Abstract
NUT carcinoma is a rare and highly aggressive malignancy characterized by rapid progression, resistance to conventional therapies, and an extremely poor prognosis. This report presents a 36-year-old patient with stage IIIB primary pulmonary NUT carcinoma who achieved remarkable clinical outcomes with NHWD-870 monotherapy, a novel BET inhibitor. After just 1 month of treatment, imaging revealed a partial response, and a complete response was achieved within 5 months. Postoperative pathologic examination confirmed no residual cancer cells, and the patient has remained disease-free without recurrence or metastasis to date. To explore the underlying mechanisms of this therapeutic response, single-cell RNA sequencing was performed on the tumor tissue, revealing enhanced activity of immune cells, particularly effector CD8+ T-cells, within the tumor microenvironment. This suggests that NHWD-870 exerts its effects through both direct tumor suppression and modulation of the immune microenvironment. This case highlights the exceptional efficacy of BET inhibitors in the treatment of NUT carcinoma, as evidenced by the first report of complete response achieved with BET inhibitor monotherapy, and supports their potential as a personalized therapeutic strategy.
Keywords: Case report, NUT carcinoma, BET Inhibitor, Single-cell sequencing, Tumor microenvironment
Introduction
Nuclear protein in testis (NUT) carcinoma is a rare and highly aggressive malignancy characterized by nuclear protein in testis (NUT) midline carcinoma family member 1 (NUTM1) (15q14) gene rearrangement. It predominantly affects young individuals and typically involves the head, neck, and thoracic regions1 Owing to its early metastatic propensity and poor response to conventional chemoradiotherapy, the median survival is only 6.7 months. Bromodomain and extra-terminal domain (BET) inhibitors and hitone deacetylase inhibitors may offer promising therapeutic options.2
Case presentation
A 36-year-old female with no substantial medical history, no family history, and no history of smoking presented in December 2023 with symptoms of cough and dyspnea. Chest and abdominal computed tomography (CT) revealed a right middle lobe mass (maximum diameter of 5.12 cm) accompanied by obstructive atelectasis, pneumonia, and metastasis to the right hilar and mediastinal lymph nodes (the largest with a short axis of 3.91 cm), whereas brain magnetic resonance imaging and other examinations revealed no evidence of distant metastasis. Bronchoscopic biopsy confirmed a diagnosis of small cell carcinoma, with immunohistochemistry findings indicating CK(+), CK-L(+), NUT(+), P40(+), P63(+), TTF-1(-), Syn(-), CgA(-), CD56(-), RB1(+), Ki-67(+70%), P53(+20%),(K5/6(-), Vimentin(-), NKX2.2(-), C099(+), INSWI(-), INI-1(+), SMARCA4/BRGI(+), LCA(-), TdT(-), CD3(-), CD20(-), and CD34(-). The comprehensive diagnosis was right lung NUT carcinoma (cT4N2M0, stage IIIB). On January 5, 2024, treatment with the BET inhibitor NHWD-870 (2 mg daily for 5 days followed by 2 days off) was initiated (Fig. 1A–E). Follow-up enhanced chest CT on February 2, 2024 revealed a reduction in lesion size (Fig. 1F–J), and further imaging on May 30, 2024 revealed additional shrinkage. The overall therapeutic response was assessed as a complete response after 4 months, with the size of the pulmonary lesion and lymph nodes becoming nearly undetectable (Fig. 1K–O). On June 30, 2024, the patient underwent radical surgery for right lung cancer (Fig. 1P–T). Postoperative pathologic examination revealed no residual cancer cells in the tumor or lymph nodes (0/16) after targeted therapy. Immunohistochemistry revealed CD163 (+), CD20 (+), CD3 (+), NUT (-), and programmed death-ligand 1 expression at 0%, and as of January 12, 2025, imaging studies revealed no evidence of recurrence or metastasis. During the course of targeted therapy, no grade 3 or higher adverse events were observed. More comprehensive treatment details, a detailed chronological timeline, and a summary of the patient’s clinical course—including diagnostic procedures, imaging findings, surgical outcomes, and follow-up—are provided in Supplementary Material 1. The study protocol and the informed consent form were reviewed and approved by the Institutional Review Board (Ethics Approval No. 2025-KELUN-007) before implementation. Written informed consent was obtained from the patient for publication of this case report.
Figure 1.
Sequential radiological changes of thoracic lesions. (A–E) Baseline CT imaging illustrating initial lesions; (F–J) Follow-up imaging after 1 month of NHWD 870 treatment; (K–O) Follow-up imaging after 4 months of NHWD 870 treatment; (P–T) Postoperative imaging findings. CT, computed tomography.
Single-Cell Sequencing Reveals the Microenvironmental State of T-cell Activation
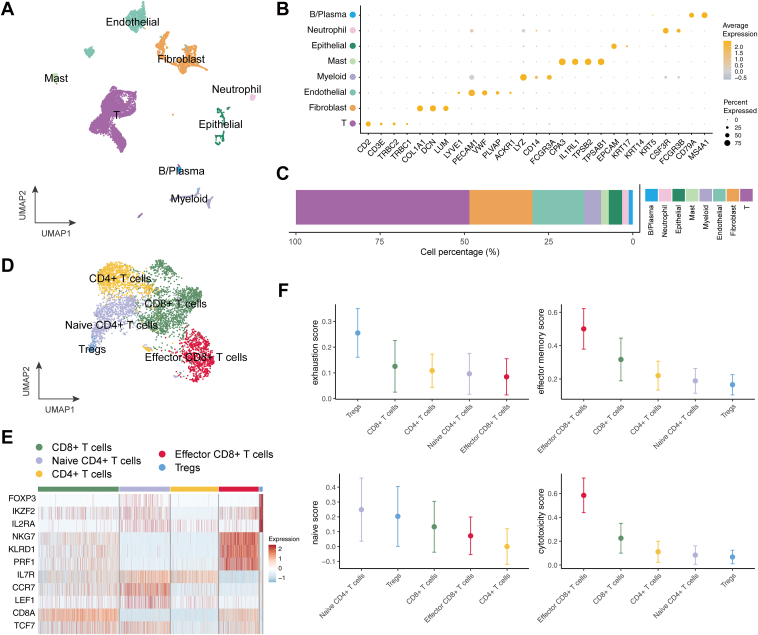
After BET inhibitor treatment, tumor tissue was obtained from the patient and analyzed using the SeekOne DD (Proteigene, Normandy, France) digital droplet platform for single-cell transcriptome sequencing. After stringent quality control, filtering, and normalization, eight distincT-cell subsets were identified on the basis of canonical gene markers (Fig. 2A and B): T-cells (n = 4479), fibroblasts (n = 1627), endothelial cells (n = 1326), myeloid cells (n = 444), masT-cells (n = 200), epithelial cells (n = 342), neutrophils (n= 173), and B and plasma cells (n = 102). Notably, epithelial cells represented only 4% of the population (Fig. 2C), supporting the effectiveness of the treatment. More importantly, over 50% of the cells in the microenvironment were T-cells (Fig. 2C), indicating an immune-enriched (immune-hot) tumor microenvironment.
Figure 2.
Tumor microenvironment of NUT carcinoma. (A) UMAP visualization of the major cell populations identified in the tumor microenvironment. (B) Dot plot displaying the expression of canonical marker genes for each major cell population. (C) Proportional distribution of each major cell population within the tumor microenvironment. (D) UMAP visualization of T and NK cells, highlighting distinct T-cell subsets. (E) Heatmap of selected T-cell-related gene expression, illustrating variation among T-cell subsets. (F) Functional scoring of T-cell subsets: median values are shown as points, with lines indicating median ± SD. NK, natural killer cells; Tregs, regulatory T-cells; UMAP, uniform manifold approximation and projection.
We further characterized the T-cell population by subdividing it into five subsets on the basis of classical marker genes (Fig. 2D and E): naive CD4+ T-cells (n = 991), CD4+ T-cells (n = 938), regulatory T-cells (n = 63), CD8+ T-cells (n = 1585), and effector CD8+ T-cells (n = 784). Among these, effector CD8+ T-cells exhibited particularly high expression of genes associated with cytotoxicity and activation, including NKG7, KLRD1, and PRF1, and accounted for 18% of the total T and natural killer cells.
To assess the functional state of these T-cell subsets, we scored cells on the basis of gene sets related to exhaustion (CXCL13, HAVCR2, PDCD1, TIGIT, LAG3, CTLA4, LAYN, RBPJ, VCAM1, TOX), effector memory (PRF1, IFNG, CCL4, HLA-DQA1, GZMK, GZMA, GZMH, CD44), naive state (CCR7, TCF7, LEF1, SELL), and cytotoxicity (PRF1, IFNG, GNLY, NKG7, GZMA, GZMH, KLRK1). Notably, regulatory T cells had the highest exhaustion scores, Naive CD4+ T-cells had the highest naive scores, and effector CD8+ T-cells had the highest effector memory and cytotoxicity scores (Fig. 2F). These findings suggest that BET inhibitor treatment may have promoted the emergence of highly active effector CD8+ T-cells, which likely played a critical role in tumor cell killing.
Furthermore, the fibroblast population was subdivided into two matrix cancer-associated fibroblasts (matCAF) clusters (matCAF_1 and matCAF_2) and one myofibroblastic CAF cluster on the basis of canonical marker gene expression, highlighting the cellular and functional heterogeneity of the tumor stroma (Fig. 3A and B). Cell-cell interaction analysis further indicated that matCAF_1 may contribute to the formation of an immune-hot tumor microenvironment by recruiting T-cells through the CXCL2–CXCR2 and CXCL12–CXCR4 signaling pathways (Fig. 3C–F). Together, these results indicate that NHWD-870 treatment not only enhances the functional activation of T-cells but may also facilitate their recruitment into the tumor microenvironment by means of CAF-mediated signaling.
Figure 3.
Subclustering of fibroblasts and analysis of cell-cell interactions with T-cells in the tumor microenvironment after NHWD-870 treatment. (A) UMAP plot illustrating T-cells and fibroblast subclusters. (B) Dot plot illustrating the expression of canonical marker genes defining fibroblast subgroups (matCAF_1, n = 959; matCAF_2, n = 85; myoCAF, n = 583). (C) Heatmap depicting the inferred interaction strength between T-cells and fibroblast subtypes by means of the CXCL signaling pathway. (D,E) Predicted ligand-receptor pairs indicating CXCL2-CXCR2 and CXCL12-CXCR4 signaling axes between matCAF_1 and T-cells. (F) Violin plots displaying the expression levels of key ligands (CXCL2, CXCL12) in fibroblasts and their corresponding receptors (CXCR2, CXCR4) in T-cells. CAF, cancer-associated fibroblasts; matCAF, matrix-producing CAF; myoCAF, myofibroblastic CAF; Tregs, regulatory T-cells; UMAP, uniform manifold approximation and projection.
Discussion
A major limitation of this report is the lack of next-generation sequencing data to identify the specific NUT fusion partner, and the lack of single-cell sequencing analysis before treatment. In addition, we did not perform single-cell T-cell receptor sequencing because of insufficient remaining tumor tissue for further library preparation. This prevents us from directly analyzing the clonality and tumor-reactivity of infiltrating T-cells. Future studies integrating both next-generation sequencing and single-cell T-cell receptor sequencing will be necessary to validate the functional expansion and specificity of these effector T-cell populations and to provide a more comprehensive molecular characterization of the tumor.
BET inhibitors exert their therapeutic effects by competitively blocking the interaction between BRD4 protein and chromatin, thereby modulating gene transcription and cell division. Given that various NUT carcinomas, including those with non-BRD4::NUTM1 fusions, are dependent on BRD4 for their oncogenic properties and are typically resistant to chemotherapy and immunotherapy, clinical trials have reported that BET inhibitors can achieve tumor control rates markedly surpassing conventional.1 NHWD-870, a novel small-molecule BET inhibitor,3 is currently under investigation in clinical trials (NCT06073938, NCT06527300). The mechanisms of action of BET inhibitors and a summary of phase I clinical trial progress are detailed in the Supplementary Material.
In this case, a patient with stage IIIB (T4N2M0) pulmonary NUT carcinoma achieved CR through NHWD-870 monotherapy, with no signs of recurrence 1 year after diagnosis. This remarkable outcome suggests that early administration of BET inhibitors may substantially improve patient prognosis, particularly given the generally poor response of NUT carcinoma to conventional chemotherapy and immunotherapy. Furthermore, single-cell analysis revealed the absence of cancer cells in both the primary tumor and its margins after NHWD-870 treatment, along with abundant infiltration of immune cells, including natural killer cells. In addition to this prominent immune activation, our cell-cell communication analysis suggested that specific fibroblast subsets, particularly matCAF_1, may actively contribute to the formation of an immune-enriched tumor microenvironment by recruiting T-cells through chemokine-mediated signaling pathways. This potential interplay between stromal fibroblasts and infiltrating T-cells implies that BET inhibitors may reshape not only tumor-intrinsic transcriptional programs but also the broader cellular ecosystem to favor effective antitumor immunity. These findings indicate that NHWD-870 not only directly inhibits cancer cell growth but also exerts antitumor effects by modulating the tumor immune microenvironment. This dual mechanism suggests the potential to transform NUT carcinoma from an immunologically "cold" to a "hot" tumor,4 implying that combining NHWD-870 with immunotherapy might achieve enhanced antitumor efficacy.5
Conclusion
This case report revealed the remarkable efficacy of NHWD-870, a novel BET inhibitor, in achieving pathological CR in a patient with stage IIIB pulmonary NUT carcinoma. The dual mechanism of NHWD-870, combining direct tumor suppression with immune microenvironment modulation, as revealed by single-cell sequencing, suggests potential advantages over traditional therapies. Although further clinical validation is needed, these findings support the early implementation of BET inhibitors in NUT carcinoma treatment and provide rationale for exploring combination strategies with immunotherapy, potentially transforming the therapeutic landscape for this aggressive malignancy.
CRediT Authorship Contribution Statement
Zhuomiao Ye: Conceptualization, Writing - original draft, Visualization, Writing - review & editing.
Xin Li: Data curation, Writing - original draft, Visualization, Funding acquisition, Formal analysis.
Minghui Zhang: Conceptualization, Writing - review & editing.
Fei Xie: Resources.
Xiangwen Luo: Visualization.
Chao Deng: Validation.
Dan Yang: Investigation.
Mingzhu Yin: Conceptualization, Supervision, Project administration.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by Chongqing Wanzhou Municipal Science and Health Joint Medical Research Project Key Program (Grant No. wzstc-kw2023001); Chongqing Wanzhou PhD “through train” Research Project (Grant No.Wzstc20230402); Chongqing Natural Science Foundation (Grant No. CSTB2023NSCQ-MSX0658); Chongqing Wanzhou PhD “through train” Research Project (Grant No. wzstc-20240006); and basic scientific research funds for central universities (Grant No. 2024IAIS-ZD001). The patient provided written informed consent for publication of this case report. The authors thank all the members of the NUT Carcinoma Department and the dedicated healthcare professionals who tirelessly support individuals affected by NUT carcinoma. The study protocol and the informed consent form were reviewed and approved by the Institutional Review Board (Ethics Approval No. 2025-KELUN-007) before implementation.
Footnotes
Drs. Ye and Li contributed equally to this work.
Cite this article as: Ye Z, Li X, Zhang M, et al. Complete response to BET inhibitor in primary pulmonary NUT carcinoma with single-cell sequencing-based analysis: a case report. JTO Clin Res Rep 2025;6:100885.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2025.100885.
Supplementary Data
References
- 1.Chau N.G., Ma C., Danga K., et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. 2019;4 doi: 10.1093/jncics/pkz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Z., LI X., Xie F., et al. Expert consensus and current landscape of NUT carcinoma: a comprehensive strategy from diagnosis to treatment tumor discovery tumor discovery. Tumor Discov. 2024;3:4904. [Google Scholar]
- 3.Shi M., He J., Weng T., et al. The binding mechanism of NHWD-870 to bromodomain-containing protein 4 based on molecular dynamics simulations and free energy calculation. Phys Chem Chem Phys. 2022;24:5125–5137. doi: 10.1039/d1cp05490b. [DOI] [PubMed] [Google Scholar]
- 4.Yin M., Guo Y., Hu R., et al. Potent BRD4 inhibitor suppresses cancer cell-macrophage interaction. Nat Commun. 2020;11:1833. doi: 10.1038/s41467-020-15290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu S., Wang Z., Li C., et al. The whole treatment process and thinking of a patient with NUT carcinoma of the parotid gland: a case report. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1094770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.