Abstract
Introduction
Postoperative dry eye disease (DED) remains a frequent complication that can reduce patient satisfaction and surgical outcomes. Low-level light therapy (LLLT) is a non-invasive technology that has shown positive outcomes in managing DED. This study aimed to assess the prophylactic application of perioperative LLLT for improving ocular surface parameters and symptoms in consecutive patients undergoing cataract surgery.
Methods
In this prospective, double-masked, randomized sham-controlled study, patients scheduled for cataract surgery were randomized to receive either periocular LLLT or sham treatment 1 week before and 1 week after surgery. Ocular surface assessments, including Ocular Surface Disease Index (OSDI) questionnaire, tear film break-up time (BUT), Schirmer test type I, tear osmolarity, and corneal fluorescein staining (Oxford score), were performed preoperatively before the first treatment/sham session (T0), and postoperatively 1 week (T1) and 1 month postoperatively (T2). All patients received the same postoperative therapy.
Results
Out of 98 patients randomized to LLLT (50 patients) or sham treatment (48 patients), 89 patients (45 males, 44 females; mean age of 73.75 ± 7.95 years) completed the study. Unlike controls, the LLLT group showed significant improvements from T0 to T1 and T2 for OSDI scores (respectively, from 26.62 ± 15.42 to 15.53 ± 12.04 and 13.36 ± 11.69; p < 0.001) and BUT values (respectively, from 5.76 ± 3.99 to 6.69 ± 4.48 and 8.38 ± 4.53; p = 0.002), and from T0 to T2 for tear osmolarity (respectively, from 300.69 ± 14.19 mOsm/l to 296.11 ± 12.30 mOsm/l; p = 0.048). No significant differences were found in Schirmer test values within or between the two groups. No adverse effects were reported.
Conclusions
Perioperative LLLT is a safe, well-tolerated, and effective treatment for preventing iatrogenic DED in cataract surgery. Integrating LLLT into the routine perioperative care may enhance patient satisfaction and overall outcomes in the setting of cataract surgery.
Clinical Trial Registration Number
NCT07067294, retrospectively registered on 05.07.2025.
Keywords: Dry eye disease, Low-level light therapy, Ocular surface, Cataract surgery
Key Summary Points
| Why carry out this study? |
| Cataract surgery often induces or worsens dry eye, compromising visual quality and patient satisfaction. |
| Device-based therapies such as low-level light therapy (LLLT) are gaining interest in dry eye disease (DED) management, but data on their prophylactic use in real-world patients with cataracts are limited. |
| Previous studies focused on healthy populations; this study evaluated LLLT in an unselected, real-life surgical cohort. |
| What was learned from the study? |
| Prophylactic LLLT significantly improved Ocular Surface Disease Index (OSDI) scores and tear-film stability in patients with cataracts. |
| LLLT was effective in preventing postoperative increases in tear osmolarity and ocular surface damage. |
| The therapy was safe, easy to implement perioperatively, and could be incorporated into standard cataract surgery protocols. |
Introduction
Cataract surgery is the most commonly performed ophthalmic procedure worldwide, and advances in surgical technology have dramatically accelerated visual rehabilitation, raising patient expectations accordingly [1]. However, dry eye disease (DED) is a frequent iatrogenic condition following cataract surgery that can significantly reduce overall outcomes [2]. DED after cataract surgery is driven by multiple factors, including topical medications, corneal incisions that disrupt nerve fibers, phototoxicity from the operating microscope, increased inflammatory mediators, loss of conjunctival goblet cells, and meibomian‐gland dysfunction (MGD), among others [3, 4]. Since many patients with cataracts have undiagnosed DED or MGD already before surgery, early detection with routine preoperative ocular surface assessment optimizes surgical outcomes by improving biometry accuracy and reducing the risk of postoperative exacerbation [5, 6]. Indeed, tear film instability significantly affects the quality of the refractive surface and changes dramatically between blinks in DED patients. Therefore, treating the ocular surface before ocular biometry (e.g., instillation of tear substitutes or secretagogue ophthalmic suspension) has been shown to significantly modify biometric parameters including axial length and central corneal thickness [7, 8].
Dry eye symptoms typically worsen during the first postoperative weeks and may persist for several months [9, 10]. Treatment options range from tear substitutes and eyelid hygiene/warm compresses to short-term topical corticosteroids or immunomodulators such as cyclosporine or lifitegrast [2, 11]. Recent research has increasingly shown that optimizing the ocular surface prior to surgery can be beneficial for patients with existing ocular surface impairment, as well as for those without it, as the surgery itself may harm the ocular surface and lead to DED after the procedure [12, 13].
In addition to medical therapy, device-based approaches such as low-level light therapy (LLLT) have recently gained interest for DED management [14, 15]. LLLT increases adenosine triphosphate (ATP) production by enhancing the absorption of red or near-infrared light by cytochrome c oxidase in the mitochondria through photobiomodulation [16]. This increase in energy activates transcription factors and signaling pathways that promote cell regeneration, anti-inflammatory response, tissue repair, and wound healing [17, 18]. A recent study on healthy patients demonstrated that prophylactic LLLT before senile cataract surgery preserves the ocular surface status and improves patient comfort [19]. However, a gap in understanding the effects of LLLT in a real-world surgical population is still present. Based on these findings, our study aims to evaluate the effects of two perioperative sessions of LLLT on ocular surface parameters and ocular discomfort symptoms in consecutive patients scheduled for senile cataract surgery.
Methods
Study Design and Participants
This randomized, double-masked, sham-controlled prospective study was conducted at the Cai Ferate Clinical Hospital (Iasi, Romania). The study was approved by the local Ethics Committee of the Cai Ferate Clinical Hospital of Iasi, Romania, (approval number: 54/29.07.2024) and retrospectively registered at ClinicalTrials.gov (NCT07067294) on 05.07.2025. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all participants prior to inclusion in the study.
Consecutive adult patients (aged ≥ 60 years) scheduled for routine phacoemulsification surgery were screened for eligibility and considered for enrolment between January and March 2025. Exclusion criteria included a prior diagnosis of ocular surface disease (OSD) or dry eye disease DED, any history of ocular surgery in either eye, ocular comorbidities, and the current or regular use of topical ocular treatments (e.g., lubricants, corticosteroids, or cyclosporine), instrumental therapies, or oral supplements for OSD or DED, systemic treatments known to be associated with DED (such as diuretics, antidepressants, antihistamines, or hormone replacement therapy), autoimmune diseases (e.g., Sjögren's syndrome), regular contact lens wear, and any intraoperative or postoperative complications that could interfere with ocular surface assessment. One eye of each patient was included in the study. Participants were randomly assigned in a 1:1 ratio to receive either perioperative LLLT or a sham procedure, using a computer-generated sequence (http://www.sealedenvelope.com). Allocation was concealed by means of sealed, opaque envelopes to ensure blinding. The study was double-blinded: neither the participants nor the outcome assessors were aware of the treatment allocation, ensuring unbiased assessment of clinical outcomes.
Interventions
Participants assigned to the treatment group received periocular LLLT sessions using the eye-light® device (Espansione Marketing S.p.A, Bologna, Italy) with wavelengths of 633 ± 10 nm. Each session lasted 15 min and was conducted 7 days before and 7 days after cataract surgery. Sham-treated controls underwent the same procedure using the device’s demo light mode, which delivered less than 30% of the full treatment power, effectively simulating a standard LLLT session for patients without providing therapeutic exposure, as previously described.
All surgeries were performed by the same experienced surgeon using the standard microincisional phacoemulsification technique. Only eyes without intraoperative or postoperative complications were included in the final analysis.
Postoperative therapy was based on clinical practice evidence and was identical for all patients [20]. It included a combination of topical dexamethasone and netilmicin for 1 week, followed by tapering dexamethasone alone over two additional weeks; in parallel topical ketorolac three times daily for 1 month was administered; additionally, preservative-free artificial tears (cross-linked hyaluronic acid, trehalose, sterilamine in liposomes) was prescribed three times daily for 3 months.
Outcome Measures
Clinical assessments were conducted at three time points: 1 week preoperatively before the first LLLT/sham session (T0), 1 week postoperatively before the second LLLT/sham session (T1), and 1 month postoperatively (T2). At each visit, a standardized, non-invasive ocular surface evaluation was carried out. The outcome measures included: Ocular Surface Disease Index (OSDI) score, tear film breakup time (BUT), Schirmer test type 1 (without anesthesia), tear osmolarity (TearLab™ osmometer), and ocular surface staining (Oxford score). All assessments were performed under consistent environmental conditions by the same experienced examiner, who was masked to the treatment allocation to ensure unbiased data collection. Figure 1 illustrates the timeline of ocular surface evaluations and treatment interventions.
Fig. 1.
Schematic overview of the timing of ocular surface assessments and treatments. Patients underwent an ocular surface evaluation 1 week before and 1 week after cataract surgery, followed by either low-level light therapy (LLLT, left panel) or sham treatment (right panel). A final ocular surface examination was performed in both groups 1 month postoperatively
Statistical Analysis
Statistical analyses were performed using SPSS for Macintosh (version 30.0.0.0, SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as mean ± standard deviation (SD), and the Shapiro–Wilk test was used to assess data normality. Between-group comparisons for continuous variables were conducted using independent t tests or Mann–Whitney U tests, depending on data distribution. Categorical variables were compared using Fisher’s exact test. To evaluate interaction effects between treatment and time, a two-way mixed ANOVA was applied. Within-group changes were analyzed using repeated-measures ANOVA, and one-way ANOVA was used to compare groups at each follow-up time point.
A post hoc power analysis was performed using G*Power software (version 3.1.9.6), based on the primary outcome (OSDI score) difference between the treatment and control groups. The estimated effect size (Cohen’s d) was 0.93. With a two-sided test at an alpha level of 0.05, the calculated statistical power was approximately 0.99 (99%), confirming that the sample size (n = 89) was sufficient to detect the observed difference. A p value < 0.05 was considered statistically significant.
Results
Study Population
A total of 315 patients were evaluated for eligibility during the study treatment. Among these, 217 were excluded (208 did not meet the inclusion or exclusion criteria, six declined to participate, and three for other unspecified reasons). The remaining 98 patients were randomized, with 50 assigned to the LLLT group and 48 to the sham group. During the study period, five patients were lost to follow-up (three in the LLLT group and two in the sham group) primarily due to scheduling conflicts or other health issues, while four were excluded due to postoperative complications unrelated to the study treatment. Ultimately, 89 patients (45 males, 44 females; mean age of 73.75 ± 7.95 years) completed the entire study and were included in the final analysis. Given the low and balanced dropout rate, the power of the study remained above 80%, as originally calculated. No significant differences in baseline demographics were observed between the groups in terms of age (p = 0.636) or sex distribution (p = 1.000). The patient recruitment and allocation process is illustrated in Fig. 2.
Fig. 2.
CONSORT flow diagram of the randomized controlled trial. This diagram illustrates the number of patients assessed for eligibility, randomized, allocated to intervention groups, and followed throughout the study period. CONSORT consolidated standards of reporting trials, LLLT low-level light therapy
Ocular Surface Parameters
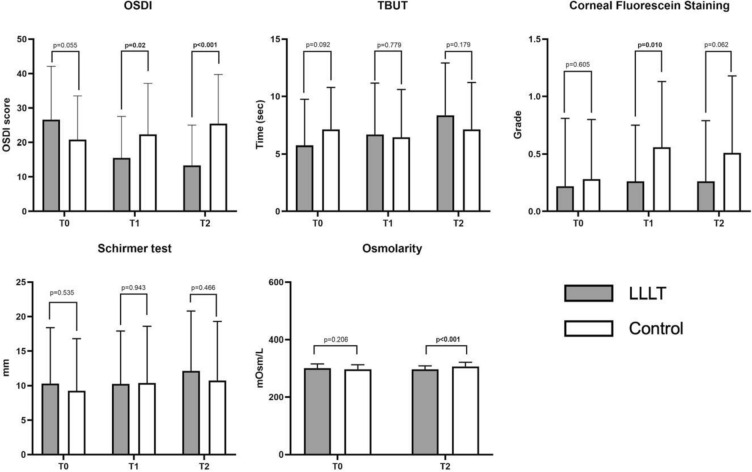
A comprehensive assessment of ocular surface health was performed at baseline (T0), 1 week (T1), and 1 month (T2) after cataract surgery to evaluate the effect of perioperative LLLT on both subjective symptoms and objective clinical indicators of DED. Time-dependent changes within each group and comparisons between the treatment groups at each time point were analyzed to identify statistically and clinically meaningful effects, as summarized in Table 1. The detailed results for each parameter are described below and reported in Fig. 3.
Table 1.
Ocular parameters measured during each time point in patients undergoing cataract surgery and randomised to receive low-level light therapy or sham treatment
| Parameter | Group | T0 | T1 | T2 | p value* |
|---|---|---|---|---|---|
| OSDI | LLLT | 26.62 ± 15.42 | 15.53 ± 12.04 | 13.36 ± 11.69 | < 0.001 |
| Control | 20.80 ± 12.67 | 22.32 ± 14.86 | 25.50 ± 14.26 | 0.033 | |
| p value† | 0.055 | 0.02 | < 0.001 | ||
| BUT (s) | LLLT | 5.76 ± 3.99 | 6.69 ± 4.48 | 8.38 ± 4.53 | 0.002 |
| Control | 7.14 ± 3.65 | 6.45 ± 4.15 | 7.14 ± 4.08 | 0.416 | |
| p value† | 0.092 | 0.799 | 0.179 | ||
| Corneal staining (Oxford) | LLLT | 0.22 ± 0.59 | 0.26 ± 0.49 | 0.26 ± 0.53 | 0.770 |
| Control | 0.28 ± 0.52 | 0.56 ± 0.57 | 0.51 ± 0.67 | 0.003 | |
| p value† | 0.605 | 0.010 | 0.062 | ||
| Schirmer test (mm/5′) | LLLT | 10.29 ± 8.10 | 10.27 ± 7.62 | 12.11 ± 8.71 | 0.129 |
| Control | 9.25 ± 7.59 | 10.39 ± 8.20 | 10.77 ± 8.52 | 0.430 | |
| p value† | 0.535 | 0.943 | 0.466 | ||
| Tear osmolarity (mOsm/l) | LLLT | 300.69 ± 14.19 | – | 296.11 ± 12.30 | 0.048 |
| Control | 296.66 ± 15.64 | – | 306.20 ± 14.59 | 0.002 | |
| p value† | 0.206 | – | < 0.001 |
ANOVA analysis of variance, LLLT low-level light therapy, OSDI Ocular Surface Disease Index, BUT break-up time. Bold indicates statistical significance
*Time effect in each group; repeated-measures ANOVA
†Treatment effects at each time point; one-way ANOVA
Fig. 3.
Comparison of ocular-surface parameters at each follow-up in patients with cataracts randomized to LLLT versus sham treatment. LLLT low-level light therapy, OSDI Ocular Surface Disease Index, TBUT tear break-up time, T0 1 week before surgery, T1 1 week after surgery, T2 1 month after surgery. Bold p values indicate statistical significance (p < 0.05)
Ocular Surface Disease Index (OSDI)
A significant interaction between treatment and time was observed for OSDI scores (p < 0.001). In the LLLT group, OSDI scores improved substantially from T0 (26.62 ± 15.42) to T1 (15.53 ± 12.04) and T2 (13.36 ± 11.69), with a significant within-group time effect (p < 0.001). Conversely, the control group exhibited a worsening trend, with OSDI scores increasing from T0 (20.80 ± 12.67) to T1 (22.32 ± 14.86) and T2 (25.50 ± 14.26), also showing a significant time effect (p = 0.033). Between-group comparisons revealed statistically significant differences at both T1 (15.53 ± 12.04 vs. 22.32 ± 14.86; p = 0.02) and T2 (13.36 ± 11.69 vs. 25.50 ± 14.26; p < 0.001), indicating a higher improvement of subjective symptoms in patients treated with LLLT.
Tear Film Break-Up Time (BUT)
BUT demonstrated a significant treatment-time interaction (p = 0.002). Patients in the LLLT group showed a steady increase in BUT from T0 (5.76 ± 3.99 s) to T1 (6.69 ± 4.48 s), reaching 8.38 ± 4.53 s at T2. This improvement was statistically significant within the group (p = 0.002). In contrast, the control group showed no significant change across time points (T0: 7.14 ± 3.65 s, T1: 6.45 ± 4.15 s, T2: 7.14 ± 4.08 s; p = 0.416). However, between-group comparisons did not reach statistical significance at any time point (p > 0.05), possibly due to inter-individual variability and a relatively small sample size.
Tear Osmolarity
Tear osmolarity levels significantly improved in the LLLT group, with a reduction from 300.69 ± 14.19 mOsm/l at T0 to 296.11 ± 12.30 mOsm/l at T2 (p = 0.048). In contrast, the control group experienced a significant increase in osmolarity, from 296.66 ± 15.64 mOsm/l at T0 to 306.20 ± 14.59 mOsm/l at T2 (p = 0.002). The between-group difference at T2 was statistically significant (p < 0.001), highlighting the beneficial effect of LLLT in stabilizing tear film osmolarity postoperatively.
Corneal Fluorescein Staining
Corneal fluorescein staining scores (Oxford scale) remained stable in the LLLT group across all time points (from 0.22 ± 0.59 at T0 to 0.26 ± 0.49 at T1 and 0.26 ± 0.53 at T2; p = 0.770), suggesting no significant postoperative worsening of corneal epithelium integrity. In contrast, the control group showed a substantial increase in staining scores, indicating damage of the corneal epithelium (from 0.28 ± 0.52 at T0 to 0.56 ± 0.57 at T1 and 0.51 ± 0.67 at T2; p = 0.003). A significant between-group difference was noted at T1 (p = 0.010), emphasizing the protective effect of LLLT on the ocular surface during the early postoperative period.
Schirmer Test
No statistically significant interaction or differences were found in Schirmer test (type 1) values within or between the two groups. In the LLLT group, mean values were 10.29 ± 8.10 mm/5′ at T0, 10.27 ± 7.62 mm/5′ at T1, and 12.11 ± 8.71 mm/5′ at T2 (p = 0.129). Control group values were 9.25 ± 7.59 mm/5′ at T0, 10.39 ± 8.20 mm/5′ at T1, and 10.77 ± 8.52 mm/5′ at T2 (p = 0.430). These data indicate that tear production, as measured by the Schirmer test, was not significantly affected by LLLT in this cohort.
Safety and Tolerability
No adverse events related to the treatment sessions were reported in either group throughout the treatment and follow-up periods. The procedure was well tolerated and did not interfere with the standard postoperative course.
Discussion
The present study demonstrated that prophylactic LLLT administered one week before and one week after cataract surgery in consecutive patients significantly improves ocular discomfort symptoms and ocular surface parameters; in contrast, both subjective and objective parameters worsened postoperatively in the control group because of the detrimental effects related to surgery. These findings suggest that LLLT may enhance postoperative comfort and increase overall patient satisfaction with surgical recovery.
In the LLLT arm, BUT and OSDI improved markedly, whereas corneal staining remained unchanged, indicating that the integrity of corneal epithelium was preserved despite the surgical injury. On the contrary, in the control group, there was a significant worsening of symptoms as well as an increase in corneal fluorescein staining score. Notably, tear osmolarity decreased significantly in the LLLT group at 1 month postoperatively, whereas it increased in the control group. Schirmer test values did not change from baseline in either group after surgery. This is not surprising, since it mainly measures aqueous tear volume, whereas iatrogenic DED involves multiple mechanisms beyond aqueous deficiency [21]. The selective improvement in tear osmolarity highlights the multifactorial nature of postoperative dry eye and suggests that LLLT may exert effects beyond tear volume restoration, potentially modulating inflammatory pathways and epithelial cell homeostasis. In addition, from a clinical perspective, this finding highlights the value of tear osmolarity as a sensitive biomarker for both ocular surface stress and recovery, potentially guiding personalized therapeutic strategies during the perioperative period.
Giannaccare et al. reported for the first time the prophylactic use of LLLT before cataract surgery to avoid the occurrence of iatrogenic DED in otherwise healthy patients. In their double-masked, randomized study, 131 healthy patients received LLLT or sham treatment [19]. Consistent with our findings, BUT and OSDI improved only in the LLLT arm in their study [19], while tear-meniscus height (TMH), redness score and meibomian gland loss were assessed showing no changes after surgery. Unlike that study, the present one focused on a more heterogeneous, real-world population of consecutive patients reflecting the routine clinical setting characterized by a wide patient variability; furthermore, tear osmolarity was evaluated herein for the first time. Elevated tear osmolarity is recognized as a trigger for ocular surface inflammation and cellular stress and is therefore considered an important process in the development, progression, and aggravation of DED [22, 23]. In addition, tear hyperosmolarity has been associated with poorer surgical outcomes and lower patient satisfaction in cataract surgery candidates with mild ocular surface disease [24, 25]. According to the most recent TFOS III report, there may be a relationship between changes in tear osmolarity and iatrogenic effects, and osmolarity may be linked to certain DED subtypes [26]. To date, no previous studies have specifically analyzed the impact of LLLT alone on this parameter. While Marta et al. reported an increase in tear osmolarity following combined intense pulsed light (IPL) and LLLT in 31 patients with MGD [27], Pérez-Silguero et al. observed a significant reduction in tear osmolarity in a larger cohort of 156 patients treated with the same combination therapy [28]. In contrast, our study was the first to evaluate the independent effect of prophylactic LLLT on tear osmolarity in a surgical setting. Notably, we provided novel evidence that LLLT alone may exert a protective role in preventing the typical postoperative rise in tear osmolarity, thereby supporting its potential utility in maintaining ocular surface stability after cataract surgery.
The increasing popularity of device-based treatments in ophthalmology has led to their use both in DED management and ocular surface optimization before cataract surgery. In this context, several studies examining vectored thermal pulsation therapy applied preoperatively have reported significant improvements in meibomian gland function and tear film stability [29–32]. Indeed, vectored thermal pulsation therapy is an invasive procedure that specifically targets the meibomian glands by applying synchronized thermal and mechanical energy to the eyelids, which increases gland temperature and promotes meibum expression [29–32]. Moreover, even in patients without preoperative MGD, the application of this treatment prevented the occurrence of MGD postoperatively and favorably affected tear-film parameters [30]. Also IPL, another device-based treatment, targets the meibomian glands and uses high-intensity heat with photocoagulation to improve meibum flow. Only a few studies have examined the preoperative use of IPL before cataract surgery, focusing mainly on patients with MGD-related DED [33, 34]. In contrast to the above-mentioned technologies, LLLT is gaining increasing attention in the armamentarium of methods used to treat the ocular surface, thanks to its non-invasive nature, crucial for the prophylactic use. Literature assessing LLLT as a stand-alone treatment for DED is growing, reporting improvements in both lacrimal secretion and meibomian gland function [35–39]. Iatrogenic DED after cataract surgery is caused by multiple intertwined mechanisms including surgery-induced inflammation and cytotoxic effects of peri-operative medications among other. LLLT represents a promising approach for the management of multifactorial, iatrogenic DED after cataract surgery due to its anti-inflammatory, tissue-repairing, and analgesic effects as well as its ability to increase meibum flow from the meibomian glands through endogenous heating [40]. A major advantage of this therapy is its non-invasive and ease-of-use nature, with no need for operator presence during the entire treatment. The availability of compact, portable LLLT devices further supports its practicality even for home use. Additionally, the application of a standardized protocol and the minimal training required for its application make LLLT a suitable option for integration into routine clinical practice as part of standard perioperative care [39].
The relatively short follow-up period of 1 month limits the assessment of the long-term sustainability and durability of treatment effects, particularly for chronic conditions like DED. Future studies should incorporate longer follow-up intervals of at least 3–6 months to monitor prolonged therapeutic benefits. Additionally, stratified analyses based on baseline disease severity according to specific ocular surface parameters could help identify subgroups that derive the greatest benefit from LLLT and establish more tailored treatment algorithms. Multicenter trials involving larger cohorts are warranted to strengthen the evidence and validate these findings.
Conclusions
In conclusion, this prospective double-masked randomized controlled clinical trial supports the perioperative use of LLLT as a safe and non-invasive modality that can be easily incorporated in the workflow of routine cataract surgery as an effective supportive treatment for the prevention of iatrogenic DED.
Acknowledgements
The authors would like to extend their heartfelt gratitude to all the colleagues and staff from the Cai Ferate Clinical Hospital of Iasi, Romania for their invaluable support and collaboration throughout the research period. Furthermore, the authors wish to express their deepest appreciation to all the participants who generously contributed to this research.
Author Contribution
Conceptualization, Mihaela-Madalina Timofte-Zorila, Nicoleta Vlas, Giuseppe Giannaccare and Daniel Constantin Branisteanu; Data curation, Mihaela-Madalina Timofte-Zorila, Filippo Lixi, Nicoleta Vlas and Daniel Constantin Branisteanu; Formal analysis, Mariana Pavel-Tanasa, Filippo Lixi, Mario Troisi, Gamze Özkan and Giulia Coco; Investigation, Mihaela-Madalina Timofte-Zorila, Daniel Constantin Branisteanu, Cristina Preda, Mariana Pavel-Tanasa and Sinziana Istrate; Methodology, Mihaela-Madalina Timofte-Zorila, Daniel Constantin Branisteanu, and Giuseppe Giannaccare; Project administration, Mihaela-Madalina Timofte-Zorila, Daniel Constantin Branisteanu, Giuseppe Giannaccare, and Mariana Pavel-Tanasa; Software, Filippo Lixi, Mario Troisi, Gamze Özkan and Assem Namazbayeva; Supervision, Mihaela-Madalina Timofte-Zorila, Nicoleta Vlas, Giuseppe Giannaccare, and Daniel Constantin Branisteanu; Visualization, Mihaela-Madalina Timofte-Zorila, Giuseppe Giannaccare, Giulia Coco, Filippo Lixi, and Mario Troisi; Writing—original draft, Mihaela-Madalina Timofte-Zorila, Filippo Lixi, Mario Troisi, Gamze Özkan, and Sinziana Istrate; Writing—review and editing, Mihaela-Madalina Timofte-Zorila, Filippo Lixi, Mario Troisi, Giuseppe Giannaccare, Giulia Coco, Assem Namazbayeva, Mariana Pavel-Tanasa and Daniel Constantin Branisteanu. All authors have read and agreed to be accountable for the content of the work.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
Mihaela-Madalina Timofte-Zorila, Filippo Lixi, Nicoleta Vlas, Mario Troisi, Gamze Özkan, Mariana Pavel-Tanasa, Sinziana Istrate, Cristina Preda, Giulia Coco, Assem Namazbayeva, Giuseppe Giannaccare and Daniel Constantin Branisteanu declare no conflict of interest. Patients were not involved in the design of the study or the dissemination of the results.
Ethical Approval
The study was approved by the local Ethics Committee of the Cai Ferate Clinical Hospital of Iasi, Romania (approval number: 54/29.07.2024) and retrospectively registered at ClinicalTrials.gov (NCT07067294) on 05.07.2025. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all participants prior to inclusion in the study.
References
- 1.Chen X, Xu J, Chen X, Yao K. Cataract: advances in surgery and whether surgery remains the only treatment in future. Adv Ophthalmol Pract Res. 2021;1(1): 100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. Tfos dews II iatrogenic report. Ocul Surf. 2017;15(3):511–38. [DOI] [PubMed] [Google Scholar]
- 3.Sutu C, Fukuoka H, Afshari NA. Mechanisms and management of dry eye in cataract surgery patients. Curr Opin Ophthalmol. 2016;27(1):24–30. [DOI] [PubMed] [Google Scholar]
- 4.Oh T, Jung Y, Chang D, Kim J, Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012;56(2):113–8. [DOI] [PubMed] [Google Scholar]
- 5.Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090–6. [DOI] [PubMed] [Google Scholar]
- 6.Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC, Yeu E, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669–84. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Li M, Chen J, Zhao J, Pazo EE, Qin G, He X. To evaluate the effects of artificial tears on ocular biological parameters in dry eye and non-dry eye patients. Sci Rep. 2025;15(1): 12392. 10.1038/s41598-025-95801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teshigawara T, Meguro A, Mizuki N. The effect of rebamipide on refractive accuracy of cataract surgery in patients with dry eye. Ophthalmol Ther. 2022;11(2):603–11. 10.1007/s40123-022-00457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park Y, Hwang HB, Kim HS. Observation of influence of cataract surgery on the ocular surface. PLoS ONE. 2016;11(10): e0152460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Gouvea L, Mimouni M, Trinh T, Santaella G, Cohen E, et al. Treatment of dry eyes with lifitegrast 5% before cataract surgery: A prospective trial. Pan-Am J Ophthalmol. 2024;6(3):98. 10.4103/pajo.pajo_34_24.
- 12.Fogagnolo P, Favuzza E, Marchina D, Cennamo M, Vignapiano R, Quisisana C, et al. New therapeutic strategy and innovative lubricating ophthalmic solution in minimizing dry eye disease associated with cataract surgery: a randomized, prospective study. Adv Ther. 2020;37(4):1664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue F, Zhou Y. Illuminating eye care: the promise and future of red light therapy in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2025. 10.1007/s00417-025-06800-1. [DOI] [PubMed] [Google Scholar]
- 15.Tan NRX, Chan KE, Lim BXH, Giannaccare G, Najjar RP, Lim CHL. Photobiomodulation: evidence and applications in ophthalmology. Curr Opin Ophthalmol. 2025. 10.1097/ICU.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 16.Glass GE. Photobiomodulation: a review of the molecular evidence for low-level light therapy. J Plast Reconstr Aesthet Surg. 2021;74(5):1050–60. [DOI] [PubMed] [Google Scholar]
- 17.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, et al. Photobiomodulation—underlying mechanism and clinical applications. J Clin Med. 2020;9(6): 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannaccare G, Rossi C, Borselli M, Carnovale Scalzo G, Scalia G, Pietropaolo R, et al. Outcomes of low-level light therapy before and after cataract surgery for the prophylaxis of postoperative dry eye: a prospective randomised double-masked controlled clinical trial. Br J Ophthalmol. 2024;108(8):1172–6. [DOI] [PubMed] [Google Scholar]
- 20.Rossi T, Romano MR, Iannetta D, Romano V, Gualdi L, D’Agostino I, Ripandelli G. Cataract surgery practice patterns worldwide: a survey. BMJ Open Ophthalmol. 2021;6(1): e000464. 10.1136/bmjophth-2020-000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SW, Hsu SL, Hsu CC. Real-world practice patterns for dry eye diagnosis: a multicenter observational study in Taiwan. Jpn J Ophthalmol. 2025;69(3):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell CR, Feulner L, Djonov V, Pavlovic D, Volarevic V. The molecular mechanisms responsible for tear hyperosmolarity-induced pathological changes in the eyes of dry eye disease patients. Cells. 2023;12(23): 2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troisi M, Troisi S, Strianese D, Rinaldi M, Turco MV, Costagliola C. Clinical and biological evaluation of NAAGA versus azelastine eye drops in patients with allergic conjunctivitis and tear film dysfunction: a randomized controlled trial. Ophthalmol Ther. 2025;14(7):1581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolffsohn JS, Benítez-Del-Castillo J, Loya-Garcia D, Inomata T, Iyar G, Liang L, et al. TFOS Collaborator Group. TFOS DEWS III Diagnostic Methodology. Am J Ophthalmol. 2025;S0002-9394(25)00275-2. 10.1016/j.ajo.2025.05.033. [DOI] [PubMed]
- 25.Sullivan BD, Palazón de la Torre M, Yago I, Duarte R, Schallhorn JM, Nijm LM, et al. Tear film hyperosmolarity is associated with increased variation of light scatter following cataract surgery. Clin Ophthalmol. 2024;18:2419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton F, Argüeso P, Asbell P, Azar D, Bosworth C, Chen W, et al. TFOS DEWS III digest report. Am J Ophthalmol. 2025. [DOI] [PubMed]
- 27.Marta A, Baptista PM, Heitor Marques J, Almeida D, José D, Sousa P, Barbosa I. Intense pulsed plus low-level light therapy in Meibomian gland dysfunction. Clin Ophthalmol. 2021;15:2803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Silguero MA, Pérez-Silguero D, Rivero-Santana A, Bernal-Blasco MI, Encinas-Pisa P. Combined intense pulsed light and low-level light therapy for the treatment of dry eye: a retrospective before-after study with one-year follow-up. Clin Ophthalmol. 2021;15:2133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Li J, Xue K, Xie J, Xie G, Gu S. Preoperative management of MGD with vectored thermal pulsation before cataract surgery: a prospective, controlled clinical trial. Semin Ophthalmol. 2021;36(1–2):2–8. [DOI] [PubMed] [Google Scholar]
- 30.Park J, Yoo YS, Shin K, Han G, Arita R, Lim DH, et al. Effects of lipiflow treatment prior to cataract surgery: a prospective, randomized, controlled study. Am J Ophthalmol. 2021;230:264–75. [DOI] [PubMed] [Google Scholar]
- 31.Mencucci R, Mercuri S, Cennamo M, Morelli A, Favuzza E. Efficacy of vector thermal pulsation treatment in reducing postcataract surgery dry eye disease in patients affected by meibomian gland dysfunction. J Cataract Refract Surg. 2023;49(4):423–9. [DOI] [PubMed] [Google Scholar]
- 32.Matossian C, Chang DH, Whitman J, Clinch TE, Hu J, Ji L, et al. Preoperative treatment of meibomian gland dysfunction with a vectored thermal pulsation system prior to extended depth of focus IOL implantation. Ophthalmol Ther. 2023;12(5):2427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawagoe T, Mizuki Y, Akaishi M, Takeuchi M, Yabuki K, Hata S, et al. Effect of preoperative dry eye treatment with intense pulsed light with meibomian gland expression on the refractive accuracy of cataract surgery in patients with meibomian gland dysfunction-related dry eye: a single-center, prospective, open-label study. J Clin Med. 2025. 10.3390/jcm14082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teshigawara T, Akaishi M, Mizuki Y, Takeuchi M, Yabuki K, Hata S, et al. Dry eye treatment with intense pulsed light for improving visual outcomes after cataract surgery with diffractive trifocal intraocular lens implantation. J Clin Med. 2024. 10.3390/jcm13226973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antwi A, Nti AN, Ritchey ER. Thermal effect on eyelid and tear film after low-level light therapy and warm compress. Clin Exp Optom. 2024;107(3):267–73. 10.1080/08164622.2023.2206950. [DOI] [PubMed] [Google Scholar]
- 36.Giannaccare G, Pellegrini M, Carnovale Scalzo G, Borselli M, Ceravolo D, Scorcia V. Low-level light therapy versus intense pulsed light for the treatment of meibomian gland dysfunction: preliminary results from a prospective randomized comparative study. Cornea. 2023;42(2):141–4. 10.1097/ICO.0000000000002997. [DOI] [PubMed] [Google Scholar]
- 37.Park Y, Kim H, Kim S, Cho KJ. Effect of low-level light therapy in patients with dry eye: a prospective, randomized, observer-masked trial. Sci Rep. 2022;12(1): 3575. 10.1038/s41598-022-07427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antwi A, Schill AW, Redfern R, Ritchey ER. Effect of low-level light therapy in individuals with dry eye disease. Ophthalmic Physiol Opt. 2024;44(7):1464–71. 10.1111/opo.13371. [DOI] [PubMed] [Google Scholar]
- 39.Giannaccare G, Vaccaro S, Pellegrini M, Borselli M, Carnovale Scalzo G, Taloni A, Pietropaolo R, Odadi AS, Carnevali A. Serial sessions of a novel low-level light therapy device for home treatment of dry eye disease. Ophthalmol Ther. 2023;12(1):459–68. 10.1007/s40123-022-00619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markoulli M, Chandramohan N, Papas EB. Photobiomodulation (low-level light therapy) and dry eye disease. Clin Exp Optom. 2021;104(5):561–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.