Abstract
To analyze the contribution of vesicular trafficking pathways in cellular cholesterol transport we examined the effects of selected endosomal Rab proteins on cholesterol distribution by filipin staining. Transient overexpression of Rab11 resulted in prominent accumulation of free cholesterol in Rab11-positive organelles that sequestered transferrin receptors and internalized transferrin. Sphingolipids were selectively redistributed as pyrene-sphingomyelin and sulfatide cosequestered with Rab11-positive endosomes, whereas globotriaosyl ceramide and GM2 ganglioside did not. Rab11 overexpression did not perturb the transport of 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine-perchlorate–labeled low-density lipoprotein (LDL) to late endosomes or the Niemann-Pick type C1 (NPC1)-induced late endosomal cholesterol clearance in NPC patient cells. However, Rab11 overexpression inhibited cellular cholesterol esterification in an LDL-independent manner. This effect could be overcome by introducing cholesterol to the plasma membrane by using cyclodextrin as a carrier. These results suggest that in Rab11-overexpressing cells, deposition of cholesterol in recycling endosomes results in its impaired esterification, presumably due to defective recycling of cholesterol to the plasma membrane. The findings point to the importance of the recycling endosomes in regulating cholesterol and sphingolipid trafficking and cellular cholesterol homeostasis.
INTRODUCTION
Cholesterol is an essential constituent of membranes in mammalian cells and a precursor for steroid hormone and bile acid synthesis. Cellular cholesterol levels are tightly regulated at the level of synthesis, esterification, and exchange with plasma lipoproteins (Brown and Goldstein, 1999; Simons and Ikonen, 2000). The route of low-density lipoprotein (LDL)-cholesterol uptake is hitherto the best characterized cellular cholesterol-trafficking pathway. The role of the LDL receptor in LDL internalization, the breakdown of the lipoprotein particle in acidic organelles, and the homeostatic mechanisms regulating the LDL-receptor levels have been unraveled (Brown and Goldstein, 1986). However, the contribution of other endocytic routes on cholesterol transport and balance and their interplay with the LDL-receptor route are so far poorly understood at the molecular level.
The endocytic organelles have been mainly defined based on the flow of different cargo molecules to early, recycling, and late compartments. Internalized molecules are initially transported to early endosomes (also termed sorting endosomes) from where they can be delivered to late endosomes and lysosomes for degradation or become recycled to the plasma membrane either directly or via a recycling endosomal membrane system (Gruenberg and Maxfield, 1995; Mellman, 1996). Recycling endosomes are considered to be cholesterol enriched (Gagescu et al., 2000; Hao et al., 2001). The cholesterol content of the early or late endocytic membranes has not been determined, but late endocytic circuits are considered to be important for the regulation of the cellular free cholesterol content. This is highlighted in the late endosomal/lysosomal cholesterol storage disorder Niemann-Pick type C (NPC) disease. In this disease, cholesterol as well as other lipids and proteins accumulate in late endocytic organelles due to mutations in either of two recently cloned gene products, NPC1 or NPC2/HE1 (Carstea et al., 1997; Naureckiene et al., 2000). Consequently, cholesterol homeostatic responses in the endoplasmic reticulum fail, as manifested by defective cholesterol esterification and inappropriately high cholesterol synthesis (Liscum et al., 1989). NPC1 is a polytopic membrane protein of late endocytic membranes, whereas NPC2 is a cholesterol-binding soluble protein that is also targeted to the late endocytic organelles. The precise functions and trafficking itineraries of both NPC1 and NPC2 remain to be elucidated.
We have recently reported that the clearance of lysosomal cholesterol deposits can be inhibited by Rab-GDP dissociation inhibitor (Hölttä-Vuori et al., 2000). This protein controls multiple vesicular transport pathways by sequestering GDP-bound (inactive) forms of the small GTPases of the Rab family in the cytoplasm. Rab proteins and their effectors coordinate consecutive stages of membrane transport, such as vesicle formation, movement, and tethering of vesicles to their target compartment (Zerial and McBride, 2001). To gain further insight into the Rab-dependent endosomal cholesterol-trafficking mechanisms, we screened the potential contribution of selected endosomal Rab proteins 1) morphologically by using filipin staining and 2) biochemically by measuring cholesterol esterification. Based on the results obtained, our further analyses focused on the role of Rab11 in controlling endocytic cholesterol routing. We provide evidence that Rab11-dependent membrane trafficking modulates endosomal cholesterol levels independent of LDL uptake and serves as an important regulator of cellular cholesterol balance.
MATERIALS AND METHODS
Antibodies and Reagents
Mouse monoclonal anti-transferrin receptor (TfR) and rabbit polyclonal anti-Rab11 antibodies were from Zymed Laboratories (South San Francisco, CA), mouse monoclonal anti-lysosome-associated membrane protein (lamp) 1 antibody was from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), and mouse monoclonal anti-LDL receptor antibodies (C7) were from American Type Culture Collection (Manassas, VA). IgG antibodies against sulfatide (Fredman et al., 1988) and IgM antibodies against globotriaosyl ceramide and GM2 (Fredman et al., 1990) were generous gifts from Jan-Eric Månsson (Sahlgrenska University Hospital, Mölndal, Sweden); anti-lysobisphosphatidic acid (LBPA) antibody (Kobayashi et al., 1998) was from Jean Gruenberg (University of Geneva, Geneva, Switzerland), and anti-HE1/NPC2 antibody (Okamura et al., 1999) was from Naomichi Okamura (University of Tsukuba, Tusbuba, Japan). Anti-NPC1 antibody has been described previously (Lusa et al., 2001). Fluorescein isothiocyanate (FITC)- and tetramethylrhodamine B isothiocyanate-conjugated anti-IgG secondary antibodies were from Immunotech (Marseille, France), and Cy3-conjugated streptavidin and tetramethylrhodamine B isothiocyanate-conjugated secondary antibodies against mouse IgM were from Jackson Immunoresearch Laboratories (West Grove, PA). FuGENE6 transfection reagent was from Roche Applied Science (Indianapolis, IN). Filipin, FITC lentil lectin, methyl-β-cyclodextrin (mβ-CD), fatty-acid free bovine serum albumin (BSA), chymostatin, leupeptin, antipain, and pepstatin A, cell culture media, cholesterol, and other unlabeled lipids were from Sigma-Aldrich (St. Louis, MO). [9,10(n)-3H]Oleic acid (specific activity 7.5 Ci/mmol), cholesteryl[1-14C]oleate (specific activity 57 mCi/mmol), [4-14C]cholesterol (specific activity 55 mCi/mmol), and [3H]acetic acid (specific activity 9 Ci/mmol) were from Amersham Biosciences (Piscataway, NJ). Alexa 568-conjugated anti-IgG secondary antibodies, Texas Red transferrin, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine-perchlorate–labeled low-density lipoproteins (DiI-LDLs), and Alexa 594-conjugated cholera toxin subunit B (CTxB) were from Molecular Probes (Eugene, OR). Pyrenyldecanoylsphingomyelin (Pyr10SM) was prepared as described previously (Via et al., 1985; Tanhuanpaa and Somerharju, 1999). γ-Cyclodextrin (γ-CD) was from Cyclodextrin Technologies Development (High Springs, FL), and 3-β-[2-(diethylamino)ethoxy]-androst-5-en-17-one (U18666A) was from Upjohn (Puurs, Belgium). Biotin-2xFYVE (Gillooly et al., 2000) was a generous gift from Harald Stenmark.
Cell Culture and Transfections
COS-1 cells were cultured in DMEM containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. F92-99 control fibroblasts and 93.41 NPC fibroblasts were obtained and cultured as described previously (Hölttä-Vuori et al., 2000). Cells were transfected using FuGENE6 according to the manufacturer's instructions and used for experiments at 40–48 h (COS-1 cells) or 65–72 h (human primary fibroblasts) posttransfection.
DNA Constructs
pEGFP-C3 was from CLONTECH (Palo Alto, CA). Green fluorescent protein (GFP)-wtRab5, GFP-wtRab6, GFP-wtRab7, and GFP-wtRab11 were as described previously (White et al., 1999; Sonnichsen et al., 2000; Feng et al., 2001). GFP-Rab5Q79L, GFP-Rab11Q70L, and GFP-Rab11S25N were generous gifts from Marino Zerial (Max Planck Institute for Molecular Cell Biology and Genetics, Dresden, Germany). The human TfR cDNA (Zerial et al., 1986) was in pCDNA3.1. Human LDL-receptor cDNA in pCB6 (Hunziker et al., 1991) and human NPC1 in pCR3.1 have been described previously (Carstea et al., 1997).
Cholesterol Esterification Assays
Cells on 12-well plates were transfected, and the following day the medium was replaced with fresh culture medium. Alternatively, to deplete cholesterol the cells were incubated with medium containing 5% lipoprotein-deficient serum (LPDS), prepared as in Goldstein et al. (1983) for 24 h before labeling.
To analyze esterification in the presence of LDL, cells grown in culture medium were washed with phosphate-buffered saline (PBS) and labeled with [3H]oleic acid (5 μCi/ml) in serum-free, 2% defatted BSA medium supplemented with 50 μg/ml LDL for 4 h. After labeling, the cells were washed with ice-cold PBS on ice and scraped into PBS, harvested by centrifugation, and resuspended in 2% NaCl. Aliquots were removed for determining the protein concentration. A chromatography recovery standard was added (2.5–5 nCi of [14C]cholesteryl oleate) and the lipids extracted with 2 ml of methanol and 1 ml of chloroform as described previously (Bligh and Dyer, 1959). After subsequent centrifugation, 1/10 of the supernatant was removed for liquid scintillation counting to determine the [14C]cholesteryl oleate radioactivity. The extracted lipids were separated by thin layer chromatography on silica gel plates by using hexane/diethyl ether/acetic acid (80:20:1) as the solvent. The cholesteryl ester band was determined based on the comigration of a cholesteryl ester standard, scraped, and 3H and 14C radioactivity measured by liquid scintillation counting. The results were corrected for the volume and procedural losses based on the recovery of 14C radioactivity and plotted against the total amount of protein in the sample. The protein concentration was determined according to Lowry et al. (1951).
To analyze esterification in delipidated cells, cells grown in 5% LPDS medium for 24 h were washed with PBS and labeled with [3H]oleic acid (5 μCi/ml) in serum-free, 2% defatted BSA medium for 4 h. Lipids were analyzed as described above.
To analyze esterification in cells loaded with cholesterol/mβ-CD-complex the cells were initially delipidated as described above and labeled with [3H]oleic acid (5 μCi/ml) in serum-free, 2% defatted BSA medium for 4 h. During the labeling, cholesterol/mβ-CD-complex prepared as described previously (Leppimaki et al., 2000) was added at 50 μg/ml concentration of cholesterol at staggered time points to yield the final loading times indicated. The basal rate of esterification as determined by samples labeled without cholesterol/mβ-CD-complex was subtracted from the values at all time points.
Western Blot Analysis
Cells were harvested in 1% Nonidet-P40 in PBS supplemented with protease inhibitors (chymostatin, leupeptin, antipain, and pepstatin, at 25 μg/ml each). Aliquots of the cell lysate (20 μg of protein) were separated by SDS-PAGE, and the proteins were transferred to Hybond-C Extra membrane (Amersham Biosciences). After blocking with 5% nonfat milk in Tris-buffered saline containing 0.2% Tween 20 for 1 h at 37°C, the membrane was incubated overnight at 4°C with rabbit polyclonal anti-GFP antibodies (CLONTECH). The membrane was then washed and incubated with horseradish peroxidase-conjugated anti-IgG secondary antibodies (Bio-Rad, Hercules, CA). The staining was visualized using enhanced chemiluminescence Western blotting detection reagent (Amersham Biosciences).
Immunocytochemistry
The cells were fixed with 4% paraformaldehyde for 20 min and quenched with 50 mM NH4Cl for 10 min. Cells were permeabilized either with 0.1% Triton X-100 for 4 min and blocked with 10% FBS for 30 min at 37°C, or alternatively the blocking solution was supplemented with 0.05% filipin to permeabilize the cells. The primary antibodies were diluted in 5% FBS and incubated for 1 h at 37°C or overnight at 4°C and the secondary antibodies for 30 min at 37°C. For filipin staining only, the fixed and quenched cells were incubated with 0.05% filipin in PBS for 15 min and washed with PBS. The coverslips were mounted with Mowiol and the antifading reagent 1,4 diazobicyclo-(2.2.2) octane and viewed with TCS SP confocal microscope (Leica, Deerfield, IL), Axiophot photomicroscope (Carl Zeiss, Thornwood, NY), or IX70 inverted microscope (Olympus, Tokyo, Japan) equipped with a Polychrome IV monochromator (TILL Photonics, Eugene, OR) with appropriate filters.
Labeling with Texas Red Transferrin
Cells double transfected with the indicated cDNAs and TfR were starved in serum-free culture medium for 1 h at 37°C. Cells were then incubated with 50 μg/ml Texas Red transferrin in Eagle's minimum essential medium supplemented with 0.2% BSA, 0.35 g/l NaHCO3, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM HEPES, pH 7.4, for 30 min on ice at 4°C. After labeling the cells were incubated in serum-free culture medium supplemented with 0.2% BSA for 30 min at 37°C, fixed, and processed for immunofluorescence microscopy as described above.
Labeling with Biotin-2xFYVE
Cells fixed and quenched as described above were blocked and permeabilized with 10% FBS supplemented with 0.05% filipin for 30 min. Cells were then incubated with 50 μg/ml biotin-2xFYVE in 10% FBS for 30 min at room temperature, washed 3 × 5 min with PBS, and further incubated with 1 μg/ml Cy3-conjugated streptavidin in 10% FBS for 30 min, washed 3 × 5 min with PBS, and mounted.
Labeling with Alexa 594-conjugated CTxB
Cells were incubated with 2 μg/ml Alexa 594-conjugated CTxB in Eagle's minimum essential medium supplemented with 0.01% BSA, 0.35 g/l NaHCO3, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM HEPES, pH 7.4, for 1 h on ice at 4°C. After labeling the cells were incubated in serum-free culture medium supplemented with 0.01% BSA for 2 h at 37°C and fixed.
Labeling with Pyr10SM
To prepare the Pyr10SM/γ-CD-complex the lipid was dried under argon and desiccated in the vacuum for 30 min. γ-CD (100 mM in PBS) was added on the lipid film in a molar ratio of 1000:1, and the suspension was sonicated 3 × 2 min (Tanhuanpää and Somerharju, 1999). Cells were labeled with Pyr10SM/γ-CD-complex at 10 nmol/ml concentration of the lipid for 10 min at 37°C and incubated in culture medium for 2 h at 37°C before fixation. The fluorescence was excited at 345 nm and visualized at 480/80 nm. The degradation rate of Pyr10SM in COS-1 cells was determined by high-performance liquid chromatography using on-line fluorescence detection (Kasurinen and Somerharju, 1992).
Electron Microscopy
Cells were fixed with 4% paraformaldehyde in 0.25 M HEPES pH 7.4, scraped, and infiltrated in 1.75 M sucrose in 0.25 M HEPES containing 4% paraformaldehyde for 48 h at 4°C. Droplets of cells in sucrose were mounted on pins and frozen in liquid nitrogen. Ultrathin cryosections were labeled with polyclonal rabbit anti-GFP antibody (a generous gift from Graham Warren (Yale University School of Medicine, New Haven, CT) and David Shima (Imperial Cancer Research Fund, London, UK), followed by protein A coupled to 10-nm gold particles. Sections were examined and photographed at 80 kV with a 1200 EX electron microscope (JEOL, Tokyo, Japan).
Labeling with DiI-LDL
Cells were incubated in medium containing 5% LPDS for 24 h then labeled with 10 μg/ml DiI-LDL in serum-free medium for 15 min at 37°C. After washing with PBS, the cells were either fixed or further incubated in serum-free medium for 2 h at 37°C before fixation.
Cholesterol Biosynthesis
Cells on six-well plates were transfected and at 6 h posttranfection the culture medium was changed to medium supplemented with 5% LPDS and [14C]cholesterol (100 nCi/ml) for 41 h. The cells were washed with PBS, pulse labeled with [3H]acetic acid (250 μCi/ml) in serum-free medium for 15 min at 37°C, and chased in serum-free medium supplemented with 10 μM lovastatin and 25 mM mevalonate for 90 min at 37°C. The cells were washed with ice-cold PBS on ice, scraped into PBS, harvested by centrifugation, and resuspended in 2% NaCl. Aliquots were removed for determining the protein concentration. Lipids were extracted as described above, separated by thin layer chromatography, and analyzed by high-performance liquid chromatography as described previously (Heino et al., 2000). Nascent cholesterol was quantified as 3H radioactivity in the cholesterol peak, corrected for the volume and procedural losses based on the recovery of 14C radioactivity, and plotted against the amount of protein in the sample.
RESULTS
Redistribution of Free Cholesterol and Inhibition of Cholesterol Esterification upon Overexpression of Endosomal Rab Proteins
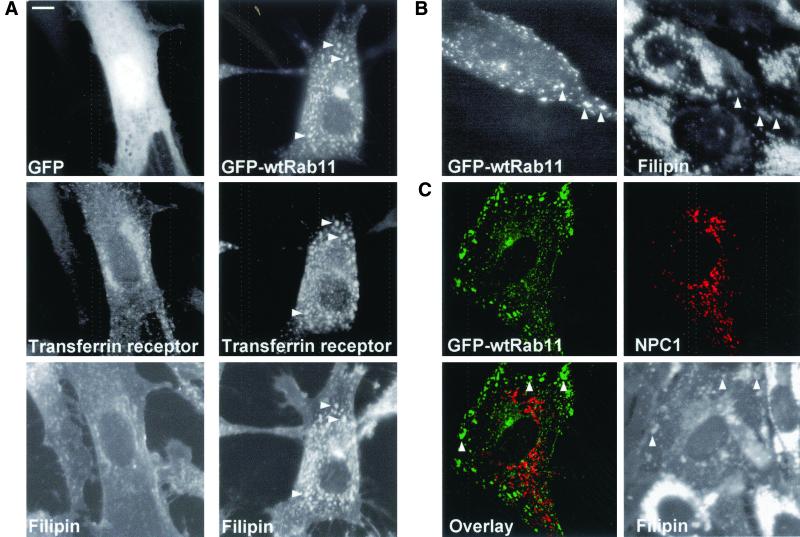
COS-1 cells were transiently transfected for 40–48 h with GFP-fusions of Rab proteins reported to regulate early, late, or recycling endocytic transport events, represented by Rab5, 7, and 11, respectively. Rab5 promotes homotypic fusion of early endosomes (Stenmark et al., 1994). Overexpression of the late endosomal Rab7, on the other hand, has been shown to affect early-to-late endosomal transport and lysosome biogenesis (Press et al., 1998; Bucci et al., 2000), whereas Rab11 regulates the function of the recycling endosomes (Ullrich et al., 1996; Ren et al., 1998; Wilcke et al., 2000). Rab6 that is involved in retrograde trafficking in the Golgi (White et al., 1999), and soluble GFP were used as controls. To visualize the distribution of free cholesterol, the cells were fixed and stained with the fluorescent sterol-binding antibiotic filipin.
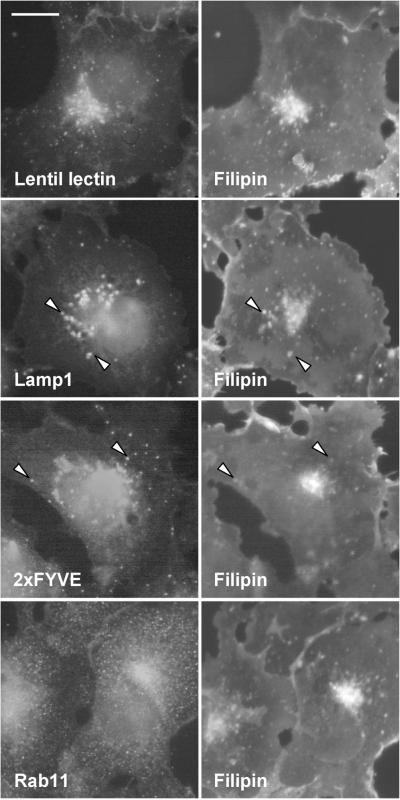
In COS cells, the perinuclear area of the cell is strongly filipin positive. In addition, the plasma membrane and punctate peripheral structures are stained, albeit at lower intensity (Figure 1). The prominent perinuclear filipin staining colocalizes with a Golgi marker lentil lectin but several filipin-positive punctae also colocalize with lysosomal or early endosomal markers as visualized by antibodies against lysosomal membrane protein lamp1, or labeling with peptide 2xFYVE that binds the early endosomal phosphatidylinositol-(3)-phosphate (PI-3-P) (Gillooly et al., 2000). The perinuclear aspect of endogenous Rab11 staining also partially overlaps with that of filipin. However, the small peripheral Rab11-positive dots are not resolved by filipin staining. The filipin staining pattern characteristic of untransfected cells was also seen in cells expressing soluble GFP, Rab6, or Rab7 (Figure 2). In Rab5-overexpressing cells, numerous brightly filipin-positive peripheral dots were observed. These structures were also positive for Rab5, indicating that they represent early endosomes (Figure 2). The most pronounced redistribution of filipin staining was seen in Rab11-overexpressing cells. In these cells, intensely filipin stained and Rab11-positive tubular elements extending to the cell periphery were observed (Figure 2). For Rab5, the effects of the GTPase-deficient mutant (Rab5Q79L) are significantly more pronounced than that of the wild-type protein, resulting in massive enlargement of early endosomes (Stenmark et al., 1994). Considering the moderate effect of wtRab5 on filipin staining pattern we also analyzed the effect of Rab5Q79L. In cells expressing this protein, early endosomes became heavily enlarged and their membranes were intensely filipin positive (Figure 2).
Figure 1.
Distribution of free cholesterol in COS-1 cells. Left, cells were stained with FITC-conjugated lentil lectin, anti-lamp1 antibodies, biotin-2xFYVE, and Cy3-conjugated streptavidin or anti-Rab11 antibodies. Right, filipin stainings of the respective cells. The arrowheads indicate colocalization of endosomal markers with filipin staining. Images were obtained using wide-field microscope. Bar, 8 μm.
Figure 2.
Distribution of free cholesterol in COS-1 cells overexpressing soluble GFP, GFP-fused wtRab5, wtRab6, wtRab7, wtRab11, and Rab5Q79L. Left, cells overexpressing GFP or GFP-fused Rab proteins. Right, filipin stainings of the respective cells. The arrowheads indicate free cholesterol redistributed in GFP-Rab–positive organelles. Images were obtained using wide-field microscope. Bar 8, μm.
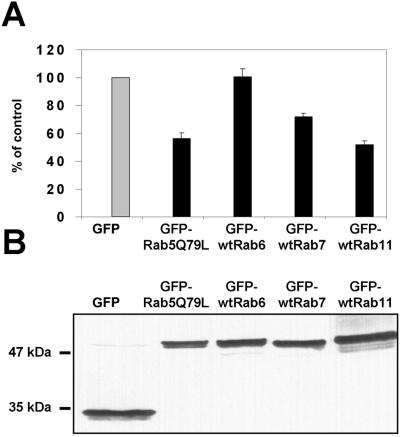
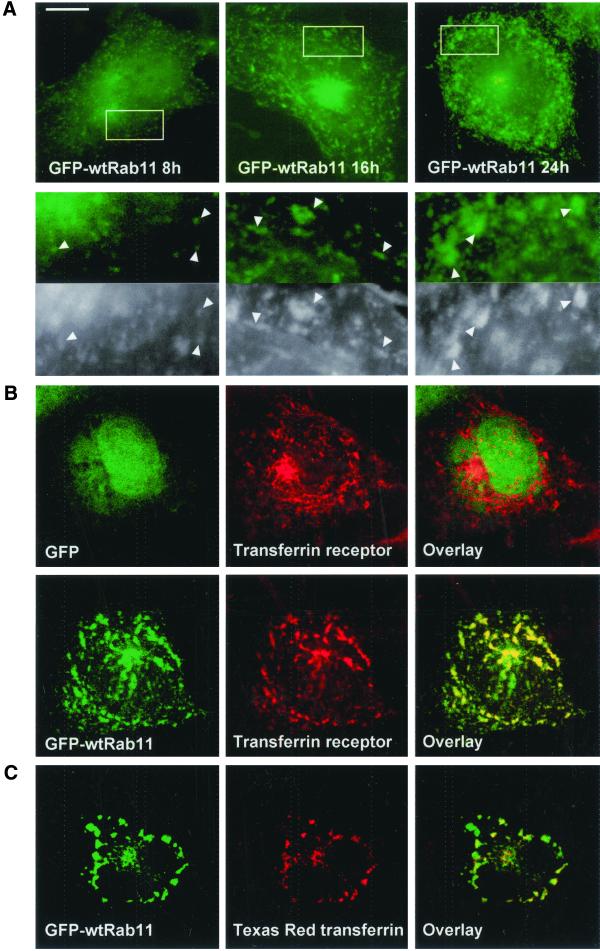
To test whether overexpression of the Rab proteins was accompanied by biochemical effects on cholesterol homeostasis, we analyzed cholesterol esterification by measuring the incorporation of [3H]oleic acid into cholesteryl esters at 40–48 h posttransfection. The values obtained with Rab overexpressions were compared with those obtained with overexpressed GFP alone. Strikingly, overexpression of the Rabs with the most pronounced effects on filipin distribution, Rab5Q79L and Rab11, also caused the strongest inhibition in cholesterol esterification (∼50% inhibition with both; Figure 3A). Overexpression of Rab7 was slightly inhibitory (25–30% inhibition), whereas Rab6 overexpression was without effect. The expression levels of the individual Rabs were closely similar with the 50–70% transfection frequencies obtained, as assessed by Western blotting with anti-GFP antibodies (Figure 3B). The effects of the individual Rabs on cholesterol esterification could be observed already at 24 h posttransfection (our unpublished data). Furthermore, the Rab11-induced redistribution of cholesterol was morphologically apparent already at 8 h posttransfection, at a stage when the GFP-Rab11 decorated small punctate structures throughout the cytoplasm (Figure 4A). By 16 h of transfection, the Rab11- and filipin-positive organelles had attained a more tubular appearance and by 24 h, larger vesicular and tubular profiles containing both Rab11 and cholesterol were generated (Figure 4A).
Figure 3.
Rate of cholesterol esterification in COS-1 cells overexpressing soluble GFP, GFP-fused Rab5Q79L, wtRab6, wtRab7, and wtRab11. (A) Transfected cells grown in culture medium were pulsed for 4 h with [3H]oleic acid in the presence of 50 μg/ml LDL. The rate of esterification is shown as percentage of esterification in cells transfected with GFP alone. Each bar represents three to nine samples from one to four individual experiments; the SEs are indicated. The mean rate of esterification in the GFP control sample was 34 dpm/μg protein/h. (B) Western blot analysis of GFP and different GFP-fused Rab proteins. The cells were transfected for 40 h, and the cell lysate was separated by SDS-PAGE and analyzed by immunoblotting with anti-GFP antibodies.
Figure 4.
(A) Formation of GFP-Rab11–positive organelles with increasing expression times. COS-1 cells overexpressing GFP-wtRab11 were fixed 8, 16, or 24 h posttransfection and stained with filipin. The areas indicated in the top panels are shown in the bottom panels. The arrowheads indicate GFP-wtRab11– and filipin-positive organelles. (B) Distribution of TfR and Texas Red transferrin in COS-1 cells coexpressing GFP-wtRab11 and TfR. Cells overexpressing GFP or GFP-wtRab11 (left) and TfR as visualized by anti-TfR antibody. (C) Localization of transferrin in the cells overexpressing GFP-wtRab11 and TfR (unpublished data). The cells were labeled with Texas Red transferrin for 30 min on ice and transferrin internalized for 30 min at 37°C. Images in A were obtained using wide-field microscope. Images in B and C are confocal and represent a single focal plane. Bar, 8 μm.
Rab11-positive Organelles Accumulate Transferrin Receptor and Internalized Transferrin
In baby hamster kidney and Chinese hamster ovary cells, Rab11 localizes with internalized transferrin in the pericentriolar recycling compartment (Ullrich et al., 1996) and in HeLa cells, Rab11 overexpression leads to morphological alterations of the TfR-containing compartments (Wilcke et al., 2000). We therefore analyzed the effect of Rab11 overexpression on the distribution of TfR and its ligand in COS cells. Cells were cotransfected with wtRab11 and TfR cDNAs, and at 40 h posttransfection Texas Red transferrin was bound to the cells for 30 min on ice, followed by 30-min internalization at 37°C. In control cells expressing soluble GFP and TfR, the receptor was localized in the perinuclear region and in small punctate and tubular structures throughout the cell (Figure 4B). In Rab11-expressing cells, the TfR staining was concentrated in larger tubular structures that also contained Rab11 (Figure 4B). Moreover, labeled transferrin accumulated readily in these structures (Figure 4C). These results suggest that also in COS cells, Rab11 regulates the dynamics of the endosomal recycling compartment as probed by using TfR and its ligand as markers.
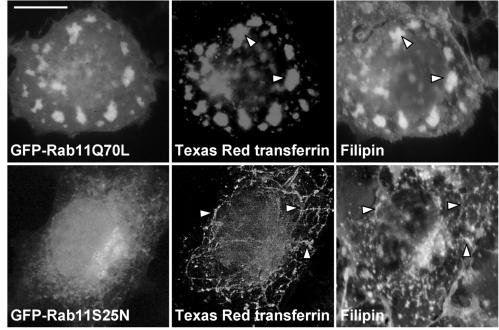
Both GTPase-deficient and Dominant Negative Mutants of Rab11 Alter TfR Distribution, Cholesterol Distribution, and Cholesterol Esterification
The GTPase-deficient Rab11 mutant (Rab11Q70L) and the dominant negative mutant (Rab11S25N) differentially affect TfR distribution in HeLa cells (Wilcke et al., 2000). To further characterize the effect of Rab11 on endocytic recycling in COS cells, we studied the distribution of Texas Red transferrin in cells coexpressing TfR and mutant Rab11 proteins. To analyze whether cholesterol distribution was affected, the transfected cells were also stained with filipin. In Rab11Q70L cells, Texas Red transferrin and filipin colocalized in extended tubulovesicular structures that were also strongly positive for the mutant Rab11 (Figure 5). These structures were reminiscent of those observed upon overexpression of wtRab11. Rab11S25N gave a predominantly cytosolic staining pattern in accordance with previous results (Ren et al., 1998). In these cells, thinner transferrin-positive tubular elements forming a perinuclearly concentrated network were visualized. This meshwork was also visualized with filipin (Figure 5). We then measured [3H]oleic acid incorporation into cholesteryl esters upon expression of the GTPase-deficient or dominant negative Rab11 mutants. This revealed that both Rab11Q70L and Rab11S25N markedly inhibited cholesterol esterification. The extent of inhibition by either mutant did not differ significantly from that observed with wtRab11 (our unpublished data).
Figure 5.
Effect of GFP-Rab11Q70L and GFP-Rab11S25N on the distribution of Texas Red transferrin and free cholesterol in COS-1 cells. The cells coexpressing GFP-Rab11Q70L or GFP-Rab11S25N (left) and TfR (unpublished data) were labeled with Texas Red transferrin for 30 min on ice and transferrin internalized for 30 min at 37°C (middle). Right, filipin stainings of the respective cells. The arrowheads indicate redistribution of free cholesterol in tubular organelles. Images were obtained using wide-field microscope. Bar, 8 μm.
Localization of Other Lipids Enriched in Plasma Membrane and Endosomal Compartments in Rab11-overexpressing Cells
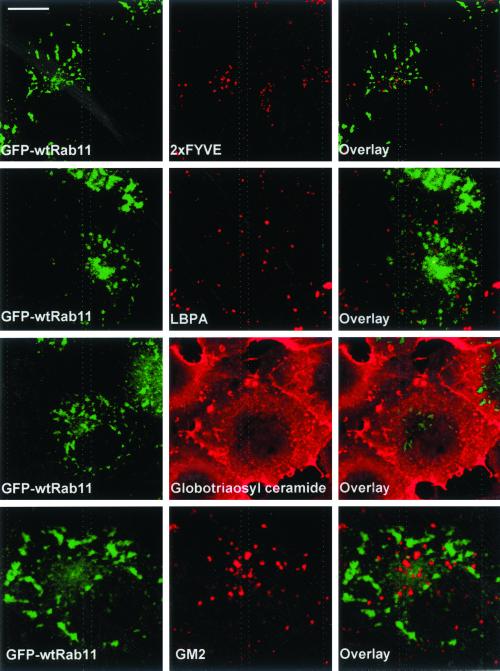
Given the Rab11-induced redistribution of cholesterol, we next analyzed whether Rab11 would also affect the subcellular localization of other endosomal lipids. Rab11-expressing cells were labeled with the PI-3-P binding peptide 2xFYVE (Gillooly et al., 2000). We found that the distribution of PI-3-P, typically in small vesicular structures characteristic of early endosomes, was not altered in Rab11-expressing cells and did not overlap with that of Rab11 (Figure 6). We then analyzed the distribution of the late endosomal acidic phospholipid LBPA by antibody staining. Also this staining was similar in Rab11-expressing cells compared with nonexpressing cells and did not colocalize with Rab11 (Figure 6).
Figure 6.
Localization of PI-3-P, LBPA, globotriaosyl ceramide, and GM2 ganglioside is not altered in GFP-wtRab11–expressing COS-1 cells. Cells overexpressing GFP-wtRab11 (left) were permeabilized with filipin and stained with biotin-2xFYVE and Cy3-conjugated streptavidin, anti-LBPA, anti-globotriaosyl ceramide, or anti-GM2 antibodies. Images were obtained using confocal microscope and represent a single focal plane. Bar, 8 μm.
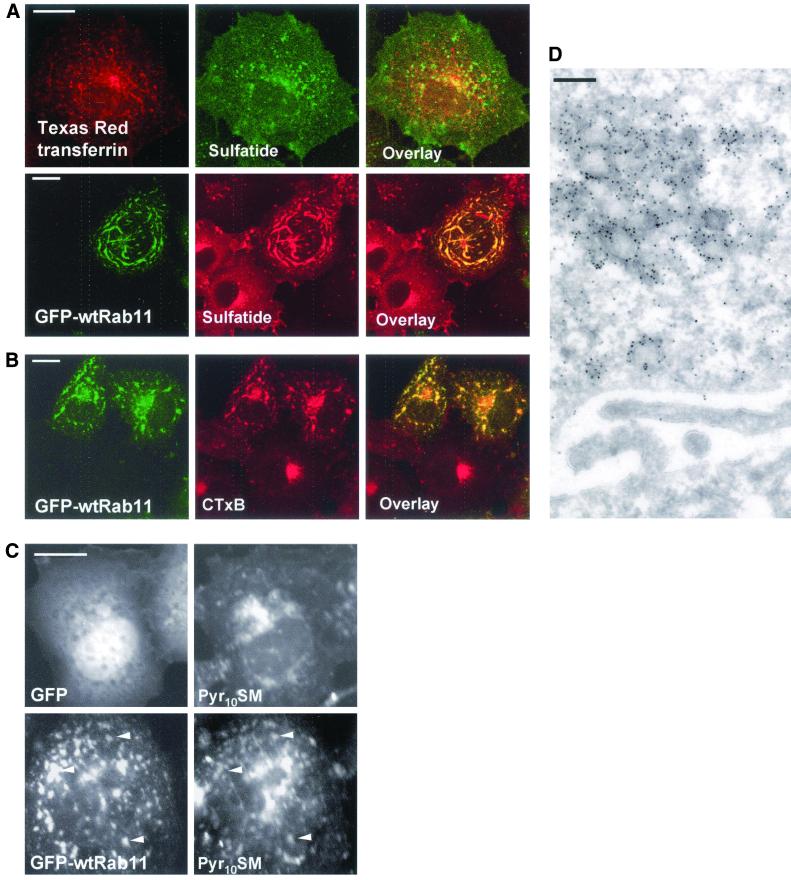
Because cholesterol is thought to associate with sphingolipids in membranes, we analyzed the localization of select glycolipids as well as sphingomyelin in Rab11-expressing cells. Monoclonal antibodies against globotriaosyl ceramide gave prominent plasma membrane staining, and this pattern was not altered upon Rab11 expression (Figure 6). GM2 ganglioside is found in late endosomes (Zhang et al., 2001), and the punctate anti-GM2 staining did not overlap with that of Rab11 (Figure 6). In contrast, the antibody against the glycosphingolipid sulfatide revealed that in some of the Rab11-overexpressing cells, this lipid was redistributed to Rab11-positive organelles as shown in Figure 7A. This labeling pattern was observed in 57% of the Rab11-overexpressing cells (n = 200). The glycolipid redistribution upon Rab11 overexpression was not as marked as that of cholesterol that consistently colocalized with GFP-Rab11 in the same cells (our unpublished data). In control cells, the anti-sulfatide antibodies visualized the plasma membrane and perinuclear organelles. Some of this labeling may correspond to recycling endosomes as the anti-sulfatide staining partially colocalized with transferrin in control cells (Figure 7A).
Figure 7.
Effect of GFP-wtRab11 on the distribution of sulfatide, CTxB, and Pyr10SM in COS-1 cells. (A) Cells overexpressing TfR (unpublished data) were labeled with Texas Red transferrin for 30 min on ice and transferrin internalized for 30 min at 37°C. The cells were then permeabilized with filipin and stained with anti-sulfatide antibodies (top). GFP-wtRab11 and nonexpressing cells were permeabilized with filipin and stained with anti-sulfatide antibodies (bottom). (B) Cells overexpressing GFP-wtRab11 and nonexpressing cells were labeled with Alexa-conjugated CTxB for 1 h on ice and CTxB internalized for 2 h at 37°C. (C) Cells overexpressing GFP or GFP-wtRab11 were labeled with Pyr10SM for 10 min at 37°C and incubated for further 2 h at 37°C in the absence of the lipid. The arrowheads indicate GFP-wtRab11–positive organelles containing Pyr10SM. (D) Electron micrograph of GFP-Rab11–overexpressing COS-1 cell. The anti-GFP antibodies decorate clustered tubulovesicular membranes. Images in A and B are confocal and represent a single focal plane. Images in C were obtained using wide-field microscope. Bars, 8 μm (A–C) and 200 nm (D).
To visualize the distribution of GM1 ganglioside, we labeled the cells with Alexa 594-conjugated CTxB for 1 h on ice followed by 2 h internalization at 37°C. This resulted in prominent perinuclear staining in control cells (Figure 7B), in accordance with the transport of the toxin to the Golgi (Lencer et al., 1999). On Rab11 overexpression CTxB was largely redistributed to GFP-Rab11–positive organelles (Figure 7B). To visualize sphingomyelin, cells were labeled with the fluorescent Pyr10SM for 10 min and chased for 2 h before fixation. At this time point Pyr10SM was not significantly degraded because >95% of the label was still associated with sphingomyelin. In GFP-expressing cells, the staining was visualized in the plasma membrane and in punctate perinuclear structures (Figure 7C), probably representing late endocytic organelles and the Golgi apparatus, analogously to the distribution of BODIPY-Sphingomyelin (Puri et al., 2001). In Rab11 cells, Pyr10SM was additionally distributed to more peripheral structures throughout the cell that colocalized to a large extent with Rab11 (Figure 7C).
These results indicate that the Rab11-induced lipid redistribution is highly selective. The GFP-Rab11–containing compartments exclude select early and late endosomal lipids while including specific sphingolipids and even distinguishing between different glycosphingolipid classes. When the ultrastructure of the Rab11-containing organelles was analyzed by electron microscopy the GFP-Rab11–positive structures were resolved as tubulovesicular clusters of membranes (Figure 7D).
Late Endosomal Cholesterol Transport in Rab11-overexpressing Cells
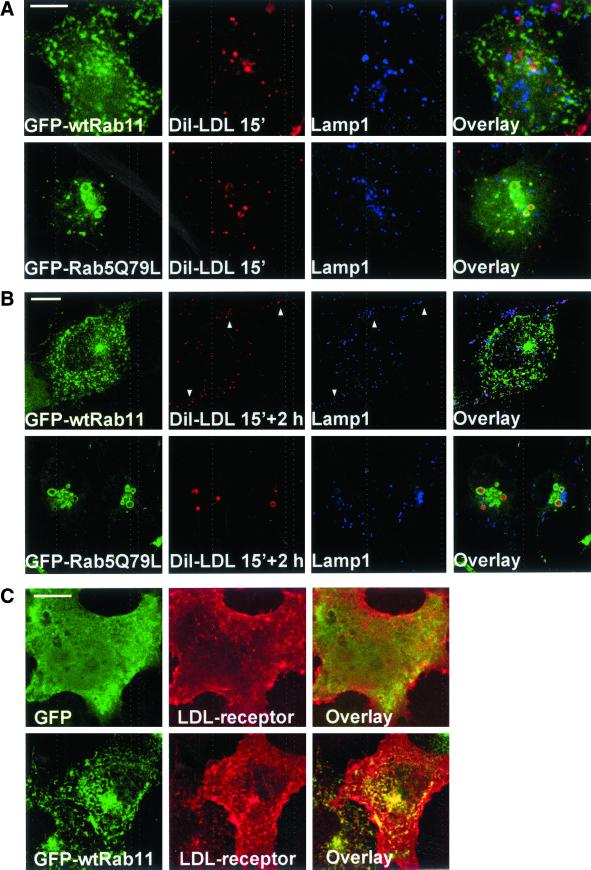
One possible explanation for the accumulation of cholesterol in Rab11-containing compartments and the inhibition in cholesterol esterification could be that Rab11 interferes with LDL-cholesterol internalization. To test this possibility, we added DiI-LDL to living cells for 15 min and either fixed the cells directly or after a 2-h chase. The distribution of DiI was visualized in cells costained with antibodies against the lysosomal membrane protein lamp1. In GFP- and in Rab11-expressing cells, the markers were segregated at 15 min, whereas at 2 h extensive colocalization was observed, indicating transport of DiI to lysosomes (Figure 8, A and B; our unpublished data). In contrast, in Rab5Q79L-expressing cells, DiI-LDL labeling was observed in the cores of enlarged early endosomes that were delineated by Rab5Q79L, at both time points (Figure 8, A and B). These data indicate that the transport of DiI-LDL to late endosomes and lysosomes was blocked in Rab5Q79L-expressing cells. However, this was not the case in Rab11-overexpressing cells.
Figure 8.
Distribution of internalized DiI-LDL in COS-1 cells overexpressing GFP-Rab5Q79L or GFP-wtRab11. Cells transfected with indicated constructs were cultured for 24 h in 5% LPDS medium, labeled with DiI-LDL for 15 min, and fixed (A) or labeled with DiI-LDL for 15 min and incubated for 2 h in serum-free medium (B). The cells were then stained with anti-lamp1 antibodies. The colocalization of DiI and lamp1 is indicated by arrowheads in respective panels and as purple color in the overlay. (C) Localization of LDL receptor in COS-1 cells double-overexpressing soluble GFP or GFP-wtRab11 and the LDL-receptor. LDL receptor was visualized by anti-LDL receptor antibodies. Images were obtained using confocal microscope and represent a single focal plane. Bars, 8 μm.
We also examined the distribution of the LDL-receptor in Rab11-overexpressing cells. The localization of the receptor was visualized in cells coexpressing the LDL receptor and Rab11 and compared with cells overexpressing the LDL receptor and soluble GFP. In both cases, anti-LDL receptor antibodies visualized surface staining and small punctate structures (Figure 8C). In the Rab11-overexpressing cells, these structures partially colocalized with Rab11. However, the prominent plasma membrane staining of the receptor was not appreciably altered by Rab11 overexpression. Together, these results suggest that the cholesterol accumulation in Rab11-containing organelles is not likely to be explained by sequestration of the LDL receptor and its ligand.
Rab11 and the NPC Phenotype
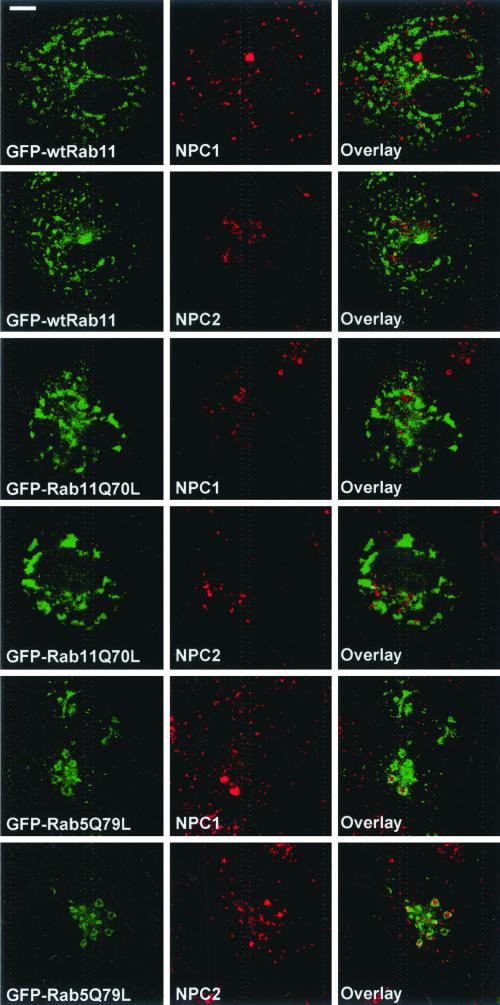
The phenotype of the Niemann-Pick type C disease cells indicates that the NPC1 and NPC2 proteins have important functions in endocytic cholesterol trafficking. When the distribution of endogenous NPC1 or NPC2 was analyzed in wtRab11 or Rab11Q70L-overexpressing cells, we found no colocalization of the proteins with the overexpressed Rab (Figure 9). Instead, NPC1 and NPC2 colocalized with late endocytic markers as reported previously (our unpublished data; Neufeld et al., 1999; Naureckiene et al., 2000). Interestingly, in Rab5Q79L-expressing cells, both NPC1 and NPC2 accumulated in the enlarged early endosomes. The proteins were often visualized inside the organelles surrounded by Rab5Q79L (Figure 9). This suggests that NPC1 and NPC2 may be more closely connected by membrane trafficking with early than with recycling endocytic compartments.
Figure 9.
Localization of NPC1 and NPC2 in COS-1 cells overexpressing GFP-wtRab11, GFP-Rab11Q70L, or GFP-Rab5Q79L. Endogenous NPC1 and NPC2 were visualized by anti-NPC1 or anti-NPC2 antibodies. Images were obtained using confocal microscope and represent a single focal plane. Bar, 8 μm.
To rule out that the cholesterol deposition in Rab11-positive organelles was a phenomenon limited to COS cells, we analyzed the cholesterol distribution upon Rab11 overexpression in primary fibroblasts cooverexpressing TfR. Also in these cells, the Rab11-positive organelles accumulated TfR as well as free cholesterol (Figure 10A). Cholesterol accumulation was not observed in cells overexpressing soluble GFP and TfR (Figure 10A). We then tested whether the Rab11-induced cholesterol accumulation could be observed in cells exhibiting a lysosomal cholesterol transport block. This was achieved by transfecting NPC patient fibroblasts with the Rab11 construct. Also in these cells, the Rab11-induced cholesterol deposits were observed (Figure 10B). These structures were typically more peripherally localized and less intensively stained with filipin compared with the lysosomal deposits. Similar accumulation of cholesterol in Rab11-containing organelles was observed in COS cells when a lysosomal cholesterol transport block was introduced pharmacologically using U18666A (our unpublished data).
Figure 10.
Effect of GFP-wtRab11 on the distribution of free cholesterol in normal and NPC fibroblasts and the complementation of NPC fibroblasts by the NPC1 protein. (A) Human control fibroblasts coexpressing TfR and soluble GFP (left) or TfR and GFP-wtRab11 (right) were stained with anti-TfR antibodies and filipin. (B) NPC fibroblasts transfected with GFP-wtRab11 and stained with filipin. (C) Human NPC fibroblast cooverexpressing GFP-wtRab11 and NPC1 (as visualized by anti-NPC1 antibodies) and filipin staining of the respective cell. The arrowheads indicate GFP-wtRab11–positive organelles containing free cholesterol. The panels in A and B and filipin panel in C were imaged using wide-field microscope. Other panels in C are confocal and represent a single focal plane. Bar, 8 μm.
We next investigated whether Rab11 overexpression would interfere with the clearance of the late endocytic cholesterol storage by the NPC1 protein. NPC fibroblasts were cotransfected with NPC1 and Rab11 and imaged 3 d posttransfection. We found that NPC1 did not colocalize with Rab11 and was capable of complementing the NPC cells as shown by the disappearance of the filipin-positive lysosomal cholesterol stores (Figure 10C). Moreover, the weaker peripheral filipin-positive staining typical to Rab11-overexpressing fibroblasts was observed in these cells. Together, these data indicate that both the buildup and the disappearance of late endocytic cholesterol accumulation can take place irrespective of Rab11 overexpression and that the Rab11 induced cholesterol deposition can be observed in cells with a late endocytic cholesterol transport block.
Rab11-induced Decrease in Cholesterol Esterification Is Not Dependent on LDL-Cholesterol and Can Be Bypassed by Adding Cholesterol in a Cyclodextrin Complex
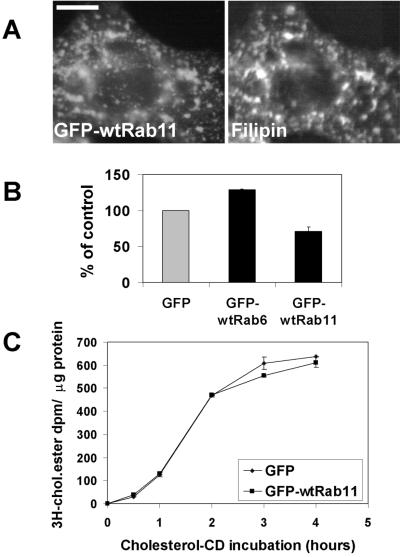
Our morphological data suggested that the cholesterol accumulation upon Rab11 overexpression may not depend on LDL-cholesterol internalization. We therefore incubated COS cells in lipoprotein-deficient medium after Rab11 transfection. Filipin staining of the cells revealed that the Rab11-containing organelles were indeed cholesterol loaded also under these conditions (Figure 11A). Moreover, the Rab11-induced decrease in cholesterol esterification was also independent of LDL-cholesterol as shown by the decrease in [3H]oleic acid incorporation into cholesteryl esters in the presence of lipoprotein-deficient medium (Figure 11B). Under delipidating conditions, the overall rate of esterification was decreased expectedly (as observed by comparing the absolute dpms to those in Figure 3), but a Rab11-induced inhibition of esterification was nevertheless clearly observed. Interestingly, under these conditions Rab6 overexpression enhanced esterification slightly compared with the GFP control (Figure 11B).
Figure 11.
(A) Distribution of free cholesterol in delipidated COS-1 cells expressing GFP-wtRab11. Cells transfected with GFP-wtRab11 were grown in 5% LPDS medium for 24 h before fixation and stained with filipin. Images were obtained using wide-field microscope. Bar, 8 μm. (B) Rate of cholesterol esterification in delipidated COS-1 cells overexpressing soluble GFP, GFP-wtRab6, or GFP-wtRab11. Cells transfected with GFP, GFP-wtRab6, or GFP-wtRab11 were cultured in 5% LPDS medium for 24 h before labeling with [3H]oleic acid for 4 h. The rate of esterification is shown as percentage of esterification in cells transfected with GFP alone. Each bar represents 5–10 samples from two to four individual experiments; the SEs are indicated. The mean rate of esterification in the GFP control sample was 5 dpm/μg protein/h. (C) Rate of cholesterol esterification in GFP-wtRab11–overexpressing COS-1 cells upon loading with cholesterol/mβ-CD-complex. Cells transfected with GFP or GFP-wtRab11 were cultured in 5% LPDS medium for 24 h before labeling with [3H]oleic acid for 4 h. Cholesterol/mβ-CD-complex was added during the labeling to yield the loading time points indicated. Values represent the average of duplicate samples from a representative experiment; the variation between samples is indicated.
To analyze the possibility that the cholesterol load in Rab11-positive organelles was due to elevated de novo cholesterol synthesis, we labeled GFP and GFP-Rab11 transfected COS cells with [3H]acetate and analyzed the amount of [3H]cholesterol formed. There was no increase in the rate of cholesterol biosynthesis in Rab11-transfected cells (207 ± 18 dpm/μg protein; SEM, n = 3) compared with GFP control (194 ± 8 dpm/μg protein; SEM, n = 3).
Considering the recycling characteristics of the Rab11-harboring organelles, we reasoned that the block in cholesterol esterification could be due to defective recycling of cholesterol from endocytic organelles to the plasma membrane. We therefore tested whether the inhibition of cholesterol esterification by Rab11 overexpression could be overcome by providing exogenous cholesterol to the plasma membrane. This was achieved by incubating cells with a cholesterol/mβ-CD-complex, which results in efficient cholesterol loading of cells (Leppimaki et al., 2000; Blom et al., 2001) and induces a rapid and massive compensatory increase in cholesterol esterification. When the increase in [3H]oleic acid incorporation upon cholesterol addition was compared between GFP- and GFP-Rab11–expressing cells no significant differences were detected at any time point analyzed (Figure 11C). This result shows that the Rab11-induced block in cholesterol esterification could be bypassed by adding cholesterol to the plasma membrane.
DISCUSSION
The role of Rab proteins as specific regulatory switches of protein transport is well appreciated. In this work, we provide the first evidence for selective regulation of cholesterol trafficking and homeostasis by endocytic Rab proteins. The well-characterized Rabs 5, 7, and 11 were chosen to encompass aspects of early, late, and recycling endocytic membrane trafficking, respectively. Yet, the precise boundaries between endocytic compartments cannot be determined by Rab proteins and to a large extent, these boundaries still remain to be established (Sonnichsen et al., 2000; Gruenberg, 2001).
Enlargement of the early endosomal compartment by the dominant active mutant of Rab5 was accompanied by sequestration of cholesterol in these organelles and a concomitant decrease in cholesterol esterification. This may be explained, at least partially by inhibition of LDL-cholesterol transport, as observed by the accumulation of DiI-LDL and the LDL-receptor in the Rab5Q79L-positive organelles (Figure 8; our unpublished data). In contrast, Rab7 overexpression did not appreciably alter cholesterol distribution as assessed by filipin staining, and its effect on cholesterol esterification was also more moderate than that of Rab5. This was somewhat surprising considering that NPC1, a key regulator of endocytic cholesterol flow, is localized in Rab7-positive late endosomes (Zhang et al., 2001) and that the cholesterol accumulation in NPC disease is most pronounced in late endocytic organelles. Because Rab5 (and Rab11; see below) overexpression instead had marked effects on cholesterol balance, one possible explanation is that the bulk of endocytic cholesterol flow normally occupies earlier endocytic compartments than the Rab7-regulated organelles. However, the transient transfection approach used and differences between individual Rabs (e.g., with respect to their GTP-GDP cycle or potential effects on LDL uptake or degradation), preclude comparisons regarding the quantitative contribution of each Rab in regulating cholesterol flow.
The most pronounced effects on cholesterol balance were observed upon Rab11 overexpression. The wild-type, GTPase-deficient, and dominant inhibitory Rab11 were all effective, analogously to the effects of Rab11 and its mutants on transferrin recycling and the transport of shiga toxin B subunit (Wilcke et al., 2000). The Rab11-regulated, cholesterol-sequestering organelles have characteristics of recycling endosomes based on the accumulation of TfR and internalized transferrin. We observed that two of the glycosphingolipids studied, sulfatide and GM1, cosequestered with cholesterol in the Rab11-positive organelles, as judged by antibody staining and cholera toxin labeling, respectively. In addition, pyrene-labeled sphingomyelin was found to colocalize with Rab11. This is in accordance with previous reports showing that recycling endosomes are enriched with sphingomyelin and cholesterol (Gagescu et al., 2000), and that these two lipids have high affinities toward each other (Ohvo-Rekila et al., 2002). On the other hand, the early endosomal PI-3-P and the late endosomal LBPA were not redistributed to Rab11 organelles. Moreover, the distributions of two other glycolipids, globotriaosyl ceramide and GM2 ganglioside, were not altered. The former localized mostly on the plasma membrane and the latter in late endocytic organelles in both Rab11-overexpressing and control cells.
Our results provide evidence that the specificity of lipid and protein transport along the endocytic pathway is maintained in Rab11-overexpressing cells. Furthermore, they reinforce the emerging concept of differential sorting of glycolipids along the endocytic pathways (Puri et al., 2001; Zhang et al., 2001). The mechanisms by which the selective accumulation of lipids upon Rab11 overexpression is generated, remain to be elucidated. It could potentially derive from selective retention in recycling endosomes or from altered sorting at an endocytic step before recycling endosomes (or a combination of both).
Because the LDL-receptor route of cholesterol internalization is well characterized the potential contribution of this route to the Rab11-induced cholesterol sequestration was studied. The following data suggest that the Rab11-regulated cholesterol transport route is separate from the LDL-cholesterol route and that the majority of the cholesterol trapped in Rab11-containing organelles is not directly derived from LDL. First, DiI-LDL was transported to lysosomes in Rab11-overexpressing cells and the bulk of the LDL-receptor was not sequestered in Rab11 organelles. Second, both the morphologically detected accumulation of free cholesterol and the inhibition of cholesterol esterification were LDL independent. Third, cholesterol deposition in Rab11-positive organelles was seen also in cells exhibiting lysosomal cholesterol accumulation. Finally, introduction of the NPC1 protein into NPC patient fibroblasts allowed correct localization of the protein and this lead to clearance of the late endocytic cholesterol deposits irrespective of Rab11 overexpression.
Entrapment of the recycling marker TfR in the Rab11-containing organelles implies that in COS cells Rab11 regulates recycling of select cargo to the plasma membrane as has been observed for other cell types (Ullrich et al., 1996; Chen et al., 1998; Ren et al., 1998; Cox et al., 2000). We therefore hypothesized that the cholesterol entrapment in Rab11 organelles may result from reduced recycling of cholesterol to the plasma membrane. This would be in accordance with recent data demonstrating that expression of a dominant negative Rme-1 retards the return of dehydroergosterol to the cell surface (Hao et al., 2001). Although recycling takes place from several stations along the endocytic route, recycling endosomes are thought to be more plastic than e.g., sorting or late endosomes (Wilcke et al., 2000) and could store accumulating cargo. Moreover, their membranes could have particularly high affinity for cholesterol (Hao et al., 2001). Even moderate stagnation in recycling may be sufficient to eventually manifest as massive deposition of cholesterol. If the decrease in cholesterol esterification upon Rab11 overexpression were due to its endosomal entrapment and inaccessibility to the plasma membrane, one should be able to bypass the effect by adding excess cholesterol on the plasma membrane. To test this, we loaded the plasma membrane with cholesterol using a cyclodextrin carrier. Indeed, the Rab11-induced inhibition of cholesterol esterification was now bypassed. However, the intensity of the plasma membrane filipin staining was not appreciably reduced upon Rab11 overexpression. It is therefore plausible that the entrapment of cholesterol in the Rab11 endosomes is by itself sufficient to explain the reduced accessibility of cholesterol for esterification.
An important concept emphasized by the present study is the modulation of cholesterol homeostasis by perturbation of recycling membrane trafficking. This effect is partially but not fully analogous to the effects seen in cholesterol balance in the NPC-related cholesterol transport block. Rab11 overexpression causes the accumulation of free cholesterol in organelles with recycling characteristics, whereas loss of NPC1 function results in the accumulation of cholesterol in late endocytic compartments. Both result in a defect in cholesterol esterification. However, in NPC cells cholesterol biosynthesis is increased, but in the Rab11 cells, cholesterol synthesis remains unaltered. Neither in NPC (Slotte et al., 1989) nor in Rab11 cells is the acyl coenzyme A-cholesterol acyltransferase (ACAT) activity reduced (as suggested by the normal esterification upon cholesterol/mβ-CD addition in Rab11 cells). This suggests that in both cases, defective esterification is due to reduced substrate availability to ACAT. This again is likely to be associated with the endocytic cholesterol transport problems.
Our data support the idea that several membrane-trafficking pathways can feed ACAT with cholesterol. The role of NPC1 in regulating membrane trafficking has been reinforced in several studies (Neufeld et al., 1999; Cruz et al., 2000; Hölttä-Vuori et al., 2000; Millard et al., 2000; Lusa et al., 2001). In this work, we demonstrate that cholesterol esterification can be modulated by a number of Rab proteins associated with distinct membrane-trafficking pathways. Interestingly, although the focus of the present study was on selected endosomal Rabs (all of which inhibited esterification to a various extent) we also noted that the Golgi-associated Rab6 enhanced cholesterol esterification when the cells were cultured in lipoprotein-deficient serum and the basal esterification rate was slow. This points to the intriguing possibility that the Rab6-regulated retrograde Golgi transport pathway may carry cholesterol back to the endoplasmic reticulum where ACAT esterifies it. This route may be physiologically relevant as the dominant negative mutant of Rab6 inhibited esterification when the cells were cultured in the presence of serum lipoproteins (our unpublished data). Interestingly, another retrograde Golgi transport route via COPI-coated vesicles is depleted of cholesterol (Brugger et al., 2000), supporting the idea that there may be preferential membrane carriers for cholesterol both along the exocytic and endocytic pathways.
In addition, nonvesicular cholesterol-trafficking itineraries operate in parallel. For instance, digestion of plasma membrane sphingomyelin by neutral sphingomyelinase has been shown to lead to plasma membrane vesiculation and stimulation of cholesterol esterification in an ATP-independent manner (Skiba et al., 1996; Zha et al., 1998). It is conceivable that cholesterol transferred to cells from the cyclodextrin complex may use such a mechanism because Rab11 does not inhibit this pathway.
In conclusion, our data reveal a novel role for the recycling endosomal circuits regulated by Rab11, in endocytic cholesterol trafficking. The effects of Rab11 are not explained by perturbations on LDL-cholesterol transport but rather point to the role of the endocytic recycling compartment in membrane cholesterol cycling and its significance in maintaining cellular cholesterol homeostasis.
ACKNOWLEDGMENTS
We thank Harald Stenmark and Vesa Olkkonen for critical reading of the manuscript; Marino Zerial for GFP-Rab5, GFP-Rab11, and the corresponding mutant cDNAs; Jamie White for GFP-Rab6; Angela Wandinger-Ness for GFP-Rab7; Walter Hunziker for LDL-receptor and Peter Penchev for NPC1 cDNA; Harald Stenmark for Biotin-2xFYVE; Jan-Eric Månsson for anti-glycolipid antibodies; Jean Gruenberg for anti-LBPA antibody; Graham Warren and David Shima for anti-GFP antibody; and Naomichi Okamura for anti-NPC2 antibody. Liisa Arala and Birgitta Rantala are acknowledged for skillful technical assistance. This work was financially supported by The Ara Parseghian Medical Research Foundation, the Academy of Finland (grants 43184 and 43668 to E.I.), Helsinki Biomedical Graduate School (to M.H.V.), and Jenny and Antti Wihuri Foundation.
Abbreviations used:
- ACAT
acyl coenzyme A-cholesterol acyltransferase
- CD
cyclodextrin
- CTxB
cholera toxin subunit B
- DiI-LDL
1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine-perchlorate–labeled low-density lipoprotein
- LBPA
lysobisphosphatidic acid
- LDL
low-density lipoprotein
- LPDS
lipoprotein-deficient serum
- mβ-CD
methyl-β-cyclodextrin
- NPC
Niemann-Pick type C
- PI-3-P
phosphatidylinositol 3-phosphate
- Pyr10SM
pyrenyldecanoylsphingomyelin
- TfR
transferrin receptor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0025. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0025.
REFERENCES
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blom TS, Koivusalo M, Kuismanen E, Kostiainen R, Somerharju P, Ikonen E. Mass spectrometric analysis reveals an increase in plasma membrane polyunsaturated phospholipid species upon cellular cholesterol loading. Biochemistry. 2001;40:14635–14644. doi: 10.1021/bi0156714. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, Helms JB, Lehmann WD, Nickel W, Wieland FT. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol. 2000;151:507–518. doi: 10.1083/jcb.151.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstea ED, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Sugii S, Yu C, Chang TY. Role of Niemann-Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2000;275:4013–4021. doi: 10.1074/jbc.275.6.4013. [DOI] [PubMed] [Google Scholar]
- Feng Y, Press B, Chen W, Zimmerman J, Wandinger-Ness A. Expression and properties of Rab7 in endosome function. Methods Enzymol. 2001;329:175–187. doi: 10.1016/s0076-6879(01)29078-8. [DOI] [PubMed] [Google Scholar]
- Fredman P, Månsson JE, Bigner SH, Wikstrand CJ, Bigner DD, Svennerholm L. Gangliosides in the human glioma cell line U-118 MG grown in culture or as xenografts in nude rats. Biochim Biophys Acta. 1990;1045:239–244. doi: 10.1016/0005-2760(90)90126-i. [DOI] [PubMed] [Google Scholar]
- Fredman P, Mattsson L, Andersson K, Davidsson P, Ishizuka I, Jeansson S, Månsson JE, Svennerholm L. Characterization of the binding epitope of a monoclonal antibody to sulfatide. Biochem J. 1988;251:17–22. doi: 10.1042/bj2510017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell. 2000;11:2775–2791. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hao M, Lin SX, Karylowski OJ, Wustner D, McGraw TE, Maxfield FR. Vesicular and non-vesicular sterol transport in living cells: the endocytic recycling compartment is a major sterol storage organelle. J Biol Chem. 2001;26:26. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci USA. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä-Vuori M, Määttä J, Ullrich O, Kuismanen E, Ikonen E. Mobilization of late-endosomal cholesterol is inhibited by Rab guanine nucleotide dissociation inhibitor. Curr Biol. 2000;10:95–98. [PubMed] [Google Scholar]
- Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Kasurinen J, Somerharju P. Metabolism of pyrenyl fatty acids in baby hamster kidney fibroblasts. Effect of the acyl chain length. J Biol Chem. 1992;267:6563–6569. [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Hirst TR, Holmes RK. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450:177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Leppimäki P, Mattinen J, Slotte JP. Sterol-induced upregulation of phosphatidylcholine synthesis in cultured fibroblasts is affected by the double-bond position in the sterol tetracyclic ring structure. Eur J Biochem. 2000;267:6385–6394. doi: 10.1046/j.1432-1327.2000.01726.x. [DOI] [PubMed] [Google Scholar]
- Liscum L, Ruggiero RM, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J Cell Biol. 1989;108:1625–1636. doi: 10.1083/jcb.108.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lusa S, Blom TS, Eskelinen EL, Kuismanen E, Månsson JE, Simons K, Ikonen E. Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane. J Cell Sci. 2001;114:1893–1900. doi: 10.1242/jcs.114.10.1893. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Millard EE, Srivastava K, Traub LM, Schaffer JE, Ory DS. Niemann-Pick type C1 (NPC1) overexpression alters cellular cholesterol homeostasis. J Biol Chem. 2000;275:38445–38451. doi: 10.1074/jbc.M003180200. [DOI] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Neufeld EB, et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem. 1999;274:9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- Ohvo-Rekilä H, Ramstedt B, Leppimäki P, Slotte JP. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- Okamura N, Kiuchi S, Tamba M, Kashima T, Hiramoto S, Baba T, Dacheux F, Dacheux JL, Sugita Y, Jin YZ. A porcine homolog of the major secretory protein of human epididymis, HE1, specifically binds cholesterol. Biochim Biophys Acta. 1999;1438:377–387. doi: 10.1016/s1388-1981(99)00070-0. [DOI] [PubMed] [Google Scholar]
- Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol. 2001;154:535–547. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of G.T.P. on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Skiba PJ, Zha X, Maxfield FR, Schissel SL, Tabas I. The distal pathway of lipoprotein-induced cholesterol esterification, but not sphingomyelinase-induced cholesterol esterification, is energy-dependent. J Biol Chem. 1996;271:13392–13400. doi: 10.1074/jbc.271.23.13392. [DOI] [PubMed] [Google Scholar]
- Slotte JP, Hedstrom G, Bierman EL. Intracellular transport of cholesterol in type C Niemann-Pick fibroblasts. Biochim Biophys Acta. 1989;1005:303–309. doi: 10.1016/0005-2760(89)90053-2. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhuanpää K, Somerharju P. γ-Cyclodextrins greatly enhance translocation of hydrophobic fluorescent phospholipids from vesicles to cells in culture. Importance of molecular hydrophobicity in phospholipid trafficking studies. J Biol Chem. 1999;274:35359–35366. doi: 10.1074/jbc.274.50.35359. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via DP, Massey JB, Vignale S, Kundu SK, Marcus DM, Pownall HJ, Gotto AM., Jr Spontaneous and plasma factor-mediated transfer of pyrenyl cerebrosides between model and native lipoproteins. Biochim Biophys Acta. 1985;837:27–34. doi: 10.1016/0005-2760(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, Melancon P, Schneider C, Garoff H. The transmembrane segment of the human transferrin receptor functions as a signal peptide. EMBO J. 1986;5:1543–1550. doi: 10.1002/j.1460-2075.1986.tb04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zha X, Pierini LM, Leopold PL, Skiba PJ, Tabas I, Maxfield FR. Sphingomyelinase treatment induces ATP-independent endocytosis. J Cell Biol. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, et al. Sterol-modulated glycolipid sorting occurs in Niemann-Pick C1 late endosomes. J Biol Chem. 2001;276:3417–3425. doi: 10.1074/jbc.M005393200. [DOI] [PubMed] [Google Scholar]