Abstract
The gut microbiome is widely accepted to play a crucial role in human health and disease. These intestinal microbiota are not only involved in gastrointestinal physiology, but they also contribute to essential immune modulation and metabolic homeostasis. Growing evidence suggests that alterations in the gut microbiota composition are linked to various metabolic disorders, including obesity and age-related diseases. Obesity, a global public health concern, is associated with shifts in microbial diversity and functionality, which influence energy extraction, fat storage, and systemic inflammation. Similarly, age-related disorders, such as neurodegenerative diseases, sarcopenia, and metabolic syndromes are linked to gut microbiome alterations that exacerbate chronic inflammation and metabolic dysfunction. Understanding the intricate relationship between the gut microbiome, obesity, and aging-related pathologies is essential for developing targeted microbiome-based interventions to mitigate these health challenges. In this review, after a brief summary of the development of the gut microbiome across the lifespan and its modulating factors, we will focus on the mechanisms underlying the relation between the gut microbiome and metabolic and aging-related disorders. Finally, the findings of interventional studies underscoring causality and the potential future directions will be discussed, with a focus on the possibility of modifying the progression of metabolic and age-related diseases through the modulation of the gut microbiome.
Keywords: fatty liver disease, gut microbiota, MASLD, senescence, obesity, aging
The heterogeneity of human aging cannot be fully explained by genetic differences alone [1]. This observation highlights the influence of exogenous factors, such as the gut microbiota, in shaping the aging process. In recent years, the gut microbiome—the vast community of microorganisms, including bacteria, fungi, viruses, and archaea, residing in the human gastrointestinal tract—has emerged as a pivotal area of study in health and disease [2, 3]. These microbial communities dynamically interact with their host, including through the production of (diet-derived) microbial metabolites that influence metabolism, immunity, and neurological function—all of which are closely tied to aging. The gut microbiome has been taken up in the hallmarks of aging, underscoring its potential role as both a marker and driver of age-related decline [4].
Despite remarkable interindividual variability—driven by factors such as diet, lifestyle, geography, and genetics—the gut microbiome exhibits certain universal patterns in composition, diversity, and functional capacity across the human lifespan [3]. Notably, compositional and functional shifts in the microbiome have been observed in obesity and other age-related conditions, such as type 2 diabetes and its complications, including kidney disease, heart disease, and metabolic dysfunction–associated steatotic liver disease (MASLD; formerly known as nonalcoholic fatty liver disease) [5-8]. These changes underscore the gut microbiome's role in metabolic (low-grade inflammation-driven) dysregulation and systemic inflammation, both of which exacerbate aging processes.
In this review, we explore the development of the gut microbiome across the human lifespan and examine how it is shaped by factors such as diet, medication use, and environmental influences, with a particular focus on its alterations in obesity and obesity-related diseases. We further explore how these microbial changes contribute to the aging process and metabolic disorders. Finally, we look into recent intervention studies and discuss future perspectives for microbiome-targeted therapies aimed at promoting healthy aging.
Temporal Development of the Human Gut Microbiome Across the Lifespan
The existence of a fetal microbiome remains controversial [9-12]; the first significant microbial colonization of the infant gut occurs at birth, influenced by the mode of delivery (vaginal vs cesarean). This is reflected in distinct gut microbial compositions between vaginally vs cesarean-delivered infants during their first months of life [13-15]. After delivery, the gut microbiome matures, marked by increased microbial diversity [15-19]. Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria dominate throughout infancy and later life [15, 19-22]. Initially, facultative anaerobes and aerobes from Proteobacteria dominate, but strict anaerobes from the other 3 phyla become dominant as the infant matures [23-25]. Breastfeeding promotes Bifidobacterium colonization [21], while weaning increases Bacteroides and Clostridium, shifting the microbiome toward an adult-like composition [19, 20]. Though this transition was postulated to more or less conclude in early childhood [26], older children and even adolescents show significant differences in gut microbiome composition and lower bacterial diversity compared to adults [27-29]. Alongside these compositional changes, the maturing infant gut microbiome diversifies with alpha diversity increasing after cessation of breastfeeding and plateauing in early adulthood [30].
In adulthood, stability prevails, as described in a study of over 6500 fecal samples from healthy individuals [31]. Bacterial species richness differed significantly only between young children and other age groups, with 50 to 70 core species (prevalence ≥50%, abundance >0.1%) across most groups, except children under 4 years of age, in whom only 7 species were identified. Pregnancy may alter this, with studies reporting temporal compositional changes [32-35] and reduced alpha diversity [33-35], although others find marginal or no change [36, 37]. With advancing age, diversity declines and structure shifts. Centenarian studies show reduced Bacteroides and Roseburia, with enrichment of Bifidobacterium and Akkermansia linked to longevity [38]. These observations have been expanded by a comprehensive 2021 study analyzing gut microbiome and phenotypic data from over 9000 individuals across 3 independent cohorts, aged 18 to 101 years [39]. This analysis demonstrated that the individual gut microbial composition becomes increasingly distinct with age, a trait linked to microbial metabolites implicated in immune modulation, inflammation, and aging processes. In healthy older adults, this drift toward uniqueness persists, whereas it diminishes or stalls in those with poorer health. Notably, healthy aging is marked by a depletion of ubiquitous core taxa like Bacteroides, and in individuals nearing extreme age, the persistence of high Bacteroides abundance combined with low microbial uniqueness correlates strongly with reduced survival [39].
Contrasting findings emerge from other cohorts. The ELDERMET study [40] reported an elevated presence of core genera such as Bacteroides, Alistipes, and Parabacteroides in older individuals compared to younger controls, alongside age-related compositional shifts associated with frailty, cognitive decline, depression, and inflammation. Another cross-population study identified consistent age-related microbial trajectories across diverse ethnicities, noting a reduction in sex-specific microbial differences and an increased abundance of health-associated species, including Akkermansia, in older adults [41]. These discrepancies suggest that physiological changes tied to aging—beyond diet and lifestyle—profoundly influence gut microbiota dynamics.
The centenarian signature is marked by increased abundance of taxa such as Clostridium, Methanobrevibacter, and Synergistetes, while Eubacterium rectale and Faecalibacterium prausnitzii are relatively depleted [42, 43]. Interestingly, it was recently shown that centenarians possess a distinct and highly diverse gut virome, which shows close associations with specific gut bacteria such as Alistipes and Clostridium [44]. In addition, the centenarian gut harbors viruses encoding specific genes that aid bacterial production of sulfide, a compound implicated in the aging process [45].
While the gut microbiome in healthy aging drifts toward uniqueness and metabolic resilience, marked by taxa like Akkermansia and beneficial metabolites, this pattern is starkly absent in obesity. Across the lifespan, obese individuals exhibit a dysbiotic microbiome that deviates from the healthy aging trajectory, marked by reduced diversity, dominance of taxa such as Bacteroides or Proteobacteria, and a pro-inflammatory metabolite profile [46]. Indeed, systematic reviews highlight these differences across pediatric [47, 48], adult [47, 49] and elderly [50] populations with obesity. In children with obesity, Eubacterium hallii (now renamed Anaerobutyricum soehngenii) and Lactobacillus spp. were found to be decreased [48]. The former is a short-chain fatty acid (SCFA)-producer [51] that was shown to improve insulin resistance in diabetic mice [52], and a first-in-human clinical trial demonstrated a small but potentially clinically relevant improvement in glucose variability in individuals with obesity and type 2 diabetes [53]. Meanwhile, certain Lactobacillus species are known for their anti-obesity properties [54] that rely on their ability to decrease leptin levels and regulate lipid metabolism [55, 56]. The abundance of Akkermansia muciniphila is decreased in children with obesity [48], as it is in both adult and elderly obese individuals [49, 50]. Recent studies have confirmed the inverse relation between Akkermansia and obesity in large cohorts [57], while multiple interventional studies in mice and humans have shed a light on the potential of A. muciniphila as a probiotic treatment of overweight and obesity [58].
It should be noted that international cohort studies have identified differences in gut microbiome composition between the sexes, which vary across the lifespan, with sex-specific microbial divergence occurring after puberty and largely disappearing in older adults [3, 59]. These patterns suggest a role for sex hormones, supported by alterations in gut microbiome composition observed in both male and female rodents following gonadectomy (ie, removal of testes or ovaries). However, recent data from both animal and human studies indicate that the sex-specific and menopausal status-related taxonomic differences are at least partially attributable to other factors, particularly diet and age [60, 61]. In addition, another recent study investigating the role of sex differences in obesity showed that male mice gained more weight than females in response to a high-fat diet, yet this disparity remained even after antibiotic depletion of the gut microbiome [62]. This suggests that sex-dependent differences in the gut microbiome play a limited role in driving metabolic outcomes.
Factors Influencing the “Healthy” Gut Microbiome Across the Lifespan
External factors dynamically shape the gut microbiome across life stages, influencing its composition, diversity, and functionality. Starting antenatally, examples of maternal factors that influence the composition of the infant gut microbiome include smoking or exposure to smoke [63], diet [64-66], obesity [67], and inflammatory bowel disease [68, 69]. Although cesarean-born infants show different bacterial colonization and lower abundance of important genera such as Bacteroides and Bifidobacterium [70, 71], these differences appear to be temporary and limited to the first few months of life [71]. Other early-life factors include both dietary as well as family-related environmental factors, as reviewed by Dong et al [72]. Among the most significant environmental factors influencing the infant gut microbiome is antenatal and early-life exposure to antibiotics. Bacterial diversity is lower in infants born to mothers exposed to antibiotics, and differences across multiple bacterial taxa are described with lower Actinobacteria and Bacteroidetes and higher Proteobacteria on the phylum level and lower Bifidobacterium and Bacteroides on the genus level [73]. Antibiotic exposure is also associated with a higher risk of obesity later in childhood [74], and longitudinal data demonstrate that multiple antibiotic courses in early childhood increase the risk of obesity later in life [75]. Interestingly, this association was not found in toddlers born from overweight mothers, while maternal body mass index (BMI) is known to influence the infant gut microbiota. Indeed, maternal pre-pregnancy BMI is associated with an altered infant microbiome at 2 days [76], 2 weeks [77], 1 month [78], 6 months [79], and 2 years of age [79].
In adulthood, the microbiome, though relatively stable, remains responsive to external inputs. Diet is a well-known modulator of the gut microbiome [80]. For example, observational and interventional data revealed that the Mediterranean diet is associated with an increase in Faecalibacterium [81], a genus with anti-inflammatory properties whose low levels are associated with inflammatory disorders such as inflammatory bowel disease [82]. The abundance of Prevotella, whose significance in human health is more ambiguous [83], also decreased with adherence to the Mediterranean diet. By contrast, the typical Western diet (high-fat, low-fiber, and ultraprocessed food) causes a gut microbiome marked by low microbial diversity, and a decrease in taxa such as Bacteroidetes and Bifidobacteria [84]. Mice fed a Western-style diet demonstrated an increase in fecal inflammatory markers and decrease in SCFA levels, with a microbiome marked by lower bacterial diversity and species richness and high abundance of pro-inflammatory species belonging to Proteobacteria [85]. Interventions involving dietary fiber lead to increases in the abundance of the genera Bifidobacterium and Lactobacillus, although they do not change gut microbial diversity [86]. In addition, numerous interventional studies show that a low carbohydrate diet is associated with taxonomical alterations, including lower relative abundance of Firmicutes and Bifidobacterium, yet higher Bacteroidetes abundance.
In addition to diet, physical exercise has been identified as another possible modifier of the adult microbiome. Two recent meta-analyses yield conflicting results on the association between exercise interventions and changes in bacterial (alpha) diversity and taxonomical compositions of the gut microbiome in adults [87, 88]. One systematic review demonstrated a positive association between exercise interventions and alpha diversity [87], whereas the other only supported this positive association for prolonged exercise and cardiorespiratory fitness while failing to demonstrate this for short-term exercise interventions [88]. In addition, while the first analysis found a significant reduction of Bacteroidetes abundance and a significant increase in Firmicutes abundance [87], the other refrained from drawing firm conclusions in view of heterogeneity from the results of the included studies [88]. However, both studies agreed on the mediating role of short-chain fatty acids (SCFAs), bacterial metabolites linked to improved glucose metabolism [89], pointing to the positive association between exercise and both SCFAs as well as specific SCFA-producing microbiota.
Yet another modulating factor of the adult gut microbiome is altitude [90]. Recent data from longitudinal studies involving healthy Chinese men who migrated from and toward a higher latitude demonstrate the profound effect of a change in altitude on the composition and function of the gut microbiome [90, 91]. In these individuals, high altitude was associated with a decrease of α-diversity and an increased abundance of anaerobes such as Blautia and Prevotella was observed, as well as alterations of multiple functional metabolic pathways.
In the elderly, one of the most critical factors influencing the gut microbiome is their living arrangement, that is, whether they are institutionalized or community-dwelling. The gut microbiome of elderly individuals can be clustered based on their housing arrangement, with the microbiota of long-time institutionalized elderly being significantly more divergent from younger controls as compared to the microbiome of their community-dwelling peers [92]. In addition, the gut microbiome of long-term institutionalized elderly is marked by significantly lower diversity and metagenomically fewer microbial genes related to SCFA-production. It remains unclear whether these institutionalization-associated signatures are reflective of a frailty phenotype or are mainly driven by environmental changes occurring upon institutionalization, such as dietary changes, as some studies suggest [93].
Irrespective of age, both antibiotic and non-antibiotic drugs can profoundly alter the composition and functionality of the gut microbiome. Short-course antibiotic treatments cause significant yet generally transient disruptions in microbial diversity and composition, depending on multiple factors of the host and microbiome itself [94, 95]. This is exemplified by patients undergoing Helicobacter pylori eradication therapy, in whom recovery of the gut microbiome is generally observed within 1 year following this aggressive antibiotic course [96]. Similarly, certain non-antibiotic drugs, such as proton pump inhibitors and antidiabetics, can mimic the effects of antibiotics on the microbiome [97]. These effects are of particular importance in the context of prolonged use and polypharmacy, which are common among elderly populations [98]. A large Italian cohort of acutely hospitalized elderly individuals possessed a gut microbiome with reduced species richness and distinct dysbiotic bacterial composition compared to nonhospitalized elderly controls without polypharmacy. In the hospitalized cohort, a significant association between polypharmacy of dysbiosis and polypharmacy was found, which was independent of other multimorbidity parameters [99]. Similar results were found in long-term residents of nursing homes, in whom non-antibiotic medications were strong independent predictors of gut microbiome composition [100].
Given the nature of polypharmacy, little is known about the reversibility of these polypharmacy-associated changes in the microbiome. However, the dramatic changes in gut microbiota observed in polypharmacy-exposed mice were largely reversed upon cessation of medication [101].
While all these factors shape the microbiome, certain influences—particularly polypharmacy, antibiotics, and Western diets—disrupt diversity and functionality in ways resembling dysbiosis, promoting pro-inflammatory states often absent in healthy aging. In contrast, factors like exercise and Mediterranean diets support microbial resilience, mirroring patterns seen in healthy longevity. This divergence likely stems from the production of microbial metabolites with signaling properties, which will be explored in the next section.
From Composition to Functionality
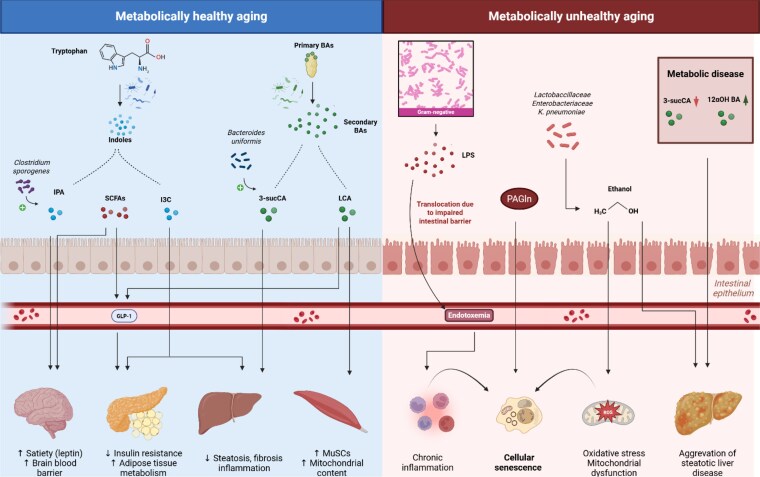
The gut microbiome exerts a profound influence on host physiology, aging, and metabolic health—primarily through its functional output rather than taxonomic composition. Microbial metabolites act as key signaling molecules, regulating inflammation, energy balance, and cellular repair. Across aging studies, metabolite profiles tend to be more consistent than microbial taxa, underscoring the importance of functionality in shaping health span and disease susceptibility (Fig. 1).
Figure 1.
Overview of various microbial metabolites and their effects on metabolic and aging-related processes.
Abbreviations: 12aOH, 12 alpha-hydroxylated; 3-sucCA, 3-succinylated cholic acids; BAs, bile acids; GLP-1, glucagon-like peptide-1; I3C, indole-3-carbinol; IPA, indole-3-propionic acid; LCA, lithocholic acid; LPS, lipopolysaccharides; MuSCs, muscle stem cells; PAGln, phenylacetylglutamine; ROS, reactive oxygen species; SCFAs, short-chain fatty acids.
Age-related microbial shifts align with several “Hallmarks of Aging” proposed by López-Otín et al [4], particularly “altered intercellular communication” and “disabled macroautophagy.” Microbial diversity supports the generation of health-promoting metabolites like SCFAs, indoles, and bile acids, which modulate immune function and maintain cellular homeostasis [102]. In healthy aging, increased microbial uniqueness and a reduction in core taxa such as Bacteroides are associated with anti-inflammatory compounds that promote autophagy and metabolic resilience [103]. In contrast, reduced diversity and sustained Bacteroides dominance correlate with higher levels of harmful metabolites such as p-cresol, which impair signaling and contribute to aging-related decline [103].
Alterations in the microbiome seen in obesity shift metabolic outputs, favoring the production of compounds such as endogenous ethanol. Ethanol disrupts mitochondrial function, promotes oxidative stress, and has been implicated in the acceleration of aging phenotypes [104]. Similarly, increased levels of lipopolysaccharide (LPS)—a component of gram-negative bacterial membranes—can enter systemic circulation due to compromised intestinal barrier function. This low-grade endotoxemia contributes to chronic inflammation and is linked to age-related and metabolic diseases such as diabetes and MASLD [105].
Disruption of gut barrier integrity further connects the microbiome to liver pathology via the gut-liver axis. When intestinal permeability increases, microbial metabolites and endotoxins leak into the portal circulation, reaching the liver and activating inflammatory pathways that contribute to steatosis and fibrosis [106]. Meta-analyses confirm significantly higher intestinal permeability in both pediatric and adult MASLD patients, which worsens with disease progression [107].
While taxonomic diversity provides a structural overview of the microbiome, its functional capacity, especially metabolite production, more accurately reflects its impact on aging [108]. Within the framework of altered intercellular communication, these microbial metabolites serve as messengers linking gut activity to systemic physiological processes [109, 110]. Understanding their biosynthesis and biological effects is essential to unraveling the microbiome's role in longevity and age-related disease.
Bile Acids
Bile acids are among the most significant microbiota-derived metabolites. Long known for their role in lipid digestion and therapeutic potential in gallstones, it was not until the early 2000s that these cholesterol-derived steroids were identified as ligands for various nuclear receptors such as the farnesoid X (FXR), vitamin D receptor (VDR), pregnate x receptor (PXR), and constitutive androstane receptor (CAR) [111]. Through the signaling pathways downstream of these receptors, bile acids influence a wide range of metabolic processes, including lipid, glucose, and drug metabolism. Accordingly, bile acids are implicated in metabolic disorders such as obesity and MASLD [112].
Bile acids show a bidirectional relationship with the intestinal microbiota. Despite their bacteriotoxic properties, bile acids are known to support microbial diversity by altering gut microbiome composition [113]. Conversely, gut bacteria regulate hepatic bile acid synthesis and synthesize enzymes crucial for the biotransformation of primary conjugated bile acids to secondary bile acids [114].
In mice, administration of lithocholic acid (LCA) ameliorated aging-related phenotype with improvement of both muscle and metabolic function. LCA was shown to induce muscle stem cells and increase mitochondrial content and respiratory function of muscle tissue, while improving insulin resistance and increasing serum glucagon-like peptide 1 (GLP-1) levels [115]. These effects are explained by the ability of LCA to activate the protein kinase AMPK, a “master regulator” of energy homeostasis that also plays an important role in regulating various aging-associated signaling pathways [116].
In humans, there is a positive correlation between BMI and total plasma bile acids [117]. In people with obesity there is a shift in the synthetic pathways of bile acids toward 12α-hydroxylated bile acids compared to lean individuals [118], and this change is also seen in metabolically unhealthy obese vs healthy obese counterparts [119]. However, data on differences in bile acid levels and compositions in humans remain scarce and should be interpreted with caution given high interindividual variety and discrepancies between portal vein and peripheral bile acid pools [112]. Indeed, it was recently shown that bile acid levels in patients with severe obesity were 7-fold higher in the portal vein compared to peripheral levels [120].
A recent study characterized the secondary bile acid 3-succinylated cholic acid and its synthesization by an enzyme expressed in Bacteroides uniformis [121]. This bile acid, which is depleted in MASLD patients, was shown to decrease hepatic steatosis, fibrosis, and inflammation by directly promoting the growth of A. muciniphila, while suppressing Clostridium sporogenes and Enterococcus hirae.
New research in the past years has uncovered a suite of biosynthetic enzymes—such as those driving 5α-reduction, 7α-dehydroxylation, and 24-reconjugation—that mediate microbial bile acid transformation [42, 122]. These processes may generate a plethora of unique bile acid structures, underscoring the complexity of microbial metabolism and signaling a need to redefine the intricate interplay between the gut microbiota and its host in shaping health and aging.
Short-Chain Fatty Acids
Numerous bacterial species inhabiting the gut are involved in the fermentation of fiber, which leads to the production of SCFAs, the most important of which are acetate, butyrate, and propionate. SCFA levels vary across the lifespan, increasing from infancy to adulthood and then declining again in elderly adults [123, 124]. These dynamics are reflective of the changes in the abundance of specific SCFA-producing taxa that occur over the lifespan [125-127]. SCFAs are ligands of receptors, most important of which belong to the G-protein-coupled receptors (GPR) family, expressed by various cells throughout the body. This explains the broad range of processes in which SCFAs are involved, including their endocrine, immunological, and neuronal effects. At the local level, SCFAs contribute to mucosal and epithelial integrity and immunity by serving as energy source for enterocytes, promoting epithelial barrier function and dampening pro-inflammatory pathways in immune cells [128]. Importantly, SCFAs are involved in systemic energy homeostasis by stimulating enteroendocrine cells to produce hormones involved in satiety, such as GLP-1 and leptin, while simultaneously influencing adipose tissue metabolism [129]. Furthermore, there is mounting evidence for the involvement of SCFAs in the “gut-brain axis,” including through their ability to reinforce the blood-brain barrier by stimulating the expression of tight junction proteins [130].
Early observations of elevated fecal SCFA levels in obese individuals led to the hypothesis that the shift in these levels are caused by obesity-related taxonomical changes in microbiota, for example the presumed altered F/B ratio [131]. However, the suggested mediating role of SCFA levels in the link between obesity and gut microbiome is not without controversy. A recent meta-analysis confirmed elevated fecal and serum concentrations of the 3 main SCFAs in obese vs non-obese individuals but failed to demonstrate phylum-level differences in microbiome composition [132]. Similarly, another recent study found that while SCFA levels were predictive for obesity in a large multinational cohort, this relationship was not mediated by gut microbiota diversity [46].
Amino Acids
Gut microbiota synthetize and metabolize several essential amino acids, notably tryptophan, which is converted to major metabolites such as serotonin, kynurenine, and indoles. Obesity alters tryptophan metabolism, shifting it away from the indole pathway as indicated by a depletion in serum indole levels in both obese children and adults compared to lean controls [133, 134].
Conversely, indoles exhibit anti-obesity effects. For example, it was recently shown that indole-3-carbinol (I3C) induced weight reduction in obese mice, reducing insulin resistance, hepatic steatosis, and systematic inflammation markers while increasing beneficial gut bacteria like Akkermansia and Eubacterium and restoring the intestinal barrier [135]. Similarly, indole-3-propionic acid (IPA) showed anti-obesity effects in diet-induced but not genetic obesity, explained by an IPA-mediated increase of sensitivity to leptin, with C. sporogenes boosting IPA levels and reversing weight gain [136].
Germ-free mice colonized with an E. coli strain incapable of converting tryptophan to indoles exhibited impaired glucose metabolism and increased food intake, lipolysis, and oxidative stress, yet paradoxically lost weight due to gastrointestinal dysfunction mimicking age-related decline [137].
Indeed, indoles are implicated in aging through their action on the aryl hydrocarbon receptor (AHR), a transcription factor involved in xenobiotic and energy metabolism, inflammation, and aging [138, 139]. Through Ahr-dependent pathways, microbiota-derived indoles increased the health span of both young and aged mice, and alleviated age-related pathological phenotypes in rodents, such as stroke [140] and osteoarthritis [141]. However, the role of the aryl hydrocarbon receptor (AHR) in the aging process remains ambiguous, with evidence suggesting both pro- and anti-aging effects depending on context and experimental conditions [139].
Another gut microbiome-derived metabolite linked to aging is phenylacetylglutamine (PAGln). Yang et al [142] showed that circulating PAGln levels increase with age and correlate with specific microbial signatures in elderly individuals. In addition, they demonstrated that PAGln induced cellular senescence, a hallmark of aging defined as a cell state marked by cell cycle arrest [4], both in vitro and in vivo.
Interventions
Diet
The connection between the gut microbiome and healthy aging suggests that it may be a modifiable factor in preventing or slowing health decline in older adults, similar to the connection between dysbiosis and obesity. However, interventions targeting the microbiome have yielded mixed results. Among dietary strategies, the most notable is the “Nu Age” trial—a year-long, multicenter study in community-dwelling older adults that tested the impact of a Mediterranean diet [143]. The intervention led to beneficial shifts in the microbiome, including an increase in species such as F. prausnitzii, which were associated with improvements in objective frailty measures like grip strength and neuropsychiatric scores. While encouraging, these effects were largely limited to disease-oriented metrics, with no significant improvement in cognition [144]. These findings highlight both the potential and the limitations of dietary interventions in modulating the microbiome for healthy aging.
Prebiotics
A prebiotic is defined as a substrate which exerts health benefits on the host after its utilization by host microbiota [145]. Common prebiotics include inulin, lactulose, and various types of oligosaccharides [146]. Inulin, a soluble dietary fiber, is known to promote beneficial microbiota like Bifidobacterium, F. prausnitzii, and Lactobacillus [147]. Its fermentation by gut microbiota releases SCFAs, which exert a range of metabolic effects as discussed previously. While it does not appear to significantly impact body weight [147], a recent placebo-controlled trial in children with obesity showed increased muscle-building biomarkers after 6 months of inulin [148]. In vitro, inulin downregulated pro-inflammatory genes (IL-1β, IL-6, TNF) in macrophages, while upregulating anti-inflammatory markers (TGF-β, FIZZ-1). However, a 12-week inulin intervention in elderly twins showed no improvement in muscle strength or function [149].
Recently, resistant starch, which evades small intestine digestion and is fermented in the colon, has emerged as a promising prebiotic. In a large randomized controlled trial, a 4-month resistant starch intervention in MASLD patients significantly improved hepatic steatosis and lipid profile and reduced body weight, BMI, adipose tissue, and systemic inflammatory markers [150]. Another randomized controlled trial in overweight and obese individuals found similar beneficial results on body composition and improvement of insulin resistance in the resistant starch-treated group [151]. Resistant starch–induced changes in microbiota and metabolites played a key role in both studies, with its anti-obesity effects being mediated through increased Bifidobacterium adolescentis and a reduction in Bacteroides stercoris and serum levels of branched-chain amino acids, both directly linked to MASLD progression [150, 151].
Probiotics
Probiotics are defined as live microorganisms that provide health benefits to the host when administered adequately [152]. The predominant probiotic strains present in food and dietary supplements are Bifidobacterium and Lactobacillus, although various bacterial strains have been ascribed probiotic properties. While probiotics clinically are recommended for certain gastrointestinal disorders [153], they are being studied for their potential therapeutic role in a wide range of conditions, including obesity and age-related conditions.
In a 2025 systematic review and meta-analysis which included 8 randomized controlled trials using oral probiotics in obese individuals, the probiotic-treated group experienced significantly more weight loss, decrease in waist circumference, and reduction of visceral fat [154]. Paradoxically, no such effect was found for BMI.
In mice, oral administration of A. mucinphila in mice caused an increase of intestinal levels of anti-aging metabolites including SCFAs and various bile acids [155], counteracted the age-related decline of the colonic mucus barrier and dampened pro-inflammatory signaling in the colon [156]. In accelerated-aging mouse models, strains of Lactobacillus reversed induced oxidative stress [157, 158] and attenuated loss of muscle mass and strength [158]. Similar anti-sarcopenic effects have been found in humans. Two recent systematic reviews have demonstrated significant positive effects of probiotic supplementation on muscle strength and muscle mass in elderly populations [159, 160], pointing toward a possible positive role of probiotics in the setting of frailty and sarcopenia. Numerous clinical trials are underway exploring the broader anti-aging potential of these next-generation probiotics [161].
Postbiotics
Postbiotics contain lifeless microorganisms or microbial components—such as inactivated bacterial cells, cell wall fragments, metabolites, or other probiotic-derived compounds—that exhibit health benefits on the host [162]. Unlike probiotics, which require live bacteria to exert effects, postbiotics offer a nonviable alternative that can still positively influence host physiology, making them an appealing option for therapeutic applications.
Preclinical data shows that postbiotic treatments can reduce body and adipose tissue weight through various mechanisms, including regulating lipid metabolism genes, reducing oxidative stress, and downregulating pro-inflammatory gene expression [163]. For instance, postbiotics are shown to upregulate genes like PPAR-α (peroxisome proliferator-activated receptor-alpha), which promotes fat breakdown, suppresses lipogenic pathways that contribute to fat accumulation, and help mitigate cellular damage caused by reactive oxygen species, a factor often linked to obesity and metabolic disorders. They also lower levels of pro-inflammatory cytokines like TNF-α and IL-6, helping to counter inflammation-driven weight gain. Moreover, heat-killed B. adolescentis improved colonic senescence in mice by inducing proliferation of intestinal stem cells, downregulating senescence markers such as p21 and p53, and blocking the Wnt/β-catenin pathway in colonocytes [164]. This suggests that postbiotics may counteract age-related decline in gut function by promoting tissue regeneration and maintaining intestinal barrier integrity. The suppression of p21 and p53, key regulators of cellular aging, indicates a potential anti-senescence effect, while inhibition of the Wnt/β-catenin signaling pathway—a mechanism often dysregulated in cancer and aging—highlights a broader protective role in gut health.
Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) refers to the transfer of stool from a healthy donor to a recipient through oral capsules, enemas, gastrointestinal tubes, or endoscopy. While currently used mainly to treat recurrent Clostridioides difficile infections, emerging murine studies suggest FMT may have therapeutic potential for metabolic and age-related disorders.
Studies have demonstrated that the aging phenotype and associated diseases can be transferred to young recipients through FMT from aged animals, whereas transplanting microbiota from young to aged mice improves aging-associated traits. For example, FMT from aged to young mice induced translocation of microbiota-derived products and increased systemic inflammation (inflammaging) [165, 166]. Conversely, FMT from young to aging animals showed numerous beneficial effects, including improved glucose sensitivity and epithelial barrier function, and reduced systemic inflammation, hepatic injury, and gut dysbiosis [167]. Supplementation with acetate and the SCFA-producer A. muciniphila—both reduced in aged mice—yielded benefits similar to those of FMT [167].
As summarized by Novelle et al, numerous clinical trials have been published or are underway exploring the potential role of FMT in metabolic and aging-related disorders, including type 2 diabetes, metabolic syndrome, MASLD, frailty, and neurodegenerative diseases. So far, results of randomized controlled trials that examine the effect of FMT on weight loss are mixed: several found no effect on weight in obese individuals [168-170], while others reported a reduction in abdominal adiposity compared to placebo [171]. Another study observed less regain of weight and waist circumference in obese individuals after autologous FMT combined with a high-polyphenol Mediterranean diet [172].
In terms of insulin resistance, a hallmark of obesity, Vrieze et al [173] reported improved insulin sensitivity after allogenous FMT in obese patients, and several recent trials have validated this finding [174-176]. Post-FMT outcomes on lipid profiles have been inconsistent across studies [169-171, 177].
Similarly, conflicting results were found between randomized controlled trials investigating the effect of FMT in patients with MASLD. While one study observed reduced elastographically measured hepatic steatosis in MASLD patients after FMT compared to probiotic treatment [178], another trial showed no histopathological effect despite significantly altered hepatic gene expression [179]. In accordance with the latter, a third randomized controlled trial demonstrated no significant changes in magnetic resonance imaging–based liver fat quantification after FMT [180].
Future Perspectives
Growing preclinical and translational evidence supports a causal role for the gut microbiome in aging, obesity, and related metabolic disorders among others through the production of bioactive microbial metabolites. These compounds exert wide-ranging effects on host physiology, influencing immune signaling, metabolic regulation, mitochondrial function, and cellular maintenance. In doing so, they intersect with biological processes that are central to aging and contribute to the development of conditions such as insulin resistance, MASLD, and systemic low-grade inflammation.
However, translating this mechanistic insights into clinically meaningful interventions remains challenging. A central question is how to define success: are improvements in surrogate biomarkers—such as epigenetic clocks or inflammatory profiles—sufficient, or must benefits be confirmed through long-term randomized trials showing delayed disease onset, improved physical or cognitive function, or increased health span? Similar questions apply to obesity and metabolic disease: should microbiome changes be considered therapeutic only if they lead to sustained improvements in glycemic control, liver health, or body composition?
Future research should prioritize the development of more targeted and individualized microbiome interventions tailored to a person's microbial profile, diet, genetics, and metabolic state. Integrating multi-omics technologies—metagenomics, metabolomics, and transcriptomics—will be key to identifying predictive microbial signatures and therapeutic targets.
Innovative strategies, such as engineered probiotics (Table 1), postbiotics, and targeted delivery of microbial metabolites, may offer more controlled and reproducible ways to modulate host-microbiome interactions than probiotic or dietary interventions. Ultimately, progress hinges on further unraveling specific microbial pathways that influence host aging and metabolism. As we gain deeper insights into how gut-derived signals regulate immune aging, nutrient sensing, mitochondrial health, and tissue repair, we will be better positioned to design interventions that move beyond correlation and toward causation. With robust biomarkers, mechanistic clarity, and well-designed clinical trials, microbiome-based therapies may become a powerful tool in the prevention and treatment of aging-related and metabolic diseases.
Table 1.
Examples of recent studies investigating the effect of engineered probiotics in metabolic and aging-related diseases
| Type of engineered probiotic | Application in disease (model) | Subject species | Results | Article |
|---|---|---|---|---|
| Secretion of IL-22 by Lactobacillus reuteri | Obesity and MASLD | Mice (HFD) | ↓ liver weight and hepatic TG levels | Oh et al (2020) [181] |
| Expression of human proinsulin and human IL-10 by Lactococcus lactis | Type 1 diabetes | Humans | Adults: significant ↓ of C-peptide at 12 months; significant ↓of HbA1c at 3 and 6 months | Mathieu et al (2023) [182] |
| Adolescents: significant ↓ of C-peptide at 6 months | ||||
| Production of GLP-1 by Lactobacillus plantarum | Type 2 diabetes | Mice (HFD/STZ and db/db) | ↓ weight, fasting blood glucose, expression of pro-inflammatory markers in pancreatic tissue ↑ glucose tolerance |
Hu et al (2023) [183] |
| Butyric acid production by Bacillus subtilis SCK6 | Obesity | Mice (HFD) | ↓ body weight, body weight gain, food intake, serum TC, TG, and ALT | Bai et al (2020) [184] |
| Consortium of butyrate-producing Bacillus subtilis SCK6 and Bifidobacterium pseudocatenulatum JJ3 | Obesity | Mice (Ob/Ob) | ↓ body weight gain, fasting glucose, HOMA-IR, liver weight, serum TBA, ALT, AST, ALP, TC, LDL, and HDL | Chen et al (2023) [185] |
| GLP-1 production by Lactococcus lactis MG1363 | Obesity | Mice (HFD) | ↓ body weight gain, adipose tissue weight, serum and hepatic TG, hepatic steatosis | Wang et al (2021) [186] |
| Secretion of GLP-1 analog by E. coli Nissle 191 | Obesity | Mice (HFD) | ↓ body weight, body weight gain, food intake, adipose tissue weight, liver weight ↑ glucose tolerance |
Ma et al (2020) [187] |
| Expression of GLP-1 by Lactococcus lactis | AD and PD | Mice | ↓ memory impairment and motor dysfunction | Fang et al (2020) [188] |
| Expression of human p62 protein by GM Lactococcus lactis | AD | Mice | ↑ memory performance ↓ reduction of inflammation and amyloid peptides load in the brain |
Cecarini et al(2020) [189] |
Abbreviations: AD, Alzheimer's disease; ALT, alanine transaminase; AST, aspartate transaminase; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HFD, high-fat diet; LDL, low-density lipoprotein; PD, Parkinson's disease; TBA, total bile acid; TC, total cholesterol; TG, triglycerides.
↑ = higher or increase/improvement.
↓ = lower or decrease.
Abbreviations
- AHR
aryl hydrocarbon receptor
- BMI
body mass index
- FMT
fecal microbiota transplantation
- GLP-1
glucagon-like peptide 1
- LCA
lithocholic acid
- MASLD
metabolic dysfunction–associated steatotic liver disease
- SCFA
short-chain fatty acid
Contributor Information
Anouar Aznou, Department of Internal and Experimental Vascular Medicine, Amsterdam University Medical Centers, Location AMC, 1105 AZ Amsterdam, the Netherlands; Department of Gastroenterology and Hepatology, Amsterdam Gastroenterology Endocrinology Metabolism (AGEM), Amsterdam University Medical Center, Location VUMC, 1081 HV Amsterdam, the Netherlands.
Joost P H Drenth, Department of Gastroenterology and Hepatology, Amsterdam Gastroenterology Endocrinology Metabolism (AGEM), Amsterdam University Medical Center, Location VUMC, 1081 HV Amsterdam, the Netherlands.
Max Nieuwdorp, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, 1105 AZ Amsterdam, the Netherlands; Diabetes Center Amsterdam, Amsterdam 1066 EC, the Netherlands.
Abraham S Meijnikman, Email: a.s.meijnikman@amsterdamumc.nl, Department of Gastroenterology and Hepatology, Amsterdam Gastroenterology Endocrinology Metabolism (AGEM), Amsterdam University Medical Center, Location VUMC, 1081 HV Amsterdam, the Netherlands; Tytgat Institute for Liver and Intestinal Research, Amsterdam Gastroenterology Endocrinology Metabolism, Academic Medical Center, University of Amsterdam, 1105 AZ Amsterdam, the Netherlands.
Funding
M.N. is supported by a personal NWO VICI grant 2020 (09150182010020) and an ERC Advanced grant (101141346). A.M. is supported by a personal NWO VENI 2023 (09150162310148) and a DUTCH Gastroenterology and Hepatology (MDLS) foundation grant.
Disclosures
M.N. is founder and a scientific advisory board member of Caelus Pharmaceuticals and Advanced Microbiome Interventions, the Netherlands. However, none of these bear direct relevance to the content of the current paper. All other authors have nothing to disclose.
Data Availability
Not applicable.
References
- 1. McClearn GE, Johansson B, Berg S, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560‐1563. [DOI] [PubMed] [Google Scholar]
- 2. O'Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214‐1215. [DOI] [PubMed] [Google Scholar]
- 3. Zhang X, Zhong H, Li Y, et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat Aging. 2021;1(1):87‐100. [DOI] [PubMed] [Google Scholar]
- 4. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243‐278. [DOI] [PubMed] [Google Scholar]
- 5. Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279‐297. [DOI] [PubMed] [Google Scholar]
- 6. Martins D, Silva C, Ferreira AC, et al. Unravelling the gut microbiome role in cardiovascular disease: a systematic review and a meta-analysis. Biomolecules. 2024;14(6):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meijnikman AS, Aydin O, Prodan A, et al. Distinct differences in gut microbial composition and functional potential from lean to morbidly obese subjects. J Intern Med. 2020;288(6):699‐710. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Zhao J, Qin Y, et al. The specific alteration of gut microbiota in diabetic kidney diseases—a systematic review and meta-analysis. Front Immunol. 2022;13:908219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy KM, Gerlach MJ, Adam T, et al. Fetal meconium does not have a detectable microbiota before birth. Nat Microbiol. 2021;6(7):865‐873. [DOI] [PubMed] [Google Scholar]
- 10. Kuperman AA, Zimmerman A, Hamadia S, et al. Deep microbial analysis of multiple placentas shows no evidence for a placental microbiome. BJOG. 2020;127(2):159‐169. [DOI] [PubMed] [Google Scholar]
- 11. Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971‐11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reyman M, van Houten MA, van Baarle D, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10(1):4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. [Google Scholar]
- 16. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET cohort. Microbiome. 2017;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wernroth ML, Peura S, Hedman AM, et al. Development of gut microbiota during the first 2 years of life. Sci Rep. 2022;12(1):9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roswall J, Olsson LM, Kovatcheva-Datchary P, et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe. 2021;29(5):765‐776.e3. [DOI] [PubMed] [Google Scholar]
- 19. Stewart CJ, Ajami NJ, O'Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690‐703. [DOI] [PubMed] [Google Scholar]
- 21. Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81. [Google Scholar]
- 22. Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagpal R, Kurakawa T, Tsuji H, et al. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: a quantitative assessment. Sci Rep. 2017;7(1):10097‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niu J, Xu L, Qian Y, et al. Evolution of the gut microbiome in early childhood: a cross-sectional study of Chinese children. Front Microbiol. 2020;11:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng J, Ringel-Kulka T, Heikamp-de Jong I, et al. Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 2016;10(4):1002‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radjabzadeh D, Boer CG, Beth SA, et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci Rep. 2020;10(1):1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol. 2011;77(2):404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mancabelli L, Milani C, De Biase R, et al. Taxonomic and metabolic development of the human gut microbiome across life stages: a worldwide metagenomic investigation. mSystems. 2024;9(4):e0129423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan R, Guo M, Chen Y, et al. Dynamics of the gut microbiota and faecal and serum metabolomes during pregnancy-a longitudinal study. Nutrients. 2024;16(4):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin H, Martinez KA 2nd, Henderson N, et al. Partial convergence of the human vaginal and rectal maternal microbiota in late gestation and early post-partum. NPJ Biofilms Microbiomes. 2023;9(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang M, Weaver NE, Frank DN, et al. Longitudinal reduction in diversity of maternal gut microbiota during pregnancy is observed in multiple low-resource settings: results from the women first trial. Front Microbiol. 2022;13:823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112(35):11060‐11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang H, Guo R, Li S, et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. NPJ Biofilms Microbiomes. 2020;6(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biagi E, Franceschi C, Rampelli S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26(11):1480‐1485. [DOI] [PubMed] [Google Scholar]
- 39. Wilmanski T, Diener C, Rappaport N, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3(2):274‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Claesson MJ, Cusack S, O'Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(supplement_1):4586‐4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaiserman A, Romanenko M, Piven L, et al. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020;20(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato Y, Atarashi K, Plichta DR, et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. 2021;599(7885):458‐464. [DOI] [PubMed] [Google Scholar]
- 43. Wu L, Zeng T, Zinellu A, Rubino S, Kelvin DJ, Carru C. A cross-sectional study of compositional and functional profiles of gut microbiota in sardinian centenarians. mSystems. 2019;4(4):e00325-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johansen J, Atarashi K, Arai Y, et al. Centenarians have a diverse gut virome with the potential to modulate metabolism and promote healthy lifespan. Nat Microbiol. 2023;8(6):1064‐1078. [DOI] [PubMed] [Google Scholar]
- 45. Wilkie SE, Borland G, Carter RN, Morton NM, Selman C. Hydrogen sulfide in ageing, longevity and disease. Biochem J. 2021;478(19):3485‐3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ecklu-Mensah G, Choo-Kang C, Maseng MG, et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: the METS-microbiome study. Nat Commun. 2023;14(1):5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Castaner O, Goday A, Park YM, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Indiani C, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood obesity and firmicutes/bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes. 2018;14(8):501‐509. [DOI] [PubMed] [Google Scholar]
- 49. Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251‐1262. [DOI] [PubMed] [Google Scholar]
- 50. Tavassol ZH, Ejtahed HS, Atlasi R, et al. Alteration in gut microbiota composition of older adults is associated with obesity and its indices: a systematic review. J Nutr Health Aging. 2023;27(10):817‐823. [DOI] [PubMed] [Google Scholar]
- 51. Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12(1):1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Udayappan S, Manneras-Holm L, Chaplin-Scott A, et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes. 2016;2(1):16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Attaye I, Witjes JJ, Koopen AM, et al. Oral Anaerobutyricum soehngenii augments glycemic control in type 2 diabetes. iScience. 2024;27(8):110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li C-P, Chen C-C, Hsiao Y, et al. The role of Lactobacillus plantarum in reducing obesity and inflammation: a meta-analysis. Int J Mol Sci. 2024;25(14):7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choi WJ, Dong HJ, Jeong HU, et al. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci Rep. 2020;10(1):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang M, Zhang B, Hu J, Nie S, Xiong T, Xie M. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J Funct Foods. 2020;72:104078. [Google Scholar]
- 57. Zhou Q, Zhang Y, Wang X, et al. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American Gut Project. Nutr Metabol. 2020;17(1):90. [Google Scholar]
- 58. Roshanravan N, Bastani S, Tutunchi H, et al. A comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch Physiol Biochem. 2023;129(3):741‐751. [DOI] [PubMed] [Google Scholar]
- 59. de la Cuesta-Zuluaga J, Kelley ST, Chen Y, et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems. 2019;4(4):e00261-19. [Google Scholar]
- 60. Cross T-WL, Simpson AMR, Lin C-Y, et al. Gut microbiome responds to alteration in female sex hormone status and exacerbates metabolic dysfunction. Gut Microbes. 2024;16(1):2295429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vriend EMC, Galenkamp H, Herrema H, Nieuwdorp M, van den Born B-JH, Verhaar BJH. Machine learning analysis of sex and menopausal differences in the gut microbiome in the HELIUS study. NPJ Biofilms Microbiomes. 2024;10(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stapleton S, Welch G, DiBerardo L, Freeman LR. Sex differences in a mouse model of diet-induced obesity: the role of the gut microbiome. Res Sq. 2023;15(1):5. [Google Scholar]
- 63. Pérez-Castro S, D'Auria G, Llambrich M, et al. Influence of perinatal and childhood exposure to tobacco and mercury in children's gut microbiota. Front Microbiol. 2024;14:1258988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. García-Mantrana I, Selma-Royo M, González S, Parra-Llorca A, Martínez-Costa C, Collado MC. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. 2020;11(4):962‐978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chu DM, Antony KM, Ma J, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lundgren SN, Madan JC, Emond JA, et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome. 2018;6(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gilley SP, Ruebel ML, Sims C, et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr Obes. 2022;17(9):e12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sabino J, Tarassishin L, Eisele C, et al. Influence of early life factors, including breast milk composition, on the microbiome of infants born to mothers with and without inflammatory bowel disease. J Crohns Colitis. 2023;17(11):1723‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Torres J, Hu J, Seki A, et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69(1):42‐51. [DOI] [PubMed] [Google Scholar]
- 70. Princisval L, Rebelo F, Williams BL, et al. Association between the mode of delivery and infant gut microbiota composition up to 6 months of age: a systematic literature review considering the role of breastfeeding. Nutr Rev. 2021;80(1):113‐127. [DOI] [PubMed] [Google Scholar]
- 71. Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 2016;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dong TS, Gupta A. Influence of early life, diet, and the environment on the microbiome. Clin Gastroenterol Hepatol. 2019;17(2):231‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dierikx TH, Visser DH, Benninga MA, et al. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: a systematic review. J Infect. 2020;81(2):190‐204. [DOI] [PubMed] [Google Scholar]
- 74. Baron R, Taye M, Besseling-van der Vaart I, et al. The relationship of prenatal and infant antibiotic exposure with childhood overweight and obesity: a systematic review. J Dev Orig Health Dis. 2020;11(4):335‐349. [DOI] [PubMed] [Google Scholar]
- 75. Kelly D, Kelly A, O’Dowd T, Hayes CB. Antibiotic use in early childhood and risk of obesity: longitudinal analysis of a national cohort. World J Pediatrics. 2019;15(4):390‐397. [Google Scholar]
- 76. Mueller NT, Shin H, Pizoni A, et al. Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci Rep. 2016;6(1):23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lemas DJ, Young BE, Baker PR, et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome12. Am J Clin Nutr. 2016;103(5):1291‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother's weight on infant's microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy123. Am J Clin Nutr. 2010;92(5):1023‐1030. [DOI] [PubMed] [Google Scholar]
- 79. Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One. 2014;9(11):e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ross FC, Patangia D, Grimaud G, et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol. 2024;22(11):671‐686. [DOI] [PubMed] [Google Scholar]
- 81. Khavandegar A, Heidarzadeh A, Angoorani P, et al. Adherence to the Mediterranean diet can beneficially affect the gut microbiota composition: a systematic review. BMC Med Genomics. 2024;17(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martín R, Rios-Covian D, Huillet E, et al. Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol Rev. 2023;47(4):fuad039. [Google Scholar]
- 83. Precup G, Vodnar DC. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br J Nutr. 2019;122(2):131‐140. [DOI] [PubMed] [Google Scholar]
- 84. Severino A, Tohumcu E, Tamai L, et al. The microbiome-driven impact of western diet in the development of noncommunicable chronic disorders. Best Pract Res Clin Gastroenterol. 2024;72:101923. [DOI] [PubMed] [Google Scholar]
- 85. Agus A, Denizot J, Thévenot J, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6(1):19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. So D, Whelan K, Rossi M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965‐983. [DOI] [PubMed] [Google Scholar]
- 87. Min L, Ablitip A, Wang R, Luciana T, Wei M, Ma X. Effects of exercise on gut microbiota of adults: a systematic review and meta-analysis. Nutrients. 2024;16(7):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ortiz-Alvarez L, Xu H, Martinez-Tellez B. Influence of exercise on the human gut microbiota of healthy adults: a systematic review. Clin Transl Gastroenterol. 2020;11(2):e00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. He J, Zhang P, Shen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liu D, Chen D, Xiao J, et al. High-altitude-induced alterations in intestinal microbiota. Front Microbiol. 2024;15:1369627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Han Y, Liu X, Jia Q, et al. Longitudinal multi-omics analysis uncovers the altered landscape of gut microbiota and plasma metabolome in response to high altitude. Microbiome. 2024;12(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178‐184. [DOI] [PubMed] [Google Scholar]
- 94. Elvers KT, Wilson VJ, Hammond A, et al. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10(9):e035677. [Google Scholar]
- 95. Ng KM, Aranda-Díaz A, Tropini C, et al. Recovery of the gut microbiota after antibiotics depends on host diet, community context, and environmental reservoirs. Cell Host Microbe. 2019;26(5):650‐665.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Du L, Chen B, Cheng F, Kim J, Kim JJ. Effects of Helicobacter pylori therapy on gut microbiota: a systematic review and meta-analysis. Dig Dis. 2024;42(1):102‐112. [DOI] [PubMed] [Google Scholar]
- 97. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lindell AE, Zimmermann-Kogadeeva M, Patil KR. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat Rev Microbiol. 2022;20(7):431‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ticinesi A, Milani C, Lauretani F, et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7(1):11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Haran JP, Zeamer A, Ward DV, Dutta P, Bucci V, McCormick BA. The nursing home older adult gut microbiome composition shows time-dependent dysbiosis and is influenced by medication exposures, age, environment, and frailty. J Gerontol A Biol Sci Med Sci. 2021;76(11):1930‐1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gemikonakli G, Mach J, Zhang F, et al. Polypharmacy with high Drug Burden Index (DBI) alters the gut microbiome overriding aging effects and is reversible with deprescribing. J Gerontol A Biol Sci Med Sci. 2023;78(2):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Baars DP, Fondevila MF, Meijnikman AS, Nieuwdorp M. The central role of the gut microbiota in the pathophysiology and management of type 2 diabetes. Cell Host Microbe. 2024;32(8):1280‐1300. [DOI] [PubMed] [Google Scholar]
- 103. Ghosh TS, Das M, Jeffery IB, O'Toole PW. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife. 2020;9:e50240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meijnikman AS, Nieuwdorp M, Schnabl B. Endogenous ethanol production in health and disease. Nat Rev Gastroenterol Hepatol. 2024;21(8):556‐571. [DOI] [PubMed] [Google Scholar]
- 105. Mohammad S, Thiemermann C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front Immunol. 2021;11:594150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhou M, Lv J, Chen X, Shi Y, Chao G, Zhang S. From gut to liver: exploring the crosstalk between gut-liver axis and oxidative stress in metabolic dysfunction-associated steatotic liver disease. Ann Hepatol. 2025;30(1):101777. [DOI] [PubMed] [Google Scholar]
- 107. De Munck TJI, Xu P, Verwijs HJA, et al. Intestinal permeability in human nonalcoholic fatty liver disease: a systematic review and meta-analysis. Liver Int. 2020;40(12):2906‐2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Best L, Dost T, Esser D, et al. Metabolic modelling reveals the aging-associated decline of host–microbiome metabolic interactions in mice. Nat Microbiol. 2025;10(4):973‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang Y, Chen R, Zhang D, Qi S, Liu Y. Metabolite interactions between host and microbiota during health and disease: which feeds the other? Biomed Pharmacother. 2023;160:114295. [DOI] [PubMed] [Google Scholar]
- 110. Caffrey EB, Sonnenburg JL, Devkota S. Our extended microbiome: the human-relevant metabolites and biology of fermented foods. Cell Metab. 2024;36(4):684‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fiorucci S, Distrutti E, Carino A, Zampella A, Biagioli M. Bile acids and their receptors in metabolic disorders. Prog Lipid Res. 2021;82:101094. [DOI] [PubMed] [Google Scholar]
- 112. Chávez-Talavera O, Haas J, Grzych G, Tailleux A, Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: what do the human studies tell? Curr Opin Lipidol. 2019;30(3):244‐254. [DOI] [PubMed] [Google Scholar]
- 113. Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21(4):236‐247. [DOI] [PubMed] [Google Scholar]
- 114. Larabi AB, Masson HLP, Bäumler AJ. Bile acids as modulators of gut microbiota composition and function. Gut Microbes. 2023;15(1):2172671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Qu Q, Chen Y, Wang Y, et al. Lithocholic acid phenocopies anti-ageing effects of calorie restriction. Nature. 2025;643(8070):192‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jeon S-M. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):e245‐e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Prinz P, Hofmann T, Ahnis A, et al. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front Neurosci. 2015;9:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Haeusler RA, Camastra S, Nannipieri M, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab. 2016;101(5):1935‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wei M, Huang F, Zhao L, et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine. 2020;55:102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Aydin Ö, Wahlström A, de Jonge PA, et al. An integrated analysis of bile acid metabolism in humans with severe obesity. Hepatology. 2025;81(1):19‐31. [DOI] [PubMed] [Google Scholar]
- 121. Nie Q, Luo X, Wang K, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell. 2024;187(11):2717‐2734.e33. [DOI] [PubMed] [Google Scholar]
- 122. Funabashi M, Grove TL, Wang M, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582(7813):566‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tsukuda N, Yahagi K, Hara T, et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021;15(9):2574‐2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Salazar N, Arboleya S, Fernández-Navarro T, de Los Reyes-Gavilán CG, Gonzalez S, Gueimonde M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients. 2019;11(8):1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Singh V, Lee G, Son H, et al. Butyrate producers, “The Sentinel of Gut“: their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front Microbiol. 2023;13:1103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29‐41. [DOI] [PubMed] [Google Scholar]
- 127. Fusco W, Lorenzo MB, Cintoni M, et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15(9):2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700‐712. [DOI] [PubMed] [Google Scholar]
- 130. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 2020;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190‐195. [DOI] [PubMed] [Google Scholar]
- 132. Kim KN, Yao Y, Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. 2019;11(10):2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cussotto S, Delgado I, Anesi A, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Virtue AT, McCright SJ, Wright JM, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11(496):eaav1892. [Google Scholar]
- 135. Mao X, Paerhati G, Wu Y, Cheng LF. Modulation of gut microbiota, up-regulation of ZO-1, and promotion of metabolism as therapeutic mechanisms of indole-3-carbinol against obesity in mice. Front Pharmacol. 2025;15:1499142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang Z, Yang S, Liu L, et al. The gut microbiota-derived metabolite indole-3-propionic acid enhances leptin sensitivity by targeting STAT3 against diet-induced obesity. Clin Transl Med. 2024;14(12):e70053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Xing PY, Agrawal R, Jayaraman A, et al. Microbial indoles: key regulators of organ growth and metabolic function. Microorganisms. 2024;12(4):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bock KW. Aryl hydrocarbon receptor (AHR), integrating energy metabolism and microbial or obesity-mediated inflammation. Biochem Pharmacol. 2021;184:114346. [DOI] [PubMed] [Google Scholar]
- 139. Brinkmann V, Ale-Agha N, Haendeler J, Ventura N. The Aryl Hydrocarbon Receptor (AhR) in the aging process: another puzzling role for this highly conserved transcription factor. Front Physiol. 2020;10:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Peesh P, Blasco-Conesa MP, El Hamamy A, et al. Benefits of equilibrium between microbiota- and host-derived ligands of the aryl hydrocarbon receptor after stroke in aged male mice. Nat Commun. 2025;16(1):1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhuang H, Ren X, Jiang F, Zhou P. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. 2023;29(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yang H, Wang T, Qian C, et al. Gut microbial-derived phenylacetylglutamine accelerates host cellular senescence. Nat Aging. 2025;5(3):401‐418. [DOI] [PubMed] [Google Scholar]
- 143. Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Marseglia A, Xu W, Fratiglioni L, et al. Effect of the NU-AGE diet on cognitive functioning in older adults: a randomized controlled trial. Front Physiol. 2018;9:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491‐502. [DOI] [PubMed] [Google Scholar]
- 146. Wang S, Xiao Y, Tian F, et al. Rational use of prebiotics for gut microbiota alterations: specific bacterial phylotypes and related mechanisms. J Funct Foods. 2020;66:103838. [Google Scholar]
- 147. Hughes RL, Alvarado DA, Swanson KS, Holscher HD. The prebiotic potential of inulin-type fructans: a systematic review. Advances in Nutrition. 2022;13(2):492‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Visuthranukul C, Leelahavanichkul A, Tepaamorndech S, Chamni S, Mekangkul E, Chomtho S. Inulin supplementation exhibits increased muscle mass via gut-muscle axis in children with obesity: double evidence from clinical and in vitro studies. Sci Rep. 2024;14(1):11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ni Lochlainn M, Bowyer RCE, Moll JM, et al. Effect of gut microbiome modulation on muscle function and cognition: the PROMOTe randomised controlled trial. Nat Commun. 2024;15(1):1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ni Y, Qian L, Siliceo SL, et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023;35(9):1530‐1547.e8. [DOI] [PubMed] [Google Scholar]
- 151. Li H, Zhang L, Li J, et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat Metab. 2024;6(3):578‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506‐514. [DOI] [PubMed] [Google Scholar]
- 153. Su GL, Ko CW, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159(2):697‐705. [DOI] [PubMed] [Google Scholar]
- 154. Guo M, Li J, Zhang L, Chen C, Wei Y, Shen Z-a. Effects of oral supplementation of probiotics on body weight and visceral fat in obese patients: a meta-analysis and systematic review. Sci Rep. 2025;15(1):6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Grajeda-Iglesias C, Durand S, Daillère R, et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging (Albany NY). 2021;13(5):6375‐6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. van der Lugt B, van Beek AA, Aalvink S, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (-/Δ7) mice. Immun Ageing. 2019;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhao J, Tian F, Yan S, Zhai Q, Zhang H, Chen W. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food Funct. 2018;9(2):917‐924. [DOI] [PubMed] [Google Scholar]
- 158. Chen LH, Huang SY, Huang KC, et al. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging (Albany NY). 2019;11(2):756‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Besora-Moreno M, Llauradó E, Valls RM, Pedret A, Solà R. Effects of probiotics, prebiotics, and synbiotics on sarcopenia parameters in older adults: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2025;83(7):e1693‐e1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Prokopidis K, Giannos P, Kirwan R, et al. Impact of probiotics on muscle mass, muscle strength and lean mass: a systematic review and meta-analysis of randomized controlled trials. J Cachexia Sarcopenia Muscle. 2023;14(1):30‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Guarente L, Sinclair DA, Kroemer G. Human trials exploring anti-aging medicines. Cell Metab. 2024;36(2):354‐376. [DOI] [PubMed] [Google Scholar]
- 162. Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Park S-J, Sharma A, Lee H-J. Postbiotics against obesity: perception and overview based on pre-clinical and clinical studies. Int J Mol Sci. 2023;24(7):6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Qi Y, He J, Zhang Y, et al. Heat-inactivated Bifidobacterium adolescentis ameliorates colon senescence through Paneth-like-cell-mediated stem cell activation. Nat Commun. 2023;14(1):6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fransen F, van Beek AA, Borghuis T, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Parker A, Romano S, Ansorge R, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ma J, Liu Z, Gao X, et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol Res. 2023;189:106687. [DOI] [PubMed] [Google Scholar]
- 168. Allegretti JR, Kassam Z, Mullish BH, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol. 2020;18(4):855‐863.e2. [DOI] [PubMed] [Google Scholar]
- 169. Lahtinen P, Juuti A, Luostarinen M, et al. Effectiveness of fecal microbiota transplantation for weight loss in patients with obesity undergoing bariatric surgery: a randomized clinical trial. JAMA Netw Open. 2022;5(12):e2247226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yu EW, Gao L, Stastka P, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Leong KSW, Jayasinghe TN, Wilson BC, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw Open. 2020;3(12):e2030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rinott E, Meir AY, Tsaban G, et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 2022;14(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913‐916.e7. [DOI] [PubMed] [Google Scholar]
- 174. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611‐619.e6. [DOI] [PubMed] [Google Scholar]
- 175. Rinott E, Youngster I, Yaskolka Meir A, et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology. 2021;160(1):158‐173.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mocanu V, Zhang Z, Deehan EC, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27(7):1272‐1279. [DOI] [PubMed] [Google Scholar]
- 177. Ng SC, Xu Z, Mak JWY, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71(4):716‐723. [DOI] [PubMed] [Google Scholar]
- 178. Xue L, Deng Z, Luo W, He X, Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol. 2022;12:759306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Witjes JJ, Smits LP, Pekmez CT, et al. Donor fecal microbiota transplantation alters gut microbiota and metabolites in obese individuals with steatohepatitis. Hepatol Commun. 2020;4(11):1578‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Craven L, Rahman A, Nair Parvathy S, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. 2020;115(7):1055‐1065. [DOI] [PubMed] [Google Scholar]
- 181. Oh JH, Schueler KL, Stapleton DS, et al. Secretion of recombinant interleukin-22 by engineered Lactobacillus reuteri reduces fatty liver disease in a mouse model of diet-induced obesity. mSphere. 2020;5(3):e00183-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Mathieu C, Wiedeman A, Cerosaletti K, et al. A first-in-human, open-label Phase 1b and a randomised, double-blind Phase 2a clinical trial in recent-onset type 1 diabetes with AG019 as monotherapy and in combination with teplizumab. Diabetologia. 2024;67(1):27‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hu H, Luo J, Liu Y, et al. Improvement effect of a next-generation probiotic L. plantarum-pMG36e-GLP-1 on type 2 diabetes mellitus via the gut-pancreas-liver axis. Food Funct. 2023;14(7):3179‐3195. [DOI] [PubMed] [Google Scholar]
- 184. Bai L, Gao M, Cheng X, Kang G, Cao X, Huang H. Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb Cell Fact. 2020;19(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Chen X, Mengxue G, Lina W, et al. A synthetic microbial consortium protects against obesity by regulating vitamin B6 metabolism. Gut Microbes. 2024;16(1):2304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wang L, Chen T, Wang H, et al. Engineered bacteria of MG1363-pMG36e-GLP-1 attenuated obesity-induced by high fat diet in mice. Front Cell Infect Microbiol. 2021;11:595575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ma J, Li C, Wang J, Gu J. Genetically engineered Escherichia coli Nissle 1917 secreting GLP-1 analog exhibits potential antiobesity effect in high-fat diet-induced obesity mice. Obesity (Silver Spring). 2020;28(2):315‐322. [DOI] [PubMed] [Google Scholar]
- 188. Fang X, Zhou X, Miao Y, Han Y, Wei J, Chen T. Therapeutic effect of GLP-1 engineered strain on mice model of Alzheimer's disease and Parkinson's disease. AMB Express. 2020;10(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cecarini V, Bonfili L, Gogoi O, et al. Neuroprotective effects of p62(SQSTM1)-engineered lactic acid bacteria in Alzheimer's disease: a pre-clinical study. Aging (Albany NY). 2020;12(16):15995‐16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.