Abstract
The hydrogenase and formate dehydrogenase levels in Syntrophobacter fumaroxidans and Methanospirillum hungatei were studied in syntrophic propionate-oxidizing cultures and compared to the levels in axenic cultures of both organisms. Cells grown syntrophically were separated from each other by Percoll gradient centrifugation. In S. fumaroxidans both formate dehydrogenase and hydrogenase levels were highest in cells which were grown syntrophically, while the formate-H2 lyase activities were comparable under the conditions tested. In M. hungatei the formate dehydrogenase and formate-H2 lyase levels were highest in cells grown syntrophically, while the hydrogenase levels in syntrophically grown cells were comparable to those in cells grown on formate. Reconstituted syntrophic cultures from axenic cultures immediately resumed syntrophic growth, and the calculated growth rates of these cultures were highest for cells which were inoculated from the axenic S. fumaroxidans cultures that exhibited the highest formate dehydrogenase activities. The results suggest that formate is the preferred electron carrier in syntrophic propionate-oxidizing cocultures of S. fumaroxidans and M. hungatei.
Methanogenic decomposition of complex organic matter is a widespread process, which accounts for a large fraction of the global methane emission (15). Examples of natural methanogenic habitats are freshwater environments such as wetlands, sediments, and rice paddy fields, as well as intestinal tracts of higher animals and insects (4, 6, 8, 21, 28). Methanogenic processes can be applied to treat industrial wastewaters in high-rate anoxic bioreactors (16, 19, 33). The microorganisms involved in methanogenic decomposition are usually immobilized in granular aggregates or biofilms, which is essential for a high conversion rate (23) and prevents biomass from being washed out of the reactor.
The amount of energy available in methanogenic conversions is small, and therefore the microorganisms involved are forced to cooperate syntrophically (24, 28). In particular, oxidation of intermediary reduced organic compounds, such as ethanol, butyrate, and propionate, is energetically unfavorable. Nevertheless, the methanogens involved keep the concentrations of the oxidation products, acetate and H2 (or formate), low enough to create a situation in which all partners involved gain energy. To dispose of reducing equivalents, acetogens reduce protons or bicarbonate. Both H2 and formate have been proposed as electron carriers in syntrophic degradation. Several studies have provided evidence for H2 transfer by demonstrating syntrophic growth with methanogens that oxidize only H2. Schmidt and Ahring (25) studied interspecies electron transfer in granules from a mesophilic anaerobic sludge bed reactor, and concluded that formate transfer was not important during propionate and butyrate oxidation in this system. On the other hand, Thiele and Zeikus (29) provided evidence that interspecies formate transfer was the dominant mechanism in a whey-processing digester, as well as in flocs, which contained primarily Desulfovibrio vulgaris and Methanobacterium formicicum. In syntrophic propionate- and butyrate-oxidizing cultures, at least some interspecies formate transfer was indicated because the rate of H2 diffusion could not account for the measured methanogenic rate (3). Possibly, H2 transfer becomes more important with shorter interbacterial distances, while formate transfer is more favorable in suspended cultures (28).
The syntrophic propionate-oxidizing bacterium Syntrophobacter fumaroxidans is one of the Syntrophobacter subspecies in the δ subdivision of the proteobacteria (13). This organism oxidizes propionate in suspended cocultures with methanogens that utilize both H2 and formate and not with Methanobrevibacter strains which utilize only H2 (10). It has been demonstrated that S. fumaroxidans is able to produce both H2 and formate during propionate oxidation, and the organism possesses both hydrogenase and formate dehydrogenase activities (9, 12, 30). We studied the levels of hydrogenase and formate dehydrogenase in propionate-grown cocultures of S. fumaroxidans and Methanospirillum hungatei, as well as in axenic cultures of both organisms. Our results suggest that besides hydrogenases, formate dehydrogenases play important role during syntrophic propionate oxidation.
MATERIALS AND METHODS
Organisms and cultivation.
M. hungatei JF1T (DSM 864) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany. S. fumaroxidans (DSM 10017) and M. hungatei were grown at 37°C in mineral bicarbonate-buffered medium as described previously (27). However, no yeast extract was added, and the medium contained 0.5 mg of EDTA per liter. M. hungatei was cultured routinely with H2 (1.7 bar of H2-CO2, 80:20) or formate (30 mM) as the substrate, in medium amended with 1 mM acetate and 1 mM cysteine. S. fumaroxidans was cultured routinely with (concentrations are in millimolar in parentheses) fumarate (20), fumarate-propionate (30:10), fumarate (20) plus H2, fumarate-formate (20:20), propionate-sulfate (20:15), H2 (1.7 bar of H2-CO2, 80:20) plus sulfate (20), or pyruvate (20). Syntrophic cocultures were cultured routinely on 30 mM propionate. For reconstitution of syntrophic growth from axenic cultures, S. fumaroxidans was transferred (5 to 10% inoculum) into M. hungatei cultures grown on H2 and CO2. The gas phase above these cultures was changed to N2-CO2 prior to inoculation of S. fumaroxidans and addition of 30 mM propionate. For mass cultivation, substrate-adapted cultures (10 transfers or more) were transferred to 3-liter serum bottles containing 1.5 liters of medium. To monitor the H2 concentration during syntrophic growth with propionate, cells from a 1.5-liter culture in the late log phase were collected and inoculated in two 117-ml serum flasks containing 50 ml of freshly prepared medium each. The flasks were flushed with N2-CO2 to remove residual H2 and CH4, leaving 1.5 bar of N2-CO2 as the final headspace. The vials were incubated in a shaker at 30°C, and after 100 min 20 mM propionate was added. Acetate, H2, and propionate were monitored by withdrawing samples from the culture at time intervals of 30 min. The amount of CH4 produced was determined at the end of the experiment.
Preparation of cell extracts.
Centrifugation steps were carried out in airtight closed tubes, and all other procedures were carried out under strict anoxic conditions in a glove box with N2-H2 (96:4, vol/vol) as the gas phase. Traces of oxygen were removed by circulating the gas phase over a platinum catalyst column.
Cells were collected in late exponential phase by centrifugation at 16,000 × g and 4°C. The cells were washed twice by resuspending the cell pellets obtained after centrifugation in 50 mM Tris-HCl (pH 8) containing 100 μM sodium dithionite. Cells were disrupted by sonication. Cell debris was removed by centrifugation at 16,000 × g.
Percoll gradient centrifugation.
Cells from a syntrophic culture on propionate were resuspended in 50 mM sodium phosphate (pH 7.5) containing 75% Percoll (vol/vol) and 100 μM sodium dithionite. The cells were separated by generating a Percoll gradient in airtight centrifuge tubes (3 by 9 ml) at 30,000 × g and 4°C for 30 min. The separated layers containing the Syntrophobacter cells and Methanospirillum cells, respectively, were collected from each tube and washed twice with 10 mM sodium phosphate (pH 7.5) and 100 μM sodium dithionite. The number of contaminating cells in each layer was less than 1% as estimated by phase-contrast microscopy. Although additional Percoll gradients hardly improved the separation, all of the cells used for further experiments had been subjected to Percoll gradient centrifugation twice.
Enzyme activities.
Enzyme activities were routinely measured at 37°C in N2-flushed 1-ml cuvettes closed with butyl rubber stoppers. One unit of enzyme is defined as the amount of enzyme catalyzing the conversion of 1 μmol of substrate per min or catalyzing the production of 1 μmol of H2 or formate in case of proton and bicarbonate reduction. Benzyl viologen-dependent formate and H2 oxidation rates were recorded at 578 nm in 50 mM Tris-HCl (pH 8) (ɛ = 8.65 mM−1 cm−1 for the free radical). The final concentrations of benzyl viologen and formate were 1 and 10 mM, respectively, while 1 bar of H2 was added into the headspace to measure H2 oxidation rates. F420-dependent formate and H2 oxidation rates were recorded at 420 nm in 50 mM Tris-HCl (pH 8) containing 2.5 mM glutathione and 0.16 mM coenzyme F420 (ɛ = 42.5 mM−1 cm−1). Methylmalonyl coenzyme A (CoA):pyruvate transcarboxylase was measured indirectly in 50 mM of sodium phosphate (pH 7) by monitoring the NADH-dependent reduction of oxaloacetate at 340 nm (ɛ = 6.22 mM−1 cm−1). The mixture contained 0.3 mM NADH, 10 mM pyruvate, 0.2 mM methylmalonyl-CoA, 2.5 mM glutathione, and 5 μg of malate dehydrogenase from pig heart (Boehringer, Mannheim, Germany). H2 production rates were measured in 35-ml serum flasks containing 2 ml of 100 mM sodium phosphate (pH 7), 10 mM methyl viologen, and 100 mM sodium dithionite. After 15 min of incubation at 37°C, the reaction was initiated by addition of 10 to 100 μl of sample. Formate-H2 lyase activity was measured at 37°C in 35-ml serum flasks containing 5 ml of 50 mM Tris-HCl (pH 8), 200 μM sodium dithionite, and 10 to 100 μl of sample. The reaction was started by addition of 20 mM sodium formate. H2 production was monitored by withdrawing 0.5-ml samples over time and analyzing them by gas chromatography. Protein was determined with a Bio-Rad DC protein assay with bovine serum albumin as a standard. To determine the protein content of whole cells, protein was extracted by boiling for 15 min in 1 M (final concentration) NaOH.
Analytical methods.
Organic acids were measured with a Spectrasystem high-pressure liquid chromatography system equipped with an autosampler and refractomonitor. The acids were separated on a Polyspher OAHY column (30 cm by 6.5 mm; Merck, Darmstadt, Germany) in 0.01 N H2SO4 at a flow rate of 0.6 ml/min and a column temperature of 60°C. The acids eluting from the column were quantified by differential refractometry. H2 and methane were measured gas chromatographically with a Packard-Becker 417 gas chromatograph equipped with a thermal conductivity detector and 13X molecular sieve (60/80 mesh). The column temperature was 50°C, and the carrier gas was argon at a flow rate of 30 ml/min. To analyze H2 in the nanomolar range (reconstitution experiment), an RGA3 reduction gas analyzer (Trace Analytic) was used. The system was equipped with a 60/80 Unibeads precolumn and a 60/80 molecular sieve 5A column. The reduction gas detector had detector and column temperatures of 265 and 105°C, respectively. The loop size was 1 ml. The carrier gas was N2 at a flow rate of 20 ml/min.
RESULTS
Growth of S. fumaroxidans.
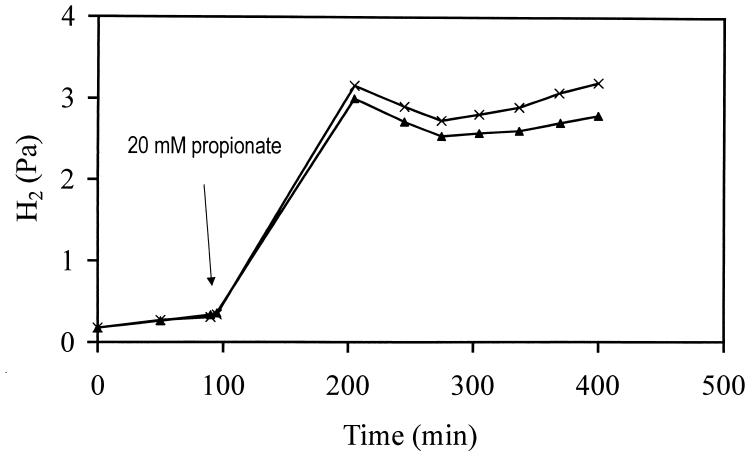
S. fumaroxidans was cultured on pyruvate, fumarate, and combinations of H2, formate, or propionate with fumarate or sulfate as an electron acceptor. The organism converted these substrates as described in previous studies (27, 30). However, cells grown on fumarate also produced low levels (1 to 3 mM) of acetate. Propionate was stoichiometrically oxidized to acetate, while fumarate was stoichiometrically reduced to succinate when H2, formate, or propionate was present as an electron donor. Fermentation of pyruvate was usually incomplete, and in these cultures several unidentified compounds were detected. H2 and formate could not be detected (H2, <0.1 μM; formate, <100 μM) during growth on most of the substrates tested. However in the mid-log phase of the culture grown on fumarate plus formate, a small amount of H2 (approximately 450 Pa [3.4 μM]) was detected in the headspace, while at the end of the log phase, formate was detected in the cultures grown on H2 plus fumarate and H2 plus sulfate (7.3 and 0.8 mM, respectively). When subsequently transferred from the logarithmic phase into fresh medium (5% inoculum), the organism converted all substrates (or combinations) within 3 weeks. In the syntrophic cultures with M. hungatei on propionate, the cells tended to aggregate in the late log phase, but most of the cells were suspended. No flocs were observed microscopically, indicating that the microbial aggregates were very weak and were disrupted as soon as samples were withdrawn from the cultures. In the resuspended syntrophic cocultures the H2 concentrations (at 30°C) decreased to 2.7 and 2.5 Pa, respectively, during syntrophic growth, which corresponds to soluble concentrations of approximately 21 and 19 nM (Fig. 1). Addition of 5 mM bromo ethane sulfonate in one of the batches resulted in a small increase of the H2 partial pressure to 3.2 Pa (25 nM) within 1 h.
FIG. 1.
H2 partial pressure during syntrophic growth of S. fumaroxidans and M. hungatei on propionate. Within the 300 min during which the H2 partial pressure was recorded after addition of propionate, 1.8 and 1.1 mM propionate were oxidized to acetate and CH4 (stoichiometrically) in batches 1 and 2, respectively. Symbols: ×, batch 1; ▴, batch 2.
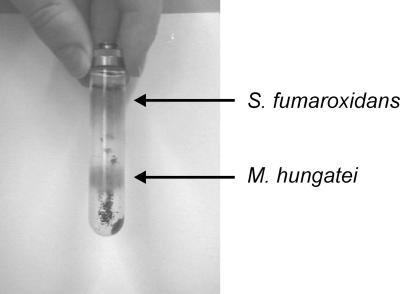
Percoll gradient centrifugation.
An amount of 1.5 g of cells collected from 4.5 liters of syntrophic coculture grown on propionate was successfully separated by Percoll gradient centrifugation. Good separations were obtained with up to 0.5 g of wet cells per 9-ml Percoll gradient (Fig. 2). For all further experiments we used cells which had been subjected to Percoll gradient centrifugation twice. The amount of contaminating protein in extracts of these cells was determined by measuring F420-dependent hydrogenase and formate dehydrogenase activities in S. fumaroxidans cell extracts and methylmalonyl-CoA:pyruvate transcarboxylase activity in M. hungatei cell extracts (Table 1). We could not detect F420-dependent activities in cell extracts of S. fumaroxidans grown axenically on fumarate, while methylmalonyl-CoA:pyruvate transcarboxylase could not be detected in cell extracts of an axenic M. hungatei culture grown on H2 plus CO2.
FIG. 2.
Separation of 0.5 g of cells from a syntrophic culture of S. fumaroxidans and M. hungatei in a 9-ml Percoll gradient. S. fumaroxidans and M. hungatei cells were recovered in the upper and lower layers, respectively.
TABLE 1.
Activities of enzymes specific for either S. fumaroxidans or M. hungatei in cell extracts of cells separated by Percoll gradient centrifugation
| Enzyme | Activity (U/mg) in:
|
|
|---|---|---|
| S. fumaroxidans | M. hungatei | |
| F420-dependent hydrogenase | 0.017 | 3.1 |
| F420-dependent formate dehydrogenase | 0.016 | 2.2 |
| Methylmalonyl-CoA pyruvate transcarboxylase | 0.29 | 0.002 |
Hydrogenase and formate dehydrogenase activities in S. fumaroxidans and M. hungatei.
Both hydrogenase and formate dehydrogenase activities were detected in all S. fumaroxidans cell extracts analyzed (Table 2). For both enzymes, the highest activities were detected in syntrophically grown cells. Only intact S. fumaroxidans cells catalyzed the interconversion of formate to H2 and CO2 (formate-H2 lyase). These activities seemed not to be related to the levels of hydrogenase and formate dehydrogenase which were measured in cell extracts (Table 2). Viologen-dependent as well as F420-dependent hydrogenase and formate dehydrogenase activities were detected in all cell extracts of M. hungatei tested (Table 3). The formate dehydrogenase activities in syntrophically grown cells were considerably higher than those in cells grown axenically with an excess H2 or formate. The hydrogenase activities in syntrophically grown cells were not higher than those in cells grown on formate, although these activities were twofold higher than those in cells grown on H2. The formate-H2 lyase activity in syntrophically grown M. hungatei cells was fivefold higher than that in cells grown on either H2-CO2 or formate.
TABLE 2.
Hydrogenase and formate dehydrogenase specific activities in cell extracts of S. fumaroxidans and formate-H2 lyase activities in intact S. fumaroxidans cellsa
| Substrate | Activity (U/mg)
|
||
|---|---|---|---|
| Formate dehydrogenase | Hydrogenase | Formate H2-lyase (cells) | |
| Propionate (syntrophic growth) | 198 | 28 | 0.037 |
| Propionate-fumarate | 60 | 16 | 0.059 |
| Propionate-sulfate | 31 | 11 | NDb |
| H2-sulfate | 16 | 12 | 0.027 |
| Fumarate-formate | 5.4 | 7.2 | ND |
| Fumarate-H2 | 6.9 | 3.5 | ND |
| Fumarate | 25 | 5.5 | 0.062 |
| Pyruvate | 103 | 32 | ND |
Syntrophically grown S. fumaroxidans cells were separated from M. hungatei cells by Percoll gradient centrifugation. Formate-H2 lyase and benzyl viologen-dependent oxidation activities were measured at 37°C and pH 8.
ND, not determined.
TABLE 3.
Hydrogenase and formate dehydrogenase specific activities in cell extracts of M. hungatei grown syntrophically and in cell extracts of cells grown axenically with H2 or formate as an electron donora
| Enzymeb | Substrate-dependent sp act (U/mg) with:
|
||
|---|---|---|---|
| Syntrophic growth | H2 | Formate | |
| BV-dependent FDH | 59 | 4.6 | 1.7 |
| F420-dependent FDH | 2.2 | 0.075 | 0.35 |
| BV-dependent H2ase | 6.0 | 2.5 | 6.5 |
| F420-dependent H2ase | 3.1 | 2.0 | 3.3 |
| H2ase (H2 evolution) | 9.1 | 3.6 | 9.6 |
| Formate-H2 lyase (cells) | 2.6 | 0.52 | 0.56 |
Syntrophically-grown M. hungatei cells were separated from S. fumaroxidans cells by Percoll gradient centrifugation. Formate-H2 lyase and oxidation activities were measured at 37°C and pH 8. Formate-H2 lyase was measured with intact cells. Methyl viologen-mediated H2 production with dithionite as an electron donor was measured at 37°C and pH 7.
FDH, formate dehydrogenase; H2ase, hydrogenase; BV, benzyl viologen.
Reconstitution of syntrophic growth from axenic cultures.
Axenic cultures of S. fumaroxidans adapted (10 transfers or more) to growth on one of the seven different growth substrates were inoculated in fresh M. hungatei cultures which were pregrown on H2 and CO2, together with 30 mM propionate. A lag phase was observed only for fumarate-grown cells and cells grown on H2 plus sulfate; all other reconstituted cultures immediately resumed syntrophic growth. The highest growth rates were measured in the cultures which were reconstituted from cells grown on propionate (syntrophically with M. hungatei), propionate plus fumarate, propionate plus sulfate, and pyruvate. The growth rates of these cultures as estimated from the CH4 production rates were between 0.18 and 0.23 day−1. The growth rates of syntrophic cultures reconstituted from cells grown axenically on fumarate, fumarate plus formate, fumarate plus H2, and H2 plus sulfate were 0.064, 0.10, 0.064, and 0.083 day−1, respectively.
DISCUSSION
To study the hydrogenase and formate dehydrogenase levels in S. fumaroxidans and M. hungatei grown in coculture, the individual organisms needed to be either separated or lysed selectively. Although selective lysis of one of the two cocultured organisms has been proven to be successful in previous studies (14, 17, 31), all of our attempts to lyse one of the organisms specifically failed (data not shown). Percoll gradient centrifugation has also been used successfully to separate cocultured organisms. Syntrophomonas wolfei was separated from M. hungatei after syntrophic growth on butyrate, resulting in a 70- to 80-fold enrichment of S. wolfei (2). Unfortunately, those authors did not provide information on the hydrogenase and formate dehydrogenase levels in these organisms. In our study we used Percoll gradient centrifugation to separate S. fumaroxidans from M. hungatei. Both organisms were enriched approximately 150-fold after two Percoll gradient centrifugations, enabling measurement of enzymes specific for either one of the separated organisms. A 9-ml gradient was sufficient to separate up to 0.5 g of wet cells.
S. fumaroxidans oxidizes propionate in suspended cocultures with methanogens which utilize both H2 and formate and not with Methanobrevibacter strains which utilize only H2 (10). To our knowledge, none of the other mesophilic syntrophic propionate-oxidizing bacteria described so far are able to grow with methanogens that utilize only H2. For several other compounds, such as ethanol and butyrate, syntrophic growth was possible with such methanogens (5, 20). Perhaps the difference lies in the oxidation steps that require extremely low H2 partial pressures during syntrophic growth. Of all the reactions involved in the oxidation of propionate, the oxidation of succinate to fumarate seems to require the lowest H2 partial pressure (24). Thermophilic syntrophic propionate oxidation, however, appeared to be possible with a methanogen that uses only H2 (26). However, it is known that H2 formation becomes energetically more favorable at higher temperatures (28), and furthermore, it was demonstrated that the growth rate was higher when a formate-utilizing methanogen was the syntrophic partner (26). Thermophilic syntrophic acetate oxidation is possible with a methanogen that utilizes only H2 (18). Recently, a syntrophic acetate oxidizer was also shown to grow better in the presence of a methanogen that uses both H2 and formate (14), while a syntrophic butyrate-oxidizing organism also appeared to require an H2- and formate-utilizing methanogen (11). This suggests that also for butyrate and acetate, formate transfer may be an important mechanism during syntrophic oxidation of these compounds. On the other hand, it is also possible that the H2- and formate-utilizing methanogens, which were used in those studies, have a lower threshold for H2 than those that were used as methanogens scavenging only H2 (7).
In syntrophic cocultures both S. fumaroxidans and M. hungatei exhibited higher levels of formate dehydrogenase than cultures grown axenically. In addition, S. fumaroxidans also exhibited higher hydrogenase levels when grown syntrophically, while the hydrogenase levels in M. hungatei were comparable to those in the cells grown axenically on formate. The two distinct formate dehydrogenases which were purified from S. fumaroxidans (unpublished results) both catalyze CO2 reduction at relatively high rates. Apparently, the extremely high formate dehydrogenase levels in S. fumaroxidans during syntrophic growth reflects the necessity to dispose of reducing equivalents via CO2 reduction. On the other hand, during syntrophic growth the hydrogenase levels were higher as well, suggesting that proton reduction also occurs.
However, if H2 is transferred between the two organisms, we expected increased levels of hydrogenase in M. hungatei as well. These levels were indeed higher than those in cells grown axenically on H2, but they were similar to the hydrogenase levels in cells grown on formate. Remarkably, the H2 evolution activity in M. hungatei cells grown syntrophically or on formate was about 2.5-fold higher than that in cells grown on H2, while the H2 uptake activity was only about 1.5-fold higher. This may suggest that the levels of a H2-evolving enzyme in M. hungatei are increased during growth on formate. The formate-H2 lyase activity in M. hungatei was also highest in cells which were grown syntrophically. In another hydrogenotrophic methanogen, Methanobacterium formicicum, formate is cleaved to H2 and HCO3− and subsequently converted to methane (32). Formate-H2 lyase activity in this organism could be reconstituted with F420-reducing hydrogenase, F420-formate dehydrogenase, and coenzyme F420 (1). The higher formate-H2 lyase activities in M. hungatei seemed not to be associated with the levels of the individual reductases during syntrophic growth (Table 3), and therefore this organism may have produced higher levels of coenzyme F420 during syntrophic growth. If we assume that M. hungatei also cleaves formate to H2 and HCO3− during growth on formate, the measured enzyme levels strongly suggest that formate is also the substrate for M. hungatei during syntrophic growth. Thus, the hydrogenase and formate dehydrogenase levels of both organisms strongly suggest that formate transfer is the more important mechanism in syntrophic cocultures of S. fumaroxidans and M. hungatei.
Additional evidence for this conclusion was provided by reconstitution of syntrophic cultures from axenic cultures. The highest conversion rates were observed in the cocultures reconstituted from cells with the highest formate dehydrogenase activities (Table 2). Only for fumarate-grown cells does this conclusion not hold, but for this compound the situation is probably more complex. When only fumarate is available, part of it is completely converted to CO2 via the acetyl-CoA cleavage pathway (coupled to fumarate reduction). S. fumaroxidans is believed to use this pathway in the opposite direction as an anaplerotic route to fix CO2 for cell synthesis during syntrophic growth (22). When an electron donor such as H2, formate, or propionate is used, fumarate is reduced only to succinate (30). Possibly the organism has to adapt to CO2 fixation again after growth on fumarate instead of using this route in the opposite direction. Accordingly, we observed that it takes several weeks for the organism to adapt to growth on fumarate when transferred from a syntrophic coculture grown on propionate (data not shown). Remarkably, most of the reconstituted cultures immediately resumed syntrophic growth, indicating that S. fumaroxidans constitutively expressed the enzymes required. This observation emphasizes that this organism is specialized in propionate oxidation in its microbial niche and that all of the substrates used by pure cultures may be “artificial substrates.”
The measured H2 level during syntrophic growth also provided evidence that formate transfer is the more important mechanism. The measured H2 concentrations were within the range of the threshold values reported for M. hungatei (7). A theoretical H2 flux can be calculated with Fick's diffusion equation by using H2 concentrations of 21 nM at the surface of M. hungatei and 52 nM at the surface of S. fumaroxidans (12) and specific parameters presented previously (12, 23). Using 2.5 · 108 cells ml−1 (counted with a Bürker-Türk counting chamber) and an average diffusion distance of 10 μm, an H2 flux of 2.5 nmol ml−1 min−1 was calculated, while the acetate production rate would correspond to an H2 production rate of 18.6 nmol ml−1 min−1. If we used an H2 partial pressure of 3.2 Pa, which was measured during syntrophic growth of the cocultures in the present study, the theoretical H2 flux was much lower, i.e., 0.3 nmol ml−1 min−1. Although we did not account for the fact that some floc formation was observed in our experiments, these calculations support our data that formate is a more important mechanism of electron transfer during syntrophic growth of S. fumaroxidans and M. hungatei on propionate.
The evidence presented in this paper, together with other published evidence, indicates that interspecies electron transfer is not exclusively by H2 transfer; although H2 transfer is not ruled out, the evidence suggests that formate is the major interspecies electron carrier.
Acknowledgments
We thank the TNO Institute of Environmental Sciences, Energy Research and Process Innovation, Apeldoorn, The Netherlands, for providing the opportunity to use their reduction gas analyzer.
REFERENCES
- 1.Baron, S. F., and J. G. Ferry. 1989. Reconstitution and properties of a coenzyme F420-mediated formate hydrogenlyase system in Methanobacterium formicicum. J. Bacteriol. 171:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaty, P. S., N. Q. Wofford, and M. J. McInerney. 1987. Separation of Syntrophomonas wolfei from Methanospirillum hungatei in syntrophic cocultures by using Percoll gradients. Appl. Environ. Microbiol. 53:1183-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boone, D. R., R. L. Johnson, and Y. Liu. 1989. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl. Environ. Microbiol. 55:1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone, D. R. 1991. Ecology of methanogenesis. p. 57-70. In J. E. Rogers and W. B. Whitman (ed.), Microbial production of greenhouse gases: methane, nitrogen oxides, and halomethanes. American Society for Microbiology, Washington, D.C.
- 5.Bryant, M. P., E. A. Wolin, M. J. Wolin, and R. S. Wolfe. 1967. Methanobacillus omelianski, a symbiotic association of two species of bacteria. Arch. Microbiol. 59:20-31. [DOI] [PubMed] [Google Scholar]
- 6.Bryant, M. P. 1977. Microbiology of the rumen, p. 287-304. In M. J. Swenson (ed.), Physiology of domestic animals, 9th ed. Cornell University Press, Ithaca, N.Y.
- 7.Cord-Ruwisch, R., H. Seitz, and R. Conrad. 1988. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch. Microbiol. 149:350-357. [Google Scholar]
- 8.Crill, P. M., R. C. Harris, and K. B. Bartlett. 1991. Methane fluxes from terrestrial wetland environments, p. 175-187. In J. E. Rogers and W. B. Whitman (ed.), Microbial production of greenhouse gases: methane, nitrogen oxides, and halomethanes. American Society for Microbiology, Washington, D.C.
- 9.Dong, X. 1994. Role of formate and hydrogen in the syntrophic degradation of propionate and butyrate. PhD thesis. Wageningen University, Wageningen, The Netherlands.
- 10.Dong, X., C. M. Plugge, and A. J. M. Stams. 1994. Anaerobic degradation of propionate by a mesophilic acetogenic bacterium in coculture and triculture with different methanogens. Appl. Environ. Microbiol. 60:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, X., G. Cheng, and A. J. M. Stams. 1994. Butyrate degradation by Syntrophospora bryantii in coculture with different methanogens and in pure culture with pentanoate as electron acceptor. Appl. Microbiol. Biotechnol. 42:647-652. [Google Scholar]
- 12.Dong, X., and A. J. M. Stams. 1995. Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation. Anaerobe 1:35-39. [DOI] [PubMed] [Google Scholar]
- 13.Harmsen, H. J. M., B. L. M. van Kuijk, C. M. Plugge, A. D. L. Akkermans, W. M. de Vos, and A. J. M. Stams. 1998. Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int. J. Syst. Bacteriol. 48:1383-1387. [DOI] [PubMed] [Google Scholar]
- 14.Hattori, S., H. Luo, H. Shoun, and Y. Kamagata. 2001. Involvement of formate as an interspecies electron carrier in a syntrophic acetate-oxidizing anaerobic microorganism in coculture with methanogens. J. Biosci. Bioeng. 3:294-298. [DOI] [PubMed] [Google Scholar]
- 15.Houghton, J. T., L. G. Meira Filho, J. Bruce, H. Lee, B. A. Callander, E. Haites, N. Harriss, and K. Maskell. 1995. Climate change 1994. Radiative forcing of climate change and an evaluation of the IPCC IS92 emissions scenarios. Cambridge University Press, Cambridge, United Kingdom.
- 16.Iza, J. 1991. Fluidized bed reactors for anaerobic wastewater treatment. Water Sci. Technol. 24:109-132. [Google Scholar]
- 17.Kiener, A., H. König, J. Winter, and T. Leisinger. 1987. Purification and use of Methanobacterium wolfei pseudomurein endopeptidase for lysis of Methanobacterium thermoautotrophicum. J. Bacteriol. 169:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, M. J., and S. H. Zinder. 1988. Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on H2-CO2. Appl. Environ. Microbiol. 54:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lettinga, G., and L. W. Hulshoff-Pol. 1991. UASB process design for various types of waste waters. Water Sci. Technol. 24:88-107. [Google Scholar]
- 20.McInerney, M. J., M. P. Bryant, R. B. Hespell, and J. W. Costerton. 1981. Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic syntrophic, fatty-acid-oxidizing bacterium. Appl. Environ. Microbiol. 41:1029-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oremland, R. S. 1988. Biogeochemistry of methanogenic bacteria, p. 641-706. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley and Sons, New York, N.Y.
- 22.Plugge, C. M., C. Dijkema, and A. J. M. Stams. 1993. Acetyl-CoA cleavage pathway in a syntrophic propionate oxidizing bacterium growing on fumarate in the absence of methanogens. FEMS Microbiol. Lett. 110:71-76. [Google Scholar]
- 23.Schink, B., and R. K. Thauer. 1988. Energetics of syntrophic methane formation and the influence of aggregation, p. 5-17. In G. Lettinga, A. J. B. Zehnder, J. T. C. Grotenhuis, and L. W. Hulshoff-Pol (ed.), Granular anaerobic sludge; microbiology and technology. Pudoc, Wageningen, The Netherlands.
- 24.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt, J. E., and B. K. Ahring. 1995. Interspecies electron transfer during propionate and butyrate degradation in mesophilic granular sludge. Appl. Environ. Microbiol. 61:2765-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stams, A. J. M., K. C. F. Grolle, C. T. M. J. Frijters, and J. B. van Lier. 1992. Enrichment of thermophilic propionate-oxidizing bacteria in syntrophy with Methanobacterium thermoautotrophicum or Methanobacterium thermoformicium. Appl. Environ. Microbiol. 58:346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stams, A. J. M., J. B. van Dijk, C. Dijkema, and C. M. Plugge. 1993. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl. Environ. Microbiol. 59:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stams, A. J. M. 1994. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Leeuwenhoek 66:271-294. [DOI] [PubMed] [Google Scholar]
- 29.Thiele, J. H., and J. G. Zeikus. 1988. Control of interspecies electron flow during anaerobic digestion: significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs. Appl. Environ. Microbiol. 54:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Kuijk, B. L. M., E. Schlösser, and A. J. M. Stams. 1998. Investigation of the fumarate metabolism of the syntrophic propionate-oxidizing bacterium strain MPOB. Arch. Microbiol. 169:346-352. [DOI] [PubMed] [Google Scholar]
- 31.Wofford, N. Q., P. S. Beaty, and M. J. McInerney. 1986. Preparation of cell-free extracts and the enzymes involved in fatty acid metabolism in Syntrophomonas wolfei. J. Bacteriol. 167:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, W. M., R. F. Hickey, M. K. Jain, and J. G. Zeikus. 1993. Energetics and regulations of formate and hydrogen metabolism by Methanobacterium formicicum. Arch. Microbiol. 159:57-65. [Google Scholar]
- 33.Young, J. C. 1991. Factors affecting the design and performance of upflow anaerobic filters. Water Sci. Technol. 24:133-155. [Google Scholar]