Abstract
Germline mutations in the BMPR2 gene encoding bone morphogenetic protein (BMP) type II receptor (BMPR-II) have been reported in patients with primary pulmonary hypertension (PPH), but the contribution of various types of mutations found in PPH to the pathogenesis of clinical phenotypes has not been elucidated. To determine the biological activities of these mutants, we performed functional assays testing their abilities to transduce BMP signals. We found that the reported missense mutations within the extracellular and kinase domains of BMPR-II abrogated their signal-transducing abilities. BMPR-II proteins containing mutations at the conserved cysteine residues in the extracellular and kinase domains were detected in the cytoplasm, suggesting that the loss of signaling ability of certain BMPR-II mutants is due at least in part to their altered subcellular localization. In contrast, BMPR-II mutants with truncation of the cytoplasmic tail retained the ability to transduce BMP signals. The differences in biological activities among the BMPR-II mutants observed thus suggest that additional genetic and/or environmental factors may play critical roles in the pathogenesis of PPH.
INTRODUCTION
Vascular development and homeostasis are regulated by a number of cytokines, including the members of the transforming growth factor-β (TGF-β) superfamily. The TGF-β superfamily includes various proteins with similar dimeric structures, e.g., activins, nodal, bone morphogenetic proteins (BMPs), and growth/differentiation factors (Massague, 1998). BMPs were originally identified as osteoinductive cytokines at extraskeletal sites in vivo (Wozney et al., 1988). Subsequently, BMPs have been shown to exhibit multifunctional activities in various types of cells. They regulate cell growth, apoptosis, and differentiation, and participate in patterning and specification of various tissues and organs (Kawabata et al., 1998a; Reddi, 1998).
BMPs transduce their signals via two types of serine/threonine kinase receptors, type I and type II receptors, both of which are required for their signal transduction (Kawabata et al., 1998a; Massague, 1998). BMPs bind to three different type II receptors, i.e., activin type II receptors (ActR-IIA and ActR-IIB) and BMPR-II (Liu et al., 1995; Nohno et al., 1995; Rosenzweig et al., 1995; Yamashita et al., 1995), and three different type I receptors, i.e., activin receptor-like kinase (ALK)-3/BMPR-IA, ALK-6/BMPR-IB, and ALK-2 (ten Dijke et al., 1994a,b; Liu et al., 1995; Macias-Silva et al., 1998; Ebisawa et al., 1999; Fujii et al., 1999). On binding of BMPs, type II receptors phosphorylate type I receptors, which in turn phosphorylate intracellular signal-transducing molecules Smad1, 5, and 8 (Heldin et al., 1997; Attisano and Wrana, 1998; Derynck et al., 1998; Massague, 1998). ALK-3 and ALK-6 activate these three Smads, whereas ALK-2 activates only Smad1 and Smad5 but not Smad8 (Aoki et al., 2001).
Recently, heterozygous germline mutations of the BMPR2 gene encoding BMPR-II were found in patients with primary pulmonary hypertension (PPH) (Deng et al., 2000; Lane et al., 2000), suggesting that BMPs may play important roles in homeostasis of the pulmonary vascular system. PPH is a disorder of the pulmonary arteries characterized by formation of plexiform lesions and obliteration of small pulmonary arteries (Rubin, 1997). Subsequently, sporadic form of PPH was also shown to be associated with germline mutations of BMPR2 in at least 26% of cases (Thomson et al., 2000).
Although BMP signals are involved in the regulation of proliferation of human pulmonary smooth muscle cells (Nakaoka et al., 1997; Morrell et al., 2001), it has not been determined whether all cases of PPH carrying mutations within the BMPR2 gene are caused by perturbation of BMP signals. Mutations are distributed throughout the coding region of the BMPR2 gene, suggesting heterogeneity of their contribution to the pathogenesis of PPH. Furthermore, many PPH kindreds carrying mutations of the BMPR2 gene do not develop any signs or symptoms, suggesting that additional environmental and/or genetic factors may be necessary for development of symptoms (Thomson et al., 2000). These findings raised the following questions: 1) whether the signaling components of BMP/Smad pathways are present in human pulmonary endothelial and smooth muscle cells, 2) whether BMP signals are impaired by all types of mutations found in PPH patients, and 3) how signal-transducing capabilities are disrupted in the BMPR-II mutant proteins.
In this study, we used various types of BMPR-II mutants found in patients with PPH to investigate their ability to transduce BMP signals and the biochemical mechanisms by which BMPR-II mutants interfere with BMP signaling. First, we showed that human pulmonary artery endothelial cells (HPAECs) and smooth muscle cells (PASMCs) expressed BMP/TGF-β signaling components, suggesting that these cells may potentially transduce their signals. Next, we showed that some BMPR-II mutants lost most signal-transducing abilities, such as transcriptional activity and phosphorylation of Smad proteins, whereas others retained most of them. Some of the mutants with defects in signaling activities were predominantly located in cytoplasm and may bind a cytoplasmic pool of type I receptors. Taken together, the findings of the present study suggest that perturbation of BMP signaling in the pulmonary vascular system by some types of mutations may be involved in the pathogenesis of PPH, whereas with other types of mutations signals can still be transduced, suggesting that additional factors may be required for the development of PPH.
MATERIALS AND METHODS
Cell Culture
HPAECs and PASMCs were obtained from Clonetics (San Diego, CA) and were maintained in EGM-2 and SmGM-2 (Clonetics), respectively. COS-7 and R-mutant mink lung epithelial cells were maintained in DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was isolated from HPAECs and PASMCs with ISOGEN (NipponGene, Tokyo, Japan), and first-strand cDNA was synthesized using the Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, CA) with random hexamer primers. Expression of various signaling components was compared by semiquantitative RT-PCR analysis. A human β-actin primer set was used to normalize the amount of total cDNA in each sample. PCR products were separated by electrophoresis in agarose gel (1%) and visualized with ethidium bromide. The primer sequences, PCR programs, and expected sizes of PCR products are available online as indicated in Table 1. As controls, RNAs from HPAECs and PASMCs were analyzed for β-actin expression without the prior generation of cDNA, and a PCR reaction for each set of primers was run against H2O.
Plasmid Construction
Plasmids of the BMPR-II, ALKs, and Smads were described previously (Beppu et al., 1997; Imamura et al., 1997). Various mutant forms of BMPR-II were constructed by a PCR-based approach. An EcoRI and an XhoI site were added to the N terminus and C terminus of the BMPR-II cDNA, respectively, and the resulting fragments were subcloned into pcDNA3-FLAG and pcDNA3-HA, which add a FLAG-tag and hemagglutinin (HA)-tag, respectively, C-terminally to the insert (Imamura et al., 1997). To increase levels of expression, inserts were subcloned into another expression vector, pcDEF3 (Goldman et al., 1996). All of the PCR products were sequenced. The sequences of the mutagenesis primers are available upon request.
Transfection, Immunoprecipitation, and Immunoblotting
COS-7 cells were transiently transfected using FuGENE6 (Roche Applied Science, Mannheim, Germany). The amounts of plasmids transfected are available online in Table 2. Immunoprecipitation and immunoblotting were performed as described previously (Kawabata et al., 1998b) using anti-HA 12CA5 (for immunoprecipitation; Roche Applied Science), anti-HA 3F10 (for immunoblotting; Roche Applied Science), anti-FLAG M2 (Sigma-Aldrich), and anti-phosphoserine antibodies (Zymed Laboratories, South San Francisco, CA).
Luciferase Assay
R-mutant mink lung epithelial cells were transiently transfected with an appropriate combination of reporter constructs, expression plasmids, and pcDNA3. Total amounts of transfected DNAs were the same in each experiment. Luciferase activities were normalized using cotransfected sea pansy luciferase activity under the control of thymidine kinase promoter.
Affinity Cross-Linking and Immunoprecipitation
Iodination of BMP-6, affinity cross-linking, and subsequent immunoprecipitation were performed as described previously (Imamura et al., 1997). Briefly, recombinant BMP-6 was iodinated using the chloramine T method, and cross-linking was performed with disuccinimidyl suberate (Pierce Chemical, Rockford, IL). Cells were lysed and subjected to immunoprecipitation with anti-FLAG antibody followed by SDS-PAGE. Cross-linked receptor complexes were visualized by using a BAS 1800 Bio-Image Analyzer (Fuji Photo Film, Tokyo, Japan).
Immunofluorescence Labeling
Immunohistochemical staining of FLAG-tagged BMPR-II in transiently transfected COS-7 cells was performed using anti-FLAG M2 antibody (Sigma-Aldrich), followed by incubation with fluorescein isothiocyanate-labeled goat anti-mouse IgG as described previously (Ebisawa et al., 1999). Nuclei of the cells were stained by 4,6-diamidino-2-phenylindole. Subcellular localization was determined by confocal laser scanning microscopy (Bio-Rad, Hercules, CA).
RESULTS
Profiles of Expression of TGF-β Superfamily Signaling Components in Pulmonary Vascular Cells
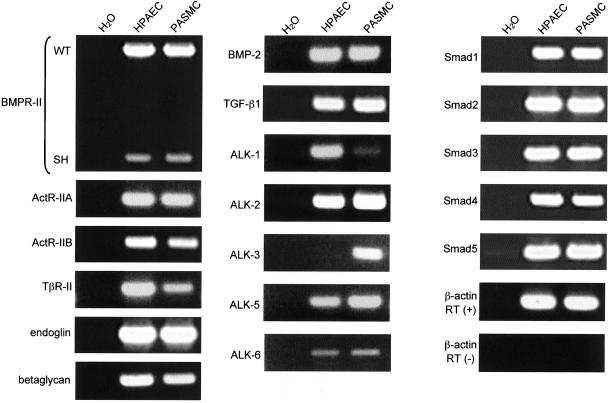
Recently, Morrell et al. (2001) showed that PASMCs express type I (ALK-1, 4, 5, and 6) and type II (TGF-β type II receptor [TβR-II], ActR-II, and BMPR-II) receptors for the TGF-β superfamily. To further evaluate the expression of TGF-β superfamily signaling components in HPAECs and PASMCs, we performed RT-PCR analysis to detect mRNA transcripts for ligands (BMP-2 and TGF-β1), type I (ALK-1, 2, 3, 4, 5, and 6), type II receptors (BMPR-II, ActR-IIA, ActR-IIB, and TβR-II), endoglin, betaglycan, and Smads (Smad1, 2, 3, 4, and 5) (Figure 1).
Figure 1.
Expression of TGF-β superfamily signaling components in HPAECs and PASMCs. RNA samples from HPAECs and PASMCs were analyzed by RT-PCR for expression of TGF-β superfamily-signaling components and the housekeeping gene β-actin. Two alternatively spliced forms, WT and SH, of BMPR-II mRNA transcripts were detected. As controls, RNAs from HPAECs and PASMCs were analyzed for β-actin expression without the prior generation of cDNA, and a PCR reaction for each set of primers was run against H2O.
Transcripts for both BMP-2 and TGF-β1 were present in HPAECs and PASMCs. Among BMP type I receptors, ALK-2 and ALK-6 were expressed in both types of cells, whereas ALK-3 was expressed only in PASMCs. ALK-1 is a TGF-β type I receptor that has been reported to be predominantly expressed in endothelial cells (Panchenko et al., 1996; Roelen et al., 1997). We detected mRNA transcripts for ALK-1 in HPAECs but only very weakly in PASMCs, whereas we detected those for ALK-5 in both types of cells. Two alternatively spliced forms of BMPR-II mRNA transcripts have been reported (Ishikawa et al., 1995; Liu et al., 1995; Rosenzweig et al., 1995). To examine which forms of BMPR-II are expressed in pulmonary vascular cells, we designed PCR primers that are able to generate distinct PCR products from the two spliced variants. As shown in Figure 1, transcripts for both the wild-type (WT) and short (SH) form of BMPR-II were detected in both types of cells, although intensities of the bands of BMPR-II (SH) were much weaker than those of BMPR-II (WT) for both types of cells. We also detected transcripts for other type II receptors, i.e., ActR-IIA, ActR-IIB, and TβR-II, and endoglin and betaglycan in both types of cells.
Finally, the expression of Smads was examined in HPAECs and PASMCs. We detected mRNA transcripts for receptor-regulated Smads specific for BMPs (Smads 1, 5, amd 8), and those for TGF-βs and activins (Smads 2 and 3), and common-partner Smad (Smad4), in both of the cell types. Thus, both HPAECs and PASMCs express transcripts for most components of BMP and TGF-β–signaling pathways, suggesting that pulmonary vascular cells are capable of responding to BMPs and TGF-βs. However, responses to these ligands may differ between HPAECs and PASMCs, because of their differences in expression profiles of type I receptors ALK-1 and ALK-3.
Construction of BMPR-II Mutants Found in Patients with PPH
Because it seemed that BMP signals are intact in pulmonary vascular cells, we attempted to characterize the biological activities of the mutant forms of BMPR-II found in patients with PPH (Deng et al., 2000; Lane et al., 2000; Thomson et al., 2000). BMPR-II has a structure essentially similar to those of other type II receptors for members of the TGF-β superfamily. However, BMPR-II (WT) has a long cytoplasmic tail, the roles of which are not well understood (Figure 2A). In addition, an alternatively spliced form (SH) lacking the cytoplasmic tail exhibited no functional differences from BMPR-II (WT) when assayed using Xenopus embryos (Ishikawa et al., 1995).
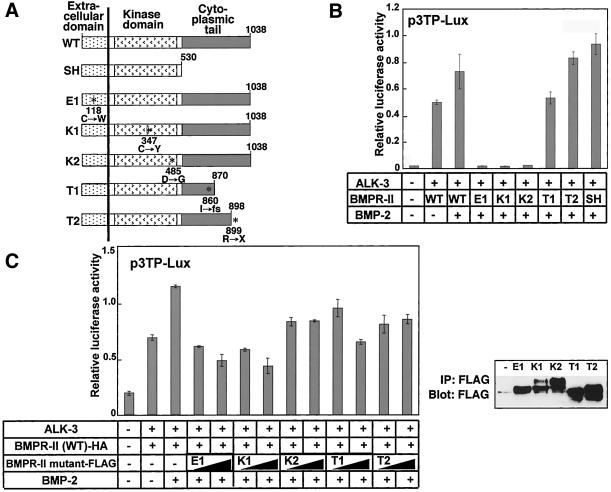
Figure 2.
Biological activities of wild-type and mutant BMPR-II. (A) Structure and location of mutations of WT, SH, and mutant BMPR-II used in the following experiments. Numbers indicate amino acid positions. Mutations are denoted by asterisks. Missense mutations in extracellular (E1) and kinase (K1 and K2) domain mutants and substituted amino acid residues are shown. Cytoplasmic tail mutants (T1 and T2) have frameshift or nonsense mutations resulting in truncated tails. (B and C) Transcriptional activation by wild-type and mutant BMPR-II. p3TP-Lux reporter gene was cotransfected into R-mutant mink lung epithelial cells with ALK-3 and wild-type and/or mutant forms of BMPR-II as indicated, and cells were stimulated with or without BMP-2 (100 ng/ml for B and 50 ng/ml for C). Luciferase activity was normalized against cotransfected sea pansy luciferase activity. Expression of cotransfected BMPR-II mutants was confirmed by immunoblotting of cell lysates with anti-FLAG antibodies (C, right).
At least four types of germline mutations of the BMPR2 gene have been reported (Machado et al., 2001). The first type (type X) has nonsense or frameshift mutations in the extracellular domain, which lead to premature truncation of the transcripts and absence of the production of transmembrane BMPR-II proteins. The second type (type E) has missense mutations in the extracellular domain, most of which involve highly conserved cysteine residues. The third type (type K) has either missense or frameshift mutations in the kinase domain. The fourth type (type T) has frameshift or nonsense mutations within the cytoplasmic tail, resulting in cytoplasmic truncation of the receptor protein. To investigate the biological activities of the BMPR-II mutants, we constructed one or two of each of the three types of BMPR-II mutant (E1, K1, K2, T1, and T2) reported by the International PPH Consortium (Figure 2A).
BMPR-II Mutants Found in PPH Patients Exhibited Differences in Transcriptional Activities
We first examined the transcriptional activities mediated by wild-type or mutant forms of BMPR-II by using p3TP-Lux, a TGF-β–responsive promoter-reporter construct, which weakly responds to BMP signals (Rosenzweig et al., 1995). Coexpression of a BMP type I receptor (ALK-3) and WT or SH of BMPR-II induced transcriptional activation of p3TP-Lux, which was further enhanced in the presence of BMP-2 (Figure 2B). None of the E1, K1, or K2 mutants induced transcriptional activation of the reporter gene. In contrast, the T1 and T2 mutants maintained the ability to induce transcription from p3TP-Lux, suggesting that truncation of the cytoplasmic tail does not efficiently disrupt the transcriptional activity of BMPR-II. Essentially similar results were obtained using 3GC2-lux (Ishida et al., 2000), a BMP-specific promoter-reporter construct (our unpublished data), suggesting that the transcriptional activities induced by BMPR-II mutants found in patients with PPH differ between the type E and K mutants and type T mutants.
Because heterozygous mutations of the BMPR2 gene were reported to cause PPH, we examined the effects of the BMPR-II mutants on the p3TP-Lux transcriptional activity induced by BMPR-II (WT) (Figure 2C, left). When the E1 or K1 mutants were cotransfected with BMPR-II (WT), they repressed the transcriptional activity induced by BMPR-II (WT) in a dose-dependent manner, suggesting that the E1 and K1 mutants behave as dominant negative mutants. In contrast, the T1 or T2 mutant that retained transcriptional activities exhibited less dominant negative effect than the E1 and K1 mutants. In addition, the K2 mutant also showed less dominant negative effect, suggesting the functional heterogeneity within the type K mutants.
BMPR-II Mutants Differentially Induce Phosphorylation of Smad5
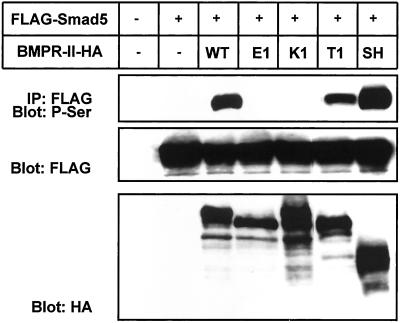
BMP receptor complexes propagate signals mainly through phosphorylation of Smads 1, 5, and 8, although there is evidence for involvement of Smad-independent pathways in this propagation (Hartsough and Mulder, 1995; Atfi et al., 1997; Hannigan et al., 1998; Liberati et al., 1999). To elucidate whether the differences in transcriptional activities induced by BMPR-II mutants involve the activation of Smads, we analyzed the phosphorylation of Smad5 cotransfected with wild-type or mutant forms of BMPR-II into COS-7 cells (Figure 3). The WT and SH forms of BMPR-II phosphorylated Smad5, whereas the E1 and K1 mutants failed to do so. Phosphorylation of Smad5 by the K2 mutant was also significantly reduced (our unpublished data). In agreement with the transcriptional activities, the T1 mutant phosphorylated Smad5, although less efficiently than BMPR-II (WT). These findings suggest that the differences in transcriptional activities mediated by BMPR-II mutants found in PPH patients are due to their abilities to activate BMP-specific Smads.
Figure 3.
Phosphorylation of FLAG-tagged Smad5 mediated by HA-tagged wild-type or mutant BMPR-IIs in transfected COS-7 cells. Top, cell lysates were immunoprecipitated (IP) with anti-FLAG antibody followed by immunoblotting with anti-phosphoserine (P-Ser) antibody. Expression of Smad5 (middle) and BMPR-II (bottom) was confirmed by immunoblotting of cell lysates with anti-FLAG and anti-HA antibodies, respectively.
Ligand-binding Abilities of E1 and K1 Mutants Are Decreased
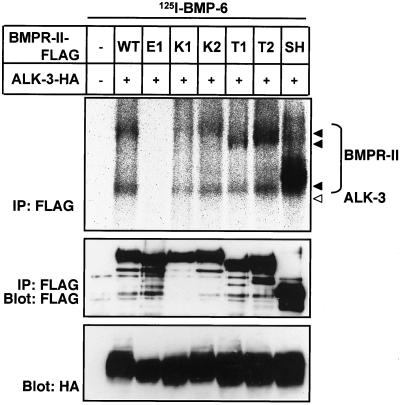
To investigate the biochemical mechanisms by which the E1 and K1 mutants lost signal-transducing abilities, we examined the ligand-binding abilities of the wild-type and mutant forms of BMPR-II. COS-7 cells were cotransfected with ALK-3 and wild-type or mutant forms of BMPR-II, affinity cross-linked using 125I-BMP-6, and subjected to immunoprecipitation by using anti-FLAG antibody for BMPR-II. As shown in Figure 4, WT, SH, K2, T1, and T2 mutant receptors bound BMP-6 efficiently in the presence of ALK-3. In contrast, the E1 mutant carrying a mutation in the extracellular ligand-binding domain did not bind BMP-6, suggesting that its loss of ligand-binding ability resulted in the loss of Smad-phosphorylating ability. Intriguingly, we also found significant reduction of the ligand-binding ability of the K1 mutant carrying a mutation in the kinase domain, which may have, at least in part, caused its loss of Smad-phosphorylating ability.
Figure 4.
Ligand-binding abilities of BMPR-II mutants. COS-7 cells were transfected with FLAG-tagged BMPR-II (BMPR-II-FLAG) and HA-tagged ALK-3 (ALK-3-HA), followed by affinity cross-linking with 125I-BMP-6, and lysates were immunoprecipitated (IP) with anti-FLAG M2 antibody. Immuno-complexes were subjected to SDS-PAGE and visualized by Fuji BAS bio-image analyzer (top). Expression of BMPR-II (middle) and ALK-3 (bottom) was confirmed by immunoblotting of cell lysates with anti-FLAG and anti-HA antibodies, respectively.
E1 and K1 Mutants Exhibit Altered Subcellular Localization
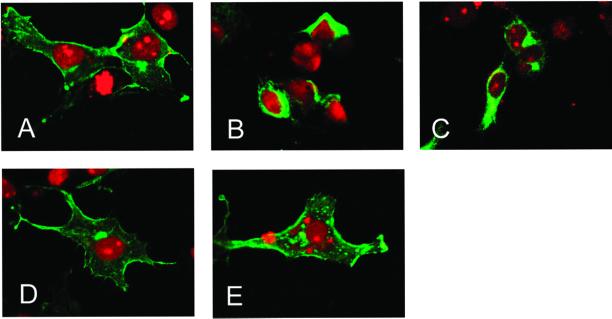
To determine how the ligand-binding abilities of the E1 and K1 mutants were reduced, we examined the subcellular localization of wild-type and mutant forms of BMPR-II. COS-7 cells transfected with the wild-type or mutant forms of BMPR-II were subjected to immunofluorescence staining. WT (Figure 5A) and the T1 mutant (Figure 5E) exhibited intense staining of the plasma membrane as well as the cytoplasm. In contrast, the E1 and K1 mutants carrying missense mutations of cysteine residues within the extracellular and kinase domains, respectively, were observed mostly in the cytoplasm (Figure 5, B and C), suggesting that reduction of the ligand binding abilities of the E1 and K1 mutants was due to their altered subcellular localization. The K2 mutant, carrying a missense mutation of aspartic acid within the kinase domain, was mainly located on the plasma membrane, suggesting that the mechanism of its loss of signal-transducing ability may be due to perturbation of kinase activity.
Figure 5.
Differential subcellular localization of wild-type and mutant BMPR-II. Subcellular distribution of FLAG-tagged wild-type (A), E1 (B), K1 (C), K2 (D), or T1 (E) mutant BMPR-II in transfected COS-7 cells. Permeabilized cells were subjected to immunofluorescence (fluorescein isothiocyanate; green) staining and observation by confocal laser scanning microscopy after nuclear staining with 4,6-diamidino-2-phenylindole (red).
E1 and K1 Mutants Are Retained in the Intracellular Compartments with Type I Receptors
Many membrane and secreted proteins are posttranslationally modified by the addition of N-linked oligosaccharides. We expected that the altered subcellular localization of E1 and K1 mutants would be confirmed by their posttranslational modification. The E1 mutant protein was observed as a fast-migrating band compared with WT (Figure 6, bottom), suggesting that the E1 protein is retained in the intracellular compartments as a glycoprotein containing high-mannose-type oligosaccharides. The K1 mutant was observed as two bands, i.e., a fast-migrating band similar to the E1 mutant and a slowly migrating band similar to the BMPR-II (WT) protein. This finding suggests that a considerable portion of the K1 mutant is also retained in the intracellular compartments.
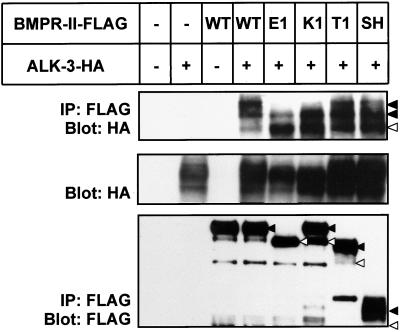
Figure 6.
Hetero-oligomerization of FLAG-tagged BMPR-II with HA-tagged ALK-3 in transfected COS-7 cells. Top, cell lysates were immunoprecipitated with anti-FLAG antibody followed by immunoblotting with anti-HA antibody. Expression of ALK-3 (middle) and BMPR-II (bottom) was confirmed by immunoblotting of cell lysates with anti-FLAG and anti-HA antibodies, respectively. Fast- and slowly migrating bands of BMPR-II and ALK-3 are indicated by open and closed triangles, respectively.
To determine how the altered subcellular localization of the BMPR-II mutants affects complex formation with type I receptors, we examined the hetero-oligomerization of BMPR-II mutants with ALK-3. COS-7 cells cotransfected with ALK-3 and wild-type or mutant forms of BMPR-II were subjected to FLAG-immunoprecipitation for BMPR-II, followed by HA-immunoblotting for ALK-3. BMPR-II (WT), BMPR-II (SH), and the T1 mutant formed complexes with slowly migrating forms of ALK-3, whereas the E1 and K1 mutants formed complexes predominantly with fast-migrating forms of ALK-3, which may contain high-mannose-type oligosaccharides (Figure 6, top). These results suggest that the E1 and K1 mutants are located in the intracellular compartments and that they may preferentially form complexes with the type I receptors located in the same compartments.
DISCUSSION
Roles of BMP and TGF-β Signaling in Maintenance of Vascular Systems
TGF-β plays important roles during yolk sac vasculogenesis as well as late stages of angiogenesis by growth inhibition and production of extracellular matrix of endothelial cells (Dickson et al., 1995; Pepper, 1997; Goumans et al., 1999). In endothelial cells, two types of TGF-β type I receptors, ALK-1 and ALK-5, mediate TGF-β signaling. ALK-5 is ubiquitously expressed in TGF-β–responsive cells and activates Smad2 and Smad3. In contrast, ALK-1 is predominantly expressed in endothelial cells and activates BMP-specific Smad1 and Smad5. These observations suggest that balance between Smad1/5/8 and Smad2/3 pathways is important in determining vascular endothelial properties during angiogenesis (Oh et al., 2000; Goumans et al., 2002). Endoglin is a dimeric glycoprotein with a short intracellular region that is structurally similar to betaglycan (also known as TGF-β type III receptor). Endoglin binds TGF-β as well as BMP-2 and BMP-7, suggesting that it may regulate both TGF-β and BMP signaling pathways (Barbara et al., 1999). Interestingly, mutations of ALK-1 and endoglin have been found in patients with hereditary hemorrhagic telangiectasia (McAllister et al., 1994; Johnson et al., 1996). Taken together with the findings that the BMPR2 gene is mutated in PPH patients, our findings suggest that TGF-β/BMP signals mediated by Smad1, 5, and 8 may play important roles in maintenance of vascular homeostasis.
Recently, Morrell et al. (2001) showed that PASMCs express receptors for TGF-β and BMPs, and that BMP suppressed the DNA synthesis and proliferation of PASMCs from patients with secondary pulmonary hypertensions, but did not suppress those from patients with PPH (Morrell et al., 2001). The present study showed that both HPAECs and PASMCs express most of the signaling components required for TGF-β/BMP signal transduction, including ligands, receptors, and Smads (Figure 1). However, response to TGF-β and BMPs may differ between HPAECs and PASMCs. Because HPAECs express both ALK-5 and ALK-1, TGF-β may activate Smad2/3 and Smad1/5 pathways, similar to other endothelial cells. Because PASMCs do not express ALK-1, the Smad1/5 pathways may not be activated by TGF-β. Intriguingly, HPAECs express ALK-2 and ALK-6, but not ALK-3, suggesting that they respond to BMP-6 and BMP-7 through ALK-2 and ALK-6, but not to BMP-4, which binds to ALK-3 (ten Dijke et al., 1994b; Ebisawa et al., 1999). In contrast, PASMCs express ALK-2, 3, and 6, suggesting that they respond to BMP-6 and -7 as well as to BMP-4.
One of the features of PPH is overproliferation of endothelial cells and smooth muscle cells. Taken together with results of previous studies showing that BMPs have growth inhibitory effects on smooth muscle cells (Nakaoka et al., 1997; Dorai et al., 2000; Morrell et al., 2001), these findings suggest that it is likely that BMP signals maintain pulmonary vascular integrity by suppressing the overproliferation of cells and that reduction of BMP signals caused by mutations of the BMPR2 gene eventually results in symptoms of PPH.
How Did Type E and K Mutants Lose Their Signal-transducing Abilities?
In the present study, we generated five BMPR-II mutants, i.e., those mutated in the extracellular domain (E1), kinase domain (K1 and K2), or cytoplasmic tail (T1 and T2) (Lane et al., 2000) (Figure 2A), to examine the biological activities of the BMPR-II mutants found in PPH patients. We found that the type E and K mutants lost their transcriptional activities, whereas the type T mutants maintained transcriptional activities although they were less potent than those of BMPR-II (WT). This suggests that these BMPR-II mutants have different biological activities.
To date, all missense mutations within the extracellular domain of BMPR-II have been found at cysteine residues in PPH patients (Machado et al., 2001). Interestingly, extracellular cysteine residues have been shown to be essential for formation of proper three-dimensional structure and to be required for membrane targeting of some receptors (Zeng et al., 1999). Consistent with this, we found that most of the E1 mutant proteins mutated at cysteine-118 were present in the cytoplasm (Figure 5B). These results suggest that loss of signal-transducing abilities due to missense mutations in the extracellular ligand-binding region is due not only to loss of ligand-binding ability of the extracellular domain but also to altered subcellular localization.
Notably, the E1 mutant protein migrated faster than the BMPR-II (WT) protein (Figure 6, bottom), implying differential posttranslational modification due to abnormal subcellular localization of the E1 protein. When ALK-3 was coexpressed with the E1 mutant, only fast-migrating protein bands of ALK-3 formed complexes with the E1 mutant proteins (Figure 6, top). Many membrane-targeted proteins are posttranslationally modified by addition of N-linked oligosaccharides during transport through the Golgi apparatus. Treatment of ALK-3 and BMPR-II with N-glycosidase F resulted in shift of slowly migrating bands of ALK-3 and BMPR-II to fast-migrating bands (our unpublished data), suggesting that the fast-migrating proteins of the E1 mutant and ALK-3 may contain high-mannose-type oligosaccharides and that they are retained in the cytoplasm as a complex. These results raised the possibility that dominant negative effects of the E1 mutant against BMPR-II (WT) may be due to sequestration of BMP type I receptors in the intracellular compartments.
On the other hand, missense mutations within the kinase region were identified at various amino acid residues, including cysteine, aspartic acid, and arginine residues (Machado et al., 2001). The K1 mutant, with substitution of cysteine-347 by tyrosine, exhibited a reduced ligand-binding ability than BMPR-II (WT). This can be explained by the distribution of mutant proteins partially in cytoplasm, as demonstrated by immunohistochemical analysis and by the presence of fast-migrating bands on immunoblot analysis (Figures 5C and 6, bottom). However, this distribution profile of the K1 mutant proteins cannot fully explain the loss of signal-transducing ability and gain of dominant negative activity by them, which were equivalent to those of the E1 mutant. Kinase activity was probably lost in the K1 mutant, resulting in the potent dominant negative effects of this mutant. In agreement with this, a BMPR-II kinase negative mutant exhibited a dominant negative effect against ActR-II in transcriptional activation activity (Liu et al., 1995). The K2 mutant, with substitution of aspartic acid-485 by glycine, exhibited normal ligand-binding ability (Figure 4) and subcellular localization (Figure 5D), but lost signal-transducing ability (Figure 2B). Kinase activity was probably lost in the K2 mutant, which may have caused the loss of transcriptional activity; however, how the K2 mutant has less dominant negative effect remains unknown.
Role of BMPR-II Mutants with Truncation of Cytoplasmic Tail in Pathogenesis of PPH
BMPR-II is structurally similar to other type II receptors of the TGF-β superfamily, e.g., TβR-II, ActR-IIA, and ActR-IIB. However, BMPR-II has a long cytoplasmic tail that is not found in other type II receptors in mammals. The functions of the cytoplasmic tail of BMPR-II are not yet clear. The fact that truncation of the cytoplasmic tail of BMPR-II was found in type T mutants from patients with PPH suggests novel functions for this region. Compared with the E1 and K1 mutants, however, the T1 mutant retained most of its biological activity with the exception that it phosphorylated Smad5 less efficiently than WT or SH forms of BMPR-II. Machado et al. (2001) analyzed the transcriptional activities of BMPR-II mutants K2 and T1 according to our nomenclature, in NMuMG cells in which endogenous BMP signaling pathways are intact. Although they concluded that both of mutants lost their signaling capabilities, their results showed that only the K2 mutant, but not the T1 mutant, inhibited endogenous BMP signals. Thus, there may be significant differences in biological activities between the K2 and T1 mutants, consistent with our results. Recently, Nohe et al. (2002) showed that BMPR-II mutants completely lacking the cytoplasmic tail were capable of transducing BMP-2 signals similar to BMPR-II (SH). Taken together with the present findings, these results suggest that the cytoplasmic tail of BMPR-II may not be essential for transduction of BMP signals through Smads, although it is possible that it has yet unidentified functions in BMP signaling. It will be important to determine whether other factors, such as additional genetic mutations and/or environmental factors, play important roles in the pathogenesis of PPH.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Y. Morishita for technical help. This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and was also supported by a grant from Boehringer Ingelheim.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0063. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0063.
REFERENCES
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci. 2001;114:1483–1489. doi: 10.1242/jcs.114.8.1483. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Mads and Smads in TGFβ signaling. Curr Opin Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-β superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- Beppu H, Minowa O, Miyazono K, Kawabata M. cDNA cloning and genomic organization of the mouse BMP type II receptor. Biochem Biophys Res Commun. 1997;235:499–504. doi: 10.1006/bbrc.1997.6816. [DOI] [PubMed] [Google Scholar]
- Deng Z, Morse JH, Slager S L, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective hematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman LA, Cutrone EC, Kotenko SV, Krause CD, Langer JA. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. Biotechniques. 1996;21:1013–1015. doi: 10.2144/96216bm10. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Zwijsen A, van Rooijen MA, Huylebroeck D, Roelen BA, Mummery CL. Transforming growth factor-β signaling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development. 1999;126:3473–3483. doi: 10.1242/dev.126.16.3473. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan M, Zhan L, Ai Y, Huang CK. The role of p38 MAP kinase in TGF-β1-induced signal transduction in human neutrophils. Biochem Biophys Res Commun. 1998;246:55–58. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Mulder KM. Transforming growth factor β activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275:6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yoshioka H, Ohuchi H, Noji S, Nohno T. Truncated type II receptor for BMP-4 induces secondary axial structures in Xenopus embryos. Biochem Biophys Res Commun. 1995;216:26–33. doi: 10.1006/bbrc.1995.2587. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary hemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998a;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998b;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips J A, 3rd, Loyd J E, Nichols W C, Trembath R C. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang XF. Smads bind directly to the Jun family of AP-1 transcription factors. Proc Natl Acad Sci USA. 1999;96:4844–4849. doi: 10.1073/pnas.96.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips III JA, Newman J, Williams D, Galie N, Manes A, NcNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, McCormick MK, Pericak-Vance MA, Heutink P, Oostra BA, Heithema T, Westerman CJJ, Porteous ME, Guttmacher AE, Letarte M, Marchuck DA. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β (1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P. TGF-β receptor-mediated signaling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka T, Gonda K, Ogita T, Otawara-Hamamoto Y, Okabe F, Kira Y, Harii K, Miyazono K, Takuwa Y, Fujita T. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest. 1997;100:2824–2832. doi: 10.1172/JCI119830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohe A, Hael S, Ehrlich M, Neubauer F, Sebald W, Henis Y, Knaus P. The mode of BMP receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko MP, Williams MC, Brody JS, Yu Q. Type I receptor serine-threonine kinase preferentially expressed in pulmonary blood vessels. Am J Physiol. 1996;270:L547–L558. doi: 10.1152/ajplung.1996.270.4.L547. [DOI] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-β: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- Roelen BA, van Rooijen MA, Mummery CL. Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn. 1997;209:418–430. doi: 10.1002/(SICI)1097-0177(199708)209:4<418::AID-AJA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH. Characterization of type I receptors for transforming growth factor-β and activin. Science. 1994a;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994b;269:16985–16988. [PubMed] [Google Scholar]
- Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC, Nichols WC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FY, Soldner A, Schoneberg T, Wess J. Conserved extracellular cysteine pair in the M3 muscarinic acetylcholine receptor is essential for proper receptor cell surface localization but not for G protein coupling. J Neurochem. 1999;72:2404–2414. doi: 10.1046/j.1471-4159.1999.0722404.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.