Abstract
Background
: Neurocognitive disorders, including dementia and mild cognitive impairment, are increasingly prevalent, demanding efficient detection and management strategies.
Objectives
: This study is part of the Puglia Region’s initiative under the Italian Dementia National Plan (DNP) and aimed to assess the capacity of the Lecce province healthcare system to identify new neurocognitive disorders cases by comparing observed cases with expected rates derived from meta-analyses and Global Burden of Disease estimates.
Design
: Using complete case ascertainment across 10 hospital-based and community centers, a total of 857 incident cases were identified in one year, including 441 Minor neurocognitive disorders (51.46 %) and 416 major neurocognitive disorders cases (48.54 %).
Setting
: Public Centers for Cognitive Disorders and Dementia (CCDDs) across hospital and community services in the Lecce province, Southern Italy.
Participants
: Eligible participants included all individuals aged between 65 and 89 residing in the Lecce province who received a diagnosis of neurocognitive disorder. 857 participants were enrolled (519 females – 338 males).
Measurements
: Incident cases of neurocognitive disorder, both minor and major, accordingly to DSM-5 criteria.
Results
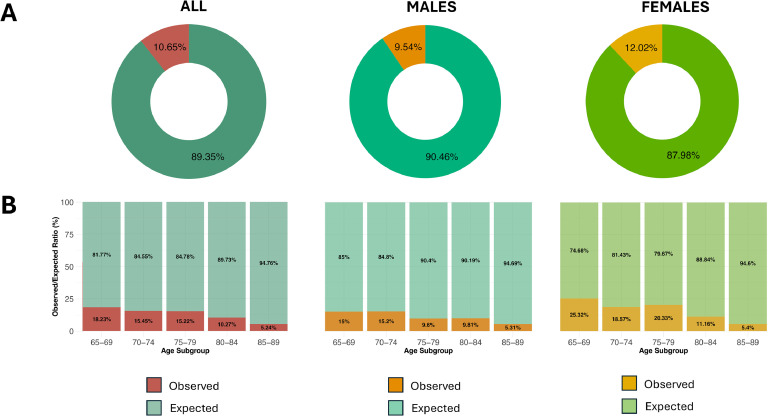
: Only 10.65 % of expected major neurocognitive disorders and 7.24 % of expected minor neurocognitive disorders cases were detected, with significant age and sex disparities, with higher underdetection rates in females. Detection rates declined with advancing age, with the observed-to-expected ratio for major neurocognitive disorders falling from 18.23 % in individuals aged 65–69 years to just 5.24 % in those aged 85–89 years. These findings were validated against Global Burden of Disease estimates.
Conclusions
: This study highlights the critical gaps in detecting neurocognitive disorders, particularly in older adults and prodromal stages such as minor neurocognitive disorders, where early intervention could yield the greatest benefits. The findings underscore the urgent need for targeted reforms to improve e diagnostic pathways and better align healthcare systems with emerging disease-modifying therapies and preventive strategies.
Keywords: Dementia, Cognitive impairment, Incidence, Healthcare system
1. Introduction
Elderly population is steadily growing (1 in 11 is over the age of 65) according to World Population Prospects 2019 (United Nations, 2019, projected to 1 in 6 in 2050)[1]. Consequently, the prevalence of age-related conditions is rapidly increasing worldwide[2] and, according to the Global Burden of Disease Study (GBD), the number of people with Dementia is expected to reach 152.8 million cases in 2050[3]. Dementia includes a wide array of conditions that can be challenging to diagnose since they require specialized neurological or geriatric expertise, a multidisciplinary approach as well as ancillary investigations. Italy is one of European country with the highest proportion of older people, accounting for the 24.0 % of the total national population, according to the 2023 Eurostat data, and with the aging index (ratio of the number of individuals aged 65 years and older to every 100 individuals younger than 14 years in a given population) estimated to almost double by 2051[4].
However, several relevant issues were still present in the management of the Dementia in Italy: 1. lack of national standards and guidelines (at the time of the study, September 2023); 2. disparity in terms of available resources and services across the Italian regions; 3. scarce integration and collaboration between primary and secondary care settings, but also with community services and home care, potentially negatively affecting the continuity of care required across different stages of disease[5]. Aiming at improving the complex process of dementia care, the first “Dementia National Plan” (DNP) was formulated in October 2014 by the Italian Ministry of Health in close cooperation with the regions (health and social sectors), the “Istituto Superiore di Sanità” (National Institute of Health) and the three major national associations of patients and carers. This represented the most important public health strategic document focusing on promoting and improving interventions in the dementia field and the support of patients and families throughout the care pathway[5]. Nevertheless, this strategy has been funded in 2021 by the institution of the “Fund for Alzheimer's and Dementia 2021–2023”, inviting all Italian regions to participate proposing regional projects on how to improve the dementia care-management.
This study, as part of the Puglia Region DNP, aimed to assess the capacity of the healthcare system in the Lecce province to identify and manage new cases of neurocognitive disorders (NCDs)[6]. Indeed, despite the country’s public healthcare system providing universal access to care, data suggest that not all individuals with cognitive disorders are adequately captured[[7], [8], [9]]. Specifically, we estimated the incidence of Major Neurocognitive Disorder (MaNCD) and Mild Cognitive Impairment (MCI), comparing observed cases with those expected based on global estimates. Dementia underdetection is a pressing concern both in low-income countries as well as in high- to medium-income countries[9,10], where despite advanced healthcare infrastructure, many cases are still unrecognized, particularly in early stages, with a rate of almost 60 %, as highlighted in previous studies and meta-analysis[8]. This may be often due to healthcare professionals' reliance on more overt symptoms or barriers such as time constraints during consultations, lack of dementia-specific training for primary care providers, and limited access to diagnostic tools[11]. This delayed detection can prevent timely interventions, which are crucial for managing symptoms and improving the quality of life for individuals affected by the disease.
Using both local data and meta-analytic estimates from published cohort studies, we sought to quantify the extent of underdiagnosis within the Lecce province public healthcare CCDDs’ system. The primary study aim was to understand how well the CCDDs healthcare system is equipped to detect all cases with specific focus on the early stages of cognitive decline, such as MCI, the prodromal clinical phase of dementia, in the context of recent approvals of disease-modifying therapies.
2. Methods
2.1. Study population and design
This study was conducted as part of the Puglia Region's DNP with the main aim to improve the diagnosis and care of patients with NCDs. The project focused on standardizing clinical tools, pathways, and training healthcare professionals to prepare the regional health system for the integration of Disease-Modifying Therapies (DMTs) for MCI. This project aims also to address the gap in evidence regarding the healthcare system’s capacity to capture and manage cognitive disorders cases in Italy. The study period spanned between September 1, 2023, and August 31, 2024.
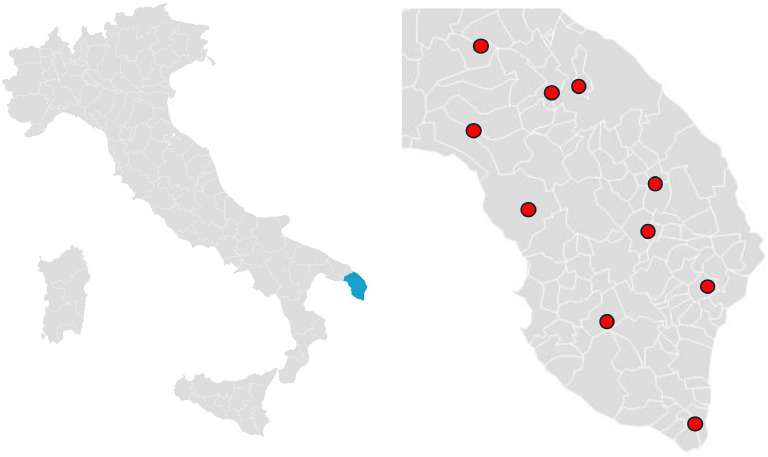
The project involved all the 10 Centers for Cognitive Disorders and Dementia (CCDDs) located across the Salento region. Those included both hospital- and community-based centers, actively operating and covering all urban and rural areas, hence ensuring best possible case ascertainment for all incident diagnoses, high data reliability and comprehensive coverage of the geographical area (Fig. 1). The Lecce province has a total population of 767.231 within an area of 2.759 km², with a population ≥65y of 200.247, an Aging Index of 229.9 (Italy- 199.8) and 497 General Practitioners-GPs actively operating in the area.
Fig. 1.
Map of Center for Dementia and Cognitive Disorders (CCDD) distribution in Lecce Province.
Cases were collected prospectively, and clinical data were anonymized for analysis. Ethical approval was obtained from the regional ethics committee (ASL LE, Verbal N° 13, 8th March 2023, protocol number: 34,441/2023), and written informed consent was secured from all participants or their legal representatives. The study adhered to the principles outlined in the Declaration of Helsinki. Data confidentiality was maintained through anonymization and secure data storage.
2.2. Participants
Eligible participants included all individuals aged between 65 and 89 residing in the Lecce province who received a new incident diagnosis of NCD throughout the CCDDs’ system of the public national healthcare system. Diagnosis included both MCI, a condition characterized by cognitive decline greater than expected for an individual's age and education level but not severe enough to interfere significantly with daily life activities, or MaNCD, a condition involving significant cognitive decline from a previous level of performance that interferes with independence in everyday activities, during the study period. Diagnoses were made according to DSM-5 criteria[6] Cases of individuals residing outside the geographical area of interest were excluded. Clinical data included demographic information including age, sex, education and residence.
2.3. Case ascertainment
Incident diagnoses were identified through the participating CCDDs. Trained neurologists and geriatricians conducted evaluations using a standardized diagnostic protocol, based on the DSM-5 criteria for Major and Minor Neurocognitive Disorders[6]. Clinical assessments included neurological examination cognitive testing (Mini-Mental State Examination (MMSE)[12] and functional scales (Basic Activities of Daily Living - BADL[13] /Instrumental Activities of Daily Living - IADL)[14], and neuroimaging (e.g., MRI, CT) when appropriate. However, specific combinations of cognitive and imaging tools may have varied across centers. Diagnoses were cross-verified by an independent panel of experts to ensure consistency and accuracy.
2.4. Statistical analysis
Descriptive statistics were calculated for demographic variables. Continuous variables were summarized as means and standard deviations or medians and interquartile ranges, while categorical variables were presented as frequencies and percentages.
Age-specific, sex-specific, and age-sex–specific incidence rates for MCI and MaNCD were computed using the reference population of Lecce province as of January 1, 2024, obtained from ISTAT demographic data[15]. Population denominators were assumed constant over the 12-month study period. Incidence rates were expressed per 1000 person-years, and 95 % confidence intervals (CIs) were calculated using the Poisson exact method.
We obtained sex-age specific pooled incidence rates for MaNCD by meta-analysing published cohort studies using data from Wolters et al.[16]. Although Wolters and colleagues presented incidence estimates from seven individual cohorts, they did not provide pooled incidence rates across studies. To address this, we extracted incidence data from the following cohorts: PAQUID[17], Rotterdam Study[18], Framingham Heart Study[19], Gothenburg Studies[20], CFAS-I[21], CFAS-II[21], Three Cities Study[22], and AGES-Reykjavik Study[23]. For MCI, meta-analytic estimates were derived from Gillis et al.[24]. Additionally, incidence estimates from the Global Burden of Disease (GBD) 2021 study were incorporated to validate findings. For this analysis, we specifically extracted age- and sex-specific incidence estimates for “Alzheimer’s disease and other dementias” referring to the Italian population, which we used as a proxy for Major Neurocognitive Disorder, in order to provide a geographically and temporally relevant comparison.
Expected cases of MaNCD and MCI were estimated by multiplying the sex-age specific pooled incidence rate times the respective population in the province of Lecce over 1000 people.
Observed-to-expected ratios were calculated for both MCI and MaNCD by comparing observed cases with expected cases across age subgroups. To verify potential differences in proportions of observed versus expected cases across age subgroups, Cochran–Armitage test for trend was conducted. In addition, we performed a sensitivity analysis using the pooled estimates from the PAQUID[17] and Three Cities Study[22] only, which were conducted in Southern France and are closer in cultural, demographic and geographic characteristics to South Italy population. Statistical significance of differences in sex proportions was assessed through chi-square tests. For the meta-analysis we used the function metagen from the meta package. All statistical analyses were performed using R software (version 4.3.1) and RStudio (version 2023.06.1).
3. Results
During the study period (September 1, 2023, and August 31, 2024), 857 total cases of neurocognitive disorders were identified in the province of Lecce, including 441 cases of MCI and 416 cases of MaNCD (Table 1). The mean age of the overall cohort was 77.13 years (75.93 years for MCI and 78.40 years for MaNCD). Females accounted for 60.56 % of the total population, with a proportion of 56.59 % in MCI and 65.06 % in MaNCD.
Table 1.
Demographic and clinical characteristics of patients with minor and major cognitive disorder in the Province of Lecce.
| Cognitive Disorder in Lecce Province |
|||
|---|---|---|---|
| Minor (N = 441) | Major (N = 416) | ALL (N = 857) | |
| Age (sd) | 75.93 (5.80) | 78.40 (5.71) | 77.13 (5.88) |
| Females % | 249 (56.59 %) | 270 (65.06 %) | 519 (60.56 %) |
| Years of educationa | 7.73 (4.41) | 6.33 (3.93) | 7.05 (4.23) |
| MMSE | |||
| All (65 – 89 years)b | 23.59 (4.96) | 16.44 (5.43) | 20.20 (6.29) |
| 65 – 69 years | 25.85 (3.07) | 17.15 (6.23) | 22.92 (6.01) |
| 70 – 74 years | 23.56 (5.22) | 16.11 (6.11) | 20.87 (6.60) |
| 75 – 79 years | 23.77 (4.38) | 16.31 (5.28) | 20.21 (6.10) |
| 80 – 84 years | 22.20 (5.39) | 17.02 (4.88) | 19.19 (5.70) |
| 85 – 89 years | 21.92 (6.26) | 15.57 (5.42) | 18.03 (6.52) |
| BADL (preserved) | |||
| All (65 – 89 years)c | 5.66 (0.89) | 4.44 (1.53) | 5.07 (1.39) |
| 65 – 69 years | 5.97 (0.18) | 4.57 (1.66) | 5.47 (1.20) |
| 70 – 74 years | 5.81 (0.56) | 4.58 (1.60) | 5.36 (1.22) |
| 75 – 79 years | 5.69 (0.89) | 4.58 (1.30) | 5.16 (1.24) |
| 80 – 84 years | 5.30 (1.29) | 4.27 (1.58) | 4.67 (1.55) |
| 85 – 89 years | 5.38 (1.07) | 4.33 (1.71) | 4.70 (1.59) |
| IADL (preserved) | |||
| All (65 – 89 years)d | 5.46 (2.12) | 2.90 (2.21) | 4.02 (2.51) |
| 65 – 69 years | 6.51 (1.43) | 2.45 (2.33) | 5.05 (2.65) |
| 70 – 74 years | 5.55 (2.12) | 2.92 (2.32) | 4.57 (2.53) |
| 75 – 79 years | 5.57 (2.02) | 3.14 (2.15) | 4.40 (2.41) |
| 80 – 84 years | 4.89 (2.22) | 2.87 (2.20) | 3.64 (2.41) |
| 85 – 89 years | 4.26 (2.37) | 2.72 (2.15) | 3.28 (2.34) |
74 subjects had missing data in years of education, 41 for minor and 33 major cognitive disorder.
51 subjects had missing data in Mini Mental Status Examination (MMSE), 17 for minor and 34 major cognitive disorder.
44 subjects had missing data in Basic Activities of Daily Living (BADL), 28 for minor and 16 major cognitive disorder.
50 subjects had missing data in Instrumental Activities of Daily Living (IADL), 33 for minor and 17 major cognitive disorder.
3.1. Observed incidence of MCI and major neurocognitive disorder in the Salento region
The incidence rates for Major Neurocognitive Disorder (MaNCD) and Mild Cognitive Impairment (MCI) varied across age and sex subgroups, as summarized in Table 2. The overall incidence rate of MaNCD in individuals aged 65–89 years was 2.23 per 1000 person-years (95 % CI: 2.02–2.46), with a significant difference between males (1.77 per 1000 person-years, 95 % CI: 1.49–2.08) and females (2.60 per 1000 person-years, 95 % CI: 2.30–2.93). Age-specific rates slightly increased with age, peaking in the 80–84 year subgroup, with an incidence of 3.98 per 1000 person-years (95 % CI: 3.31–4.75). Males in this subgroup had an incidence of 3.17 per 1000 person-years (95 % CI: 2.27–4.30), while females exhibited a higher rate of 4.56 per 1000 person-years (95 % CI: 3.63–5.65). The overall incidence rate of MCI for individuals aged 65–89 years was 2.37 per 1000 person-years (95 % CI: 2.15 – 2.60). The incidence peaked in the 75–79 year age subgroup, with a rate of 3.45 per 1000 person-years (95 % CI: 2.89–4.08), followed by the 80–84 year subgroup at 2.67 per 1000 person-years (95 % CI: 1.79–2.76). The lowest incidence was recorded in the 65–69 year subgroup, at 1.34 per 1000 person-years (95 % CI: 1.03 – 1.70). New cases and person-year of major and minor cognitive disorders in the province of Lecce by age subgroup and sex are reported in Supplemental Table 1.
Table 2.
Incidence of major and minor cognitive disorder in the province of Lecce by sex and age subgroup.
| Age subgroups | Major Cognitive Disorder |
Minor Cognitive Disorder | ||
|---|---|---|---|---|
| Males | Females | Both | Both | |
| All (65 to 89 years) | 1.77 (1.49 – 2.08) | 2.60 (2.30 – 2.93) | 2.23 (2.02 – 2.46) | 2.37 (2.15 – 2.60) |
| 65 to 69 years | 0.65 (0.36 – 1.07) | 0.76 (0.46 – 1.17) | 0.71 (0.49 – 0.98) | 1.34 (1.03 – 1.70) |
| 70 to 74 years | 1.43 (0.97 – 2.03) | 1.53 (1.09 – 2.10) | 1.49 (1.16 – 1.88) | 2.57 (2.13 – 3.07) |
| 75 to 79 years | 2.06 (1.44 – 2.85) | 3.98 (3.18 – 4.92) | 3.11 (2.58 – 3.72) | 3.45 (2.89 – 4.08) |
| 80 to 84 years | 3.17 (2.27 – 4.30) | 4.56 (3.63 – 5.65) | 3.98 (3.31 – 4.75) | 2.67 (2.12 – 3.30) |
| 85 to 89 years | 3.12 (1.98 – 4.68) | 3.44 (2.49 – 4.64) | 3.32 (2.57 – 4.23) | 1.86 (1.31 – 2.57) |
3.2. Expected incidence of major and minor cognitive disorders based on meta-analysis
The meta-analysis estimated an overall incidence of MaNCD of 16.46 per 1000 person-years (95 % CI: 9.36–23.56) for both sexes combined (Table 3). Females exhibited a higher incidence rate (21.12 per 1000 person-years, 95 % CI: 15.74–26.50) compared to males (13.00 per 1000 person-years, 95 % CI: 5.82–20.19). Age-specific rates showed a sharp increase with advancing age. In the 65–69 year subgroup, the incidence rate was 3.88 per 1000 person-years (95 % CI: 2.39–5.36). This rose to 9.63 per 1000 person-years (95 % CI: 6.19–13.08) in the 70–74 year subgroup, and further to 20.45 per 1000 person-years (95 % CI: 14.37–26.54) in the 75–79 year subgroup. The highest rates were observed in the 80–84 and 85–89 year subgroups, with incidences of 38.74 per 1000 person-years (95 % CI: 29.99–47.50) and 63.39 per 1000 person-years (95 % CI: 51.29–75.48), respectively. Males consistently had higher incidence rates than females in the 65–69, 70–74, and 75–79 age subgroups, whereas females showed higher rates compared to males in the 80–84 and 85–89 subgroups. Observed and expected cases of major cognitive disorders, and Lecce province population by sex and age subgroup based on the meta-analysis are reported in Table 4. Meta-analytic results by age and sex subgroup are shown in supplemental figures 1 to 19.
Table 3.
Incidence of major and minor cognitive disorder based on meta-analysis by sex and age subgroup.
| Age subgroups | Major Cognitive Disordera |
Minor Cognitive Disorderb | ||
|---|---|---|---|---|
| Males | Females | Both | Both | |
| All (65 to 89 years) | 13.00 (5.82 – 20.19) | 21.12 (15.74 – 26.50) | 16.46 (9.36 – 23.56) | – |
| 65 to 69 years | 4.35 (2.66 – 6.04) | 2.98 (1.71 – 4.26) | 3.88 (2.39 – 5.36) | – |
| 70 to 74 years | 9.42 (6.45 – 12.38) | 8.25 (5.60 – 10.89) | 9.63 (6.19 – 13.08) | 24.2 (12.4 – 35.9) |
| 75 to 79 years | 21.42 (13.76 – 29.09) | 19.56 (14.76 – 24.35) | 20.45 (14.37 – 26.54) | 22.5 (5.1 – 51.4) |
| 80 to 84 years | 32.24 (22.86 – 41.63) | 40.87 (34.07 – 47.66) | 38.74 (29.99 – 47.50) | 40.9 (7.7 – 97.5) |
| 85 to 89 years | 58.76 (47.25 – 70.27) | 63.68 (52.07 – 75.30) | 63.39 (51.29 – 75.48) | 60.1 (6.7 – 159.0) |
Meta-analysis of the PAQUID, Rotterdam study, Framingham Heart Study, Gothenburg studies, CFAS-I, CFAS-II, three cities’ studies and AGES-Reykjavik study using data obtained by Wolters et al. Neurology, 2020.
Meta-analysed data obtained from Gillis et al. Alzheimer’s Dement (Amst), 2019.
Table 4.
Observed cases in province of Lecce and expected cases of major cognitive disorder based on the meta-analysis data from all studies reported by Wolters et al., only those in Southern France (PAQUID and Three-City Study), and the Global Burden of disease study 2021.
| Age Range | Males |
Females |
Both |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs. | Exp. | Observed-to-expected ratio | D | Obs. | Exp. | Observed-to-expected ratio | Obs. | Exp. | Observed-to-expected ratio | |
| Meta-analysis expected data | ||||||||||
| 65–69 | 15 | 66 | 22.73 % | 20 | 98 | 20.41 % | 35 | 164 | 21.34 % | |
| 70–74 | 31 | 139 | 22.30 % | 39 | 221 | 17.65 % | 70 | 359 | 19.50 % | |
| 75–79 | 36 | 233 | 15.45 % | 85 | 386 | 22.02 % | 121 | 620 | 19.52 % | |
| 80–84 | 41 | 303 | 13.53 % | 83 | 553 | 15.01 % | 124 | 855 | 14.50 % | |
| 85–89 | 23 | 236 | 9.75 % | 43 | 507 | 8.48 % | 66 | 744 | 8.87 % | |
| Total | 146 | 977 | 14.94 % | 270 | 1765 | 15.30 % | 416 | 2742 | 15.17 % | |
| Global burden of disease study 2021 expected data | ||||||||||
| 65–69 | 15 | 100 | 15.00 % | 20 | 79 | 25.32 % | 35 | 192 | 18.23 % | |
| 70–74 | 31 | 204 | 15.20 % | 39 | 210 | 18.57 % | 70 | 453 | 15.45 % | |
| 75–79 | 36 | 375 | 9.60 % | 85 | 418 | 20.33 % | 121 | 795 | 15.22 % | |
| 80–84 | 41 | 418 | 9.81 % | 83 | 744 | 11.16 % | 124 | 1207 | 10.27 % | |
| 85–89 | 23 | 433 | 5.31 % | 43 | 796 | 5.4 % | 66 | 1259 | 5.24 % | |
| Total | 146 | 1′530 | 9.54 % | 270 | 2′247 | 12.02 % | 416 | 3906 | 10.65 % | |
| Sensitivity Analysis: Meta-analysis expected data (Southern France) | ||||||||||
| 65–69 | 15 | 72 | 20.98 % | 20 | 47 | 42.14 % | 35 | 110 | 31.75 % | |
| 70–74 | 31 | 152 | 20.44 % | 39 | 145 | 26.92 % | 70 | 297 | 23.60 % | |
| 75–79 | 36 | 318 | 11.31 % | 85 | 425 | 19.99 % | 121 | 756 | 16.01 % | |
| 80–84 | 41 | 315 | 13.02 % | 83 | 717 | 11.58 % | 124 | 1030 | 12.04 % | |
| 85–89 | 23 | 362 | 6.36 % | 43 | 789 | 5.45 % | 66 | 1190 | 5.54 % | |
| Total | 146 | 1311 | 11.14 % | 270 | 2219 | 12.17 % | 416 | 3562 | 11.68 % | |
Exp: Expected cases; Obs: Observed cases; y: Years.
For MCI, age-specific rates increased with advancing age. In the 70–74 year subgroup, the incidence rate was 24.2 per 1000 person-years (95 % CI: 12.4–35.9). This slightly decreased to 22.5 per 1000 person-years (95 % CI: 5.1–51.4) in the 75–79 year subgroup but rose again in older age subgroups. The 80–84 year subgroup had an incidence rate of 40.9 per 1000 person-years (95 % CI: 7.7–97.5), with the highest rate observed in the 85–89 year subgroup at 60.1 per 1000 person-years (95 % CI: 6.7–159.0). Observed and expected cases of minor cognitive disorders based on the meta-analysis of Gillis et al. 2019 are reported in supplemental Table 2.
3.3. Observed-to-expected ratio
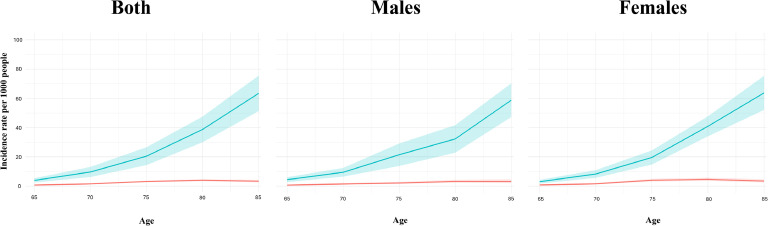
Fig. 2 illustrates the incidence rates of both MaNCD and MCI, expressed per 1000 individuals, stratified by age and sex, in the Lecce Province and based on a meta-analysis of published cohort studies. The observed-to-expected ratios for both MaNCD and MCI in are presented in Figs. 3 and 4, respectively. These ratios are expressed as percentages and provide insights into the extent of case identification and underdiagnosis across different age subgroups. For MaNCD, the overall observed-to-expected ratio was 10.65 % observed, with 89.35 % of cases remaining undiagnosed. Age-specific analysis revealed a sharp decline in the observed-to-expected ratio with advancing age. Among individuals aged 65–69 years, 18.23 % of expected MaNCD cases were observed, but this proportion decreased to 15.45 % in the 70–74 year subgroup and further declined to 15.22 % in the 75–79 year subgroup. The ratio fell to almost negligible levels in the oldest age subgroups, with only 10.27 % observed in the 80–84 year subgroup and 5.24 % in the 85–89 year subgroup (test for trend: p = 0.003).
Fig. 2.
Incidence rate of both MaNCD and MCI per 1000 by age in Lecce Province and based on meta-analysis of cohort studies by age and sex.
Fig. 3.
Observed-to-expected ratio express in percentage for major cognitive disorder cases in the province of Lecce by sex and age subgroup.
Fig. 4.
Observed-to-expected ratio express in percentage for minor cognitive disorder cases in the province of Lecce by sex and age subgroup.
Considering the expected cases derived from the Global Burden of Disease (GBD) 2021 study (Table 4), the overall observed-to-expected ratio for Major Neurocognitive Disorder (MaNCD) in the province of Lecce reflects substantial underdiagnosis (Supplemental figure 20), consistent with the patterns observed in local data. For the entire population aged 65–89 years, the observed-to-expected ratio for MaNCD was 15.17 %. This underdiagnosis was consistent across both males and females, with females (15.3 %) showing a slightly higher observed-to-expected ratio than males (14.94 %). Age-specific analysis showed a sharp decline in the observed-to-expected ratio with age. In the 65–69 year subgroup, the ratio was 21.34 %. This dropped to 19.5 % in the 70–74 year subgroup, stay steady in the 75–79 year subgroup, and dropped again to 14.5 % in the 85–89 year subgroup and to 8.87 in the 85 to 89 subgroup. The test for trend was statistically significant (p = 0.011), indicating a clear decrease with age.
For MCI, the observed-to-expected ratio was significantly lower, with only 7.24 % of expected cases observed and 92.76 % undiagnosed (Fig. 4). In the age groups, the ratio showed a consistent downward trend. In the 70–74 year subgroup, 10.62 % of expected cases were observed, followed by 13.11 % in the 75–79 year subgroup, and 5.35 % in the 80–84 year subgroup. The lowest observed-to-expected ratio was found in the 85–89 year subgroup, with only 2.53 % of expected cases being identified.
3.4. Sensitivity analysis
Observed-to-expected ratio using pooled data from the PAQUID[17] and Three Cities Study[22] showed similar total ratios with respect to pooled data from all studies reported by Wolters et al.[25] In addition, there was a similar trend in cases detection decline with aging in both sexes (Table 4, supplemental figure 21). The only difference identified was in the observed-to-expected ratio of females between 65–69 years which double the ratio of males and the ratio with all studies from Wolters et al. Results from the meta-analysis for PAQUID[17] and Three Cities Study[22] including age-sex specific incidence rates are present in supplemental figure 22–38 and supplemental Table 3 respectively.
4. Discussion
The capacity of the CCDDs public healthcare system in the Salento Region to identify new cognitive disorders diagnoses remains particularly low, with approximately 85 % to 90 % of expected cases going undetected. Comparisons between observed cases and expected estimates derived from multiple meta-analytic sources consistently reveal a significant underdetection, with detection rates declining progressively with advancing age. Additionally, detection rates are notably lower in males compared to females, further highlighting disparities in diagnostic practices. Of particular concern is the system’s even greater difficulty in identifying patients with MCI, a condition that marks the early and prodromal stage of dementia, where early intervention could play a crucial role in slowing disease progression.
Our findings align closely with previous literature that highlights significant underdiagnosis of dementia across various populations. A recent meta-analysis conducted by Lang et al. [8] reported a global undetected dementia rate of 61.7 %, with higher rates in community settings compared to residential care. The meta-analysis also identified notable geographic and demographic disparities, with underdiagnosis rates being particularly pronounced in low- and middle-income countries, among males, and in individuals diagnosed by general practitioners. Similarly, another study emphasized that approximately 40 % of older adults with dementia in the US remain undiagnosed, identifying disparities related to sex, education level, and healthcare access[7]. Furthermore, a recent study by Bynum et al. highlighted the rate of variation in newly identified cases of Alzheimer’s and related Dementias (ADRD) diagnoses across the United States showing significant differences in the country[26]. However, a distinct divergence is noted in the age-related patterns in our study; while Lang et al. and Bynum et al.[26], observed higher underdiagnosis in younger populations[8], our findings suggest a progressive decline in detection rates with advancing age, underscoring potential systemic gaps in addressing dementia in the elderly. Similar incidence and underdetection rates were also found when using pooled estimates from two studies conducted in southern France[17,22], which shares more cultural and geographic characteristics compared to pooled estimates in all studies, including those made in USA and Northern Europe, further confirming our findings.
Moreover, the underdiagnosis of MCI, the prodromal stage of dementia, represents an additional critical gap in clinical practice. MCI offers a pivotal window for intervention, yet detection rates remain suboptimal. Liu et al.[27] reported that primary care settings in the United States exhibit particularly low MCI detection rates, despite the condition’s significance as an early indicator of neurodegenerative processes. The reliance on non-specialist clinicians and the absence of standardized screening protocols likely contribute to this diagnostic gap. Our findings echo this concern, emphasizing that the failure to recognize MCI not only delays potential therapeutic interventions but also diminishes opportunities for patients and families to plan for future care needs. Additionally, although observed cases of Minor Neurocognitive Disorder were recorded in individuals aged 65–69 years, this subgroup was excluded from observed-to-expected ratio analyses due to the lack of age-specific incidence estimates in the reference meta-analysis[24], which only includes individuals aged 70 and above. This highlights the need for broader epidemiological benchmarks that encompass younger age groups where early prodromal detection is increasingly relevant.
Interestingly, our findings indicate that the ability of the CCDDs public health system to capture new dementia diagnoses decreases consistently with advancing age. While this trend may be partially explained by factors such as the presence of comorbidities, increased difficulty accessing diagnostic services, or wrong ascription of clinical symptoms to normal aging, it could also reflect the influence of a structural form of prejudice known as ageism, which remains underexplored in the context of dementia diagnosis[28,29].
Ageism, defined as stereotyping, prejudice, and discrimination against individuals based on age, constitutes a significant yet often under-recognized factor influencing the diagnosis, diagnostic delay, treatment, and management of NCDs. The phenomenon of ageism in neurocognitive disorders is deeply embedded across individual, social, clinical, and structural levels[30]. At a socio-cultural level, pervasive stereotypes equate cognitive decline with “normal aging,” contributing to the normalization and under-recognition of early symptoms of NCDs. In parallel, the stigma associated both with aging and with cognitive impairment may discourage individuals and families from seeking timely medical evaluation or disclosing symptoms. On the other hand, internalized ageism may cause older adults to minimize or dismiss cognitive symptoms, delaying care-seeking behavior[31]. Within the healthcare system, ageist biases can lead to diagnostic overshadowing, wherein clinicians attribute cognitive and behavioral changes to aging rather than pursuing differential diagnosis for neurocognitive pathology, broadly defining it as “senile dementia”, which conflates normal aging with pathological processes, and carries a stigma that also contribute to delayed medical evaluation. Moreover, older patients are frequently subject to therapeutic nihilism, resulting in less diagnostic efforts or limited treatment options, and reduced inclusion in clinical trials[32]. Addressing this multifactorial bias requires efforts to promote awareness, challenge stereotypes, educate healthcare professionals, and implement policies that ensure equitable care and opportunities for older adults living with NCDs.
Despite the National Health System emphasis on primary care with almost 500 GPs operating in the Salento area, our findings highlight a significant gap in the system’s ability to detect prodromal cases, such as MCI. This poses a critical challenge, particularly as disease-modifying therapies targeting early stages of dementia are becoming available[33,34]. These therapies have the potential to alter the trajectory of disease progression, but their effectiveness hinges on early and accurate identification of individuals in the early stage of the disease. Furthermore, growing evidence supports the idea that dementia is not inevitable but rather preventable, with progression being modifiable through risk factor management and lifestyle changes[35]. The inability of the current system to detect these prodromal cases suggests a mismatch between emerging therapeutic and preventive paradigms and the preparedness of healthcare systems. Urgent efforts are needed to develop screening strategies and integrate systematic approaches to identify early cases, ensuring timely intervention and maximizing the benefits of advances in dementia care.
This study has several notable strengths. First, it utilized complete ascertainment of all diagnosed cases identified in the CCDDs public healthcare system, actively involving all hospital- and community-based centers across the Lecce province, ensuring comprehensive coverage of observed cases. Second, for expected cases, we adopted a meta-analytic approach, leveraging data from well-established international cohort studies, ensuring robust comparisons between observed and expected incidence rates. Additionally, the incorporation of estimates from the Global Burden of Disease (GBD) study, one of the most extensive global epidemiological initiatives, enhances the validity and generalizability of our findings. Finally, all neurologists and geriatricians participating in case identification received rigorous training, ensuring consistency and accuracy in diagnoses.
Despite these strengths, some limitations must be acknowledged. First, the study period was restricted to a single year, which may not fully capture annual variations or long-term trends in incidence rates. However, as the project is ongoing, future analyses will include data from subsequent years. Furthermore, one limitation of this study is the implicit assumption that cognitive disorders diagnosis, including MCI and MaNCD, occur exclusively within CCDDs. Although this may reflect the optimal diagnostic pathway, in routine clinical practice, dementia is sometimes diagnosed in alternative settings, such as geriatric or neurological outpatient services. Moreover, a greater proportion of individuals in the advanced stages of dementia are likely to be cared for within the nursing home system, which may lead to an underrepresentation of these cases in the available data, contributing to a possible overestimate of the cognitive impairment underdetection estimate in our study. Despite these limitations, our study may serve as a valuable indicator of the capacity of the public healthcare system to address cognitive impairment care.
Additionally, population-based studies on MaNCD and MCI estimates are lacking for the region. To address this, we utilized robust estimates derived from a meta-analysis of European studies, which provide reliable and comparable data representative of the European population. While the estimates derived from a meta-analysis of studies offer reliable and comparable data representative of the European population, it is important to acknowledge that these studies were conducted in different geographic regions and across various time periods. To account for this limitation, we also incorporated updated and comprehensive data from the GBD study for MaNCD, which yielded consistent results, thereby further reinforcing the robustness of our findings.
In conclusion, this study highlights critical gaps in the Lecce province CCDDs public healthcare system's ability to detect neurocognitive disorders, with only a small fraction of expected cases identified. Detection rates were particularly low among older adults, women, and individuals in the prodromal stages. These findings suggest structural biases, including ageism, which hinder equitable diagnostic practices. As disease DMTs and prevention strategies emerge, the failure to identify early-stage cases represents a missed opportunity for timely intervention and risk modification. Addressing these gaps will require systemic reforms, including enhanced diagnostic protocols, professional training, and better integration of diagnostic with preventive strategies. This study should prompt further investigations to assess detection capabilities of other regions’ health care systems too. Leveraging the National Dementia Plan offers a crucial opportunity to improve this scenario for individuals with cognitive disorder in the entire Italian area.
Funding
This work has been supported with the funding of Regione Puglia within the national “Fund for Alzheimer's and Dementia 2021-2023″ (Piano Regionale Demenze 2021/2023 - D.G.R. 1284/2022) and with the funding of Regione Puglia for Tecnopolo per la Medicina di Precisione. D.G.R. n. 2117 of 21.11.2018 (B84I18000540002)
Acknowledgements
We are deeply grateful to all the participants in the study for the collaboration, to the Puglia Region “Department of health promotion, social well-being and sport for all” for administrative and management support to the project and to all the CCDDs staff involved:
Dr. Gaetano Barbagallo, MD, PhD, ASL Lecce
Dr. Nicoletta Trevisi, MD, ASL Lecce
Dr. Renato Sambati, MD, ASL Lecce
Dr. Marina Nuzzo, MD, ASL Lecce
Dr. Maria Luigia Fulgido, MD, ASL Lecce
Dr. Giuseppe De Santis, MD, ASL Lecce
Dr. Gianluigi Calabrese, MD, ASL Lecce
Dr. Nadia Rita Panico, MD, ASL Lecce
Dr. Maria Alessandria, MD, ASL Lecce
Dr. Angelo Zenzola, MD, ASL Lecce
Dr. Francesca Fiorillo, PhD, ASL Lecce
Dr. Maria Elisa Frisullo, PhD, ASL Lecce
Data availability statement
Datasets are available from the corresponding author upon reasonable request.
Consent statement
Ethical approval was obtained from the regional ethics committee (ASL LE, Verbal N° 13, 8th March 2023, protocol number: 34,441/2023), and written informed consent was secured from all participants or their legal representatives. The study adhered to the principles outlined in the Declaration of Helsinki. Data confidentiality was maintained through anonymization and secure data storage.
CRediT authorship contribution statement
Davide Vilella: Writing – original draft, Methodology, Data curation, Conceptualization. Daniele Urso: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Agnese Valguarnera: Writing – review & editing, Data curation. Giuseppe Volpe: Writing – review & editing, Data curation. Valentina Gnoni: Writing – review & editing, Data curation. Eleonora Rollo: Writing – review & editing, Data curation. Alessia Giugno: Writing – review & editing, Data curation. Marcella Caggiula: Writing – review & editing, Data curation. Brigida Coluccia: Writing – review & editing, Data curation. Annamaria Mauro: Writing – review & editing, Data curation. Roberta Barone: Writing – review & editing, Data curation. Miriam Accogli: Writing – review & editing, Data curation. Marzia Leopizzi: Writing – review & editing, Data curation. Alessandro Introna: Writing – review & editing, Data curation. Marco Musio: Writing – review & editing, Data curation. Stefano Giannoni-Luza: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Giancarlo Logroscino: Writing – original draft, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tjpad.2025.100295.
Appendix. Supplementary materials
References
- 1.United Nations DoE, Social Affairs PD. World Population Prospects 2019. 2019.
- 2.Logroscino G., Urso D., Savica R. Descriptive epidemiology of neurodegenerative diseases: what are the critical questions? Neuroepidemiology. 2022;56(5):309–318. doi: 10.1159/000525639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2) doi: 10.1016/S2468-2667(21)00249-8. e105-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Istituto Nazionale di S. Rapporto annuale 2014: la situazione del Paese: ISTAT; 2014.
- 5.Di Fiandra T., Canevelli M., Di Pucchio A., Vanacore N. The Italian dementia national plan. Annali dell'Istituto Superiore di Sanità. 2015;51:261–264. doi: 10.4415/ANN_15_04_02. [DOI] [PubMed] [Google Scholar]
- 6.Sachdev P.S., Blacker D., Blazer D.G., Ganguli M., Jeste D.V., Paulsen J.S., et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014;10(11):634–642. doi: 10.1038/nrneurol.2014.181. [DOI] [PubMed] [Google Scholar]
- 7.Amjad H., Roth D.L., Sheehan O.C., Lyketsos C.G., Wolff J.L., Samus Q.M. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33(7):1131–1138. doi: 10.1007/s11606-018-4377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang L., Clifford A., Wei L., Zhang D., Leung D., Augustine G., et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(2) doi: 10.1136/bmjopen-2016-011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Y., Zeng Q.T., Chen K.K., Shutes-David A., Thielke S.M. Tsuang DW. Detection of probable dementia cases in undiagnosed patients using structured and unstructured electronic health records. BMC Med Inform Decis Mak. 2019;19(1):128. doi: 10.1186/s12911-019-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly A., Gaehl E., Martin H., Morris J., Purandare N. Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011;15(8):978–984. doi: 10.1080/13607863.2011.596805. [DOI] [PubMed] [Google Scholar]
- 11.Prince M., Wimo A., Guerchet M., Ali G.-C., Wu Y.-T., Prina M. World Alzheimer Report 2015. The global impact of dementia: An analysis of prevalence, incidence, cost and trends. 2015 [Google Scholar]
- 12.Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Katz S., Downs T.D., Cash H.R., Grotz R.C. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 14.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. Pt 1. [PubMed] [Google Scholar]
- 15.Istituto Nazionale di S. Demo ISTAT – Dati Demografici. 2025.
- 16.Wolters F.J., Chibnik L.B., Waziry R., Anderson R., Berr C., Beiser A., et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States. Neurology. 2020;95(5) doi: 10.1212/WNL.0000000000010022. e519-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dartigues J.F .GM., Michel P., et al. The Paquid research program on the epidemiology of dementia. Methods and initial results. Rev. Neurol. (Paris) 1991;147(3):225–230. [PubMed] [Google Scholar]
- 18.Hofman A., Brusselle G.G.O., Murad S.D., van Duijn C.M., Franco O.H., Goedegebure A., et al. The Rotterdam Study: 2016 objectives and design update. Eur. J. Epidemiol. 2015;30(8):661–708. doi: 10.1007/s10654-015-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawber T.R., Meadors G.F., Moore F.E., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bengtsson C., Blohmé G., Hallberg L., Hällström T., Isaksson B., Korsan-Bengtsen K., et al. The study of women in Gothenburg 1968-1969–a population study. General design, purpose and sampling results. Acta Med Scand. 1973;193(4):311–318. doi: 10.1111/j.0954-6820.1973.tb10583.x. [DOI] [PubMed] [Google Scholar]
- 21.Matthews F.E., Stephan B.C., Robinson L., Jagger C., Barnes L.E., Arthur A., et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7 doi: 10.1038/ncomms11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group C.S. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22(6):316–325. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 23.Harris T.B., Launer L.J., Eiriksdottir G., Kjartansson O., Jonsson P.V., Sigurdsson G., et al. Age, gene/environment susceptibility-Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillis C., Mirzaei F., Potashman M., Ikram M.A., Maserejian N. The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement (Amst) 2019;11:248–256. doi: 10.1016/j.dadm.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolters F.J., Chibnik L.B., Waziry R., Anderson R., Berr C., Beiser A., et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer Cohorts Consortium. Neurology. 2020;95(5) doi: 10.1212/WNL.0000000000010022. e519-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bynum J.P.W., Benloucif S., Martindale J., O'Malley A.J., Davis M.A. Regional variation in diagnostic intensity of dementia among older U.S. adults: an observational study. Alzheimers Dement. 2024;20(10):6755–6764. doi: 10.1002/alz.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y., Jun H., Becker A., Wallick C., Mattke S. Detection rates of mild cognitive impairment in primary care for the United States Medicare population. J Prev Alzheimers Dis. 2024;11(1):7–12. doi: 10.14283/jpad.2023.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacsu J.D., Kortzman A., Fraser S., Chasteen A.L., MacDonald J., O'Connell M.E. Understanding intersectional ageism and stigma of dementia: protocol for a scoping review. JMIR Res Protoc. 2023;12 doi: 10.2196/46093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adkins-Jackson P.B., George K.M., Besser L.M., Hyun J., Lamar M., Hill-Jarrett T.G., et al. The structural and social determinants of Alzheimer's disease related dementias. Alzheimers Dement. 2023;19(7):3171–3185. doi: 10.1002/alz.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen J.O., Solway E., Kirch M., Singer D., Kullgren J.T., Moise V., et al. Experiences of everyday ageism and the health of older US adults. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradford A., Kunik M.E., Schulz P., Williams S.P., Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen J.O. Ageism as a risk factor for chronic disease. Gerontologist. 2016;56(4):610–614. doi: 10.1093/geront/gnu158. [DOI] [PubMed] [Google Scholar]
- 33.Logroscino G., Urso D., Gnoni V., Giugno A., Vilella D., Castri A., et al. Mild cognitive impairment and early Alzheimer's disease eligibility for disease modification therapies in a tertiary centre for cognitive disorders: a simultaneous real-word study on aducanumab and lecanemab. Eur J Neurol. 2024 doi: 10.1111/ene.16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urso D., Introna A., Gnoni V., Giugno A., Vilella D., Zecca C., et al. Donanemab eligibility in early Alzheimer's disease: a real-world study. J Alzheimers Dis. 2025;105(3):745–750. doi: 10.1177/13872877251331243. [DOI] [PubMed] [Google Scholar]
- 35.Livingston G., Huntley J., Liu K.Y., Costafreda S.G., Selbæk G., Alladi S., et al. Dementia prevention, intervention, and care: 2024 report of the <em>Lancet</em>standing Commission. The Lancet. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets are available from the corresponding author upon reasonable request.