Abstract
The importin α family of nuclear-cytoplasmic transport factors mediates the nuclear localization of proteins containing classical nuclear localization signals. Metazoan animals express multiple importin α proteins, suggesting their possible roles in cell differentiation and development. Adult Caenorhabditis elegans hermaphrodites express three importin α proteins, IMA-1, IMA-2, and IMA-3, each with a distinct expression and localization pattern. IMA-2 was expressed exclusively in germ line cells from the early embryonic through adult stages. The protein has a dynamic pattern of localization dependent on the stage of the cell cycle. In interphase germ cells and embryonic cells, IMA-2 is cytoplasmic and nuclear envelope associated, whereas in developing oocytes, the protein is cytoplasmic and intranuclear. During mitosis in germ line cells and embryos, IMA-2 surrounded the condensed chromosomes but was not directly associated with the mitotic spindle. The timing of IMA-2 nuclear localization suggested that the protein surrounded the chromosomes after fenestration of the nuclear envelope in prometaphase. Depletion of IMA-2 by RNA-mediated gene interference (RNAi) resulted in embryonic lethality and a terminal aneuploid phenotype. ima-2(RNAi) embryos have severe defects in nuclear envelope formation, accumulating nucleoporins and lamin in the cytoplasm. We conclude that IMA-2 is required for proper chromosome dynamics in germ line and early embryonic mitosis and is involved in nuclear envelope assembly at the conclusion of mitosis.
INTRODUCTION
The regulated distribution of proteins between the nucleus and the cytoplasm is critically important for maintenance of the cell cycle, differentiation of cells and tissues, and the development of a complete organism (Koepp and Silver, 1998; Affolter et al., 1999). The bidirectional movement of proteins between the cytoplasm and nucleus is due to intrinsic peptide sequences in each protein. The importin β/karyopherin β family of proteins specifically recognizes many of these sequences and chaperones the proteins between the two compartments through the nuclear pore complex (Adam, 1999). A subset of nuclear import factors known as the importin αs recognizes proteins containing classical nuclear localization sequences (cNLSs) (Conti et al., 1998; Conti and Kuriyan, 2000; Fontes et al., 2000). This family of proteins shares two common structural features: a central armadillo repeat-containing domain that recognizes the cNLS and an amino terminal importin β binding (IBB) domain that binds to the cargo carrier importin β1 (for review, see Chook and Blobel, 2001; Conti and Izaurralde, 2001). The interaction of importin α with importin β allows cNLS-containing proteins to be translocated across the nuclear pore complex, thus the importin α family can be thought of as a set of adapter proteins for transport. Various studies suggest that there is both redundancy and specificity in the recognition of cNLSs by the importin α family (Nadler et al., 1997; Prieve et al., 1998; Hu and Jans, 1999; Kohler et al., 1999).
The number of importin α genes increases with the complexity of the organism; budding yeast have a single gene, whereas humans have at least eight. Phylogenetic analyses group the importin αs into three conserved clades, although several proteins cannot be assigned to any clades (Malik et al., 1997; Kohler et al., 1999; Mathe et al., 2000; Mason et al., 2002). The restriction of the α2 and α3 clades to animals suggests that these importins have specific roles in animal development (Mason et al., 2002). Recently, we described the identification of three importin α proteins in Caenorhabditis elegans: IMA-1, IMA-2, and IMA-3 (an α3) (Geles and Adam, 2001). IMA-1 and IMA-2 are among the small group of unusual importin αs that cannot yet be classified into distinct clades.
The key to regulation of the nuclear-cytoplasmic transport system is the small GTPase Ran and its associated factors that modulate nucleotide binding and hydrolysis (Macara, 2001). The Ran-GTPase network also regulates DNA replication, the exit from mitosis, microtubule polymerization, and accurate chromosome segregation in mitosis and meiosis (Kusano et al., 2001; Moore, 2001). Although some aspects of this regulation may be simply a requirement to transport the necessary factors into the nucleus, other nontransport roles for Ran are now evident. Recent studies in Xenopus laevis egg extracts have shown that Ran-GTP modulates the release of factors that control mitotic spindle formation from importin α and importin β (Gruss et al., 2001; Nachury et al., 2001; Wiese et al., 2001) and can direct nuclear envelope (NE) formation (Zhang and Clarke, 2000).
Conditional mutations or depletion of the Saccharomyces cerevisiae importin α Srp1p result in a mitotic cell cycle arrest at G2/M accompanied by chromosome condensation and segregation defects (Kussel and Frasch, 1995b; Loeb et al., 1995). Mutations in one of the two Schizosaccharomyces pombe importin αs, cut15, lead to mitotic progression without chromosome condensation, resulting in the septum bisecting the nuclear material, a characteristic “cut” phenotype (Matsusaka et al., 1998). cut15-85 mutants do not have gross defects in nuclear protein import, suggesting that the cut phenotype is not due to failure in protein import. The Drosophila melanogaster importin α2 pendulin may also have a direct role in mitosis because it accumulates in embryonic nuclei at the onset of mitosis (Kussel and Frasch, 1995a; Torok et al., 1995). Although these results have been suggestive of a nontransport role in mitosis for members of the importin α family, only the sequestration and release of TPX2 from importin α to regulate mitotic spindle assembly has been directly implicated in a mitotic function (Gruss et al., 2001).
MATERIALS AND METHODS
General Procedures and Nematode Strains
The wild-type N2 Bristol strain was maintained at 20°C on NGM plates seeded with Escherichia coli OP50.
Antibody Production
A polymerase chain reaction product encoding amino acids 512–531 of IMA-2 was inserted in frame with the glutathione S-transferase (GST) protein of the pGEX-4T-1 vector (Amersham Biosciences, Piscataway, NJ). Recombinant GST-IMA-2 protein induction and purification on glutathione-Sepharose were performed as described in the manufacturer's instructions (Amersham Biosciences). HTI Bio-Products (Ramona, CA) prepared rabbit antiserum for the GST fusion protein. Anti-IMA-2 antibodies were affinity purified on full-length IMA-2 S peptide-tagged fusion proteins immobilized on polyvinylidene difluoride membranes. The specificity of the affinity-purified antibodies was determined by immunoblotting whole worm lysates and bacterial lysates containing expressed recombinant IMA proteins (Geles and Adam, 2001).
Indirect Immunofluorescence Microscopy
Detection of IMA proteins in wild-type and RNA-mediated gene interference (RNAi) germ lines was performed on extruded gonads fixed in 1% paraformaldehyde for 4 min (Crittenden and Kimble, 1999). All subsequent staining and washing steps were performed in Tris-buffered saline containing 0.1% Triton X-100 (TBST). Nucleoporins were detected with MAb414 diluted 1:4000 in TBST (Covance Research Products, Richmond CA). Affinity-purified anti-IMA-2 antibodies were diluted 1:200 in TBST. No staining was observed with the anti-IMA-2 antibodies in the presence of the immunogen. β-Tubulin was localized with the monoclonal antibody (mAb) E7 as described previously (Skop and White, 1998). The E7 mAb developed by Michael Klymkowsky was obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA) developed under the auspices of the National Institutes of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA). Embryos extended from gravid hermaphrodites were fixed and stained with mAb414 and rat anti-LMN-1 (a generous gift of Drs. Kathy Wilson, Johns Hopkins University School of Medicine, Baltimore, MD, and Yosef Gruenbaum, The Hebrew University of Jerusalem, Jerusalem, Israel) as described previously (Lee et al., 2000). DNA was stained by inclusion of 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) or 1 μg/ml TOTO-3 iodide (Molecular Probes, Eugene, OR) in the final wash buffer.
Immunofluorescence images were obtained with an LSM510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY). Images of DAPI-stained nuclei were obtained with either Eclipse E800 (Nikon, Melville, NY) or Axioskop (Carl Zeiss) microscopes equipped with digital cameras. The digital images were processed in MetaMorph, version 4.0 (Universal Imaging, Downington, PA) and Photoshop, version 5.0 (Adobe Systems, Mountain View, CA).
RNA Interference Assays
Double stranded RNA (dsRNA) was generated from linearized plasmids of full-length EST yk96a12 in pBluescript II SK(−) by in vitro transcription with T3 and T7 RNA polymerases. After annealing, the double-stranded RNAs were microinjected into the intestines of L4 larvae at a concentration of either 0.5 or l mg/ml with equivalent results (Fire et al., 1998). As a control, distilled H2O was injected into the intestines of L4 larvae. Between 24 and 72 h postinjection, gonads were extruded and processed as described previously (Kawasaki et al., 1998). For RNAi soaking experiments, RNA was prepared as described and L3 and L4 worms were soaked for 24 h at 18°C (Maeda et al., 2001). After soaking, the worms were washed in water and transferred to OP50-seeded plates. The P0s were transferred to new plates every 24 h. Embryos were obtained from the adults 24 h after soaking.
RESULTS
Localization of IMA-2
The three C. elegans importin α genes (ima-1, ima-2, and ima-3) are differentially expressed during development and each protein has a unique germ line localization. IMA-3 is expressed in both somatic and germ cells and reduced expression of IMA-3 stops the progression of germ cells through pachytene of meiotic prophase I (Geles and Adam, 2001). To understand the role of IMA-2 in the germ line, we first examined the localization of the protein in the adult hermaphrodite. Previously, we have demonstrated that ima-2 mRNA was weakly expressed in embryonic and larval stages but expression increased in L4 and adult animals. A mutant strain of C. elegans [glp-1(q224ts)] that do not develop a germ line as adults and did not express ima-2 mRNA when grown at the nonpermissive temperature indicated that ima-2 is a germ line intrinsic gene (Geles and Adam, 2001). Two independent genome-wide analyses of germ-line gene expression subsequently confirmed our identification of ima-2 as a germ line intrinsic gene (Reinke et al., 2000; Hanazawa et al., 2001).
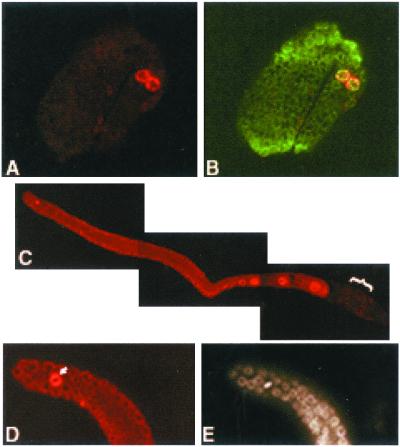
Affinity-purified antibodies to IMA-2 detected the protein only in germ line cells (Figure 1). In embryos the maternal IMA-2 was diluted during early cell divisions and was expressed at detectable levels only in the germ line precursor cells Z2 and Z3, not in the somatic cells (Figure 1, A and B). We have not determined when in the germ cell lineage ima-2 expression was activated. In the adult hermaphrodite germ line, IMA-2 was present within all germ cells from the distal end of the germ line to the proximal oocyte (Figure 1C). Note that IMA-2 was not detected in sperm (Figure 1C), consistent with the absence of an NE in these cells. In distal germ cells, IMA-2 was predominantly cytoplasmic and NE associated. However, in the developing oocytes IMA-2 was predominantly cytoplasmic and intranuclear with no apparent enrichment at the NE. When distal germ cells in prometaphase and metaphase were evident by DAPI staining of the DNA, IMA-2 was enriched at the region immediately surrounding the condensed chromosomes (Figure 1, D and E). IMA-3 was dispersed throughout the mitotic cells in the distal germ line with no obvious enrichment near the chromosomes (our unpublished data).
Figure 1.
Immunofluorescence localization of IMA-2 in embryos and adults. IMA-2 was localized by indirect immunofluorescence with affinity-purified antibodies to IMA-2. (A) IMA-2 localization in red in a tadpole stage embryo. (B) Overlay of IMA-2 staining in red and nucleoporin staining with MAb414 in green. (C) The two cells positive for IMA-2 are the germ line precursor cells Z2 and Z3. An extruded hermaphrodite germ line with IMA-2 is shown in red. The distal end is to the left and the spermatheca is to the right (indicated by the bracket). IMA-2 is cytoplasmic and nuclear in all germ cells but is not detected in sperm. IMA-2 (D) and DNA (E) show a higher magnification of a distal germ line with a germ cell in metaphase (arrow) to highlight the nuclear localization of IMA-2 at mitosis.
IMA-2 Expression in Eggs and Early Embryos
Because early embryonic cells are larger than germ cells, we localized IMA-2 in early embryos by indirect immunofluorescence to better define the timing of nuclear association for IMA-2. The oocyte nucleus is in diakinesis of meiotic prophase I, completing meiosis only upon fertilization. After fertilization, the oocyte nucleus is positioned in the anterior end of the embryo and completes its two meiotic divisions, producing two polar bodies. The female and male pronuclei then form and move toward each other, meeting in the posterior half of the embryo. After the two pronuclei make contact and move back toward the center of the egg, the mitotic spindle rotates onto the A-P axis, the NEs break down, and the chromosomes move to the metaphase plate. Mitosis progresses through anaphase and is completed with NEs reforming around each set of chromosomes at telophase (Strome, 1989).
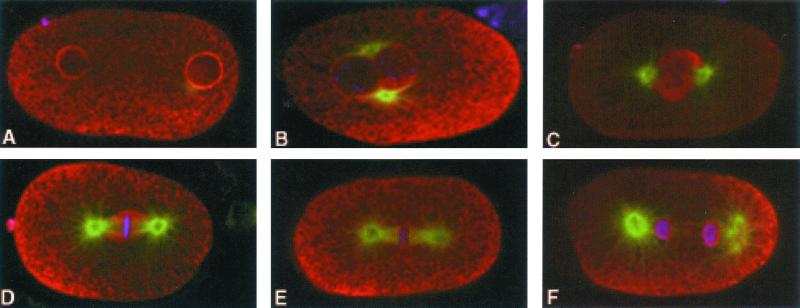
In the fertilized egg before pronuclear migration, IMA-2 was predominantly cytoplasmic and NE associated in both pronuclei (Figure 2). The intranuclear IMA-2 in the proximal oocyte had dispersed into the oocyte cytoplasm at NE breakdown (our unpublished data), but returned to the NE upon NE reformation around the pronuclei. Because sperm do not contain IMA-2 (Figure 1), the male pronuclear IMA-2 must have originated in the egg cytoplasm. As the pronuclei became associated, the intensity of IMA-2 staining at the NE decreased. After pronuclear fusion, IMA-2 completely filled the space surrounding the chromosomes but did not seem to be enriched on the surface of the condensed chromosomes (Figure 2C). This perichromosomal localization persisted during congression of the chromosomes toward the metaphase plate but had decreased by the time of metaphase plate formation. Early in anaphase as chromosome separation first became apparent, IMA-2 staining could still be seen in the region surrounding the chromosome, but was significantly decreased compared with earlier stages. Later in telophase, IMA-2 again became associated with the NE as soon as the structure was detectable around the daughter nuclei. IMA-2 staining persisted at the NE through cytokinesis and into interphase of the next cell cycle. The mitotic IMA-2 staining was not coincident with β-tubulin staining in the spindle microtubules or the spindle poles.
Figure 2.
Immunofluorescence localization of IMA-2 in fertilized zygotes. IMA-2 localization is shown in red, tubulin in green, and DNA in blue. Each panel is a different embryo with the anterior end of the embryo oriented to the left. The series shows the progression through pronuclear formation after fertilization (A), pronuclear meeting and spindle rotation (B), pronuclear fusion/prometaphase (C), metaphase (D), early anaphase (E), and telophase and NE reformation (F). The embryos shown are representative of typical localization patterns.
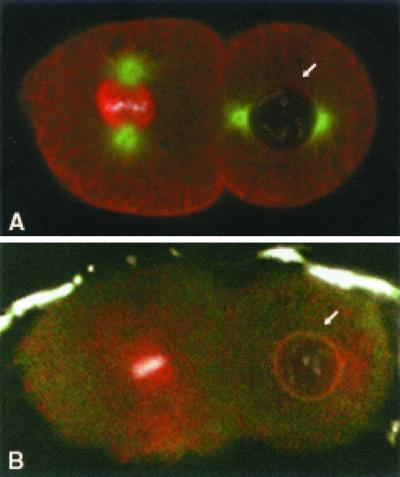
We further investigated the localization of IMA-2 in two-cell embryos to see whether the mitotic localization of IMA-2 was preserved in later cell divisions. Two mechanisms could explain the accumulation of IMA-2 around the chromosomes at mitosis. IMA-2 could enter the nucleus before NE breakdown by entry through the nuclear pores or it could surround the chromosomes by interaction with another component after NE breakdown. Mitosis in C. elegans is unique in that the NE becomes permeable to proteins only in late prometaphase and only fully disassembles during anaphase in early embryos (Lee et al., 2000). To define the timing of NE breakdown in our experiments, we immunolocalized IMA-2 along with nucleoporins or β-tubulin in two-cell embryos. As seen in Figure 3, IMA-2 surrounded the chromosomes only in nuclei that were in late prometaphase or later (Figure 2C). Nuclei containing fully condensed chromosomes, but in which the microtubules had not penetrated, excluded IMA-2 (Figure 3A). Note, however, that by this point IMA-2 was only weakly detected at the NE. Nuclei in which peripheral nucleoporin staining could be detected did not accumulate IMA-2 around the chromosomes (Figure 3B). The localization patterns for IMA-2 presented in Figures 2 and 3 suggest that IMA-2 entered the nucleus only after the point when the NE was permeable to proteins.
Figure 3.
Immunofluorescence localization of IMA-2 in two-cell embryos. (A) Fixed and permeabilized embryos stained for IMA-2 in red, tubulin in green, and TOTO-3–stained DNA in white. (B) IMA-2 is red, nucleoporins are in green, and DNA is in white. In A, the cell to the left is in metaphase and the cell to the right is in late prophase (arrow), and the microtubules have not penetrated the NE. In B, the cell to the left is in early anaphase and the cell to the right (arrow) is in late prophase.
IMA-2 RNA Interference
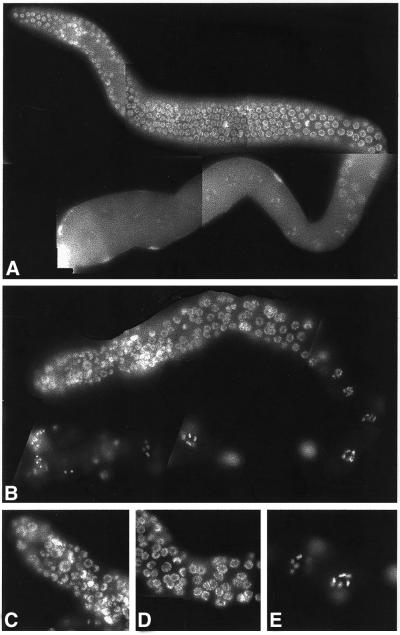
Gene expression can be specifically down-regulated in C. elegans by dsRNA interference (Fire et al., 1998). We reduced the expression of ima-2 by injection of the dsRNA into the intestines of L4 or young adult worms. Between 24 and 72 h postinjection, fixed and extruded germ lines were stained with DAPI to visualize the chromosomes (Figure 4). The hermaphrodite germ line is organized with the cells most distal to the uterus forming a mitotic stem cell population. Between 10 and 20 cell diameters away from the distal tip cell, the germ cells initiate meiosis I in a region called the transition zone. Proximally to the transition zone, germ cells progress from pachytene through diakinesis of meiotic prophase with some of the cells eventually developing into oocytes (Schedl, 1997). ima-2(RNAi) had a dramatic effect on germ line morphology in 25% of the injected animals. In these affected animals, the distal germ line was highly disorganized with DAPI-staining material occupying the central core. The germ line contained fewer germ cells than control animals and the germ cell chromatin in the distal arm had an abnormal appearance. The nuclei in the distal end seemed to have become aneuploid with both larger and smaller than normal DAPI-staining bodies present. In spite of this dramatic effect on the mitotic germ line, some germ cells seemed to enter meiosis normally. These germ cells had likely entered the meiotic cell cycle before the full effect of the RNAi. Proximal to the transition zone, most of the germ cells had normal pachytene morphology with condensed cable-like chromosomes at the nuclear periphery, although several small highly condensed DAPI-staining masses were also interspersed throughout this region. The developing oocytes had the normal complement of six bivalent chromosomes and both the nuclear and cytoplasmic oocyte volumes increased normally. These observations and the fewer number of germ cells in the ima-2(RNAi) worms compared with control animals suggested that germ cells that initiated meiosis before full penetrance of the ima-2(RNAi) were fertilized but not fully replenished. The majority of injected germ lines had no obvious morphological nuclear defects but produced nonviable embryos with aneuploid nuclei (see below).
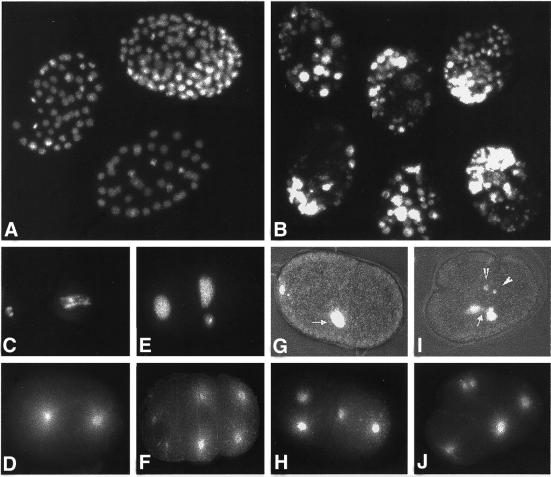
Figure 4.
Phenotype of ima-2(RNAi). Extruded germ lines from control injected (A) or ima-2(RNAi) (B–E) germ lines were stained with DAPI to visualize the DNA. (C–E) Higher magnifications of the mitotic region, pachytene region, and oocytes, respectively. The nuclei in the ima-2(RNAi) germ line contain unequal amounts of DNA. Note also that the regular organization of the mitotic germ cells is disrupted.
The F1 progeny from the injected hermaphrodites exhibited 97% embryonic lethality before the 200-cell stage (Table 1). A small number of progeny produced shortly after RNA injection died as larvae (0.1%) or survived to adulthood (3%). All of the embryos that developed into adults were produced in the first 18 h post dsRNA injection, suggesting that the RNAi phenotype was fully penetrant by 18 h. Of the worms that survived to adulthood, 27% (55/204) were phenotypically wild type. The remainder of the F1 adults had germ line mitotic chromatin defects similar to the injected animals and produced embryos with aneuploid nuclei.
Table 1.
Phenotypes of ima-2(RNAi) F1 Progeny
| Hours post-injection | 0–8 h | 8–18 h | 18–30 h | 30–46 h | 46–70 h | Percent of total |
|---|---|---|---|---|---|---|
| Embryonic lethal (n = 6670) | 2 ± 1 | 24 ± 10 | 51 ± 14 | 75 ± 21 | 97 ± 35 | 97 |
| Larval lethal (n = 7) | 1 ± 0.4 | 0.1 | ||||
| Adults (n = 204) | 2 ± 2 | 2 ± 1 | 3 |
The numbers represent the average (± SD) number of progeny extruded from 27 injected L4 hermaphrodites during the indicated time interval. The phenotypes of all progeny were scored 5 days post-extrusion. n indicates the total number of progeny scored in each class.
Embryos obtained from adults treated for RNAi by soaking exhibited greater than 99% lethality either before or early in gastrulation. The terminal phenotype of the F1 embryos was characterized by severe aneuploidy (Figure 5, A and B) making it difficult to determine the precise point of arrest. The nuclei displayed unequal amounts of DNA and some areas of the embryos seemed to lack DNA entirely, indicative of a chromosome segregation defect. We localized microtubules and DNA in very early embryos with anti-β tubulin antibodies and with DAPI to determine the state of the chromatin and organization of the mitotic spindles. Most embryos contained disorganized chromosome masses between the spindle poles (Figure 5, C–F). The DNA in these embryos was not uniformly stained with DAPI, suggesting different states of chromosome condensation and we rarely observed clearly individualized chromosomes. At telophase, some chromosome masses failed to separate to daughter nuclei and the chromosomes remained as single masses of chromatin. In some embryos, two unequal chromosome masses separated, frequently connected by a chromatin bridge or trailing a strand of chromatin. We observed a small number of embryos with the chromosomes either peripherally associated with the spindle or completely separated from the spindle (Figure 5, G–J). In these embryos, the DNA mass was frequently bisected by the plasma membrane between two daughter cells. In most ima-2(RNAi) embryos examined, the mitotic spindles seemed structurally normal and were properly oriented, although an occasional embryo was seen with a chromosome mass associated with multiple spindles (our unpublished data). Some asynchrony in the well-defined embryonic cell division pattern may have also occurred resulting in early embryos with an abnormal appearance (Figure 5, E and F and I and J).
Figure 5.
Terminal phenotype of ima-2(RNAi) embryos. Fixed and permeabilized embryos were stained with DAPI to visualize the DNA. (A) Embryos from hermaphrodites soaked in RNAi buffer alone. (B) ima-2(RNAi) embryos. Embryos in A and B were obtained from age-matched hermaphrodites and were extruded 24 h postsoaking. The embryos in C–J were obtained from ima-2(RNAi)-injected hermaphrodites 48 h postinjection. C and D, E and F, G and H, and I and J are four different embryos stained for DNA with DAPI (C, E, G, and I) or tubulin (D, F, H, and J). Note that in G and I, the DNA is not associated with the spindles (arrows). In I, the arrow indicates a chromatin bridge, and the arrowheads indicate what seem to be individual chromosomes.
The disorganized state of the chromatin in the ima-2(RNAi) embryos led us to examine the NE by indirect immunofluorescence to determine whether each chromatin mass was surrounded by an NE. ima-2(RNA)i embryos obtained by soaking were collected 24 h after treatment. Immunostaining with antibodies to nucleoporins (Figure 6, A–D) or the C. elegans lamin LMN-1 (Figure 6, E–F) revealed incompletely formed NEs and mislocalized nucleoporins and lamin in the embryos. Some chromatin masses seemed to be completely surrounded by nucleoporins, whereas others had very weak perinuclear nucleoporin staining or were associated with large immunoreactive spots and patchy nuclear staining. In control embryos, the NE as defined by nucleoporin or lamin localization is closely apposed to the surface of the chromatin (Figure 6, A and E). Frequently when nuclei in ima-2(RNAi) embryos had what seemed to be a complete NE, the NE was detached from the surface of the chromatin mass (Figure 6B). Immunostaining with rat anti-LMN-1 antibodies showed a similar lack of a complete NE around individual chromatin masses. These results indicate that IMA-2 is involved in NE assembly after mitosis.
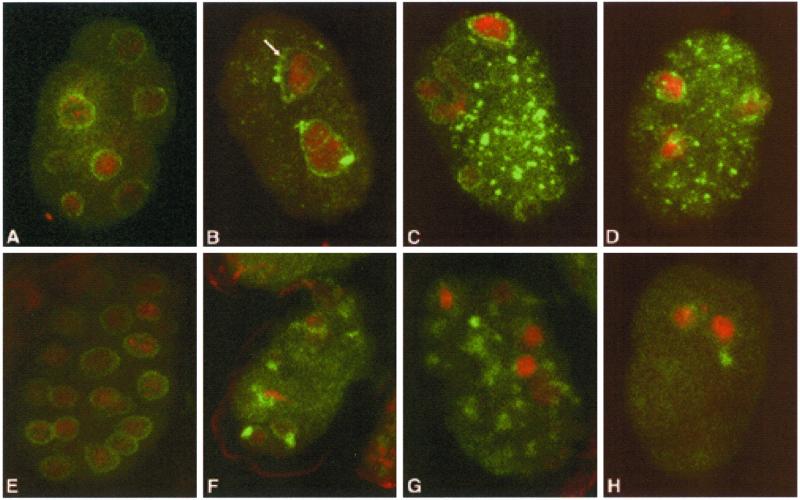
Figure 6.
Nuclear envelope assembly in ima-2(RNAi) embryos. Fixed and permeabilized embryos were stained for nucleoporins with mAb414 (A–D) or lamin with rat anti-LMN-1 (E and F). Nucleoporins and lamin are in green. DNA was stained with TOTO-3 in red. A and E are representative wild-type embryos. B–D and F–H are representative ima-2(RNAi) embryos. The arrow in B points to a separation of the nuclear envelope from the chromatin. Both nucleoporins and lamin accumulate in the cytoplasm of the treated embryos. Note also the more intense DNA stain in nuclei of ima-2(RNAi) embryos.
DISCUSSION
IMA-2 Has Multiple Roles in Mitotic Cell Cycle
Adult C. elegans express three importin α proteins: IMA-1, IMA-2, and IMA-3. IMA-3 is expressed in somatic and germ line cells, whereas IMA-1 and IMA-2 expression is confined to the germ line (Geles and Adam, 2001). The expression of multiple members of the importin α family of nuclear transporters within the same cells and tissues suggests a redundancy of function as has been described for the importin β family in yeast (Rout et al., 1997). This redundancy could mask certain phenotypes in RNAi or other loss of function experiments, but also may reveal unique roles for each protein. Within the germ line, IMA-2 is present in mitotic and meiotic germ cells, suggesting that it has a role in transporting proteins involved in both types of cell cycle. We did not observe any defects in meiosis in our experiments, suggesting that either IMA-2 does not play a critical role in meiosis or that IMA-1 or IMA-3 can compensate for the reduction in IMA-2 levels. In contrast, ima-3(RNAi) leads to a block early in meiosis but does not have a discernable effect on mitosis (Geles and Adam, 2001).
The dramatic defects in the mitosis resulting from ima-2(RNAi) were most apparent in embryos. The terminal embryonic phenotype was arrest before or early in gastrulation characterized by severe aneuploidy of the embryonic cells. The unequal amounts of DNA between cells as well as the appearance of chromatin bridges and lagging chromosomes are consistent with a chromosome segregation defect. In the most extremely affected embryos, the partially condensed DNA mass could be seen in a single cell, completely separated from any spindles. At this time, we cannot determine whether the chromosome segregation defect is due to a failure in the nuclear transport function of IMA-2 or is related to its localization at mitosis, or a combination of the two. ima-2 has also been a target in two large-scale analyses of gene function by RNA interference. These studies have also found that ima-2(RNAi) results in embryonic lethality before the 200-cell stage (Fraser et al., 2000; Hanazawa et al., 2001).
Conservation of Importin α Functions
Phylogenetic analyses of importin α protein sequences from plants, fungi, and animals group the proteins into three clades. IMA-2 cannot be placed into any of the three clades of importin α genes, indicating the protein sequence has likely diverged from the importin αs of other metazoans (Malik et al., 1997; Kohler et al., 1999; Mathe et al., 2000; Mason et al., 2002). Despite this apparent divergence in sequence, some functional characteristics of IMA-2 may have been conserved with importin α proteins in other organisms. Drosophila melanogaster pendulin (an α2) and S. pombe Cut15p (an α1) exhibit a similar mitotic nuclear localization. Pendulin accumulates rapidly in the nucleus at the onset of prophase of early embryos (Kussel and Frasch, 1995a; Torok et al., 1995). The intensity of nuclear staining decreases through metaphase and anaphase and increases again at telophase. During decondensation of the chromatin as the cells enter interphase, pendulin redistributes into the cytoplasm. The S. pombe Cut15 polypeptide has a similar transient nuclear localization (Matsusaka et al., 1998). Cut15-GFP has strong NE localization throughout the cell cycle with intranuclear localization increasing through mitosis, reaching a maximum at prometaphase/metaphase. The intranuclear Cut15p then decreases through the remainder of mitosis. IMA-2 is distributed between the cytoplasm and the NE during interphase in the C. elegans embryo. The NE-associated protein disappears early in prophase followed by a rapid accumulation of IMA-2 in the region surrounding the mitotic chromosomes during prometaphase. Through metaphase and anaphase the localization of IMA-2 around the chromosomes decreases until telophase when IMA-2 again localizes strongly to the NE.
A mutant allele of cut15, cut15-85, and the reduction of IMA-2 expression both result in chromosome segregation defects (Matsusaka et al., 1998). The phenotype of cut15-85 cells is similar to the phenotypes of top2 and cut14 mutant cells, suggesting that Cut15p might interact with topoisomerase II or condensin inside the nucleus during mitosis. The cut15-85 allele is synthetically lethal with three alleles of cut3, a condensin subunit, supporting a direct role for Cut15p in chromosome condensation. Cut3p is localized to the nucleus in cut15-85 cells at the nonpermissive temperature, supporting the interpretation that Cut15p may interact with the condensin complex inside the nucleus. RNAi of the C. elegans Cut3 homolog F35G12.8 results in a “cross-eyed” phenotype in which the daughter nuclei stay close to the central cortex and multiple nuclei form in each daughter blastomere (Gonczy et al., 2000). This phenotype is associated with lagging chromosomal material that retains daughter nuclei together at the central cortex. A similar chromatin morphology is seen in some ima-2(RNAi) embryos. The single S. cerevisiae homolog Srp1p has been reported to have a role in regulation of the ubiquitin-proteasome system distinct from its role in nuclear protein import (Tabb et al., 2000). The srp1-31 allele arrests at the G2/M boundary and arrested cells are unable to degrade the cell cycle regulator Clb2p (Loeb et al., 1995). Depletion of Srp1p results in a terminal phenotype very similar to the terminal phenotype of IMA-2 depletion; abnormal appearance of mitotic chromatin, and dissociation of the chromosomes from the mitotic spindle (Kussel and Frasch, 1995b). Together, the mitotic localization of the yeast, nematode, and fly importin αs and the phenotypes associated with depletion or mutation of these proteins argue for a direct role for the importin αs in mitosis distinct from their protein transport functions. Our results presented herein show that a role for importin α proteins in mitotic chromosome behavior is conserved in animals and yeast.
Association of IMA-2 with Nuclear Mitotic Structures
The localization of IMA-2 around mitotic chromosomes in prometaphase and later suggests that IMA-2 may have a mitotic role distinct from its role as a nuclear transporter. Permeabilization of the NE in C. elegans embryos occurs late in prophase without significant loss of nucleoporins or lamin from the NE (Lee et al., 2000). Because IMA-2 is not detected around the chromosomes until prometaphase, it is likely that the protein is entering the nucleus by diffusion after early fenestration of the NE. The accumulation of IMA-2 around the chromosomes at metaphase cannot simply be due to constraining the protein within the partially disassembled NE. Lee et al. (2000) have reported that nucleoporins and lamins are present in the NE of early embryos until anaphase; however, in our hands, nucleoporins and lamin are undetectable in the NE by metaphase (Figures 2 and 3; our unpublished data). Alternatively, the rapid appearance of IMA-2 in the prometaphase nucleus could be the result of rapid accumulation of the protein during a transport event immediately before fenestration of the envelope, similar to the accumulation of cdc2/cyclin B1 (Hagting et al., 1998; Toyoshima et al., 1998; Yang et al., 1998). The sudden nuclear accumulation and gradual dissipation of the accumulated IMA-2 to the cytoplasm during metaphase and anaphase suggest that IMA-2 may associate with other factors important for mitosis that are “anchored” near the mitotic spindle. Recently, roles for importin β and importin α in regulating promoting spindle formation have been described previously (Gruss et al., 2001; Nachury et al., 2001; Wiese et al., 2001). A local high concentration of RanGTP near the mitotic chromosomes is believed to release the spindle-promoting factors from the importins (Kalab et al., 1999). However, we do not observe any defects in mitotic spindle formation when IMA-2 levels are reduced. If IMA-2 has a direct role in sequestering factors that regulate mitosis, this role must be distinct from a role in spindle formation. The localization of IMA-2 near the condensing chromosomes at the onset of mitosis suggests that the protein is important for an early mitotic event, possibly interacting with another factor or factors involved in chromosome condensation or kinetochore formation. The localization and timing of IMA-2 appearance around the mitotic chromosomes is in some ways similar to Skeletor, a protein that has been suggested to be a component of a spindle matrix (Walker et al., 2000). Whether IMA-2 is associated with a spindle matrix-type structure will be an area for active future investigation.
IMA-2 in Nuclear Envelope Formation
One of the most dramatic effects of the ima-2(RNAi) was the inability to reform correct NE structures after mitosis. Immunolocalization of nucleoporins and lamins demonstrated that both NE components are mislocalized in the ima-2(RNAi) embryos. This could be the result of the failure to transport the sole C. elegans lamin LMN-1 into the forming nucleus at telophase and into interphase. Because nuclear envelope assembly is a coordinated process to assemble a lamina, membrane, and pore complexes, failure to assemble an adequate lamina would also result in a failure to assemble pore complexes, resulting in their accumulation in the cytoplasm. A failure to import an adequate amount of LMN-1 to maintain a functional lamina could also result in a failure to complete replication or organize the nucleus correctly, leading to defects in chromosome condensation and segregation (Moir et al., 2000). Videomicroscopic analysis of ima-2(RNAi) embryo phenotypes identified a possible defect in NE formation in early embryos (Zipperlen et al., 2001). The NE defects observed could also be an indirect effect of the failure to transport other factors that are required upstream of nuclear envelope assembly. In support of a direct role for IMA-2 in LMN-1 transport, we identified a fragment of LMN-1 containing the putative NLS in a two-hybrid screen with IMA-2. In vitro binding assays indicate that both IMA-2 and IMA-3 are capable of directly binding the NLS of LMN-1 in solution (our unpublished data). lmn-1(RNAi) results in similar chromosomal and pore complex organizational defects as seen for ima-2(RNAi) (Liu et al., 2000).
The Ran GTPase network is required for NE assembly at the conclusion of mitosis (Clarke and Zhang, 2001). It has recently been demonstrated that importin β is required for NE assembly induced by Ran in Xenopus egg nuclear assembly assays (Zhang et al., 2002). Additionally, the Drosophila Ketel dominant negative mutations in the importin β gene block the formation of the NE in cleavage nuclei (Timinszky et al., 2002). Importin β provides a link between nuclear transport, mitotic spindle formation, and NE assembly all tied to regulation by the RanGTPase network. The importins are believed to act as chaperones to sequester spindle assembly factors or NE components until they can be released at the proper time by Ran-GTP (for review, see Moore, 2001). Because the importin αs are complexed to importin β through their IBB domain, they are a component of this general mechanism. A role for an importin α in sequestering the spindle assembly factor TPX2 in Xenopus has been described recently (Gruss et al., 2001). It is likely that IMA-2 is operating in mitosis through a similar mechanism. Because IMA-2 cannot be grouped into a phylogenetic clade with any of the other identified importin αs, it will be interesting to determine whether any of the importin αs from higher animals have retained the localization pattern and functions of IMA-2.
ACKNOWLEDGMENTS
We thank Drs. Elizabeth Goodwin and James Kramer for assistance and helpful discussions. We also thank Drs. Kathy Wilson and Yosef Gruenbaum for antiserum to Lmn-1. This work was supported by National Institutes of Health Grant GM-47866 (to S.A.A.) and a National Institutes of Health Carcinogenesis Training Grant and a U.S. Army Breast Cancer Training Grant (to K.G.G.).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0069. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0069.
REFERENCES
- Adam SA. Transport pathways of macromolecules between the nucleus and the cytoplasm. Curr Opin Cell Biol. 1999;11:402–406. doi: 10.1016/S0955-0674(99)80056-8. [DOI] [PubMed] [Google Scholar]
- Affolter M, Marty T, Vigano MA. Balancing import and export in development. Genes Dev. 1999;13:913–915. doi: 10.1101/gad.13.8.913. [DOI] [PubMed] [Google Scholar]
- Chook Y, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11:703–715. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Clarke PR, Zhang C. Ran GTPase: a master regulator of nuclear structure and function during the eukaryotic cell division cycle? Trends Cell Biol. 2001;11:366–371. doi: 10.1016/s0962-8924(01)02071-2. [DOI] [PubMed] [Google Scholar]
- Conti E, Izaurralde E. Nucleocytoplasmic transport enters the atomic age. Curr Opin Cell Biol. 2001;13:310–319. doi: 10.1016/s0955-0674(00)00213-1. [DOI] [PubMed] [Google Scholar]
- Conti E, Kuriyan J. Crystallographic analysis of the specific yet versatile recognition of distinct nuclear localization signals by karyopherin α. Structure Fold Des. 2000;8:329–338. doi: 10.1016/s0969-2126(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell. 1998;94:193–204. doi: 10.1016/s0092-8674(00)81419-1. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Kimble J. Confocal methods for Caenorhabditis elegans. Methods Mol Biol. 1999;122:141–151. doi: 10.1385/1-59259-722-x:141. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans [see comments] Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fontes MR, Teh T, Kobe B. Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-α. J Mol Biol. 2000;297:1183–1194. doi: 10.1006/jmbi.2000.3642. [DOI] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Geles KG, Adam SA. Development 128, 1817–1830. 2001. Germline and developmental roles of the nuclear transport factor importin α3 in C. elegans. [DOI] [PubMed] [Google Scholar]
- Gonczy P, et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Hagting A, Karlsson C, Clute P, Jackman M, Pines J. MPF localization is controlled by nuclear export. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa M, Mochii M, Ueno N, Kohara Y, Iino Y. Use of cDNA subtraction and RNA interference screens in combination reveals genes required for germ-line development in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2001;98:8686–8691. doi: 10.1073/pnas.141004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jans DA. Efficiency of importin alpha/beta-mediated nuclear localization sequence recognition and nuclear import. Differential role of ntf2. J Biol Chem. 1999;274:15820–15827. doi: 10.1074/jbc.274.22.15820. [DOI] [PubMed] [Google Scholar]
- Kalab P, Pu RT, Dasso M. The ran GTPase regulates mitotic spindle assembly. Curr Biol. 1999;9:481–484. doi: 10.1016/s0960-9822(99)80213-9. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Shim YH, Kirchner J, Kaminker J, Wood WB, Strome S. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–645. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- Koepp DM, Silver PA. Nucleocytoplasmic transport and cell proliferation. Biochim Biophys Acta. 1998;1377:M39–M47. doi: 10.1016/s0304-419x(97)00036-x. [DOI] [PubMed] [Google Scholar]
- Kohler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Gorlich D, Hartmann E. Evidence for distinct substrate specificities of importin α family members in nuclear protein import. Mol Cell Biol. 1999;19:7782–7791. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano A, Staber C, Ganetzky B. Nuclear mislocalization of enzymatically active RanGAP causes segregation distortion in Drosophila. Dev Cell. 2001;1:351–361. doi: 10.1016/s1534-5807(01)00042-9. [DOI] [PubMed] [Google Scholar]
- Kussel P, Frasch M. Pendulin, a Drosophila protein with cell cycle-dependent nuclear localization, is required for normal cell proliferation. J Cell Biol. 1995a;129:1491–1507. doi: 10.1083/jcb.129.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussel P, Frasch M. Yeast Srp1, a nuclear protein related to Drosophila and mouse pendulin, is required for normal migration, division, and integrity of nuclei during mitosis. Mol Gen Genet. 1995b;248:351–363. doi: 10.1007/BF02191602. [DOI] [PubMed] [Google Scholar]
- Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis [In Process Citation] Mol Biol Cell. 2000;11:3089–3099. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ben-Shahar TR, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes [In Process Citation] Mol Biol Cell. 2000;11:3937–3947. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JD, Schlenstedt G, Pellman D, Kornitzer D, Silver PA, Fink GR. The yeast nuclear import receptor is required for mitosis. Proc Natl Acad Sci USA. 1995;92:7647–7651. doi: 10.1073/pnas.92.17.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara IG. Transport into and out of the nucleus. Microbiol Mol Biol Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11:171–176. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Malik HS, Eickbush TH, Goldfarb DS. Evolutionary specialization of the nuclear targeting apparatus. Proc Natl Acad Sci USA. 1997;94:13738–13742. doi: 10.1073/pnas.94.25.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DA, Fleming RJ, Goldfarb DS. Drosophila melanogaster Importin alpha1 and α3 can replace importin α2 during spermatogenesis but not oogenesis. Genetics. 2002;161:157–170. doi: 10.1093/genetics/161.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathe E, Bates H, Huikeshoven H, Deak P, Glover DM, Cotterill S. Importin-α3 is required at multiple stages of Drosophila development and has a role in the completion of oogenesis. Dev Biol. 2000;223:307–322. doi: 10.1006/dbio.2000.9743. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Imamoto N, Yoneda Y, Yanagida M. Mutations in fission yeast Cut15, an importin α homolog, lead to mitotic progression without chromosome condensation. Curr Biol. 1998;8:1031–1034. doi: 10.1016/s0960-9822(07)00425-3. [DOI] [PubMed] [Google Scholar]
- Moir RD, Spann TP, Herrmann H, Goldman RD. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J Cell Biol. 2000;149:1179–1192. doi: 10.1083/jcb.149.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD. The Ran-GTPase and cell-cycle control. Bioessays. 2001;23:77–85. doi: 10.1002/1521-1878(200101)23:1<77::AID-BIES1010>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K. Importin β is a mitotic target of the small GTPase Ran in spindle assembly. Cell. 2001;104:95–106. doi: 10.1016/s0092-8674(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Nadler SG, Tritschler D, Haffar OK, Blake J, Bruce AG, Cleveland JS. Differential expression and sequence-specific interaction of karyopherin α with nuclear localization sequences. J Biol Chem. 1997;272:4310–4315. doi: 10.1074/jbc.272.7.4310. [DOI] [PubMed] [Google Scholar]
- Prieve MG, Guttridge KL, Munguia J, Waterman ML. Differential importin-α recognition and nuclear transport by nuclear localization signals within the high-mobility-group DNA binding domains of lymphoid enhancer factor 1 and T-cell factor 1. Mol Cell Biol. 1998;18:4819–4832. doi: 10.1128/mcb.18.8.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V, et al. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–616. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Rout MP, Blobel G, Aitchison JD. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Schedl T. Developmental genetics of the germ line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 241–269. [PubMed] [Google Scholar]
- Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol. 1998;8:1110–1116. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S. Generation of cell diversity during early embryogenesis in the nematode Caenorhabditis elegans. Int Rev Cytol. 1989;114:81–123. doi: 10.1016/s0074-7696(08)60859-1. [DOI] [PubMed] [Google Scholar]
- Tabb MM, Tongaonkar P, Vu L, Nomura M. Evidence for separable functions of srp1p, the yeast homolog of importin α (karyopherin α): role for srp1p and sts1p in protein degradation [In Process Citation] Mol Cell Biol. 2000;20:6062–6073. doi: 10.1128/mcb.20.16.6062-6073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timinszky G, Tirian L, Nagy FT, Toth G, Perczel A, Kiss-Laszlo Z, Boros I, Clarke PR, Szabad J. The importin-β P446L dominant-negative mutant protein loses RanGTP binding ability and blocks the formation of intact nuclear envelope. J Cell Sci. 2002;115:1675–1687. doi: 10.1242/jcs.115.8.1675. [DOI] [PubMed] [Google Scholar]
- Torok I, Strand D, Schmitt R, Tick G, Torok T, Kiss I, Mechler BM. The overgrown hematopoietic organs-31 tumor suppressor gene of Drosophila encodes an importin-like protein accumulating in the nucleus at the onset of mitosis. J Cell Biol. 1995;129:1473–1489. doi: 10.1083/jcb.129.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Wang D, Jin Y, Rath U, Wang Y, Johansen J, Johansen KM. Skeletor, a novel chromosomal protein that redistributes during mitosis provides evidence for the formation of a spindle matrix. J Cell Biol. 2000;151:1401–1412. doi: 10.1083/jcb.151.7.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y. Role of importin-β in coupling Ran to downstream targets in microtubule assembly. Science. 2001;291:653–656. doi: 10.1126/science.1057661. [DOI] [PubMed] [Google Scholar]
- Yang J, Bardes ES, Moore JD, Brennan J, Powers MA, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Clarke PR. Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science. 2000;288:1429–1432. doi: 10.1126/science.288.5470.1429. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hutchins JR, Muhlhausser P, Kutay U, Clarke PR. Role of importin-β in the control of nuclear envelope assembly by Ran. Curr Biol. 2002;12:498–502. doi: 10.1016/s0960-9822(02)00714-5. [DOI] [PubMed] [Google Scholar]
- Zipperlen P, Fraser AG, Kamath RS, Martinez-Campos M, Ahringer J. Roles for 147 embryonic lethal genes on C. elegans chromosome I identified by RNA interference and video microscopy. EMBO J. 2001;20:3984–3992. doi: 10.1093/emboj/20.15.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]