Abstract
Objectives
The relationship between abdominal obesity and cognitive impairment is not fully understood. The lipid accumulation product (LAP) is a convenient and cost-effective indicator for abdominal obesity. In the present study, we investigated the association between the LAP and cognitive impairment in a community-based 4-year prospective cohort study.
Methods
A total of 1349 (≥ 40 years) participants without cognitive impairment from the village of Xi’an, China were followed for 4 years. Cognitive impairment was diagnosed using a three-step protocol. The LAP was calculated using waist circumference and serum triglyceride levels. Multivariate logistic regression analysis and interaction analysis were used to assess the relationship between the LAP and cognitive impairment.
Results
The mean age of the participants at baseline was 55.0 ± 9.3 years, 816(60.5%) were female, and 46 (3.4%) were diagnosed as cognitive impairment during the 4-year follow-up. Multivariate logistic regression analysis revealed that lnLAP was not associated with the cognitive impairment in the total population. After stratification by sex, cognitive impairment was associated with lnLAP (OR = 1.85, 95% CI: 1.058–3.257, P = 0.031) in females but not in males (OR = 1.142, 95% CI: 0.503–2.594, P = 0.751). In the females, cognitive impairment was 4.09-fold greater in the highest LAP quartile than that in the lowest LAP quartile (OR = 4.098, 95% CI: 1.135–14.792, P = 0.031).
Conclusion
High LAP was associated with cognitive impairment in females but not in males. These findings indicate that the effects of abdominal obesity on cognitive impairment may differ between males and females.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-025-03143-z.
Keywords: Alzheimer's disease, Cognitive impairment, Cohort study, Lipid accumulation product, Risk factors
Introduction
Cognitive impairment and dementia are among the leading causes of disability and death in older adults and will be the most significant public health crisis in the coming decades. Therefore, it is necessary to actively identify and address risk factors to prevent cognitive impairment [1–3].
Obesity, as a social problem and major contributor to the global burden of disease, has been shown to increase the risk of dementia in the past [4, 5]. Notably, abdominal obesity may be even more closely associated with cognitive impairment [6–11]. Studies in which abdominal fat was measured via magnetic resonance imaging, have reported that greater amounts of abdominal adipose tissue appear to have a negative effect on brain structure and cognitive function in healthy older adults [12] and that abdominal obesity is associated with reduced cognitive function [13]. However, imaging examinations are expensive and not suitable for large-scale screening, and although body mass index (BMI) is a convenient and inexpensive measure of obesity, it is not a good measure of abdominal obesity [14]. Whereas the combination of waist circumference (WC) and triglycerides, the lipid accumulation product (LAP), a new visceral index, could be more feasibly used to reflect anatomical and physiological changes associated with excessive lipid accumulation in adults.14 Associations of changes in the LAP with metabolic syndrome, type 2 diabetes and coronary artery disease have also been identified [15–19]. However, the relationships between the LAP and cognitive impairment have not been determined. Recently, a cross-sectional study involving 220 type 2 diabetes patients reported that the LAP was inversely correlated with cognitive function and that a high LAP was an independent risk factor for mild cognitive impairment [20]. In addition, a cross-sectional study of 5,542 normal-weight Chinese hypertensive patients reported that the positive association between the LAP and cognitive impairment was stronger in older females than in males [21]. However, cohort studies linking the LAP to cognitive impairment are lacking. Moreover, the role of sex on the relationship between the LAP and cognitive impairment have not been clarified.
In the present study, we investigated the relationship between the LAP and cognitive impairment in a community-based longitudinal study.
Methods
Study population
This was a community-based prospective cohort study involving middle-aged and elderly people in two villages in rural Xi’an. The inclusion criteria were as follows: (1) permanent residents of two natural villages for ≥ 3 years, (2) baseline age ≥ 40 years, (3) no cognitive impairment at baseline, and (4) willingness to participate in the study and sign the informed consent form. The exclusion criteria were as follows: (1) lost to follow-up or died; (2) had severe visual disorder or hearing loss, which affects cognitive assessment; (3) had diseases that could affect cognitive function during follow-up (e.g., central nervous system infection, severe traumatic brain injury, Parkinson’s disease, organic psychosis, anxiety, depression, or congenital intellectual disability); and (4) had missing information.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2014LSK-111).
Standard interview
All participants underwent a face‒face interview in 2014 and were followed-up in 2018. The survey was conducted by qualified neurologists and graduate students with at least one week of training. The questionnaire included general information (age, sex, education level and employment status), lifestyle habits (alcohol abuse, smoking history, physical activity level, and sleep), and medical history (hypertension, diabetes, hyperlipidemia, cardiovascular disease, transient ischemic attack, stroke, and cognitive impairment).
All the subjects received a systemic and neurologic examination, including blood pressure, heart rate, body mass index (BMI, which was calculated as [weight (kg)]/[height (m)2]), WC, etc.
Diagnosis of cognitive impairment
Participants completed a standardized cognitive assessment battery in 2014 and were followed-up in 2018.The diagnosis of cognitive impairment followed a three-step protocol. First, we used the Chinese version of the Mini-Mental State Examination (C-MMSE) for global cognitive function assessment. The cutoff values were set on the basis of educational level (score ≤ 17 for illiterate; score ≤ 20 for primary school-educated subjects; and ≤ 24 for those educated at the junior high school level or above). Second, participants with a C-MMSE score below the cutoff needed further neuropsychological testing to assess different cognitive domains, including the Clock Drawing Task, Fuld Object Memory Evaluation, Rapid Verbal Retrieval, Digit Span Test, and Activity of Daily Living Scale. Third, the diagnosis was made based on the medical history and neuropsychological test by a neurologist. The cognitive impairment includes mild cognitive impairment and dementia, and the final diagnosis of mild cognitive impairment or dementia was made according to the NIA-AA criteria by repeated interviews and examination 6 months later for the tracked person.
Laboratory evaluation
After fasting overnight, 3 ml of cubital venous blood was collected from all the subjects between 8 and 10 am and placed in an anticoagulant tube. The sample was sent immediately to the laboratory of the First Affiliated Hospital of Xi’an Jiaotong University for biochemical tests. Measurements included fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c) levels.
The formula for the LAP is as follows: LAP = [WC (cm)-65] × TG (mmol/L) for males and [WC (cm) − 58] × TG (mmol/L) for females [15].
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (Inc., IBM, Chicago, IL, USA), and R software version 4.1.0 (R Foundation, Vienna, Austria). A two-sided P value of less than 0.05 was considered to indicate statistical significance.
First, normality tests were performed to unify continuous variables. Approximately normally distributed continuous variables included age, WC, systolic blood pressure (SBP), diastolic blood pressure (DBP), TC, HDL-c, and LDL-c. The mean (standard deviation) is used to represent these variables. Severely skewed variables included years of education, LAP, FBG, and TG, with the median (interquartile range) representing the distribution. The categorical data included sex, smoking, drinking, lack of exercise, hypertension, dyslipidemia, diabetes, stroke, and cognitive impairment, expressed as frequencies (percentages).
When comparing the baseline characteristics of the data in the univariate analysis, we used the independent sample t test or Analysis of Variance for approximately normally distributed data. For severely skewed data, we used a nonparametric test (Mann‒Whitney U test), and categorical data were compared using the chi‒square test. Since the LAP data had a nonnormal distribution, a natural log transformation was performed to convert it to a normal distribution, denoted “lnLAP”. Furthermore, lnLAP was considered a categorical variable by quartiles, and were divided into three groups (Quartile1, Quartile2-3, Quartile4).
Restricted cubic splines were used to assess whether there was a nonlinear relationship between lnLAP and cognitive impairment. Next, multivariate logistic regression analysis was utilized. Logistic regression models were established with lnLAP as the independent variable and cognitive dysfunction as the dependent variable in the total population. Model 1 was adjusted for age, sex and education. Model 2 was further adjusted for age, sex, education, smoking, drinking, lack of exercise, hypertension, dyslipidemia, and diabetes.
When stratified by sex, the multivariate logistic regression model was the same as that used for the total population, and interaction analyses were performed.
In sensitivity analyses, we further included confounders in the aforementioned models: lipid-lowering drug use and a history of stroke. The analyses were also repeated after they were winsorized. (Winsorize the top and bottom 2.5% of values to mitigates skewness in statistical analyses)
Results
Characteristics of participants at baseline
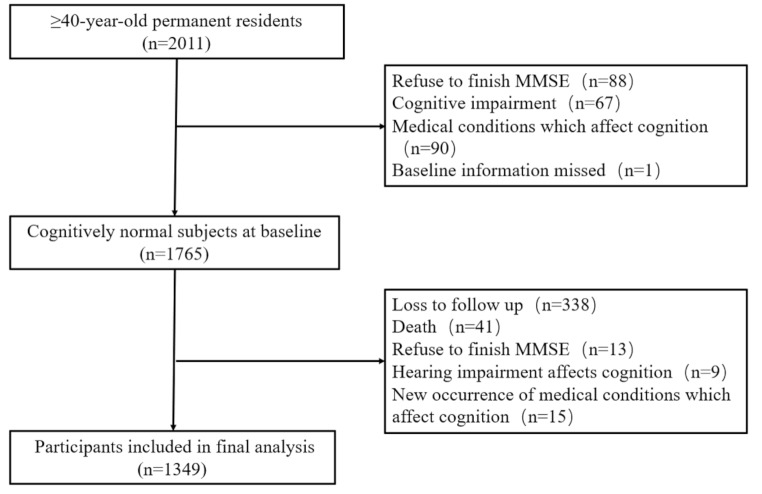
At the baseline in 2014, there were 2,011 individuals enrolled. A total of 1,765 participants without cognitive impairment were followed up. After the follow-ups were conducted in 2018, finally, there were 1349 subjects included in the final analyses (Fig. 1).
Fig. 1.
Flowchart of participant selction
There were 816 female (60.5%) with a mean age of 55 ± 9.27 years. During 4 years follow-up, 46 (3.4%) met the criteria of cognitive impairment, including 10 participants with dementia and 36 with mild cognitive impairment. The participants were divided into three groups according to the LAP quartile: group 1 (< 21.07), group 2 (21.07–54.37), and group 3 (> 54.37).
As shown in Table 1, the highest LAP group was older, more female, more higher blood pressure and dyslipidemia, and increased SBP, DBP, BMI, WC, FBG, TC, TG, HDL and LDL. (Table 1)
Table 1.
Characteristics of participants according to quartiles of LAP
| Lipid accumulation product | Total (N = 1349) |
P | |||
|---|---|---|---|---|---|
| Group1 (N = 337) |
Group 2 (N = 675) |
Group 3 (N = 337) |
|||
| Age, years, mean (SD) | 53.8 (9.3) | 55.3 (9.4) | 55.5 (8.9) | 55.0 (9.3) | 0.022 |
| Female, n(%) | 175 (51.9%) | 417 (61.8%) | 224 (66.5%) | 816 (60.5%) | 0.000 |
| Education, years, M(IQR) | 7(4) | 7(5) | 7(4) | 7(4) | 0.830 |
| Lifestyle, n(%) | |||||
| Smoking | 108 (32.0%) | 184 (27.3%) | 94 (27.9%) | 386 (28.6%) | 0.268 |
| Drinking | 56 (16.6%) | 89 (13.2%) | 40 (11.9%) | 185 (13.7%) | 0.158 |
| Lack of exercise | 44 (13.1%) | 104 (15.4%) | 67 (19.9%) | 215 (15.9%) | 0.046 |
| Comorbidity, n(%) | |||||
| Hypertension | 114 (33.8%) | 330 (48.9%) | 227 (67.4%) | 671 (49.7%) | <0.001 |
| Stroke | 16 (4.7%) | 34 (5.0%) | 21 (6.2%) | 71 (5.3%) | 0.643 |
| Dyslipidemia | 136 (40.4%) | 336 (49.8%) | 222 (65.9%) | 694 (51.4%) | <0.001 |
| Diabetes | 29 (8.6%) | 83 (12.3%) | 46 (13.6%) | 158 (11.7%) | 0.101 |
| Lipid-lowering use, n(%) | 5 (1.5%) | 31 (4.6%) | 14 (4.2%) | 50 (3.7%) | 0.042 |
| LAP | 14.28(8.0) | 34.16(16.0) | 74.34 (34.2) | 34.1 (33.3) | <0.001 |
| BMI, kg/m2, mean (SD) | 22.9 (2.3) | 25.4 (2.6) | 27.8 (3.5) | 25.4 (3.3) | <0.001 |
| Waist, cm, mean (SD) | 77.1 (6.1) | 85.3 (6.4) | 93.3 (8.0) | 85.2 (8.9) | <0.001 |
| SBP, mmHg, mean (SD) | 126 (15.8) | 131 (17.6) | 138 (17.8) | 132 (17.8) | <0.001 |
| DBP, mmHg, mean (SD) | 78.5 (8.99) | 82.0 (10.0) | 86.3 (11.5) | 82.2 (10.6) | <0.001 |
| Laboratory tests | |||||
| FBG, mmol/L, M(IQR) | 5.26(0.6) | 5.4 (0.7) | 5.55 (1.1) | 5.39 (0.7) | <0.001 |
| TC, mmol/L, mean (SD) | 4.65 (0.8) | 4.97 (0.9) | 5.45 (1.0) | 5.01 (1.0) | <0.001 |
| TG, mmol/L, M(IQR) | 0.9(0.4) | 1.41(0.6) | 2.4(1.1) | 1.43(1.0) | <0.001 |
| HDL-c, mmol/L, mean (SD) | 1.54 (0.3) | 1.39 (0.3) | 1.25 (0.3) | 1.40 (0.3) | <0.001 |
| LDL-c, mmol/L, mean (SD) | 2.92 (0.8) | 3.29 (0.9) | 3.68 (0.9) | 3.30 (0.9) | <0.001 |
| MMSE at baseline, M(IQR) | 27(4) | 27(4) | 27(4) | 27(4) | 0.651 |
| Cognitive impairment, n(%) | 8 (2.4%) | 26 (3.9%) | 12 (3.6%) | 46 (3.4%) | 0.467 |
Abbreviations: M(IQR), median (interquartile range); BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; FBG, fasting blood glucose
Relationships between the LAP and cognitive impairment
In the total population, the prevalence of cognitive impairment had no difference among the LAP groups. We performed RCS analysis to visualize the relationship between the LAP and cognitive impairment. After multivariable adjustment, LAP did not show a nonlinear relationship with cognitive impairment (Poverall=0.238, Pnon−linear=0.335) (Figure S1).
Since LAP has a non-normal distribution, we convert LAP to lnLAP. Multivariate logistic regression analysis were performed to adjust for confounders. When lnLAP was used as a continuous variable in the logistic regression analysis, the independent variable was lnLAP, and the dependent variable was cognitive impairment, after adjusting for all confounding factors, including age, sex, education, smoking, drinking, lack of exercise, hypertension, dyslipidemia, and diabetes, lnLAP wasn’t associated with cognitive impairment either (Model 3, OR = 1.466, 95% CI: 0.911–2.360, P = 0.115) (Table 2).
Table 2.
Multivariable logistic regression analysis of LAP and cognitive impairment
| lnLAP | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Continuous | 1.326 | 0.875–2.010 | 0.184 | 1.278 | 0.827–1.975 | 0.268 | 1.466 | 0.911–2.360 | 0.115 |
| Categories | |||||||||
| Group 1 | Ref | Ref | Ref | ||||||
| Group 2 | 1.648 | 0.738–3.679 | 0.223 | 1.550 | 0.686–3.503 | 0.292 | 1.640 | 0.711–3.784 | 0.246 |
| Group 3 | 1.518 | 0.613–3.763 | 0.367 | 1.462 | 0.579–3.692 | 0.422 | 1.828 | 0.687–4.864 | 0.227 |
Model 1, Unadjusted
Model 2, adjusted for age, gender and education
Model 3, adjusted for age, sex, education, smoking, drinking, lack of exercise, hypertension, dyslipidemia, and diabetes
When lnLAP was used as a categorical variable according to its interquartile range, multivariable logistic regression analysis showed that, compared to the group 1, group 2 and group 3 were not associated with impairment(Model 3, OR = 1.64, 95% CI: 0.711–3.784, P = 0.246; OR = 1.828, 95% CI: 0.687–4.864, P = 0.227 respectively) (Table 2).
The effects of sex on the association between the LAP and cognitive impairment
Considering sex differences in abdominal obesity, we performed sex-stratified analysis. Female group (n = 816) were younger (54.7 ± 9.12 years), had lower levels of education, smoking, drinking, exercise, and increased WC, LAP, TC, and HDL-C (Table 3).
Table 3.
Comparison of the female and male at baseline
| Female (N = 816) |
Male (N = 533) |
P | |
|---|---|---|---|
| Age, years, mean (SD) | 54.7 (9.1) | 55.4 (9.5) | 0.170 |
| Education, years, M(IQR) | 7(5) | 8(4) | 0.023 |
| Lifestyle, n(%) | |||
| Smoking | 153 (18.8%) | 233 (43.7%) | <0.001 |
| Drinking | 14 (1.7%) | 171 (32.1%) | <0.001 |
| Lack of exercise | 153 (18.8%) | 62 (11.6%) | <0.001 |
| Comorbidity, n(%) | |||
| Hypertension | 399 (48.9%) | 272 (51.0%) | 0.443 |
| Stroke | 40 (4.9%) | 31 (5.8%) | 0.462 |
| Dyslipidemia | 421 (51.6%) | 273 (51.2%) | 0.893 |
| Diabetes | 102 (12.5%) | 56 (10.5%) | 0.266 |
| Lipid-lowering drugs, n(%) | 33 (4.0%) | 17 (3.2%) | 0.417 |
| LAP | 36.16 (35.4) | 30.8 (28.9) | <0.001 |
| BMI, kg/m2, mean (SD) | 25.5 (3.5) | 25.3 (2.9) | 0.220 |
| Waist, cm, mean (SD) | 84.0 (8.9) | 87.2 (8.5) | <0.001 |
| SBP, mmHg, mean (SD) | 132 (18.5) | 132 (16.7) | 0.990 |
| DBP, mmHg, mean (SD) | 81.9 (10.6) | 82.7 (10.5) | 0.159 |
| Laboratory tests | |||
| FBG, mmol/L, M(IQR) | 5.39(0.8) | 5.4(0.7) | 0.137 |
| TC, mmol/L, mean (SD) | 5.09 (1.0) | 4.89 (0.9) | <0.001 |
| TG, mmol/L, M(IQR) | 1.44(1.0) | 1.41(1.1) | 0.511 |
| HDL-c, mmol/L, mean (SD) | 1.46 (0.3) | 1.30 (0.3) | <0.001 |
| LDL-c, mmol/L, mean (SD) | 3.33 (0.9) | 3.25 (0.8) | 0.135 |
| Cognitive impairment, n(%) | 33 (4.0%) | 13 (2.4%) | 0.112 |
Abbreviations: M(IQR), median (interquartile range); BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; FBG, fasting blood glucose
The prevalence of cognitive impairment had no significant difference between females and males (4.0% vs. 2.4%, P = 0.112) (Table 3).
Association between the LAP and cognitive impairment after stratification by sex
In order to exclude the influence of confounding factors on the relationships between LAP and cognitive impairment, the multivariate analysis was conducted. When lnLAP was used as continuous variable, logistic regression analysis revealed that lnLAP was associated with cognitive impairment even after adjustment for all confounding factors in the female group (Model 3, OR = 2.064, 95% CI: 1.097–3.883, P = 0.025), but not in the male group (Model 3, OR = 1.142, 95% CI: 0.503–2.594, P = 0.751) (Table 4).
Table 4.
Multivariable analysis of the LAP and cognitive impairment after stratified by sex
| lnLAP | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | ||
| Female | Continuous | 1.624 | 0.947–2.783 | 0.078 | 1.856 | 1.058–3.257 | 0.031 | 2.064 | 1.097–3.883 | 0.025 |
| Categories | ||||||||||
| Group 1 | Ref | Ref | Ref | |||||||
| Group 2 | 1.817 | 0.602–5.479 | 0.289 | 2.180 | 0.705–6.743 | 0.176 | 2.240 | 0.700-7.162 | 0.174 | |
| Group 3 | 2.420 | 0.767–7.637 | 0.132 | 3.431 | 1.030–11.42 | 0.045 | 4.098 | 1.135–14.79 | 0.031 | |
| Male | Continuous | 0.867 | 0.446–1.687 | 0.675 | 0.827 | 0.402–1.705 | 0.608 | 1.142 | 0.503–2.594 | 0.751 |
| Categories | ||||||||||
| Group 1 | Ref | Ref | Ref | |||||||
| Group 2 | 1.428 | 0.432–4.714 | 0.559 | 1.239 | 0.365–4.202 | 0.731 | 1.879 | 0.478–7.389 | 0.367 | |
| Group 3 | 0.996 | 0.996 | 0.996 | |||||||
Model 1, Unadjusted
Model 2, adjusted for age, education;
Model 3, adjusted for age, education, smoking, drinking, lack of exercise, hypertension, dyslipidemia, and diabetes
When LAP was used as a categorical variable, the results showed that compared to the group 1, the group 2 was not associated with cognitive impairment, while the group 3 was significantly associated with cognitive impairment in female even after adjustment for all confounding factors (Model 3, OR = 4.098, 95% CI: 1.135–14.792, P = 0.031), but not in male (Model 3, OR = 1.887, 95% CI: 0.478–7.389, P = 0.367) (Table 4).
Interaction analysis revealed that sex and the LAP tended to have an interaction effect on cognitive impairment, but this effect was not statistically significant (sex, OR = 1.362, 95%CI: 0.397–4.673, P=0.623; lnLAP, OR = 1.778, 95%CI: 1.006–3.142, P = 0.048; lnLAP×sex, OR = 0.374, 95%CI: 0.132–1.059, P = 0.064) (Table 5).
Table 5.
The interaction effect of sex and LAP on cognitive impairment
| B | S. E | Wald | P | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Model 1 | sex | 1.764 | 1.741 | 1.027 | 0.311 | 5.836 | 0.192–176.962 |
| lnLAP | 0.627 | 0.303 | 4.271 | 0.039 | 1.872 | 1.033–3.391 | |
| lnLAP by sex | -0.672 | 0.483 | 1.934 | 0.164 | 0.511 | 0.198–1.317 | |
| Model 2 | sex | 0.309 | 0.629 | 0.242 | 0.623 | 1.362 | 0.397–4.673 |
| LAP* | 0.576 | 0.290 | 3.926 | 0.048 | 1.778 | 1.006–3.142 | |
| LAP* by sex | -0.984 | 0.531 | 3.428 | 0.064 | 0.374 | 0.132–1.059 | |
Model 1, adjusted for age, education, smoking, drinking, lack of exercise, hypertension, dyslipidemia, diabetes, sex, LAP(continuous variable), and LAP(continuous variable) by sex
Model 2, adjusted for age, education, smoking, drinking, lack of exercise, hypertension, dyslipidemia, diabetes, sex, lnLAP*( categorical variable), and lnLAP*( categorical variable) by sex
Sensitivity analysis
In the sensitivity analysis, we prove the robustness of the results in female. The results were consistent with the above analysis, when stroke and using lipid-lowering drugs were further adjusted in the multivariate logistic regression analyses (Table S1). When lnLAP was used as continuous variable, logistic regression analysis revealed that lnLAP was associated with cognitive impairment (Model 1 OR = 2.097, 95% CI: 1.104–3.983, P = 0.024) in the female group. When LAP was used as a categorical variable, the results showed that compared to the group 1, the group 3 was significantly associated with cognitive impairment (Model 1, OR = 4.257, 95% CI: 1.155–15.692, P = 0.030) (Table S1). The results still did not change when the data was winsorized in model 2 (Table S1).
Discussion
In this community-based longitudinal cohort study, the middle-aged and elderly adults were followed -up for 4 years. We found that no significant association between the LAP and cognitive impairment in the total population. However, in the sex stratification analysis, a higher LAP was positively associated with cognitive impairment in females but not in males.
The LAP is an indicator of the combination of abdominal obesity and dyslipidemia status. The LAP was first proposed by Kahn in the early 2000s as a new obesity-related measure of fat accumulation in the abdomen [15]. However, the relationship between the LAP and cognitive impairment had not yet been determined. A cross-sectional study in people with diabetes revealed that a high LAP was more strongly associated with MCI than WC and BMI were [20]. Another cross-sectional study revealed that a greater LAP was associated with better cognitive function in people with normal-weight hypertension [21]. The reason for these different results has not been determined.
Unlike previous studies, the present study is the first prospective cohort study to examine the association between the LAP and cognitive impairment in a community-based cohort population and revealed that the LAP was positively correlated with cognitive impairment in females but not in males. Cognitive impairment was more significant in the highest LAP quartile in females. In our previous study [22], we reported that a high LAP was associated with cognitive decline in females with normal blood pressure but not in those with high blood pressure or in males over a 4-year follow-up. Taken together, these findings indicate that the relationship between the LAP and cognitive impairment may be affected by sex.
A study from North America revealed that females had more brain atrophy with increased visceral fat than males did [23]. However, a British study suggested that the volume of visceral fat is associated with better brain health in females in midlife [24]. The protective effects of estrogen on female cognitive function are lost after menopause, Estrogen may protect against Aβ-induced neuronal loss [25], against excitotoxic injury induced by glutamate, and another neuroprotective action of estrogen is inhibition of pathological tau hyperphosphorylation [26]. and later life stage is associated with an increased risk of cognitive decline [26, 27]. Other researches examining sex differences in the association between obesity and cognitive function also found that female exhibit greater susceptibility to the detrimental cognitive effects of obesity [28], and compared to males [29], obesity inflicts greater disruption to the blood-brain barrier in females, and with stronger pro-inflammatory effects [30, 31]. However, the precise underlying mechanisms require further studies.
The mechanism by which abdominal obesity relate to cognitive impairment is poorly understood. Metabolic inflammation and lipotoxicity, as possible mechanisms of obesity-induced cognitive decline, have been reported. The massive increase in white adipose tissue in the abdominal region leads to abnormal adipose tissue function, infiltration of inflammatory cells, secretion of inflammatory cytokines by adipose tissue, and a chronic low-grade inflammatory response [32, 33]. It induces changes in vascular permeability, endothelial function, and microvascular structure, all of which may contribute to the pathogenesis of cerebrovascular disease and affect white matter integrity [34, 35]. In addition to vascular damage, inflammatory responses such as neuroglial reactions [36], insulin resistance [37], and increased Aβ burden in the brain contribute to cognitive decline [38].
The study has several limitations. First, the LAP level was only measured at baseline. The LAP level may have changed dynamically during the 4-year follow-up. Second, the sample size was small, and the follow-up time was short, which resulted in a small number of patients with new-onset cognitive impairment. Third, we did not determine the cause of cognitive impairment. Higher LAP level may be a risk factor for all-cause cognitive impairment. Although multivariate regression models were employed to adjust for age, education, smoking status, drinking status and comorbidities, the possibility of residual confounding from unmeasured variables (e.g., socioeconomic status or dietary patterns) persists.
In conclusion, in this cohort follow-up study, we found that the LAP was significantly associated with cognitive impairment in females but not in males. These findings indicate that the relationships among hyperlipidemia, obesity, and cognitive impairment may be affected by sex.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
Wang Yanyu and Liu Jie collected information, performed the statistical analysis and wrote the manuscript. Zhou Rong, Dang Liangjun, Gao Ling, Wei Shan, Shang Suhang, Wang Jin, Wang Jingyi collected the data. Qu Qiumin and Deng Yongning developed the idea, provided technical guidance and made revisions.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2014LSK-111).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wang Yanyu and Liu Jie contributed equally to this work.
Contributor Information
Qu Qiumin, Email: quqiumin@xjtufh.edu.cn.
Deng Yongning, Email: dengyongning@xjtufh.edu.cn.
References
- 1.GBD 2016 Dementia Collaborators (2019) Global, regional, and National burden of alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 18:88–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL et al (2011) National estimates of the prevalence of alzheimer’s disease in the united States. Alzheimers Dement J Alzheimers Assoc 7:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gale SA, Acar D, Daffner KR (2018) Dement Am J Med 131:1161–1169 [DOI] [PubMed] [Google Scholar]
- 4.Alford S, Patel D, Perakakis N, Mantzoros CS (2018) Obesity as a risk factor for alzheimer’s disease: weighing the evidence. Obes Rev Off J Int Assoc Study Obes 19:269–280 [DOI] [PubMed] [Google Scholar]
- 5.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2011) Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology 76:1568–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang Z, Jin W, Huang L, Chen H (2024) Body mass index, waist circumference, hip circumference, abdominal volume index, and cognitive function in older Chinese people: a nationwide study. BMC Geriatr 24:925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafson DR, Bäckman K, Waern M, Ostling S, Guo X, Zandi P et al (2009) Adiposity indicators and dementia over 32 years in Sweden. Neurology 73:1559–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudala K, Bansal D, Schifano F, Bhansali A (2013) Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig 4:640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K (2007) Body mass index in midlife and risk of alzheimer disease and vascular dementia. Curr Alzheimer Res 4:103–109 [DOI] [PubMed] [Google Scholar]
- 10.Tezapsidis N, Smith MA, Ashford JW (2009) Central obesity and increased risk of dementia more than three decades later. Neurology 72:1030–1031 author reply 1031 [PubMed] [Google Scholar]
- 11.Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S et al (2018) Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II study. Alzheimers Dement J Alzheimers Assoc 14:178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang N, Zhai H, Han B, Li Q, Chen Y, Chen Y et al (2016) Visceral fat dysfunction is positively associated with hypogonadism in Chinese men. Sci Rep 6:19844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon DH, Choi SH, Yu JH, Ha JH, Ryu SH, Park DH (2012) The relationship between visceral adiposity and cognitive performance in older adults. Age Ageing 41:456–461 [DOI] [PubMed] [Google Scholar]
- 14.Tchernof A, Després J-P (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93:359–404 [DOI] [PubMed] [Google Scholar]
- 15.Kahn HS (2005) The lipid accumulation product performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord 5:26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazidi M, Kengne A-P, Katsiki N, Mikhailidis DP, Banach M (2018) Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J Diabetes Complications 32:266–270 [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi I, Daimon T (2014) A strong association between lipid accumulation product and diabetes mellitus in Japanese women and men. J Atheroscler Thromb 21:282–288 [DOI] [PubMed] [Google Scholar]
- 18.Tian T, Pei H, Chen Z, Hailili G, Wang S, Sun Y et al (2020) Comparison of lipid accumulation product and body mass index as indicators of diabetes diagnosis among 215,651 Chinese adults. PeerJ 8:e8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimento-Ferreira MV, Rendo-Urteaga T, Vilanova-Campelo RC, Carvalho HB, da Paz Oliveira G, Paes Landim MB et al (2017) The lipid accumulation product is a powerful tool to predict metabolic syndrome in undiagnosed Brazilian adults. Clin Nutr Edinb Scotl 36:1693–1700 [DOI] [PubMed] [Google Scholar]
- 20.Yu Z-W, Li X, Wang Y, Fu Y-H, Gao X-Y (2020) Association between lipid accumulation product and mild cognitive impairment in patients with type 2 diabetes. J Alzheimers Dis JAD 77:367–374 [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Li J, Yu G, Zhou X, Zhou W, Zhu L et al (2021) Association between lipid accumulation product and cognitive function in hypertensive patients with normal weight: insight from the China H-type hypertension registry study. Front Neurol 12:732757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Wei S, Zhou R, Shang S, Dang L, Gao L et al (2021) The relationships between lipid accumulation product levels and cognitive decline over 4 years in a rural area of xi’an, China. Front Aging Neurosci 13:761886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raji CA, Meysami S, Hashemi S, Garg S, Akbari N, Gouda A et al (2024) Visceral and subcutaneous abdominal fat predict brain volume loss at midlife in 10,001 individuals. Aging Dis 15:1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran C, Herson J, Than S, Collyer T, Beare R, Syed S et al (2024) Interactions between age, sex and visceral adipose tissue on brain ageing. Diabetes Obes Metab 26:3821–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barron AM, Pike CJ (2012) Sex hormones, aging, and alzheimer’s disease. Front Biosci Elite Ed 4:976–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike CJ, Carroll JC, Rosario ER, Barron AM (2009) Protective actions of sex steroid hormones in alzheimer’s disease. Front Neuroendocrinol 30:239–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Than S, Moran C, Beare R, Vincent AJ, Collyer TA, Wang W et al (2021) Interactions between age, sex, menopause, and brain structure at midlife: A UK biobank study. J Clin Endocrinol Metab 106:410–420 [DOI] [PubMed] [Google Scholar]
- 28.Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G et al (2006) Vascular risk factors for incident alzheimer disease and vascular dementia: the cache County study. Alzheimer Dis Assoc Disord 20:93–100 [DOI] [PubMed] [Google Scholar]
- 29.Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K (2007) Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med 262:643–650 [DOI] [PubMed] [Google Scholar]
- 30.Mascarenhas-Melo F, Marado D, Palavra F, Sereno J, Coelho Á, Pinto R et al (2013) Diabetes abrogates sex differences and aggravates cardiometabolic risk in postmenopausal women. Cardiovasc Diabetol 12:61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canon ME, Crimmins EM (2011) Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J Nutr Health Aging 15:695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M et al (2010) Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 299:E506–515 [DOI] [PubMed] [Google Scholar]
- 33.Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Park Y, Wu J, Chen X, ping, Lee S, Yang J et al (2009) Role of TNF-alpha in vascular dysfunction. Clin Sci Lond Engl 1979 116:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M et al (2010) Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74:1022–1029 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen JCD, Killcross AS, Jenkins TA (2014) Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci 8:375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring H-U (2016) Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96:1169–1209 [DOI] [PubMed] [Google Scholar]
- 38.Farris W, Mansourian S, Leissring MA, Eckman EA, Bertram L, Eckman CB et al (2004) Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol 164:1425–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.