ++ | ++ | + | + | + |
| Butyrate | + | − | − | − | + | W | − | + | + | + |
| Citrate | + | − | − | + | + | W | W | + | + | − |
| Malate | − | − | − | + | + | + | + | + | + | + |
| Succinate | − | − | + | + | + | + | + | + | + | − |
| Lactate | − | − | + | + | + | − | ++ | + | + | + |
| Formate | − | − | − | − | − | − | − | − | − | − |
| Fructose | + | ++ | − | − | ++ | ++ | ++ | ++ | ++ | + |
| Glucose | + | + | + | ++ | ++ | ++ | ++ | + | ++ | ++ |
| Ethanol | − | − | − | − | + | + | + | ++ | + | + |
| Methanol | − | − | − | − | − | − | − | − | − | − |
| Yeast extract | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Hydrolysis of: | ||||||||||
| Starch | + | + | − | − | + | + | + | + | + | + |
| Gelatin | + | − | − | − | + | − | − | + | − | − |
| Tween 60 | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ |
| Agar | ++ | − | − | − | − | − | − | ++ | − | − |
| Antibiotic sensitivity to: | ||||||||||
| Chloramphenicol | + | + | + | + | + | + | + | + | + | + |
| Penicillin G | + | − | − | − | − | + | − | − | − | + |
| Streptomycin | + | + | + | + | − | − | + | + | − | − |
| Polymixin B | + | + | + | + | + | + | + | + | + | + |
| Tetracycline | + | + | − | − | − | + | + | + | − | − |
| Ampicillin | + | − | − | − | + | + | − | − | − | − |
| Kanamycin | + | + | + | + | − | + | + | + | − | + |
| Nalidixic acid | + | + | − | + | + | + | + | + | − | + |
| Reduction of metalloid oxyanions with: | ||||||||||
| K2TeO3 (μg/ml) | ||||||||||
| MIC | 1,500 | 2,000 | >2,500 | >2,500 | >2,500 | 2,000 | 1,500 | >2,500 | >2,500 | >2,500 |
| Maximum reduction | 1,300 | 1,500 | 2,000 | 2,000 | 1,300 | 1,300 | 1,300 | 1,300 | 1,300 | 2,000 |
| Na2SeO3 (μg/ml) | ||||||||||
| MIC | 7,000 | 6,000 | 7,000 | >7,000 | 5,000 | 6,000 | 6,000 | 7,000 | 6,000 | 6,000 |
| Maximum reduction | 5,000 | 5,000 | 6,000 | >7,000 | 2,000 | 4,000 | 4,000 | 5,000 | 4,000 | 4,000 |
+, substrate is utilized substrate is hydrolyzed or strain is antibiotic sensitive; ++, substrate is utilized for very good growth; −, substrate is not utilized, substrate is not hydrolyzed, or strain is antibiotic resistant W, weak growth. Maximum reduction is the concentration at which growth and the production of black coloration (in the case of strains grown in the presence of K2TeO3) or red coloration (in the presence of Na2SeO3) was optimal.
All strains grew well on plates containing Na2SeO3, up to a concentration of 5,000 μg/ml, and most strains showed high levels of SeO32− reduction, resulting in the accumulation of elemental Se at selenite concentrations as high as 4,000 μg/ml. Only strain Se-1-1-or was unable to reduce selenite in excess of 2,000 μg/ml. Strain Te-2-2 expressed the greatest resistance to SeO32−, growing well and showing high levels of reduction up to 7,000 μg of Na2SeO3 per ml, the highest concentration tested.
To determine the viability of cells after exposure to high concentrations of either K2TeO3 or Na2SeO3 (2,000 μg/ml) for 5 days, cells were removed from plates and streaked onto fresh D/O medium plates free of metalloids (31). The strains Te-2-1, Te-2-2, and Se-1-2-red (which all showed good growth and strong reduction at both 2,000 μg of K2TeO3 per ml and 2,000 μg of Na2SeO3 per ml) continued to grow when they were transferred to the metalloid-free medium, with no visible black or red color, indicating that cells remained viable despite the accumulation of elemental Te or Se.
Strain Te-1-2 (which gave poor growth on 2,000 μg of Na2SeO3 per ml) produced only a few colonies when it was transferred to the metalloid-free medium, indicating that although this concentration was toxic, some cells remained viable over the period of exposure.
Strain Se-6-1-or did not grow on plates containing 2,000 μg of K2TeO3 per ml, but still reduced a small amount of tellurite to elemental Te, resulting in a grey appearance of the inoculum. When transferred to metalloid-free plates, these cells formed only a few isolated colonies, indicating that some cells remained viable even after exposure to concentrations that inhibited growth.
The ability to use various oxyanions of Se as a terminal electron acceptor for anaerobic respiration has been described previously (23, 35). To evaluate the ability of our strains to anaerobically respire using either tellurite or selenite as an electron acceptor, tests were performed in both anaerobic tubes and on plates in an anaerobic jar. K2TeO3 and Na2SeO3 concentrations of 5, 100, and 1,000 μg/ml were tested. None of the strains was capable of growth under anaerobic conditions. However, when grown in anaerobic tubes, strains Se-1-1-or and Se-1-3-red reduced a very small amount of tellurite to elemental Te and strains Te-2-1, Te-2-2, and Se-1-1-or reduced a small amount of selenite to elemental Se. This indicates that metalloid reduction proceeds independently of growth and that reduction activity is retained by these strains even under suboptimal conditions. On plates incubated in an anaerobic jar, no growth and no reduction of either K2TeO3 or Na2SeO3 was observed.
Diagnostic growth and physiological properties.
The major physiological properties of the isolates are summarized in Table 2. All strains were catalase and oxidase positive and hydrolyzed Tween 60. Most strains hydrolyzed starch, and three strains hydrolyzed gelatin. Strains Te-1-1 and Se-1-2-or formed large clear halos on agar media that resulted from agar hydrolysis.
All strains were capable of growth over a wide range of pH values. Five strains (Se-1-1-or, Se-1-2-or, Se-1-2-red, Se-1-3-red, and Se-6-2-red) grew at all pH values tested from 5.0 to 11.0. Three strains (Te-1-1, Te-1-2, and Te-2-2) were incapable of growth below pH 5.5, and two strains (Se-6-1-or and Te-2-1) grew over the slightly more narrow pH range of pH 5.5 to 10.0 (Table 2). Growth over a wide range of pH values might be indicative of an adaptation to the vicinity of hydrothermal vents, where low-pH vent fluids mix with more-alkaline surrounding waters, creating steep gradients in pH (8). The isolates also grew over a wide range of temperatures (Table 2), with most strains growing at temperatures from 5 to 45°C. Again, this may indicate an adaptation to the environmental conditions at the site of isolation, where high-temperature fluids mix with cold surrounding waters, creating steep temperature gradients (8).
All strains grew over a wide range of NaCl concentrations (up to 10%). Strain Se-1-1-or was capable of growth at 15% NaCl, and strains Se-1-3-red and Se-6-2-red grew in the presence of 20% NaCl, the highest concentration tested. All strains required NaCl concentrations of ⩾0.5% for growth. Strain Se-1-2-or showed the strongest requirement for salt, not growing below 1.5% NaCl (Table 2). Tolerance for high salt concentrations is not surprising, as chloride concentrations in hydrothermal fluids have been reported as 30 to 200% of the levels found in the surrounding seawater (3) and high numbers of halotolerant bacteria in samples collected from the Endeavor segment of the Juan de Fuca Ridge have been reported (17).
The isolates utilized a wide variety of organic carbon sources as the sole source of carbon for aerobic heterotrophic growth. The best carbon sources for most of the strains were glutamate, glucose, and fructose. Anaerobic growth was not observed, although strain Se-6-1-or produced acid from glucose, and strains Te-1-2, Se-1-2-or, and Se-6-1-or produced acid from fructose under microaerophilic conditions. Nitrate was not reduced by any of the strains. None of the strains required vitamins, although strain Te-2-2 showed slightly reduced growth in media lacking thiamine. The ability to utilize a wide range of organic carbon sources and the lack of growth factor requirements may represent adaptations to the relatively oligotrophic waters found in the deep ocean, where less favorable nutrients must be used when more favorable nutrient sources become depleted.
The strains showed different responses to antibiotics (Table 2), varying from that of strain Te-1-1, which was susceptible to all antibiotics tested, to that of strain Se-1-3-red, which was resistant to penicillin G, streptomycin, tetracycline, ampicillin, kanamycin, and nalidixic acid.
Phylogenetic analyses.
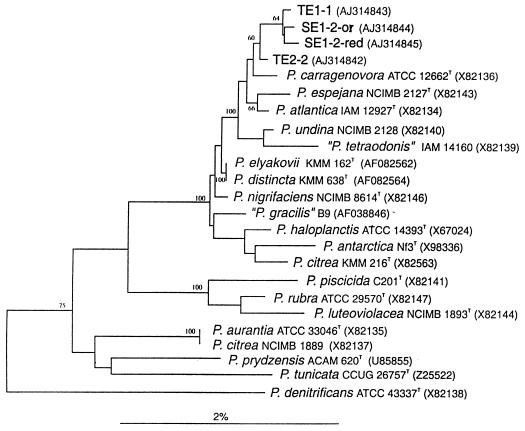
16S rDNA phylogenetic analyses were performed on four representative strains: Te-1-1, Te-2-2, Se-1-2-or, and Se-1-2-red. The sequences, covering 1,143 (Se-1-2-red), 1,468 (Te-2-2), 1,500 (Se-1-2-or), and 1,504 (Te-1-1) nucleotides, were compared with each other and with those of members of the class Proteobacteria. These four isolates share high 16S rDNA sequence identities, ranging between 99.7 and 99.9%. Similarly high values are found for these isolates and the majority of type strains of species of the genus Pseudoalteromonas, a member of the γ-3 subclass of the Proteobacteria, which is composed of obligately marine, gram-negative, obligately aerobic, straight or curved rod-shaped bacteria (11).
The Pseudoalteromonas genus contains several distinct phylogenetic clusters (Fig. 4), one of which embraces the four isolates as well as P. carragenovora, P. espejana, P. atlantica, P. undina, P. elyakovii, P. distincta, P. nigrificans, P. haloplanctis, P. antarctica, P. citrea, and some taxonomically invalid species. These organisms share higher than 98.5% sequence similarity, which explains the low statistical significance of the majority of branching points, as indicated by mostly low bootstrap values.
FIG. 4.
Unrooted tree showing the phylogenetic positions of four selected strains among members of the obligately marine genus Pseudoalteromonas, within the γ-subclass of the Proteobacteria.
The high degree of sequence similarity among the majority of Pseudoalteromonas species makes it difficult to evaluate the taxonomic status of the four isolates. As demonstrated with P. elyakovii and P. distincta two different species may share 100% 16S rDNA identity.
Concluding remarks.
The vicinity of deep-ocean hydrothermal vents along the Main Endeavor segment of the Juan de Fuca Ridge harbors a large number of bacteria resistant to high concentrations of Te and/or Se oxides. This high level of resistance may represent adaptations to the varied dissolved metal and metalloid ions present in hydrothermal-vent fluids. Our results show that strains isolated from this extreme environment are resistant to very high concentrations of the toxic Te and Se oxyanions and reduce them to less toxic elemental forms.
Four of the ten isolates were shown to be closely related to members of the genus Pseudoalteromonas on the basis of 16S rDNA sequence analyses. The major physiological properties of strains isolated in this study are in agreement with those described for the genus Pseudoalteromonas (11), although no member of this genus has yet been reported to resist or reduce oxyanions of either Te or Se. In contrast, members of the genus Pseudoalteromonas were described as having weak or irregular catalase activity (11), whereas our isolates all vigorously released O2 when they were exposed to hydrogen peroxide, indicating a strong catalase activity.
The ability to reduce toxic Te or Se oxyanions to their less toxic elemental forms could play an important future role in bioremediation of highly polluted effluents from industrial and mining operations. In addition, bacteria capable of reducing tellurite and selenite could prove useful in the applied biometallurgy of Te and Se, rare and expensive metals used extensively for their properties as semiconductors.
Acknowledgments
This work was supported by grants from the NSERC (Canada).
We gratefully acknowledge C. L. Van Dover for giving us the chance to participate in the ALISS cruise and to collect samples for this research. This cruise was funded by an NSF grant (Geology and Geophysics) to C. L. Van Dover and A. Chave. We thank Elaine Humphrey (University of British Columbia, Vancouver, Canada) for generous help with electron microscopy preparations.
REFERENCES
- 1.Avazeri, C., R. J. Turner, J. Pommier, J. H. Weiner, G. Giordano, and A. Vermeglio. 1997. Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 143:1181-1189. [DOI] [PubMed] [Google Scholar]
- 2.Bagnall, K. W. 1975. Selenium, tellurium, and polonium, p. 935-1008. In M. Schmidt, W. Siebert, and K. W. Bagnall (ed.), The chemistry of sulfur, selenium, tellurium and polonium. Pergamon Press, New York, N.Y.
- 3.Butterfield, D. A., R. E. McDuff, M. J. Mottl, M. D. Lilley, J. E. Lupton, and G. J. Massoth. 1994. Gradients in the composition of hydrothermal fluids from the Endeavour segment vent field: phase separation and brine loss. Geophys. Res. 99:9561-9583. [Google Scholar]
- 4.Conde, J. E., and M. Sanz Alaejos. 1997. Selenium concentrations in natural and environmental waters. Chem. Rev. 97:1979-2003. [DOI] [PubMed] [Google Scholar]
- 5.Daniels, L. A. 1996. Selenium metabolism and bioavailability. Biol. Trace Elem. Res. 54:185-199. [DOI] [PubMed] [Google Scholar]
- 6.DeSoete, G. 1983. A least squares algorithm for fitting additive trees to proximity data. Psychometrika 48:621-626. [Google Scholar]
- 7.Drews, G. 1983. Mikrobiologisches Praktikum. Springer Verlag, Berlin, Germany.
- 8.Feely, R. A., M. Lewison, G. J. Massoth, G. Robert-Baldo, J. W. Lavelle, R. H. Byrne, K. L. Von Damm, and H. C. Curl. 1987. Composition and dissolution of black smoker particulates from active vents on the Juan de Fuca Ridge. J. Geophys. Res. 92:11347-11363. [Google Scholar]
- 9.Felsenstein, J. 1993. PHYLIP (phylogenetic inference package) version 3.5.1. Department of Genetics, University of Washington, Seattle.
- 10.Fletcher, M., and G. D. Floodgate. 1973. An electron-microscopic demonstration of acidic polysaccharide involved in the adhesion of a marine bacterium to solid surfaces. J. Gen. Microbiol. 74:325-334. [Google Scholar]
- 11.Gauthier, G., M. Gauthier, and R. Christen. 1995. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int. J. Syst. Bacteriol. 45:755-761. [DOI] [PubMed] [Google Scholar]
- 12.Geesy, G. G., P. J. Bremer, J. J. Smith, M. Muegger, and L. K. Jang. 1992. Two-phase model for describing the interactions between copper ions and exopolymers from Alteromonas atlantica. Can. J. Microbiol. 38:785-793. [Google Scholar]
- 13.Gerrard, T. L., J. N. Telford, and H. H. Williams. 1974. Detection of selenium deposits in Escherichia coli by electron microscopy. J. Bacteriol. 119:1057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregersen, T. 1978. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 5:123-127. [Google Scholar]
- 15.Holmstrom, C., and S. Kjelleberg. 1999. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol. Ecol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 16.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 17.Kaye, J. Z., and J. A. Baross. 2000. High incidence of halotolerant bacteria in Pacific hydrothermal-vent and pelagic environments. FEMS Microbiol. Ecol. 32:249-260. [DOI] [PubMed] [Google Scholar]
- 18.Kellenberger, E., A. Ryter, and J. Sechaud. 1958. Electron microscope study of DNA-containing plasms. J. Biophys. Biochem. Cytol. 4:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessi, J., M. Ramuz, E. Wehrli, M. Spycher, and R. Bachofen. 1999. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 65:4734-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klevay, L. M. 1976. Pharmacology and toxicology of heavy metals: tellurium. Pharmacol. Ther. 1:223-229. [Google Scholar]
- 21.Knott, R., A. E. Fallick, D. Rickard, and H. Backer. 1995. Mineralogy and sulfur isotope characteristics of a massive sulphide boulder, Galapagos Rift, 85°55′W, p. 207-222. In L. M. Parson, D. R. Dixon, and C. L. Warker (ed.), Hydrothermal vents and processes. Geological Society, London, United Kingdom.
- 22.Lauchli, A. 1993. Selenium in plants: uptake, function and environmental toxicity. Bot. Acta 106:455-468. [Google Scholar]
- 23.Laverman, A. M., J. S. Blum, J. K. Schaefer, E. J. P. Phillips, D. R. Lovley, and R. S. Oremland. 1995. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl. Environ. Microbiol. 61:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, D. S., and J. M. Edmond. 1985. Tellurium species in seawater. Nature 313:782-785. [Google Scholar]
- 25.Lloyd-Jones, G., A. M. Osborn, D. A. Ritchie, P. Strike, J. L. Hobman, N. L. Brown, and D. A. Rouch. 1994. Accumulation and intracellular fate of tellurite in tellurite-resistant Escherichia coli: a model for the mechanism of resistance. FEMS Microbiol. Lett. 118:113-120. [DOI] [PubMed] [Google Scholar]
- 26.Losi, M. E., and W. T. Frankenberger, Jr. 1997. Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl. Environ. Microbiol. 63:3079-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macy, J. M., S. Rech, G. Auling, M. Dorsch, E. Stackebrandt, and L. I. Sly. 1993. Thauera selenatis gen. nov., sp. nov., a member of the beta subclass of Proteobacteria with a novel type of anaerobic respiration. Int. J. Syst. Bacteriol. 43:135-142. [DOI] [PubMed] [Google Scholar]
- 28.Madigan, M. T., J. M. Martinko, and J. Parker. 1997. Brock biology of microorganisms, 8th ed. Prentice Hall, Upper Saddle River, N.J.
- 29.Maidak, B. L., G. L. Olsen, N. Larsen, R. Overbeek, M. J. McCaughey, and C. R. Woese. 1996. The ribosomal database project. Nucleic Acids Res. 24:82-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Measures, C. I., and J. D. Burton. 1980. The vertical distribution and oxidation states of dissolved selenium in the northeast Atlantic Ocean and their relationship to biological processes. Earth Planet Sci. Lett. 46:385-396. [Google Scholar]
- 31.Moore, M. D., and S. Kaplan. 1992. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J. Bacteriol. 174:1505-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, M. D., and S. Kaplan. 1994. Members of the family Rhodospirillaceae reduce heavy-metal oxyanions to maintain redox poise during photosynthetic growth. ASM News 60:17-23. [Google Scholar]
- 33.O'Gara, J. P., M. Gomelsky, and S. Kaplan. 1997. Identification and molecular genetic analysis of multiple loci contributing to high-level tellurite resistance in Rhodobacter sphaeroides 2.4.1. Appl. Environ. Microbiol. 63:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlendorf, H. M., and G. M. Santolo. 1994. Kesterson Reservoir—past, present and future: an ecological risk assessment, p. 69-117. In J. R. Frankenberger and S. Benson (ed.), Selenium in the environment. Marcel Dekker, New York, N.Y.
- 35.Oremland, R. S. 1994. Biogeochemical transformation of selenium in anoxic environments, p. 389-420. In J. R. Frankenberger and S. Benson (ed.), Selenium in the environment. Marcel Dekker, New York, N.Y.
- 36.Pearion, C. T., and P. E. Jablonski. 1999. High level, intrinsic resistance of Natronococcus occultus to potassium tellurite. FEMS Microbiol. Lett. 174:19-23. [Google Scholar]
- 37.Petragnani, N., and W. L. Lo. 1998. Organometallic reagents for synthetic purposes: tellurium. J. Braz. Chem. Soc. 9:415-425. [Google Scholar]
- 38.Pimenova, M. N., N. N. Grechushkina, L. G. Azova, E. V. Semenova, and S. I. Mylnikova. 1983. Practical microbiology. Moscow State University, Moscow, Russia.
- 39.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 40.Sabaty, M., C. Avazeri, D. Pignol, and A. Vermeglio. 2001. Characterization of the reduction of selenate and tellurite by nitrate reductases. Appl. Environ. Microbiol. 67:5122-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawabe, T., H. Makino, M. Tatsumi, K. Nakano, K. Tajima, M. M. Iqbal, I. Yumoto, Y. Ezura, and R. Christen. 1998. Pseudomonas bacteriolytica sp. nov., a marine bacterium that is the causative agent of red spot disease of Laminaria japonica. Int. J. Syst. Bacteriol. 48:769-774. [DOI] [PubMed] [Google Scholar]
- 42.Summers, A. O., and G. A. Jacoby. 1977. Plasmid-determined resistance to tellurium compounds. J. Bacteriol. 129:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzina, N. E., V. I. Duda, L. A. Anisimova, V. V. Dmitriev, and A. M. Boronin. 1995. Cytological aspects of resistance to potassium tellurite conferred on Pseudomonas cells by plasmids. Arch. Microbiol. 163:282-285. [DOI] [PubMed] [Google Scholar]
- 44.Switzer-Blum, J., A. B. Bindi, J. Buzzelli, J. F. Stolz, and R. S. Oremland. 1998. Bacillus arsenicoselenatis sp. nov., and Bacillus selenitireducens, sp. nov.: two haloalkaliphiles from Mono Lake, California, which respire oxyanions of selenium and arsenic. Arch. Microbiol. 171:19-30. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, D. E., E. G. Walter, R. Sherburne, and D. P. Bazett-Jones. 1988. Structure and location of tellurium deposited in Escherichia coli cells harboring tellurite resistance plasmids. J. Ultrastruct. Mol. Struct. Res. 99:18-26. [DOI] [PubMed] [Google Scholar]
- 47.Tomei, F. A., L. L. Barton, C. L. Lemanski, T. G. Zocco, N. H. Fink, and L. O. Sillerud. 1995. Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J. Ind. Microbiol. 14:329-336. [Google Scholar]
- 48.Van Fleet-Stalder, V., H. Gurleyuk, R. Bachofen, and T. G. Chasteen. 1997. Effects of growth conditions on production of methyl selenides in cultures of Rhodobacter sphaeroides. Ind. Microbiol. Biotechnol. 19:98-103. [Google Scholar]
- 49.Vincent, P., P. Pignet, F. Talmont, L. Bozzi, B. Fournet, J. Guezennec, C. Jeanthon, and D. Prieur. 1994. Production and characterization of an exopolysaccharide excreted by a deep-sea hydrothermal vent bacterium isolated from the polychaete annelid Alvinella pompejana. Appl. Environ. Microbiol. 60:4134-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Dam, K. L., J. M. Edmond, C. I. Measures, and B. Grant. 1985. Chemistry of submarine hydrothermal solutions at Guaymas Basin, Gulf of California. Geochim. Cosmochim. Acta 49:2221-2237. [Google Scholar]
- 51.Von Dam, K. L., J. M. Edmond, B. Grant, and C. I. Measures. 1985. Chemistry of submarine hydrothermal solutions at 21°N, East Pacific Rise. Geochim. Cosmochim. Acta 49:2197-2220. [Google Scholar]
- 52.Walter, E. G., and D. E. Taylor. 1989. Comparison of tellurite resistance determinants from the IncPa plasmid RP4Ter and the IncHII plasmid pHH1508a. J. Bacteriol. 171:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrangstadh, M., P. L. Conway, and S. Kjelleberg. 1986. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch. Microbiol. 145:220-227. [DOI] [PubMed] [Google Scholar]
- 54.Yamada, A., M. Miyashita, K. Inoue, and T. Matsunaga. 1997. Extracellular reduction of selenite by a novel marine photosynthetic bacterium. Appl. Microbiol. Biotechnol. 48:367-372. [DOI] [PubMed] [Google Scholar]
- 55.Yurkov, V., J. Jappe, and A. Vermeglio. 1996. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl. Environ. Microbiol. 62:4195-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yurkov, V., E. Stackebrandt, A. Holmes, J. A. Fuerst, P. Hugenholtz, J. Golecki, N. Gad'on, V. M. Gorlenko, E. I. Kompantseva, and G. Drews. 1994. Phylogenetic positions of novel aerobic, bacteriochlorophyll a-containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp. nov. Int. J. Syst. Bacteriol. 44:427-434. [DOI] [PubMed] [Google Scholar]
- 57.Yurkov, V., and H. van Gemerden. 1993. Abundance and salt tolerance of obligately aerobic, phototrophic bacteria in a microbial mat. Neth. J. Sea Res. 31:57-62. [Google Scholar]
- 58.Yurkov, V. V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62:695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]