Abstract
The influence of atmosphere composition on the metabolism of Brochothrix thermosphacta was studied by analyzing the consumption of glucose and the production of ethanol, acetic and lactic acids, acetaldehyde, and diacetyl-acetoin under atmospheres containing different combinations of carbon dioxide and oxygen. When glucose was metabolized under oxygen-free atmospheres, lactic acid was one of the main end products, while under atmospheres rich in oxygen mainly acetoin-diacetyl was produced. The proportions of the total consumed glucose used for the production of acetoin (aerobic metabolism) and lactic acid (anaerobic metabolism) were used to decide whether aerobic or anaerobic metabolism predominated at a given atmosphere composition. The boundary conditions between dominantly anaerobic and aerobic metabolisms were determined by logistic regression. The metabolism of glucose by B. thermosphacta was influenced not only by the oxygen content of the atmosphere but also by the carbon dioxide content. At high CO2 percentages, glucose metabolism remained anaerobic under greater oxygen contents.
Modified atmosphere packaging extends the shelf life of raw meat and fish by suppressing or slowing down the growth of gram-negative psychrotrophic bacteria, mainly pseudomonad-like organisms, which cause rapid spoilage of food stored in contact with air as a result of the presence of end products from aerobic metabolism. Different combinations of carbon dioxide, nitrogen, and oxygen have been used in modified-atmosphere-packaged (MAP) meat and fish. The commercial approach to optimizing the bright red color of MAP fresh red meat consists in enriching the atmosphere in both carbon dioxide and oxygen, usually to ca. 20% CO2 and ca. 80% O2 (1, 19, 23). Color also plays an important role as a limiting factor in the shelf life of some species of scombroids, such as tuna and related species, stored under modified atmospheres. It has been reported elsewhere that 40% CO2 and 60% O2 are the most effective mixture to extend the shelf life of tuna, in which Shewanella putrefaciens was one of the dominant bacteria when stored in contact with air (18). This higher concentration of CO2 recommended for MAP tuna (18) is due to the greater resistance of S. putrefaciens than of Pseudomonas spp. to CO2 (20) and also to the relatively high pH of tuna, at which S. putrefaciens is able to grow (14).
Brochothrix thermosphacta is one of the dominant microorganisms in CO2- and CO2-O2-enriched atmospheres. Therefore, this bacterium plays an important role in the spoilage of MAP meat (1, 6, 16, 28) and fish (8, 17, 18, 24). The main factor limiting the shelf life of refrigerated MAP food is offensive odor development resulting from microbial metabolism (28). Gill and Newton (11) found that glucose was the substrate preferentially used by B. thermosphacta when growing in meat. No utilization of glucose-6-phosphate, lactic acid, and nucleotides was detected. Moreover, the range of amino acids that this bacterium could use was very restricted, and the resulting growth was slower. The main metabolites resulting from consumption of glucose by B. thermosphacta under anaerobic conditions are l-(+)-lactic acid and ethanol, but no acetoin, and only small or no amounts of short-chain fatty acids have been detected (7, 13). Under aerobic conditions, B. thermosphacta produces acetoin; acetic, isobutyric, 2-methylbutyric, and isovaleric acids; and 3-methylbutanol (3, 4, 5). The main sign of spoilage in meat inoculated with B. thermosphacta is the sour-sweet offensive odor mainly associated with acetoin and, to a lesser degree, with the above-mentioned acids (7). Therefore, the spoilage pattern associated with aerobic metabolism is faster developing and more offensive than that associated with anaerobic metabolism, which yields mainly lactic acid and ethanol as end products.
The results of Ordóñez et al. (22, 24) suggest that the inclusion of oxygen in CO2-enriched atmospheres could enhance the aerobic metabolism of B. thermosphacta. It is therefore of interest to determine which combinations of CO2 and O2 delay the oxidation of myoglobin but also ensure that the metabolism of B. thermosphacta remains dominantly anaerobic. The purpose of this work was to study the extension of the shelf life of MAP products by controlling microbial metabolism rather than growth rates. The conditions that determined the boundary between dominantly anaerobic and dominantly aerobic glucose metabolisms were estimated by studying selected end products of glucose metabolism under different combinations of CO2 and O2. The proportion of the total consumed glucose that was metabolized in aerobic conditions was estimated from the amount of acetoin-diacetyl formed, while the amount of lactic acid was used to estimate the proportion of glucose anaerobically metabolized. These proportions were used to decide whether the metabolism was dominantly aerobic or anaerobic for a given atmosphere composition.
MATERIALS AND METHODS
Strains.
B. thermosphacta MR 165, originally isolated from fresh bacon and kindly supplied by the Reading Laboratory of the Institute of Food Research, Reading, United Kingdom, was maintained at −20°C. Immediately before the experiments, it was subcultured in tryptone soy broth (Oxoid/Unipath CM129) and incubated at 25°C for 24 h three consecutive times.
Preparation of the cultures.
Amounts of 200 ml of tryptone soy broth (previously adjusted to pH 6) were dispensed in glass bottles (volume, 1 liter) with rectangular sides and screw caps. Bottles were autoclaved at 121°C for 15 min. The caps had a sampling hole, covered by a silicone disk located between the bottle mouth and the cap.
Open bottles were placed inside laminated film bags (25 by 50 cm) of low gas permeability (Cryovac BB4L; O2 diffusion coefficient, 35 cm3/24 h/m2/105 Pa, and CO2 diffusion coefficient, 150 cm3/24 h/m2/105 Pa). A EUVAC 65 packaging machine was used to produce the modified atmospheres inside the bags. After the air was removed and the selected gas mixture was injected, the bags containing the open bottles were heat sealed. Caps were firmly screwed onto the bottles, inside the sealed bags. The gas composition inside the bottle was determined indirectly by measuring the composition inside the sealed bag with a CO2-O2 analyzer (Abiss model GT12). Finally, the bottles were removed from the bags and stored at 5°C until inoculation.
The different atmospheres at which growth and metabolite production were measured are given in Table 1. They were obtained by mixing CO2-N2 (80/20, vol/vol), O2-N2 (80/20, vol/vol), and N2 (100%) gases, supplied by Carburos de España S.A.
TABLE 1.
Lactic acid and acetoin-diacetyl production rates, glucose consumption rates, total ethanol and acetic acid concentrations, and maximum specific growth rates of B. thermosphacta at 5°C with different percentages of oxygen and carbon dioxide in the atmospherea
| % CO2b | % O2b | μ | SE (μ) | βL × 108 | SE (βL × 108) | βAD × 108 | SE (βAD × 108) | βG × 108 | SE(βG × 108) | Teth | SD(Teth) | Tacac | SD(Tacac) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0.0656 | 0.00421 | 0.436 | 0.0597 | 0.0167 | 0.00541 | 1.10 | 0.254 | 3.86 | 2.65 | 116 | 64.7 |
| 0 | 8.5 | 0.0695 | 0.00348 | 0.509 | 0.0643 | 0.0175 | 0.00541 | 1.26 | 0.191 | 2.22 | 2.05 | 116 | 49.9 |
| 0 | 17.7 | 0.0714 | 0.00373 | 0.180 | 0.0228 | 0.110 | 0.00978 | 1.11 | 0.357 | 10.4 | 3.11 | 111 | 34.4 |
| 0 | 26 | 0.0721 | 0.00550 | 0.119 | 0.0157 | 0.143 | 0.0130 | 0.916 | 0.191 | 2.08 | 1.01 | 94.9 | 38.5 |
| 0 | 49 | 0.0700 | 0.00233 | 0.376 | 0.103 | 0.690 | 0.156 | 1.18 | 0.299 | 3.33 | 3.47 | 165 | 56.1 |
| 0 | 80 | 0.0670 | 0.00180 | 0.472 | 0.0514 | 1.11 | 0.244 | 1.53 | 0.479 | 2.20 | 1.53 | 183 | 53.7 |
| 17 | 33 | 0.0652 | 0.00366 | 0.560 | 0.0809 | 0.592 | 0.120 | 1.71 | 0.331 | 2.80 | 1.19 | 157 | 66.0 |
| 17 | 66 | 0.0537 | 0.00183 | 0.738 | 0.102 | 0.791 | 0.276 | 1.54 | 0.527 | 1.50 | 0.335 | 164 | 35.0 |
| 18.5 | 7.7 | 0.0691 | 0.00334 | 0.541 | 0.0873 | 0.111 | 0.0114 | 0.770 | 0.152 | 1.83 | 0.586 | 188 | 85.8 |
| 21 | 0 | 0.0629 | 0.00511 | 0.698 | 0.0739 | 0.0389 | 0.00996 | 1.28 | 0.553 | 1.75 | 0.898 | 151 | 62.8 |
| 22 | 14 | 0.0525 | 0.00329 | 0.399 | 0.177 | 0.258 | 0.0638 | 0.961 | 0.216 | 1.58 | 0.638 | 159 | 64.7 |
| 23 | 20 | 0.0546 | 0.00288 | 0.412 | 0.0687 | 0.173 | 0.0278 | 1.43 | 0.185 | 3.10 | 2.78 | 177 | 58.1 |
| 23 | 52 | 0.0463 | 0.00190 | 0.460 | 0.142 | 0.540 | 0.159 | 1.21 | 0.132 | 3.36 | 1.25 | 170 | 24.1 |
| 33 | 41 | 0.0451 | 0.00290 | 0.478 | 0.0684 | 0.439 | 0.0364 | 1.45 | 0.161 | 1.67 | 0.511 | 200 | 56.9 |
| 37 | 52 | 0.0437 | 0.00225 | 0.488 | 0.0380 | 0.538 | 0.0644 | 1.22 | 0.0899 | 1.75 | 0.627 | 214 | 52.8 |
| 39 | 0 | 0.0410 | 0.00230 | 0.751 | 0.134 | 0.0249 | 0.00198 | 0.897 | 0.137 | 2.08 | 0.511 | 184 | 51.0 |
| 39 | 6 | 0.0435 | 0.00405 | 0.866 | 0.0800 | 0.0788 | 0.00884 | 0.624 | 0.137 | 1.39 | 0.702 | 197 | 71.7 |
| 35 | 33 | 0.0467 | 0.00382 | 0.903 | 0.198 | 0.321 | 0.0355 | 0.632 | 0.0286 | 2.52 | 0.819 | 195 | 76.9 |
| 40 | 25 | 0.0412 | 0.00364 | 0.928 | 0.243 | 0.249 | 0.0557 | 0.861 | 0.156 | 2.06 | 1.14 | 159 | 45.8 |
| 41 | 13 | 0.0481 | 0.00472 | 0.995 | 0.267 | 0.221 | 0.0434 | 0.914 | 0.203 | 1.83 | 0.699 | 148 | 49.7 |
| 55 | 0 | 0.0318 | 0.00237 | 0.589 | 0.121 | 0.111 | 0.0117 | 1.35 | 0.813 | 1.99 | 0.310 | 158 | 20.2 |
| 58 | 6 | 0.0334 | 0.00251 | 0.859 | 0.119 | 0.161 | 0.0289 | 1.03 | 0.183 | 2.00 | 0.408 | 142 | 33.6 |
| 55 | 30 | 0.0408 | 0.00364 | 0.840 | 0.189 | 0.237 | 0.0402 | 1.31 | 0.313 | 2.21 | 0.731 | 160 | 66.9 |
| 65 | 20 | 0.0359 | 0.00345 | 0.626 | 0.169 | 0.235 | 0.0573 | 0.598 | 0.0667 | 2.57 | 1.13 | 181 | 84.4 |
| 80 | 0 | 0.0341 | 0.00286 | 0.789 | 0.0749 | 0.0829 | 0.00122 | 0.844 | 0.183 | 1.29 | 0.922 | 112 | 58.6 |
| 73 | 10 | 0.0355 | 0.00253 | 0.675 | 0.117 | 0.214 | 0.0222 | 0.887 | 0.204 | 4.22 | 1.10 | 162 | 48.7 |
| 62 | 12 | 0.0373 | 0.00265 | 0.903 | 0.123 | 0.119 | 0.00936 | 0.565 | 0.123 | 2.47 | 1.35 | 134 | 44.6 |
μ, maximum specific growth rate; Teth, total ethanol concentration; Tacac, total acetic acid concentration
Plus nitrogen up to a complete 100%.
Each bottle was inoculated with 1 ml of the appropriate dilution of the subculture of B. thermosphacta, to reach a final concentration of ca. 103 CFU/ml. The inoculum was injected with a sterile syringe through the silicone disk of the cap. Bottles were incubated at 5°C lying on one of their sides.
Sampling, bacterial counts, and metabolite concentration determinations.
At each sampling time, a 3-ml sample was removed from each bottle with a sterile syringe through the silicone disk of the cap. Bacterial counts were determined by plating samples on tryptone soy agar (Oxoid/Unipath CM131). Bacteria were counted following incubation of tryptone soy agar for 48 h at 25°C. The remaining volume of the sample was centrifuged, filtered (0.45-μm pore size; Millipore), and stored at −20°C until chemical analyses were performed.
Analyses of l-(+)-lactic acid, acetic acid, ethanol, acetaldehyde, and glucose were carried out with Roche/Atom kits, according to the manufacturer's instructions. The acetoin-diacetyl concentration was measured as previously described (29).
After the last sampling time, the composition of the atmosphere in each bottle was determined.
Modeling.
The growth of the population was fitted to the Baranyi and Roberts model (2). The specific metabolite production rate was defined as
 |
(1) |
where x is the cell concentration (CFU per milliliter), CP is the concentration of the metabolite P (milligrams per 100 ml), and t is time (hours).
It was observed that, during the stationary phase of bacterial growth, lactic acid and acetoin-diacetyl were produced at a constant rate, independently of the conditions. Therefore, their non-growth-associated specific production rate was constant: βP(t) = βP. This parameter was expressed as rate (milligrams 100 ml−1 hour−1) per 108 cells (βP × 108).
Glucose consumption showed the opposite trend. Therefore, the specific glucose consumption rate was defined as
 |
(2) |
where CG is the concentration of glucose G (milligrams per 100 ml) and is expressed as the rate of glucose consumption (milligrams 100 ml−1 hour−1) per 108 cells (βG × 108).
No increases in the concentrations of ethanol and acetic acid were detected during the stationary phase. The total concentration (milligrams per 100 ml) of these metabolites under each atmosphere was calculated as the average of the measurements obtained at the different sampling times.
The natural logarithms of the maximum specific growth rates and those of the non-growth-associated rates of lactic acid and acetoin-diacetyl production and glucose consumption were described as a function of the percentage of CO2 and O2 in the atmosphere by second-order polynomials. The relationship between the composition of the atmosphere and the natural logarithm of the total concentration of acetic acid and ethanol was also studied by using quadratic polynomials.
F tests were used to determine the significance of the effects of oxygen and/or carbon dioxide on the modeled bacterial responses. Initially, the effect of the variable (oxygen or carbon dioxide) which accounted for the greatest variability of the bacterial responses was modeled. Then an F test was used to decide whether the effect of the other variable was significant.
Boundary atmosphere composition for aerobic-anaerobic metabolism.
A discriminant function was developed to establish the atmosphere compositions that determine the boundary between dominantly aerobic and dominantly anaerobic metabolisms of B. thermosphacta. This boundary was defined as the conditions at which the microorganism population could carry out aerobic or anaerobic metabolism with equal probability (0.5). A similar approach was used to determine the boundary growth conditions of Shigella flexneri in reference 27.
Assuming that B. thermosphacta has a homofermentative metabolism, i.e., it produces two molecules of lactic acid per molecule of glucose, the proportion of the total consumed glucose that was anaerobically metabolized yielding lactic acid, Gluanaer, can be estimated as half the ratio between the produced lactic acid molecules and the consumed glucose molecules:
 |
(3) |
where MWL is the molecular weight of lactic acid, L, and MWg is the molecular weight of glucose, g.
Likewise, assuming that acetoin is formed via α-acetolactate, the proportion of glucose that was aerobically metabolized to yield acetoin-diacetyl as end product, Gluaer, was estimated as
 |
(4) |
where MWA is the molecular weight of acetoin, A.
When the proportion of glucose anaerobically metabolized, Gluanaer, was greater than the proportion of glucose aerobically metabolized, Gluaer, the metabolism of the population was considered to be dominantly anaerobic. When the opposite occurred, the metabolism was considered dominantly aerobic.
A logistic model (SAS/STAT User's Guide, version 8; SAS Institute Inc., Cary, N.C.) was used to describe the probability of anaerobic metabolism as a function of the percentage of CO2 and O2 in the atmosphere. The metabolism of glucose was considered to be a random variable following the Bernoulli distribution, with a value of 1 when the metabolism was anaerobic and 0 when it was aerobic. The fitted model was
 |
(5) |
where p is the parameter of the Bernoulli distribution (probability of anaerobic metabolism) and a, b, and c are the parameters estimated by the maximum likelihood method.
To determine the boundary between both forms of metabolism, a discriminant function was obtained from equation 5 by imposing the p = 0.5 condition:
 |
(6) |
RESULTS
Acetaldehyde was not detected under any of the experimental conditions. Ethanol and acetic acid were detected under all of the tested atmospheres, but no consistent pattern of accumulation was observed during the stationary phase. However, lactic acid and acetoin-diacetyl were produced and glucose was consumed at constant rates during the stationary phase. The data in Fig. 1 show the behavior of the population under two different atmospheres: 0/80/20 and 0/0/100 ratios of CO2-O2-N2, respectively. At 0% oxygen, consumption of glucose was slower than at 80%. The rate of acetoin-diacetyl production was much higher at 80% oxygen than at 0%, while no noticeable changes were observed in the production of lactic acid. For B. thermosphacta, Table 1 reports the growth rate, production rates of lactic acid and acetoin-diacetyl, glucose consumption rate, and total concentrations of acetic acid and ethanol calculated for each experimental condition.
FIG. 1.
Growth (•); production of lactic acid (×), acetoin-diacetyl (□), acetic acid (○), and ethanol (◊); and consumption of glucose (▵) by B. thermosphacta at 5°C under atmospheres with 0/80/20 (a) and 0/0/100 (b) ratios of CO2-O2-N2, respectively.
Table 2 reports the final estimates of the different models. Note that models have been developed to study which factor, oxygen or carbon dioxide, had a significant influence on metabolite production and glucose depletion rather than to give predictions. For those models with low coefficients of determination (R2), predictions may be unreliable. The coefficients in Table 2 refer to variables that significantly affected bacterial response. The higher the oxygen concentration in the atmosphere, the greater the acetoin-diacetyl production rate (Fig. 2b), the total concentration of acetic acid (Fig. 2c), and the rate of glucose consumption (Fig. 2d). However, no effect of oxygen on lactic acid production was observed (Fig. 2a). Likewise, greater concentrations of carbon dioxide in the atmosphere yielded higher production rates of lactic acid (Fig. 2a) and lower production rates of acetoin-diacetyl (Fig. 2b). No significant changes were observed in the consumption of glucose, and production of acetic acid did not show a consistent trend: the total concentration of this acid increased as CO2 was increased from 0 to 20 to 40% and decreased as CO2 was increased from 20 to 40 to 80%. Only small amounts of ethanol were detected. Ethanol concentration was not influenced by either oxygen or carbon dioxide percentages. The maximum specific growth rate of B. thermosphacta was not affected by the percentage of oxygen, but it decreased when the concentration of carbon dioxide was increased (Fig. 2e).
TABLE 2.
Models for the natural logarithms of the maximum specific growth rate of B. thermosphacta, of the production rates of lactic acid and acetoin-diacetyl, of the consumption rate of glucose, and of the total concentration of acetic acida
| Term | In(μ)
|
In(βL × 108)
|
In(βAD × 108)
|
In(βG × 108)
|
In(Tacac)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fitted value | SE | Fitted value | SE | Fitted value | SE | Fitted value | SE | Fitted value | SE | |
| Intercept | −2.64 | 0.0356 | −1.17 | 0.136 | −4.37 | 0.281 | −0.0512 | 0.101 | 4.75 | 0.0967 |
| CO2 | −0.0145 | 0.00230 | 0.0326 | 0.00878 | 0.0556 | 0.0137 | 0.0194 | 0.00471 | ||
| CO22 | 0.0000583 | 0.0000313 | −0.000283 | 0.000120 | −0.000377 | 0.000158 | −0.000236 | 0.0000546 | ||
| O2 | 0.127 | 0.0174 | −0.000748 | 0.00772 | −0.000498 | 0.00599 | ||||
| O22 | −0.000898 | 0.000202 | 0.000104 | 0.000109 | 0.0000694 | 0.0000696 | ||||
| CO2*O2 | −0.00109 | 0.000281 | −4.75 × 10−6 | 0.0000970 | ||||||
| Adjusted R2 | 0.88 | 0.48 | 0.88 | 0.16 | 0.57 | |||||
| Standard error of fit | 0.093 | 0.35 | 0.39 | 0.28 | 0.14 | |||||
μ, maximum specific growth rate; Tacac, total concentration of acetic acid.
FIG. 2.
Lactic acid production rate (a), acetoin-diacetyl production rate (b), total amount of acetic acid (c), glucose consumption rate (d), and maximum specific growth rate (e) of B. thermosphacta at 5°C and different percentages of oxygen and carbon dioxide in the atmosphere.
The data in Fig. 3 show the effect of atmosphere composition on the metabolism of glucose by B. thermosphacta. The proportion of glucose transformed into acetoin-diacetyl clearly increased with increased oxygen concentration (Fig. 3a). Consequently, the increase of the acetoin-diacetyl production rate with oxygen (Fig. 2b) is attributable to two main effects: higher consumption rate of glucose (Fig. 2d) and greater acetoin-diacetyl yield (Fig. 3a), i.e., more glucose was consumed under high oxygen concentrations, a greater proportion of which was transformed into acetoin-diacetyl. The proportion of glucose transformed into lactic acid decreased at high oxygen percentages (>40 to 50%) (Fig. 3b); however, lactic acid production rates were not affected by oxygen concentration (Fig. 2a). This result may be explained by the fact that, although the presence of oxygen decreased the anaerobic metabolism of glucose, it increased the glucose consumption rate (Fig. 2d), i.e., more glucose was consumed, yielding greater amounts of end products.
FIG. 3.
Proportion of glucose consumed aerobically (a) and anaerobically (b) under different percentages of oxygen and carbon dioxide in the atmosphere.
The data in Fig. 3 demonstrate a greater transformation of glucose into lactic acid (Fig. 3b) and a lower yield of acetoin-diacetyl (Fig. 3a) as the CO2 concentration was increased. Increased carbon dioxide enhanced the anaerobic metabolism of glucose but did not increase its consumption rate, which accounted, in turn, for increases in the lactic acid production rates and decreases in the acetoin-diacetyl rates (Fig. 2a and b, respectively).
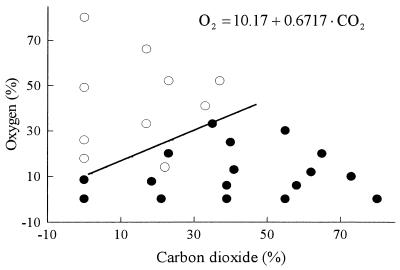
The data in Fig. 4 show the dominant metabolism in each of the modified atmospheres. The metabolism of B. thermosphacta was dominantly aerobic in atmospheres with high oxygen content, i.e., more glucose was aerobically metabolized, yielding acetoin-diacetyl, than anaerobically metabolized, yielding lactic acid. High percentages of CO2 in the atmosphere favored anaerobic metabolism. The effect of oxygen on the metabolism of B. thermosphacta was related to the amount of CO2; under atmospheres with relatively high percentages of CO2 the metabolism was predominantly aerobic only at higher concentrations of O2 (Fig. 4). In the absence of CO2, the metabolism was predominantly aerobic in the presence of 9 to 17% O2. However, at ca. 35% CO2, more than 30% O2 was required to obtain the same effect (Fig. 4).
FIG. 4.
Atmosphere compositions that determine the boundary between dominantly aerobic (○) or dominantly anaerobic (•) metabolism in B. thermosphacta.
The sum of proportions of aerobically and anaerobically metabolized glucose (Gluaer + Gluanaer) should not exceed 1.0 (Fig. 3). Sums of proportions greater than 1 are attributable to the magnification of the experimental error. The experimental error was magnified by adding up proportions that were calculated as the rates between two estimations, βP and βG. The influence of this error on the estimation of the boundary between anaerobic and aerobic metabolism was minimized by transforming proportions into binary data, 1 when Gluanaer was greater than Gluaer and 0 otherwise, as described above.
Because of the interaction between the effects of the two gases, the percentage of oxygen in the atmospheres for which anaerobic and aerobic metabolisms in the population were equally probable was a function of the CO2 percentage, as follows:
 |
(7) |
Notice that equation 7 is derived from equation 6 after estimating the coefficients and solving for the oxygen percentage. When the percentage of oxygen is higher than the solution of this equation for a given CO2 concentration, the metabolism of B. thermosphacta is predominantly aerobic, and it is predominantly anaerobic when the percentage of oxygen is lower than the solution (Fig. 4).
DISCUSSION
The greater yield of lactic acid under anaerobic conditions, the greater yield of acetoin-diacetyl under aerobic conditions, and the fact that no acetaldehyde was detected suggest that the metabolism of B. thermosphacta could have certain similarities with that of Lactococcus lactis subsp. lactis var. diacetylactis. Under aerobic conditions, this organism produces acetoin from diacetyl, which comes from pyruvate via α-acetolactate, yielding one molecule of acetoin per molecule of glucose, while under anaerobic conditions it transforms glucose mainly into lactic acid, yielding two molecules of lactic acid per molecule of glucose (12, 25). Similar results have been reported for food, such as those for refrigerated MAP pork (22), where B. thermosphacta was identified as the dominant bacterium. Greater acetoin-diacetyl production under high oxygen concentration and no effect of oxygen on production of l-lactic acid were reported. B. thermosphacta was also the dominant microorganism in MAP hake (24) and salmon steaks (8), and a greater accumulation of lactic acid was detected at high CO2 concentrations.
Acetic acid and ethanol did not accumulate during the stationary phase; these metabolites may have been used as substrates for other reactions. The total amount of acetic acid produced was greater under higher concentrations of oxygen. Acetic acid has been identified elsewhere as an end product of aerobic metabolism of B. thermosphacta (3, 4, 5). Production of 3-methylbutanol and isobutyric and 2-methylbutyric acid by B. thermosphacta was also reported previously (3, 4, 5). These metabolites are derived from acetic acid (25), which could explain why acetic acid did not accumulate during the stationary phase. The relationship between CO2 percentage and the total concentration of acetic acid was not clear. The observations for hake (24) followed a trend similar to that found in this study, with production of acetic acid being lowest in the absence of CO2 and greater at 20 than at 40% CO2. In similar experiments with salmon (8), no differences were observed in acetic acid production at 0, 20, and 40% CO2.
It has been suggested elsewhere that B. thermosphacta behaves as a heterofermentative organism, yielding lactic acid, ethanol, and CO2 from glucose (13). Whether the ethanol comes from this pathway or whether its formation is concomitant with other organic acids, acetaldehyde is required as an intermediate metabolite (12, 25). In the present work, acetaldehyde was not detected at any of the sampling times and under any atmosphere, which suggests either that ethanol is not produced via these pathways or, more likely, that the yield of ethanol is very small and the yield of acetaldehyde is not detectable. This result corroborates the idea that the anaerobic metabolism of glucose by B. thermosphacta is homofermentative. Ethanol may not accumulate in the presence of CO2 and O2 because it is transformed into fatty acid ethyl esters, as observed with other bacteria, such as the family Micrococcaceae (21).
Oxygen and carbon dioxide mainly affected glucose metabolism. Glucose consumption rates were affected only under atmospheres with high oxygen percentages, atmospheres at which higher rates were detected. In situations of oxygen stress, the growth rate of Escherichia coli was limited by the intracellular level of superoxide dismutase, which provides effective protection against superoxide ion toxicity (9). Furthermore, high percentages of O2 did not affect the growth rate of E. coli when the level of superoxide dismutase was higher and the respiration rate was higher (9). The lack of influence of oxygen on the exponential growth rates of B. thermosphacta was likely associated with higher respiration rates. The higher glucose consumption rates detected during the stationary phase at high O2 percentages were also probably related to the higher respiration rate required under oxygen stress.
The exponential growth was not affected by the level of oxygen in the atmosphere. Oxygen does not influence the rate of food spoilage in terms of the growth of B. thermosphacta; however, spoilage is not determined by growth per se but by the microbial metabolism. Equation 6 estimates the maximum percentage of O2 as a function of the percentage of CO2 that a modified atmosphere should contain in order to avoid a shift from dominantly anaerobic to dominantly aerobic metabolism. This metabolic switch would cause a change in the end metabolites, from mainly lactic acid (less offensive odor) to acetoin-diacetyl, short-chain fatty acids, etc. (more offensive odor), and thus an earlier rejection of the product by consumers.
Red meats (e.g., beef and pork) and scombroids (e.g., tuna) require atmospheres enriched in O2, in order to maintain the bright red color of oxymyoglobin, and in CO2, in order to inhibit the growth of aerobic gram-negative bacteria. Atmospheres with a 20/80 proportion of CO2-O2 are typically used for red meat with a normal pH of ca. 5.5 (1, 19, 23). If the metabolism of B. thermosphacta is considered a limiting factor, in order to avoid the change from predominantly anaerobic to aerobic metabolism, based on this model (equation 6), the maximum concentration of oxygen should be 46% oxygen with the remaining 54% of the volume being carbon dioxide. Thus, it seems necessary to modify the composition of the previously reported CO2-O2 mixtures for use in red meat which include 80% oxygen (1, 19, 23). Percentages of CO2 as high as 54% could produce a slight, but probably perceptible, acidification of the meat that would cause undesirable exudates. The recommended approach to solving this problem is increasing the CO2 percentage up to 35 to 40% and decreasing the O2 concentration from 80 to 40%, according to equation 6, and completing the mixture with nitrogen. Regarding meat color, it has been reported elsewhere that consumers reject meat with ca. 60% metmyoglobin (1). Assuming that metmyoglobin formation is proportional to time and interpolating from previously reported data (1, 18, 23), the time needed to reach ca. 60% metmyoglobin with ca. 40% oxygen is 20 days or longer. This is at least twice the shelf life of meat stored at refrigeration temperatures (4 to 5°C) in contact with air.
However, the recommended atmosphere for storage of tuna contains higher percentages of CO2 (40%) to inhibit the growth of S. putrefaciens (18). Although a 40/60 mixture of CO2-O2 is close to the boundary described by equation 6, a slight decrease in the O2 percentage, from 60 to 45 or 50%, as well as a slight increase in the CO2 concentration, from 40 to 55 or 50%, would ensure a predominantly anaerobic metabolism. The acidification in fish due to the high CO2 percentage would not produce the adverse results that would be expected for red meat, because the difference between the pH of tuna (ca. 6.0) and the isoelectric point of the muscle proteins is greater than that of meat. Oxygen is also used in packaging fish with very low myoglobin content such as hake, salmon, sole, etc. These fish, with relatively high pHs, are packaged in atmospheres with 40 to 50% CO2 without O2 (8, 18, 24). However, some researchers have reported on the value of including oxygen at low concentrations, e.g., 5%, to avoid the growth of Clostridium botulinum type E (10, 26). Therefore, 40 to 50/5 to 10/55 to 60 ratios of CO2-O2-N2 are also recommended to package these kinds of fish (15). According to equation 6, the content of oxygen in these atmospheres is much lower than the minimum content required for B. thermosphacta to carry out aerobic metabolism.
Acknowledgments
We thankfully acknowledge the support of the Comisión Interministerial de Ciencia y Tecnología (CICYT, Spain), project ALI99-0405 and project AGL0692.
REFERENCES
- 1.Asensio, M. A., J. A. Ordóñez, and B. Sanz. 1988. Effect of carbon dioxide- and oxygen-enriched atmospheres on the shelf life of refrigerated pork packed in plastic bags. J. Food Prot. 51:356-360. [DOI] [PubMed] [Google Scholar]
- 2.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 3.Dainty, R. H., and C. M. Hibbard. 1980. Aerobic metabolism of Brochothrix thermosphacta growing on meat surface and in laboratory media. J. Appl. Bacteriol. 48:387-396. [DOI] [PubMed] [Google Scholar]
- 4.Dainty, R. H., and C. M. Hibbard. 1983. Precursors of the major end products of aerobic metabolism of Brochothrix thermosphacta. J. Appl. Bacteriol. 55:127-133. [DOI] [PubMed] [Google Scholar]
- 5.Dainty, R. H., and F. J. K. Hofman. 1983. The influence of glucose concentration and culture incubation time on end-product formation during aerobic growth of Brochothrix thermosphacta. J. Appl. Bacteriol. 55:233-239. [Google Scholar]
- 6.Dainty, R. H., and B. M. Mackey. 1992. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Bacteriol. 73:103S-104S. [DOI] [PubMed] [Google Scholar]
- 7.Dainty, R. H., B. G. Shaw, C. D. Harding, and S. Michanie. 1979. The spoilage of vacuum-packed beef by cold-tolerant bacteria, p. 83-100. In A. D. Russell and R. Fuller (ed.), Cold-tolerant microbes in spoilage and the environment. Academic Press, London, United Kingdom.
- 8.De la Hoz, L., D. E. López-Gálvez, M. Fernández, E. Hierro, and J. A. Ordóñez. 2000. Use of carbon dioxide-enriched atmospheres in the refrigerated storage (2°C) of salmon (Salmo salar) steaks. Eur. Food Res. Technol. 210:179-188. [Google Scholar]
- 9.Fridovich, I. 1978. The biology of oxygen radicals. Science 201:875-880. [DOI] [PubMed] [Google Scholar]
- 10.García, G. W., C. Genigeorgis, and S. Lindroth. 1987. Risk of growth and toxin production by Clostridium botulinum nonproteolytic types B, E, and F in salmon fillets stored under modified atmospheres at low and abused temperatures. J. Food Prot. 50:330-336. [DOI] [PubMed] [Google Scholar]
- 11.Gill, C. O., and K. G. Newton. 1977. The development of aerobic spoilage flora on meat stored at chill temperatures. J. Appl. Bacteriol. 43:189-195. [DOI] [PubMed] [Google Scholar]
- 12.Gottschalk, G. (ed.). 1986. Bacterial metabolism. Springer-Verlag, New York, N.Y.
- 13.Hitchener, B. J., A. F. Egan, and P. J. Rogers. 1979. Energetics of Microbacterium thermosphactum in glucose-limited continuous culture. Appl. Environ. Microbiol. 37:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood, D. E., and G. C. Mead. 1993. Modified atmosphere storage of fresh meat and poultry, p. 269-298. In R. T. Parry (ed.), Principles and applications of modified atmosphere packaging. Blackie Academic and Professional, Glasgow, United Kingdom.
- 15.Hotchkiss, J. H. 1988. Experimental approaches to determining the safety of food packaged in modified atmospheres. Food Technol. 42:55-66. [Google Scholar]
- 16.Kakauri, A., and G. J. E. Nychas. 1994. Storage of poultry meat under modified atmospheres or vacuum packs: possible role of microbial metabolites as indicator of spoilage. J. Appl. Bacteriol. 76:163-172. [DOI] [PubMed] [Google Scholar]
- 17.López-Gálvez, D. E., L. de la Hoz, M. Blanco, and J. A. Ordóñez. 1998. Refrigerated storage (2°C) of sole (Solea solea) fillets under CO2-enriched atmospheres. J. Agric. Food Chem. 46:1143-1149. [Google Scholar]
- 18.López-Gálvez, D. E., L. de la Hoz, and J. A. Ordóñez. 1995. Effect of carbon dioxide- and oxygen-enriched atmospheres on microbiological changes in refrigerated tuna (Thunnus alalunga) steaks. J. Agric. Food Chem. 43:483-490. [Google Scholar]
- 19.McDougall, D. B., and A. A. Taylor. 1975. Color retention in fresh meat stored in oxygen—a commercial-scale trial. J. Food Technol. 10:339-347. [Google Scholar]
- 20.Molin, G., and I.-M. Stenström. 1983. Effect of the temperature on the microbial flora of herring fillets stored in air or carbon dioxide. J. Appl. Bacteriol. 56:275-282. [DOI] [PubMed] [Google Scholar]
- 21.Montel, M. C., J. Reitz, R. Talon, J. L. Berdagué, and S. Rousset-Akrim. 1996. Biochemical activities of Micrococcaceae and their effects on the aromatic profiles and odors of a dry sausage model. Food Microbiol. 13:489-499. [Google Scholar]
- 22.Ordóñez, J. A., B. de Pablo, B. Pérez de Castro, M. A. Asensio, and B. Sanz. 1991. Selected chemical and microbiological changes in refrigerated pork stored in carbon dioxide and oxygen-enriched atmospheres. J. Agric. Food Chem. 39:668-672. [Google Scholar]
- 23.Ordóñez, J. A., and D. A. Ledward. 1977. Lipid and myoglobin oxidation in pork stored in oxygen- and carbon dioxide-enriched atmospheres. Meat Sci. 1:41-48. [DOI] [PubMed] [Google Scholar]
- 24.Ordóñez, J. A., D. E. López-Gálvez, M. Fernández, E. Hierro, and L. de la Hoz. 2000. Microbial and physicochemical modifications of hake (Merluccius merluccius) steaks stored under carbon dioxide-enriched atmospheres. J. Sci. Food Agric. 80:1831-1840. [Google Scholar]
- 25.Parés, R., and A. Juárez (ed.). 1997. Bioquímica de los microorganismos. Reverté, Barcelona, Spain.
- 26.Post, L. S., D. A. Lee, M. Solberg, D. Furang, J. Specchio, and C. Graham. 1985. Development of botulinum toxin and sensory deterioration during storage of vacuum and modified atmosphere packaged fish fillets. J. Food Sci. 50:990-996. [Google Scholar]
- 27.Ratkowsky, D. A., and T. Ross. 1995. Modelling the bacterial growth/no growth interface. Lett. Appl. Microbiol. 20:29-33. [Google Scholar]
- 28.Sheridan, J. J., A. M. Doherty, P. Allen, D. A. McDowell, I. S. Blair, and D. Harrington. 1997. The effect of vacuum and modified atmosphere packaging on the shelf-life of lamb primals, stored at different temperatures. Meat Sci. 45:107-117. [DOI] [PubMed] [Google Scholar]
- 29.Westerfield, W. W. 1945. A colorimetric determination of blood acetoin. J. Biochem. Chem. 161:495-502. [PubMed] [Google Scholar]