Abstract
Porins allow exchanges between bacteria and their environment. In the gram-negative food-borne pathogen Campylobacter jejuni two porins, major outer membrane protein (MOMP) and Omp50, have been identified. MOMP is synthesized at a very high level under laboratory culture conditions, suggesting that its promoter functions very efficiently under these conditions. In Campylobacter samples, we observed that MOMP porin expression increased at a high temperature (42°C) or a high pH (pH 8.5) compared to expression at a low temperature (31°C) or an acidic pH (pH 5.5). To study the regulation of MOMP expression at the transcriptional level, we constructed an momp-gfp fusion in which gfp expression was put under the control of the momp promoter. Interestingly, we observed the same pattern of regulation in Escherichia coli, as monitored by green fluorescent protein production, that was found in Campylobacter. The ranges of pH and temperature tested are physiologically relevant, because they can be found in the digestive tracts of both birds and humans, which are both colonized by Campylobacter. Our results suggest that a component of the regulatory mechanism is conserved in C. jejuni and E. coli. However, medium osmolarity and sodium salicylate did not have a significant effect on C. jejuni momp promoter activity in E. coli, suggesting that major regulatory elements of E. coli porin expression do not participate in MOMP regulation. In contrast, mechanisms involving DNA supercoiling may be involved, as shown by DNA gyrase inhibition assays. These findings are a step towards determining the role of outer membrane proteins in the adaptation of C. jejuni to its environment.
The gram-negative bacterium Campylobacter jejuni is a commensal organism of many animal species, particularly birds (32). It is one of the major causes of human enteritis in developed and developing countries (36, 37). One of its main reservoirs is the chicken digestive tract, and most human C. jejuni infections in industrialized countries are acquired by eating undercooked poultry and, to a lesser extent, by consuming contaminated unpasteurized milk and water. Because C. jejuni can colonize such different environments, it is thought that this bacterium must be able to adapt its behavior in response to external conditions.
Much is known about how bacteria respond to different media, and one way that they respond is to regulate membrane permeability. In gram-negative bacteria, porins are outer membrane proteins which govern the exchanges between bacteria and their environment. These pore-forming proteins allow hydrophilic molecules to cross the outer membrane. OmpF and OmpC of Escherichia coli are the most-studied models with regard to the regulation of porin expression, and many regulatory systems are well characterized; the EnvZ-OmpR two-component regulatory system (23) and the marRAB operon (3) both regulate E. coli major porin expression. For these porins, osmoregulation was initially described (21), but pH and temperature have since been shown to modify their expression (2, 38); more recently, new mechanisms for porin regulation in response to pH and temperature have been described (13, 31). Moreover, mechanisms that include promoter regulation by variation in DNA supercoiling have been described for E. coli OmpF (15).
Two porins have been characterized for C. jejuni: major outer membrane protein (MOMP) (6, 17, 24) and, more recently, Omp50 (7). MOMP is a large-channel porin (11) that must favor large exchanges of solutes across the outer membrane. This porin is produced at very high levels under the laboratory culture conditions tested to date, as assessed by the pseudocrystalline network visualized in electron micrographs of intact outer membrane fragments (1). However, high membrane permeability can be unfavorable for bacteria in hostile circumstances, such as a medium containing toxic agents, detergents, or antibiotics. Omp50, which is a smaller-channel porin (7), may be an alternative to MOMP, serving to decrease membrane permeability while allowing a sufficient level of nutrient uptake.
Our knowledge of the mechanisms governing the regulation of gene expression in Campylobacter and their responses to the environment is very limited. Although several studies have demonstrated that Campylobacter species are able to respond to various stresses, such as iron limitation or heat (25), the influence of the environment on both the structure and the function of the outer membrane has not been clearly determined to date. For Campylobacter species, protein expression studies under different growth conditions have given controversial results for porins. Heat shock and alkaline pH were found to stimulate MOMP expression (41), but later studies on thermoregulation did not confirm these results (8, 18). Moreover, study of MOMP expression in Campylobacter involving slight modifications in the composition of the medium is difficult due to the stringent growth condition requirements of the organism.
In order to study MOMP regulation, we constructed a gene fusion between the momp promoter region and the gfp gene coding for the green fluorescent protein (GFP). The GFP chromophore is very useful as a reporter for gene expression and offers several advantages (10, 27): (i) it exhibits low toxicity; (ii) it is continuously synthesized, so that there is no dilution of the fluorescence signal during bacterial replication; and (iii) it can be easily imaged and quantified and has already been successfully used with members of a number of bacterial genera, including E. coli (9), as well as Yersinia, Salmonella, and Mycobacterium species (39). Moreover, studies in which differential fluorescence induction (40) was used to monitor the regulatory effects of growth conditions on gene expression have recently been described (5, 20, 28).
Despite numerous studies of MOMP, little is known about its regulation. Analyses of the synthesis of MOMP under different culture conditions are important to our understanding of how this protein is involved in the adaptation of C. jejuni to its environment. To this end, we constructed an momp-gfp gene fusion to monitor momp promoter activity. In this study, we experimented with this gene fusion in E. coli. Interestingly, we demonstrated that as in C. jejuni, the momp promoter is regulated by growth conditions, such as pH and temperature. Therefore, we investigated the putative involvement of the known mechanisms of E. coli porin regulation and of DNA topology, which is a regulatory mechanism conserved between species. Our results show that the momp promoter does not respond to general E. coli porin regulatory pathways but that its regulation does involve DNA supercoiling.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemicals.
Strains and plasmids used in this study are described in Table 1. Campylobacter strains were routinely cultivated at 42°C under microaerophilic conditions (5% O2, 10% CO2, 85% N2) on Columbia agar or Mueller-Hinton agar (MH) supplemented or not supplemented with 5% sheep blood (Bio-Mérieux). For protein expression, Campylobacter strains were cultivated until the exponential growth phase at 32, 37, and 42°C in MH or MH buffered from pH 5.5 to 8 with sodium phosphate buffer. E. coli strains were cultivated at 37°C in Luria broth (LB) under aerobic conditions. For regulation assays, E. coli strains were grown in LB with the same modifications of pH and temperature. Where indicated below, isopropyl-beta-d-thiogalactopyranoside (IPTG) (50 μg/ml), salicylate (5 mM), NaCl (300 mM), and novobiocin (final concentration, 5, 10, or 20 μg/ml) were used. The pUC18 cloning vector, the Klenow fragment of DNA polymerase, T4 DNA ligase, alkaline phosphatase, and restriction endonucleases were purchased from New England Biolabs and used as recommended by the manufacturer. The pGFP cloning vector, in which GFP expression is under control of the lac promoter, was purchased from Clontech Laboratories, Inc. (Palo Alto, Calif.) and was used as a standard. This plasmid was also used as a coding sequence source for momp-gfp fusion, giving the pBDES1 plasmid constructed in this study. Unless specified otherwise, chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.).
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| TG1 | E. coli cloning strain | 30 |
| TG1-GFP | TG1 with pGFP | This study |
| TG1-S1-GFP | TG1 with pBDES1 | This study |
| 79AH | C. jejuni nonpathogenic isolate | 14 |
| 85H | C. jejuni pathogenic isolate | 14 |
| Plasmids | ||
| pUC18 | Ampr, cloning vector | 42 |
| pGB43 | Ampr, pUC 18 cut with HindIII, contains HindIII 720-bp fragment of momp promoter | 19 |
| pGFP | Ampr, cloning vectors, lac promoter-gfp fusion, also used as fluorescent standard | Clontech |
| pBDES1 | Ampr, pGFP cut with HindIII-XbaI, contains HindIII-SpeI 490-bp fragment of pGB43 | This study |
Construction of momp promoter-gfp fusions.
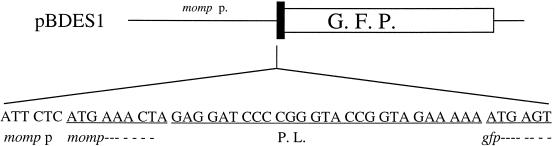
pUC18 (9) was used as a vector for cloning a previously described HindIII 720-bp fragment of the C. jejuni 85H MOMP promoter (19), giving plasmid pGB43 (Table 1). The pGFP cloning vector was then used to clone a 490-bp HindIII-SpeI fragment of the 720-bp momp promoter sequence, giving plasmid pBDES1 (Table 1 and Fig. 1).
FIG. 1.
momp-gfp fusion construct. pBDES1 contains the 478-bp fragment of the 85H momp promoter sequence (momp p) and the sequence coding for the two N-terminal amino acids of MOMP inserted in the pGFP polylinker (P.L.) upstream of the gfp gene.
Bacterial transformation procedures.
E. coli TG1 was transformed by using the calcium chloride protocol (4). Competent cells (100 μl) were transformed with 10 ng of DNA and selected with the appropriate antibiotics. For plasmid-carrying strains, tetracycline (10 μg/ml), ampicillin (100 μg/ml), or kanamycin (50 μg/ml) was used.
Protein analysis.
C. jejuni strains 85H and 79AH (14) were cultivated under different conditions, and electrophoresis was performed by using a standard sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) protocol, as previously described (6). Protein profiles of these two strains are presented in this paper in order to show the most separated signal obtained for MOMP under various growth conditions. For immunodetection assays, previously described polyclonal rabbit antibodies were used for MOMP (6) and Omp50 (7). Polyclonal GFP antibodies were kindly provided by L. F. Wu (UPR 9043, CNRS, Marseilles, France). Monoclonal OmpF and OmpC antibodies have been described previously (12).
Fluorescence microscopy.
An overnight culture of TG1 harboring plasmids was diluted 1:100 in LB supplemented with IPTG and ampicillin and grown at 32 and 37°C until the A600 reached 0.7. Cells were centrifuged, resuspended in phosphate-buffered saline, and immediately examined with a Zeiss PhoMi III fluorescence microscope equipped with a filter set for fluorescein isothiocyanate. Images were captured by using a charge-coupled device camera (Color Cool View; Photonic Sciences) and image Pro-Plus software.
Fluorescence spectrometry.
The fluorescence levels in crude extracts were quantified by using a Spex Fluorolog III fluorimeter equipped with a cooled detector (Jobin-Yvon) for GFP detection. A minimum of three independent assays was performed for each strain under each set of conditions.
RESULTS
C. jejuni MOMP porin expression under different growth conditions.
During a previous study of MOMP porin purification, we obtained better yields when bacteria were cultivated at temperatures above 40°C and pH 7.5 (Bolla et al., unpublished data). The overproduction suggested that the momp promoter might be upregulated under these conditions and should therefore be downregulated under other conditions encountered in the environment. In order to further investigate this hypothesis, C. jejuni strain 79H was cultivated under different growth conditions, and total protein fractions were analyzed by electrophoresis and Coomassie blue staining. C. jejuni strains grow at a minimum temperature of 32°C and a maximum temperature of 44°C. We decided to experiment at temperatures ranging from 32 to 42°C. We observed that MOMP expression increased twofold when the growth temperature was increased (Fig. 2A). Signal specificity was confirmed by immunodetection assays (Fig. 2B). In addition, we observed that at 42°C there was induction of a 64-kDa heat shock protein, as previously described by Wu et al. (41). In order to test the effect of pH on MOMP expression, C. jejuni strain 85H was cultivated at 32°C in different media having pH values ranging from 5.5 to 8.5. Electrophoresis profiles were compared after Coomassie blue staining. We observed that MOMP expression increased between two- and threefold at more alkaline pH values (Fig. 2C). This result was confirmed by immunodetection with a specific antibody (Fig. 2D). The 64-kDa protein was also an alkaline-induced protein (41). A comparison of the protein profile of bacteria cultivated at an acidic pH and 32°C with the protein profile of bacteria cultivated at an alkaline pH and 42°C revealed the greatest variations (data not shown).
FIG. 2.
Porin expression analysis. (A) Coomassie blue-stained SDS-PAGE profile from extracts of strain 79AH cultivated at neutral pH at 32, 37, and 42°C. (B) Western blot with MOMP antibodies. (C) Coomassie blue-stained SDS-PAGE profile of extracts of strain 85H cultivated at 32°C at pH 5.5, 7, and 8.5. (D) Western blot with MOMP antibodies.
For thermal regulation, many gene regulatory circuits act at the transcriptional level. Furthermore, by analogy with the regulation of porin synthesis in E. coli, the C. jejuni MOMP porin would be expected to be regulated at several stages in the expression process, including transcription, which we initially analyzed in E. coli using GFP as a reporter gene.
Monitoring momp promoter-gfp fusion expression.
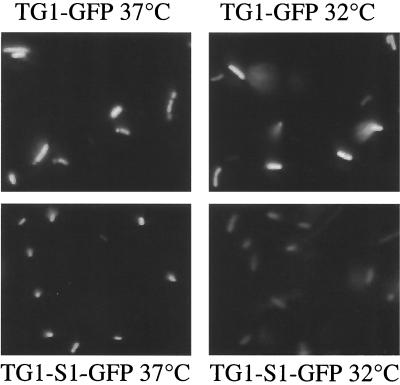
To monitor momp promoter activity, we constructed an momp-gfp gene fusion (as described in Materials and Methods). Expression of the gene fusion resulted in S1-GFP, which is a GFP with two amino acids of the MOMP signal sequence at the N terminus (Fig. 1). We observed that the momp promoter sequence induced strong GFP expression in E. coli (Fig. 3). We compared the fluorescence of the TG1-S1-GFP strain with that of the fluorescence control strain TG1-GFP under different culture conditions. In a preliminary screening, direct observation of bacterial colonies under UV illumination detected differences in the level of fluorescence (data not shown). To monitor GFP fluorescence emission at the cellular level, bacteria in the exponential growth phase were observed with a fluorescence microscope. As shown in Fig. 3, TG1-GFP, producing GFP under the control of the lac promoter, showed the same fluorescence intensity at 32 and 37°C, while the TG1-S1-GFP strain fluorescence significantly decreased when the organism was cultivated at 32°C. We also determined that in the same culture all bacteria in the same focal plane were equally fluorescent. A high GFP expression level was found at 42°C by immunodetection (data not shown). However, fluorescence intensity was not measured because GFP is known to misfold at this temperature (16).
FIG. 3.
Comparison of fluorescence intensities of bacterial populations at different growth temperatures as determined by fluorescence microscopy. The positive control (TG1-GFP) and the momp-gfp fusion (TG1-S1-GFP) grown at 32 and 37°C were compared.
Quantification of fluorescence intensity.
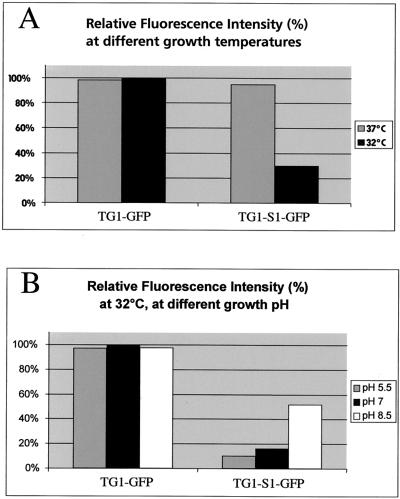
In order to quantify fluorescence intensity as a function of growth temperature, we performed spectrofluorimetric assays. The TG1-GFP and TG1-S1-GFP strains were cultivated at 32 or 37°C, and the concentrations of bacteria were adjusted to the same value by measuring the optical density at 600 nm. Fluorescent emission spectra were then measured, as described in Materials and Methods. For the TG1-S1-GFP strain, growth at 32°C decreased fluorescent expression by approximately threefold compared to the expression at 37°C, while TG1-GFP fluorescence was not significantly altered under the same conditions (Fig. 4A). The experiment was performed with the same strains cultivated at 32°C in media with pH values ranging from 5.5 to 8.5 (Fig. 4B). We had already found that at 32°C the fluorescence intensity of S1-GFP was lower than that of control GFP, and in this experiment we observed that a decrease in the growth medium pH from pH 8.5 to 5.5 decreased the S1-GFP fluorescence intensity by a factor of approximately 3 (Fig. 4B). The maximum differences were observed between pH 7 and 8.5. In contrast to the differences at different temperatures, no differences in GFP folding at different pH values have been reported to date, and our controls gave stable values under the conditions described here.
FIG. 4.
Effect of environmental conditions on MOMP-GFP expression as measured by spectrofluorimetry. (A) Effect of the growth temperature at neutral pH. (B) Effect of the growth pH at 32°C.
Mechanism of MOMP porin regulation in E. coli.
momp-gfp fusion expression was regulated in E. coli, and we investigated whether the major regulatory elements of E. coli porins were involved. In E. coli, sodium salicylate interacts with the marRAB operon, activating micF antisense mRNA, which decreases the OmpF expression level (29). In the same way, medium osmolarity influences the expression of E. coli major porins via the EnvZ-OmpR two-component regulatory system, which also acts through micF (21). The TG1-GFP and TG1-S1-GFP strains were cultivated with or without sodium salicylate or in media with various osmolarities (150 and 300 mM NaCl) for different periods of time, and immunodetection assays were performed by using OmpF-, OmpC-, and GFP-specific antibodies. We observed that neither the addition of sodium salicylate (Fig. 5) nor an increase in osmolarity (data not shown) had an effect on S1-GFP synthesis, while the expression of the E. coli major OmpC and OmpF porins, analyzed as a control, was downregulated. These results indicate that the micF cascade does not regulate S1-GFP expression, in agreement with the absence of sequence homology between micF or other E. coli regulatory sequences and momp promoter sequences. Moreover, these results suggest that neither E. coli EnvZ-OmpR nor the marRAB operons were able to regulate the momp promoter and that another mechanism must be involved. No fluorescence assays were performed, because sodium salicylate exhibits a fluorescent signal under UV light.
FIG. 5.
Effect of sodium salicylate on E. coli major porin expression and on S1-GFP expression. Immunodetection was performed by using OmpF (A), OmpC (B), and GFP (C) antibodies. TG1-GFP and TG1-S1-GFP were cultivated in LB until the A600 reached 0.2, and 5 mM sodium salicylate was added. Samples were collected at different times, as follows: lanes 1, controls; lanes 2, 1 h; lanes 3, 2 h; lanes 4, 3 h.
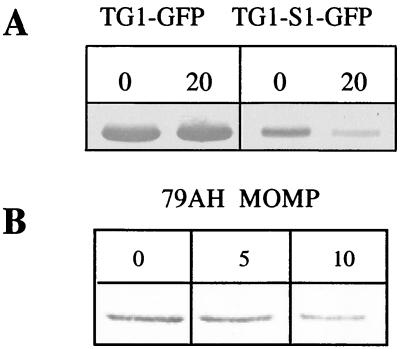
C. jejuni and E. coli are very different bacteria. Nevertheless, we observed the same pattern of regulation for MOMP in both bacteria and demonstrated that the E. coli major porin regulatory pathways do not efficiently regulate expression of C. jejuni momp. Therefore, we investigated whether the degree of DNA superhelicity variation, which is a highly conserved mechanism of regulation in different bacterial species and genera, could be involved. To address this question, we tested the effect of a DNA gyrase inhibitor on GFP production by TG1-GFP and TG1-S1-GFP and performed immunodetection with GFP antibodies. The antibiotic concentration used had no effect on the growth rate. We observed that a sublethal novobiocin concentration decreased S1-GFP production (Fig. 6A) and had no effect on control GFP production. In order to check MOMP regulation by DNA supercoiling in C. jejuni, we performed DNA gyrase inhibition assays with C. jejuni cultures and analyzed MOMP production by immunodetection assays. To perform DNA gyrase inhibition assays without altering bacterial growth, we had to use antibiotic concentrations fourfold lower than the MIC. In E. coli, we tested novobiocin at a concentration of 20 μg/ml, which is a suitable sublethal concentration. C. jejuni is more sensitive than E. coli to novobiocin, so we used lower concentrations. We observed almost no effect at a concentration of 5 μg/ml and a twofold decrease in MOMP production at a concentration of 10 μg/ml (Fig. 6B). Our results showed that changes in DNA topology regulate momp promoter activity.
FIG. 6.
Effect of DNA gyrase inhibitor. (A) Effect on momp-gfp expression. TG1-GFP and TG1-S1-GFP were cultivated in medium containing sublethal concentrations of novobiocin (0 and 20 μg/ml). (B) Effect on MOMP production by C. jejuni 79AH cultivated with novobiocin (0, 5, and 10 μg/ml).
DISCUSSION
Porins allow exchanges between gram-negative bacteria and their environment. As has been shown for the well-studied OmpC and OmpF porins of E. coli, bacteria change their porin synthesis in response to environmental changes, such as changes in osmotic pressure, pH, temperature, and nutrient concentration. The MOMP porin of C. jejuni is one of the most extensively studied proteins in this species (6, 17, 24, 43). Several functions have been assigned to this protein, including a structural role and the crucial pore-forming activity that allows hydrophilic molecules to cross the outer membrane barrier.
We observed that Campylobacter MOMP porin expression increased if the pH or the temperature increased. In order to study MOMP porin promoter regulation, we constructed a gene fusion using gfp as a reporter gene. Here we report analysis of the expression of this chimeric gene in E. coli under various culture conditions. The pBDES1 construct expresses the S1-GFP protein and allowed us to investigate the regulation of MOMP at the transcriptional level. The S1-GFP-producing strain showed a high level of GFP expression, with strong fluorescence of bacterial colonies under UV light. This result confirmed that the C. jejuni MOMP promoter has strong activity in E. coli. Of the numerous parameters tested, we found that pH and temperature regulated GFP expression under the control of the momp promoter. Our results showed that regulation most probably occurs at the transcriptional level and suggested that similar regulatory systems exist in Campylobacter and E. coli. There could be a domain in the momp promoter which is recognized by an E. coli regulatory component or a highly conserved regulatory mechanism, such as variation in DNA superhelicity.
In order to characterize this regulatory response, we tested the conditions that regulate E. coli major porin expression. The regulatory cascades are complex, and several pathways have been described (2, 3, 21, 23, 29, 31, 38). If sodium salicylate and medium osmolarity had had the same effect on both E. coli major porins and S1-GFP fusion, this would have suggested that the regulatory systems involved had a role in the regulation of our fusion construct. However, we observed repression of OmpF with no effect on S1-GFP, suggesting that other regulatory systems are involved. Our results showed that the E. coli major porin regulatory elements, EnvZ-OmpR, the marRAB operon, and micF, had no effect on the momp promoter sequence. Several regulatory systems may be involved. As in temperature-regulated S1-GFP expression, other regulators, such as StpA or another H-NS-like protein, could be involved, as has been shown for E. coli (13). The published complete C. jejuni NCTC 11168 genome sequence suggests that a small number of putative regulatory elements may be involved in the regulation of gene expression in response to various environmental changes (26). In addition, in the C. jejuni genome sequence, we found no homology to any of the large number of genes that are known to regulate porin expression in other species.
Moreover, the fact that we did not observe any regulation of MOMP after the osmolarity or the carbon source in the medium was modified suggested that there are large differences between E. coli porin regulation and C. jejuni porin regulation. The mechanism that is most probably conserved in such different genera is DNA supercoiling. DNA gyrase inhibition assays showed that DNA topology variations regulate momp promoter activity. We observed a greater effect on a multicopy plasmid than on the simple chromosomal gene copy. This mechanism has been found to regulate OmpF expression in E. coli, which is also regulated by growth temperature. The same mechanism was found in Enterobacter and in Pseudomonas for the fleQ gene, which is involved in motility (34), and also for a flagellin gene, flaB, in a microorganism relatively similar to C. jejuni, Helicobacter pylori (35). However, this type of mechanism has not been described to date for a Campylobacter promoter sequence.
We observed that MOMP was regulated by pH and temperature. In contrast to regulation of porins by osmolarity, oxidative stress, detergents, ethanol, medium compounds, and antibiotics, little is known about the thermoregulation of porins in E. coli. Thermoregulation may act as a signal for bacteria when they pass from the external environment into a host (33). Our results indicated that there was environmental regulation of MOMP expression by medium, pH, and temperature. Expression increased at higher pH values and temperatures, reaching a maximum at the physiological values in its natural ecological location, the digestive tract of chickens. These results agreed with the hypothesis that large exchanges between bacteria and medium are possible in favorable conditions. First, Campylobacter colonizes the intestines but not the stomach or the acidic vacuoles in eucaryotic cells, where the pH is lower. Second, Campylobacter preferentially colonizes birds whose body temperature is 42°C and cannot growth at low temperatures outside the body. In the host, expression of a large-channel porin gives a bacterium a growth advantage over the local flora.
The momp-gfp fusion can now be used to study the mechanisms involved in porin regulation in Campylobacter. GFP expression in Campylobacter gives fluorescence, as described by Miller et al. (22). Due to its strong induction, a single chromosomal copy of the momp-gfp fusion may be used. Moreover, because of its efficiency, the momp promoter may become a useful tool for overproduction of proteins in Campylobacter species by using shuttle vectors. Further research on the regulation of Campylobacter porin expression is required, and additional studies focusing on MOMP regulation in the genus Campylobacter and a study of Omp50 are now required. These studies should help us to better understand regulation of Campylobacter porin expression and should allow us to gain information about the global phenomena of outer membrane permeability that are controlled by these bacteria.
Acknowledgments
We thank A. Bernadac for performing fluorescence microscopy, J. Sturgis for performing spectrofluorimetry, L. F. Wu for providing GFP antibodies, and V. Mejean for commenting on the manuscript.
This work was supported by INSERM France, by the Université de la Méditerranée, and by grant 99.34.036.00.470.75.65 from the Ministère de la défense DGA/DSP/STTC (Direction Générale de l'Armement/Direction des Systèmes de Forces et de la Prospective/Service Technique des Technologies Communes).
REFERENCES
- 1.Amako, K., N. Baba, N. Suzuki, S. N. Wai, and A. Umeda. 1997. A structural analysis of the regularly arranged porin on the outer membrane of Campylobacter jejuni based on correlation averaging. Microbiol. Immunol. 41:855-859. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J., S. A. Forst, K. Zhao, M. Inouye, and N. Delihas. 1989. The function of micF RNA. micF RNA is a major factor in thermal regulation of OmpF protein in Escherichia coli. J. Biol. Chem. 264:17961-17970. [PubMed] [Google Scholar]
- 3.Ariza, R. R., Z. Li, N. Ringstad, and B. Demlpe. 1995. Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J. Bacteriol. 177:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F., R. Brent, R. Kingston, D. Moore, J. G. Seidman, J. Smit, and K. Struhl. 1992. Current protocols in molecular biology, p. 1.8.1-1.8.8. John Wiley and Sons, New York, N.Y.
- 5.Bartilson, M., A. Marra, J. Christine, J. S. Asundi, W. P. Sneider, and A. E. Hromockyi. 2001. Differential fluorescence induction reveals Streptococcus pneumoniae loci regulated by competence stimulatory peptide. Mol. Microbiol. 39:126-135. [DOI] [PubMed] [Google Scholar]
- 6.Bolla, J. M., E. Loret, M. Ladzunski, and J.-M. Pagès. 1995. Conformational analysis of the Campylobacter jejuni porin. J. Bacteriol. 177:4266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolla, J. M., E. Dé, A. Dorez, and J.-M. Pagès. 2000. Purification, characterization and sequence analysis of Omp50, a new porin isolated from Campylobacter jejuni. Biochem. J. 352:637-643. [PMC free article] [PubMed] [Google Scholar]
- 8.Bras, A. M., S. Chatterjee, B. W. Wren, D. G. Newell, and J. Ketley. 1999. A novel Campylobacter two-component regulatory system important for temperature-dependent growth and colonization. J. Bacteriol. 181:3298-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha, H. J., R. Srivastava, V. N. Vakharia, G. Rao, and W. E. Bentley. 1999. Green fluorescent protein as a noninvasive stress probe in resting Escherichia coli cells. Appl. Environ. Microbiol. 65:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfie, M., Y. Tu, G. Euskirchen, W. W. Ward, and D. C. Prasher. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802-805. [DOI] [PubMed] [Google Scholar]
- 11.Dé, E., M. Jullien, G. Labesse, J.-M. Pagès, G. Molle, and J. M. Bolla. 2000. MOMP (major outer membrane protein) of Campylobacter jejuni: a versatile pore-forming protein. FEBS Lett. 469:93-97. [DOI] [PubMed] [Google Scholar]
- 12.Dé, E., A. Baslé, M. Jaquinod, N. Saint, M. Malléa, G. Molle, and J.-M. Pagès. 2001. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 41:189-198. [DOI] [PubMed] [Google Scholar]
- 13.Deighan, P., A. Free, and C. J. Dorman. 2000. A role of the E. coli H-NS-like protein StpA in OmpF porin expression though modulation of micF RNA stability. Mol. Microbiol. 38:126-139. [DOI] [PubMed] [Google Scholar]
- 14.Fauchère, J. L., A. Rosenau, M. Véron, E. N. Moyen, N. Richard, and A. Pfister. 1986. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect. Immun. 54:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graeme-Cook, K. A., G. May, E. Bremer, and C. F. Higgins. 1989. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol. Microbiol. 3:1287-1294. [DOI] [PubMed] [Google Scholar]
- 16.Heim, R., D. C. Prasher, W. M. Westler, and R. Y. Tsien. 1994. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 91:12501-12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huyer, M., T. R. Parr, R. E. W. Hancock, and W. J. Page. 1986. Outer membrane porin protein in Campylobacter jejuni. FEMS Microbiol. Lett. 37:247-250. [Google Scholar]
- 18.Konkel, M. E., B. J. Kim, J. D. Klena, C. R. Young, and R. Ziprin. 1998. Characterization of thermal stress response of Campylobacter jejuni. Infect. Immun. 66:3666-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labesse, G., E. Garnotel, S. Bonnel, C. Dumas, J.-M. Pages, and J. M. Bolla. 2001. MOMP, a divergent porin from Campylobacter: cloning and primary structural characterization. Biochem. Biophys. Res. Commun. 280:280-387. [DOI] [PubMed] [Google Scholar]
- 20.Lowder, M., A. Unge, N. Maraha, J. K. Jansson, J. Swiggett, and J. D. Oliver. 2000. Effect of starvation and the viable-but-nonculturable state on green fluorecsent protein (GFP) fluorescence in GFP-tagged Pseudomonas fluorescens A506. Appl. Environ. Microbiol. 66:3160-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lugtenberg, B., and W. Van Alphen. 1977. Influence of osmolarity of the growth medium on the OMP pattern of E. coli. J. Bacteriol. 131:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, W. G., A. H. Bates, S. T. Horn, M. T. Brandl, M. R. Wachtel, and R. E. Mandrell. 2000. Detection on surfaces of Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno, T., E. T. Wurtzel, and M. Inouye. 1982. Cloning of the regulatory genes (OmpR and EnvZ) for the matrix proteins of the E. coli outer membrane. J. Bacteriol. 150:1462-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page, W. J., G. Huyer, M. Huyer, and E. A. Woromec. 1989. Characterization of the porins of Campylobacter jejuni and Campylobacter coli and implication for antibiotic susceptibility. Antimicrob. Agents Chemother. 33:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, S. F. 2000. Environmental regulatory genes, p. 423-440. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 26.Parkhill, J., B. Wren, K. Mungall, J. Ketley, C. Churcher, D. Basham, T. Chillinggworth, T. Feltwell, S. Holroyd, K. Jagels, A. Karlyshev, S. Moule, M. Pallen, C. Penn, M. Quail, M. Rajandream, K. Rutherford, A. van Vliet, S. Whitehead, and B. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 27.Prasher, D. C., V. K. Eckenrode, W. W. Ward, F. G. Prendergast, and M. J. Cormier. 1992. Primary structure of the Aequrea victoria green fluorescent protein. Gene 111:229-233. [DOI] [PubMed] [Google Scholar]
- 28.Resto-Ruiz, S. I., D. Sweger, R. H. Widen, N. Valkov, and B. E. Anderson. 2000. Transcriptional activation of the htrA (high-temperature requirement A) gene from Bartonella henselae. Infect. Immun. 68:5970-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosner, J. L., T. J. Chai, and J. Foulds. 1991. Regulation of OmpF porin expression by salicylate in Escherichia coli. J. Bacteriol. 173:5631-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Sato, M., K. Machida, E. Arikado, H. Saito, T. Kakekawa, and H. Kobayashi. 2000. Expression of outer membrane protein in Escherichia coli growing at acid pH. Appl. Environ. Microbiol. 66:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skirrow, M. B., and M. J. Blaser. 1992. Clinical and epidemiologic consideration, p. 3-8. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 33.Snyder, L., and W. Champness. 1997. Regulation of porin synthesis, p. 314-317. In Molecular genetics of bacteria, American Society for Microbiology, Washington, D.C.
- 34.Soutourina, O. A., E. A. Semenova, V. V. Parfenova, A. Danchin, and P. Bertin. 2001. Control of bacterial motility by environmental factors in polarly flagellated and peritrichous bacteria isolated from Lake Baikal. Appl. Environ. Microbiol. 67:3852-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spohn, G., and V. Scarlato. 1999. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J. Bacteriol. 181:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tauxe, R. V. 1992. Epidemiology of Campylobacter jejuni: infections in the United States and other industrialized nations, p. 9-19. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 37.Taylor, D. E. 1992. Campylobacter infections in developing countries, p. 20-30. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 38.Thomas, A. D., and I. R. Booth. 1992. The regulation of expression of the porin OmpC by acid pH. J. Gen. Microbiol. 138:1829-1835. [DOI] [PubMed] [Google Scholar]
- 39.Valdivia, R. D., A. E. Hromockyi, D. Monack, L. Ramakrishnaa, and S. Falkow. 1996. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173:47-52. [DOI] [PubMed] [Google Scholar]
- 40.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of S. typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 41.Wu, Y. L., L. H. Lee, D. M. Rollins, and W. M. Ching. 1994. Heat shock- and alkaline pH-induced proteins of Campylobacter jejuni: characterization and immunological properties. Infect. Immun. 62:4256-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanish-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning and host strains: nucleotide sequences of the M13mp18 and pUC 19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Q., J. C. Meitzler, S. Huang, and T. Morishita. 2000. Sequence polymorphism, predicted secondary structures, and surface-exposed conformational epitopes of Campylobacter major outer membrane protein. Infect. Immun. 68:5679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]