Abstract
Background
Endometriosis and body mass index (BMI) are known to influence reproductive outcomes, but their combined impact on in vitro fertilization (IVF) success remains uncertain.
Objective
To evaluate the relationship between BMI and the risk of endometriosis, and to assess how endometriosis—with or without prior surgical treatment—affects IVF outcomes, including oocyte yield, maturity, clinical pregnancy rates, and live birth rates.
Methods
This meta-analysis included 19 studies identified through comprehensive searches of databases including PUBMED, MEDLINE, and EMBASE. Primary outcomes assessed were the association between BMI and endometriosis risk, number of oocytes retrieved, and number of mature oocytes (MII). Secondary outcomes included clinical pregnancy rates, live birth rates, and the influence of prior surgery on IVF outcomes in women with endometriosis.
Results
BMI significantly influenced the risk of endometriosis, with obese women showing an increased risk (OR: 2.28) and normal-weight women showing a protective effect (OR: 0.45). Women with endometriosis had fewer total oocytes (mean difference [MD]: −2.06) and mature oocytes (MD: −2.07) compared to women without endometriosis undergoing IVF. While clinical pregnancy rates were not significantly different between groups (OR: 1.03), live birth rates were significantly lower in the endometriosis group (OR: 0.87). In a subgroup analysis of women who underwent prior surgical treatment for endometriosis, no improvement was observed in clinical pregnancy rates (OR: 0.79), and live birth rates were further reduced (OR: 0.67).
Conclusions
Higher BMI is associated with an increased risk of endometriosis, which negatively affects IVF outcomes by reducing oocyte yield and live birth rates. Prior surgical treatment does not appear to enhance IVF success and may further compromise live birth outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00404-025-08137-w.
Keywords: Endometriosis, BMI, IVF, Endometriosis removal
Introduction
Endometriosis is a chronic estrogen-dependent disorder marked by the presence of tissue resembling the endometrium growing outside the uterus [8]. It is a significant gynecological disorder that affects approximately 15% of women of reproductive age. Various risk factors for endometriosis have been identified, with body mass index (BMI) being a key concern. Among women experiencing infertility, the prevalence of endometriosis is notably higher, estimated to range from 25 to 50%, and 30% to 50% of women with endometriosis are infertile [6]. The underlying correlation between infertility and endometriosis has driven interest, and women with endometriosis may require assisted reproductive techniques to achieve a pregnancy. Given tremendous articles suggests that BMI significantly impacts ovarian receptivity, we aim to explore whether endometriosis independently affects ovarian receptivity by examining the relationship between BMI and endometriosis.
Assisted reproductive technology (ART), and more specifically in vitro fertilization (IVF), has significantly transformed infertility treatment. Although it has seen widespread application and success, its primary outcomes remain a subject of ongoing discussion. These outcomes include clinical pregnancy rates, live birth rates, as well as the quality and quantity of oocytes, particularly in women affected by endometriosis. The relationship between endometriosis and IVF outcomes is an area of considerable debate in the field.
Endometriosis has a profound and complex impact on reproductive health. Multiple studies have indicated that endometriosis elevates inflammatory cytokine levels and oxidative stress within the pelvic cavity, which can negatively affect the success of IVF treatments [18]. Both in vitro and in vivo research have demonstrated that endometriosis causes local inflammation and oxidative stress in the ovaries, particularly within granulosa cells [29]. This inflammatory environment can result in the early activation of primordial follicles, disrupting normal follicular development [37]. What’s more, endometriosis interferes with normal hormonal regulation, leading to abnormal follicle maturation [11]. Human studies have shown that women with endometriosis and reduced ovarian reserve are more likely to experience poorer IVF outcomes due to this compromised ovarian reserve [9, 17, 27, 30, 39]. However, some meta-analyses and retrospective studies have suggested that the presence of endometriosis may not significantly affect pregnancy outcomes [16, 32, 40]. BMI has been shown to be related with ovarian response. By evaluating the impact of different BMI levels on endometriosis, we can also determine if ovarian response is a key factor contributing to the detrimental effects on pregnancy outcomes.
Surgical intervention is a primary treatment for women with severe endometriosis. However, there have been no randomized controlled trials specifically addressing this issue. Compelling evidence suggests that surgery may not have a beneficial effect on ovarian reserve [7, 22, 33], one study suggests that serum Anti-Müllerian Hormone (AMH) levels often decrease after surgery, which can ultimately impact cumulative live birth rates per retrieval [23]. Despite this, surgery may still be recommended for patients who experience significant pain after discontinuing hormonal therapies or during ovarian stimulation, and other studies suggest that the excision of endometriosis can improve fertility outcomes [4, 5], highlighting the ongoing debate about its role in enhancing reproductive success. Therefore, further research is needed to clarify the benefits of surgical intervention in improving IVF outcomes for women with endometriosis.
A systematic literature search was conducted, restricted to randomized controlled trials (RCTs), population-based, cross-sectional, and retrospective studies. Databases including PUBMED, MEDLINE, EMBASE, Web of Science, and the Cochrane Library, along with clinical trial registries (e.g., ClinicalTrials.gov and WHO International Clinical Trials Registry), were explored for studies published to date. The search focused on the effects of BMI on endometriosis, IVF outcomes in patients with and without endometriosis, and the impact of surgical intervention on IVF outcomes. The search strategy combined terms related to “endometriosis,” “BMI,” “IVF outcomes,” “oocyte quality,” “surgical treatment,” and “reproductive outcomes”.
Method
Systematic search and strategy
This systematic review and meta-analysis was carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [20]. As the study did not include any human interventions, it was not subject to approval by an institutional review board. The study protocol was registered on the PROSPERO website (http://www.crd.york.ac.uk/PROSPERO/) with ID CRD420250650285 before the quantitative analysis began.
Search strategy and participants
A thorough search was carried out to locate studies examining the relationship between BMI and endometriosis, differences in IVF outcomes between women with and without endometriosis, and the effect of surgical treatment on IVF success in endometriosis patients. The review focused on articles published between 2019 and 2024. The databases searched included PUBMED, MEDLINE, EMBASE, Web of Science, Cochrane Library, as well as clinical trial registries like ClinicalTrials.gov and the WHO International Clinical Trials Registry.
Inclusion and exclusion criteria
The inclusion criteria for this meta-analysis are randomized controlled trials (RCTs), population-based studies, cross-sectional studies, and retrospective studies published in English within the last 10 years. Studies must involve women diagnosed with endometriosis undergoing IVF/ICSI treatment, comparing IVF outcomes (e.g., oocyte quality, clinical pregnancy rate, live birth rate) in relation to BMI or surgical interventions. Exclusion criteria include nonhuman studies, studies lacking clear comparison groups, those not reporting relevant IVF outcomes, studies involving confounding conditions (e.g., PCOS). Only studies with well-defined BMI categories and the data on surgical treatments for endometriosis are considered. We conduct risk of bas analysis, articles with more than 3 unclear risks or with 1 high risk is not selected for further analysis.
Data extraction and quality assessment
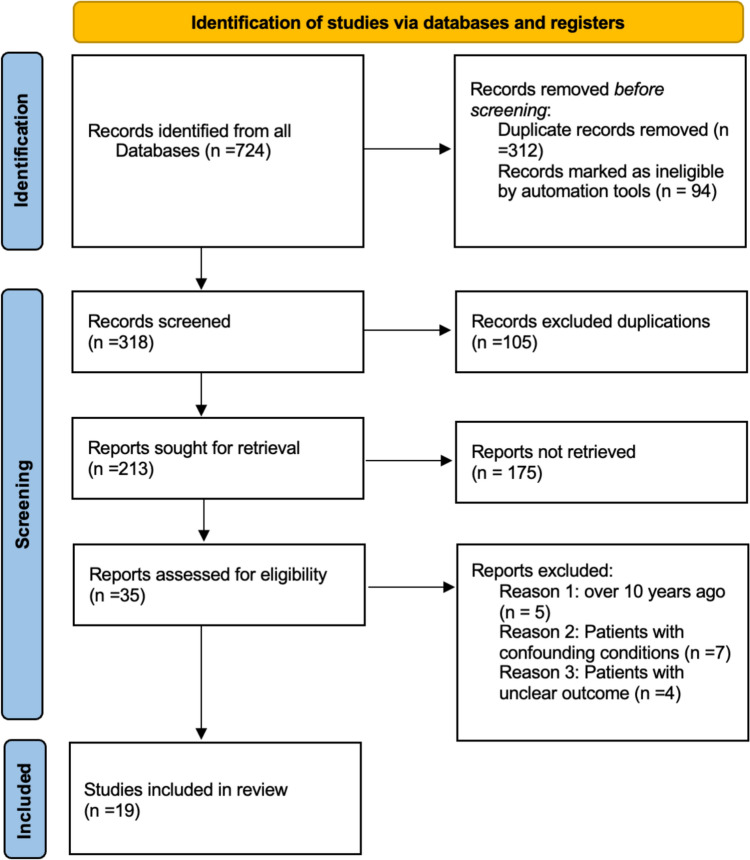
Two independent reviewers (LL and SJ) evaluated the risk of bias in each study using the Cochrane Risk of Bias Tool 2.0, specifically designed for randomized trials. The quality of the overall evidence was assessed and rated following the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [15]. Figure 1 shows that PRISMA flowchart that describing the included/excluded literature.
Fig. 1.
PRISMA flowchart of this study
Statistical analysis
Data management, including relevancy checks, removal of duplicates, and eligibility assessments, was carried out following PRISMA guidelines using Microsoft Excel. Statistical analyses were performed with Review Manager 5.4. The pooled odds ratio (OR) was calculated for dichotomous data, and the weighted mean difference (WMD) for continuous data, both with a 95% confidence interval (CI). Heterogeneity was evaluated by examining the percentage of total variation across studies (I2).
Subgroup and sensitivity analysis
Sensitivity analysis was performed by excluding studies with a high risk of bias (i.e., judged high risk on at least one domain or unclear risk on two or more domains based on the risk of bias assessment tool). Subgroup analyses were conducted to examine the impact of different BMI categories on IVF outcomes in women with endometriosis. In addition, we performed subgroup analyses comparing IVF outcomes in patients with endometriosis who underwent surgical intervention versus those who did not.
Results
Our systematic search consists of 724 records. After removing duplications, 213 references were screened by analyzing the titles and abstracts. After analyzing the full text of 35 studies, we selected 19 studies to be included in the meta-analysis. Prisma checklist details our search results and summary table for the studies included can be found in supporting material. Figure 2 is the graph of risk of bias. The summary of the risk of bias is also attached in the supplementary material.
Fig. 2.
Risk of bias graph for all selected articles
Primary outcome
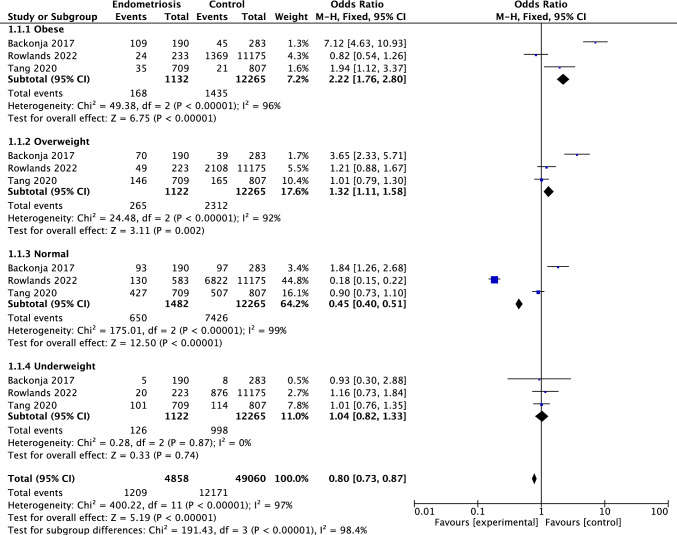
This study is based on the cross-sectional data and includes women scheduled for gynecologic laparoscopy or laparotomy, regardless of the surgical indication. BMI categories were classified as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a fixed-effects model. Significant heterogeneity was observed across studies (I2 = 98.4%). Subgroup analysis demonstrated a significant association between BMI and the risk of endometriosis. Obese individuals had a markedly increased risk of endometriosis (OR: 2.22, 95% CI: 1.76–2.80), followed by overweight individuals (OR: 1.32, 95% CI: 1.11–1.58). In contrast, normal-weight individuals showed a significantly reduced risk (OR: 0.45, 95% CI: 0.40–0.51), suggesting a protective effect. Underweight individuals showed no significant association with endometriosis risk (OR: 1.04, 95% CI: 0.82–1.33) (Fig. 3).
Fig. 3.
Forest plot illustrating the association between body mass index (BMI) categories and the risk of endometriosis
The forest plot displays the mean difference and 95% CIs for the number of retrieved oocytes between endometriosis patients and normal IVF patients undergoing ART treatment. Endometriosis patients had significantly fewer retrieved oocytes compared to normal IVF patients, with a pooled MD of -2.03 (95% CI: − 3.01, − 1.05, P < 0.00001) (Fig 4).
Fig. 4.
Forest plot comparing the number of retrieved oocytes between endometriosis patients and normal IVF patients
This forest plot displays the mean difference and 95% CIs for the number of MII oocytes between endometriosis and normal IVF patients undergoing ART treatment. Endometriosis patients had significantly fewer MII oocytes compared to normal IVF patients, with a pooled MD of -2.07 (95% CI: − 2.86, − 1.28, P < 0.00001) (Fig 5).
Fig. 5.
Forest plot comparing the number of MII oocytes between endometriosis patients and normal IVF patients
This forest plot shows the ORs and CIs for clinical pregnancy rates in endometriosis patients versus normal IVF patients. The pooled OR is 1.03 (95% CI: 0.70, 1.52, P = 0.89), indicating a insignificant difference in clinical pregnancy rates between the two groups (Fig 6).
Fig. 6.
Forest plot comparing clinical pregnancy rates between endometriosis patients and normal IVF patients
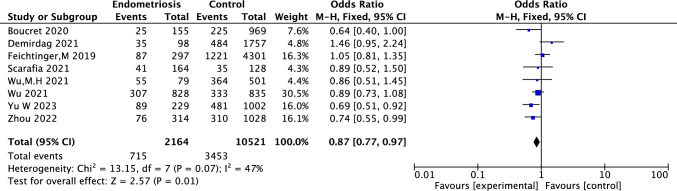
This forest plot presents the ORs and 95% CIs for live birth rates in endometriosis patients versus normal patients in their ART treatment. The pooled OR is 0.87 (95% CI: 0.77, 0.97, P = 0.01), indicating a statistically significant reduction in live birth rates for endometriosis patients who undergoing ART treatment compared to normal IVF patients. Heterogeneity among studies was low to moderate (I2 = 47%, P = 0.07), supporting the use of a fixed-effects model (Fig 7).
Fig. 7.
Forest plot comparing live birth rates between endometriosis patients and normal IVF patients in their ART treatment
This forest plot presents ORs and 95% CIs for clinical pregnancy rates in endometriosis patients undergoing ART treatment with prior surgery (experimental group) compared to those without surgery (control group). The pooled OR is 0.79 (95% CI: 0.49, 1.25, P = 0.31), indicating no statistically significant difference in clinical pregnancy rates between the two groups. The findings suggest that prior surgical treatment does not significantly impact clinical pregnancy rates as compared to no surgery in endometriosis patients (Fig 8).
Fig. 8.
Forest plot comparing clinical pregnancy rates between endometriosis patients undergoing ART treatment with prior surgery and those without surgery
This forest plot shows the ORs and 95% Cis for live birth rates rates in endometriosis patients undergoing ART treatment with prior surgery as compared to those without surgery. The pooled OR is 0.67 (95% CI: 0.50, 0.91, P = 0.010), indicating a statistically significant reduction in live birth rates for patients who underwent surgical treatment. These results suggest that surgical treatment in endometriosis patients may be associated with a modestly reduced likelihood of achieving a live birth following ART (Fig 9).
Fig. 9.
Forest plot comparing live birth rates in endometriosis patients with and without surgical treatment
Discussion
Body mass index (BMI) has long been associated with ovarian receptivity and reproductive outcomes, and our findings further reinforce this relationship. Specifically, we observed that obesity significantly increases the risk of endometriosis, aligning with prior research indicating that adiposity impairs endometrial stromal cell decidualization, disrupts hormonal homeostasis, and promotes an inflammatory uterine environment conducive to the development of endometriotic lesions [13]. Conversely, individuals with a normal BMI appeared to exhibit a protective effect, likely due to optimal endocrine function and reduced systemic inflammation [14]
The most widely accepted mechanism linking BMI to reproductive dysfunction, including endometriosis, involves obesity-induced disruption of the hypothalamic–pituitary–ovarian (HPO) axis [31]. Leptin, a hormone secreted by adipose tissue, plays a regulatory role in this axis. In the context of obesity, persistently elevated leptin levels may impair leptin signaling, leading to reduced secretion of gonadotropin-releasing hormone (GnRH) and subsequent dysregulation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormonal disturbances contribute to menstrual irregularities and diminished fertility. Furthermore, recent evidence suggests that leptin not only reflects adiposity but actively contributes to the pathogenesis of endometriosis by enhancing the proliferation, survival, and invasiveness of ectopic endometrial cells [38]. Excess leptin, along with pro-inflammatory cytokines, drives chronic inflammation, abnormal cell proliferation, and angiogenesis—mechanisms central to the progression of endometriosis [24].
Our findings also demonstrate a strong association between endometriosis and reduced oocyte yield, decreased number of metaphase II (MII) oocytes, and lower live birth rates. This suggests that while fertilization and implantation may still occur, endometriosis may impair the ability to sustain a pregnancy, thereby compromising overall reproductive success. A key mechanism underlying these outcomes is the inflammatory and oxidative stress environment induced by ovarian endometriotic lesions [35]. Elevated reactive oxygen species (ROS) levels result in oxidative damage to DNA, proteins, and lipids, thereby reducing both oocyte quality and quantity [1]. In addition, the pro-inflammatory milieu within the peritoneal cavity can impair sperm function and disrupt sperm–oocyte interaction, further diminishing fertility potential [26]. The altered follicular environment—characterized by increased cytokines such as TNF-α and IL-6—contributes to oxidative stress, which ultimately compromises oocyte maturation and developmental competence [25].
The impact of endometriosis on clinical pregnancy rate remains a subject of debate. Several studies report no significant difference in clinical pregnancy rates between women with and without endometriosis [19, 21], while others suggest a negative association [10, 30]. These conflicting findings underscore the complexity of the reproductive system and may reflect variations in disease staging. Endometriosis severity ranges from stage I (minimal) to stage IV (severe), and studies that fail to stratify by stage may obscure potential associations. Advanced-stage disease is typically associated with greater hormonal dysregulation and anatomical distortion, which may contribute to lower clinical pregnancy rates. However, emerging evidence suggests that the negative effect of endometriosis is more pronounced on live birth rates than on clinical pregnancy rates, likely due to its sustained impact on the reproductive environment. Future research should ensure consistent staging criteria to accurately evaluate the effect of endometriosis severity on clinical outcomes.
Given our findings that endometriosis adversely affects IVF outcomes, we further examined whether treatment strategies for endometriosis influence reproductive success. Hormonal therapy remains the recommended first-line treatment for endometriosis [3, 34], primarily due to its noninvasive nature and efficacy in controlling disease progression. However, the variability in treatment modalities complicates the evaluation of their individual impacts on ART outcomes. Our data suggest that surgical excision of endometriotic lesions, particularly ovarian endometriomas, may impair ovarian reserve and subsequently reduce IVF success rates. This aligns with previous studies reporting that surgical removal of ovarian endometriomas leads to a substantial decline in AMH levels, with a 38% overall decrease—44% in bilateral cystectomies and 30% in unilateral procedures [28, 32, 36]. Other reviews have similarly concluded that although surgical treatment may improve certain symptoms, it may also diminish ovarian reserve and negatively impact IVF outcomes [12].
Emerging evidence suggests that combining surgical and hormonal therapies may offer enhanced disease control and symptom relief [2]. However, the effect of this combined approach on IVF outcomes remains insufficiently understood and warrants further investigation.
Limitations
This study has several limitations that should be addressed in future research. First, stricter control of patient age is essential, as age significantly influences ovarian response and overall IVF success. Second, more precise classification of endometriosis severity is needed to evaluate stage-specific effects on reproductive outcomes. Third, our outcome measures could be expanded to include implantation rate, miscarriage rate, and other gynecological comorbidities for a more comprehensive assessment. Lastly, greater attention should be paid to treatment type and uniformity, as different therapeutic approaches may exert varying effects on ovarian function and IVF success.
Supplementary Information
Below is the link to the electronic supplementary material.
Author's contribution
Liting Liao, Zhijian Pan and Yanjuan Li were the main contributors to writing the manuscript and designing the work.
Funding
This paper was produced without financial support.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liting Liao, Email: 317700549@qq.com.
Zhijian Pan, Email: 474339269@qq.com.
Yanjuan Li, Email: liyanjuan77@163.com.
References
- 1.Afzal S, Abdul Manap AS, Attiq A, Albokhadaim I, Kandeel M, Alhojaily SM (2023) From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front Pharmacol 14:1269581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaire C, Bedaiwy MA, Yong PJ (2023) Diagnosis and management of endometriosis. CMAJ 195:E363–E371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andres MP, Mendes RFP, Hernandes C, Araújo SEA, Podgaec S (2019) Hormone treatment as first line therapy is safe and relieves pelvic pain in women with bowel endometriosis. Einstein. 10.31744/einstein_journal/2019AO4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendifallah S, Roman H, Mathieu d’Argent E, Touleimat S, Cohen J, Darai E, Ballester M (2017) Colorectal endometriosis-associated infertility: should surgery precede ART? Fertil Steril 108:525–31.e4 [DOI] [PubMed] [Google Scholar]
- 5.Bianchi PH, Pereira RM, Zanatta A, Alegretti JR, Motta EL, Serafini PC (2009) Extensive excision of deep infiltrative endometriosis before in vitro fertilization significantly improves pregnancy rates. J Minim Invasive Gynecol 16:174–180 [DOI] [PubMed] [Google Scholar]
- 6.Bulletti C, Coccia ME, Battistoni S, Borini A (2010) Endometriosis and infertility. J Assist Reprod Genet 27:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capelle A, Lepage J, Langlois C, Lefebvre C, Dewailly D, Collinet P, Rubod C (2015) Surgery for deep infiltrating endometriosis before in vitro fertilization: no benefit for fertility? Gynecol Obstet Fertil 43:109–116 [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Liu Y, Zhong Z, Wei C, Liu Y, Zhu X (2023) Peritoneal immune microenvironment of endometriosis: role and therapeutic perspectives. Front Immunol 14:1134663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coelho Neto MA, Martins Wde P, Luz CM, Jianini BT, Ferriani RA, Navarro PA (2016) Endometriosis, ovarian reserve and live birth rate following in vitro fertilization/intracytoplasmic sperm injection. Rev Bras Ginecol Obstet 38:218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahimpoor M, Firouzabadi RD, Javaheri A, Shamsi F, Dashti S (2024) The impact of endometriosis on reproductive outcomes in ART cycles. Adv Biomed Res 13:89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan W, Yuan Z, Li M, Zhang Y, Nan F (2023) Decreased oocyte quality in patients with endometriosis is closely related to abnormal granulosa cells. Front Endocrinol (Lausanne) 14:1226687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrero S, Gazzo I, Crosa M, Rosato FP, Barra F, Maggiore ULR (2024) Impact of surgery for endometriosis on the outcomes of in vitro fertilization. Best Pract Res Clin Obstet Gynaecol 95:102496 [DOI] [PubMed] [Google Scholar]
- 13.Galio L, Bernet L, Rodriguez Y, Fourcault C, Dieudonné MN, Pinatel H, Henry C, Sérazin V, Fathallah K, Gagneux A, Krupova Z, Vialard F, Santos ED (2023) The effect of obesity on uterine receptivity is mediated by endometrial extracellular vesicles that control human endometrial stromal cell decidualization and trophoblast invasion. J Extracell Biol 2:e103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonnella F, Konstantinidou F, Donato M, Gatta DMP, Peserico A, Barboni B, Stuppia L, Nothnick WB, Gatta V (2024) The molecular link between obesity and the endometrial environment: a starting point for female infertility. Int J Mol Sci. 10.3390/ijms25136855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ (2008) What is “quality of evidence” and why is it important to clinicians? BMJ 336:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Invernici D, Reschini M, Benaglia L, Somigliana E, Galati G, La Vecchia I, Vigano P, Vercellini P (2022) The impact of endometriosis on IVF efficacy: qualitative and quantitative assessment of ovarian response and embryo development. Reprod Biomed Online 45:275–281 [DOI] [PubMed] [Google Scholar]
- 17.Mappa I, Page ZP, Di Mascio D, Patelli C, D’Antonio F, Giancotti A, Gebbia F, Mariani G, Cozzolino M, Muzii L, Rizzo G (2024) The effect of endometriosis on in vitro fertilization outcomes: a systematic review and meta-analysis. Healthcare 12:2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Máté G, Bernstein LR, Török AL (2018) Endometriosis is a cause of infertility. Does reactive oxygen damage to gametes and embryos play a key role in the pathogenesis of infertility caused by endometriosis. Front Endocrinol. 10.3389/fendo.2018.00725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzemaekers J, Lust E, Rhemrev J, Van Geloven N, Twijnstra A, Van Der Westerlaken L, Jansen FW (2021) Prognosis in fertilisation rate and outcome in IVF cycles in patients with and without endometriosis: a population-based comparative cohort study with controls. Facts Views Vis Obgyn 13:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morcel K, Merviel P, Bouée S, Le Guillou M, Carlier M, James P, Drapier H, Beauvillard D (2024) What is the impact of endometriosis and the AFS stage on cumulative pregnancy rates in IVF programs? Reprod Health 21:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mounsambote L, Cohen J, Bendifallah S, d’Argent EM, Selleret L, Chabbert-Buffet N, Ballester M, Antoine JM, Daraï E (2017) Deep infiltrative endometriosis without digestive involvement, what is the impact of surgery on in vitro fertilization outcomes? A retrospective study. Gynecol Obstet Fertil Senol 45:15–21 [DOI] [PubMed] [Google Scholar]
- 23.Nankali A, Kazeminia M, Jamshidi PK, Shohaimi S, Salari N, Mohammadi M, Hosseinian-Far A (2020) The effect of unilateral and bilateral laparoscopic surgery for endometriosis on anti-mullerian hormone (AMH) level after 3 and 6 months: a systematic review and meta-analysis. Health Qual Life Outcomes 18:314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oală IE, Mitranovici MI, Chiorean DM, Irimia T, Crișan AI, Melinte IM, Cotruș T, Tudorache V, Moraru L, Moraru R, Caravia L, Morariu M, Pușcașiu L (2024) Endometriosis and the role of pro-inflammatory and anti-inflammatory cytokines in pathophysiology: a narrative review of the literature. Diagnostics (Basel). 10.3390/diagnostics14030312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orisaka M, Mizutani T, Miyazaki Y, Shirafuji A, Tamamura C, Fujita M, Tsuyoshi H, Yoshida Y (2023) Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front Endocrinol (Lausanne) 14:1324429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potiris A, Moustakli E, Trismpioti E, Drakaki E, Mavrogianni D, Matsas A, Zikopoulos A, Sfakianakis A, Tsakiridis I, Dagklis T, Zachariou A, Christopoulos P, Domali E, Drakakis P, Stavros S (2025) From inflammation to infertility: how oxidative stress and infections disrupt male reproductive health. Metabolites 15:267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu H, Du Y, Yu Y, Wang M, Han T, Yan L (2022) The effect of endometriosis on IVF/ICSI and perinatal outcome: a systematic review and meta-analysis. J Gynecol Obstet Hum Reprod 51:102446 [DOI] [PubMed] [Google Scholar]
- 28.Raffi F, Metwally M, Amer S (2012) The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab 97:3146–3154 [DOI] [PubMed] [Google Scholar]
- 29.Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, Greco P, Nappi L (2017) Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev 2017:7265238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senapati S, Sammel MD, Morse C, Barnhart KT (2016) Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the society for assisted reproductive technologies database. Fertil Steril 106:164–71.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvestris E, de Pergola G, Rosania R, Loverro G (2018) Obesity as disruptor of the female fertility. Reprod Biol Endocrinol 16:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somigliana E, Piani LL, Paffoni A, Salmeri N, Orsi M, Benaglia L, Vercellini P, Vigano P (2023) Endometriosis and IVF treatment outcomes: unpacking the process. Reproduct Biol Endocrinol. 10.1186/s12958-023-01157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surrey ES, Schoolcraft WB (2003) Does surgical management of endometriosis within 6 months of an in vitro fertilization-embryo transfer cycle improve outcome? J Assist Reprod Genet 20:365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vannuccini S, Clemenza S, Rossi M, Petraglia F (2022) Hormonal treatments for endometriosis: the endocrine background. Rev Endocr Metab Disord 23:333–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Yang R, Lan J, Lin H, Jiao X, Zhang Q (2021) Ovarian endometrioma negatively impacts oocyte quality and quantity but not pregnancy outcomes in women undergoing IVF/ICSI treatment: a retrospective cohort study. Front Endocrinol (Lausanne) 12:739228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yılmaz Hanege B, Güler Çekıç S, Ata B (2019) Endometrioma and ovarian reserve: effects of endometriomata per se and its surgical treatment on the ovarian reserve. Facts Views Vis Obgyn 11:151–157 [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T, He M, Zhang J, Tong Y, Chen T, Wang C, Pan W, Xiao Z (2023) Mechanisms of primordial follicle activation and new pregnancy opportunity for premature ovarian failure patients. Front Physiol 14:1113684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng L, Yang L, Guo Z, Yao N, Zhang S, Pu P (2023) Obesity and its impact on female reproductive health: unraveling the connections. Front Endocrinol (Lausanne) 14:1326546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong C, Gao L, Shu L, Hou Z, Cai L, Huang J, Liu J, Mao Y (2021) Analysis of IVF/ICSI outcomes in endometriosis patients with recurrent implantation failure: influence on cumulative live birth rate. Front Endocrinol (Lausanne) 12:640288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann A, Faust C, Miquel L, Berbis J, Perrin J, Courbiere B (2023) Impact of moderate-to-severe endometriosis on IVF cumulative live birth rate: a retrospective matched cohort study. Reprod Biomed Onlin 47:103186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.