Abstract
An exopolymer (slime)-producing soil bacterium Pseudomonas sp. (strain PS+) rapidly clogged sand-filled columns supplied with air-saturated artificial groundwater containing glucose (500 mg liter−1) as a sole carbon source and nitrate (300 mg liter−1) as an alternative electron acceptor. After 80 days of operation under denitrifying conditions, the effective porosity and saturated hydraulic conductivity (permeability) of sand in these columns had fallen by 2.5- and 26-fold, respectively. Bacterial biofilms appeared to induce clogging by occluding pore spaces with secreted exopolymer, although there may also have been a contribution from biogas generated during denitrification. The bacterivorous soil flagellate Heteromita globosa minimized reductions in effective porosity (1.6-fold) and permeability (13-fold), presumably due to grazing control of biofilms. Grazing may have limited growth of bacterial biomass and hence the rate of exopolymer and biogas secretion into pore spaces. Evidence for reduction in biogas production is suggested by increased nitrite efflux from columns containing flagellates, without a concomitant increase in nitrate consumption. There was no evidence that flagellates could improve flow conditions if added once clogging had occurred (60 days). Presumably, bacterial biofilms and their secretions were well established at that time. Nevertheless, this study provides evidence that bacterivorous flagellates may play a positive role in maintaining permeability in aquifers undergoing remediation treatments.
Bacterial growth in natural porous media frequently leads to clogging through a combination of factors involving biomass accumulation, exopolymeric slime secretion, and insoluble biogas formation. Many operations, including wastewater disposal, microbe-enhanced oil recovery, groundwater recharge, and in situ bioremediation are variously affected by this process (for a review, see reference 2). Since groundwater aquifers provide a significant proportion of the world population with a potable supply of water, their contamination with organic pollutants poses a serious risk both to health and the environment. At present, in situ bioremediation is considered to be the most cost-effective and least invasive strategy available to remediate an organically contaminated aquifer (12). This approach relies on maintaining good hydraulic conductivity (permeability) in the saturated subsurface to permit adequate groundwater flow through the affected area. Nutrients and/or oxidants may then be injected upstream of the contaminant to biostimulate the biodegradative abilities of the resident microorganisms. However, these water injection wells are frequent foci for partial clog formation in the subsurface (17). Consequently, bacterial clogging may have an adverse effect on the rate and extent of in situ bioremediation owing to reduced permeability in the aquifer.
Bacterivorous protozoa are primary grazers on bacteria in numerous environments, and comparatively large populations coexist with bacteria in variously contaminated aquifers (8, 14, 25, 36). The impact of protozoa on in situ bioremediation is presently unknown but may be influenced by their ability to selectively graze on and control the biomass of the bacterial community (16, 24). Grazing protozoa are known to remineralize growth-limiting nutrients (for a review, see reference 21), which may directly stimulate bacterial metabolism and hence biodegradation. Furthermore, it is possible that grazing protozoa may indirectly stimulate the rate of in situ bioremediation by controlling bacterial clogging and therefore improving permeability (25). Soil acanthamoebae have already been shown to have a positive short-term effect on permeability in laboratory sand-filled columns undergoing bacterial clogging (5). Alternatively, it has been suggested that grazing protozoa may exert an adverse effect in contaminated aquifers by critically reducing the biomass of bacteria available for biodegradation (13).
The purpose of this study was to investigate the impact of grazing by the common soil flagellate Heteromita globosa on the development and hydraulic properties of a clog formed during rapid growth of a slime-secreting Pseudomonas sp. (strain PS+). A model was developed in which small sand-filled columns inoculated with these organisms were perfused with an artificial groundwater medium (AGW) containing glucose as a sole carbon source and nitrate as an oxidant (C/N ratio = 4.0). Columns were allowed to develop denitrifying redox conditions typical of many organically contaminated aquifers undergoing remediation. It is anticipated that the findings could provide an impetus to further studies addressing interactions between bacteria and protozoa in contaminated aquifers and perhaps information to assist in the enhancement of existing strategies for in situ bioremediation.
MATERIALS AND METHODS
Organisms and media.
Pseudomonas sp. strain PS+ was isolated from a diesel-contaminated aquifer near Studen, Switzerland. This strain was selected for use based on its abilities to produce exopolymeric slime, perform denitrification, and provide a food source for soil flagellates. Strain PS+ was deposited with the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) (DSM 12877). The soil flagellate H. globosa was isolated from a petroleum hydrocarbon-contaminated aquifer at an abandoned oil refinery near Hünxe, Germany (36). An enrichment culture was prepared by suspending sediment in soil extract-salts medium (19) with strain PS+ at 25°C. Single flagellates were isolated from dilutions of the culture using a micromanipulator and refed with strain PS+. The isolation procedure was repeated (three times) to ensure purity of the isolate. H. globosa was deposited with the American Type Culture Collection (Manassas, Va.) (ATCC 50780).
Sand-filled columns were perfused with AGW containing (in milligrams liter−1): NaHCO3 (500), NaNO3 (412), CaCl2·2H2O (40), MgSO4·7H2O (30), KH2PO4 (15), H3BO3 (2.86), MnCl2·4H2O (1.81), CoCl2·6H2O (0.04), CuSO4·5H2O (0.04), Na2MoO4·2H2O (0.03), and ZnCl2 (0.02), and 1 N HCl (1.25 ml liter−1). After autoclaving, CaCl2·2H2O and KH2PO4 were added separately through a sterile luer lock filter (0.2-μm pore size; Sartorius AG, Göttingen, Germany) and the pH was readjusted to 7.4. Glucose (500 mg liter−1) was added to the medium as a sole carbon source (C/N ratio = 4.0) on which bacteria but not flagellates were able to grow. All chemicals were of the highest purity (>99%) and were supplied by Wako Pure Chemical Industries Ltd., Tokyo, Japan, unless otherwise stated. All aqueous solutions were made using MilliQ pure water (Millipore, Tokyo, Japan) unless otherwise stated.
Column apparatus.
The arrangement used is shown in Fig. 1. All construction materials were supplied by Iuchi (Tokyo, Japan) unless otherwise stated. Steel columns (height, 10 cm; inside diameter, 4 cm) were each perforated with three side ports through which stainless steel needles (gauge 16) were inserted and secured with adjustable compression fittings (Naruse, Tokyo, Japan). Needles with multiple perforations were used to ensure efficient water and pressure sampling across each column. Columns were sealed at both ends using rubber stoppers having nonperforated needles preinserted to form top and base ports. Twelve columns were mounted upright and secured between two circular plates forming a torus capable of rotating on a spindle around a fixed central axis. Sterile AGW was supplied to the base port, and sterile AGW containing glucose was supplied to one side port of each column from separate reservoir canisters (20 liters) via microtube peristaltic pumps (Eyela, Tokyo, Japan). Negative control columns were supplied with sterile AGW from a separate reservoir canister to minimize risk from contamination. Some 24 flow lines supplying 12 columns were each connected to separate pump channels fitted with Tygon tubing (inside diameter, 1.59 mm). All connections to columns and pumps were made using PTFE tubing (inside diameter, 1.59 mm) and assorted polypropylene luer lock fittings, unless otherwise stated. Sterile luer lock filters (0.2-μm pore size) were inserted between column inlet ports and flow lines in order to minimize bacterial growth and contamination of reservoir canisters upstream. All filters were replaced regularly, and flow lines were flushed with hydrogen peroxide (6%) to sterilize. Effluent emerging from the upper port of each column could be sampled or diverted to a waste canister via a three-way luer-fitting stopcock (Sigma, Tokyo, Japan) attached to the stopper needle. The two remaining side ports on each column (located on either side of the nutrient inlet) were each connected via a three-way luer-fitting stopcock to glass tubes (length, 100 cm; inside diameter, 4.0 cm) using low-gas-permeability Viton tubing. Glass tubes functioned as piezometers for measuring hydraulic head and were oriented vertically and secured to the spindle adjacent to a ruled scale. Stopcocks were opened to allow piezometers to equilibrate several hours prior to reading. Fine quartz sand with a grain size of 80 to 150 μm (Koso Chemicals, Tokyo, Japan) was treated with sodium acetate (1 M, pH 5.0) and hydrogen peroxide (6%) to remove adsorbed carbonates and organics, respectively. Treated sand was rinsed thoroughly with deionized water and oven dried. Sand was then autoclaved and redried before use. Columns were packed with dry sand (120 g) using an unaligned steel mesh and mild sonication (100 W) to randomly disperse grains and were then flushed with CO2 and sealed. Displacing resident air with more soluble CO2 minimized the initial impact of gas bubbles on the effective porosity (θm) of the sand. Columns were resterilized in situ by perfusion with sodium azide (0.05%, 8 h) and were then flushed with AGW prior to inoculation with organisms. Packed sand was estimated to have a pore diameter range of 12 to 23 μm based on the assumption of cylindrical pore spaces.
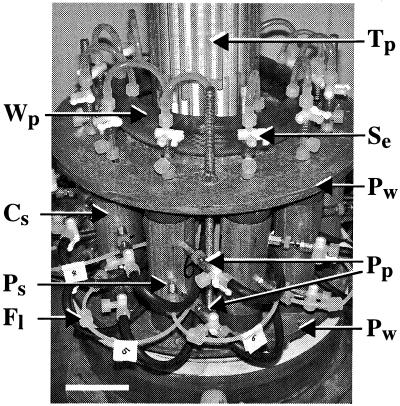
FIG. 1.
Photograph showing sand-filled column apparatus. Steel columns (Cs) were oriented vertically around a central axis and secured between two plates (Pw). AGW was supplied through the base (not shown) and side port (Ps) on each column, while effluent was diverted to a waste pipe (Wp) or sampled via a stopcock (Se) mounted on the upper port. Luer lock fittings (Fl) for the inline attachment of filters with side ports (Ps) are also shown. Saturated hydraulic conductivity (sand permeability) was measured as a function of water height in glass piezometer tubes (Tp) that were operated through stopcocks connected to side ports (Pp). Scale bar = 5 cm.
Experimental design.
Columns were divided into four groups (A to D) with three replicates in each. Groups A and C were designated positive controls for clogging and were inoculated with strain PS+ at 0 days. Group B replicates were designated treatments and were inoculated with strain PS+ and H. globosa at 0 days. This group was used to determine the impact of grazing flagellates on clogging. Group D replicates were designated abiotic negative controls and were neither inoculated with organisms nor supplied with glucose. After 60 days at a Ks/Ks0 of 0.1 (detailed below), group C members were inoculated with H. globosa as described previously. This group was used to determine if a formed clog could be reversed by grazing flagellates. Appropriate columns were inoculated with strain PS+ (108 cells ml−1) and H. globosa (105 cells ml−1) in AGW equivalent to 2 pore volumes. Pumping was stopped overnight to facilitate establishment of organisms and was again resumed at day 0. Continuous flow was maintained through columns for 80 days at an average initial flow volume of 12.0 ± 0.3 ml h−1. The apparatus was maintained at an ambient temperature of 21 ± 0.3°C during this period.
Breakthrough curves.
Transport properties in sand were measured for each column at the beginning and end of the experiment. A pulse of NaBr (10 mM, 2 min) in MilliQ water was injected in the flow line to the base port of each column while flow to the side port was stopped. Effluent samples were collected at regular time intervals equivalent to a total of 3 pore volumes, and the flow volume (Q, in milliliters hour−1) was determined by weight. Bromide concentrations were measured using a TOA Ion Analyzer (IA-100) equipped with an auto sample injector (TOA Electronics Ltd., Tokyo, Japan). Data were used to solve the advection-dispersion equation for coefficients of partitioning (β) and longitudinal dispersion (D) using the program CXTFIT 2.1 (31). β was used to determine the effective porosity of water-saturated sand in the equation θm = β·θ, where θ is the total porosity (0.43 ± 0.002), determined gravimetrically. D was used to determine the Peclet number (Pe) in the equation Pe = v·x/D, where x is the height of the sand column (7 cm), v (equivalent to Q/A·θ) is the average pore water velocity (in centimeters hour−1), and A is the cross-sectional area of the column (11.95 cm2). Pe is a dimensionless ratio that relates the effectiveness of mass transport by advection to that by dispersion or diffusion (6).
Saturated hydraulic conductivity (permeability).
Darcy's Law (equation 1) was used to determine changes in the permeability (Ks, in centimeters hour−1) in each column as follows:
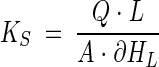 |
(1) |
where L is the vertical distance between piezometers (5 cm) and ∂HL is the change in hydraulic head between piezometers (in centimeters). The ratio Ks/Ks0 expressing differences in the permeability between time t and 0 days was used as an indicator of clogging.
Analytical methods.
Dissolved oxygen (DO) was measured using an azide modification of the Winkler technique (28). Unfiltered reservoir canister and effluent samples (2 ml) were collected in a nitrogen-filled, gastight syringe (5 ml; SGE International Pty. Ltd., Ringwood, Australia). The syringe was sealed with a rubber septum through which 15 μl each of two solutions (A and B) was injected into the sample followed by immediate mixing. Solution A contained (in grams per 10 ml) MnCl2·4H2O (8.0), and solution B contained (in grams per 10 ml) NaOH (3.6), KI (2.0), and NaN3 (0.05). Phosphoric acid (85%; 50 μl) was subsequently added with immediate mixing. Soluble starch solution (1%; 20 μl) was added to the sample and titrated to colorlessness against freshly prepared Na2S2O3 (0.5 mM). The detection sensitivity for the method was approximately 40 μg of DO liter−1.
Anions (Br−, NO2−, NO3−, PO42−, and SO42−) were measured in a reservoir canister sterilized by filtration (0.2-μm pore size) and effluent samples (1 ml) using a TOA Ion Analyzer as described previously. The detection sensitivity was 0.1 mg of anion liter−1.
Glucose was measured spectrophotometrically using a chromogen reagent containing glucose oxidase (EC 1.1.3.4; 3,600 U), peroxidase (EC 1.11.1.7; 250 U), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS; 165 mg), and NaN3 (3.0 mg) in phosphate buffer (0.1 M; 300 ml; pH 7.0). Phosphate buffer contained NaH2PO4·2H2O (15.6 g liter−1). Samples (60 μl) of effluent, standards, or from reservoir canisters were sterilized by filtration (0.2-μm pore size) and added to chromogen reagent (3.0 ml). After incubation (40 to 50°C; 30 min), the absorbance at 415 nm was measured using a HACH DR/2000 spectrophotometer (HACH Co., Loveland, Colo.). Glucose standard contained d-glucose (200 mg liter−1) and benzoic acid (400 mg liter−1) as a preservative. The detection sensitivity was approximately 120 μg of glucose liter−1.
Cell enumeration.
Samples (0.5 ml) of unfiltered effluent were briefly sonicated (100 W; 30 s) and were fixed with an equal volume of glutaraldehyde (1%) in phosphate buffer (pH 7.0). Phosphate buffer contained (in grams liter−1) NaCl (0.12), MgSO4·7H2O (0.04), CaCl2·6H2O (0.04), Na2HPO4 (1.42), and KH2PO4 (1.36). Fixed samples were enumerated in a Neubauer chamber (Sigma) examined at a magnification of ×320 under interference-contrast microscopy (Nikon Optiphot; Nikon, Tokyo, Japan). Samples were diluted with phosphate buffer as appropriate, and bacterial counts were averaged for 20 ruled units (volume, 2.5 × 10−7 ml each).
Scanning electron microscopy (SEM).
Sand cores were collected in copper cylinders (diameter, 0.8 by 1.0 cm) and were capped at both ends with a nylon mesh (100-μm pore size) secured with silicon tubing. Cores were prefixed (2 h, 4°C) with freshly prepared glutaraldehyde (2%) in sodium cacodylate buffer (0.05 M; pH 7.0), containing l-lysine (0.05 M) to stabilize exopolymers. Main fixation with glutaraldehyde in sodium cacodylate buffer (8 h, 4°C) was followed by washing using the same buffer (8 h). Postfixation with osmium tetroxide (1%) in cacodylate buffer (2 h, 4°C) was followed by washing using the same buffer (2 h) and dehydration to 95% ethanol. Cores were dehydrated in absolute ethanol and were critical point dried with CO2 in a Hitachi HCP-2 (Hitachi, Tokyo, Japan). Sand was carefully extruded from each cylinder and was then mounted on aluminum stubs and sputtered with platinum using a Hitachi E102 Ion sputter coater. Stubs were examined using a Hitachi S-2500 scanning electron microscope operating at 15 kV.
Statistical analysis.
Data were transformed as log10 (X + 1) prior to analysis and were compared using one-way analysis of variance, linear regression, and Student's t test (26). Values are presented as sample means with standard errors unless otherwise stated.
RESULTS
Physical evidence of bioclogging.
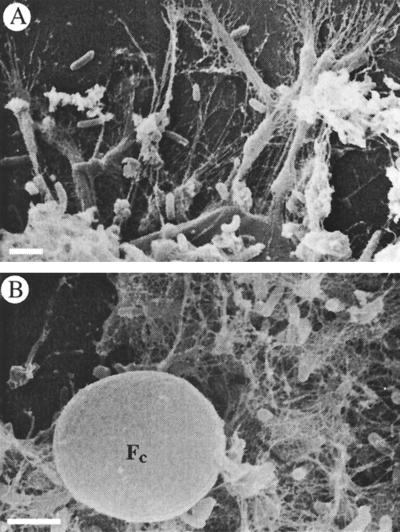
Sand removed from all columns except abiotic negative controls (group D) showed direct evidence of deposition of exopolymeric slime on the surface of grains observed under SEM (Fig. 2A). Amorphous condensates of exopolymer were randomly distributed and appeared anchored to the surface of grains by a network of fine filaments (diameter, 40 ± 5 nm). These filaments encompassed and appeared to originate from microcolonies of rod-shaped bacteria (0.7 ± 0.04 by 0.3 ± 0.01 μm) residing on the surface (Fig. 2B). Slime secretion was first noted in the column effluent after 20 days.
FIG. 2.
SEM micrographs showing exopolymer of Pseudomonas sp. (strain PS+) apparently attached to sand grains (A) and bacteria enmeshed in a filamentous network (B). A cyst of H. globosa (Fc) is also shown. Scale bars = 1 μm.
A comparison of data for θm indicated significant differences over time and between groups of columns (Table 1). All columns except the abiotic negative controls showed significantly reduced values for θm compared with corresponding values at 0 days (P < 0.002). Columns containing only bacteria (group A) also showed significantly reduced values (P < 0.05) for θm (2.5-fold) in comparison with those containing both bacteria and flagellates (group B; 1.6-fold). Similarly, data for Pe indicated significant differences over time and between groups of columns. Columns containing only bacteria showed significantly reduced values for Pe in comparison with abiotic negative controls (P < 0.05) and also with values at 0 days (P < 0.05). However, Pe remained above 6 in all cases.
TABLE 1.
Determining θm and Pe in sand-filled columns using the program CXTFIT 2.1 (31) to model bromide breakthrough dataa
| Experimental conditions | Column group | Q (cm3 h−1) | v (cm h−1) | β | θm | D (cm2 h−1) | Pe | Data fit to model (r2) |
|---|---|---|---|---|---|---|---|---|
| Preinoculation (t = 0 days) | 12.0 ± 0.25) | 2.3 ± 0.05 | 0.7 ± 0.04 | 0.3 ± 0.02 | 0.3 ± 0.03 | 63.5 ± 5.83 | ≥0.91 | |
| Bacteria only (t = 80 days)b | A | 11.9 ± 0.61 | 2.3 ± 0.12 | 0.3 ± 0.03 | 0.1 ± 0.01 | 1.7 ± 0.81 | 14.4 ± 4.84 | ≥0.97 |
| Bacteria with flagellates | B | 13.6 ± 0.34 | 2.7 ± 0.07 | 0.4 ± 0.03 | 0.2 ± 0.01 | 1.2 ± 0.45 | 22.2 ± 9.74 | ≥0.98 |
| Bacteria with flagellates added after 60 days | C | 12.1 ± 0.31 | 2.4 ± 0.06 | 0.3 ± 0.02 | 0.1 ± 0.01 | 0.6 ± 0.11 | 29.1 ± 5.83 | ≥0.97 |
| Abiotic control | D | 13.3 ± 0.42 | 2.6 ± 0.08 | 0.8 ± 0.02 | 0.3 ± 0.01 | 0.3 ± 0.10 | 63.8 ± 16.80 | ≥0.97 |
Data are shown as mean ± standard error of three replicates.
t = 80 days for results for groups A to D.
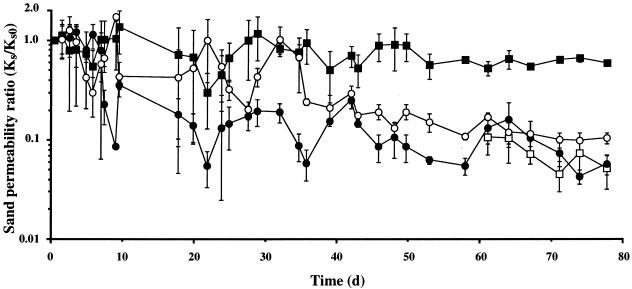
Sand permeability was significantly different between (but not within) all groups of columns in which it also decreased with time (P < 0.0001) (Fig. 3). Decreasing permeability was marked by oscillations in magnitude at periodic intervals. The largest sustained reduction in permeability (26-fold) occurred in columns containing only bacteria (group A) and was significantly lower (P < 0.01) than in those containing bacteria and flagellates (group B; 13-fold). The permeability in columns containing only bacteria was similar to that measured in group C (P > 0.9) and remained unchanged despite the addition of flagellates to the latter after 60 days (P > 0.7). Permeability reduction in abiotic negative controls was less than twofold.
FIG. 3.
Changes in sand permeability with respect to time (in days) for groups A (flagellates absent; closed circles), B (flagellates present; open circles), C (flagellates added after 60 days; open squares), and D (abiotic control; closed squares). Data are shown as mean ± standard error of three replicates.
Biological populations in columns.
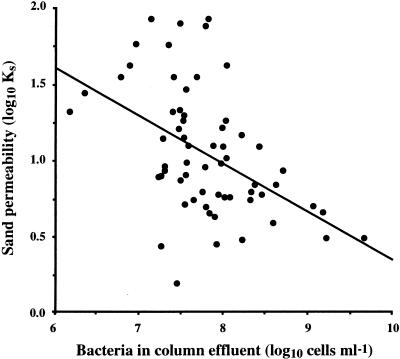
Bacterial numbers measured in the effluent from columns containing only bacteria and those containing both bacteria and flagellates were similar (P > 0.1) and generally increased with time. Superimposed on this increase were fluctuations in numbers that also appeared to increase in amplitude with time. Similar observations were made on numbers of flagellates exiting group B, though no relationship was found with corresponding numbers of bacteria (P < 0.0001). Variation within groups of columns was not found to be significant (P, >0.05 to 0.9) and probably had minimal impact on this effect. Interestingly, numbers of bacteria exiting these columns (Fig. 4) were inversely related with permeability (P < 0.001), whereas no relationship was found with numbers of flagellates (P > 0.1). Furthermore, numbers of bacteria exiting columns in groups A and C were not significantly different even after the addition of flagellates to the latter (P > 0.05). Bacterial aggregates (up to 500 μm in diameter) were observed in the effluent emerging from each column, whereas elongated bacterial cells (up to 12 μm in length) emerged only from columns containing both bacteria and flagellates.
FIG. 4.
Relationship between sand permeability and numbers of bacteria in effluent from group B. The solid line shows the linear regression of the log10 data (r2 = 0.236, n = 63, P < 0.001).
Bacterial numbers in sediment were also not significantly different for columns containing only bacteria and those containing both bacteria and flagellates (6.2 × 109 ± 0.5 × 109 per g [dry weight]). However, numbers within columns were reduced below the glucose inlet (2.0 × 109 ± 1.0 × 109 per g [dry weight]). Interestingly, flagellates (group B) were more numerous in sediment below the glucose inlet (1.0 × 107 ± 0.6 × 107 per g [dry weight]) than they were above glucose (2.1 × 106 ± 0.4 × 106 per g [dry weight]).
Substrate utilization.
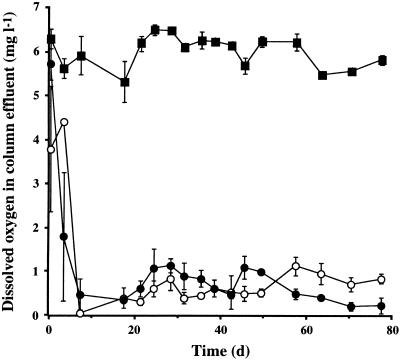
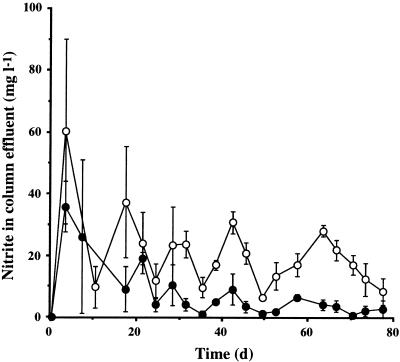
Glucose measured in the effluent from columns containing only bacteria and from those containing both bacteria and flagellates was significantly depleted from 500 mg liter−1 (reservoir) to approximately 1.0 to 100 mg liter−1 after 10 days. Similarly, DO exiting these columns was also rapidly depleted from 7.0 ± 0.1 mg liter−1 (reservoir) to less than 1.0 mg liter−1 after 8 days (Fig. 5). Nitrate exiting these columns was also significantly depleted from the reservoir concentration. However, no significant differences were found either within or between columns containing only bacteria and those containing both bacteria and flagellates with respect to glucose, DO, or nitrate emerging in the effluent (P, >0.4 to 0.9). Interestingly, nitrite emerging from columns containing both bacteria and flagellates (18.2 ± 2.3 mg liter−1) was significantly higher (P < 0.001) than from those containing only bacteria (7.2 ± 1.6 mg liter−1) (Fig. 6). Again, no significant variation within groups of columns was found (P, >0.05 to 0.3).
FIG. 5.
Effluent concentrations of DO from groups A (flagellates absent; closed circles), B (flagellates present; open circles), and D (abiotic control; closed squares) with respect to time (in days). Data are shown as mean ± standard error of three replicates.
FIG. 6.
Effluent concentrations of nitrite from groups A (flagellates absent; closed symbols) and B (flagellates present; open symbols) with respect to time (in days). Data are shown as mean ± standard error of three replicates.
DISCUSSION
Saturated hydraulic conductivity (permeability) measures the ability of a porous matrix to conduct water. While permeability is determined by porosity of the matrix, the latter may be influenced by microorganisms residing in pore spaces. Bacteria may reduce porosity and lead to clogging through one or more factors, including biomass accumulation, secretion of exopolymers, alteration of the redox environment, and production of insoluble biogas (for a review, see reference 2). Permeability has proven difficult to measure under field conditions, so laboratory models have been used to examine the dynamics of clogging in various porous matrices under different redox conditions (5, 20, 23, 29, 30, 32-35).
Physical conditions affecting clogging.
Porosity is related to the volume and contiguity of adjoining pores, which are both directly affected by grain size. Despite smaller grain size, the porosity of fine sand used in this study was higher (0.43 ± 0.002) than that for medium-to-coarse aquifer sediment (0.31 ± 0.002) from which the flagellates were isolated (36). The neck diameter of contiguous pores in this fine sand was estimated to be two to four times the width of these flagellates and probably afforded them maximum mobility for grazing on bacteria.
Anaerobic conditions have traditionally been considered necessary for inducing large reductions in permeability (for a review, see reference 2). However, porous matrices loaded with aerobic effluents often showed higher rates and magnitudes of clogging than did those charged with anaerobic effluents (4, 18, 20). Even so, rapid proliferation of bacteria at aerobic inlets often induced steep nutrient (33) and DO (23) gradients that precluded growth in these matrices. Alternatively, facultative anaerobic bacteria were found to produce more extensive clogging throughout the same matrices (23). Similar observations were made during this study, where a facultative denitrifying Pseudomonas sp. (strain PS+) proliferated and induced clogging throughout sand columns supplied with glucose and nitrate at non-growth-limiting concentrations. Typically the initial C/N ratio (4.0) was low in order to simulate conditions in the subsurface at sites undergoing wastewater disposal treatment or in situ bioremediation with nitrate injection. The maximum sustained permeability reduction (26-fold) due to column clogging lay within the range previously reported for aerobic-anaerobic conditions (18).
Bacterial biomass accumulation and clogging.
The surface area for potential colonization by bacteria is normally higher in a fine-grained matrix, although the observed pattern is often sparse and heterogeneous. Indeed, permeability fell by 3 orders of magnitude when a non-exopolymer-forming Arthrobacter sp. occupied only 8.5% of pore volume (33). It has been suggested that reductions in permeability occur either from a strategically located biofilm that alters the geometry of pore necks (3) or by accumulation of bacterial aggregates lodged in those pore necks (33, 34). Bacterial aggregates are frequently formed as a defensive strategy towards grazing by protozoa (5, 10) and were formed by strain PS+ in response to H. globosa in batch cultures. However, aggregates occurred in the presence and/or absence of flagellates in columns and may have resulted from exopolymer secretion rather than in response to grazing. Presumably, aggregates formed in response to grazing would adversely affect permeability (5). That this did not occur in these columns may be partly due to the predatory efficiency of flagellates as discussed later. However, filaments formed by strain PS+ in the presence of flagellates were considered a strategy to avoid predation (9).
Bacterial exopolymer and biogas affect clogging.
Bacteria may secrete exopolymers as an adherent capsule and/or loosely associated slime layers. Interestingly, exopolymer secretion by strain PS+ was not found in batch cultures and may have resulted from direct contact with sand as reported previously (35). Bacterial exopolymers are mostly hydrated polysaccharides with the ability to reduce water-conducting spaces and hence permeability in porous matrices (4, 20, 23, 27, 32, 33, 35). Indeed, exopolymer secretion was associated with reduction in the θm of a microbially fouled gravel pack (4). Similarly, a reduction in θm in sand columns colonized by strain PS+ was deduced from bromide breakthrough data (Table 1). Two possible mechanisms could account for bacterial clogging in these columns. Firstly, clogging could result from exopolymer secretion by bacterial biofilms residing in pore spaces. Progressive accumulation of exopolymer could reduce θm (for a review, see reference 2) without having a major impact on the Pe. Secondly, clogging could result from strategic lodging of bacterial aggregates and/or small insoluble biogas bubbles in the necks between contiguous pore spaces (22, 33, 34). Blockage of pore necks would not have a significant effect on θm but would instead create stagnant zones in the matrix (22). Such zones would cause a shift from advective to dispersive flow with a consequent reduction in Pe (to ≤6) according to Fetter (6). This second possibility was considered less likely since strain PS+ significantly reduced θm, while Pe remained above 6 in all cases.
Permeability reductions in sand columns containing strain PS+ also showed periodic oscillations in magnitude. A similar phenomenon linked with bacterial plug development and propagation was interpreted using a three-stage pressure model (27). The model assumed three successive phases of exopolymer induction, plugging, and plug propagation. Exopolymer induction was associated with development of contiguous bacterial colonies throughout the matrix and had minimal impact on pore volume. Plugging occurred when exopolymers were secreted into pore spaces and coincided with a reduction in matrix permeability. Advective water flow was diverted from channels in the matrix to those within the biofilm in the region of plugging. Biofilms were more restrictive to flow and permeability was reduced further until their sloughing point was reached. Shearing forces responsible for biofilm sloughing opened new flow channels, allowing partial restoration of permeability to occur (27). Although these forces have largely been attributed to changes in flow velocity within pore spaces (3, 27), it is possible that sloughing was assisted by biogas production in columns. Fine bubbles may have become entrapped in the network of exopolymer secreted by underlying bacteria and weakened the cohesiveness of the biofilm (1, 11). This would probably reduce θm in pore spaces and facilitate biofilm sloughing at lesser shearing forces. Subsequent deposition of sloughed biofilm in pore necks led to further reductions in permeability until at maximum pressure these plugs were swept downstream (27). This propagation phase was associated with periodic oscillations in permeability and repeated plug breakthrough.
Reductions in sand permeability were also associated with higher numbers of bacteria released in column effluents. Presumably, the greater shearing forces associated with lower permeability caused increased numbers of bacteria to be sloughed into pore spaces prior to plug breakthrough. Interestingly, numbers of flagellates in column effluents were independent of permeability and suggested that these motile protozoa may have more control over their position in the mobile water phase than does nonmotile strain PS+.
Although biogas was formed as a product of denitrification, there was no evidence to indicate that large gas pockets deleterious to permeability (11, 22) were permanently trapped in these sand columns. Gas bubbles were naturally vented from sand under prevailing hydrostatic pressures, and piezometers were serviced for potential gas locks several hours before measurements were made.
Flagellates reduce bacterial clogging.
Bacterivorous protozoa (especially heterotrophic flagellates) coexist with bacteria thriving on organic contaminants in the subsurface (8, 14, 16, 25, 36). One important consequence of protozoan grazing is the remineralization of bacterial nutrients (for a review, see reference 21). Circumstantial evidence suggested that protozoa feeding on bacteria in a polycyclic aromatic hydrocarbon (PAH)-contaminated aquifer may have remineralized PAH-derived carbon (8). Indeed, evidence for the reincorporation of carbon from biodegraded [14C]toluene into biomass by grazing flagellates (maximum 5%) was reported in batch culture (15). Circumstantial evidence also suggested that protozoa may play a role in maintaining hydraulic conductivity in aquifers undergoing biotreatment of degradable organics (25). This hypothesis was examined in sand columns inoculated with bacteria (nonmotile, non-exopolymer-secreting Bacillus sp.) and acanthamoebae (5). Despite repeated inoculation of columns with acanthamoebae, these protozoa were only able to maintain favorable permeability over a short period. Subsequent examination of the sand matrix revealed that bacteria had migrated up a nutrient gradient (against downward water flow) and away from the acanthamoebae, which had subsequently encysted to survive adverse grazing conditions (5). Columns used in the present study were operated with an upward water flow to reduce preferential flow paths in the sand matrix and to discourage nutrient gradients against gravity. Furthermore, these columns were inoculated only once with flagellates that were smaller and more mobile than were amoebae and were perhaps more representative of aquifer protozoa (16, 36). Protozoa are regarded as being more sensitive than bacteria to lower concentrations of DO (25), and this could limit their role in aquifers where anaerobic degradation predominates. Interestingly, denitrifying conditions did not appear to adversely affect the spatial distribution of flagellates above the glucose inlet in columns during the present study. However, flagellate cysts tended to accumulate at the base of these columns as previously observed for acanthamoebae (5). Nevertheless, these flagellates maintained a significantly higher sand permeability (13-fold reduction over controls) than did the columns from which they were absent (26-fold reduction). Corroborative evidence was also provided by higher θm in columns containing flagellates. The main impact of flagellates on permeability may be in reducing the ability of bacteria to accumulate exopolymers and biogas in pore spaces rather than in grazing bacteria strategically blocking pore necks. In addition, flagellate grazing may directly form water-conducting channels in the developing biofilm. Supporting evidence showed that flagellates added after 60 days were unable to restore θm or sand permeability. Presumably, bacterial biofilms and their secretions were well established by this stage.
Surprisingly, higher concentrations of nitrite were detected in the effluent from columns with flagellates than from those containing only bacteria. Indeed, certain protozoa have been reported to perform dissimilatory nitrate reduction to provide an additional source of electron acceptors under anoxic conditions (7). However, there was no evidence to suggest a corresponding increase in nitrate consumption in the presence of these flagellates. Nevertheless, the data could suggest that flagellates restrict the potential for biogas formation by strain PS+ in sand. The role and importance of soil flagellates in the processes of nitrate reduction and denitrification are presently being investigated in our laboratory.
Acknowledgments
We thank A. E. Wheeler and Y. Inomata for valuable assistance in constructing and operating the column apparatus.
We also thank the New Energy and Industrial Technology Development Organization (NEDO) “Bioconsortia project” and Time Machine Bio (TMB) for funding this study and also NEDO for providing a personal fellowship to R.G.M.
REFERENCES
- 1.Battersby, N. S., D. J. Stewart, and A. P. Sharma. 1985. Microbiological problems in the offshore oil and gas industries. J. Appl. Bacteriol. Symp. 1985(Suppl.):227S-235S. [Google Scholar]
- 2.Baveye, P., P. Vandevivere, B. L. Hoyle, P. C. DeLeo, and D. S. de Lozada. 1998. Environmental impact and mechanisms of the biological clogging of saturated soils and aquifer materials. Crit. Rev. Environ. Sci. Technol. 28:123-191. [Google Scholar]
- 3.Characklis, W. G., A. B. Cunningham, A. Escher, and D. Crawford. 1987. Biofilms in porous media, p. 57-78. In D. R. Cullimore (ed.), Proceedings of the International Symposium on Biofouled Aquifers: Prevention and Restoration. American Water Resources Association technical publication series. American Water Resources Association, Bethesda, Md.
- 4.Cullimore, D. R., and N. Mansuy. 1987. A screen arc model well to simulate iron bacterial biofouling. J. Microbiol. Methods 7:225-232. [Google Scholar]
- 5.DeLeo, P. C., and P. Baveye. 1997. Factors affecting protozoan predation of bacteria clogging laboratory aquifer microcosms. Geomicrobiol. J. 14:127-149. [Google Scholar]
- 6.Fetter, C. W. 1992. Contaminant hydrogeology. Macmillan Publishing Co., New York, N.Y.
- 7.Finlay, B. J., A. S. W. Span, and J. M. P. Harman. 1983. Nitrate respiration in primitive eukaryotes. Nature 303:333-336. [Google Scholar]
- 8.Ghiorse, W. C., J. B. Herrick, R. L. Sandoli, and E. L. Madsen. 1995. Natural selection of PAH-degrading bacterial guilds at coal-tar disposal sites. Environ. Health Perspect. 103:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn, M. W., E. R. B. Moore, and M. G. Höfle. 1999. Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl. Environ. Microbiol. 65:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn, M. W., E. R. B. Moore, and M. G. Höfle. 2000. Role of microcolony formation in the protistan grazing defense of the aquatic bacterium Pseudomonas sp. MWH1. Microb. Ecol. 39:175-185. [DOI] [PubMed] [Google Scholar]
- 11.Harremöes, P., J. C. Jansen, and G. H. Kristensen. 1980. Practical problems related to nitrogen bubble formation in fixed film reactors. Prog. Water Technol. 12:253-269. [Google Scholar]
- 12.Hart, S. 1996. In situ bioremediation: defining the limits. Environ. Sci. Technol. 30:398A-401A. [DOI] [PubMed] [Google Scholar]
- 13.Kota, S., R. C. Borden, and M. A. Barlaz. 1999. Influence of protozoan grazing on contaminant biodegradation. FEMS Microbiol. Ecol. 29:179-189. [Google Scholar]
- 14.Madsen, E. L., J. L. Sinclair, and W. C. Ghiorse. 1991. In situ biodegradation: microbiological patterns in a contaminated aquifer. Science 252:830-833. [DOI] [PubMed] [Google Scholar]
- 15.Mattison, R. G., and S. Harayama. 2001. The predatory soil flagellate Heteromita globosa stimulates toluene biodegradation by a Pseudomonas sp. FEMS Microbiol. Lett. 194:39-45. [DOI] [PubMed] [Google Scholar]
- 16.Novarino, G., A. Warren, H. Butler, G. Lambourne, A. Boxshall, J. Bateman, N. E. Kinner, R. W. Harvey, R. A. Mosse, and B. Teltsch. 1997. Protistan communities in aquifers: a review. FEMS Microbiol. Rev. 20:261-275. [DOI] [PubMed] [Google Scholar]
- 17.Oberdorfer, J. A., and F. L. Peterson. 1985. Waste-water injection: geochemical and biogeochemical clogging processes. Ground Water 23:753-761. [Google Scholar]
- 18.Okubo, T., and J. Matsumoto. 1983. Biological clogging of sand and changes of organic constituents during artificial recharge. Water Res. 17:813-821. [Google Scholar]
- 19.Page, F. C. 1988. A new key to freshwater and soil gymnamoebae. Freshwater Biological Association, Ambleside, Cumbria, United Kingdom.
- 20.Raiders, R. A., M. J. McInerney, D. E. Revus, H. M. Torbati, R. M. Knapp, and G. E. Jenneman. 1986. Selectivity and depth of microbial plugging in Berea sandstone cores. J. Ind. Microbiol. 1:195-203. [Google Scholar]
- 21.Ratsak, C. H., K. A. Maarsen, and S. A. L. M. Kooijman. 1996. Effects of protozoa on carbon mineralization in activated sludge. Water Res. 30:1-12. [Google Scholar]
- 22.Ronen, D., B. Berkowitz, and M. Magaritz. 1989. The development and influence of gas bubbles in phreatic aquifers under natural flow conditions. Transport Porous Med. 4:295-306. [Google Scholar]
- 23.Shaw, J. C., B. Bramhill, N. C. Wardlaw, and J. W. Costerton. 1985. Bacterial fouling in a model core system. Appl. Environ. Microbiol. 49:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherr, B. F., E. B. Sherr, and J. McDaniel. 1992. Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Appl. Environ. Microbiol. 58:2381-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinclair, J. L., D. H. Kampbell, M. L. Cook, and J. T. Wilson. 1993. Protozoa in subsurface sediments from sites contaminated with aviation gasoline or jet fuel. Appl. Environ. Microbiol. 59:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokal, R. R., and F. J. Rohlf. 1981. Biometry: the principles and practice of statistics in biological research. W. H. Freeman, San Francisco, Calif.
- 27.Stewart, T. L., and H. S. Fogler. 2001. Biomass plug development and propagation in porous media. Biotechnol. Bioeng. 72:353-363. [DOI] [PubMed] [Google Scholar]
- 28.Suter, M. 1995. Abschätzung des Einflusses eines Azoarcus-Stammes auf den Abbau von Dieselöl in Säulensystemen. Diplomarbeit thesis. Institute for Terrestrial Ecology, ETH, Zurich, Switzerland.
- 29.Taylor, S. W., and P. R. Jaffé. 1990. Biofilm growth and the related changes in the physical properties of a porous medium. I. Experimental investigation. Water Resour. Res. 26:2153-2159. [Google Scholar]
- 30.Torbati, H. M., R. A. Raiders, E. C. Donaldson, M. J. McInerney, G. E. Jenneman, and R. M. Knapp. 1986. Effect of microbial growth on pore entrance size distribution in sandstone cores. J. Ind. Microbiol. 1:227-234. [Google Scholar]
- 31.Toride, N., F. J. Leij, and M. T. van Genuchten. 1999. The CXTFIT code for estimating transport parameters from laboratory or field tracer experiments. Version 2.1. Research report no. 137. U.S. Salinity Laboratory, U.S. Department of Agriculture, Riverside, Calif.
- 32.Vandevivere, P., and P. Baveye. 1992. Effect of bacterial extracellular polymers on the saturated hydraulic conductivity of sand columns. Appl. Environ. Microbiol. 58:1690-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandevivere, P., and P. Baveye. 1992. Saturated hydraulic conductivity reduction caused by aerobic bacteria in sand columns. Soil Sci. Soc. Am. J. 56:1-13. [Google Scholar]
- 34.Vandevivere, P., and P. Baveye. 1992. Relationship between transport of bacteria and their clogging efficiency in sand columns. Appl. Environ. Microbiol. 58:2523-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandevivere, P., and D. L. Kirchman. 1993. Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl. Environ. Microbiol. 59:3280-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarda, B., G. Mattison, A. Hess, D. Hahn, P. Höhener, and J. Zeyer. 1998. Analysis of bacterial and protozoan communities in an aquifer contaminated with monoaromatic hydrocarbons. FEMS Microbiol. Ecol. 27:141-152. [Google Scholar]