Abstract
Epithelial tight junctions (TJs) provide an important route for passive electrolyte transport across airway epithelium and provide a barrier to the migration of toxic materials from the lumen to the interstitium. The possibility that TJ function may be perturbed by airway inflammation originated from studies reporting (1) increased levels of the proinflammatory cytokines interleukin-8 (IL-8), tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), and IL-1β in airway epithelia and secretions from cystic fibrosis (CF) patients and (2) abnormal TJ strands of CF airways as revealed by freeze-fracture electron microscopy. We measured the effects of cytokine exposure of CF and non-CF well-differentiated primary human airway epithelial cells on TJ properties, including transepithelial resistance, paracellular permeability to hydrophilic solutes, and the TJ proteins occludin, claudin-1, claudin-4, junctional adhesion molecule, and ZO-1. We found that whereas IL-1β treatment led to alterations in TJ ion selectivity, combined treatment of TNF-α and IFN-γ induced profound effects on TJ barrier function, which could be blocked by inhibitors of protein kinase C. CF bronchi in vivo exhibited the same pattern of expression of TJ-associated proteins as cultures exposed in vitro to prolonged exposure to TNF-α and IFN-γ. These data indicate that the TJ of airway epithelia exposed to chronic inflammation may exhibit parallel changes in the barrier function to both solutes and ions.
INTRODUCTION
Tight junctions (TJs) are characteristically located at the apicolateral borders of adjacent epithelial cells and are responsible for the selective regulation of the passage of ions and neutral molecules through the paracellular space. In addition to their key role in maintaining barrier function of the epithelium, TJs have been implicated in the pathogenesis of several diseases. One group of disorders, inflammatory bowel disease, displays a highly deregulated barrier function that appears to be a fundamental event in disease pathogenesis (Schmitz et al., 1999a, 2000). Whether the alteration in TJ barrier function is a primary phenomenon associated with the disease or an acquired response caused by the high degree of inflammation associated with the disease is unclear. If, indeed, the TJ response is acquired, this would suggest that other diseases known to be associated with a high level of inflammation may display a characteristic change in TJ barrier function.
Cystic fibrosis (CF) serves as an excellent model for the study of the effects of inflammation on TJ barrier function. The airway disease of CF is characterized by an inflammatory response with a marked influx of neutrophils and chronic bacterial infection with Pseudomonas aeruginosa. Proinflammatory cytokines detected in CF airways serve in part to perpetuate this inflammatory response. High levels of the proinflammatory cytokines tumor necrosis factor α (TNF-α), interleukin (IL)-8, and IL-1β as well as the soluble intercellular adhesion molecule (ICAM)-1 have been measured in the airways of patients with CF (Salva et al., 1996; Bonfield et al., 1999; Osika et al., 1999). The airway epithelium of patients with CF also expresses an increased level of signal transducer and activator of transcription-1 (STAT-1), a component of the interferon γ (IFN-γ) signaling cascade, indicating that IFN-γ may also be increased in CF airways (Kelley and Elmer, 2000). In addition, increased levels of IFN-γ mRNA have also been detected in the airway epithelium of CF patients (Wojnarowski et al., 1999). Freeze-fracture electron microscopy (EM) has shown previously that the TJ strands of inflamed CF airways appear to be altered compared with those from non-CF patients (Carson et al., 1990).
The cytokines IL-1, IL-4, IL-10, IL-13, TNF-α, and IFN-γ have all been shown to regulate the TJ of both epithelia and endothelia (Youakim and Ahdieh, 1999; Ahdieh et al., 2001; Oshima et al., 2001). In addition, IL-1β has been shown to alter TJ permeability through an effect on the claudin family of transmembrane proteins thought to be important in maintaining junctional integrity in astrocytes (Duffy et al., 2000). However, these studies have not focused on the effects of chronic cytokine exposure on airway epithelial TJ function.
The TJ is a complex structure composed of several protein components, some of whose function(s) remain largely unclear. The claudin family of transmembrane proteins are thought to be key components of the TJ, but the growing number of members of this family impedes a complete understanding of their function. Several other components, such as occludin, junctional adhesion molecule (JAM), and more recently the coxsackievirus and adenovirus 2/5 receptor, have been localized to the TJ (Cohen et al., 2001). Occludin, the first transmembrane TJ protein identified, has been localized to the TJ strand with claudins. JAM has been identified as a component of the TJ necessary to allow the transmigration of monocytes through the paracellular space, which makes it a likely target for inflammation-induced alterations in TJ function. Which of these components is significant in maintaining the integrity of the airway TJ during inflammation is unknown.
The regulation of TJ function is incompletely understood and may vary among different cell types. Several protein kinase C (PKC) isoforms have been associated with changes in paracellular permeability. Previous studies have shown a correlation between the level of membrane-associated PKCα and the extent of paracellular permeability, and PKCβ has been shown to play a role in the regulation of endothelial TJs (Clarke et al., 2000). In addition, the atypical PKC isoforms PKCζ have been localized to the TJ of MDCK cells (Dodane and Kachar, 1996). Atypical PKC isotype-specific interacting protein may also localize to the TJ via interactions with PKCι/λ and PKCζ, forming a complex that is tethered to the junction by a direct interaction with JAM (Ebnet et al., 2001). Although PKC isoforms appear to play a functional role in the TJ, factors regulating their expression remain unclear, in particular, how these isoforms are affected by biological stimuli, including inflammation.
The difference in the morphology of CF airways as determined by freeze-fracture EM suggests that the environment of the CF airway might lead to disruption of the barrier function of the TJ. To determine whether the chronic inflammation of CF airways leads to modulation of airway TJs, we exposed CF and non-CF well-differentiated (WD) primary human airway epithelial (HAE) cells to cytokines that are upregulated in CF, including IL-1β, TNF-α, and IFN-γ. We then assessed the effects of this treatment on TJ barrier function and on components of the TJ. To correlate the effects of cytokines on primary HAE cells in vitro with the effects seen in vivo, we performed immunofluorescence localization of TJ components in freshly excised human CF and non-CF large airways.
MATERIALS AND METHODS
Chemicals and Antibodies
TNF-α and IFN-γ were purchased from Sigma Chemical Inc. (St. Louis, MO). Rabbit polyclonal antibodies to ZO-1, claudin-1, and occludin and mouse monoclonal antibodies (mAbs) to occludin, ZO-1, and ICAM-1 were purchased from Zymed Laboratories (San Francisco, CA). Rabbit polyclonal and goat polyclonal antibodies to ICAM-1 were purchased from Santa Cruz Biochemical (Santa Cruz, CA). The 3D8 mouse mAb to JAM was kindly provided by Dr. Kenji Ishii (Kyoto, Japan). Rabbit polyclonal antibodies to both PKCι/λ and PKCζ were purchased from Santa Cruz. The mouse anti-claudin-4 mAb was generously provided by James M. Anderson (Yale University, New Haven, CT). The FITC-conjugated dextrans were purchased from Sigma. The PKC inhibitor H7 and the tyrosine kinase inhibitor genistein were purchased from Sigma. Chelerythrine chloride was purchased from Alexis Corp. (San Diego, CA).
Cell Culture
Primary airway cells from human subjects were obtained in accordance with guidelines approved by the Committee on the Protection of the Rights of Human Subjects. Bronchial cells of normal (non-CF) and CF type were isolated from surgical specimens, plated at a density of 2 × 105 cells/12-mm Transwell-Col (0.4-μm pore size) insert, and maintained in a 50:50 mixture of LHC Basal Medium (Biofluids, Rockville, MD) and DMEM-H medium supplemented with growth factors, retinoic acid, and BSA as previously described (Yankaskas et al., 1985). After cultures reached confluence, medium was aspirated from the apical surface, and cells were maintained at an air-liquid interface for 4 wk. Cultures with 10% cilia as determined by microscopy and a transepithelial resistance (RT) of 600 Ω-cm2 measured with an ohmmeter (EVOM; World Precision Instruments, Sarasota, FL) were selected for experiments. Cultures were exposed to cytokines on the basolateral surface for 24, 48, or 72 h.
Transepithelial Cell Permeability
Permeation of FITC-dextrans of 10 and 2000 kDa across primary HAE cells was measured after 24, 48, or 72 h exposure to cytokines. Compounds were added to the apical surface (200 μL of a 5-mg/mL solution in HEPES-buffered Ringer's solution containing 1.3 mM CaCl2), and samples (10 μL) were removed from the apical and basolateral compartments at 10, 20, 30, 40, and 60 min. The rate of permeation was determined by measuring the sample fluorescence at 496 nm in a 96-well fluorescent plate reader. The paracellular permeability to hydrophilic solutes (Papp) coefficients were calculated as previously described (Stutts et al., 1981).
Electrophysiological Measurements of Dilution Potential
Polarized CF primary HAE cultures were treated with IL-1β for 72 h and mounted in modified Ussing chambers interfaced with an electrometer, in which transepithelial potential difference (VT) and RT were measured continuously under open-circuit conditions. The temperature of all solutions was maintained at 37°C, and pH was regulated by bubbling with 95% O2–5% CO2. Basal VT, RT, and current were recorded across cultures in a buffer containing high NaCl (in mM: 120 NaCl, 10 HEPES at pH 7.4, 10 NaHCO3, 1.2 CaCl2, 5 KCl, and 1 MgSO4) and 10−4 M amiloride. The change in VT (ΔVT) in response to luminal substitution with a buffer containing low NaCl (in mM: 60 NaCl, 120 mannitol, 10 HEPES at pH 7.4, 10 NaHCO3, 1.2 CaCl2, 5 KCl, and 1 MgSO4) and 10−4 M amiloride was subsequently recorded (Van Itallie et al., 2001). Blank filters were used to determine background and subtracted from subsequent measurements. Dilution potentials (ΔVT) and PCl−/PNa+ were calculated as previously described (Gorodeski et al., 1996). All measurements were performed in a minimum of six total cultures isolated from two patients.
Immunofluorescence Labeling and Confocal Microscopy
Cells were permeabilized with methanol at −20°C for 30 min. Antibodies to ZO-1, occludin, claudin-1, claudin-4, PKCι/λ, and JAM diluted to 1:1000 were added to the luminal surface for 1 h. Cells were washed with PBS, and Texas Red–labeled secondary antibodies (Amersham), diluted 1:600 in 10% goat serum/PBS, were added to the luminal surface. For occludin and claudin-1 double labeling, a mouse mAb to occludin conjugated to FITC was incubated with rabbit polyclonal antibody to claudin-1 and incubated for 1 h at room temperature. For JAM and ZO-1 double labeling, 3D8 mouse anti-JAM and rabbit anti-ZO-1 were added to the culture. After washing, anti-rabbit Texas Red and antimouse FITC were incubated in 10% goat serum/PBS for 1 h at room temperature. Cells were postfixed with 4% paraformaldehyde. Transwell-Col inserts were excised and mounted on slides with 100 μL Vectashield (Vector Laboratories, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole (DAPI). Images were captured with a confocal laser-scanning microscope (Leica, Exton, PA).
Freshly excised human bronchi from non-CF and CF (ΔF/ΔF) subjects were embedded in OCT, and frozen sections (10 μm) were cut. Sections were incubated in 95% ethanol followed by a brief wash in PBS. After incubation in acetone, sections were washed in 0.1% Triton X-100 in PBS, and then primary and secondary antibodies added as described above.
Western Blotting
Lysates of primary HAE cells were prepared with 0.1% Triton X-100 extraction buffer containing phenylmethanesulfonyl fluoride (PMSF) and dithiothreitol (DTT). Equal amounts of protein (30 μg) were loaded onto Tris-glycine gels (Novex, San Diego, CA). After electrophoresis for 1 h at 200 V, protein was transferred to nitrocellulose or polyvinylidine difluoride (PVDF) membrane at 33 V and blocked in 5% fat-free milk. Membranes were probed at antibody dilutions of 1:1000 in Tris-buffered saline–Tween-20 (TBS-T). Protein was visualized with a peroxidase-conjugated secondary antibody (1:20,000) by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) or Supersignal west femto maximum sensitivity substrate for JAM blotting (Pierce, Rockford, IL). For immunoprecipitation of PKCι/λ and ζ and JAM, lysates were incubated with rabbit polyclonal antibodies to PKCι/λ or ζ or mouse mAb to JAM for 2 h (PKC) or overnight (JAM) at 4°C, and Sepharose G beads were added for an additional 1 h. After centrifugation, beads were washed in cell lysis buffer and then heated at 95°C for 15 min. Loading buffer was added and Western blotting performed as described above. Blots were stripped with Restore Western blot stripping buffer according to the manufacturer's protocol (Pierce).
Human bronchi from non-CF and CF (ΔF/ΔF) lungs were dissected immediately after removal from patients undergoing clinical lung transplantation (freshly excised) and placed in ice-cold PBS, and epithelium was removed with a scalpel. Protein was isolated in 7 M urea buffer containing SDS and β-mercaptoethanol and sonicated for 30 s. Protein concentration was determined with the RC/DC Protein Assay (Bio-Rad, Hercules, CA), 50 μg total protein run on Tris-glycine gels and transferred to PVDF membrane at 33 V for 2 h. Western blotting was performed as described above. For Western blotting of JAM, membranes were incubated with 3D8 antibody overnight at 4°C. Occludin Western blotting was performed with the rabbit polyclonal antibody as described above. Densitometry was performed with Scion Image for Windows (Scion, Frederick, MD) to determine average band intensity and percent difference between non-CF and CF. The intensity of lanes containing no bands was set to the background level.
RT-PCR and Quantitative RT-PCR
Total RNA was isolated with Qiagen RNeasy Protect according to the manufacturer's protocol (Qiagen, Valencia, CA). RNA was then treated with DNase (Ambion, Austin, TX). For complementary DNA synthesis, 1 μg total RNA was used in a 20-μL reaction containing 1 mM deoxynucleotide triphosphates (dNTPs), 2.5 mM random hexamers or oligo dT, 1000 U/ml RNase inhibitor, 0.1 volume 10X buffer (supplied by manufacturer), and 2500 U/ml murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). The reverse transcription (RT) reaction was carried out at 1 cycle in a thermal cycler at 42°C for 50 min, followed by 15 min incubation at 70°C. For competitive quantitative RT-PCR (cQRT-PCR), an internal standard was generated containing a 60-nucleotide deletion that would be recognized by the same primers as the target sequence (Ho et al., 1989). RNA for this internal standard was generated by ligation of a T7 promoter followed by in vitro transcription. Before RT, the standard was added at a concentration range of 0.0001 to 1 ng, and cDNA was generated. For semiquantitative RT-PCR (QRT-PCR), primers to the gene of interest and those to GAPDH were added simultaneously to the PCR reaction. PCR was carried out with Taq DNA polymerase for 25 cycles. PCR products were separated on a 1.5% agarose gel containing ethidium bromide. The relative band intensities were then determined with Scion Image, and the amount of JAM mRNA expression was calculated as previously described (Kaufmann et al., 1999). The amount of standard added to the reaction (in ng) versus the ratio of standard (ΔJAM) to transcript was plotted on a double logarithmic plot. The amount of transcript was then determined by measuring the point on the plot at which the y-intercept equals 1, indicating an equal ratio of standard to transcript, and then calculating the x-intercept.
Enzyme-linked Immunosorbent Assay
Primary HAE cells were treated with cytokines for 24, 48, or 72 h, and total protein was isolated as described above. For ICAM-1, a 96-well assay plate (Costar) was coated overnight with mouse monoclonal ICAM-1, then lysate was added at 1, 3, and 10 μg and incubated for 2 h. After washing, rabbit polyclonal anti-ICAM-1 was added for 2 h, followed by horseradish peroxidase (HRP)-labeled secondary antibody. For JAM, plates were coated overnight with 1, 3, 10, or 30 μg lysate and incubated with mouse monoclonal anti-JAM (3D8) for 2 h and visualized with antimouse HRP. Protein was detected by addition of a 1:1 ratio of H2O2:tetramethylbenzidine. Optical density was determined at 450 nm. Data are expressed as percent change in JAM or ICAM-1 relative to vehicle controls.
Freeze-Fracture EM
Cultures treated with vehicle or cytokines were fixed in 2% glutaraldehyde/2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) at 4°C overnight. The epithelium was gently removed from the Transwell-Col with a scalpel and rinsed in phosphate buffer containing 0.2 M sucrose at room temperature, followed by a 25% glycerol cryoprotectant solution. The epithelium was sandwiched between gold double-replica mounts and frozen in liquid nitrogen–cooled Freon. Specimens were fractured in a Balzers BAF 400T freeze-fracture plant at a stage temperature of −100°C, and replicas were made at a 30o angle with platinum/carbon shadowing. The replicas and adherent tissue were removed by placing in distilled water, followed by transferring to a solution of 5% sodium dichromate in 50% sulfuric acid for cleaning. Replicas were then moved again to distilled water, where they were retrieved and placed onto standard copper microscopy grids. The replicas were examined, and fields exhibiting TJs at a plate magnification of 20,000× were photographed with a Zeiss EM-10A at an accelerating voltage of 60 kV. Morphometric analysis of TJ strands was performed as previously described (Carson et al., 1988). Photomicrographs were enlarged to a final magnification of 60,000×, and TJs were transected by lines perpendicular to the luminal border with adjacent transects no closer than 1 μm apart. Strand depth was calculated at the transected lines by measuring the distance from the most luminal strand to the most distal strand.
Statistics
Data are presented as mean ± SEM. A one-way analysis of variance (ANOVA) and Bonferroni's correction for multiple comparisons were used to determine statistical significance (p < 0.05).
RESULTS
Effect of Cytokine Treatment on RT and Papp
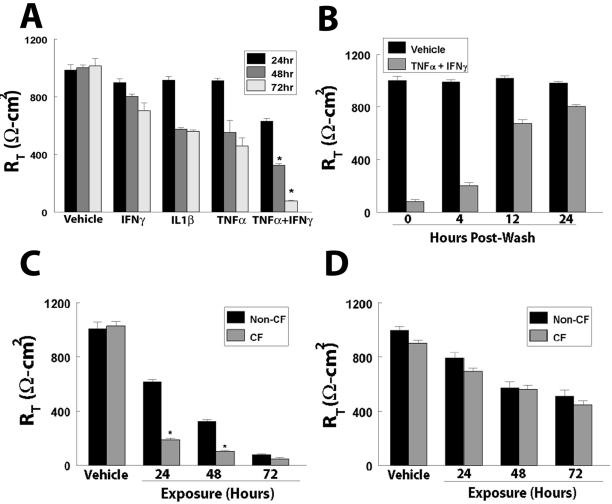
WD primary HAE cells of non-CF and CF types were exposed to cytokines involved in CF inflammation, IL-1β, IFN-γ, and TNF-α. To determine whether treatment with either cytokine could elicit effects on airway TJ permeability, we exposed the basolateral surface of HAE cells to IL-1β (100 ng/ml), TNF-α (10 ng/ml), or IFN-γ (100 ng/ml) alone or in combination and determined the effects on RT and Papp at 24, 48, and 72 h. These concentrations were chosen because they are similar to levels detected in the airways of CF patients (Osika et al., 1999). After 24 h exposure of non-CF WD HAE to IL-1β, TNF-α, or IFN-γ alone, there were no significant effects on Rt. However, in cultures exposed to combined treatment of TNF-α and IFN-γ, RT at 24 h decreased to 65% of control (vehicle-treated) cultures (Figure 1A). After 48 h exposure, cultures exposed to IFN-γ alone exhibited RT values that were 80% of vehicle cultures, whereas RT in those exposed to TNF-α or IL-1β alone decreased to 60%. The RT of HAE cells treated with a combination of TNF-α and IFN-γ decreased to 30% of control cultures (Figure 1A). The effect of cytokines on RT was even more dramatic after 72 h exposure, with RT of cultures treated with TNF-α and IFN-γ in combination falling to 8% of control cultures (Figure 1A), whereas exposure to IL-1β, TNF-α, or IFN-γ alone decreased RT to ∼60% of vehicle controls. Exposure of cultures with TNF-α, IFN-γ, and IL-1β simultaneously did not alter the kinetics or magnitude of either RT or Papp (data not shown). Upon removal of cytokines at 72 h, RT returned to control levels by 24 h posttreatment (Figure 1B).
Figure 1.
Effect of cytokines on RT. (A) RT of non-CF WD primary HAE cells exposed to TNF-α, IFN-γ, or IL-1β alone, or TNF-α and IFN-γ in combination. (B) Recovery of RT after removal of TNF-α and IFN-γ at 4, 12, and 24 h after washing. (C) Effect of combined TNF-α and IFN-γ treatment on RT in non-CF and CF primary HAE cells. (D) Effect of IL-1β on RT in non-CF and CF primary HAE cells. *Significantly different from (A) vehicle and TNF-α, IFN-γ, or IL-1β alone or from (C) non-CF controls, at p < 0.001. Data presented are a minimum of n = 12 cultures from at least three patients.
CF cultures displayed a different kinetic profile of cytokine-induced changes in RT than did non-CF cultures. Whereas the RT of non-CF cultures was only modestly affected by cytokine treatment at 24 h (61% of vehicle), the RT of CF cultures exposed to combined TNF-α and IFN-γ treatment was reduced to 18% of vehicle-treated controls (Figure 1C). This trend continued after 48 h exposure, with RT in non-CF cultures reduced to 32%, compared with 10% of vehicle controls in CF cultures. However, by 72 h exposure, the RT in both non-CF and CF cultures was reduced to 5–10% of vehicle controls (Figure 1C). In contrast, there was no significant difference between non-CF and CF cultures after exposure to IL-1β at any time point (Figure 1D).
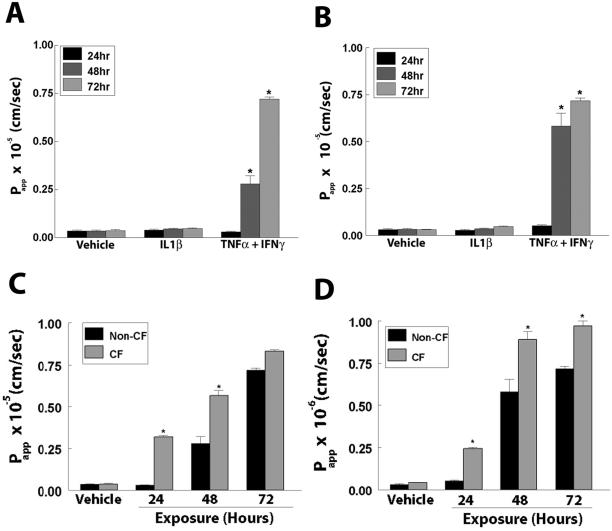
To correlate the changes in RT induced after cytokine exposure to alterations in the barrier function of the TJ, the permeability coefficients of cultures to both a small solute, a 10-kDa FITC-labeled dextran, and a larger solute, 2000-kDa FITC-labeled dextran, were measured after cytokine exposure. In non-CF cultures, there was no difference in the Papp to the 10- and 2000-kDa dextrans by 24 h (Figure 2, A and B) in cultures treated with TNF-α and IFN-γ in combination. However, pronounced increases in Papp after 48 and 72 h exposure were detected. The Papp to the 10-kDa dextran increased by 8-fold and that to the 2000-kDa 19-fold when treated with TNF-α and IFN-γ in combination for 48 h. By 72 h after treatment, Papp to the 10-kDa dextran was increased 17-fold and that to the 2000-kDa dextran 25-fold (Figure 2, A and B, respectively). There were no differences in the Papp between cultures treated with vehicle and those treated with IL-1β alone at any time point (Figure 2, A and B). There was also no difference in cultures exposed to TNF-α or IFN-γ alone (data not shown). To provide evidence that cytokines were increasing Papp via passive mechanisms typical of the paracellular route, the permeability to solutes from the basolateral to apical compartments were measured and were equal to those from the apical to basolateral compartments (data not shown).
Figure 2.
Effect of cytokines on Papp. Papp to (A) 10-kDa or (B) 2000-kDa FITC-labeled dextran in WD primary HAE exposed to IL-1β alone or TNF-α and IFN-γ in combination for 24, 48, or 72 h. (C, D) Comparison of the effects of TNF-α and IFN-γ combined treatment on Papp in non-CF vs. CF WD primary HAE cells to (C) 10-kDa or (D) 2000-kDa FITC-labeled dextran. *Significantly different from vehicle (A, B) or from non-CF (C, D) controls at p < 0.05. Data presented are a minimum of n = 12 cultures from at least three patients.
The kinetics of the changes in Papp of CF cultures treated with TNF-α and IFN-γ in combination were more significant than those of non-CF cultures. After 24 h exposure, Papp of CF cultures was 10-fold greater than both vehicle-control and non-CF cultures. At 48 h, the Papps of CF cultures were ∼twofold greater than those of non-CF cultures. However, like RT, Papp after 72 h exposure was equal between non-CF and CF cultures (Figure 2C). In addition, Papp to the 2000-kDa dextran was also significantly increased in CF versus non-CF cultures (Figure 2D). Although there was no increase in Papp in non-CF cultures after 24 h, there was a fivefold increase in CF cultures. In addition, at 48 and 72 h, there is an ∼1.5-fold greater increase in Papp of CF cultures than non-CF (Figure 2D).
IL-1β Effects on Na+ and Cl− Permeability
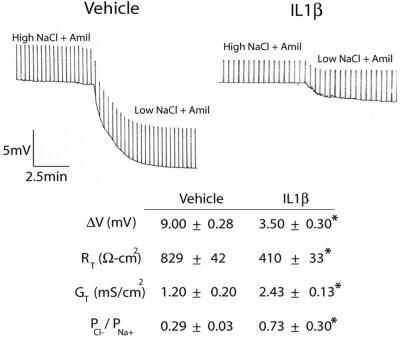
Because treatment of primary HAE cells with IL-1β did not induce alterations in Papp to dextrans but did lead to a modest decrease in RT, we evaluated more subtle effects on TJ function by measuring the relative ion selectivity of the paracellular path in CF cultures at 72 h after IL-1β exposure. The effect of NaCl ion substitution on the transepithelial dilution potentials were determined and used to calculate the ratio of the relative permeabilities of Cl− to Na+ (PCl−/PNa+). To exclude ion permeation via the transcellular pathway, experiments were performed in CF cultures that do not express the CF transmembrane conductance regulator (CFTR) channel on the apical membrane, thereby excluding Cl− permeation via the transcellular route. To block Na+ transcellular permeation, all luminal solutions contained amiloride (10−4 M).
Cultures treated with IL-1β relative to vehicle exhibited a decrease in ΔVT in response to dilute NaCl solutions from 9.00 ± 0.28 mV (vehicle) to 3.50 ± 0.30 mV (IL-1β), indicating an effect on ion permeation (Figure 3). This reduction in ΔVT correlated with an increase in PCl−/PNa+ in cultures exposed to IL-1β for 72 h, from 0.29 ± 0.03 to 0.73 ± 0.30 (Figure 3). The twofold increase in PCl−/PNa+ in IL-1β–treated cultures also correlated with a ∼twofold increase in conductance (GT), an increase from 1.20 ± 0.20 mS/cm2 (vehicle) to 2.43 ± 0.13 mS/cm2 (IL-1β). Because IL-1β led to increased GT and a hyperpolarization of VT while exhibiting a net increase in PCl−/PNa+, it can be concluded that IL-1β leads to a selective increase in paracellular permeability to Cl−.
Figure 3.
Effect of IL-1β on relative ion selectivity. Polarized CF WD primary HAE cells were exposed to IL-1β for 72 h. Electrophysiological measurements and dilution potential (ΔVT) were measured and permeability ratio of Cl−/Na+ (PCl−/PNa+) calculated as described in MATERIALS AND METHODS. Shown are the transepithelial voltage recordings with intermittent current pulses in amiloride (Amil)-treated cultures before and after luminal substitution with a low-NaCl solution. Current pulses and voltage scales are the same. Summary data are presented in the table. Data presented are representative of recordings from 12 cultures from 2 patients. *Significantly different from vehicle-treated controls.
Cytokines and TJ Morphology
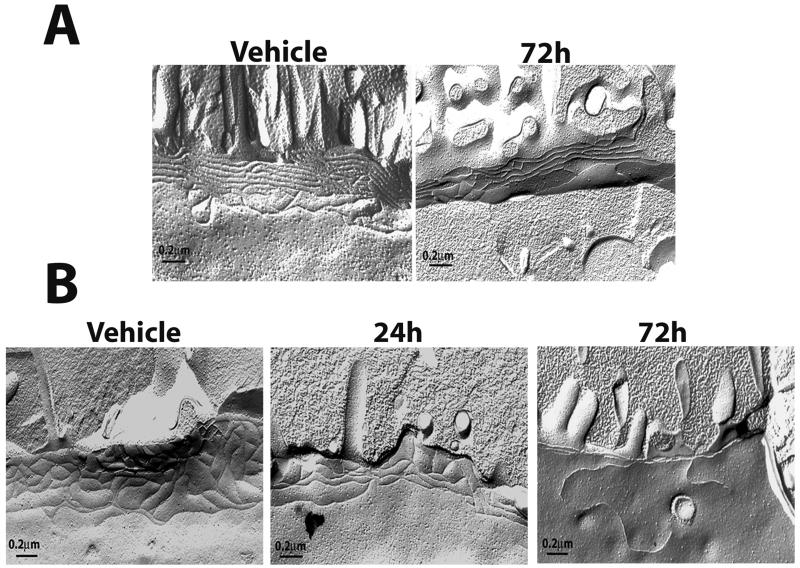
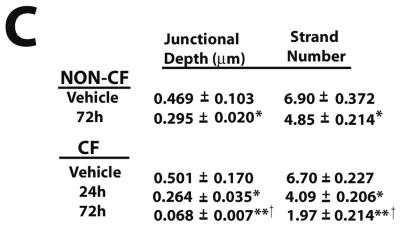
To determine whether the effects of TNF-α and IFN-γ cotreatment on the distribution of TJ proteins also affected the morphology of TJ strands, we performed freeze-fracture EM and quantified the effect of cytokine treatment on junctional depth and strand number. Freeze fracture splits the cell membrane along a hydrophobic core, thereby allowing an en face view of the strands of the TJ. Electron micrographs of freeze-fracture replicas from non-CF cultures treated with TNF-α and IFN-γ for 72 h revealed that treatment decreased overall junctional depth and number of strands (Figure 4, A and C). In cultures exposed to cytokines, junctional depth was measured at 0.295 ± 0.020 μm, whereas that of vehicle controls was 0.469 ± 0.103 μm as determined by morphometric analysis (Figure 4C). Strand number was decreased from 6.90 ± 0.372 in vehicle controls to 4.85 ± 0.214 in cultures exposed to TNF-α and IFN-γ.
Figure 4.
Morphology of TJs of primary HAE cells exposed to TNF-α and IFN-γ. Freeze-fracture analysis of TJ strands of WD non-CF (A) or CF (B) HAE cells exposed to vehicle or cytokines for the indicated times. (C) Quantification of strand number and depth. Micrographs are representative of 40 μm (vehicle non-CF), 20 μm (vehicle CF), 70 μm (non-CF 72 h), 60 μm (CF 24 h), and 50 μm (CF 72 h) of TJ analyzed. Significantly different from vehicle controls (*), vehicle controls and 24 h exposure (**), and from non-CF 72 h (†) at p < 0.05.
Unlike non-CF cultures, which were unaffected by cytokines at 24 h, the effect of TNF-α and IFN-γ on RT of CF cultures was pronounced by 24 h after treatment (Figure 1C). For this reason, freeze-fracture analysis was also performed on CF cultures at 24 h. After exposure for 24 h, CF cultures displayed a significant change in both depth and strand number, with changes similar to those seen in non-CF cultures after 72 h cytokine exposure (Figure 4B). By 72 h, the number of strands had decreased to 1.97 ± 0.214 and junctional depth to 0.068 ± 0.007 μm, compared with a strand number of 6.7 ± 0.227 and a depth of 0.501 ± 0.170 μm in vehicle-controls. Although the RT and Papp of CF cultures at 72 h after cytokine exposure do not differ significantly from non-CF, TJ morphology differs significantly, suggesting an increased sensitivity of CF cultures to TNF-α and IFN-γ.
Cytokine Effects on the Integrity of TJ-Associated Proteins
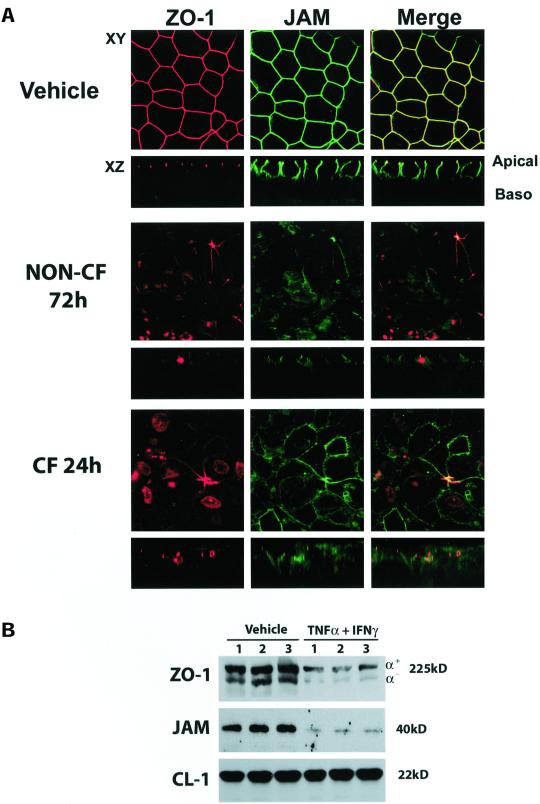
To determine whether exposure to TNF-α and IFN-γ disrupted the organization of TJ-associated proteins, we performed immunofluorescence localization of ZO-1, JAM, claudin-1, claudin-4, and occludin. When double-immunofluorescence localization was performed for the cytoplasmic protein ZO-1 and the integral membrane protein JAM, we saw redistribution of both proteins at 48 h and 72 h (Figure 5A), with less obvious alterations at 24 h (data not shown). The apparent disruption was detected only in cultures treated with the combination of TNF-α and IFN-γ and not in cultures treated with IL-1β, TNF-α, or IFN-γ alone (data not shown). CF cultures at 24 h exposure to TNF-α and IFN-γ exhibited a similar pattern of reorganization of ZO-1 and JAM as non-CF cultures at 72 h (Figure 5A). CF cultures exposed to cytokines for 72 h displayed a similar relocalization of ZO-1 and JAM as non-CF at 72 h (data not shown). The apparent change in localization of ZO-1 and JAM shown by immunofluorescence was also associated with a decrease in ZO-1 and JAM expression by Western blot analysis of non-CF cultures exposed to TNF-α and IFN-γ for 72 h (Figure 5B).
Figure 5.
Effect of TNF-α and IFN-γ treatment on expression and localization of JAM and ZO-1. (A) Immunofluorescent staining for JAM and ZO-1 in non-CF and CF primary HAE cells exposed to TNF-α and IFN-γ for 72 or 24 h. Red staining (left) represents ZO-1, green staining (middle) is JAM, and (right) merged image of both ZO-1 and JAM. Areas of colocalization appear as yellow. Images are representative of a minimum of n = 6 cultures from two patients. (B) Western blotting of ZO-1 (top) and immunoprecipitation of JAM (middle) in non-CF cultures. Western blot for ZO-1 was stripped and reprobed with an antibody specific for claudin-1 to control for loading (bottom).
However, immunofluorescence localization of claudin-1 showed no apparent change in either the intensity or distribution of fluorescent staining for either protein (Figure 6A). Occludin exists in both a high-molecular-weight (HMW) and low-molecular-weight (LMW) form. Although immunofluorescence did not reveal a change in the expression or localization of occludin, the antibody used recognized only the LMW form of the protein (Figure 6A). To reconcile whether there were TNF-α and IFN-γ–induced effects on either occludin expression or phosphorylation, we performed Western blots with two antibodies, one recognizing only the LMW and one recognizing both forms. Samples isolated from cultures exposed to TNF-α and IFN-γ for 72 h and probed with an antibody recognizing both the LMW and HMW forms appeared to express more of the 73-kDa occludin than vehicle-controls (Figure 6B, lanes 4–6). However, when the membrane was reprobed with an antibody that recognizes only the LMW form of the protein, there was no change in the 65-kDa occludin band after treatment (Figure 6C). This finding would indicate that the relative increase seen correlates with a shift from the LMW form to the HMW, indicating an increase in the phosphorylation state of the protein. Although there appeared to be effects on occludin levels, claudin-1 expression remained unchanged (Figure 6C). The distribution and expression of claudin-4, the other claudin species identified in our culture system, was also unaffected by TNF-α and IFN-γ treatment (Figure 6, B and C). The finding with regard to claudin-4 was confirmed by comparison of relative fluorescence intensity of immunofluorescence images and by densitometry of Western blots (data not shown).
Figure 6.
TNF-α and IFN-γ effect on occludin, claudin-1, and claudin-4 distribution and expression. (A) Immunofluorescent staining for occludin and claudin-1 in primary HAE cells exposed to TNF-α and IFN-γ for 72 h. Occludin staining in green (middle), claudin-1 in red (left), and merged image (right). Colocalization appears as yellow. (B) Immunofluorescence staining of claudin-4 in vehicle-treated cultures and in cultures exposed to TNF-α and IFN-γ for 72 h. (C) Western blot analysis of occludin, claudin-1, and claudin-4. Top, Probed with an antibody recognizing both the HMW and LMW forms of occludin. This blot was then stripped and reprobed with an antibody recognizing only the LMW form of the protein. Claudin-1 and claudin-4 expression are below. Lanes 1–3, vehicle; 4–6, exposed to TNF-α and IFN-γ for 72 h. Images are representative of a minimum of n = 6 cultures from two patients.
Cytokine Exposure and JAM and ICAM-1 Expression
Redistribution of JAM by cytokines (Figure 5A) would be expected to decrease monocyte transmigration across the airway, whereas increased ICAM-1 expression would be required for neutrophil transmigration (Ozaki et al., 1999; Kidney and Proud, 2000). Because neutrophils are the predominant inflammatory cell in the CF airway, we investigated whether treatment of primary non-CF HAE cells with TNF-α and IFN-γ altered the levels of expression of JAM and ICAM-1. After exposure of cultures for 24, 48, or 72 h with TNF-α and IFN-γ, the level of JAM and ICAM-1 mRNA was determined by cQRT-PCR or semiquantitative RT-PCR (QRT-PCR), respectively, and compared with the expression in untreated cultures. Two methods of mRNA quantification were chosen because more subtle changes were expected in JAM expression level. The level of JAM mRNA decreased to 58 ± 12% of vehicle controls after 72 h cytokine exposure as determined by cQRT-PCR (Figure 7A). Similar results were found after QRT-PCR (data not shown). In contrast, ICAM-1 mRNA expression increased with increasing duration of exposure to cytokines (Figure 7B). By 48 and 72 h exposure, the level of ICAM-1 mRNA was significantly greater in cytokine-treated than in control cultures. No ICAM-1 was detectable in vehicle-treated HAE cultures, consistent with previously published data (Striz et al., 1999). After 24 h exposure to cytokines, there was a significant increase in ICAM-1 mRNA expression, which increased further to 214 ± 14.5% of the 24-h level by 48 h and 242 ± 8.5% at 72 h.
Figure 7.
Coordinate regulation of JAM and ICAM-1 in response to TNF-α and IFN-γ exposure. (A) JAM mRNA expression as determined by cQRT-PCR with an internal standard (ΔJAM) at the indicated concentration (1 to 0.0001 ng). Left, Vehicle, and right, cultures exposed to TNF-α and IFN-γ for 72 h. (B) Semiquantitative RT-PCR (QRT-PCR) analysis of ICAM-1 mRNA expression normalized to GAPDH. Top, ICAM-1 expression in vehicle or at 24, 48, or 72 h after exposure to TNF-α and IFN-γ. Bottom, GAPDH expression. (C, D) JAM (C) and ICAM-1 (D) protein expression determined by ELISA at 24, 48, and 72 h after exposure to TNF-α and IFN-γ. Data are from a minimum of n = 6 cultures from at least three patients.
To correlate the changes in mRNA expression after cytokine exposure with protein levels, we performed an enzyme-linked immunosorbent assay (ELISA) on vehicle and cytokine-treated cultures for 24, 48, or 72 h (Figure 7). The level of JAM expression decreased to 80 ± 10%, 70 ± 10%, and 40 ± 4% of control cultures at 24, 48, and 72 h, respectively. In contrast, ICAM-1 expression increased to 141 ± 9%, 227 ± 7%, and 356 ± 22% of controls at 24, 48, and 72 h, respectively. Thus, TNF-α and IFN-γ downregulate JAM expression while upregulating ICAM-1 expression.
Mechanism of Cytokine-induced Alterations in Permeability
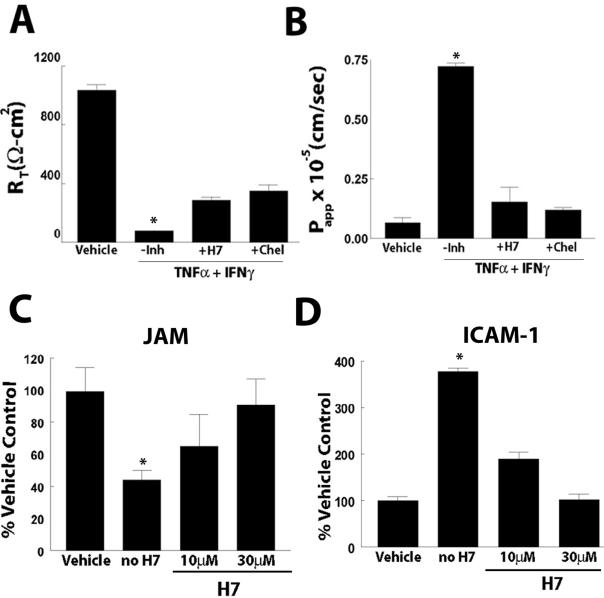
Cytokine-induced changes in TJ permeability have previously been linked to alterations in tyrosine kinases and PKC (Ratcliffe et al., 1999; Schmitz et al., 1999b). We examined the ability of a tyrosine kinase inhibitor, genistein, and the nonspecific PKC inhibitor H7 to block the effect of TNF-α and IFN-γ on RT and Papp to small and large solutes, respectively. Cultures were pretreated bilaterally with the inhibitor at concentrations ranging from 1 to 300 μM for 1 h. After this initial incubation, TNF-α and IFN-γ in the presence of the inhibitor were incubated for 72 h, the time point at which the greatest effect of cytokine was observed (Figure 1). Genistein did not inhibit the effect of cytokines on RT or Papp at any concentration (data not shown), whereas H7 (10 and 30 μM) significantly inhibited the effect on both RT and Papp. At 72 h, H7 (10 μM) displayed partial inhibition of the TNF-α and IFN-γ–induced changes in RT (Figure 8A).
Figure 8.
Effect of selective and nonselective inhibitors of PKC on cytokine-induced changes in RT, Papp, and JAM and ICAM-1 expression. Cultures were exposed to TNF-α and IFN-γ in the presence or absence of H7 (nonselective) or chelerythrine chloride (selective) inhibitors of PKC. (A) RT and (B) Papp to 10-kDa FITC-dextran. JAM (C) and ICAM-1 (D) protein expression by ELISA in cultures treated with TNF-α and IFN-γ for 72 h in the absence or presence of 10 μM or 30 μM H7. *Significantly different from vehicle at p < 0.05. Data presented are a minimum of n = 12 cultures from at least three patients.
We next determined whether H7 would also induce a partial inhibition of the cytokine-mediated increase in Papp by measuring Papp of the 10-kDa FITC-dextran at 72 h after cytokine exposure. Papp in cultures treated in the absence of H7 was increased 17-fold over vehicle controls. In contrast, cultures treated with TNF-α and IFN-γ in the presence of H7 (10 μM) displayed a negligible increase in Papp, with Papp to the FITC-dextran only twofold greater than that of vehicle controls (Figure 8B).
Because the nonspecific inhibitor H7 may also inhibit protein kinase A (PKA), we also evaluated the effect of the specific PKC inhibitor chelerythrine, which inhibits the catalytic domain of all PKC isoforms on RT and Papp. Like H7, chelerythrine (1 μM) inhibited the cytokine-induced decrease in RT and the associated increase in Papp to a similar degree as H7 (Figure 8, A and B), suggesting that the effects on RT and Papp were mediated through PKC signaling pathways.
Effect of H7 on Cytokine-induced Alterations JAM and ICAM-1 Expression
In addition to eliciting a profound effect on RT and Papp, exposure of WD primary HAE cells to combined treatment of TNF-α and IFN-γ caused changes in the expression of JAM and ICAM-1 (Figure 7). To determine whether cotreatment of H7 with cytokines could inhibit the effect on JAM and ICAM-1 protein expression, we performed ELISA analysis after 72 h exposure to TNF-α and IFN-γ in the absence or presence of H7. JAM and ICAM-1 expression were 44 ± 6% and 378 ± 7% of vehicle controls after treatment in the absence of H7 (Figure 8, C and D). However, JAM expression in cultures treated in the presence of H7, 10 and 30 μM, was 65 ± 20% and 90 ± 16% of control, respectively (Figure 8C). ICAM-1 expression in the presence of H7 also remained at or near control levels, 189 ± 15% and 102 ± 15% in cultures treated with 10 and 30 μM H7, respectively (Figure 8D).
Effect of PKC Inhibitors on Distribution of TJ-associated Components
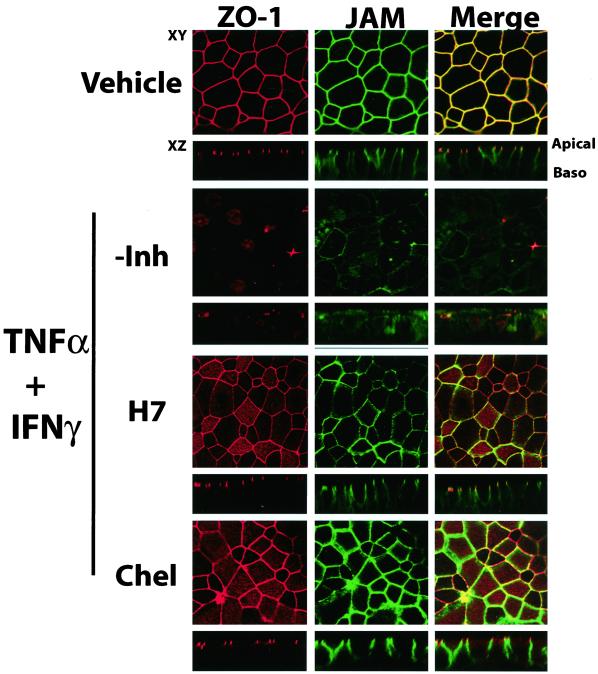
The nonspecific (H7) and specific (chelerythrine) PKC inhibitors prevented the cytokine-induced changes in junctional barrier function (as assessed by RT and Papp measurements) and the changes in the reciprocal regulation of JAM and ICAM-1 expression (Figure 8). To determine whether these inhibitors could also inhibit the changes in the distribution of ZO-1 and JAM, which occurred after 72 h TNF-α and IFN-γ treatment, we performed immunofluorescence localization after treatment of cultures in the presence of H7 and chelerythrine. Although a subtle redistribution of JAM and ZO-1 was detected after cytokine exposure in the presence of H7 or chelerythrine, the extent of this relocalization was less pronounced than with cytokine exposure alone (Figure 9). Claudin-1 and occludin distribution was not effected either in the presence or absence of the inhibitors (data not shown).
Figure 9.
Effect of H7 and chelerythrine on the reorganization of ZO-1 and JAM. Immunofluorescent staining for JAM and ZO-1 in primary HAE cells exposed to TNF-α and IFN-γ for 72 h in the presence or absence of H7 (10 μM) or chelerythrine (10 μM). Red staining (left) represents ZO-1, green staining (middle) is JAM, and right is a merged image of both ZO-1 and JAM. Areas of colocalization appear yellow.
Characterization of PKC Isomers Mediating Cytokine-induced Changes in TJ Permeability
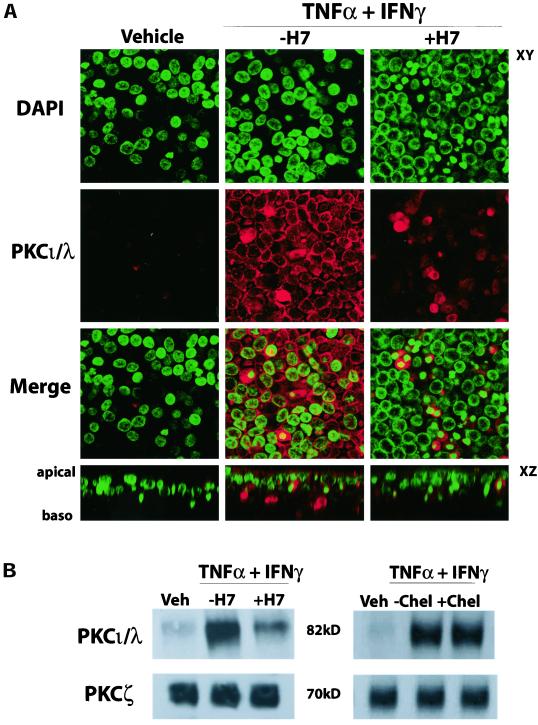
Because H7 and chelerythrine inhibited the effects of TNF-α and IFN-γ on RT and Papp, we determined which PKC isoform was involved in the cytokine-mediated alteration of the TJ. We focused on the atypical PKC isoforms ι/λ and ζ because of their link to the TJ (Ebnet et al., 2001). In particular, because JAM expression is highly regulated by cytokine exposure, we examined whether expression of PKCι/λ, an isoform thought to bind directly to JAM, would be altered in the same manner. After 72 h exposure, PKCι/λ protein expression was greatly increased in cultures exposed to TNF-α and IFN-γ (Figure 10). Immunofluorescence revealed an increase in PKCι/λ expression, correlating with enhanced staining of the nuclei, plasma membrane, and the apical portion of the cytoplasm (Figure 10A). In addition, immunoprecipitation of PKCι/λ and PKCζ followed by Western blot analysis demonstrated an increase in the PKCι/λ isoform after cytokine exposure, but not PKCζ (Figure 10B). Treatment of cells with cytokines in the presence of H7, which prevented the changes in barrier function and JAM and ICAM-1 expression (Figures 7 and 9), also partially inhibited the increase in PKCι/λ at 72 h (Figure 10B). In contrast, the specific PKC inhibitor chelerythrine, which prevented the cytokine-induced alteration in RT and Papp (Figure 7), did not inhibit the increase in PKCι/λ expression (Figure 10B), suggesting that the reduction in PKC ι/λ expression by H7 was mediated through PKA. PKA is known to regulate expression of aPKCs (see below) in cells that express adenosine receptors (Huang et al., 2001b).
Figure 10.
Cytokine effects on PKC isomer expression. WD primary HAEs were exposed to TNF-α and IFN-γ in the absence or presence of H7, and expression of PKCι/λ and PKCζ was determined. (A) Immunofluorescence localization of PKCι/λ after 72 h exposure to TNF-α and IFN-γ, with or without H7. Increased staining in cultures exposed to TNF-α and IFN-γ is evident. (B) Immunoprecipitation followed by Western blot analysis of PKCι/λ (top) and PKCζ (bottom) after 72 h exposure to TNF-α and IFN-γ, with or without H7 (left) or chelerythrine (right). Images and gel are representative of a minimum of n = 6 cultures from at least 3 patients.
Expression of Junctional Components and ICAM-1 In Vivo
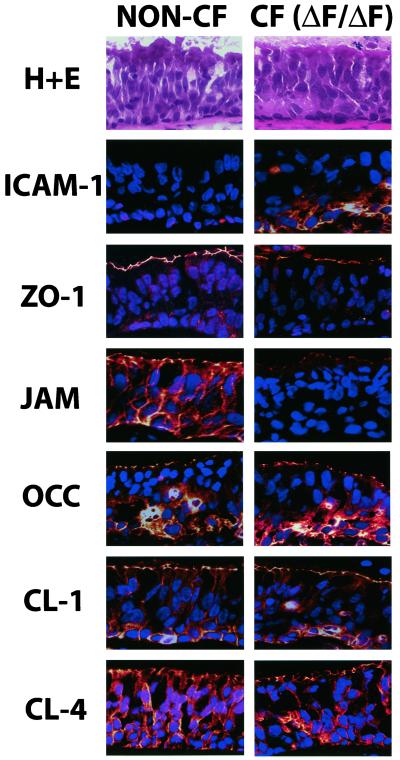
We have demonstrated that prolonged cytokine treatment induces alteration in the barrier function of the TJ, presumably via PKC-induced changes in protein components of the TJ. Because the airways of CF patients are chronically exposed to an inflammatory milieu, we examined the expression of these same TJ components in large airways excised from non-CF and CF patients after lung transplantation. We found that the changes in ICAM-1, JAM, and ZO-1 induced by cytokine treatment in our in vitro system were also present in vivo. Bronchi from CF patients expressed increased ICAM-1, particularly at the basolateral surface, and decreased ZO-1 and JAM staining as assessed by immunofluorescence (Figure 11). However, no apparent change in the fluorescence intensity of occludin, claudin-1, or claudin-4 was detected.
Figure 11.
In vivo localization of ICAM-1 and junctional components in freshly excised non-CF and CF (ΔF/ΔF) bronchus. Bronchi from non-CF and CF patients were isolated and prepared for staining as described in MATERIALS AND METHODS. Frozen sections were stained for ICAM-1, ZO-1, JAM, claudin-1, occludin, and claudin-4. Blue, DAPI-stained nuclei and red, positive protein staining. Images are representative of sections from a total of four patients of non-CF and CF (ΔF/ΔF) type each. Top, Hematoxylin and eosin (H+E) staining of a representative section of non-CF or CF type to illustrate section morphology.
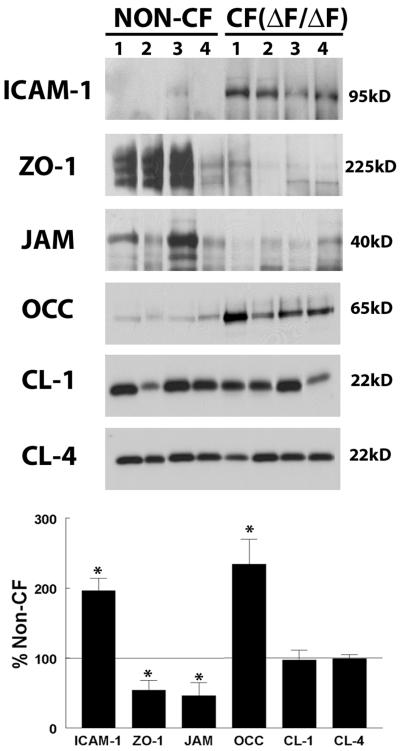
To further quantify these changes, we performed Western blot analysis of protein isolated from bronchi of non-CF donors and CF patients after transplant. We found that, as occurs with cytokine exposure in our in vitro system, bronchial epithelia from CF patients in vivo exhibit a decrease in ZO-1 and JAM and a marked increase in ICAM-1 and occludin (Figure 12). CF bronchial epithelia in vivo expressed 46 ± 14% less ZO-1 and 54 ± 19% less JAM than non-CF donors. In contrast, CF bronchial epithelia in vivo demonstrated a 196 ± 18% increase in ICAM-1 expression over non-CF. These data are similar to those of previously published reports measuring ICAM-1 expression in CF and non-CF epithelium (Hubeau et al., 2001). Similarly, occludin Western blotting showed an increase of 234 ± 36% over non-CF donor epithelium. When the polyclonal antibody to occludin was used in Western blotting, only a single band was detected in the range of 65–73 kDa. There was no significant change in the expression of either claudin-1 or -4 in CF epithelium (97 ± 14% and 96 ± 6% of non-CF, respectively), consistent with our in vitro data.
Figure 12.
In vivo expression of ICAM-1 and junctional components in non-CF and CF (ΔF/ΔF) freshly excised bronchus. Total protein isolated from the bronchi of non-CF and CF patients after transplant was subjected to Western blot analysis of ICAM-1 and TJ components as described in MATERIALS AND METHODS. Band intensity was analyzed and expression in CF patients presented as a percentage of non-CF (bottom). *Significantly different from non-CF (p < 0.05).
DISCUSSION
We hypothesized that the chronic inflammation in the CF lung may lead to changes in the integrity of the TJ in CF patients, leading to decreased barrier function and alterations in ion selectivity. To address this hypothesis, we exposed non-CF and CF WD primary HAE cultures to cytokines known to be upregulated in CF. We found that exposure of WD primary HAE to TNF-α and IFN-γ combined, but not separately, induced significant changes in the barrier function and rearrangement of structural components of the TJ. In addition, it appears that these cytokine-induced changes may be regulated via a signaling cascade involving upregulation of PKCι/λ, an atypical PKC isoform.
Prolonged exposure of the airway to a combination of TNF-α and IFN-γ led to significant changes in TJ barrier properties, with significant alterations in both RT and Papp (Figures 1 and 2). However, there was a less pronounced effect when cultures were treated with either of these cytokines alone or with IL-1β. In addition, the kinetics of the effects of TNF-α and IFN-γ on RT and Papp were different in CF WD primary HAE cells from those in non-CF controls. Although cultures of the non-CF type were resistant to the effects of cytokine treatment until 48 h of exposure, CF cultures showed a significant decrease in RT and an increase in Papp by 24 h (Figure 2, C and D), indicating some properties of the CF epithelium make it respond more rapidly to the effects of cytokines on the TJ. Perhaps primary cells isolated from CF airways are already sensitized to the effects of cytokines because of retention of changes in gene expression induced by the inflammatory environment present in vivo.
Our data measuring the effects of cytokines on RT and Papp for noncharged solutes and dilution potentials allowed us to identify differences in TJ responses to individual cytokines. For example, IL-1β treatment modestly decreased RT, while exhibiting no effect on Papp to small or large noncharged solutes, suggesting that the barrier function of the TJ to noncharged solutes was unchanged, whereas the ion permeability of the junction was altered. Measurements of the effects of 72 h IL-1β treatment on GT and transepithelial dilution potentials suggested that the increase in GT (or reduction in RT) could be accounted for solely by the increase in relative permeability for Cl− across the paracellular pathway (Figure 3). Previously published data have established a pattern of ion transport defects in CF epithelium both in vitro and in vivo (for review, see Boucher, 1994a, 1994b). Although CF epithelia are impermeable to Cl− ions via the transcellular pathway, because of the CFTR defect, they exhibit an increased rate of Cl− absorption in vivo, which would reflect increased permeability of the paracellular pathway to Cl− ions in CF (Boucher et al., 1986; Willumsen and Boucher, 1989). Because IL-1β levels have been shown to be markedly increased in CF sputum, our data showing increased PCl−/PNa+ ratio induced by IL-1β may in part explain some of the enhanced absorption of Cl− ions in CF epithelia. Isotonic transcellular hyperabsorption of sodium (Na+), which occurs because of absent or defective apical membrane CFTR Cl− channels that are responsible for inhibiting epithelial sodium channels, is accompanied by hyperabsorption of Cl− via the paracellular path in CF epithelia and has been proposed as a mechanism by which defective cellular Cl− transport leads to decreased airway surface liquid height and volume, decreased mucociliary clearance, bacterial infection, and bronchiectasis in CF (Matsui et al., 1998; Wine, 1999; Tarran et al., 2001). This finding might also suggest the induction of a claudin with selective Cl− permeability, which is in contrast to the recently reported decreased Na+ permeability induced by expression of claudin-4 (Van Itallie et al., 2001). In contrast, the large changes in RT and in large solute permeability (Papp) induced by TNF-α and IFN-γ suggest that these cytokines are acting via a distinct pathway that either degrades the TJ barrier function greatly or alters the properties of a claudin member with very large equivalent pore radii.
The claudin family of transmembrane proteins play a critical role in maintaining TJ integrity and may be responsible for the selective passage of ions and molecules through the paracellular space (for review, see Tsukita and Furuse, 1999; Tsukita et al., 2001). However, claudin-1 and claudin-4 do not appear to be affected by the exposure of primary HAE cells to TNF-α and IFN-γ (Figure 6), suggesting that claudin-1 and -4 are not involved in the regulation of the TJ mediated by cytokines in the airway epithelium.
Freeze-fracture EM revealed that as a correlate to altered barrier properties of the TJ to ions and solutes, there was also an effect on the morphology of the TJ strands. Non-CF cultures exposed to cytokines had a decrease in the extent of their junctional depth and a modest decrease in strand number (Figure 4A). These data are consistent with previously published data suggesting that cytokine exposure altered TJ ultrastructure in intestinal cell lines (Schmitz et al., 1999b). In contrast to non-CF cultures, those of the CF type appear to exhibit a unique kinetic profile, as evidenced by a significant decrease in RT and Papp after 24 h TNF-α and IFN-γ treatment (Figure 1, C and D). The more rapid effect of cytokines on CF cultures was also seen in freeze-fracture EM at 24 and 72 h after treatment. At 24 h after treatment, the depth and strand number of CF strands resembled those of the non-CF type exposed to cytokines for 72 h. Similarly, JAM and ZO-1 redistribution in CF cultures at 24 h was similar to that of non-CF cultures at 72 h (Figure 5).
To determine whether TNF-α and IFN-γ exposure of primary HAE cells altered the organization of TJ components, we performed QRT-PCR, Western blotting, ELISA, and immunofluorescence studies. We found that several components of the TJ are affected by cytokine exposure. In particular, ZO-1 and JAM were downregulated and redistributed after cytokine exposure (Figure 5). Previous studies of the effects of cytokines on proteins involved in the maintenance of TJ integrity have focused primarily on cell lines of intestinal origin. Previous work in HT-29/B6 cells, a human intestinal cell line, suggested that the effects of TNF-α on the TJ are mediated via downregulation of occludin (Mankertz et al., 2000). Occludin exists in both an LMW and an HMW form, with the HMW form corresponding to an increased state of phosphorylation. In our study, the expression of the LMW form of occludin remained unchanged, whereas the expression of the HMW form increased, indicating a role for occludin phosphorylation in the cytokine-mediated effects on the TJ, perhaps as an attempt to reseal leaky junctions (Wong, 1997).
JAM is a member of the immunoglobulin superfamily localized to the TJ and may play a key role in monocyte transmigration across the paracellular pathway (Ozaki et al., 1999). Therefore, redistribution from the TJ and decreased expression of JAM after TNF-α and IFN-γ exposure may suggest a decreased ability of monocytes to penetrate to the lumen and perpetuate the inflammatory response. Because CF airways are filled with neutrophils, we postulated that reciprocal regulation of JAM and ICAM-1 might occur with cytokine treatment. Chronic TNF-α and IFN-γ treatment increased ICAM-1 expression at both the mRNA and protein levels, with a relative decrease in JAM (Figure 7). These data indicated a shift in the epithelium chronically exposed to TNF-α and IFN-γ, from that which allows the migration of monocytes to one that is much more prone to the passage of neutrophils via the paracellular pathway. This observation was supported by published data showing increased levels of neutrophils and soluble ICAM-1 in CF sputum, consistent with a predominant influx of neutrophils through the paracellular pathway into the lumen (Salva et al., 1996). The in vivo results presented here in CF freshly excised airways are consistent with this hypothesis (Figures 11 and 12).
The effect of TNF-α and IFN-γ on the airway TJ appears to reflect widespread effects on several protein components. To investigate which signaling pathways activated by the combined treatment of TNF-α and IFN-γ may be relevant to TJ function, we tested agents reported to inhibit two pathways known to regulate TJ permeability, tyrosine kinases and PKC. The tyrosine kinase inhibitor genistein was ineffective in blocking the cytokine-mediated effect on TJ barrier function. In contrast, the PKC inhibitors H7 and chelerythrine significantly inhibited the increase in Papp to both intermediate- and large-sized molecules induced by cytokines (Figure 8). However, the inhibition of the decrease in RT was less complete, indicating that whereas PKC is involved in the pathway regulating the cytokine-induced effects on permeability to solutes, it may have limited effects on ionic permeability. These data support a model of cytokine regulation of TJ barrier function in which there are distinct properties for the regulation of permeability to solutes and ions, one involving PKC.
The family of aPKC kinases consists of two distinct members, PKCι/λ and PKCζ, that are insensitive to Ca2+, diacylglycerol, and phorbol esters, which are known to be potent activators of the calcium-dependent (cPKCs) and novel (nPKCs) subfamilies but are instead regulated by lipid cofactors (Dempsey et al., 2000). We focused on the effects of TNF-α and IFN-γ on the expression of both PKCι/λ and PKCζ, because of its reported interaction with JAM and our data indicating that cytokine exposure affected JAM localization and expression. After cytokine treatment, there was an increase in the expression of PKCι/λ, as revealed by Western blot analysis and immunofluorescence, while PKCζ remained constant (Figure 10). Of note, the diffuse cytoplasmic staining for PKCι/λ in the apical region of the cells is consistent with studies of PKCι/λ in MDCK cells (Dodane and Kachar, 1996). In addition, this increase was blocked when cells were cotreated with H7, presumably because of the chronic exposure of the epithelium to H7 for 72 h. Because H7 also inhibits PKA, we also examined the effect of chelerythrine on the increase in PKCι/λ expression. Whereas H7 partially inhibited the increase in expression of PKCι/λ, chelerythrine did not, presumably indicating the inhibition of PKA by H7 upstream of PKCι/λ. This conclusion is supported by published data implicating a role for PKA-mediated regulation of aPKCs by adenosine receptors (Huang et al., 2001a), which are known to be expressed in HAE cells.
The aPKC isoform PKCι/λ has also been identified as a potential regulator of apoptosis in several cell types, and inhibition of this isoform may be necessary for the induction of apoptosis (Murray and Fields, 1997; Xie et al., 2000). We did detect a modest increase in lactate dehydrogenase (LDH) levels after treatment with TNF-α and IFN-γ in combination (data not shown), consistent with the possible apoptotic events. However, the reversible effect of TNF-α and IFN-γ treatment, with RT levels returning to normal levels within 24 h (Figure 1B) and specific effects on TJ proteins, would suggest a role for PKCι/λ independent of apoptosis in the cytokine-induced alteration at the TJ, perhaps via its interactions with JAM. In addition, LDH levels were similar in cultures treated with TNF-α and IFN-γ alone to those treated with TNF-α and IFN-γ in combination, although only combined treatment elicits effects on junctional components (data not shown).
In summary, we examined the effects on TJ structure and function of exposure of primary HAE cultures to cytokines known to be present in CF. Chronic exposure of TNF-α and IFN-γ caused significant effects on both RT and Papp. The mechanism by which TNF-α and IFN-γ altered TJ permeability may involve signaling via PKCι/λ, because inhibitors of PKC, H7 and chelerythrine, inhibited the effects of cytokines on RT and Papp and prevented the increase in expression of ICAM-1 and decrease in JAM. Although the majority of our studies were conducted in a model system of WD polarized airway epithelium, changes in expression of JAM, ZO-1, and ICAM-1 induced by cytokines correlated with the expression of these proteins in chronically inflamed excised CF airways. In addition, the distinct effects induced by TNF-α and IFN-γ synergistically compared with IL-1β suggest that epithelium exposed to a mixture of cytokines, as is the CF airway, may exhibit extreme alterations in the barrier of the TJ to solutes and ion species.
In our study, the cytokine-induced alteration in the TJ may help explain several associated findings in CF, namely, increased Cl− absorption through the paracellular pathway in response to accelerated Na+ absorption and the ability to increase neutrophil transmigration caused by increased ICAM-1 levels. With regard to other inflammatory diseases, the pattern of cytokine-induced alteration of TJs may similarly contribute to the severity and type of inflammation observed. Given the ability of H7 and chelerythrine to inhibit TJ effects on permeability and ICAM-1 expression, a clearer understanding of cytokine effects on TJs may therefore permit the ability to pharmacologically modulate the severity of chronic inflammation.
ACKNOWLEDGMENTS
We thank Zhaoquing Zhou, Ph.D., C. William Davis, Ph.D., and Carla Ribeiro, Ph.D., for helpful discussions. We also thank the Cystic Fibrosis Pulmonary Research Center's Tissue Culture Core (Scott H. Randell, Ph.D., Director) for providing human airway cells and surgical specimens for our studies. This work was supported by grant HL-54832 from the National Heart, Lung, and Blood Institute.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–03–0134. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–03–0134.
REFERENCES
- Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029–C2038. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104:72–78. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Human airway ion transport. Part one. Am J Respir Crit Care Med. 1994a;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Human airway ion transport. Part two. Am J Respir Crit Care Med. 1994b;150:581–593. doi: 10.1164/ajrccm.150.2.8049852. [DOI] [PubMed] [Google Scholar]
- Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia: abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Collier AM, Gambling TM, Knowles MR, Boucher RC. Ultrastructure of airway epithelial cell membranes among patients with cystic fibrosis. Hum Pathol. 1990;21:640–647. doi: 10.1016/s0046-8177(96)90011-8. [DOI] [PubMed] [Google Scholar]
- Carson JL, Collier AM, Gambling TM, Leigh MW, Boucher RC, Hu SC, Boat TF. Development, organization, and function of tight junctional complexes in the tracheal epithelium of infant ferrets. Am Rev Respir Dis. 1988;138:666–674. doi: 10.1164/ajrccm/138.3.666. [DOI] [PubMed] [Google Scholar]
- Clarke H, Marano CW, Peralta Soler A, Mullin JM. Modification of tight junction function by protein kinase C isoforms. Adv Drug Deliv Rev. 2000;41:283–301. doi: 10.1016/s0169-409x(00)00047-8. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- Dodane V, Kachar B. Identification of isoforms of G proteins and PKC that colocalize with tight junctions. J Membr Biol. 1996;149:199–209. doi: 10.1007/s002329900020. [DOI] [PubMed] [Google Scholar]
- Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodeski GI, Peterson DE, De Santis BJ, Hopfer U. Nucleotide receptor-mediated decrease of tight-junctional permeability in cultured human cervical epithelium. Am J Physiol. 1996;270:C1715–C1725. doi: 10.1152/ajpcell.1996.270.6.C1715. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Huang NK, Lin YW, Huang CL, Messing RO, Chern Y. Activation of protein kinase A and atypical protein kinase C by A(2A) adenosine receptors antagonizes apoptosis due to serum deprivation in PC12 cells. J Biol Chem. 2001a;276:13838–13846. doi: 10.1074/jbc.M008589200. [DOI] [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA. 2001b;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubeau C, Lorenzato M, Couetil JP, Hubert D, Dusser D, Puchelle E, Gaillard D. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann D, Bartelt B, Hoffmeyer S, Muller R. Posttranslational regulation of neurofibromin content in melanocytes of neurofibromatosis type 1 patients. Arch Dermatol Res. 1999;291:312–317. doi: 10.1007/s004030050415. [DOI] [PubMed] [Google Scholar]
- Kelley TJ, Elmer HL. In vivo alterations of IFN regulatory factor-1 and PIAS1 protein levels in cystic fibrosis epithelium. J Clin Invest. 2000;106:403–410. doi: 10.1172/JCI9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney JC, Proud D. Neutrophil transmigration across human airway epithelial monolayers: mechanisms and dependence on electrical resistance. Am J Respir Cell Mol Biol. 2000;23:389–395. doi: 10.1165/ajrcmb.23.3.4068. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113:2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- Murray NR, Fields AP. Atypical protein kinase C iota protects human leukemia cells against drug-induced apoptosis. J Biol Chem. 1997;272:27521–27524. doi: 10.1074/jbc.272.44.27521. [DOI] [PubMed] [Google Scholar]
- Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter, Jennings PS, Granger DN, Joh T, Alexander JS. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- Osika E, Cavaillon JM, Chadelat K, Boule M, Fitting C, Tournier G, Clement A. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur Respir J. 1999;14:339–346. doi: 10.1034/j.1399-3003.1999.14b17.x. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–557. [PubMed] [Google Scholar]
- Ratcliffe MJ, Smales C, Staddon JM. Dephosphorylation of the catenins p120 and p100 in endothelial cells in response to inflammatory stimuli. Biochem J. 1999;338:471–478. [PMC free article] [PubMed] [Google Scholar]
- Salva PS, Doyle NA, Graham L, Eigen H, Doerschuk CM. TNF-alpha, IL-8, soluble ICAM-1, and neutrophils in sputum of cystic fibrosis patients. Pediatr Pulmonol. 1996;21:11–19. doi: 10.1002/(SICI)1099-0496(199601)21:1<11::AID-PPUL2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999a;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Barmeyer C, Gitter AH, Wullstein F, Bentzel CJ, Fromm M, Riecken EO, Schulzke JD. Epithelial barrier and transport function of the colon in ulcerative colitis. Ann NY Acad Sci. 2000;915:312–326. doi: 10.1111/j.1749-6632.2000.tb05259.x. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999b;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- Striz I, Mio T, Adachi Y, Heires P, Robbins RA, Spurzem JR, Illig MJ, Romberger DJ, Rennard SI. IL-4 induces ICAM-1 expression in human bronchial epithelial cells and potentiates TNF-alpha. Am J Physiol. 1999;277:L58–L64. doi: 10.1152/ajplung.1999.277.1.L58. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Boucher RC, Bromberg PA, Gatzy JT. Effects of ammonium and nitrate salts on ion transport across the excised canine trachea. Toxicol Appl Pharmacol. 1981;60:91–105. doi: 10.1016/0041-008x(81)90139-3. [DOI] [PubMed] [Google Scholar]
- Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, Boucher RC. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol. 1989;256:C1054–C1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- Wine JJ. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103:309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnarowski C, Frischer T, Hofbauer E, Grabner C, Mosgoeller W, Eichler I, Ziesche R. Cytokine expression in bronchial biopsies of cystic fibrosis patients with and without acute exacerbation. Eur Respir J. 1999;14:1136–1144. doi: 10.1183/09031936.99.14511369. [DOI] [PubMed] [Google Scholar]
- Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- Xie J, Guo Q, Zhu H, Wooten MW, Mattson MP. Protein kinase C iota protects neural cells against apoptosis induced by amyloid beta-peptide. Brain Res Mol Brain Res. 2000;82:107–113. doi: 10.1016/s0169-328x(00)00187-x. [DOI] [PubMed] [Google Scholar]
- Yankaskas JR, Cotton CU, Knowles MR, Gatzy JT, Boucher RC. Culture of human nasal epithelial cells on collagen matrix supports: a comparison of bioelectric properties of normal and cystic fibrosis epithelia. Am Rev Respir Dis. 1985;132:1281–1287. doi: 10.1164/arrd.1985.132.6.1281. [DOI] [PubMed] [Google Scholar]
- Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]