Abstract
A standardized disinfectant test for Staphylococcus aureus cells in biofilms was developed. Two disinfectants, the membrane-active compound benzalkonium chloride (BAC) and the oxidizing agent sodium hypochlorite, were used to evaluate the biofilm test. S. aureus formed biofilms on glass, stainless steel, and polystyrene in a simple system with constant nutrient flow that mimicked as closely as possible the conditions used in the current standard European disinfectant test (EN 1040). The biofilm that was formed on glass contained cell clumps and extracellular polysaccharides. The average surface coverage was 60%, and most (92%) of the biofilm cells were viable. Biofilm formation and biofilm disinfection in different experiments were reproducible. For biofilms exposed to BAC and hypochlorite the concentrations needed to achieve 4-log killing were 50 and 600 times higher, respectively, than the concentrations needed to achieve this level of killing with the European phase 1 suspension test cells. Our results show that a standardized disinfectant test for biofilm cells is a useful addition to the current standard tests.
Every year food-borne diseases cause millions of illnesses worldwide (17, 28, 35). One way that food can be contaminated with pathogens is through contact with food-processing equipment. Therefore, it is of the utmost importance that this equipment be cleaned and disinfected regularly and sufficiently. Thus, an effective disinfectant should be used, and an appropriate concentration of this disinfectant should be applied. A concentration that is too low increases the risk of food contamination and the risk of acquisition of resistance to the disinfectant, and a concentration that is too high increases the cost and the environmental burden.
The procedure used for testing candidate disinfectants in Europe consists of three phases. In phase 1 the basic activity of the product is tested with a suspension test. Phase 2 consists of two steps. In the first step the product is tested with a suspension test under conditions that are representative of practical conditions. The second step consists of other laboratory tests (e.g., hand washing, hand rubbing, and surface tests simulating practical conditions). Phase 3 consists of field tests under practical conditions (3, 4, 5). As in Europe, in the United States disinfectants are tested predominantly by using cell suspensions (10). Concerns have been expressed about the European phase 1 and phase 2 step 1 tests for bactericidal testing (26, 27, 31), and suggestions have been made for improvement (26, 27). However, there are still some concerns. A good test must be able to predict the value of the disinfectant in practice (34), and in practice cells are found much more frequently on surfaces than in suspension. Thus, the question is whether suspension test cells are really representative of cells under practical conditions. In this light, in the United States the AOAC hard surface carrier test method is used (7, 8, 9). In this surface test a suspension of cells is put on a surface and dried for 45 min. Then the disinfectant is applied. In Europe a new surface test is being developed for phase 2 step 2 (12, 25), in which a similar procedure is used. These surface tests are already a step forward compared to suspension tests. Still, there can be some concern about the suitability of the surface tests. The cells in a surface test only have time to attach to the surface and not time to grow, whereas it is known that attached cells that are allowed time to grow form biofilms. Biofilms are much more resistant to antimicrobial agents than free-living cells (14, 18, 39) and may act as continuous sources of food spoilage bacteria and pathogens that contaminate food if this increased resistance is not taken into account during disinfection. Therefore, there is a need for a standard disinfectant test for biofilm cells.
Several techniques have been described for antimicrobial agent (predominantly antibiotic) testing with biofilms. Most of these techniques are medically orientated. They often use the MIC to assess antibiotic efficacy (20, 44). However, disinfectant efficacy has to be assessed by viable counting, since growth- inhibited cells can still contaminate food and cells can regrow after recovery. Very often batch systems are used for biofilm formation. In these systems coupons are placed in inoculated rich medium, and sometimes the medium is replaced several times. Alternatively, inoculated medium is used for development of biofilms on the surfaces of microtiter plate wells or Erlenmeyer flasks (11, 16, 29, 33). A disadvantage of these batch methods is that since little or no shear force is applied to them, the cells are very loosely attached to the surface and thus are not representative of biofilms in practice. In some of the studies in which these kinds of systems are used shear force is applied by shaking the surface during biofilm formation (13, 15). Still, the biofilms grown in batch cultures and on rich media are not representative of biofilms in the food industry. An interesting method for biofilm formation is to trap planktonic cells in a poloxamer hydrogel that is liquid at temperatures below 15°C and solid at temperatures above 15°C (23). With this method the viability of cells can be easily analyzed. However, the cells do not have the biofilm physiology. Another method is to apply cells to a filter and place the filter on solid medium (38) or perfuse the filter with liquid medium (21). The resulting biofilms are different from natural biofilms on inert surfaces because the cells receive their nutrients from the surface side and not from the air or bulk liquid side like food industry biofilms do. The biofilms that come closest to biofilms in the food industry are the ones that are formed in special reactors that apply a certain shear force to the biofilm cells while they are growing and that continuously provide the cells with relatively poor medium as a food source. Examples of this kind of reactor are the Robbins device (1), a chemostat with coupons in it (45), the concentric cylinder reactor (40), and the constant-depth film fermentor (36). However, all these methods are very sophisticated and require expensive equipment, and thus they are not very suitable for disinfectant testing, since the requirements for a disinfectant test are that it should be as simple as possible and not require specialized or expensive pieces of laboratory equipment. Furthermore, a standard disinfectant test for biofilm cells should resemble as closely as possible the current suspension tests in order to make comparisons between the results possible.
The aim of this study was to develop a standard disinfectant test for biofilm cells that meets all of the requirements mentioned above. For this study we used Staphylococcus aureus ATCC 6538 because it is used as the representative of gram-positive bacteria in the United States and European standard tests (4, 5, 10) and it is able to form biofilms (2, 22, 30). The membrane-active compound benzalkonium chloride (BAC) and the oxidizing agent hypochlorite were used; both of these compounds are commonly used in the food industry as disinfectants (32, 43).
MATERIALS AND METHODS
Bacterial strain and growth conditions.
S. aureus DSM 799 (corresponding to ATCC 6538) stock cultures were kept at −80°C with 25% (wt/vol) glycerol added. Cells were grown at 30°C. Following the procedure of the European phase 1 suspension test (5), we grew cells aerobically on tryptone soy agar (TSA) for 24 h and transferred them to fresh TSA for another 24 h of incubation. Then cells were suspended in peptone physiological salt solution (PPS) (1 g of neutralized bacteriological peptone per liter, 8.5 g of NaCl per liter) to an optical density at 620 nm corresponding to a concentration of 1.5 × 108 to 5 × 108 CFU ml−1. This is the phase 1 standard test suspension. Cells that were used for comparisons of their diameters to the diameters of biofilm cells were first statically grown overnight in tryptone soy broth (TSB). This culture was used to inoculate (2% [vol/vol] inoculum) 10 ml of 10-fold-diluted TSB (1/10 TSB). For aerobically grown cells this new culture was transferred to a 100-ml Erlenmeyer flask and shaken in a gyratory incubator at 130 rpm. For anaerobically grown cells the culture was transferred to a 60-ml closed vessel, and resazurin (1 mg liter−1) was added as an indicator of the presence of oxygen. Oxygen was depleted after 3 h. Samples were taken 2.5, 8, and 24 h after inoculation.
Chemicals and disinfectants.
The disinfectants used in this study were 50% alkyl-benzyl-dimethylammonium chloride (alkyl distribution from C8H17 to C16H33) (BAC) (Lamers & Pleuger, Den Bosch, The Netherlands) and sodium hypochlorite with 130 g of active chlorine per liter (Acros, Geel, Belgium). For both disinfectants 10- and 100-fold dilutions in demineralized water were prepared from the stock solutions before each experiment and used immediately. TSB, TSA, and neutralized bacteriological peptone were obtained from Oxoid (Basingstoke, United Kingdom). Glycerol was obtained from Fluka Chemie AG (Buchs, Switzerland), lecithin from soybeans was obtained from BDH Laboratory Supplies (Poole, England), Congo red was obtained from Aldrich Chemical Co. (Milwaukee, Wis.), sodium lactate was obtained from PURAC Biochem BV (Gorinchem, The Netherlands), and resazurin was obtained from Janssen (Geel, Belgium). Quinolinium 1,1′-{1,3-propanediylbis[(dimethyliminio)-3,1-propanediyl]}bis{4-[(3-methyl-2(3H)-benzothiazolylidene) methyl]}-tetraiodide (TOTO) was obtained from Molecular Probes Europe BV (Leiden, The Netherlands). All other chemicals were obtained from Merck KGaA (Darmstadt, Germany).
Biofilm production.
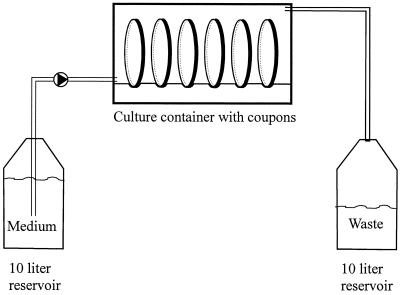
Biofilms were grown in a simple apparatus (Fig. 1) that consisted of a vessel containing 1/10 TSB, a pump (Masterflex model 7521-10; Cole-Parmer Instrument Co., Chicago, Ill.), a culture container (perfusion culture container 4702; Minucells und Minutissue, Bad Abbach, Germany), and a vessel with waste, all connected by silicon tubing. Twenty-three coupons (diameter, 13 mm), each held by a coupon carrier (Minusheet; Minucells und Minutissue), were placed in the culture container and used as surfaces for biofilm formation. In most experiments we used glass coupons (Deckglaeser; Menzel Glaeser, Braunsweig, Germany); the exceptions were when we used polystyrene coupons (Thermanox plastic coverslips; Nalge Nunc Int., Naperville, Ill.) or stainless steel coupons (custom made from austenitic stainless steel 304 AISI, werkstofnr 1.4301; ODS, Barendrecht, The Netherlands). All coupons and supports were cleaned with 70% ethanol and autoclaved before use. Before inoculation the coupons were placed in the culture container, and 1/10 TSB was pumped (dilution rate, 17 h−1) through the system for 1 h. The coupons were then inoculated by removing 9 ml of medium from the culture container, pipetting 9 ml of the phase 1 standard test suspension (see above) into the container, waiting for 30 min, removing the cell suspension, and adding 9 ml of fresh medium. Then the pump was started again, and the biofilms were allowed to develop for 24 h at 30°C with a constant nutrient flow. Then the pump was stopped, the coupons were removed from the culture container, the supports were removed with tweezers, and the coupons were washed by dipping them in PPS once, which removed all unattached cells; then the coupons were ready for analysis or exposure to disinfectants.
FIG. 1.
Schematic representation of the practical setup used for biofilm formation in the culture container (external dimensions, 12.5 by 4 by 3.5 cm; internal volume, ca. 22.5 ml). In reality, the culture container contained 23 coupons (diameter, 13 mm), each held by a coupon carrier.
Biofilm characteristics.
To determine the pH and the concentrations of glucose, acetic acid, and lactic acid, 10-ml samples were taken from the medium vessel and from the tube connecting the culture container and the waste vessel at 24 h. Both samples were filter sterilized (pore size, 0.2 μm), and the pH was measured with a PHM240 pH-ion meter from Radiometer (Copenhagen, Denmark). The concentrations of glucose, acetic acid, and lactic acid were determined by high-performance liquid chromatography (HPLC) of a 20-μl sample by comparison with standards. For HPLC we used an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad, Veenendaal, The Netherlands) and elution at 40°C with a solution containing 5 mol of H2SO4 per liter at a rate of 0.6 ml min−1. The eluate was monitored with a refractive index detector.
Photomicrographs of S. aureus cells were taken at magnifications of ×400 and ×1,000 with an MC80 camera (Carl Zeiss, Oberkochen, Germany) mounted on an Axioscope phase-contrast microscope. Extracellular polysaccharide formation was monitored by incubating biofilms for 40 min with a 0.1% Congo red solution (which stained polysaccharides [19]) and washing them twice in phosphate-buffered saline (0.2 g of KCl per liter, 0.2 g of KH2PO4 per liter, 1.5 g of Na2HPO4 per liter, 8.0 g of NaCl per liter; adjusted to pH 7.2 with HCl). To determine the cell diameter, at least 50 cells were measured on photomicrographs, and the values were corrected for the difference in magnification between the photomicrographs and the images observed with the microscope. To determine the percentage of viable cells, biofilms were incubated in 9 ml of phosphate-buffered saline containing 0.3 μmol of TOTO per liter for 15 min and examined with an Axioscope microscope equipped with a 50-W mercury arc lamp and a fluorescein isothiocyanate filter set (excitation at 450 to 490 nm, emission at >520 nm). To confirm the viability results, a biofilm cell suspension was prepared by swabbing the coupon surface and vortexing (in 3 ml of PPS) as described below. The concentration of viable cells was determined by diluting the cell suspension in PPS and counting the cells by plating them on TSA after incubation for 48 h at 30°C. The total concentration of cells was determined by counting the cell suspension under a phase-contrast microscope by using a Bürker-Türk counting chamber with a depth of 0.01 mm at a magnification of ×1,000. For each sample 63 0.0025-mm2 squares were counted. The percentage of viable cells was calculated by dividing the concentration of viable cells by the total concentration of cells and multiplying by 100%. Experiments were performed in quadruplicate. The percentage of surface coverage was calculated by dividing the average level of biofilm formation (in CFU per square centimeter) by the percentage of viable cells and multiplying the result by the area covered by one biofilm cell (πr2, where r is 0.5 × average diameter). Extracellular polymeric substances were not included when the diameter was determined.
Killing experiments.
All killing experiments were done at 20°C. Biofilms were grown and washed as described above. One coupon was added to 3 ml of disinfectant or, for the control, to 3 ml of demineralized water in a closed 50-ml tube. Three milliliters was chosen to achieve approximately the same cell concentration per milliliter of disinfectant as in the phase 1 test. After 5 min, 27 ml of neutralizer was added, which consisted of 10 ml of a buffer containing 34 g of KH2PO4 per liter adjusted to pH 7.2 with NaOH, 3 g of lecithin from soybeans per liter, 30 ml of Tween 80 per liter, 5 g of Na2S2O3 per liter, and 1 g of l-histidine per liter. After another 5 min, the coupon, which was still in the liquid, was swabbed (polyester fiber-tipped applicator swab; Becton Dickinson and Company, Sparks, Md.) twice with the same swab on both sides, and the tube containing the swab and the coupon was vortexed at full speed for 30 s to remove all biofilm cells from the swab and the surface. We tried several other methods to remove the biofilm cells from the coupons, including shaking or vortexing with glass beads, vortexing, and sonication. The swab-vortex method gave the best removal from the surface and the highest number of CFU per square centimeter (results not shown). An appropriate dilution of the neutralized suspension was made in PPS, and the sample was enumerated by spiral plating on TSA immediately after dilution. In the original suspension test pour plates are used, but Langsrud and Sundheim (26) showed that the use of pour plates reduced the number of surviving S. aureus cells exposed to BAC significantly. The plates were incubated at 30°C, and the colonies were counted after 48 h. Killing experiments were done in quadruplicate and in a way that prevented bias in the results due to the position of the coupon in the culture container. The position of the coupon in the culture container might influence the amount of biofilm formed on the coupon or the physiological status of the cells. Therefore, the 20 coupons used for one killing experiment were divided into four groups (coupons 1 to 5, coupons 6 to 10, etc.). For each of the five treatments a coupon was taken at random from each of the four groups.
To kill suspended biofilm cells, cells were first removed from the surface and placed in 3 ml of demineralized water as described above, and then a small volume of concentrated disinfectant (or demineralized water for the control) was added. After 5 min, 27 ml of neutralizer was added (see above). Further analysis was done as described above for biofilm cells. Phase 1 test cells were grown and killed as prescribed by the phase 1 test as described previously (27). All data from the killing experiments were statistically analyzed with a paired Student's t test with two-tailed distribution and a 0.05 confidence level. The null hypothesis was that there was no significant difference between the viabilities of the cells that were treated differently. In addition, the biofilm killing results for most disinfectant concentrations were analyzed by calculating the repeatability standard deviation for the log reduction values (45); the exceptions were the concentrations that resulted in a more-than-5-log reduction in one or both independent experiments. The log reduction after exposure to a disinfectant concentration was calculated by subtracting the average log10 CFU per square centimeter for disinfectant-exposed biofilms from the average log10 CFU per square centimeter for control biofilms. The (repeatability) standard deviation was calculated by using the log reduction values obtained in the independent experiments.
RESULTS
In this study we developed a standard test to assess the efficacy of candidate disinfectants against S. aureus growing as a biofilm on glass. Besides glass (negatively charged), polystyrene (hydrophobic) and stainless steel (negatively charged) also supported the growth of biofilms. Biofilm formation, as determined by plate counting, did not differ significantly on the three types of surfaces (data not shown). We used glass because it is negatively charged, like stainless steel, which is used widely in the food industry. Furthermore, glass was used because it is easy to observe formation, removal, and staining of biofilms on this surface by phase-contrast microscopy.
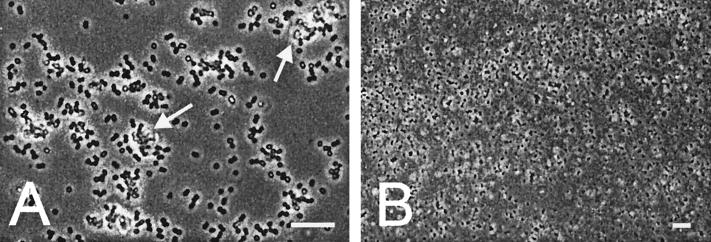
Cells growing as biofilms were characterized microscopically (Fig. 2). At a high magnification we observed single cells, diplococci, and big clumps of cells attached to the surface (Fig. 2A). The cells in the clumps were surrounded by yellowish material that represented extracellular polymeric substances. This was confirmed by staining the biofilm with the polysaccharide stain Congo red (results not shown). A lower magnification showed that the biofilm fully covered the glass surface over a wide area (Fig. 2B). This observation was confirmed by the calculated surface coverage, 60%. This percentage was calculated by using the average biofilm concentration (8.1 × 107 ± 4.4 × 107 CFU cm−2), the percentage of viable cells in the biofilm (92% ± 27%), and the average diameter of the biofilm cells (Table 1). To further characterize the biofilm cells, their diameters were compared to those of cells grown under various other conditions (Table 1). The longer the planktonic cells were grown, the smaller they became. This was true for anaerobically and aerobically grown cells. The diameters of biofilm cells were most comparable to the diameters of cells that were grown aerobically for 8 and 24 h in 1/10 TSB. Analysis of inflowing medium (influent) and outflowing waste (effluent) gave an indication of the nutrient supply and nutrient consumption in the biofilms (Table 2). At 24 h, the biofilm cells were consuming nearly all of the glucose present in the influent and converting it to the end products of S. aureus aerobic (acetic acid) and anaerobic (lactic acid) glucose metabolism at a proportion of 10 to 7. Acid formation was confirmed by the decrease in the pH of about 1 pH unit.
FIG. 2.
Light microscopic image of a 24-h S. aureus biofilm on glass after it was washed in PPS. Images were taken at magnifications of ×1,000 (A) and ×400 (B). Bars = 10 μm. The bright material accompanying the clumps of cells is extracellular polymeric substances (arrows).
TABLE 1.
Diameters of S. aureus cells cultured under a variety of conditions
| Cell growth | Medium | Growth conditions | Time (h) | Cell diam (μm)a |
|---|---|---|---|---|
| Planktonic | 1/10 TSB | Aerobic | 2.5 | 1.12 ± 0.12 |
| Planktonic | 1/10 TSB | Aerobic | 8 | 0.97 ± 0.13 |
| Planktonic | 1/10 TSB | Aerobic | 24 | 0.86 ± 0.12 |
| Agar (phase 1 test cells) | TSA | Aerobic | 24 | 1.08 ± 0.19 |
| Planktonic | 1/10 TSB | Anaerobic | 2.5 | 1.11 ± 0.13 |
| Planktonic | 1/10 TSB | Anaerobic | 8 | 1.04 ± 0.18 |
| Planktonic | 1/10 TSB | Anaerobic | 24 | Cell lysis |
| Biofilm | 1/10 TSB | Unknown | 24 | 0.93 ± 0.24 |
The values are averages ± standard deviations based on at least 50 cells.
TABLE 2.
Characteristic parameters of the influent and the effluent of the culture container after 24 h of continuous feeding with 1/10 TSBa
| Solution | Concn (mmol liter−1) of:
|
pH | ||
|---|---|---|---|---|
| Glucose | Acetic acid | Lactic acid | ||
| Influent | 1.2 | 0 | 0 | 7.5 |
| Effluent | 0.29 | 1.27 | 0.87 | 6.6 |
The values are averages for six experiments.
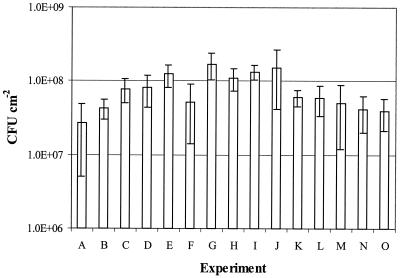
Figure 3 shows that the biofilm formed by using the new technique is reproducible. The concentrations in the biofilms formed in the 15 independent experiments were between 4 × 107 and 1.3 × 108 CFU cm−2. The error bars in Fig. 3 demonstrate that the biofilm concentrations on the different coupons in one experiment varied. To prevent errors in the results of the disinfectant tests due to these or other variations in biofilm formation, we used a robust statistical method for coupon sampling during disinfectant testing (see Materials and Methods).
FIG. 3.
Average concentrations of S. aureus in biofilms after 24 h for 15 independent experiments. The values are averages for at least four coupons. The error bars indicate standard deviations.
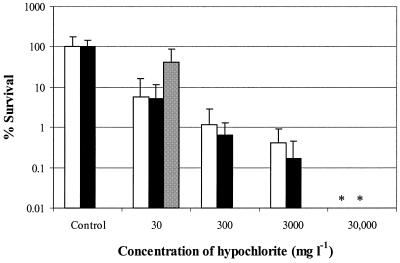
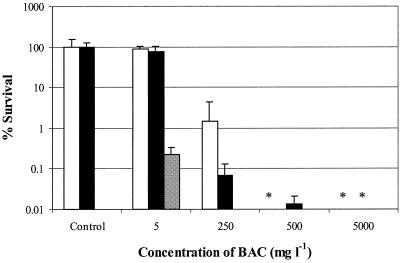
The results of the disinfectant tests are shown in Fig. 4 and 5. Both of these figures present the results of two separate biofilm experiments and a separate planktonic cell experiment. The levels of survival of biofilm cells and phase 1 test cells exposed to 30 mg of hypochlorite per liter did not differ significantly (1.2-, 1.3-, and 0.38-log reductions [Fig. 4]). To obtain a more-than-4-log reduction, a concentration of 30,000 mg of hypochlorite per liter was needed. For phase 1 cells, only 50 mg liter−1 was needed to obtain a more-than-5-log reduction (data not shown). The repeatability standard deviations for the log reduction values for 30, 300, and 3,000 mg of hypochlorite per liter were 0.98, 0.23, and 0.64, respectively. BAC at a concentration of 5 mg liter−1 had almost no effect on the viability of biofilm cells (0.041-log and 0.12-log reductions), whereas phase 1 test cells showed a 2.6-log reduction when they were exposed to the same concentration of BAC (Fig. 5). To obtain at least a similar reduction for biofilm cells, a BAC concentration of 250 mg liter−1 was needed. A concentration of 500 mg liter−1 gave almost a 4-log reduction. For phase 1 cells, only 10 mg liter−1 was needed to obtain a 5-log reduction (data not shown). The repeatability standard deviations for the log reduction values for 5 and 250 mg of BAC per liter were 0.11 and 0.23, respectively.
FIG. 4.
Survival of S. aureus biofilm cells after 5 min of exposure to 0, 30, 300, 3,000, and 30,000 mg of sodium hypochlorite per liter in two separate experiments (open and solid bars) and survival of S. aureus phase 1 test suspension cells exposed to 30 mg of sodium hypochlorite per liter (gray bar) (separate results, taken from reference 27). The values are averages for at least four coupons. The error bars indicate standard deviations. An asterisk indicates that the level of survival was below the detection limit (0.01%).
FIG. 5.
Survival of S. aureus biofilm cells after 5 min of exposure to 0, 5, 250, 500, and 5,000 mg of BAC per liter in two separate experiments (open and solid bars) and survival of S. aureus phase 1 test suspension cells exposed to 5 mg of BAC per liter (gray bar) (separate results, taken from reference 27). The values are averages for at least four coupons. The error bars indicate standard deviations. An asterisk indicates that the level of survival was below the detection limit (0.01%).
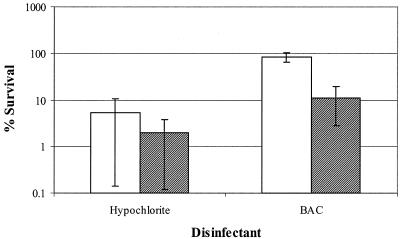
To find out whether attachment to the surface contributes to resistance, biofilm cell survival and suspended biofilm cell survival were determined (Fig. 6) Biofilm cell survival after exposure to hypochlorite did not differ from the survival of suspended biofilm cells. For biofilm cells exposed to BAC, survival was about eight times greater (0.88-log unit difference) than the survival of suspended biofilm cells.
FIG. 6.
Survival of S. aureus biofilm cells (open bars) and suspended biofilm cells (cross-hatched bars) after 5 min of exposure to 30 mg of sodium hypochlorite per liter or 5 mg of BAC per liter. The error bars indicate standard deviations. The experiment was performed in quadruplicate.
DISCUSSION
In this paper we propose a standard test to assess the activity of candidate disinfectants against S. aureus biofilm cells. To our knowledge, this is the first complete description of a standard biofilm test for disinfectants that are used for disinfection of food industry equipment. Our biofilm test meets all requirements of a standard test: the biofilm-forming system is very simple and relatively cheap, the test can be performed in 1 week, and all equipment and material is commercially available. Furthermore, the test is as similar as possible to the phase 1 suspension test to make comparison of the results to phase 1 test results easier, and the test is reproducible and repeatable within the limits set for the phase 1 test. The phase 1 test allows the concentration of cells to be tested to vary between 1.5 × 108 and 5 × 108 CFU ml−1 (a 3.3-fold difference in cell concentration). Our results are within this limit. In the context of standardized biofilm formation, Jackson et al. (24) found a 50-fold difference in biofilm cell concentration for mixed biofilms of Pseudomonas fluorescens, Pseudomonas aeruginosa, and Klebsiella pneumoniae. When they used a specific number of viable cells, the difference was reduced to fivefold. Ceri et al. (15) found an approximately threefold difference in biofilm cell concentrations for P. aeruginosa. The reproducibility of disinfection in this test was the same as that found for the phase 1 suspension test (27). Zelver et al. (45) found in a literature survey of standard antimicrobial suspension and dried surface tests that the repeatability standard deviation of log reduction values varied between 0.2 and 1.2. They found a repeatability standard deviation of 0.66 for P. aeruginosa biofilms exposed to chlorine (45). Our results showed similar values (0.11 to 0.98).
The following criterion was used for a candidate disinfectant to pass the test: more than a 4-log reduction in 5 min in a biofilm with a cell concentration (4 × 107 to 1.3 × 108 CFU cm−2) that falls within the 3.3-fold variation allowed in the current suspension tests. Other authors (43) have proposed that for a biofilm test only a 3-log reduction is necessary, but this is too small a reduction for biofilms which can contain up to 1.3 × 108 CFU cm−2.
In addition to the general requirements for a disinfectant test, the specific requirements for a biofilm test are also met by our test. To obtain firmly attached biofilm cells, like those that occur under practical conditions, we used a system for biofilm formation with constant shear stress. Furthermore, the biofilm cells were supplied with a continuous flow of relatively poor medium (1/10 TSB). In this system S. aureus formed a genuine biofilm (37) after 24 h as clumps of cells with extracellular material could be observed. The number of cells on the surface was quite high. Other authors found S. aureus concentrations in biofilms that were between 5 × 106 and 8 × 107 CFU cm−2 after 24 to 48 h of incubation in batch systems in rich media at temperatures ranging from 35 to 37°C (2, 22, 30). Other characteristics of the biofilm cells were that after 24 h they did not have enough oxygen to grow completely aerobically but were not glucose limited. The biofilm cells were quite large (diameter, 0.93 μm). Several authors observed smaller cells (diameters, 0.5 to 0.7 μm) in S. aureus biofilms after 12 to 24 h (30, 41, 46). This might have been caused by growth of the biofilm cells in a batch system in which nutrient limitation and the accumulation of waste start much earlier than they do in a continuous-flow system. The size of planktonic cells appeared to be related to the growth rate, as was previously described by Williams et al. (41). These authors concluded that this was also true for biofilm cells. We found that biofilm cell size was most similar to the size of aerobically grown cells in the late logarithmic phase. The large variation in size indicated that there was variety in the growth phases of the biofilm cells.
The biofilm cells in our proposed test appear to be much less susceptible to disinfectants than phase 1 test cells, especially at the concentration needed to reduce the viability of the cells more than 4 log units. Furthermore, our results show that the increased resistance of biofilm cells is only partially caused by attachment to a surface. Other factors that may be responsible for the increased resistance of the biofilm cells are the presence of extracellular polymeric substances, the different physiology of the biofilm cells due to attachment and quorum sensing, and the variation in the physiologies of the biofilm cells due to variations in the growth phase and oxygen concentration. It is known that S. aureus biofilm cells are more resistant to antibiotics than free-living cells (2, 6, 42). Oie et al. (30) showed that that this is also true for disinfectants. They exposed 24-h biofilms of methicillin-resistant S. aureus grown in TSB on silicone disks in a batch system to several disinfectants. To obtain more than a 4-log reduction in biofilm cell viability in 10 min, 10,000 mg of BAC per liter or 1,000 mg of hypochlorite per liter was needed. Some preliminary results of the surface test that is being developed for phase 2 step 2 (25) show that in this test about 100 times more BAC is needed to achieve the same killing level for surface test cells as for phase 1 test cells; these results are comparable to our results. However, for hypochlorite only 20 times more disinfectant is needed to achieve the same killing level, whereas for our biofilm test cells we needed 600 times more hypochlorite. Thus, a standard biofilm test is a useful addition to the current standard tests. We propose that the test could replace the phase 2 step 1 suspension test or be performed as a replacement for or an addition to the phase 2 step 2 tests.
In conclusion, we have developed a standard biofilm test that confirms that biofilm cells are less susceptible to disinfectants than suspension test cells. This test may be used with other bacteria, such as P. aeruginosa. This test not only may help to better predict the efficacy of a disinfectant in practice but may also help researchers find new disinfectants or to select existing disinfectants that are particularly effective against biofilms, since now candidate disinfectants are not tested on biofilm cells.
Acknowledgments
This work was supported by a grant from the Dutch Soap Association (NVZ).
We thank Birgit Hasenack for helping with the HPLC analysis and Christine Bunthof, Wilma Hazeleger, and Gilma Chitarra for help with the killing experiments.
REFERENCES
- 1.Adams, J. L., and R. J. C. McLean. 1999. Impact of rpoS deletion on Escherichia coli biofilms. Appl. Environ. Microbiol. 65:4285-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama, H., O. Yamasaki, H. Kanzaki, J. Tada, and J. Arata. 1998. Effects of sucrose and silver on Staphylococcus aureus biofilms. J. Antimicrob. Chemother. 42:629-634. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1997. Chemical disinfectants and antiseptics-hygienic handwash—test method and requirement (phase 2, step 2). EN-1499. Nederlands Normalistatie-Instituut, Delft, The Netherlands.
- 4.Anonymous. 1997. Chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas—test method and requirement (phase 2, step 1). EN-1276. Nederlands Normalistatie-Instituut, Delft, The Netherlands.
- 5.Anonymous. 1997. Chemical disinfectants and antiseptics—basic bactericidal activity—test method and requirement (phase 1). EN-1040. Nederlands Normalistatie-Instituut, Delft, The Netherlands.
- 6.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Eradication of biofilm cells of Staphylococcus aureus with tobramycin and cephalexin. Can. J. Microbiol. 38:618-625. [DOI] [PubMed] [Google Scholar]
- 7.AOAC International. 1995. AOAC official method 991.47. Testing disinfectants against Salmonella choleraesuis. Hard surface carrier test method. Official methods of analysis of AOAC International. AOAC International, Arlington, Va.
- 8.AOAC International. 1995. AOAC official method 991.48. Testing disinfectants against Staphylococcus aureus. Hard surface carrier test method. Official methods of analysis of AOAC International. AOAC International, Arlington, Va.
- 9.AOAC International. 1995. AOAC official method 991.49. Testing disinfectants against Pseudomonas aeruginosa. Hard surface carrier test method. Official methods of analysis of AOAC International. AOAC International, Arlington, Va.
- 10.AOAC International. 1995. Official methods of analysis of AOAC International. AOAC International, Arlington, Va.
- 11.Becker, P., W. Hufnagle, G. Peters, and M. Herrmann. 2001. Detection of differential gene expression in biofilm-forming versus planktonic populations of Staphylococcus aureus using micro-representational-difference analysis. Appl. Environ. Microbiol. 67:2958-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloomfield, S. F., M. Arthur, B. V. Klingeren, W. Pullen, J. T. Holah, and R. Elton. 1994. An evaluation of the repeatability and reproducibility of a surface test for the activity of disinfectants. J. Appl. Bacteriol. 76:86-94. [DOI] [PubMed] [Google Scholar]
- 13.Brooun, A., S. Liu, and K. Lewis. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpentier, B., and O. Cerf. 1993. Review: biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 75:499-511. [DOI] [PubMed] [Google Scholar]
- 15.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke, E. M. 1990. Epidemiology of foodborne illness: UK. Lancet 336:790-793. [DOI] [PubMed] [Google Scholar]
- 18.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 45:711-745. [DOI] [PubMed] [Google Scholar]
- 19.Das, J. 1996. The effect of attachment to surfaces on bacterial susceptibility to biocides. Ph.D. thesis. University of Manchester, Manchester, United Kingdom.
- 20.Das, J. R., M. Bhakoo, M. V. Jones, and P. Gilbert. 1998. Changes in the biocide susceptibility of Staphylococcus epidermidis and Escherichia coli cells associated with rapid attachment to plastic surfaces. J. Appl. Microbiol. 84:852-858. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert, P., D. G. Allison, D. J. Evans, P. S. Handley, and R. W. Brown. 1989. Growth rate control of adherent bacterial populations. Appl. Environ. Microbiol. 55:1308-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracia, E., A. Fernández, P. Conchello, J. L. Alabart, M. Pérez, and B. Amorena. 1999. In vitro development of Staphylococcus aureus biofilms using slime-producing variants and ATP-bioluminescence for automated bacterial quantification. Luminescence 14:23-31. [DOI] [PubMed] [Google Scholar]
- 23.Härkönen, P., S. Salo, T. Matilla-Sandholm, G. Wirtanen, D. G. Allison, and P. Gilbert. 1999. Development of a simple in vitro test system for the disinfection of bacterial biofilms. Water Sci. Technol. 39:219-225. [Google Scholar]
- 24.Jackson, G., H. Beyenal, W. M. Rees, and Z. Lewandowski. 2001. Growing reproducible biofilms with respect to structure and viable cell counts. J. Microbiol. Methods 47:1-10. [DOI] [PubMed] [Google Scholar]
- 25.Klingeren, B. V., W. Koller, S. F. Bloomfield, R. Böhm, A. Cremieux, J. Holah, R. Reybrouck, and H. J. Rödger. 1998. Assessment of the efficacy of disinfectants on surfaces. Int. Biodeterior. Biodegrad. 41:289-296. [Google Scholar]
- 26.Langsrud, S., and G. Sundheim. 1998. Factors influencing a suspension test method for antimicrobial activity of disinfectants. J. Appl. Microbiol. 85:1006-1012. [DOI] [PubMed] [Google Scholar]
- 27.Luppens, S. B. I., F. M. Rombouts, and T. Abee. 2002. The effect of the growth phase of Staphylococcus aureus on resistance to disinfectants in a suspension test. J. Food Prot. 65:124-129. [DOI] [PubMed] [Google Scholar]
- 28.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monzon, M., C. Oteiza, J. Leiva, and B. Amorena. 2001. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 48:793-801. [DOI] [PubMed] [Google Scholar]
- 30.Oie, S., Y. Huang, A. Kamiya, H. Konishi, and T. Nakazawa. 1996. Efficacy of disinfectants against biofilm cells of methicillin-resistant Staphylococcus aureus. Microbios 85:223-230. [PubMed] [Google Scholar]
- 31.Payne, D. N., J. R. Babb, and C. R. Bradley. 1999. An evaluation of the suitability of the European suspension test to reflect in vitro activity of antiseptics against clinically significant organisms. Lett. Appl. Microbiol. 28:7-12. [DOI] [PubMed] [Google Scholar]
- 32.Payne, K. R. 1988. Industrial biocides, vol. 23. Society of Chemical Industry, John Wiley & Sons, Chichester, United Kingdom.
- 33.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reybrouck, G. 1998. The testing of disinfectants. Int. Biodeterior. Biodegrad. 41:269-272. [Google Scholar]
- 35.Todd, E. C. D. 1992. Foodborne disease in Canada—a 10-year summary from 1975 to 1984. J. Food Prot. 55:123-132. [DOI] [PubMed] [Google Scholar]
- 36.Vroom, J. M., K. J. D. Grauw, H. C. Gerritsen, D. J. Bradshaw, P. D. Marsh, G. K. Watson, J. J. Birmingham, and C. Allison. 1999. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl. Environ. Microbiol. 65:3502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watnick, P., and R. Kolter. 2000. Minireview: biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wentland, E. J., P. S. Stewart, C. T. Huang, and G. A. McFeters. 1996. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 12:316-321. [DOI] [PubMed] [Google Scholar]
- 39.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 40.Willcock, L., P. Gilbert, J. Holah, G. Wirtanen, and D. G. Allison. 2000. A new technique for the performance evaluation of clean-in-place disinfection of biofilms. J. Ind. Microbiol. Biotechnol. 25:235-241. [Google Scholar]
- 41.Williams, I., F. Paul, D. Lloyd, R. Jepras, I. Critchley, M. Newman, J. Warrack, T. Giokarini, A. J. Hayes, P. F. Randerson, and W. A. Venables. 1999. Flow cytometry and other techniques show that Staphylococcus aureus undergoes significant physiological changes in the early stages of surface-attached culture. Microbiology 145:1325-1333. [DOI] [PubMed] [Google Scholar]
- 42.Williams, I., W. A. Venables, D. Lloyd, F. Paul, and I. Critchley. 1997. The effects of adherence to silicone surfaces on antibiotic susceptibility in Staphylococcus aureus. Microbiology 143:2407-2413. [DOI] [PubMed] [Google Scholar]
- 43.Wirtanen, G. 1995. Biofilm formation and its elimination from food processing equipment. Ph.D. thesis. Helsinki University of Technology, Helsinki, Finland.
- 44.Wright, T. L., R. P. Ellen, J. M. Lacroix, S. Sinnadurai, and M. W. Mittelman. 1997. Effects of metronidazole on Porphyromonas gingivalis biofilms. J. Periodontal Res. 32:473-477. [DOI] [PubMed] [Google Scholar]
- 45.Zelver, N., M. Hamilton, D. Goeres, and J. Heersink. 2001. Development of a standardized antibiofilm test. Methods Enzymol. 337:363-376. [DOI] [PubMed] [Google Scholar]
- 46.Zoltai, P. T., E. A. Zottola, and L. L. McKay. 1981. Scanning electron microscopy of microbial attachment to milk contact surfaces. J. Food Prot. 44:204-208. [DOI] [PubMed] [Google Scholar]