Abstract
When wild-type Saccharomyces cerevisiae strains pregrown in maltose-limited chemostat cultures were exposed to excess maltose, release of glucose into the external medium was observed. Control experiments confirmed that glucose release was not caused by cell lysis or extracellular maltose hydrolysis. To test the hypothesis that glucose efflux involved plasma membrane glucose transporters, experiments were performed with an S. cerevisiae strain in which all members of the hexose transporter (HXT) gene family had been eliminated and with an isogenic reference strain. Glucose efflux was virtually eliminated in the hexose-transport-deficient strain. This constitutes experimental proof that Hxt transporters facilitate export of glucose from S. cerevisiae cells. After exposure of the hexose-transport-deficient strain to excess maltose, an increase in the intracellular glucose level was observed, while the concentrations of glucose 6-phosphate and ATP remained relatively low. These results demonstrate that glucose efflux can occur as a result of uncoordinated expression of the initial steps of maltose metabolism and the subsequent reactions in glucose dissimilation. This is a relevant phenomenon for selection of maltose-constitutive strains for baking and brewing.
The disaccharide maltose is an important carbon source for Saccharomyces cerevisiae during beer fermentation and leavening of dough (3, 10, 20, 42). In addition to having applied significance, the maltose regulon in S. cerevisiae serves as a paradigm for metabolic regulation in eukaryotes (18, 23, 36, 37).
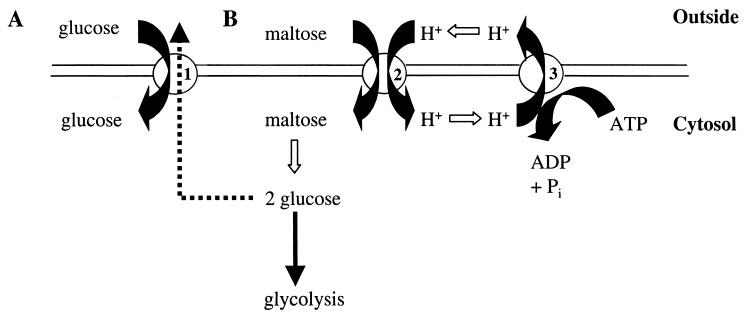
In S. cerevisiae, an intracellular maltase (α-glucosidase; EC 3.2.1.20) hydrolyzes maltose to glucose. The transport of maltose over the cell membrane differs from the transport of glucose (Fig. 1). In S. cerevisiae, glucose uptake occurs exclusively via facilitated diffusion (29, 43). Facilitated diffusion of glucose involves the 17 members of the HXT gene family (61), which encode hexose transporters that differ with respect to kinetic properties (12), transcriptional regulation, and intracellular localization (39). As Hxt-mediated glucose transport does not require input of metabolic energy, alcoholic fermentation of glucose by S. cerevisiae results in a net yield of two ATP molecules per glucose molecule (30). Conversely, maltose is taken up via a maltose-one-proton symport mechanism (49). Extrusion of the symported proton via the plasma membrane ATPase costs one ATP molecule per proton (53, 59). Consequently, the net ATP yield from alcoholic fermentation of one maltose molecule is only three ATP molecules (21).
FIG. 1.
Schematic representation of glucose and maltose transport in S. cerevisiae. (A) Facilitated diffusion of glucose, driven by the concentration gradient of the sugar. (B) Maltose-proton symport driven by the proton motive force and the sugar concentration gradient. ATP hydrolysis by the plasma membrane ATPase is required to expel the protons that enter the cell together with the maltose. For each maltose molecule transported into the cell, one ATP molecule is hydrolyzed (53, 59). 1, Hxt transporter; 2, maltose permease; 3, H+-ATPase complex.
The genes encoding the maltose permease are located in five highly homologous loci (MAL1, MAL2, MAL3, MAL4, and MAL6) (2, 8). The number and identity of MAL loci is strain dependent (35). Each MAL locus consists of three genes. The first gene (MALx1) encodes the maltose-proton symporter (9). Maltase is encoded by the MALx2 gene (13, 22). The third MAL gene (MALx3) encodes a DNA-binding, maltose-dependent transcriptional activator that specifically controls expression of the MALx1 and MALx2 genes (7, 21).
Maltose metabolism in S. cerevisiae is strongly downregulated by glucose. At the transcriptional level, glucose represses transcription of the MALx1 and MALx2 genes via binding of the transcriptional repressor Mig1p in the MAL intergenic region (26, 27, 62). Moreover, glucose causes rapid catabolite inactivation of maltose permease activity (5). This glucose-induced inactivation can involve two different signaling pathways (32). The first pathway uses Rgt2p as a sensor of extracellular glucose and induces degradation of the maltose permease protein. This degradation requires ubiquitination and endocytic internalization of the maltose transporter protein to the vacuole, where proteolysis takes place (32-34, 45). The second pathway depends on glucose transport and causes very rapid inactivation of maltose transport activity, followed by degradation of the maltose permease (24, 25). Which signal triggers this catabolite inactivation is still a matter of debate. Some authors have proposed that hexose transport via Hxt transporters is required for this pathway (24, 25), whereas other authors have stated that galactose and even maltose can also elicit catabolite inactivation (40, 46). In addition, trehalose and/or trehalose 6-phosphate have recently been mentioned as possible signals for catabolite inactivation (4). The glucose-induced loss of maltose transport activity is generally much faster than the loss expected from mere proteolytic degradation of the maltose transporter. This observation has been explained by glucose-induced phosphorylation of the maltose transporter that precedes proteolytic breakdown and immediately reduces transport activity (5).
Despite this multilayer regulation of maltose metabolism, several reports have indicated that S. cerevisiae has difficulty coping with sudden changes in the extracellular maltose concentration. Exposure of aerobic, maltose-limited chemostat cultures to excess maltose has even been reported to result in maltose-accelerated death (41). The loss of viability, accompanied by the release of glucose into the medium, was interpreted to be caused by nonrestricted maltose uptake and hydrolysis, with concomitant rapid intracellular accumulation of glucose and protons leading to cell death and lysis (41). Release of glucose upon exposure to excess maltose has also been observed in S. cerevisiae mutants defective in glucose catabolite repression (5, 11, 26).
The aim of the present study was to investigate the mechanism responsible for glucose release during maltose fermentation by S. cerevisiae. Special attention was paid to a possible role of the HXT-encoded glucose transporters in mediating glucose efflux.
MATERIALS AND METHODS
Strains and maintenance.
The strains used in this study (Table 1) were grown to the stationary phase in shake flask cultures on synthetic medium (55) adjusted to pH 6.0 and containing 2% (wt/vol) glucose. After addition of sterile glycerol (30%, vol/vol), 2-ml aliquots were stored in sterile vials at −80°C. These frozen stock cultures were used to inoculate precultures for chemostat cultivation.
TABLE 1.
S. cerevisiae strains used in this study
| S. cerevisiae strain | Relevant genotype and/or phenotype | Source | Reference |
|---|---|---|---|
| CEN.PK113-7D | MATa, prototrophic | P. Kötter | 50 |
| DS28911 | Aneuploid, prototrophic | DSM Bakery Ingredients, Delft, The Netherlands | 51 |
| CBS 8066 | HO/HO, prototrophic | CBSa | 50 |
| CEN.PK2-1C | MATα leu2-3,112 ura3-52 trp1-289 his3-Δ1 hxt17Δ | E. Boles | 61 |
| EBY.VW.4000 | hxt1 through -17Δ::loxP gal2Δ::loxP stl1Δ::loxP agt1Δ::loxP ydl247wΔ::loxP yjr160cΔ::loxP | E. Boles | 61 |
CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
Media.
Synthetic medium containing mineral salts and vitamins was prepared and sterilized as described previously (55). To meet the auxotrophic requirements of strains CEN.PK.2-1C and EBY.VW.4000, media for cultivation of these strains were supplemented with uracil (113 mg · liter−1), l-histidine (45 mg · liter−1), l-leucine (180 mg · liter−1), and l-tryptophan (27 mg · liter−1). Auxotrophic requirements were calculated as described by Oura (38), and the required concentrations were multiplied by 2 to prevent limitation by nutrients other than the sugar substrate. For chemostat cultivation, the glucose or maltose concentration in reservoir media was 7.5 g · liter−1 (0.25 mol of C · liter−1). Media for maltose-limited cultivation of strains CEN.PK.2-1C and EBY.VW.4000 were also supplemented with ethanol (5% on a total carbon basis) to eliminate the persistent metabolic oscillations (6, 28) in maltose-limited chemostat cultures of these strains.
Chemostat cultivation.
Aerobic chemostat cultivation was performed at a dilution rate of 0.10 h−1, at pH 5.0, and at 30°C in laboratory fermentors (Applikon, Schiedam, The Netherlands) as described previously (51). Steady-state data are data for cultures without detectable oscillations. A maximum culture age of 170 h (25 generations) was used to minimize selection of mutants.
Off-gas analysis.
The exhaust gas was cooled in a condenser (2°C) and dried with a Perma Pure dryer (type PD-625-12P). O2 and CO2 concentrations were determined with a Rosemount NGA2000 analyzer. The exhaust gas flow rate was determined and specific rates of CO2 production and O2 consumption were calculated as described previously (54, 60).
Determination of culture dry weights.
Culture dry weights were determined by filtration of samples with nitrocellulose filters and drying in a microwave oven as described previously (51).
Extracellular metabolite analysis.
Glucose, maltose, ethanol, glycerol, acetate, and pyruvate concentrations in the supernatants of chemostat cultures were determined by high-performance liquid chromatography (HPLC) analysis by using an HPX-87H Aminex ion-exchange column (300 by 7.8 mm; Bio-Rad) at 60°C. The column was eluted with 5 mM sulfuric acid at a flow rate of 0.6 ml · min−1. Pyruvate and acetate were detected at 214 nm with a Waters 441 UV meter coupled to a Waters 741 data module. Glucose, maltose, ethanol, and glycerol were detected with an ERMA type ERC-7515A refractive index detector coupled to a Hewlett-Packard type 3390A integrator. Glucose and maltose in reservoir media were also analyzed by HPLC.
Anaerobic fermentation assays.
Samples containing exactly 200 mg (dry weight) of biomass were harvested from a steady-state chemostat culture by centrifugation (5,000 × g, 3 min) and were resuspended in 10 ml of fivefold-concentrated synthetic medium (pH 5.6). Subsequently, the cell suspensions were introduced into a thermostat-controlled (30°C) vessel. The volume of each suspension was adjusted to 40 ml with demineralized water. After 10 min of incubation, 10 ml of a maltose solution (100 g · liter−1) was added, and samples (two 1-ml samples) were taken at appropriate time intervals for 2 h. The 10-ml headspace was continuously flushed with water-saturated carbon dioxide at a flow rate of approximately 30 ml · min−1. Sugar concentrations and metabolite levels in the supernatants were determined by HPLC analysis. The ethanol concentration in the supernatant was determined by a colorimetric assay (56) by using partially purified alcohol oxidase from Hansenula polymorpha (a kind gift from Bird Engineering, Rotterdam, The Netherlands). At the end of the experiments (after 2 h) some growth had taken place (data not shown). Consequently, the levels of carbon recovery were only ca. 90% if growth of biomass was not taken into account.
Intracellular metabolite measurements.
Biomass samples (4 ml of a 4-g [dry weight]/liter suspension) were taken from an anaerobic fermentation assay mixture and immediately quenched with 20 ml of 60% methanol at −40°C. After the cells were washed twice with cold 60% methanol, intracellular metabolites were extracted by resuspending the cell pellets in 5 ml of boiling 75% ethanol and incubating them for 3 min at 80°C (19). Cell debris and intracellular metabolites were dried at room temperature with a vacuum evaporator (type AES1010 Savant Automatic Environmental SpeedVac system). Finally, 0.5 ml of demineralized water was added to each preparation. The resulting suspension was stored at −20°C. Before metabolite analysis the suspension was centrifuged. The ATP content was determined by a commercial bioluminometric luciferase assay (catalog no. 1699695; Roche, Almere, The Netherlands). Glucose 6-phosphate was measured by monitoring its conversion by NADP+-dependent glucose-6-phosphate dehydrogenase (obtained from Roche). The reaction was carried out in the presence of 0.04 mM NADP+ and 0.05 U of glucose-6-phosphate dehydrogenase. Microtiter plates with total volumes of 100 and 60 μl were used for analysis of ATP and quantification of NADPH formation, respectively, in the glucose 6-phosphate measurements. A Mediators PHL luminometer (Mediators Diagnostic Systems, Vienna, Austria) was used to analyze the bioluminescence, and a Perkin-Elmer HTS 7000 Plus bioassay reader was used to quantify NADPH formation. Glucose contents were determined with a commercial kit (catalog no. 716251; Boehringer) based on conversion of glucose via hexokinase and NADP+-dependent glucose-6-phosphate-dehydrogenase. Glucose assays were performed with an Amersham Pharmacia Biotech NovaspecII spectrophotometer by using 1-ml cuvettes. Intracellular maltose contents were determined by HPLC analysis by using an Aminex HPX-87K column (300 by 7.8 mm; Bio-Rad) at 85°C. The column was eluted with demineralized water at a flow rate of 0.5 ml · min−1. Maltose was detected with a TSP type RI-150 refractive index detector. Intracellular metabolite concentrations were calculated based on the assumption that 1 g of biomass has a cellular volume of 2 ml.
Fluorescent staining for yeast viability.
A commercial LIVE/DEAD yeast viability kit (L-7009; Molecular Probes, Leiden, The Netherlands) was used to estimate the fractions of dead cells in samples obtained from anaerobic fermentation assays. FUN-1 and Calcofluor White M2R cell stain were added to yeast cell suspensions (106 to 107 cells/ml) at final concentrations of 5 to 20 and 25 μM, respectively. After staining, the suspensions were mixed thoroughly and incubated in the dark at 30°C for 30 min. Five microliters of a stained yeast suspension was trapped between a coverslip and an object slide and analyzed with a fluorescence microscope (Zeiss Axioplan 2 Imaging, Weesp, The Netherlands) by using appropriate filter sets (fluorescein isothiocyanate, Zeiss 09 450-490 FT510 LP515; and 4′,6′-diamidino-2-phenylindole [DAPI], Zeiss 02 G365 FT395 LP420).
Maltase activity assay.
As a check for extracellular maltase activity, a standard anaerobic fermentation assay was performed. At 0, 30, and 60 min, a sample (2 ml) was centrifuged. Each supernatant (1 ml) was incubated at 30°C. Samples were taken at different times and analyzed for glucose by using the UV method (Boehringer kit no. 716251). A 10% (wt/vol) maltose solution in water was used as a negative control.
Protein concentration determination.
Protein concentrations in the supernatants of anaerobic fermentation assay mixtures and in cell extracts were estimated by the method of Lowry et al. (31). Dried bovine serum albumin (fatty acid free; obtained from Sigma, Zwijndrecht, The Netherlands) was used as a standard.
Determination of viable counts.
Viable counts of S. cerevisiae CEN.PK113-7D were determined on 2% (wt/vol) YPD agar plates. This complex medium contained (per liter) 10 g of yeast extract (Difco, Detroit, Mich.), 20 g of peptone from casein (Merck, Darmstadt, Germany), 20 g of d-glucose, and 20 g of agar (Difco). After appropriate dilution of the culture and plating (which yielded 50 to 400 colonies per plate), colonies were counted following 48 h of incubation at 30°C. At least 1,000 colonies were counted to calculate viable counts.
RESULTS
Release of glucose during maltose fermentation.
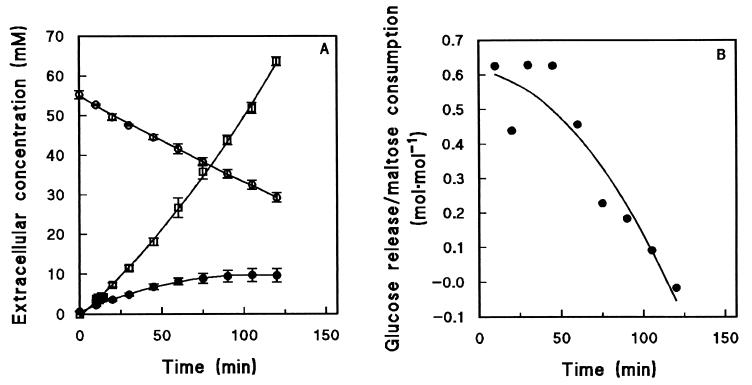
When anaerobic maltose fermentation was studied with S. cerevisiae CEN.PK113-7D pregrown in aerobic, maltose-limited chemostat cultures, substantial amounts of glucose were produced in addition to fermentation products like ethanol and glycerol (Fig. 2A). The highest rate of glucose production (dglucose/dt) took place during the first 45 min after maltose addition. During this period, ca. 0.6 mol of glucose was released for each 1 mol of maltose consumed (Fig. 2B), corresponding to 30% of the maltose carbon. After 45 min, the amount of glucose released gradually decreased, and after ca. 2 h no further net production of glucose occurred (Fig. 2). When similar experiments were performed with cells pregrown in aerobic, glucose-limited chemostat cultures, the initial maltose consumption rates were low. No glucose release was observed during the slow induction of maltose-fermenting capacity (data not shown).
FIG. 2.
Anaerobic fermentation of maltose by cells harvested from an aerobic, maltose-limited chemostat culture of S. cerevisiae CEN.PK113-7D and subsequently exposed to excess maltose. The values are averages ± mean deviations for three experiments with cells from independent chemostat cultures. (A) Extracellular metabolite concentrations as determined by pulse assays of three independent cultures. Symbols: ○, maltose; •, glucose; □, ethanol. (B) Amount of glucose released per mole of maltose consumed during the anaerobic maltose fermentation experiment, calculated by dividing the slopes of the glucose and maltose curves in panel A.
To investigate whether glucose release also occurs in other wild-type S. cerevisiae strain backgrounds, anaerobic maltose fermentation experiments were performed with maltose-grown cultures of the industrial baker's yeast strain S. cerevisiae DS28911 (51, 52) and the laboratory strain S. cerevisiae CBS8066. Qualitatively, these two strains exhibited product formation profiles that were very similar to that of the CEN.PK113-7D strain (data not shown). The maximum glucose concentrations observed under standardized conditions were 9, 4, and 12 mM for strains CEN.PK.113-7D, DS28911, and CBS8066, respectively.
These results indicate that glucose release during maltose fermentation is a general phenomenon in S. cerevisiae cultures but that the amount of glucose released is strain dependent.
Glucose release is not caused by cell lysis.
Postma et al. (41) observed cell death and release of proteins after exposure of aerobic, maltose-limited chemostat cultures of S. cerevisiae CBS8066 to excess maltose. To investigate whether the glucose release observed during anaerobic incubation of maltose-grown S. cerevisiae CEN.PK113-7D with excess maltose (Fig. 2) was due to cell lysis, possibly accompanied by the release of maltase into the extracellular medium, several control experiments were performed.
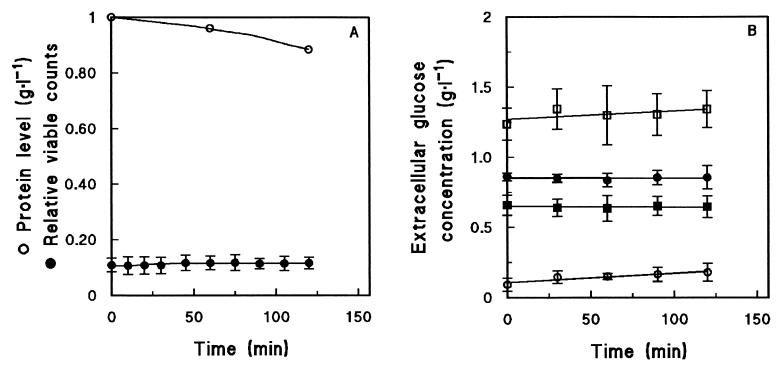
Plating on complex medium did not reveal a marked decrease in viable counts during anaerobic maltose fermentation (Fig. 3A). During the first 1 h of the experiments, during which glucose release was most pronounced (Fig. 2), the viable counts were reduced by only ca. 4% (Fig. 3A). These results were corroborated by a fluorescent live-dead staining technique, which indicated that throughout the pulse experiments virtually all cells remained metabolically active (data not shown). As a further indicator of possible cell lysis, protein concentrations were analyzed in culture supernatants (29). No significant increase in the extracellular protein concentration was observed during the pulse (Fig. 3A). More specifically, the possibility that the extracellular glucose encountered during anaerobic maltose fermentation was due to an extracellular maltase was investigated. Incubation of supernatant samples taken during the maltose fermentation experiments did not reveal any extracellular maltase activity (Fig. 3B).
FIG. 3.
Controls for cell integrity and viability during anaerobic fermentation of maltose by cells harvested from aerobic, maltose-limited chemostat cultures of S. cerevisiae CEN.PK113-7D. (A) Extracellular protein concentrations and fraction of viable cells as determined from plate counts. The values are averages ± mean deviations for two experiments performed with cells from independent chemostat cultures. (B) Extracellular maltase activity, plotted as glucose concentration during incubation of supernatant samples taken at different times during anaerobic fermentation experiments. Symbols: ○, zero-time supernatant; •, 30-min supernatant; □, 60-min supernatant; ▪, maltose control (100 g · liter−1), revealing contamination of commercially available maltose with glucose. The values are averages ± mean deviations for two experiments performed with cells from independent chemostat cultures.
Absence of glucose release during maltose fermentation in an hxt null strain.
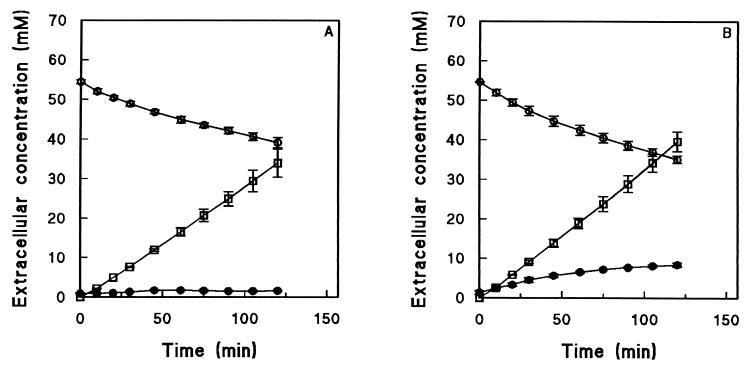
To investigate whether glucose release during anaerobic maltose fermentation was mediated by plasma membrane glucose transporters (Fig. 1), anaerobic maltose fermentation experiments were performed with the hxt null strain S. cerevisiae EBY.VW.400. In this strain, all members of the HXT gene family have been deleted, which eliminates glucose uptake via facilitated diffusion (58). S. cerevisiae EBY.VW.4000 is a member of the CEN.PK strain family, but in contrast to the prototrophic CEN.PK113-7D strain, it carries four auxotrophic markers. Duplicate control experiments performed with cells from independent maltose-grown chemostat cultures of the isogenic, auxotrophic reference strain CEN.PK2-1C yielded the same glucose and ethanol profiles as the experiments performed with the prototrophic CEN.PK113-7D strain (data not shown). In contrast, hardly any extracellular glucose (maximum concentration, 1.6 ± 0.1 mM) was found during anaerobic maltose fermentation experiments performed with the hxt null strain (Fig. 4). The concentrations of other extracellular metabolites (ethanol, glycerol, and acetate) were comparable to those in the reference strains (Fig. 2A and 4).
FIG. 4.
Anaerobic fermentation of maltose by cells harvested from aerobic, maltose-limited chemostat cultures of S. cerevisiae EBY.VW.4000 (Δhxt1 through -17 Δgal2 Δagt1 ΔYDL247w ΔYJR160c) (A) and S. cerevisiae CEN.PK2-1C (reference strain) (B). Extracellular concentrations of maltose (○), glucose (•), and ethanol (□) are shown. The values are averages ± mean deviations for two experiments performed with cells from independent chemostat cultures.
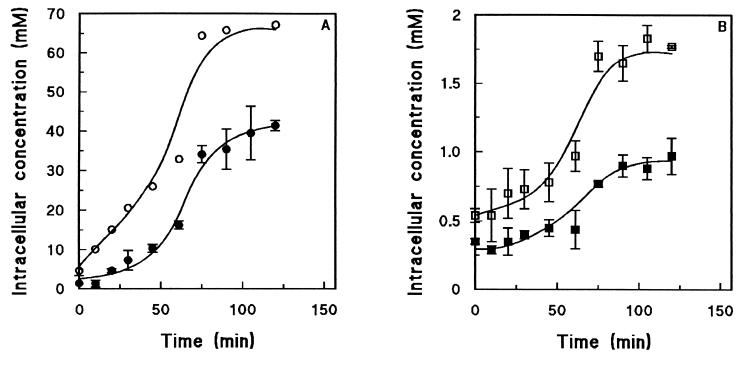
The strongly reduced glucose release by the hxt null strain suggests that one or more HXT-encoded hexose transporters are involved in glucose efflux. To investigate whether the absence of functional hexose transporters led to intracellular accumulation of glucose, intracellular metabolite assays were performed during anaerobic maltose fermentation by S. cerevisiae EBY.VW.4000. In this experiment, the intracellular glucose levels increased to 41.4 ± 1.3 mM during anaerobic maltose fermentation by the hxt null strain (Fig. 5A). The intracellular concentrations of glucose 6-phosphate and ATP were 1.8 ± 0.1 and 1.0 ± 0.1 mM, respectively (Fig. 5B). High concentrations of intracellular maltose (ca. 130 mM) accumulated during anaerobic maltose fermentation, suggesting that maltase activity was saturated under the experimental conditions.
FIG. 5.
Intracellular metabolite levels during anaerobic maltose fermentation by maltose-pregrown cells of S. cerevisiae EBY.VW.4000 (Δhxt1 through -17 Δgal2 Δagt1 ΔYDL247w ΔYJR160c). (A) Symbols: ○, maltose; •, glucose. (B) Symbols: □, glucose 6-phosphate; ▪, ATP. Most of the values are averages ± mean deviations for two experiments performed with cells from independent chemostat cultures; the only exceptions are the maltose values, which are values from a single experiment.
DISCUSSION
The results presented in this paper demonstrate that one or more HXT-encoded glucose transporters are involved in glucose efflux during exposure of S. cerevisiae to excess maltose. The reversibility of glucose transport has been demonstrated in previous studies with kinase-less mutants (14, 15). However, to our knowledge the present study provides the first experimental proof that Hxt transporters are involved in glucose export. There is no reason to assume that the ability to export glucose is confined to one or a few members of the Hxt family. However, as has been demonstrated for glucose uptake via the individual Hxt transporters, it is likely that the kinetic properties of the Hxt-encoded transporters for glucose efflux are different (44, 57).
The simultaneous uptake of maltose and efflux of glucose result in reduced ATP yields from maltose dissimilation. When the protons symported with maltose are expelled via the plasma membrane ATPase complex, the combination of maltose uptake via proton symport and glucose efflux via facilitated diffusion results in a net hydrolysis of ATP (Fig. 1). The physiological response of S. cerevisiae to excess maltose reported here is less dramatic than that reported in a previous study (41), in which exposure to excess maltose resulted in a loss of viability and cell lysis. The reason for this difference is not known but may be related to the use of a different strain background. Furthermore, the experimental conditions were different; in contrast to the data obtained in the present study, the data reported by Postma et al. (41) were obtained with aerobic, respiring cultures.
The detrimental effects of an imperfect balance between maltose uptake and glucose dissimilation are likely to be relevant for the development of maltose-constitutive S. cerevisiae strains for baker's yeast production and brewing. For example, constitutive overproduction of maltose permease and maltase, which has been proposed as a means to increase fermentative capacity with maltose as the substrate, is likely to result in an imbalance between maltose uptake and glycolysis. This complication may also occur in other cases where disaccharides are transported via proton symport and hydrolyzed intracellularly. A relevant example is the metabolic engineering of S. cerevisiae for lactose fermentation by constitutive expression of a lactose-proton symporter and intracellular beta-galactosidase (1, 47, 48).
To prevent the dissipation of metabolic energy that results from noncoordinated uptake and hydrolysis of disaccharides, two regulatory mechanisms can be envisaged. One possible mechanism would involve downregulation of disaccharide hydrolysis (for instance, by glucose inhibition of the disaccharide hydrolase). Although maltase in S. cerevisiae is not known to be regulated via glucose inhibition or glucose catabolite inactivation, transcription of the MALx2 genes is subject to glucose repression (16, 17).
The second possibility is downregulation of maltose uptake to match the uptake rate to the glycolytic activity of the cells (for example, by glucose repression of the synthesis of maltose permease or glucose-induced inactivation of the maltose carriers). Albeit with different time scales, both mechanisms should lead to a situation where the level of maltose permease is adapted to the capacity of glucose metabolism. The intricate mechanisms described for glucose repression and glucose inactivation of the S. cerevisiae maltose permease are generally explained by considering glucose the preferred carbon source (32, 62). Such a preferred status of glucose can be explained by the lower ATP yield from maltose due to an energy requirement for maltose uptake (49, 59). The present study offers an alternative, additional explanation: these control systems may have evolved to prevent ATP dissipation via simultaneous energy-dependent maltose uptake and glucose efflux.
Acknowledgments
We thank Hans van Dijken for many stimulating discussions and Eckhard Boles for providing the hexose-transport-negative strain.
This work was financially supported by the Dutch Ministry of Economic Affairs via the EET program.
REFERENCES
- 1.Adam, A. C., J. A. Prieto, M. Rubio-Texeira, and J. Polaina. 1999. Construction of a lactose-assimilating strain of baker's yeast. Yeast 15:1299-1305. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, J. A. 1981. The utilization of disaccharides and some other sugars by yeasts. Adv. Carbohydr. Chem. Biochem. 39:347-404. [DOI] [PubMed] [Google Scholar]
- 3.Bell, P., V. J. Higgins, and P. V. Attfield. 2001. Comparison of fermentative capacities of industrial baking and wild-type yeasts of the species Saccharomyces cerevisiae in different sugar media. Lett. Appl. Microbiol. 32:224-229. [DOI] [PubMed] [Google Scholar]
- 4.Brondijk, T. H. C., W. N. Konings, and B. Poolman. 2001. Regulation of maltose transport in Saccharomyces cerevisiae. Arch. Microbiol. 176:96-105. [DOI] [PubMed] [Google Scholar]
- 5.Brondijk, T. H. C., M. E. van der Rest, D. Pluim, Y. de Vries, K. Stingl, B. Poolman, and W. N. Konings. 1998. Catabolite inactivation of wild-type and mutant maltose transport proteins in Saccharomyces cerevisiae. J. Biol. Chem. 273:15352-15357. [DOI] [PubMed] [Google Scholar]
- 6.Cazzador, L. 1991. Analysis of oscillations in yeast continuous cultures by a new simplified model. Bull. Math. Biol. 53:685-700. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y. S., R. A. Dubin, E. Perkins, D. Forrest, C. A. Michels, and R. B. Needleman. 1988. MAL63 codes for a positive regulator of maltose fermentation in Saccharomyces cerevisiae. Curr. Genet. 14:201-209. [DOI] [PubMed] [Google Scholar]
- 8.Charron, M. J., E. Read, S. R. Haut, and C. A. Michels. 1989. Molecular evolution of the telomere-associated MAL loci of Saccharomyces. Genetics 122:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, Q., and C. A. Michels. 1991. MAL11 and MAL61 encode the inducible high-affinity maltose transporter of Saccharomyces cerevisiae. J. Bacteriol. 173:1817-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dequin, S. 2001. The potential of genetic engineering for improving brewing, wine-making and baking yeasts. Appl. Microbiol. Biotechnol. 56:577-588. [DOI] [PubMed] [Google Scholar]
- 11.Diderich, J. A., L. M. Raamsdonk, A. L. Kruckeberg, J. A. Berden, and K. van Dam. 2001. Physiological properties of Saccharomyces cerevisiae from which hexokinase II has been deleted. Appl. Environ. Microbiol. 67:1587-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diderich, J. A., M. Schepper, P. van Hoek, M. A. H. Luttik, J. P. van Dijken, J. T. Pronk, P. Klaassen, H. F. M. Boelens, M. J. Teixeira de Mattos, K. van Dam, and A. L. Kruckeberg. 1999. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J. Biol. Chem. 274:15350-15359. [DOI] [PubMed] [Google Scholar]
- 13.Dubin, R. A., R. B. Needleman, D. Gossett, and C. A. Michels. 1985. Identification of the structural gene encoding maltase within the MAL6 locus of Saccharomyces carlsbergenesis. J. Bacteriol. 164:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entian, K. D. 1980. A defect in carbon catabolite repression associated with uncontrollable and excessive maltose uptake. Mol. Gen. Genet. 179:169-175. [DOI] [PubMed] [Google Scholar]
- 15.Entian, K. D., and M. C. Loureiro-Dias. 1990. Misregulation of maltose uptake in a glucose repression defective mutant of Saccharomyces cerevisiae leads to glucose poisoning. J. Gen. Microbiol. 136:855-860. [DOI] [PubMed] [Google Scholar]
- 16.Federoff, H. J., T. R. Eccleshall, and J. Marmur. 1983. Carbon catabolite repression of maltase synthesis in Saccharomyces carlsbergensis. J. Bacteriol. 156:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira, J. C., A. D. Panek, and P. S. de Araujo. 2000. Inactivation of maltose permease and maltase in sporulating Saccharomyces cerevisiae. Can. J. Microbiol. 46:383-386. [DOI] [PubMed] [Google Scholar]
- 18.Goldenthal, M. J., M. Vanoni, B. Buchferer, and J. Marmur. 1987. Regulation of MAL gene expression in yeast: gene dosage effects. Mol. Gen. Genet. 209:508-517. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez, B., J. François, and M. Renaud. 1997. A rapid and reliable method for metabolite extraction in yeast using boiling buffered ethanol. Yeast 13:1347-1356. [DOI] [PubMed] [Google Scholar]
- 20.Hammond, J. R. M. 1995. Genetically-modified brewing yeasts for the 21st century. Progress to date. Yeast 11:1613-1627. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, V. J., M. Braidwood, P. Bell, P. Bissinger, I. W. Dawes, and P. V. Attfield. 1999. Genetic evidence that high noninduced maltase and maltose permease activities, governed by MALx3-encoded transcriptional regulators, determine efficiency of gas production by baker's yeast in unsugared dough. Appl. Environ. Microbiol. 65:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong, S. H., and J. Marmur. 1986. Primary structure of the maltase gene of the MAL6 locus of Saccharomyces carlsbergenesis. Gene 41:75-84. [DOI] [PubMed] [Google Scholar]
- 23.Hu, Z., A. W. Gibson, J. H. Kim, L. A. Wojciechowicz, B. Zhang, and C. A. Michels. 1999. Functional domain analysis of the Saccharomyces MAL-activator. Curr. Genet. 36:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, H., I. Medintz, and C. A. Michels. 1997. Two glucose sensing/signaling pathways stimulate glucose-induced inactivation of maltose permease in Saccharomyces. Mol. Biol. Cell 8:1293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang, H., I. Medintz, B. Zhang, and C. A. Michels. 2000. Metabolic signals trigger glucose-induced inactivation of maltose permease in Saccharomyces. J. Bacteriol. 182:647-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein, C. J. L., L. Olsson, B. Ronnow, J. D. Mikkelsen, and J. Nielsen. 1996. Alleviation of glucose repression of maltose metabolism by MIG1 disruption in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 62:4441-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein, C. J. L., J. L. Rasmussen, B. Ronnow, L. Olsson, and J. Nielsen. 1999. Investigation of the impact of MIG1 and MIG2 on the physiology of Saccharomyces cerevisiae. J. Biotechnol. 68:197-212. [DOI] [PubMed] [Google Scholar]
- 28.Kompala, D. S. 1999. Cybernetic modeling of spontaneous oscillations in continuous cultures of Saccharomyces cerevisiae. J. Biotechnol. 71:267-274. [DOI] [PubMed] [Google Scholar]
- 29.Kruckeberg, A. L. 1996. The hexose transporter family of Saccharomyces cerevisiae. Arch. Microbiol. 166:283-292. [DOI] [PubMed] [Google Scholar]
- 30.Lagunas, R. 1986. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast 2:221-228. [DOI] [PubMed] [Google Scholar]
- 31.Lowry, O. H., H. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 32.Medintz, I., H. Jiang, E. Han, and W. Cui. 1996. Characterization of the glucose-induced inactivation of maltose permease in Saccharomyces cerevisiae. J. Bacteriol. 178:2245-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medintz, I., H. Jiang, and C. A. Michels. 1998. The role of ubiquitin conjugation in glucose-induced proteolysis of Saccharomyces maltose permease. J. Biol. Chem. 273:34454-34462. [DOI] [PubMed] [Google Scholar]
- 34.Medintz, I., X. Wang, T. Hradek, and C. A. Michels. 2000. A PEST-like sequence in the N-terminal cytoplasmic domain of Saccharomyces maltose permease is required for glucose-induced proteolysis and rapid inactivation of transport activity. Biochemistry 39:4518-4526. [DOI] [PubMed] [Google Scholar]
- 35.Naumov, G. I., E. S. Naumova, and C. A. Michels. 1994. Genetic variation of the repeated MAL loci in natural populations of Saccharomyces cerevisiae and Saccharomyces paradoxus. Genetics 136:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needleman, R. B. 1991. Control of maltase synthesis in yeast. Mol. Microbiol. 5:2079-2084. [DOI] [PubMed] [Google Scholar]
- 37.Needleman, R. B., D. B. Kaback, R. A. Dubin, E. L. Perkins, N. G. Rosenberg, K. A. Sutherland, D. B. Forrest, and C. A. Michels. 1984. MAL6 of Saccharomyces: a complex genetic locus containing three genes required for maltose fermentation. Proc. Natl. Acad. Sci. USA 81:2811-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oura, E. 1972. Reactions leading to the formation of yeast cell material from glucose and ethanol. Ph.D. thesis. University of Helsinki, Helsinki, Finland.
- 39.Özcam, S., and M. Johnston. 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63:554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penalver, E., P. Lucero, E. Moreno, and R. Lagunas. 1998. Catabolite inactivation of the maltose transporter in nitrogen-starved yeast could be due to stimulation of general protein turnover. FEMS Microbiol. Lett. 166:317-324. [DOI] [PubMed] [Google Scholar]
- 41.Postma, E., C. Verduyn, A. Kuiper, W. A. Scheffers, and J. P. van Dijken. 1990. Substrate-accelerated death of Saccharomyces cerevisiae CBS 8066 under maltose stress. Yeast 6:149-158. [DOI] [PubMed] [Google Scholar]
- 42.Randez-Gil, F., P. Sanz, and J. A. Prieto. 1999. Engineering baker's yeast: room for improvement. Tibtech 17:237-244. [DOI] [PubMed] [Google Scholar]
- 43.Reifenberger, E., E. Boles, and M. Ciriacy. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur. J. Biochem. 245:324-333. [DOI] [PubMed] [Google Scholar]
- 44.Reifenberger, E., K. Freidel, and M. Ciriacy. 1995. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol. Microbiol. 16:157-167. [DOI] [PubMed] [Google Scholar]
- 45.Riballo, E., M. Herweijer, D. H. Wolf, and R. Lagunas. 1995. Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J. Bacteriol. 177:5622-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson, K. S., K. Lai, T. A. Cannon, and P. McGraw. 1996. Inositol transport in Saccharomyces cerevisiae is regulated by transcriptional and degradative endocytic mechanisms during the growth cycle that are distinct from inositol-induced regulation. Mol. Biol. Cell 7:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubio-Texeira, M., M. Arevalo-Rodriguez, J. L. Lequerica, and J. Polaina. 2000. Lactose utilization by Saccharomyces cerevisiae strains expressing Kluyveromyces lactis LAC genes. J. Biotechnol. 84:97-106. [DOI] [PubMed] [Google Scholar]
- 48.Rubio-Texeira, M., J. A. Castrillo, A. C. Adam, U. O. Ugalde, and J. Polaina. 1998. Highly efficient assimilation of lactose by a metabolically engineered strain of Saccharomyces cerevisiae. Yeast 14:827-837. [DOI] [PubMed] [Google Scholar]
- 49.Serrano, R. 1977. Energy requirements for maltose transport in yeast. Eur. J. Biochem. 80:97-102. [DOI] [PubMed] [Google Scholar]
- 50.van Dijken, J. P., J. Bauer, L. Brambilla, P. Duboc, J. Francois, C. Gancedo, M. L. F. Giuseppin, J. J. Heijnen, M. Hoare, H. C. Lange, E. A. Madden, P. Niederberger, J. Nielsen, J. L. Parrou, T. Petit, D. Porro, M. Reuss, N. van Riel, M. Rizzi, H. Y. Steensma, C. T. Verrips, J. Vindelov, and J. T. Pronk. 2000. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 26:706-714. [DOI] [PubMed] [Google Scholar]
- 51.van Hoek, P., J. P. van Dijken, and J. T. Pronk. 1998. Effect of specific growth rate on fermentative capacity of baker's yeast. Appl. Environ. Microbiol. 64:4226-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hoek, P., J. P. van Dijken, and J. T. Pronk. 2000. Regulation of fermentative capacity and levels of glycolytic enzymes in chemostat cultures of Saccharomyces cerevisiae. Enzyme Microb. Technol. 26:724-736. [DOI] [PubMed] [Google Scholar]
- 53.van Leeuwen, C. C. M., R. A. Weusthuis, E. Postma, P. J. A. van den Broek, and J. P. van Dijken. 1992. Maltose/proton co-transport in Saccharomyces cerevisiae. Biochem. J. 284:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Urk, H., P. R. Mak, W. A. Scheffers, and J. P. van Dijken. 1988. Metabolic responses of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 upon transition from glucose limitation to glucose excess. Yeast 4:283-291. [DOI] [PubMed] [Google Scholar]
- 55.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous study on regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 56.Verduyn, C., J. P. van Dijken, and W. A. Scheffers. 1984. Colorimetric alcohol assays with alcohol oxidase. J. Microbiol. Methods 2:15-25. [Google Scholar]
- 57.Walsh, M. C., H. P. Smits, M. Scholte, and K. van Dam. 1994. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J. Bacteriol. 176:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wendell, D. L., and L. F. Bisson. 1993. Physiological characterization of putative high-affinity glucose transport protein Hxt2 of Saccharomyces cerevisiae by use of anti-synthetic peptide antibodies. J. Bacteriol. 175:7689-7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weusthuis, R. A., H. Adams, W. A. Scheffers, and J. P. van Dijken. 1993. Energetics and kinetics of maltose transport in Saccharomyces cerevisiae: a continuous culture study. Appl. Environ. Microbiol. 59:3102-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weusthuis, R. A., M. A. H. Luttik, W. A. Scheffers, J. P. van Dijken, and J. T. Pronk. 1994. Is the Kluyver effect in yeast caused by product inhibition? Microbiology 140:1723-1729. [DOI] [PubMed] [Google Scholar]
- 61.Wieczorke, R., S. Krampe, T. Weierstall, K. Freidel, C. P. Hollenberg, and E. Boles. 1999. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464:123-128. [DOI] [PubMed] [Google Scholar]
- 62.Yao, B., P. Sollitti, X. Zhang, and J. Marmur. 1994. Shared control of maltose induction and catabolite repression of the MAL structural genes in Saccharomyces. Mol. Gen. Genet. 243:622-630. [DOI] [PubMed] [Google Scholar]