Abstract
We characterized the intracellular symbiotic bacteria of the hematophagous glossiphoniid leeches Placobdelloides siamensis and a Parabdella sp. These leeches have a specialized structure called an “esophageal organ,” the cells of which harbor bacterial symbionts. From the esophageal organ of each species, a 1.5-kb eubacterial 16S rRNA gene segment was amplified by PCR, cloned, and sequenced. Diagnostic PCR detected the symbiont in the esophageal organ and intestine. Phylogenetic analysis of the 16S rRNA gene(s) demonstrated that the symbionts from the leeches formed a monophyletic group in a well-defined clade containing endosymbiotic bacteria of plant sap-feeding insects in the γ-subdivision of the Proteobacteria. The nucleotide compositions of the 16S rRNA gene from the leech symbionts were highly AT biased (53.7%).
Many animals that feed exclusively on vertebrate blood throughout their life possess symbiotic microorganisms. Bloodsucking arthropods such as ticks, lice, bedbugs, reduviid bugs, and tsetse flies usually harbor symbiotic microorganisms inside a highly developed symbiotic organ, called a “mycetome” (or “bacteriome”), consisting of a number of mycetocytes (or bacteriocytes) (2). Since these symbionts are yet unculturable outside the host cells and the hosts become sterile or die with antibiotic treatment, the host-microorganism relationships have been believed to be mutualistic in general (1). In some cases, the symbionts are suggested to provide their host with B complex vitamins (thiamine, pantothenic acid, pyridoxine, folic acid, and biotin) that are scarce in vertebrate blood (20).
Leeches (Annelida: Hirudinea) include many bloodsucking species, some of which have endosymbiotic microorganisms (2, 23). The class Hirudinea is divided into two orders, Rhynchobdellida and Arhynchobdellida, and most species of the former and a part of the latter are hematophagous. Endosymbionts and mycetocytes have been described mainly from the Rhynchobdellida. From hematophagous species of the Arhynchobdellida, gut bacteria such as Aeromonas hydrophila and Aeromonas veronii were reported to be mutualistic symbionts (9, 14).
The order Rhynchobdellida consists of three families: Ozobranchidae, Piscicoridae, and Glossiphoniidae. Most species of the first two families have a pair of well-developed bulbous mycetomes, each connected to the esophagus through a very narrow duct (23). In the family Glossiphoniidae, organization of their mycetome is impressively diverse. (i) In the genus Theromyzon, enlarged endothelial cells of anterior two crop ceca house symbionts (26). (ii) In the genera Placobdelloides, Marspiobdella, and Batracobdella, as well as others, large mycetocytes surrounding the lumen of the esophagus form a tube-like structure called an “esophageal organ” (21, 27) (Fig. 1). (iii) In the genera Placobdella and Desserobdella, mycetomes are a pair of evaginated sacs joining the esophagus at their narrowed ends (10, 11). (iv) In the genus Haementeria, mycetomes are arranged in two pairs of large bulbous structures, each of which is connected to the esophagus through a very narrow duct (23). Some hematophagous members of the Glossiphoniidae are without mycetocytes (e.g., Hemicrepsis marginata) (13), and species living on invertebrate body fluid totally lack symbiotic microorganisms (e.g., Glossiphonia complanata and Helobdella stagnalis) (23). Rickettsial intercellular symbionts or parasites were also discovered from the hematophagous glossiphoniid leeches Torix tagoi and H. marginata (15). These diverse forms of mycetomes in the Glossiphoniidae would provide us with an opportunity to comparatively study the evolution of the endosymbiotic system in leeches. However, the microbiological nature of the mycetocyte symbionts has been very poorly understood, except for the histological descriptions mentioned above.
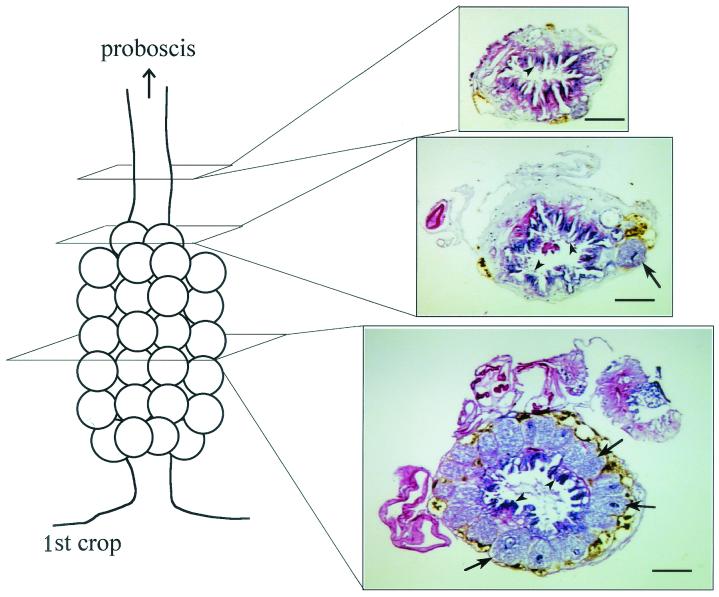
FIG. 1.
Structure of the esophageal organ of P. siamensis. The tissue was fixed in alcoholic formalin (3:1 ethanol-formalin), processed into paraffin sections, and stained with hematoxylin-eosin. Arrows and arrowheads indicate mycetocytes and luminal villi, respectively. Bars, 100 μm.
Here we report the first molecular characterization of symbiotic bacteria associated with the esophageal organ of two hematophagous glossiphoniid leeches.
Bacterial 16S rRNA gene sequences from the esophageal organ.
Five individuals of Placobdelloides siamensis were collected from Akamatsu Pond, Tottori, Japan, on 6 August 2001, and two individuals of the Parabdella sp. were collected from Uchi Pond, Miyagi, Japan, on 24 May 2001. The Parabdella sp. is an undescribed species closely related to Parabdella quadrioculata (18). These leeches were brought to the laboratory alive, relaxed in carbonated water, and dissected to obtain tissues of the esophageal organ. The tissues were repeatedly washed with distilled water to minimize possible contamination and preserved in acetone until molecular analyses (6).
Total DNA was extracted from the esophageal organs by standard Tris-sodium dodecyl sulfate-proteinase K digestion and phenol-chloroform extraction procedures. The PCR products, amplified with primers for the eubacterial 16S rRNA gene 16SA1 and 16SB1 (7), were subjected to cloning, restriction fragment length polymorphism (RFLP) typing, and DNA sequencing as described previously (8, 15).
RFLP profiles of the 16S rRNA gene clones (10 from P. siamensis and 5 from the Parabdella sp.) exhibited the same patterns with endonucleases that cut 4 bases, such as HaeIII and RsaI (data not shown), indicating that a single bacterial species is dominantly associated with the esophageal organ of each species. Of course the data do not exclude the possibility that some minor microbial associates might exist in addition to the principal symbiont.
Three clones of the 16S rRNA gene from each species were sequenced. The sequences were 1,483 bp long and completely identical between clones in each species, whereas a low level of difference (0.61% [9 of 1,483]) was found between the species. These sequences exhibited an AT-biased nucleotide composition (53.7%). Through the BLAST search of the DDBJ, EMBL, and GenBank DNA databases, they showed the highest sequence similarity (around 89%) to the 16S rRNA genes from Buchnera aphidicola, the primary endosymbiont of aphids.
Tissue localization.
Localization of the symbiont in the host leeches was examined by diagnostic PCR with primer 16SA1 and the leech symbiont-specific primer LS16SR (5′-AATTCTACCCCCCTCTAT-3′). Tissues of the esophageal organ, epidermis, salivary gland, intestine, ovisac, and testisac were dissected out from individuals of P. siamensis maintained in the laboratory and were repeatedly washed with distilled water. The total DNA samples extracted from these tissues were subjected to diagnostic PCR. Amplification of the mitochondrial cytochrome oxidase I gene of the leech with primers LCO1490 and HCO2198 (15) was used as a positive control of the DNA preparation. PCR was conducted according to the following temperature profile: 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 1 min and annealing at 55°C and 50°C for 1 min for the primer sets 16SA1-LS16SR and LCO1490-HCO2198, respectively, followed by extension at 72°C for 2 min.
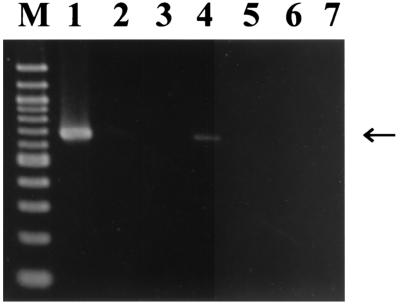
In the diagnostic PCR with the specific primer set for the 16S rRNA gene of the symbionts, strong signal was detected from the esophageal organ, while a slight band was detected from the intestine (Fig. 2). These results indicated that the main location of the symbiont is in the esophageal organ, and a small symbiont population may be found in the intestine.
FIG. 2.
Localization of the symbiont in tissues of P. siamensis detected by specific PCR with primers 16SA1 and LS16SR. Lanes: 1, esophageal organ; 2, epidermis; 3, salivary gland; 4, intestine; 5, ovisac; 6, testisac; 7, no-template control; M, DNA size markers (1,500, 1,300 1,000, 900, 800, 700, 600, 500, 400, 300, and 200 bp from top to bottom). An arrow indicates specific PCR products (approximately 660 bp).
Phylogenetic analysis.
Multiple alignment of 16S rRNA gene sequences was performed with the Clustal W program package (25). A neighbor-joining tree (22) was constructed with Kimura's two-parameter distance (16) by using Clustal W (25). A bootstrap test (5) was conducted with 1,000 resamplings.
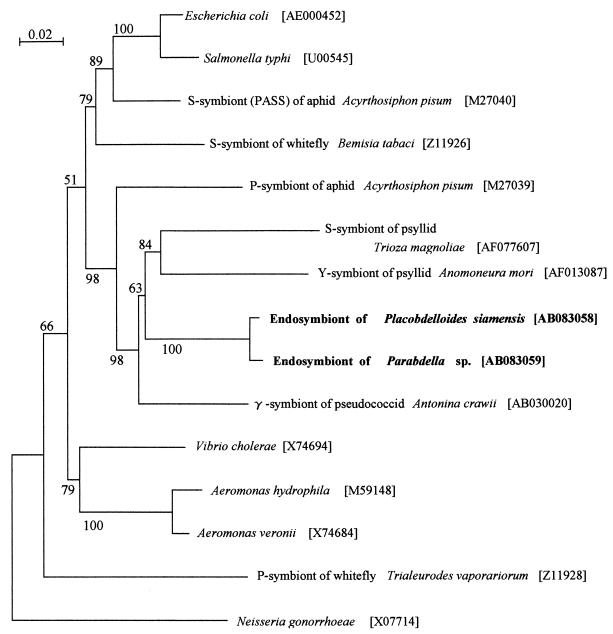
In the phylogenetic tree, the symbionts from P. siamensis and the Parabdella sp. formed a monophyletic group in the clade of the insect endosymbiotic bacteria in the γ-subdivision of the Proteobacteria (Fig. 3). In particular, the leech symbionts showed a phylogenetic affinity to secondary symbionts of psyllids, Trioza magnoliae and Anomoneura mori, and the γ-symbiont of the pseudococcid Antonina crawii. The leech symbionts were not related to Aeromonas spp., which were regarded as digestive tract, not intercellular, symbionts of the medical leech Hirudo medicinalis (Arhynchobdellida: Hirudinidae) (9, 14).
FIG. 3.
Phylogenetic position of the symbionts from P. siamensis and the Parabdella sp. based on their 16S rRNA gene sequences in the γ-subdivision of the Proteobacteria. A total of 1,473 aligned nucleotide sites were subjected to the analysis. A neighbor-joining tree is shown. The bootstrap values obtained with 1,000 resamplings are shown at the nodes.
Vertical transmission.
Glossiphoniid leeches carry their eggs and hatchlings under their dorso-ventrally flattened body (23). We found an individual of P. siamensis carrying 28 eggs, 10 of which were subjected to PCR detection of the symbiont with the primers 16SA1 and LS16SR. From all of the eggs, the symbiont was detected (data not shown), indicating that the symbiont is vertically transmitted through generations of the leeches. The process of vertical transmission and the exact location of the symbionts in the eggs require further investigation.
Association between host and symbiont.
The glossiphoniid leeches examined in this study possess a well-developed symbiotic system, the esophageal organ consisting of a number of mycetocytes harboring the symbionts (Fig. 1 and 2). The symbionts of P. siamensis and the Parabdella sp. are closely related to each other and form a distinct monophyletic group in the γ-subdivision of the Proteobacteria (Fig. 3). At least in P. siamensis, the symbionts might be vertically transmitted through generations. All of these results relate directly to the idea that the symbiont was acquired by the common ancestor of P. siamensis and the Parabdella sp. and has been stably inherited in the leech lineage by vertical transmission over an evolutionary time. To confirm this idea, however, more extensive sampling of glossiphoniid leeches and further molecular phylogenetic analyses of both symbionts and hosts are needed.
AT-biased nucleotide composition.
Notably, 16S rRNA genes from the leech symbionts were extremely AT biased (53.7%). It has been suggested that small population size and lack of effective recombination in vertically transmitted microorganisms result in the accumulation of mildly deleterious mutations, which could be detected as faster sequence evolution and a shift in base composition that reflects mutational bias (19). In fact, essential primary symbionts of insects such as Buchnera aphidicola in aphids and Carsonella ruddii in psyllids exhibit highly AT-biased genomes (4, 24). In this context, the AT-biased nucleotide composition may favor the idea that the glossiphoniid leeches and the symbionts have been in an intimate endosymbiotic association over a long evolutionary time.
The leech symbionts showed a phylogenetic affinity to endosymbionts of plant sap-feeding homopteran insects (Fig. 3). To explain the phylogenetic relationship, several hypotheses are conceivable. One possibility, although unlikely, is horizontal transfer between glossiphoniid leeches and homopteran insects. Another possibility is that the leech symbionts have been derived from a group of enteric bacteria that are prevalent in the intestinal environments of arthropods and annelids in common. However, a more likely explanation is that the phylogenetic affinity is due to parallel AT-directed molecular evolution in these symbiont lineages, on the grounds that all 16S rRNA genes of the insect symbionts in the clade exhibited extreme AT contents (54.5% for the symbiont of the psyllid Anomoneura mori, 56.9% for the symbiont of the psyllid Trioza magnoliae, and 51.9% for the symbiont of the pseudococcid Antonina crawii).
Biological function.
At present, the biological function of the symbionts for the host leeches is totally unknown. The leech symbionts might provide their host with B complex vitamins, as has been suggested in bloodsucking arthropods (20). In general, hematophagous leeches store a large amount of concentrated paste of blood in their crop ceca for a long time (17). Therefore, the possibility that the symbiotic bacteria produce antibiotics that prevent the growth of contaminant bacteria and accordingly retard putrefaction has been proposed (3, 23). The bacterial symbionts might be involved in digestion of the ingested blood meal (12). To test these ideas, it is pivotal to make a comparison between symbiotic and aposymbiotic leeches that could be experimentally produced by antibiotic treatments.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of the symbionts from Placobdelloides siamensis and the Parabdella sp. have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession numbers AB083058 and AB083059, respectively.
Acknowledgments
We thank O. Kitade and S. Izawa for leech samples; A. Sugimura, H. Ouchi, S. Tatsuno, and K. Sato for technical and secretarial assistance; and J. Kojima for advice.
This research was supported by the Industrial Science and Technology Frontier Program “Technological Development of Biological Resources in Bioconsortia” of the Ministry of International Trade and Industry of Japan.
REFERENCES
- 1.Baumann, P., and N. A. Moran. 1997. Non-cultivable microorganisms from symbiotic associations of insects and other hosts. Antonie Leeuwenhoek 72:38-48. [DOI] [PubMed] [Google Scholar]
- 2.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, N.Y.
- 3.Büsing, K.-H. 1951. Pseudomonas hirudinis, ein backterieller Darmsymbiont des Blutegels (Hirudo officinalis). Zentbl. Bakteriol. 157:478-485. [PubMed] [Google Scholar]
- 4.Clark, M. A., L. Baumann, M. L. L. Thao, N. A. Moran, and P. Baumann. 2001. Degenerative minimalism in the genome of a psyllid endosymbiont. J. Bacteriol. 183:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17:368-376. [DOI] [PubMed] [Google Scholar]
- 6.Fukatsu, T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935-1945. [DOI] [PubMed] [Google Scholar]
- 7.Fukatsu, T., and N. Nikoh. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 64:3599-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graf, J. 1999. Symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medical leech: a novel model for digestive tract associations. Infect. Immun. 67:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassé, P. P. 1959. Traite de zoologie. Masson et Cie, Paris, France.
- 11.Hemingway, E. E. 1912. The leeches of Minnesota. II. The anatomy of Placobdella pediculata. Geol. Nat. Hist. Surv. Minnesota Zool. 5:29-63. [Google Scholar]
- 12.Hornbostel, H. 1942. Ueber die bacteriologischen Eigenschaften des Darmsymbionten beim medizinischen Blutegel (Hirudo officinalis) nebst Bemerkungen zur Symbiosefrage. Zentbl. Bakteriol. 148:36-47. [Google Scholar]
- 13.Hotz, H. 1938. Protocclepsis tesselata (O. F. Müller). Ein Beitrag zur Kenntnis von Bau und Lebensweise der Hirudineen. Rev. Suisse Zool. 45:1-380. [Google Scholar]
- 14.Jennings, J. B., and V. M. Ven Der Lande. 1967. Histochemical and bacteriological studies on digestion in nine species of leeches (Annelida: Hirudinea). Biol. Bull. 133:166-183. [Google Scholar]
- 15.Kikuchi, Y., S. Sameshima, O. Kitade, J. Kojima, and T. Fukatsu. 2002. Novel clade of Rickettsia spp. from leeches. Appl. Environ. Microbiol. 68:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 17.Lent, C. M., K. H. Filegner, E. Freedman, and M. H. Dickinson. 1988. Ingestive behavior and physiology of the medicinal leech. J. Exp. Biol. 137:513-527. [DOI] [PubMed] [Google Scholar]
- 18.Moore, J. P. 1930. Leeches (Hirudinea) from China with descriptions of new species. Proc. Acad. Nat. Sci. Philadelphia 82:169-192. [Google Scholar]
- 19.Moran, N. A. 1996. Accelerated evolution and Muller's ratchet in endosymbiotic bacteria. Proc. Natl. Acad. Sci. USA 93:2873-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogge, G. 1981. Significance of symbionts for the maintenance of an optimal nutritional state for successful reproduction in haematophagous arthropods. Parasitology 82:299-304. [Google Scholar]
- 21.Oosthuizen, J. H. 1979. Redescription of Placobdella multistriata (Johansson, 1909) (Hirudinea: Glossiphoniidae). Koedoe 22:61-79. [Google Scholar]
- 22.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer, R. T. 1986. Leech biology and behavior. Oxford University Press, Oxford, United Kingdom.
- 24.Shigenobu, S., H. Watanabe, M. Hattori, Y. Sakaki, and H. Ishikawa. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81-86. [DOI] [PubMed] [Google Scholar]
- 25.Thompson, J. D., D. G. Higgins, and J. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Der Lande, V. M. 1983. Observations on the growth and development of the duck leech, Theromyzon tessulatum (Hirudinea: Glossiphoniidae), as a function of feeding. J. Zool. 201:377-393. [Google Scholar]
- 27.Van Der Lande, V. M., and R. C. Tinsley. 1976. Studies on the anatomy, life history and behavior of Marsupiobdella africana (Hirudinea: Glossiphoniidae). J. Zool. (London) 180:537-563. [Google Scholar]