Abstract
Nephrogenesis starts with the reciprocal induction of two embryonically distinct analages, metanephric mesenchyme and ureteric bud. This complex process requires the refined and coordinated expression of numerous developmental genes, such as inv. Mice that are homozygous for a mutation in the inv gene (inv/inv) develop renal cysts resembling autosomal-recessive polycystic kidney disease. The gene locus containing inv has been proposed to serve as a common modifier for some human and rodent polycystic kidney disease phenotypes. We generated polyclonal antibodies to inversin to study its subcellular distribution, potential binding partners, and functional aspects in cultured murine proximal tubule cells. A 125-kDa inversin protein isoform was found at cell-cell junctions. Two inversin isoforms, 140- and 90-kDa, were identified in the nuclear and perinuclear compartments. Plasma membrane allocation of inversin is dependent upon cell-cell contacts and was redistributed when cell adhesion was disrupted after incubation of the cell monolayer with low-calcium/EGTA medium. We further show that the membrane-associated 125-kDa inversin forms a complex with N-cadherin and the catenins. The 90-kDa nuclear inversin complexes with β-catenin. These findings indicate that the inv gene product functions in several cellular compartments, including the nucleus and cell-cell adhesion sites.
INTRODUCTION
Organogenesis of the mammalian kidney involves the coordinated regulation of gene expression that occurs during the reciprocal induction of two embryonically distinct analages, mesenchymal metanephric blastema and epithelial ureteric bud. Successful nephrogenesis and maturation of renal tubules requires a combination of growth pattern control, cell fate determination, and cell cycle control. A relatively common derangement of tubule maturation is the growth of fluid-filled epithelial cysts. Mutations in one of several genetic loci can lead to a cystic phenotype characterized by epithelial cell proliferation, reversal of cell polarity, and alterations in apoptosis, extracellular matrix, and transepithelial transport of fluids and electrolytes (for review, see Grantham et al., 2000).
Rodent models have proved valuable in elucidating the mechanisms underlying renal epithelial cyst formation. One such model, the inversion of embryonic turning (inv/inv) mutant mouse was created by insertional mutagenesis and exhibits an autosomal recessive form of situs inversus and renal cysts (Mochizuki et al., 1998; Morgan et al., 1998), the latter resembling autosomal recessive polycystic kidney disease (PKD). The predicted sequence of inversin, the inv gene product, has 15 Ank/Swi6 motifs arranged in a tandem array located toward the amino-terminal side of the protein (Mochizuki et al., 1998). The C-terminal one-half of inversin has no similarity to other proteins (Mochizuki et al., 1998). Subcellular distribution and potential binding partners of inversin have yet to be discovered.
Although the function of the inv gene product is unknown, inversin's potential contribution to renal cystogenesis is supported by quantitative trait localization data. Quantitative trait localization indicates that inv is one of many genes within a locus that contains one or more modifying genes in the pcy, cpk, jck, and bpk mouse models of human PKD. (Woo et al., 1997; Guay-Woodford et al., 2000; Kuida and Beier, 2000; D.D. Woo, personal communication). Modifying genes have been proposed to explain the diversity of PKD phenotypes observed in humans and animal models (Koptides and Deltas, 2000). The wide range of PKD phenotypes in humans and animal models, combined with the numerous gene defects underlying cyst formation, suggests there is an abundance of proteins that participate in a common pathway of cystogenesis. Inversin appears to be one of these proteins.
In this paper, the subcellular distribution of inversin is characterized. In a murine S1 proximal tubule cell line, affinity-purified antibodies raised against inversin's c-terminal domain show nuclear, perinuclear, and plasma membrane labeling by confocal microscopy and the detection of 140-, 125-, and 90-kDa isoforms on immunoblots. The 140- and 90-kDa isoforms were enriched in nuclear and cytoplasmic extracts, whereas the 125-kDa isoform was most abundant in membrane fractions. Inversin antibodies immunoprecipitated N-cadherin and α, β, and γ-catenins, but not E-cadherin, β1-integrin, or vinculin. Reverse immunoprecipitations with antibodies to N-cadherin and β-catenin confirmed that inversin forms a stable complex with these integral membrane proteins. Like inversin, N-cadherin has been implicated in defective left-right asymmetry (Garcia-Castro et al., 2000), and transgenic mice overexpressing β-catenin develop cystic kidneys (Saadi-Kheddouci et al., 2001). Inversin's localization at the lateral membrane is dependent upon cell-cell contacts and can be disrupted by incubating the monolayer with low-calcium/EGTA medium. These findings suggest that isoforms of inversin may participate in intercellular junction biogenesis and gene transcription similar to β-catenin's cellular function.
MATERIALS AND METHODS
Cell Culture
An immortalized cell line derived from the early segment (S1) of the proximal tubule (S1 cells) was a kind gift from Dr. G.T. Nagami (UCLA School of Medicine, Los Angeles, CA; Kaunitz et al., 1993). Cells were maintained in a 50:50 mixture of Hams-F12:DMEM media supplemented with 2 mM l-glutamine, 10 mM Na-HEPES, 2 mM sodium pyruvate, insulin, sodium-selenite, and sodium-bicarbonate, and 7% fetal calf serum, penicillin, and streptomycin (Kaunitz et al., 1993, 2000). Cells were grown on dishes, coverslips, and polyester membrane filters (0.4-μm pore size; Transwell, Costar, Cambridge, MA) coated with type I rat-tail collagen (Invitrogen, Carlsbad, CA; Hammerton et al., 1991).
Reagents and Antibodies
Cell media, fetal calf serum, and reagent grade chemicals were supplied by Sigma (St. Louis, MO). Mouse monoclonal antibody to histone H1 was obtained from StressGen Biotechnologies (Victoria, British Columbia, Canada). Mouse-monoclonal antibodies to α-, β-, and γ-catenin, rat monoclonal antibody to E-cadherin, and polyclonal rabbit antibody to β-catenin were obtained from Zymed Laboratories (South San Francisco, CA). Pan-cadherin antibody was purchased from Sigma. Polyclonal rabbit antibody to N-cadherin was obtained from Calbiochem (La Jolla, CA). Mouse monoclonal antibodies to vinculin, β1-integrin, and E- and N-cadherin were purchased from BD Transduction Laboratories (Franklin Lakes, NJ). All secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). 4′,6-Diamidino-2-phenyindole (DAPI) nucleic acid stain, rhodamine-phalloidin, and SYPRO protein gel stain were purchased from Molecular Probes (Eugene, OR). All other chemicals and reagents were supplied by Fisher Scientific (Pittsburgh, PA).
Inversin Polyclonal Antibody
An expressed sequence tag (EST; NCBI accession no. AI790867) was obtained from Incyte Genomics (St. Louis, MO; Suzuki et al., 1997) and was sequenced to confirm homology to the 3′ end of the inv gene (Lark Technologies, Houston, TX). A 459-base pair (bp) segment of the EST clone was ligated into pRSETB (Invitrogen) and was sequenced to confirm the clone was in-frame with a polyhistidine tag. The recombinant plasmid was transfected into Escherichia coli strain BL21(DE3)pLysS (Invitrogen) to synthesize a bacterial fusion-protein containing the C-terminal 153 amino acids of the EST clone. After induction with 1 mM isopropyl-β-d-thiogalactopyranose, the recombinant protein was expressed and isolated from bacterial lysate by Ni2+ affinity chromatography (ProBond Resin; Invitrogen). Bound protein was eluted with 500 mM NaCl and 20 mM NaPO4, pH 4.0, and was then dialyzed against 50 mM Tris-Cl, pH 8.0, 1 mM CaCl2, and 0.1% Tween-20, after which the histidine tag was cleaved by enterokinase digestion (EKMax; Invitrogen). Digest products were separated on a 15% SDS-PAGE gel, and protein was recovered by gel excision under visualization using SYPRO protein gel stain. Recombinant protein (17 kDa) was electroeluted from gel slices using an electroeluter (Bio-Rad Laboratories, Richmond, CA). Successful cleavage of the histidine tag was confirmed by immunoblotting with a mouse monoclonal antibody specific for histidine epitope tags (Anti-HisG; Invitrogen). Rabbits were immunized with the purified 17-kDa inversin miniprotein by Covance Research Products (Denver, PA). Antibodies were purified by immunoaffinity chromatography using the inversin mini protein coupled to activated beads (Affigel 10; Bio-Rad Laboratories) according to the manufacturer's directions. Antibody was eluted in 100 mM glycine, pH 2.4, and the eluate was neutralized with Tris base.
Protein Extraction
For total protein extracts, confluent S1 cells were washed with cold phosphate-buffered saline (PBS), scraped from the dish with a rubber-policeman, and pelleted by centrifugation at 850 × g for 5 min at 4°C. Cell pellets were resuspended and homogenized using a ball-bearing homogenizer in extraction buffer (150 mM NaCl, 50 mM Tris-Cl, pH 8.0, 4 mM EDTA, 1 mM phenyl methyl sulfonyl fluoride [PMSF], and protease inhibitor cocktail [Sigma] with or without Triton X-100 at vol/vol of 1.0%). Cell lysates were centrifuged at 15,000 × g for 10 min at 4°C. Supernatants were mixed with Laemmli buffer (2% SDS, 100 mM Tris-Cl, pH 6.8, 25% [vol/vol] glycerol, 10 mM dithiothreitol, 0.001% [wt/vol], and bromphenol blue) and boiled for 10 min (Laemmli, 1970).
SDS-PAGE and Immunoblot Analysis
Proteins were separated on 7.5% SDS-PAGE gels and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ; Laemmli, 1970). Membranes were blocked with 3% newborn calf serum (NCS) dissolved in Tris-buffered saline (TBS) containing 0.1% (vol/vol) Tween-20 and incubated for 45 min with primary antibodies diluted in 3% NCS in TBS. Membranes were washed in TBS-Tween 20, incubated with horseradish peroxidase-conjugated secondary antibodies in 3% NCS in TBS for 45 min and washed as above. Chemiluminescence was used for detection (Pierce, Rockford, IL).
Mass Spectrometry Analysis of Inversin Immunoprecipitate
Total protein extracts from cultured S1 cells were immunoprecipitated with the inversin antibody in 1% (vol/vol) Triton X-100, 150 mM NaCl, 10 mM Tris-Cl, pH 8.0, 1 mM EDTA, 1 mM EGTA, 0.2 mM Na3VO4, 2 mM PMSF, and protease inhibitor cocktail, 1:100. Precipitates were recovered by protein A conjugated to magnetic beads (Dynal, Lake Success, NY). Beads were collected in a magnetic particle concentrator and were washed with three buffers featuring different ionic strengths (buffer I: 150 mM NaCl, 50 mM Tris-Cl, pH 8.0, and 1% [vol/vol] Triton X-100; buffer II: 500 mM NaCl and 50 mM Tris-Cl, pH 8.0; and buffer III: 50 mM Tris-Cl, pH 8.0). Precipitates were resuspended in Laemmli buffer and boiled for 10 min to elute complexes. Proteins were separated by 7.5% SDS-PAGE and visualized with Coomassie stain (Pierce). The 140-kDa band was excised, trypsin digested, and analyzed with a Finnigan (Thermoquest) LCQ mass spectrometer (Biochemistry Biotechnology Facility, Indiana University School of Medicine, Indianapolis, IN). A free-ware program was used to compute and map the cleavage fragments obtained from a virtual trypsin digest of the inv sequence (Client Paws; ProteoMetrics, New York, NY).
Immunohistochemistry and Fluorescence Microscopy
Cells grown on filters were fixed in 4% paraformaldehyde in PBS for 10 min. The fixation reaction was quenched in 100 mM NH4Cl dissolved in PBS. Samples were incubated in blocking buffer (1% bovine serum albumin and 0.1% Triton X-100 in PBS) for 10 min before labeling. Cells were incubated in primary antibodies and rhodamine-phalloidin, followed by incubation with fluorescein- or Cy5-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and washing in PBS. Nuclei were labeled with DAPI diluted in blocking buffer for 10 min. Filters were placed in 2% PFA in PBS, mounted with Mowoil (Calbiochem), and examined with a LSM 510 laser scanning microscope (Carl Zeiss North America, Thornwood, NY) equipped with a UV argon laser, a visible argon laser, and two helium-neon lasers. Images were collected sequentially and processed by Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Iodixanol Gradient Fractionation
Iodixanol subcellular fractionation was performed as previously described (Grindstaff et al., 1998). Briefly, confluent S1 cells were homogenized in isotonic sucrose buffer [0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM HEPES-KOH, pH 8.0, 2 mM PMSF, 0.5 mM 4-(2-aminoethyl)benzensulfonyl-fluoride, and protease inhibitor cocktail 1:100] by repeated passage through a ball-bearing homogenizer. Eight volumes of postnuclear supernatant were combined with 10 volumes of a 54% iodixinol solution that was prepared from a 60% stock solution [diluted with 1/10 vol of 900 mM KOAc, 20 mM Mg(OAc)2, and 200 mM HEPES-KOH, pH 8.0]. Samples were centrifuged at 354,000 × g for 1 h at 4°C in a TLN-100 rotor (Beckman Instrument, Fullerton, CA). Fractions were collected and proteins were separated by 7.5% SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with antibodies specific for β-catenin, pan-cadherin, and inversin.
Extraction of Nuclear Protein Complexes
Nuclear proteins were extracted by stepwise lysis of cells to ensure minimal cross-contamination, as described earlier (Dignam et al., 1983). Briefly, confluent S1 cells were washed with cold PBS, scraped in PBS from the dish with a rubber-policeman, and pelleted by centrifugation at 500 × g for 3 min at 4°C. Using NE-PER nuclear extraction reagents (Pierce), cells were chemically lysed and nuclei were isolated after centrifugation (16,000 × g for 5 min) and resuspension per the manufacturer's instructions. Nuclear cell debris and DNA were removed by centrifugation (16,000 × g for 5 min) and nuclear proteins were mixed with Laemmli buffer, boiled for 10 min, and loaded on 7.5% SDS-PAGE gels.
Extraction of Membrane Protein Complexes
Plasma membranes were purified by the technique of aqueous two-phase partition (Morre and Morre, 1989; Ohlendieck, 1996). Briefly, confluent S1 cells were washed with cold PBS, scraped from the dish with a rubber-policeman, and pelleted by centrifugation at 500 × g for 3 min at 4°C. Using Mem-PER extraction reagents (Pierce), cells were chemically lysed and membrane proteins were solubilized. The mixture was incubated at 37°C and centrifuged at 10,000 × g for 2 min to separate hydrophobic from hydrophilic proteins. The viscous phase containing the membrane protein fraction was collected and proteins were purified by precipitation with 10% trichloroacetic acid. Recovered proteins were mixed with Laemmli buffer, boiled for 10 min, and loaded on 7.5% SDS-PAGE gels.
Ca2+ Switch
S1 cells were grown in normal-calcium (1.8 mM Ca2+) medium (NCM, see cell culture for formula). Low-calcium medium (LCM) was prepared by supplementing NCM with EGTA to a final concentration of 4 mM, as previously described (Citi, 1992). The pH was adjusted to its initial value with NaOH, and the medium was sterile filtered. S1 cells were washed with PBS, and NCM was replaced with LCM. At 45 and 90 min of incubation in LCM, cells were fixed and processed for immunofluorescence microscopy.
Immunoprecipitation
Confluent S1 cells grown on collagen I-coated tissue culture dishes were washed with PBS and incubated on ice for 30 min in CSK buffer [50 mM NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 3 mM MgCl2, 0.5% (vol/vol) Triton X-100, 2 mM PMSF, 0.5 mM 4-(2-aminoethyl)benzensulfonylfluoride, and protease inhibitor cocktail 1:100; Torres and Nelson, 2000]. Cells were scraped from dishes and insoluble material was removed by centrifugation at 10,000 × g for 10 min at 4°C. Cell extracts were incubated with primary rabbit polyclonal antibodies for 1 h. Immune complexes were recovered by incubation with protein A-Sepharose (Amersham) for 1 h. Protein A-Sepharose beads were washed three times in extraction buffer before protein complexes were released by boiling in Laemmli buffer for 10 min. Precipitated proteins were separated by SDS-PAGE followed by immunoblotting.
RESULTS
Characterization of the Polyclonal Inversin Antibody
A 17-kDa recombinant protein fragment was expressed as a fusion protein containing 153 amino acids from the C-terminal portion of the EST that lacks significant homology with any other protein in the available databases (Morgan et al., 1998). Affinity-purified antibody detected three bands estimated at 140, 125, and 90 kDa on immunoblots containing proteins from confluent S1 cells extracted in 1% Triton X-100 (Figure 1A, left lane). Antibody recognition of both bands was competitively blocked when the affinity-purified antibody was preincubated with the immunizing inversin miniprotein (Figure 1A, right lane).
Figure 1.

Characterization of inversin antibody. (A) Total protein extracted from confluent S1 cells (1% Triton X-100 buffer) was separated by 7.5% SDS-PAGE, transferred to membranes, and incubated with affinity-purified inversin antibody. Three bands were detected at 140, 125, and 90 kDa with anti-inversin alone, but no bands were detected when inversin antibody was preincubated with the immunizing recombinant protein. (B) Postnuclear homogenate from confluent S1 cells was immunoprecipitated with affinity-purified inversin antibody and was resolved by 7.5% SDS-PAGE. The 140-kDa band was excised, trypsin digested in situ, and analyzed by mass spectrometry. Table shows peak values measured from mass spectrum as compared with calculated fragment masses after trypsin digestion of inversin. Differences between these values ranged in the expected variability.
Mass Spectrometry Confirmation of Antibody Specificity
Mass spectrometry analysis of the 140-kDa protein detected by the affinity-purified antibody confirmed that the antibody specifically bound to inversin. Proteins from S1 cell extracts were immunoprecipitated with inversion antibody and separated by SDS-PAGE. The 140-kDa band was digested with trypsin and the resultant fragments were analyzed by mass spectrometry (Figure 1C). Measured mass-to-charge ratios (m/z) of tryptic peptides significantly correlated with calculated fragment m/z ratios obtained from computed analysis of the inversin protein sequence. Masses of fragments were also compared with the masses of a peptide database calculated from NCBI's nr protein database (Zhang and Chait, 2000). The inversin protein was identified as the top ranking of the candidate proteins based on its calculated posterior probability (Zhang and Chait, 2000). The next top nine candidate proteins did not produce significant alignments to the inversin protein sequence (Altschul et al., 1997). Similarly, the antibody to inversin precipitated two proteins of 165- and 90-kDa that matched inversin when analyzed by mass spectrometry (unpublished data; Zhang and Chait, 2000).
Subcellular Localization of Inversin: Laser Confocal Microscopy
Subconfluent (Figure 2, A, C, and E) and confluent (Figures 2, B, D, and F, and 3) S1 cells were grown on filters and stained with inversin antibody (Figures 2, A and B and 3B) and DAPI, a nucleic acid stain (Figures 2, C and D and 3A). To show the cellular architecture, the actin cytoskeleton was labeled with rhodamine phalloidin (Figures 2, E and F and 3C; Small et al., 1999). In subconfluent cells, inversin was predominantly distributed in nuclei (compare Figure 2, A with C). Weak perinuclear and membrane staining was also observed (Figure 2A). No signal was detected when cells were incubated with the inversin antibody in the presence of the immunizing peptide or the secondary antibody alone (Figure 2A, insets). In separate experiments, perinuclear inversin staining colocalized with anti-KDEL antibody staining, suggesting that inversin is in the rough endoplasmic reticulum (unpublished data). When incubated with anti-inversin, confluent cells showed distinct labeling of cell membranes (Figures 2B and 3B) that was only weakly apparent (Figure 2A, arrowheads) in subconfluent cells with adjacent cell borders (Figure 2E). A vertical image section (X-Z axis) of confluent S1 cells shows a lateral distribution of inversin (Figure 3B). Similar labeling by inversin antibody was also observed in other cultured renal epithelial cell lines such as MDCK cells or cells derived from the distal convoluted tubule (unpublished data; Kroning et al., 2000), indicating that various cell lines carry this protein in a membrane-associated fashion.
Figure 2.
Confocal microscopy of subconfluent (A, C, and E) and confluent (B, D, and F) S1 cells fixed in paraformaldehyde and triple-labeled with inversin antibody (A and B), DAPI (C and D), and phalloidin-rhodamine (E and F). In both subconfluent (A) and confluent (B) S1 cells, the inversin antibody stained nuclei and less intensely the perinuclear compartment. Cell membranes appeared uniformly stained in confluent cells (B), but were focally stained in subconfluent cells at early cell-cell contacts (A, arrowheads). Staining seen with inversin antibody (A and B) was absent in cells stained with inversin antibody preincubated with immunizing protein, and secondary antibody alone (A, insets).
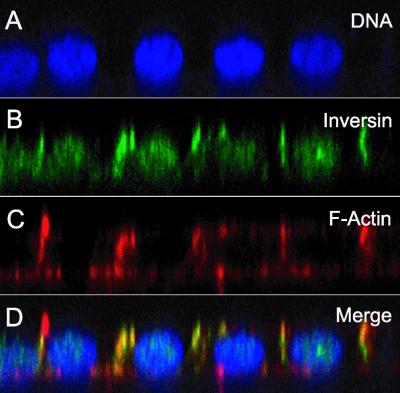
Figure 3.
Vertical sections (X-Z axis) of inversin staining. Confluent S1 cells were grown on filters, fixed in paraformaldehyde, and triple-labeled with DAPI (A), inversin antibody (B), and phalloidin-rhodamine (C). The vertical sections (X-Z axis) demonstrate distribution of inversin to membranes of cell-cell contacts, but not to apical or basal plasma membranes. (D) overlay of all three channels.
Subcellular Distribution of Inversin: Iodixanol Gradient Fractionation
To identify which inversin isoforms (i.e., 140-, 125-, or 90-kDa bands) are responsible for the membrane staining patterns, S1 cell extracts were fractionated by detergent-free iodixanol density gradient centrifugation (Grindstaff et al., 1998). In iodixanol gradients, low-density plasma membrane and cytoplasmic particles (<1.1 g/ml) are recovered in the top layer of the gradient (fraction 1), while cytosolic proteins exhibiting a higher density (1.26 g/ml) migrate to the bottom of the gradient during centrifugation (fraction 12). Postnuclear supernatants of confluent S1 cells were fractionated by iodixanol gradient, and fractions were subsequently analyzed by immunoblotting with antibodies to inversin, pan-cadherin, and β-catenin. Figure 4A shows the immunoblot analysis of fractions collected from the iodixanol gradient. The inversin antibody detected a band at 140 kDa in all fractions, with the greatest intensity in fractions rich in cytosolic proteins. The weakest 140-kDa bands were in the fractions rich in plasma membrane particles. Additionally, in fraction 1, the inversin antibody detected a 125-kDa band that was not detected in any other fraction. The 90-kDa inversin signal was detected in all fractions with progressively increasing signal intensity from fraction 1 to fraction 12. When the immunoblots were subjected to extended exposure times, a 165-kDa band was detected in the cytoplasmic-enriched fractions 11 and 12 (unpublished data). Pan-cadherin and β-catenin antibodies detected bands in fractions 1 and 2 containing low-density plasma membrane particles, in agreement with previous findings (Grindstaff et al., 1998). Taken together, these data suggest that the 125-kDa band is only found in the fraction that is enriched for plasma membranes, whereas the 90-kDa isoform is found in all fractions.
Figure 4.
Subcellular distribution of inversin. (A) Iodixinol subcellular fractionations were collected from the lightest (top fraction 1) to the heaviest (bottom fraction 12) of the gradient. Equivalent fraction volumes were resolved by 7.5% SDS-PAGE and immunoblotted with antibodies to inversin, pan-cadherin, or β-catenin. Bands (140- and 90-kDa) were detected with anti-inversin in all fractions, but a 125-kDa band was detected only in membrane fraction 1. The bands detected by anti-pan-cadherin and anti-β-catenin were restricted to the lightest fractions. The accompanying graph expresses phosphorimager values for each band as a percentage of the total volume of all fractions measured for each antibody. (B) Nuclear and membrane protein extracts from confluent S1 cells were separated by 7.5% SDS-PAGE and were immunoblotted with anti-inversin. The inversin antibody detected only one band of 125 kDa in the membrane protein extract and two bands of 140- and 90- kDa in the nuclear protein extracts. (C) Total protein was extracted from confluent S1 cells with and without 1% Triton X-100 followed by inversin immunoblot analysis. Anti-inversin detected bands at 140 and 90 kDa in both extracts, but only in the presence of Triton X-100 did the inversin antibody detect a band at 125 kDa.
Immunoblot Analysis
To confirm the enrichment of the 125-kDa inversin protein in cell membranes, immunoblots were performed on membrane protein complexes extracted from confluent S1 cells using aqueous two-phase partitioning. The inversin antibody detected a single band at 125 kDa (Figure 4B, right lane) migrating at the same molecular weight as the 125-kDa band in fraction 1 of the iodixanol gradient (Figure 4A).
Nuclear protein complexes were isolated from confluent S1 cells, separated by SDS-PAGE. and transferred to membranes for immunoblot analysis. Successful extraction of nuclear proteins was confirmed on immunoblots by monoclonal antibody detection of histone H1, an exclusive nuclear protein (unpublished data). Inversin antibody detected bands at 90 and 140 kDa in immunoblots of nuclear extracts (Figure 4B, left lane), but the 125-kDa band was not detectable.
To confirm that the 125-kDa inversin protein is restricted to detergent-soluble membrane fractions, protein was extracted from confluent S1 cells in a solution with or without 1.0% Triton X-100. The 140- and 90-kDa bands were detected on immunoblots containing proteins extracted without detergent (Figure 4C, left lane). When S1 cell proteins were extracted with 1.0% Triton X-100, bands were detected at 140, 125, and 90 kDa (Figure 4C, right lane).
Inversin Forms a Complex with Catenins and N-Cadherin
As described above, inversin localizes to the lateral plasma membrane at regions of cell-cell contacts in confluent S1 cells. To identify proteins that potentially interact with inversin, we immunoprecipitated S1 cell homogenates with the inversin antibody and probed the precipitates with mouse monoclonal antibodies to the cell adhesion proteins α-, β-, and γ-catenins, pan-, E-, and N-cadherins, vinculin, and β1-integrin. Bands of the expected molecular weights were detected with antibodies to α-, β-, and γ-catenins, and pan- and N-cadherin, but not with antibodies to E-cadherin, vinculin, or β1-integrin despite extended exposure times (Figure 5A).
Figure 5.
(A) Homogenates from confluent S1 cells were immunoprecipitated with inversin antibody and precipitates were immunoblotted with a panel of antibodies. Arrowheads indicate expected molecular weights. Bands were detected for α-, β-, and γ-catenin, and pan-, and N-cadherin, but no bands were detected for E-cadherin, B1-integrin, and vinculin despite long film exposure. (B) Confluent S1 cell homogenates were immunoprecipitated with anti-β-catenin (lane 1) and anti-N-cadherin (lanes 2 and 3), and precipitates were immunoblotted with anti-inversin or anti-β-catenin. The inversin antibody detected 125- and 90-kDa bands in both the β-catenin precipitate (left panel) and the N-cadherin precipitate (middle panel). The N-cadherin precipitate that was immunoblotted for β-catenin detected a 92-kDa band (right panel). (C) Iodixanol fractions 1 and 12 were collected as for Figure 3A, immunoprecipitated with inversin antibody, and immunoblotted with antibodies to β-catenin and N-cadherin. Inversin precipitates from fraction 1 contained β-catenin and N-cadherin (lanes 1 and 2), but no bands were detected in fraction 12 (lanes 3 and 4). Protein extracts in A, B, and C were separated by 7.5% SDS-PAGE and transferred to nitrocellulose membranes.
Inverse immunoprecipitations were performed on S1 cell homogenates. Complexes that were precipitated using polyclonal rabbit antibodies to β-catenin or N-cadherin contained inversin as detected in immunoblots using the inversin antibody (Figure 5B). Both β-catenin and N-cadherin precipitates contained the 125- and 90-kDa inversin isoforms. Protein complexes precipitated by anti-N-cadherin contained β-catenin, as shown in Figure 5B.
To confirm that protein complexes containing inversin, β-catenin, and N-cadherin are membrane associated in S1 cells, we analyzed membrane and cytosolic fractions obtained from the iodixanol density gradient as described for Figure 4A. Protein complexes from fractions enriched in membranes (fraction 1) or cytosol (fraction 12) were immunoprecipitated with anti-inversin followed by SDS-PAGE and immunoblot analysis with monoclonal antibodies to β-catenin and N-cadherin. Coprecipitation of inversin with β-catenin and N-cadherin was found only in the membrane fraction 1, but not in the cytosolic fraction 12 (Figure 5C).
Codistribution of Inversin and the Cadherin/Catenin Complex
Confluent S1 cells were double-labeled with antibodies to inversin and β-catenin or inversin and N-cadherin. Both inversin and α-catenin were located at the lateral cell membranes and in the nucleus (Figures 6, A and B). Most inversin staining colocalized with β-catenin at the plasma membrane, consistent with our findings that these proteins are part of a complex (Figure 6C). In the nucleus, the antibody labeling of β-catenin and inversin showed partial colocalization in S1 cells (Figure 6C) and was weak to absent in MDCK cells (unpublished data). Anti-N-cadherin strongly stained lateral cell membranes (Figure 6E). Figure 6F shows that membrane-associated inversin and N-cadherin colocalized exclusively at the plasma membrane (Figure 6F). No staining was observed when cells were labeled with secondary antibodies alone, confirming the specificity of the primary antibodies used (insets in Figure 6, A, B, D, and E).
Figure 6.
Colabeling of inversin with β-catenin or N-cadherin. Confluent S1 cells were double-labeled with antibodies to inversin (A) and β-catenin (B) or inversin (D) and N-cadherin (E) and were analyzed by confocal microscopy. Inversin colocalized with β-catenin at cell membranes, but there was partial overlap in nuclei (C, yellow overlap). Inversin colocalized with N-cadherin only at cell membranes.
Inversin Membrane Assembly Is Calcium Dependent
Inversin's membrane distribution may be dependent on cell-cell adhesions. To study whether this distribution is calcium dependent, confluent S1 cells were switched from medium containing 1.8 mM calcium to media containing 1.8 mM calcium plus EGTA. Cells fixed at various time points (0, 45, and 90 min) were labeled with anti-inversin, anti-β-catenin, and fluorescein isothiocyanate-conjugated phalloidin (Figure 7, A-I) or anti-inversin, anti-N-cadherin, and fluorescein isothiocyanate-conjugated phalloidin (Figure 7, J-R). In 1.8 mM calcium medium (0 min), cells were confluent, and staining for F-actin was continuous at regions of cell-cell contacts (Figure 7, G and P). Similar to the results shown in Figure 6, inversin and β-catenin localized to the cell membrane and nuclei (Figure 7, A, D, and J). Under these conditions, anti-N-cadherin also labeled cell membranes (Figure 7M). Incubating the cells in calcium-chelated media induced changes in cell shape, cell adhesion, and redistribution of analyzed proteins. After 45 min in calcium-chelated media, cell contacts were discontinuous, and phalloidin staining illustrated a retraction of the F-actin belt (Figure 7, H and Q). Membrane labeling of inversin, β-catenin, and N-cadherin was dramatically weakened, whereas nuclear staining of inversin and β-catenin appeared unchanged (Figure 7, B, E, K, and N). After 90 min in LCM, cells were spherical and contacts with neighboring cells were minimal or absent (Figure 7, I and R). Staining of inversin and β-catenin was almost exclusively confined to the nuclear compartment (Figure 7, C, F, and L). Only a few membrane segments showed residual staining of β-catenin and N-cadherin (Figure 7, F and O). These results demonstrate that membrane assembly of inversin is calcium dependent. Changes in membrane assembly of inversin occurred over the same time course as the changes in N-cadherin and β-catenin membrane assembly (unpublished data). Low calcium-induced redistribution of inversin was almost complete after 45 min. Similarly, complete redistribution of N-cadherin and β-catenin occurred at the 45-min time point. Notably, intranuclear inversin staining was unaffected by changes in extracellular calcium.
Figure 7.
Calcium depletion in confluent S1 cells by confocal microscopy. Confluent S1 cells were calcium depleted by incubating with medium containing 4 mM EGTA. At time 0, 45, and 90 min, cells were triple labeled with anti-inversin (A-C), anti-β-catenin (D-F), and phalloidin (G-I) or anti-inversin (J-L), anti-N-cadherin (M-O), and phalloidin (P-R). At 45 and 90 min of calcium depletion, cells progressively lost cell-cell contacts, as displayed by staining of the F-actin cytoskeleton (H, I, Q, and R). Calcium depletion also led to diminished staining of inversin (B, C, K and L), β-catenin (E and F), and N-cadherin (N and O) from cell membranes. Nuclear staining of inversin and β-catenin remained unchanged under low calcium conditions (C, F, and L).
DISCUSSION
The inv gene was originally identified in the OVE210 model of reversal of embryonic turning and PKD (Yokoyama et al., 1993; Mochizuki et al., 1998; Morgan et al., 1998). The activity of the inv gene product, inversin, is not known, but the defects seen in the inv/inv mouse suggest that inv plays a crucial role in the establishment of the left-right axis and maturation of epithelial structures such as renal tubules.
Using an inversin-specific polyclonal antibody, this study identifies at least three inversin proteins of 140-, 125-, and 90- kDa. The existence of multiple isoforms is supported by the identification of alternatively spliced inv transcripts. Morgan et al. (1998) described alternative splicing in exon 13 of the full-length inv sequence predicting isoforms of 99, 104, and 118 kDa. Using reverse transcriptase-polymerase chain reaction, we found splice variants lacking exon 4 (accounting for 6.2 kDa) or exon 10 (4 kDa) in mouse kidney and exon 11 (7.9 kDa) in MDCK cells (canine; unpublished data). Using the NCBI database, we identified 16 ESTs with 90% homology to inv, but when completely sequenced, none of these encoded the full-length (1062 amino acids) sequence. Fifteen of the ESTs had limited homology to exon 16 (unpublished data), but one EST (AI790867) was a splice variant that encoded a 76-kDa in-frame inversin protein that we used to generate antigen for our inversin antibody. We used web-based software to predict posttranslational modifications of inversin and we found potential phosphorylation sites (50 serine, 11 threonine, and 6 tyrosine; Blom et al., 1999), as well as several type O-glycosylation sites (11 serine and 2 threonine; Hansen et al., 1998). Some of these posttranslational modifications may account for the difference between the predicted size of the 118- and 140-kDa bands detected by the inversin antibody.
Potential Cellular Function of Inversin
Of the two lower molecular-weight inversin isoforms that precipitate with β-catenin and N-cadherin, the 125-kDa band was most enriched in the cell membrane fractions. Like the cadherin/catenin complex (Aberle et al., 1996), membrane-associated inversin is calcium dependent. Analysis of the inversin protein sequence did not reveal any known transmembrane sequence motifs; therefore, the effect of calcium depletion on inversin redistribution may be mediated via N-cadherin or β-catenin. These findings suggest that the 125-kDa inversin may play a role in regulating the molecular architecture of cell-cell junctions.
The inversin proteins responsible for the nuclear and perinuclear immunofluorescence appear to be the 140- and 90-kDa isoforms. The import of inversin into the nucleus likely involves the recognition of a nuclear localization signal sequence (NLS; Christophe et al., 2000). The full-length inversin sequence exhibits one classical type of NLS (KHRR at aa 735; Hicks and Raikhel, 1995) and two bipartite NLSs (RKDAAAKKREEENKRKE at aa 589 and KRQDRAARPRGASQKRR at aa 782; Robbins et al., 1991). Rheinhardt's method for cytoplasmic/nuclear discrimination also predicted a nuclear localization of inversin (Reinhardt and Hubbard, 1998), providing further evidence for a nuclear function of inversin. Based on PROSITE database analysis, the inversin sequence does not exhibit sequence motifs that are involved in DNA binding (Bairoch et al., 1997). Of the two inversin proteins that were enriched in nuclear fractions, only the 90-kDa protein complexed with β-catenin, the latter a protein involved in transcription regulation (Zhurinsky et al., 2000). Therefore, a nuclear inversin protein may modulate gene transcription via interaction with transcription factors such as β-catenin.
Potential Role of Inversin in Determination of the Left-Right Axis
Mice lacking a functional inv gene exhibit defects in left-right patterning (Mochizuki et al., 1998; Morgan et al., 1998), suggesting a role of inversin in the determination of the left-right axis. In the earliest stages of asymmetrical development, a nodal flow generated by motile cilia is postulated to initiate expression of genes, such as Nodal, that regulate embryonic turning (Ryan and Izpisua Belmonte, 2000). Although little is known about the function of inversin, a reduced nodal flow was observed in the inv/inv mouse (Okada et al., 1999) and has been proposed to be associated with the reversal of embryonic turning in this model (Ryan and Izpisua Belmonte, 2000). We found inversin localization at nuclei and at basolateral membranes. When MDCK cells were costained for inversin and tubulin, we did not observe inversin staining in monocilia (unpublished data). However, inversin precipitated with proteins that are involved in axis development, i.e., N-cadherin and β-catenin. N-cadherin (neural cadherin), a classical or type I cadherin, is expressed in a developmental manner (Hatta et al., 1987; Redies and Takeichi, 1993), regulates migration of cortical and neural crest cells (Akitaya and Bronner-Fraser, 1992; Nakagawa and Takeichi, 1998), and plays a role in the embryonic development of the kidney (Okada, 1988). However, N-cadherin is also involved in establishment of embryonic left-right asymmetry. Garcia-Castro et al. (2000) found that chicken embryos treated with anti-N-cadherin exhibit a randomization of left-right asymmetry. Mechanistically, N-cadherin, which is localized to the right side of the node, may restrict activation of Nodal (Rodriguez-Esteban et al., 2001), which in turn controls embryonic turning (Ryan and Izpisua Belmonte, 2000). A defective inv gene product may adversely impact N-cadherin function, resulting in defects of establishing the left-right axis.
Inversin also interacts with β-catenin, a protein that plays a crucial role in the Wnt/β-catenin signaling pathway (Zhurinsky et al., 2000). Nuclear β-catenin complexes with LEF/TCF transcription factors and subsequently activates LEF/TCF target genes (Nusse, 1999), including the promoter of Nodal (Norris and Robertson, 1999). Hence, the defect in the left-right axis in the inv/inv mouse could also result from deregulated β-catenin signaling. However, altered left-right asymmetry has not been described in transgenic mice expressing an activated mutant of the β-catenin gene (Saadi-Kheddouci et al., 2001).
Potential Role of Inversin in Kidney Development
Renal cyst formation has been proposed to result from the disruption of a multicomponent membrane-spanning polycystin complex (Calvet, 1998; Wilson, 2001). Several proteins have been identified that participate in this complex, including polycystin-1, polycystin-2, the catenins, and E-cadherin (Huan and van Adelsberg, 1999; van Adelsberg, 1999; Calvet and Grantham, 2001). Defects in these proteins and deregulation of the linked Wnt/β-catenin signaling cascade (Gumbiner, 1995; Kim et al., 1999; Zhurinsky et al., 2000) may also play a key role in the cystogenic pathway (Wilson, 2001).
Recently, Saadi-Kheddouci et al. (2001) have shown that transgenic mice expressing a mutant form of β-catenin develop polycystic kidneys (Saadi-Kheddouci et al., 2001). Similarly, overexpression of c-myc, which is a target gene of β-catenin signaling (Trudel et al., 1991), results in a cystic phenotype in transgenic mice (Trudel et al., 1991). In this study, we found inversin coimmunoprecipitating with molecules that complex with polycystin. As with other junctional proteins (Yang et al., 2001), the 125-kDa membrane-enriched inversin may modulate β-catenin function. The finding of inversin and β-catenin colocalization in nuclei provides further evidence of a partnership of these two proteins in pathways that may include cystogenesis.
ACKNOWLEDGMENTS
We thank Vincent H. Gattone, James A. Marrs, and Simon J. Atkinson for helpful discussions and critical reading of the manuscript. C.L.P. acknowledges support from the National Institutes of Health (NIH K08 DK02785), a George Schreiner MD Young Investigator Grant from the National Kidney Foundation, from the Polycystic Kidney Research Foundation (99023), from the Clarian Health Values Fund (VFR21), and from the Ralph W. and Grace M. Showalter Research Trust Fund. R.L.B. acknowledges support from the National Institutes of Health (NIH RO1 DK50141). J.N. acknowledges funding from the Deutsche Forschungsgemeinschaft (NU 118/1–1).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0195. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0195.
REFERENCES
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Calvet JP. Molecular genetics of polycystic kidney disease. J Nephrol. 1998;11:24–34. [PubMed] [Google Scholar]
- Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol. 2001;21:107–123. doi: 10.1053/snep.2001.20929. [DOI] [PubMed] [Google Scholar]
- Christophe D, Christophe-Hobertus C, Pichon B. Nuclear targeting of proteins: how many different signals? Cell Signal. 2000;12:337–341. doi: 10.1016/s0898-6568(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol. 1992;117:169–178. doi: 10.1083/jcb.117.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro MI, Vielmetter E, Bronner-Fraser M. N-Cadherin, a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science. 2000;288:1047–1051. doi: 10.1126/science.288.5468.1047. [DOI] [PubMed] [Google Scholar]
- Grantham JJ, Nair V, Winklhofer F. Cystic Diseases of the Kidney. In: Brenner BM, editor. The Kidney. Vol. 2. Philadelphia: W.B. Saunders Company; 2000. pp. 1699–1730. [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guay-Woodford LM, Wright CJ, Walz G, Churchill GA. Quantitative trait loci modulate renal cystic disease severity in the mouse bpk model. J Am Soc Nephrol. 2000;11:1253–1260. doi: 10.1681/ASN.V1171253. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction of β-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+,K+-ATPase in polarized epithelial cells. Science. 1991;254:847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Lund O, Tolstrup N, Gooley AA, Williams KL, Brunak S. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconjugates J. 1998;15:115–130. doi: 10.1023/a:1006960004440. [DOI] [PubMed] [Google Scholar]
- Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Hicks GR, Raikhel NV. Protein import into the nucleus: an integrated view. Annu Rev Cell Dev Biol. 1995;11:155–188. doi: 10.1146/annurev.cb.11.110195.001103. [DOI] [PubMed] [Google Scholar]
- Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E- cadherin and the catenins. J Clin Invest. 1999;104:1459–1468. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunitz JD, Cummins VP, Mishler D, Nagami GT. Inhibition of gentamicin uptake into cultured mouse proximal tubule epithelial cells by l-lysine. J Clin Pharmacol. 1993;33:63–69. doi: 10.1002/j.1552-4604.1993.tb03905.x. [DOI] [PubMed] [Google Scholar]
- Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- Koptides M, Deltas CC. Autosomal dominant polycystic kidney disease: molecular genetics and molecular pathogenesis. Hum Genet. 2000;107:115–126. doi: 10.1007/s004390000347. [DOI] [PubMed] [Google Scholar]
- Kroning R, Lichtenstein AK, Nagami GT. Sulfur-containing amino acids decrease cisplatin cytotoxicity and uptake in renal tubule epithelial cell lines. Cancer Chemother Pharmacol. 2000;45:43–49. doi: 10.1007/PL00006741. [DOI] [PubMed] [Google Scholar]
- Kuida S, Beier DR. Genetic localization of interacting modifiers affecting severity in a murine model of polycystic kidney disease. Genome Res. 2000;10:49–54. [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, et al. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature. 1998;395:177–181. doi: 10.1038/26006. [DOI] [PubMed] [Google Scholar]
- Morgan D, et al. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet. 1998;20:149–156. doi: 10.1038/2450. [DOI] [PubMed] [Google Scholar]
- Morre DJ, Morre DM. Preparation of mammalian plasma membranes by aqueous two-phase partition. BioTechniques. 1989;7:946–948. , 950–944, 956–948. [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Norris DP, Robertson EJ. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 1999;13:1575–1588. doi: 10.1101/gad.13.12.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. WNT targets: repression and activation. Trends Genet. 1999;15:1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K. Purification of membrane proteins. Methods Mol Biol. 1996;59:313–322. doi: 10.1385/0-89603-336-8:313. [DOI] [PubMed] [Google Scholar]
- Okada TS. The expression of cell adhesion molecules, cadherins: markers of kidney morphogenesis. Pediatr Nephrol. 1988;2:115–117. doi: 10.1007/BF00870390. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Redies C, Takeichi M. Expression of N-cadherin mRNA during development of the mouse brain. Dev Dyn. 1993;197:26–39. doi: 10.1002/aja.1001970104. [DOI] [PubMed] [Google Scholar]
- Reinhardt A, Hubbard T. Using neural networks for prediction of the subcellular location of proteins. Nucleic Acids Res. 1998;26:2230–2236. doi: 10.1093/nar/26.9.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Esteban C, Capdevila J, Kawakami Y, Izpisua Belmonte JC. Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Development. 2001;128:3189–3195. doi: 10.1242/dev.128.16.3189. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Izpisua Belmonte JC. Establishing a left-right axis in the embryo. IUBMB Life. 2000;50:1–11. doi: 10.1080/15216540050176520. [DOI] [PubMed] [Google Scholar]
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the β-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- Small J, Rottner K, Hahne P, Anderson KI. Visualizing the actin cytoskeleton. Microsc Res Technol. 1999;47:3–17. doi: 10.1002/(SICI)1097-0029(19991001)47:1<3::AID-JEMT2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S. Construction and characterization of a full length-enriched and a 5′-end-enriched cDNA library. Gene. 1997;200:149–156. doi: 10.1016/s0378-1119(97)00411-3. [DOI] [PubMed] [Google Scholar]
- Torres MA, Nelson WJ. Colocalization and redistribution of disheveled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol. 2000;149:1433–1442. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M, D'Agati V, Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- van Adelsberg JS. The role of the polycystins in kidney development. Pediatr Nephrol. 1999;13:454–459. doi: 10.1007/s004670050639. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol. 2001;12:834–845. doi: 10.1681/ASN.V124834. [DOI] [PubMed] [Google Scholar]
- Woo DD, Nguyen DK, Khatibi N, Olsen P. Genetic identification of two major modifier loci of polycystic kidney disease progression in pcy mice. J Clin Invest. 1997;100:1934–1940. doi: 10.1172/JCI119724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SZ, Kohno N, Yokoyama A, Kondo K, Hamada H, Hiwada K. Decreased E-cadherin augments β-catenin nuclear localization: studies in breast cancer cell lines. Int J Oncol. 2001;18:541–548. [PubMed] [Google Scholar]
- Yokoyama T, Copeland NG, Jenkins NA, Montgomery CA, Elder FF, Overbeek PA. Reversal of left-right asymmetry: a situs inversus mutation. Science. 1993;260:679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chait BT. ProFound: an expert system for protein identification using mass spectrometric peptide mapping information. Anal Chem. 2000;72:2482–2489. doi: 10.1021/ac991363o. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113:3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]