Abstract
Torula corallina, a strain presently being used for the industrial production of erythritol, has the highest erythritol yield ever reported for an erythritol-producing microorganism. The increased production of erythritol by Torula corallina with trace elements such as Cu2+ has been thoroughly reported, but the mechanism by which Cu2+ increases the production of erythritol has not been studied. This study demonstrated that supplemental Cu2+ enhanced the production of erythritol, while it significantly decreased the production of a major by-product that accumulates during erythritol fermentation, which was identified as fumarate by instrumental analyses. Erythrose reductase, a key enzyme that converts erythrose to erythritol in T. corallina, was purified to homogeneity by chromatographic methods, including ion-exchange and affinity chromatography. In vitro, purified erythrose reductase was significantly inhibited noncompetitively by increasing the fumarate concentration. In contrast, the enzyme activity remained almost constant regardless of Cu2+ concentration. This suggests that supplemental Cu2+ reduced the production of fumarate, a strong inhibitor of erythrose reductase, which led to less inhibition of erythrose reductase and a high yield of erythritol. This is the first report that suggests catabolite repression by a tricarboxylic acid cycle intermediate in T. corallina.
Erythritol is a four-carbon polyol with properties similar to those of other polyols presently used as food ingredients (4). Erythritol has 60 to 70% of the sweetness of sucrose in a 10% (wt/vol) solution. Erythritol has very high negative heat when dissolved in solution, providing a strong cooling effect (10). Because it is a small molecule, erythritol has strong colligative properties, including freezing point depression, boiling point elevation, and high osmotic pressure. With its low hygroscopicity and viscosity in solution, it is very useful for reducing and controlling water activity in foodstuffs (9). Because erythritol cannot be used as a fermentation substrate for caries-producing bacteria, its use in food does not promote tooth decay.
Erythritol can be produced by microbial methods using osmophilic yeasts and some bacteria (1, 16, 17, 27, 30, 32, 33). Erythritol was found to be synthesized from erythrose-4-phosphate, an intermediate of the pentose-phosphate cycle, by dephosphorylation followed by reduction of the resultant erythrose. Erythrose reductase, catalyzing this last step, is well known as a key enzyme for the biosynthesis of erythritol (15, 31). It has been reported that polyols were produced by 43 of 1,753 osmophilic yeast strains isolated from honey and pollen (29). Three of these strains of Trichosporon and Trichosporonoides (1) produced only erythritol at good yields of 27.9 to 40.7% (wt/wt). Other microorganisms that have been reported to produce erythritol include Pichia, Zygopichia, Candida, Torulopsis, Trigonopsis (13, 28), and Moniliella iomentosa var. pollis (11). Erythritol production using this strain could not be applied on an industrial scale due to by-products such as glycerol and ribitol. Erythritol has been produced commercially by using a mutant of Aureobasidium that produced erythritol at a high concentration of 175 g/liter, with a high yield of 43.8% (wt/wt) after 4 days of cultivation in a jar fermentor (14).
Recently, a novel microorganism, which is able to produce erythritol, was isolated and identified as Torula corallina (16). A mutant of this strain produced erythritol at a high concentration of 196 g/liter with a high yield of 48.9% and did not produce by-products such as glycerol and ribitol, resulting in application on an industrial scale (16, 20). It was previously reported that erythritol production was improved by controlling glucose concentration in a fed-batch culture of T. corallina (26) and by providing inositol, phytic acid (19), Mn2+, and Cu2+ supplements (20). The mechanisms by which Cu2+ increases erythritol production, however, have not yet been studied.
It has been demonstrated that many catabolic pathways are repressed in the presence of tricarboxylic acid (TCA) cycle intermediates (5, 23). In this study, we investigated the mechanism of Cu2+ enhancement of erythritol production. We determined that a major by-product of erythritol production was fumarate; we quantified its production during culture and examined its effect on the activity of erythrose reductase, a key enzyme in erythritol biosynthesis.
MATERIALS AND METHODS
Microorganisms, media, and chemicals.
T. corallina was isolated from a 40% sucrose solution at the Bolak Co. R&D Center (Osan, Korea) (16). Growth medium (200 g of glucose and 10 g of yeast extract per liter) was used for initial shake-flask cultivation at 30°C. The production medium contained 400 g of glucose and 20 g of yeast extract per liter. Fermentor experiments were carried out with and without the addition of 2 μg of CuSO4 · 5H2O/liter. Medium components were purchased from Difco (Detroit, Mich.) and Wako Pure Chemical Industries (Osaka, Japan). Organic acids such as oxalacetate, malate, succinate, fumarate, maleate, pyruvate, citrate, α-ketoglutarate, acetate, and lactate were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Culture conditions.
A single colony of T. corallina was inoculated into a 20-mm-diameter test tube containing 5 ml of growth medium and was incubated at 30°C with agitation at 250 rpm for 48 h. Following agitation, 5 ml of broth was transferred into a 500-ml baffled flask containing 100 ml of growth medium and was then cultivated at 30°C and 250 rpm for 24 h. This seed culture was then transferred into a fermentor. Fermentor experiments were performed with 5-liter jar fermentors (KoBiotech Co., Inchon, Korea) containing 3 liters of production medium. The temperature and pH of the fermentor were controlled to 34°C and 5.5, respectively. To maintain the dissolved oxygen concentration above 20%, the agitation speed was adjusted to between 500 and 850 rpm. The aeration rate was 0.5 vol/vol/min (vvm) during fermentation (16).
Preparation of cell extracts.
Cells from the culture broth were harvested by centrifugation. After being washed with 0.1 M Tris-HCl buffer (pH 7.8), cells were resuspended in disruption buffer (2) containing 20 mM Tris-HCl (pH 7.8), 10 mM MgCl2, and 1 mM phenylmethylsulfonyl fluoride. The cell suspension was disrupted by grinding with glass beads that were 0.5 mm in diameter (Sigma Chemical Co.). Cell extracts were obtained by removing the ruptured cells by centrifugation at 10,000 × g for 30 min.
Purification of erythrose reductase.
Cell extracts were fractionated by ammonium sulfate precipitation. The fraction precipitated by between 40 and 70% saturation of ammonium sulfate was collected by centrifugation and dissolved in 50 mM Tris-HCl buffer (pH 7.8). Following removal of insoluble material by centrifugation at 10,000 × g for 1 h, the enzyme solution was dialyzed against the same buffer at 4°C for 24 h. Dialyzed enzyme solution was placed on a DEAE-Toyopearl 650S column (1.4 by 20 cm) equilibrated with 50 mM Tris-HCl buffer at pH 7.8. Protein was eluted by a linear 0 to 0.5 M NaCl gradient in the same buffer. Active fractions were pooled. The enzyme solution was put on a Cibacron Blue 3GA affinity column (1.4 by 20 cm) that had been equilibrated with the same buffer. The column was eluted by a linear gradient of 0 to 1 M NaCl in the same buffer. The active fractions were pooled, concentrated, and dialyzed against the same buffer and concentrated with Centricon (Amicon, Bedford, Mass.) and were then used as a purified enzyme in the following experiments.
Erythrose reductase activity assay.
The activity of erythrose reductase was determined by monitoring the oxidation of NADPH in a spectrophotometric cuvette at 340 nm at 37°C (6). The cuvette contained 1.5 ml of 50 mM Tris-HCl buffer (pH 7.8), 0.1 ml of 0.1 M 2-mercaptoethanol, 0.2 ml of enzyme solution, and 0.2 ml of 3.4 mM NADPH. This reaction mixture was allowed to stand for 1 min to eliminate the endogenous oxidation of NADPH. The reaction was started by addition of 0.1 ml of 0.5 M d-erythrose. One unit of enzyme represents the amount of enzyme that caused an initial rate of decrease of 1 μmol of NADPH per min. Activities are expressed as units/milligram of protein.
PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (18). Native PAGE was performed on a 10% polyacrylamide gel without SDS. Protein bands were stained with Coomassie brilliant blue R250. Erythrose reductase activity staining on a polyacrylamide gel was done by use of a modification of the procedure of Birken and Pisano (5). The staining mixture used for the detection of NADP-erythritol activity consisted of 40 ml of 0.1 M Tris-HCl buffer (pH 7.8), 25 mg of nitroblue tetrazolium, 3 mg of phenazine methosulfate, 30 mg of NADP, and 2 ml of 0.5 M erythritol. Gels were incubated in appropriate activity staining solution for 15 min, washed in water, and stored in 7% acetic acid.
Analytical methods.
Dry cell weight was estimated by using a calibration curve derived from the relationship between the absorbance at 600 nm and the dry cell weight. Protein was determined by the Lowry method using bovine serum albumin as a standard. Concentrations of erythritol and glucose were determined by high-performance liquid chromatography (HPLC) coupled to a refractive index detector (model 2410; Waters, Milford, Mass.) and a KR100-10NH2 column (4.6 by 250 mm; Kromasil, Bohus, Sweden). The mobile phase was acetonitrile/water (80:20 [vol/vol]), and the flow rate was 1.5 ml/min. For identification of individual organic acids by HPLC, culture broths were compared with standard solutions of organic acids, such as oxalacetate, malate, succinate, fumarate, pyruvate, citrate, α-ketoglutarate, acetate, and lactate, on a Capcell Pak C18 MG column (4.6 by 250 mm; Shiseido, Tokyo, Japan) with a UV detector (Waters model 486). The mobile phase was 2.5% aqueous CH3CN (pH 2.5 with H3PO4), and the flow rate was 0.7 ml/min.
Mass and 1H-NMR spectroscopy.
Atmospheric pressure chemical ionization was carried out in the negative ion mode on a JEOL AX505WA mass spectrometer (JEOL Inc., Peabody, Mass.) at a 5-kV needle voltage, 250°C, 5-mA corona discharge current, and 500°C capillary temperature. 1H-nuclear magnetic resonance (NMR) measurements were recorded in the pulsed Fourier transform mode on a Bruker ARX Fourier transform spectrometer (Bruker Instruments Inc., Billerica, Mass.) operating at 400 MHz.
RESULTS
Increased erythritol production by Cu2+ supplementation.
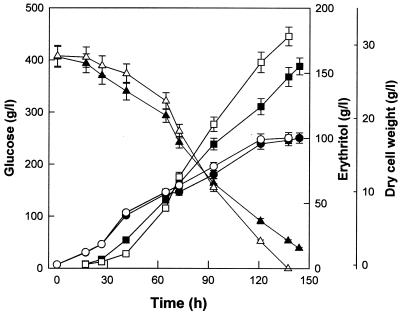
To investigate the role of Cu2+ in the metabolism of T. corallina and its stimulatory action on erythritol production, a control culture without Cu2+ and an experimental culture with 2 μg of CuSO4 · 5H2O/ml were established, as shown in Fig. 1. Maximal cell growth required about 1 μg of CuSO4 · 5H2O/ml, whereas maximal erythritol production required about 2 μg of CuSO4 · 5H2O/ml. Inhibition of cellular growth was observed with 10-μg/ml and higher concentrations of CuSO4 · 5H2O. There were no large differences between the cultures for approximately 70 h. After 70 h, cell growth, glucose consumption, and erythritol production rates (in grams/liter-hour) of cultures containing Cu2+ were higher than those of control cultures. The supplemental Cu2+ increased cellular growth slightly and significantly enhanced erythritol production. After culture for 144 h, the final concentration of erythritol produced from 400 g of glucose/liter in the control culture without Cu2+ was 155 g/liter. The concentration increased to 178 g/liter at 138 h with supplemental Cu2+. In the control culture the volumetric rate of erythritol production was 1.08 g/liter-h, and the erythritol yield from glucose was 38.3% (wt/wt). With the addition of Cu2+, these values increased to 1.29 g/liter-h and 44.5% (wt/wt), respectively.
FIG. 1.
Glucose consumption and erythritol production in a culture without and with 2.0 μg of CuSO4 · 5H2O/ml by T. corallina. Glucose consumption without (▴) and with (▵) CuSO4 · 5H2 is shown, as is erythritol production without (▪) and with (□) CuSO4 · 5H2O; dry cell weight without (•) and with (○) CuSO4 · 5H2O is also shown. Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%.
Identification of a major by-product in T. corallina culture.
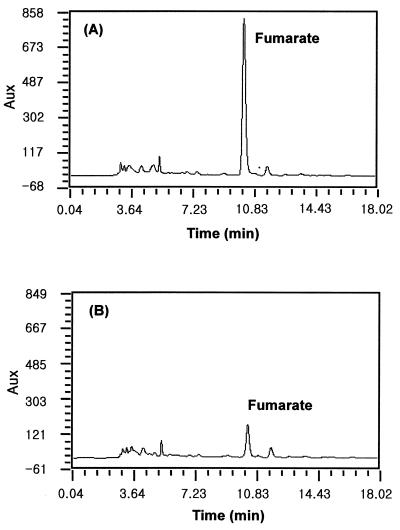
The by-products of cultures with and without Cu2+ were analyzed by using HPLC coupled to a UV detector and a Shiseido Capcell Pak C18 MG column at 215 nm (Fig. 2).
FIG. 2.
HPLC pattern of erythritol fermentation broth in a culture of T. corallina without (A) and with (B) 2.0 μg of CuSO4 · 5H2O/ml. A major by-product was identified as fumarate by instrumental analyses.
Separation of individual organic acids by HPLC revealed that an unknown compound that accumulated in cultures of T. corallina coeluted with fumarate. This major by-product was detected at a retention time of approximately 9 min, identical to that of fumarate under conditions described in Materials and Methods. To confirm that this by-product was fumarate, we purified it by preparative HPLC. Eluates containing the by-product were subjected to atmospheric pressure chemical ionization mass spectroscopy in the negative ion mode. In the total ion current chromatogram, one major peak was detected at a retention time of 2.27 min, corresponding to HPLC UV detection. In deprotonated form, this peak represented a molecular mass of 115 Da. This value corresponded well with the known mass of the fumarate pseudomolecular ion, [M-H]+. The ions with an m/z of 71 corresponded to [M-H-COOH]+. 1H-NMR analysis was also performed, and the result was identical to that for fumarate (data not shown). Taken together, these results indicate that the by-product accumulated in T. corallina culture was indeed fumarate.
Effect of Cu2+ on by-product production and its quantification.
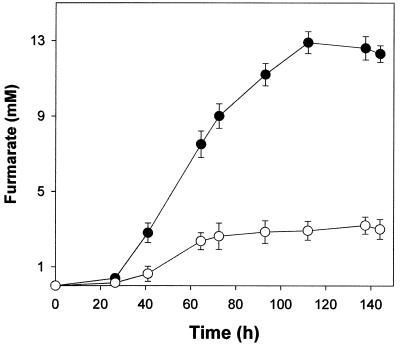
The effect of Cu2+ on fumarate production was studied by performing fumarate production time course experiments during erythritol fermentation in cultures with and without 2 μg of CuSO4 · 5H2O/ml (Fig. 3). HPLC analysis was carried out to examine the accumulation of fumarate. In the absence of exogenous Cu2+, the highest fumarate level reached was 12.9 mM. In the presence of exogenous Cu2+, the levels of fumarate accumulation significantly decreased at CuSO4 · 5H2O concentrations exceeding 1 μg/ml. The decrease in erythritol production at CuSO4 · 5H2O concentrations above 2 μg/ml was due to the inhibition of cellular growth by Cu2+. Following supplementation with 2 μg of CuSO4 · 5H2O/ml, a stimulator of erythritol production, fumarate production was reduced to about 25%. The maximum concentrations of fumarate found were 12.9 mM without Cu2+ and 3.2 mM with Cu2+. Cu2+ was found to strongly inhibit the production of fumarate, a major by-product during erythritol fermentation.
FIG. 3.
Time course of fumarate production in cultures without (•) and with (○) 2.0 μg of CuSO4 · 5H2O/ml during erythritol fermentation. Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%.
Purification of erythrose reductase.
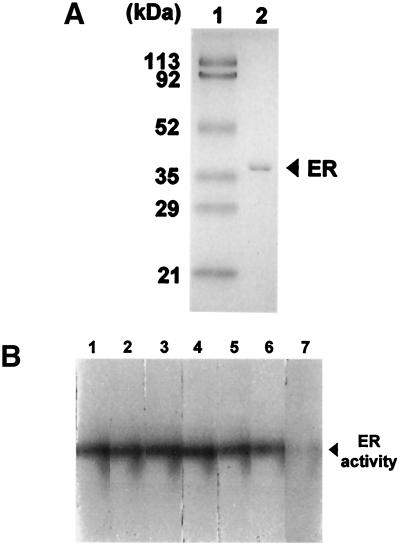
Crude extracts of T. corallina were obtained from culture at 4 days, when the enzymatic activity of erythrose reductase is high. Fractionation with ammonium sulfate increased the specific activity twofold, with 76% recovery of the erythrose reductase activity. The active fractions were applied to a DEAE-Toyopearl 650S column, and erythrose reductase was eluted by a 0 to 0.5 M NaCl linear gradient. Erythrose reductase activities were recovered in approximately 0.2 M NaCl-containing fractions. On Cibacron Blue 3GA affinity column chromatography, three peaks with protein content and one peak with erythrose reductase activity were resolved (data not shown). The first large peak and the third peak did not show erythrose reductase activity, whereas the second small peak that eluted as a single peak at approximately 0.7 M NaCl did show erythrose reductase activity. The active fractions were collected, concentrated, and dialyzed with a stirred cell (Amicon). The chromatographic procedure resulted in a 53.5-fold purification of erythrose reductase with a recovery of 10.8% and a specific activity of 1.8 U/mg. At this purification step, erythrose reductase was found to be homogeneous by SDS-PAGE (Fig. 4A). And this enzyme solution was used for an activity assay of erythrose reductase.
FIG. 4.
(A) SDS-10% PAGE shows a homogeneous enzyme preparation after the final purification step. Lane 1, molecular weight marker; lane 2, purifed erythrose reductase. (B) In vitro activity staining assay demonstrating that fumarate specifically inhibits the activity of erythrose reductase. Lane 1 contains no TCA cycle intermediates. Lanes 2 to 7 contain TCA cycle intermediates as follows: lane 2, 10 mM citrate; lane 3, 10 mM α-ketoglutarate; lane 4, 10 mM succinate; lane 5, 10 mM malate; lane 6, 10 mM oxalacetate; and lane 7, 10 mM fumarate. ER, erythrose reductase.
Effect of Cu2+ and fumarate on erythrose reductase.
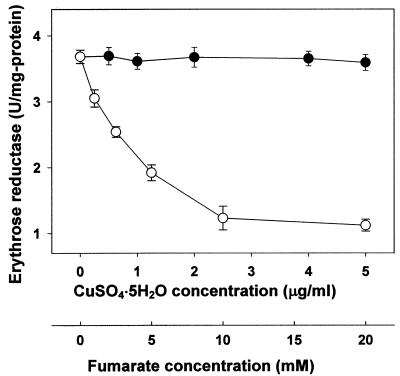
In order to investigate in detail the role of Cu2+ in erythritol production, erythrose reductase from T. corallina was purified and its in vitro activity was assayed with increasing Cu2+ concentration. Cu2+ was added as CuSO4 · 5H2O, and the Cu2+ concentration was measured by atomic absorption spectrophotometry. Cu2+ did not have a great effect on erythrose reductase activity (Fig. 5). Divalent metals such as Cu2+, Mn2+, Fe2+, Co2+, Ni2+, and Zn2+ did not affect enzyme activity at concentrations of 1 to 10 μg/ml. These results suggest that supplementation with Cu2+, Mn2+, Fe2+, Co2+, Ni2+, and Zn2+ did not directly affect the activity of erythrose reductase. The effect of fumarate on erythrose reductase was also investigated. When the fumarate concentration in purified enzyme solution was increased, the activity of erythrose reductase significantly decreased (Fig. 5). Fumarate was found to be a strong inhibitor for erythrose reductase.
FIG. 5.
Effect of Cu2+ (•) and fumarate (○) on the activity of purified erythrose reductase from T. corallina. Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%.
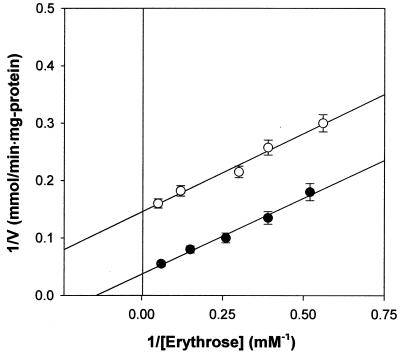
The kinetic parameters of erythrose reductase were determined by using initial rate determination followed by Lineweaver-Burk plotting. The Km and Vmax for the reduction of erythrose in the plot were determined to be about 7.12 mM and 26 mmol/min · mg of protein, respectively. The effect of fumarate concentration on erythrose reductase inhibition was studied as a Lineweaver-Burk plot. The inhibition was found to be noncompetitive, and the Ki for fumarate was about 3.9 mM. In vitro assays demonstrated that fumarate specifically inhibited erythrose reductase in a noncompetitive manner against an enzyme-substrate (erythrose reductase-erythrose) complex (Fig. 6).
FIG. 6.
Lineweaver-Burk plot of the inhibition of erythrose reductase by fumarate in T. corallina. The rate of decrease in the absorbance of NADPH at 340 nm was measured as reported in Materials and Methods. Symbols: •, without fumarate; ○, with 10 mM fumarate. Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%.
To determine if any TCA cycle intermediates directly inhibited erythrose reductase, in vitro assays were performed with activity staining (5) and either 10 mM citrate (Fig. 4B, lane 2), 10 mM α-ketoglutarate (lane 3), 10 mM succinate (lane 4), 10 mM malate (lane 5), 10 mM oxalacetate (lane 6), or 10 mM fumarate (lane 7). Lanes 2 to 6 demonstrated that samples with organic acids other than fumarate had full erythrose reductase activity. Fumarate (lane 7) was the only metabolite among tested organic acids that caused specific inhibition of erythrose reductase.
DISCUSSION
Many organisms produce small amounts of fumarate, malate, lactate, and other organic acids as metabolic by-products during oxidative metabolism. In some instances, certain fungi produce significant quantities of fumarate from glucose and carbon dioxide (8, 21, 25). Catabolite inhibition is a general phenomenon, even though the inhibitory effects vary widely from metabolite to metabolite. Interest in the inhibition of by-product production during the biosynthesis process as a way to minimize the effect of catabolite repression has been strong. During the culture of T. corallina for the production of erythritol, large amounts of a by-product were accumulated and instrumental analyses determined that it was fumarate. During erythritol fermentation, the levels of fumarate declined in Cu2+-supplemented cultures compared to those in Cu2+-deficient cultures.
It has been demonstrated that Cu2+ ions stimulate the synthesis of erythritol in T. corallina (20), and we propose that the stimulatory effect of Cu2+ on erythritol synthesis is mediated by the inhibition of fumarate, a strong inhibitor of erythrose reductase. Regulation by intracellular fumarate levels is not unique to this system. Alginate production in Pseudomonas aeruginosa (12) and phosphorylation-independent flagellar motor switching in Escherichia coli (3, 23) are both regulated by intracellular fumarate levels.
Growth on TCA cycle intermediates has been reported to cause carbon catabolite repression and to repress the expression of many operons at the transcriptional level in Pseudomonas (7, 24, 34). McFall et al. (22) suggested that the growth and metabolism of some bacteria and yeasts were repressed during growth with TCA cycle intermediates and that this repression was found at the transcriptional level.
The mechanism by which fumarate inhibits erythrose reductase is unknown. To determine whether related compounds could play either an activating or a repressing role in the activity of erythrose reductase, many analogous organic acids, including the cis isomer of fumarate, maleic acid, were added to in vitro assay mixtures. None of these compounds modulated the activity of erythrose reductase. Fumarate has a trans double bond that is unique among the TCA cycle intermediates. Although this difference may allow fumarate to act as a key signaling molecule for the metabolic status of the cell, the mechanism by which fumarate inhibits erythrose reductase in vivo has still to be investigated.
In conclusion, supplementation with Cu2+ in cultures of T. corallina reduced the production of fumarate, a strong inhibitor of erythrose reductase. As a result, erythrose reductase activity became less inhibited and a high yield of erythritol was produced. To our knowledge this is the first report that proposes the catabolite repression of a key enzyme, erythrose reductase, in the biosynthesis of a sugar alcohol by a TCA cycle intermediate, fumarate.
Acknowledgments
This research was supported by a grant from the Korean Ministry of Science & Technology.
REFERENCES
- 1.Aoki, M. A. Y., G. M. Pastore, and K. Park. 1993. Microbial transformation of sucrose and glucose to erythritol. Biotechnol. Lett. 15:383-388. [Google Scholar]
- 2.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Short protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Barak, R., and M. Eisenbach. 1992. Fumarate or a fumatate metabolite restored switching ability to rotating flagella of bacterial envelopes. J. Bacteriol. 174:643-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billaux, M. S., B. Flourie, C. Jacquemim, and B. Messing. 1991. Sugar alcohols, p. 72. In S. Marie and F. R. Pogolt (ed.), Handbook of sweeteners. Blackie Academic & Professional, Glasgow, United Kingdom.
- 5.Birken, S., and M. A. Pisano. 1976. Purification and properties of a polyol dehydrogenase from Cephalospirium chrysogenus. J. Bacteriol. 125:225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, C., and S. G. Knight. 1966. d-Xylose reductase and xylitol dehydrogenase from Penicillium chrysogenum. Methods Enzymol. 9:188-193. [Google Scholar]
- 7.Duetz, W. A., S. Marques, C. de Jong, J. Ramos, and J. G. van Andel. 1994. Inducibility of the TOL catabolic pathway in Pseudomonas putida (pWW0) growing on succinate in continuous culture: evidence of carbon catabolite repression control. J. Bacteriol. 176:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster, J. W., and S. A. Waksman. 1939. The production of fumaric acid by molds belonging to the genus Rhizopus. J. Am. Chem. Soc. 61:127-135. [Google Scholar]
- 9.Goldberg, I. 1994. Functional foods. Chapman & Hall, New York, N.Y.
- 10.Goossen, J., and H. Röper. 1994. Erythritol, a new bulk sweetener. Confectionery Prod. 24:182-188. [Google Scholar]
- 11.Hajney, G. J., J. H. Smith, and J. C. Garver. 1964. Erythritol production by yeast like fungus. Appl. Microbiol. 12:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassett, D. J., M. L. Howell, P. A. Sokol, M. L. Vasil, and G. E. Dean. 1997. Fumarase C activity is elevated in response to iron deprivation and in mucoid, alginate-producing Pseudomonas aeruginosa: cloning and characterization of fumC and purification of native FumC. J. Bacteriol. 179:1442-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattori, K., and T. Suzuki. 1974. Production of erythritol by n-alkane grown yeasts. Agric. Biol. Chem. 38:581-586. [Google Scholar]
- 14.Ishizuka, H., H. Wako, T. Kasumi, and T. Sasaki. 1989. Breeding of a mutant of Aureobasidium sp. with high erythritol production. J. Ferment. Bioeng. 68:310-314. [Google Scholar]
- 15.Ishizuka, H., K. Tokuoka, T. Sasaki, and H. Taniguchi. 1992. Purification and some properties of an erythrose reductase from an Aureobasidium sp. Biosci. Biotechnol. Biochem. 56:941-945. [DOI] [PubMed] [Google Scholar]
- 16.Kim, K. A., J. K. Lee, S. Y. Kim, and D. K. Oh. 2000. Optimization of culture conditions for erythritol production by Torula sp. J. Microbiol. Biotechnol. 10:69-74. [Google Scholar]
- 17.Kim, S. Y., K. H. Lee, J. H. Kim, and D. K. Oh. 1997. Erythritol production by controlling osmotic pressure in Trigonopsis variabilis. Biotechnol. Lett. 19:729-733. [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. K., S. Y. Kim, and D. K. Oh. 2001. Increased erythritol production in Torula sp. by inositol and phytic acid. Biotechnol. Lett. 23:497-500. [Google Scholar]
- 20.Lee, J. K., S. J. Ha, S. Y. Kim, and D. K. Oh. 2000. Increased erythritol production in Torula sp. by Mn2+ and Cu2+. Biotechnol. Lett. 22:983-986. [Google Scholar]
- 21.Margulies, M., and W. Vishniac. 1981. Dissimilation of glucose by the MX strain of Rhizopus. J. Bacteriol. 81:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFall, S. M., B. Abraham, C. G. Narsolis, and A. M. Chakrabarty. 1997. A tricarboxylic acid cycle intermediate regulating transcription of a chloroaromatic biodegradative pathway: fumarate-mediated repression of the clcABD operon. J. Bacteriol. 179:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montrone, M., D. Oesterhelt, and W. Marwan. 1996. Phosphorylation-independent bacterial chemoresponses correlate with changes in the cytoplasmic level of fumarate. J. Bacteriol. 178:6882-6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller, C., L. Petruschka, H. Cuypers, G. Burchhardt, and H. Herrmann. 1996. Carbon catabolite repression of phenol degradation in Pseudomonas putida is mediated by the inhibition of the activator protein PhiR. J. Bacteriol. 178:2030-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ningjun, C., D. Jianxin, C. S. Gong, and G. T. Tsao. 1996. Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Appl. Environ. Microbiol. 62:2926-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh, D. K., C. H. Cho, J. K. Lee, and S. Y. Kim. 2001. Increased erythritol production in fed-batch cultures of Torula sp. by controlling glucose concentration. J. Ind. Microbiol. Biotechnol. 26:248-252. [DOI] [PubMed] [Google Scholar]
- 27.Onishi, H. 1967. Production of polyalcohols by yeast. Jpn. Ferment. Technol. 25:495-506. [Google Scholar]
- 28.Onishi, H. 1960. Studies on osmophilic yeast. Bull. Agric. Chem. Soc. Jpn. 24:131-140. [Google Scholar]
- 29.Park, Y. K., M. H. Koo, and I. M. A. Oliveira. 1996. Biochemical characteristics of osmophilic yeasts isolated from pollen and honey. Biosci. Biotechnol. Biochem. 60:1872-1873. [DOI] [PubMed] [Google Scholar]
- 30.Ryu, Y. W., C. Y. Park, J. B. Park, S. Y. Kim, and J. H. Seo. 2000. Optimization of erythritol production by Candida magnoliae in fed-batch culture. J. Ind. Microbiol. Biotechnol. 25:100-103. [Google Scholar]
- 31.Tokuoka, K., H. Ishizuka, K. Wako, and H. Taniguchi. 1992. Comparison of three forms of erythrose reductase from an Aureobasidium sp. mutant. J. Gen. Appl. Microbiol. 38:145-155. [DOI] [PubMed] [Google Scholar]
- 32.Veiga-Da-Cunha, M., P. Firme, M. V. San Romão, and H. Santos. 1992. Application of 13C nuclear magnetic resonance to elucidate the unexpected biosynthesis of erythritol by Leuconostoc oenos. Appl. Environ. Microbiol. 58:2271-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veiga-da-Cunha, M., H. Santos, and E. Van Schaftingen. 1993. Pathway and regulation of erythritol formation in Leuconostoc oenos. J. Bacteriol. 175:3941-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolff, J. A., C. H. MacGregor, R. C. Eisenberg, and P. V. Phibbs, Jr. 1991. Isolation and characterization of catabolite repression control mutants of Pseudomonas aeruginosa PAO. J. Bacteriol. 173:4700-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]