Abstract
Mutations in XPB and XPD TFIIH helicases have been related with three hereditary human disorders: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. The dual role of TFIIH in DNA repair and transcription makes it difficult to discern which of the mutant TFIIH phenotypes is due to defects in any of these different processes. We used haywire (hay), the Drosophila XPB homolog, to dissect this problem. Our results show that when hay dosage is affected, the fly shows defects in structures that require high levels of transcription. We found a genetic interaction between hay and cdk7, and we propose that some of these phenotypes are due to transcriptional deficiencies. We also found more apoptotic cells in imaginal discs and in the CNS of hay mutant flies than in wild-type flies. Because this abnormal level of apoptosis was not detected in cdk7 flies, this phenotype could be related to defects in DNA repair. In addition the apoptosis induced by p53 Drosophila homolog (Dmp53) is suppressed in heterozygous hay flies.
INTRODUCTION
The TFIIH DNA repair/transcription factor provides an outstanding example of the complexity encountered in genotype–phenotype relationships. Mutations in some of the TFIIH components in humans may produce three hereditary disorders: xeroderma pigmentosum (XP), Cockayne syndrome (CS), and trichothiodystrophy (TTD) (Lehmann, 1998, 2001). XP patients present sunlight hypersensitivity, abnormal skin pigmentation, and a high skin cancer predisposition (Cleaver, 2000; de Boer and Hoeijmakers, 2000; Rolig and McKinnon, 2000; Lehmann, 2001). CS individuals have slow postnatal growth and defects in nervous system development (Nance and Berry, 1992; Rolig and McKinnon, 2000). On the other hand, TTD patients share some of the neurological problems present in CS and have the particular phenotypes of brittle hair, fragile nails and ichthyosis (Itin and Pittelkow, 1990). In addition defects in TFIIH may have a role in the generation of cancer (Lehmann, 1998; Liu et al., 2000).
TFIIH takes part in nucleotide excision repair (NER) (Buratowski, 1993; Feaver et al., 1993; Schaeffer et al., 1993; Drapkin and Reinberg, 1999; Conaway et al., 2000; Le Page et al., 2000). In eukaryotes, NER repairs many types of lesions that cause a distortion of the DNA helical structure, including pyrimidine dimers (Lehmann, 1987; Friedberg, 1996a; Wood, 1996). It has also been reported that TFIIH may participate in base excision repair (BER) when DNA suffers oxidative damage (Le Page et al., 2000), increasing the number of roles that TFIIH plays in the eukaryotic genome maintenance.
TFIIH is formed by the DNA helicases XPB and XPD; the p62, p52, p44, and p34 polypeptides; and the complex known as cyclin-dependent kinase (Cdk)-activating kinase or CAK, which is formed by three proteins: Cdk7, CycH, and Mat1. Cdk7, CycH, and Mat1 are not involved in DNA repair, and there are no reported syndromes related to defects in these genes (Feaver et al., 1994; Roy et al., 1994; Serizawa et al., 1995; Shiekhattar et al., 1995; Rossignol et al., 1997; Yankulov and Bentley, 1997). The Cdk7 kinase phosphorylates the C-terminal domain of RNA polymerase II. This phosphorylation is necessary for RNA polymerase II elongation (Gerber et al., 1995). It has been suggested that the Drosophila Cdk7 homolog may also have a role in cell cycle control (Larochelle et al., 1998). Therefore, the central role of TFIIH factor in transcription, DNA repair, and probably in cell cycle, explains the extremely pleiotropic phenotypes observed in XP, TTD, and CS disorders.
Functional analysis of TFIIH has been done using yeast, human cells, and in vitro transcription/DNA repair systems. More recently, the use of transgenic mice has allowed the generation of a TTD mouse model by introducing a mutation found in a human TTD patient into the mouse XPD gene (de Boer et al., 1998). The mouse carrying the TTD allele has shown clearly TTD phenotypes. Unfortunately, this is the only example of a mammalian model that reproduces some manifestations found in humans affected in TFIIH (de Boer et al., 1998; de Boer and Hoeijmakers, 1999; Rossi et al., 2001). On the other hand, experiments in Drosophila have demonstrated that the fly is an excellent model for understanding and assaying the developmental function of genes that encode for the TFIIH complex components (Mounkes et al., 1992; Larochelle et al., 1998; Reynaud et al., 1999; Leclerc et al., 2000). In Drosophila the XPB homolog was identified as the haywire (hay) gene (Mounkes et al., 1992). Alleles of hay mimic in the fly some of the defects found in XP and CS (Mounkes et al., 1992). In this work we identified some phenotypes caused by defects either in transcription or by deficient DNA repair in hay flies, showing that the complex phenotype–genotype relationship of TFIIH genes in Drosophila can be genetically analyzed in detail. Our results show that when TFIIH is not functional during development, a high degree of apoptosis is induced. We also found that there is a genetic interaction between Dmp53 and hay similar to the one found in human cells affected in XPB.
MATERIALS AND METHODS
Drosophila Strains
Wild-type strain in all experiments was OreR. hay alleles used in this work were haync2, haync2rv1–4, and haync2rv8 reported by Mounkes and Fuller (1999). Two new alleles, hayXPCS and hayTTD, were constructed. Deficiency Df(3L)lxd6 uncovers the hay gene (Lindsley and Zimm, 1990). cdk7P140S is a transgenic conditional negative dominant allele. Therefore, all the crosses with this allele were performed at 29°C. The genotype of the cdk7 stock is as follows: w Df(1)JB254 Pw+ [snf+, dhd+]/w Df(1)JB254 Pw+[snf+, dhd+]; +/+; Pw+[Dmcdk7P140S] Sb/TM3 Ser; Df(1)JB254 uncovers the 4F1-2 region of the X chromosome (Larochelle et al., 1998). For p53 experiments the stocks used were w118; Dmp53/CyO (Brodsky et al., 2000), w1118; Dmp53R155H; Dmp53H159N; Dmp53K259H; and Dmp53C+ (Brodsky et al., 2000; Ollman et al., 2000) and MS1096 as a GAL4 wing imaginal disk driver located in the X chromosome (Capdevila and Guerrero, 1994).
Phenotypic Analyses of Wings, Cuticles, and Bristles
Wings were dehydrated and dissected in ethanol before being mounted in Permount (Fisher Scientific, Pittsburgh, PA) and then visualized with an optic microscope. The cuticle phenotypes were observed with a stereoscopic microscope; subsequently, abdominal regions of adult flies were dissected and prepared for electron microscopy as described previously (Stathakis et al., 1999) and were examined in an EM900 transmission electron microscope (Carl Zeiss, Thornwood, NY). Bristle defects were visualized in a stereoscopic microscope. Flies were fixed in glutaraldehyde, postfixed in osmium tetroxide, dehydrated through a graded series of alcohol, and critical point dried before mounting on stubs and coated with carbon and gold. Samples were analyzed with a 5410 LV scanning electron microscope (JEOL, Tokyo, Japan).
Transgenic Flies
Mutations in the hay cDNA were introduced using a polymerase chain reaction-based method (Merino et al., 1992). For the generation of the hayTTD allele a base substitution (A-C) in position 385 on the hay cDNA (Koken et al., 1992) was made using the oligonucleotide 5′-AGTACAAACTCCCCGCATACAGTTTATATG-3′. To generate a change in the reading frame hayXPCS was made by introducing a guanine in position 2293. The oligonucleotide used was 5′-CCGACACGTCTCACCGATGCCGCCCG-3′. Mutant cDNAs were cloned by using the appropriate restriction enzymes in pCaSper hsp83 vector, and the whole genes were sequenced to corroborate the mutations. Transgenic flies were constructed following a standard protocol (Spradling and Rubin, 1982). Two hayTTD and four hayXPCS independent lines in the second chromosome were isolated.
Staining of Imaginal Discs and CNS
Third instar imaginal discs and larval CNSs were dissected in 1× phosphate-buffered saline and stained with acridine orange vital dye (Sigma-Aldrich, St. Louis, MO) or Nile blue (Sigma-Aldrich) at a concentration of 5 and 100 μg/ml, respectively (Abrams et al., 1993). Samples were analyzed with a conventional fluorescence microscope. For irradiated larvae, the imaginal discs were dissected and stained 24 h after irradiation. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed using the In Situ Cell Death Detection kit from Roche Applied Science (Indianapolis, IN) following the recommended protocol. The tissue was visualized on an MRC-600 confocal microscope (Bio-Rad, Hercules, CA).
Slot Blot Hybridization
Total RNA from adult flies was purified using the TRIzol kit (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. The integrity of the RNA was verified in a typical formamide-agarose gel. The RNA was loaded in Hybond-N+ membranes (Amersham Biosciences, Piscataway, NJ) by using a slot blot manifold (Hoeffer, San Francisco, CA). The blot was hybridized with labeled Pcp-1 and Actin probes (Roter et al., 1985; Vigoreux and Tobin, 1987) in a 50% formamide hybridization solution. After hybridization, the membrane was washed at 65°C several times in 0.2× SSC, 0.1% SDS and exposed to XAR-5 film (Eastman Kodak, Rochester, NY). As control the membrane was hybridized with total rRNA labeled with [α-32P]dCTP in a reverse transcriptase reaction. Signal quantification was performed by using the Scan Image system (Bio-Rad).
UV Irradiation
Third instar wild-type and haync2/haync2 larvae were irradiated with the wild-type half LD by using 254-nm UV light with a germicide lamp (UVP), and the irradiation was measured using a UVX radiometer (UVP).
Antibody Generation and Western Blot
A Hay-Glutathione S-transferase recombinant protein was constructed using the first 70 residues from the Hay protein. The protein was purified as described previously (Smith, 1993) and used to elicit polyclonal antibodies in Wistar rats. The antibody obtained was used in Western blot experiments. In general, total protein soluble extracts were prepared from adult flies and standardized. Samples were loaded in 12% SDS-PAGE gels (Laemmli, 1970), blotted in nitrocellulose, and immunostained (Baurnette, 1981). Hay protein was visualized using a 1:1000 dilution of the anti-Hay antibody. Peroxidase-conjugated goat anti-rat was used as secondary antibody (Zymed Laboratories, South San Francisco, CA).
RESULTS
Multiple Cuticular Defects Are Present in haywire Mutant Flies
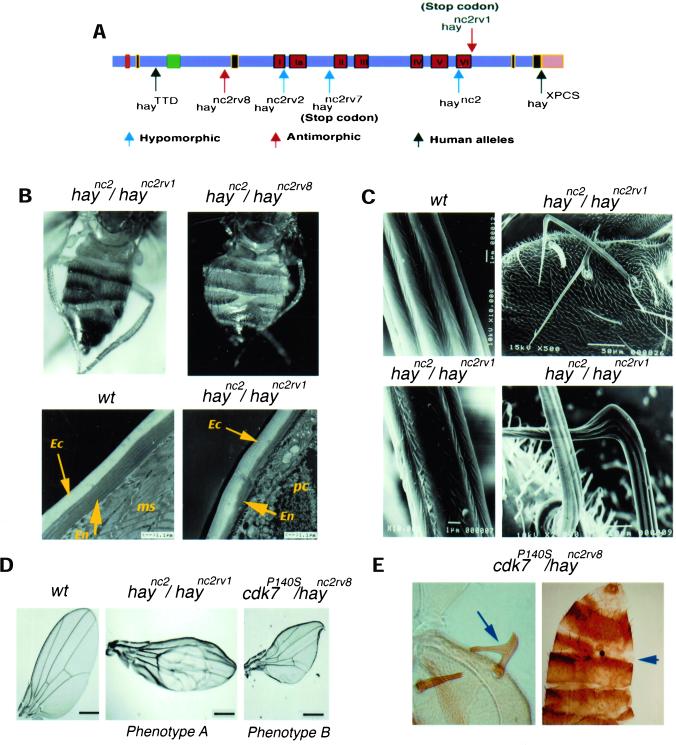
Several hypomorphic and antimorphic hay alleles have been analyzed at the genetic and molecular levels (Mounkes and Fuller, 1999). It has been reported that mutations in the hay gene produce an increase to UV light sensitivity, male sterility in combination with mutations in a particular tubulin gene, and some defects in the development of the nervous system (Mounkes et al., 1992). These phenotypes resemble some of the human manifestations produced in humans affected with XPB. However, except for the sensitivity phenotype caused by UV irradiation, which is attributed to defects in DNA repair, other phenotypes can be the consequence of DNA repair, transcriptional problems, or both. To understand the phenotypes produced by hay mutations in more detail, we reduced hay activity to its lowest viable level by making heteroallelic combinations of different antimorphic or hypomorphic alleles (Mounkes and Fuller, 1999) and two human-like alleles with the conditional hypomorphic haync2 mutation (Figure 1A). Progeny from these crosses had abdominal and wing defects, as well as deformations in the bristles (Table 1 and Figure 1). The abdominal abnormalities appeared as the loss of some cuticle portions. Electron microscopy showed that this phenotype is a consequence of a reduction of the deepest layers of the lamellate procuticle, whereas the superficial layers were not affected (Figure 1B). Both cuticular layers are derived from the same cell type (Fristrom and Liebrich, 1986), which suggest that this phenotype is due to a reduction of protein synthesis, rather than to the absence of these cells.
Figure 1.
hay gene structure, and heteroallelic and transheterozygous phenotypes of hay and cdk7 mutants. (A) Gene structure and localization of the mutations of the different hay alleles. The red boxes are the helicase domains, the black boxes are nuclear localization signals, the green box is the ATP-binding region, and the pink box is the COOH-terminal region affected in the XPCS human allele. The nucleotide sequence of the fly alleles has been reported previously (Mounkes and Fuller, 1999). The hayTTD human-like allele has a change of Ser 119 for Pro (Weeda et al., 1997), and the hayXPCS human-like allele is an insertion of one base at the intron acceptor site that produces a phase change in the open reading frame at the COOH terminus, which is described in the human allele (Weeda et al., 1990). Hypomorphic alleles are indicated with a blue arrow, antimorphic alleles with a red arrow, and the human alleles introduced in transgenic flies with a black arrow. The original haync2 allele was originally described as antimorphic in terms of its interactions with tubulin mutants (Mounkes et al., 1992); however, in our genetic analysis it behaves as hypomorphic. (B) Dorsal view of hay heteroallelic flies with cuticle deformations in abdominal tergites (top panels) and electron microscopy pictures of cuticle preparations of wild-type and mutant flies (bottom panels). The arrows indicate the exocuticular (Ec) and endocuticular (En) layers, ms is muscle, and pc is parenchyma. (C) Brittle bristle phenotype. Scanning micrographs of wild-type and brittle bristles from the scutellum of hay heteroallelic flies. (D) Wing phenotypes A and B of haync2/haync2rv1 and cdk7P140S/haync2rv8 flies. Note that both phenotypes are very different. The relevant genotypes are indicated. (E) Cuticle preparations of cdk7P140S/haync2rv8 flies showing the brittle bristle and abdominal cuticular phenotypes. Complete genotypes are given in MATERIALS AND METHODS.
Table 1.
Viability and frequency of defects in heteroallelic hay flies The data in table are percentages obtained from at least 150 individuals of each genotype. An example of each phenotype is shown in Figure 1.
| Genotype | Viability | Brittle bristlesa | Abdominal cuticular defectsb | Phenotype A wingc |
|---|---|---|---|---|
| haync2/+ | 100 | 0 | 0 | 0 |
| haync2/haync2 | 46.3 | 14 | 13 | 9 |
| haync2/haync2rv1 | 47.9 | 19.9 | 21.5 | 14.3 |
| haync2/haync2rv2 | 45.6 | 49.1 | 12.0 | 9.6 |
| haync2/haync2rv4 | 12.4 | 47.9 | 16.6 | 0 |
| haync2/haync2rv7 | 38.8 | 46.1 | 16.7 | 1.0 |
| haync2/haync2rv8 | 49.7 | 11.1 | 15.3 | 0 |
| haync2/Df(3L)lxd6 | 50.0 | 26.6 | 26.0 | 0 |
Brittle bristles phenotype consists of deformed and fragile macro and microchaete.
Abdominal cuticular defects consist of a reduction of the endocuticular layer.
Abnormal wing phenotype A consists in the lost of marginal regions and blistered wings.
Compared with wild-type flies, bristles present in hay heteroallelic flies were very fragile and deformed in shape and texture (Figure 1C). This phenotype affects both the machrochaete and the microchaete. Some of the bristles have a fork-like structure at the tip and in general the macro- and microchaete in the thorax do not show the organization found in wild-type flies. Because of their similarities with the brittle hair phenotype present in TTD patients, we named this phenotype “brittle” bristles. Although the origin, function, and structure of the hair in mammals are basically different to the fly bristles, they do have molecular similarities in their development. The integrity of both hair and bristles requires high transcription levels of genes' structural coding for proteins that assemble these complex structures (de Boer et al., 1998; Tilney et al., 2000). Genetic evidence that the brittle bristle phenotype is due to transcriptional defects is presented below.
In addition to the cuticular and brittle bristle defects, wing defects were also observed in some of the hay heteroallelic combinations (Table 1 and Figure 1D). This phenotype, which we named wing phenotype A, is not as penetrant as are the brittle bristle and abdominal cuticular phenotypes. The haync2rv8 and haync2rv4 alleles as well as the Df(3L)lxd6 with the haync2 allele do not show wing defects (Table 1). These results suggest that these wing defects were not due to the gene product dosage, but to allele-specific interactions.
Transgenic Flies Carrying Human-like Alleles with Mutations Reported in Human Patients Reproduce Defects Observed in hay Heteroallelic Flies
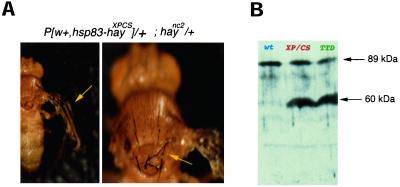
In addition to known EMS alleles (Mounkes and Fuller, 1999), we constructed two new hay alleles containing two mutations found in XPB patients. One of these mutations causes TTD, whereas the other produces both XP and CS manifestations (Weeda et al., 1990, 1997; Riou et al., 1999). These transgenes were named hayTTD and hayXPCS, respectively (Figure 1A). Although wild-type transgenes were able to rescue lethality of hay homozygous flies as well at the abdominal, bristle, and wing defects, mutant transgenes (hayTTD and hayXPCS) were not (our unpublished data). The presence of one copy of the hayTTD or hayXPCS transgenes in a haync2/haync2 background decreases fly viability dramatically (Table 2). Transgenic flies carrying one copy of hayXPCS, one copy of haync2 allele, and one copy of the wild-type gene reproduced defects observed in heteroallelic combinations of hay, but in a more severe manner. For instance, bristles were much more fragile and thinner and wings were practically amorphous (Figure 2A). In addition, locomotion impairments were observed. None of these defects were observed in transgenic flies carrying two wild-type hay alleles, demonstrating that, in individuals with this genotype, hayXPCS is not dominant. Both transgenes overexpress the corresponding RNA (our unpublished data). A polyclonal rat antibody against the N-terminal domain of the wild-type Hay protein was produced (see MATERIALS AND METHODS). By using this antibody, we analyzed the Hay protein produced by the transgenic flies harboring the hayTTD and hayXPCS constructs. We found that both transgenic lines have a band corresponding to the expected size of wild-type and mutant proteins (∼89 kDa) and a truncated product 30 kDa smaller than the wild type (Figure 2B). This result suggests that these mutant forms produce a nonstable protein that is processed.
Table 2.
Fly viability in heteroallelic and homozygous haync2flies in the presence of the hayTTD and hayXPCS alleles Data are presented as the percentage of expected individuals of each genotype and are an average of at least 500 embryos of all genotypes laid in each cross.
| Genotype | wt | haync2/haync2rv1 | haync2/haync2 | haync2/+ |
|---|---|---|---|---|
| 100 | 47.9 | 46.9 | 100 | |
| P[w, hay+] | 100 | nd | nd | |
| P[w, hayTTD] | 100 | 2.1 | 0 | 92 |
| P[w, hayXPCS] | 100 | 0.4 | 0.6 | 70 |
Figure 2.
hayXPCS allele enhances the hay phenotypes. (A) Phenotype of a haync2 heterozygous fly carrying one copy of the hayXPCS allele expressed from the fly hsp83 promoter. Similar phenotypes are presented with the hayTTD allele. haync2/+ flies have a wild-type phenotype. (B) Western analysis of soluble proteins from wild-type and transgenic third instar larvae expressing either the HayTTD and HayXPCS proteins. After Western transfer Hay proteins were visualized using a polyclonal antibody against a glutathione S-transferase fusion protein harboring the N terminus of Hay. The genotypes of the larvae are wild-type: OreR; XPCS: w118, hayXPCS/CyO; TTD: w118, hayTTD/CyO. The wild-type protein is indicated by a red arrow and the blue arrow indicates a truncated form which is observed in both mutants.
cdk7 Genetically Interacts with Hay
The observed hay phenotypes could be due to problems either in DNA repair or in transcription. It has been demonstrated that Cdk7 participates in transcription as a component of TFIIH and is also involved in the control of the cell cycle, but it does not participate in DNA repair (Feaver et al., 1994; Serizawa et al., 1995; Svejstrup et al., 1995). Thus, it is possible that cdk7 mutants would present a subset of defects observed in hay flies affected in these processes. In an attempt to dissect the causes of different phenotypes observed in hay mutants, we performed crosses of hay flies with flies harboring different doses of cdk7 wild-type gene, with or without the conditional dominant negative cdk7P140S (Larochelle et al., 1998; see MATERIALS AND METHODS). If at least some of the defects observed in single cdk7 or hay mutant flies are caused by defective transcription due to different TFIIH abnormal subunits, we should observe a genetic interaction in transheterozygous flies. The results are shown in Table 3. We found that when the only source of Cdk7 comes from the mutant allele cdk7P140S, flies incubated at the restrictive temperature presented wing, cuticular, and bristle phenotypes. The last two were similar in appearance to the ones presented by single hay homozygous or heteroallelic mutants (Table 1, and Figure 1, D and E). Most of the hay alleles show a genetic interaction with cdk7P140S increasing the penetrance of bristle and cuticular phenotypes (Table 3). A stronger interaction was found between cdk7P140S and haync2rv8 alleles. Based on the appearance of the common bristle and cuticular phenotypes by single hay and cdk7 mutants, and the enhancement of these phenotypes in transheterozygous cdk7/hay flies, we suggest that these two phenotypes were caused by transcriptional defects. These phenotypes were only observed at the restrictive temperature for cdk7P140S. In addition to this result, no interaction was observed in Df(1)JB254/+; hay/+ flies (Table 3), indicating that the bristle and cuticle defects are only due to the presence of the cdk7P140S mutant. Thus, the possibility of a second mutation that interacts with hay producing these phenotypes could be discarded. On the other hand, bristle and cuticle phenotypes emerge in homozygous Df(1)JB254 flies in the presence of one copy of the cdk7P140S allele (Table 3), reflecting that these phenotypes were due to the loss of function of cdk7.
Table 3.
Wing and bristles phenotypes in cdk7 flies and genetic interaction with hay
| Genotype | Wing phenotype B | Brittle bristles | Cuticle |
|---|---|---|---|
| Df/+ | 0 | 0 | 0 |
| Df/+; +/haync2 | 0 | 0 | 0 |
| Df/+; cdk7P140S/+ | 20.9 | 0 | 0 |
| Df/Df; cdk7P140S/TM3 | 36.3 | 16.3 | 5 |
| Df/+; cdk7P140S+/haync2 | 17.2 | 10.4 | 4.03 |
| Df/+; cdk7P140S+/haync2rv2 | 17.5 | 14.4 | 2.12 |
| Df/+; cdk7P140S+/haync2rv7 | 18.0 | 6.7 | n.d. |
| Df/+; cdk7P140S+/haync2rv8 | 49.3 | 30.0 | 5 |
Data represent percentages. Because cdk7 is located in the X chromosome, the flies used in these crosses were w Df(1)JB254 Pw+ [snf+, dhd+]/ w Df(1)JB254 Pw+ [snf+, dhd+]; +/+; Pw+ [Dmcdk7P140S] Sb/TM3 Ser. Df(I)JB254 is deficient for the cdk7, gene and for the snf and dhd genes. The snf and dhd genes are complemented by wild-type alleles carried in a P element inserted in the same X chromosome (Larochelle et al., 1998). In the table this genotype is named Df. The genetic interaction between cdk7P140S and haync2rv2 is indicated in bold. The wing phenotype B consists in shape deformations and size reduction (phenotype B; see Figure 1). The percentages of flies presenting the three phenotypes with combinations of cdk7P140S and hay alleles are significant (P < 0.0005).
In contrast, wing defects observed in cdk7P140S flies were clearly different from the ones presented by hay mutant flies, thus we named it wing phenotype B (Figure 1D). This phenotype was present even when there is a wild-type cdk7 copy, which is not the case for bristle and cuticular phenotypes. cdk7P140S flies present small and deformed wings (phenotype B), whereas wings of heteroallelic hay flies show loss of marginal regions and the presence of blisters (Figure 1D, phenotype A). All transheterozygous hay/cdk7 combinations showed the cdk7P140S wing phenotype B. Unexpectedly, the wing phenotype of cdk7P140S is not enhanced by the addition of a defective hay allele with the exception of the haync2rv8 allele, showing that the interaction between cdk7P140S and haync2rv8 is allele specific (Table 3, in bold). Lack of enhancement of cdk7P140S wing phenotype with different hay alleles, with the exception of haync2rv8 (see DISCUSSION), supports that the different wing defects in hay and cdk7 mutants (phenotype A and B) are caused by failure in different mechanisms. Thus, because Cdk7 is only required for transcription and for the progression of cell cycle, the hay wing phenotype is probably caused by a deficiency in DNA repair rather than by transcription.
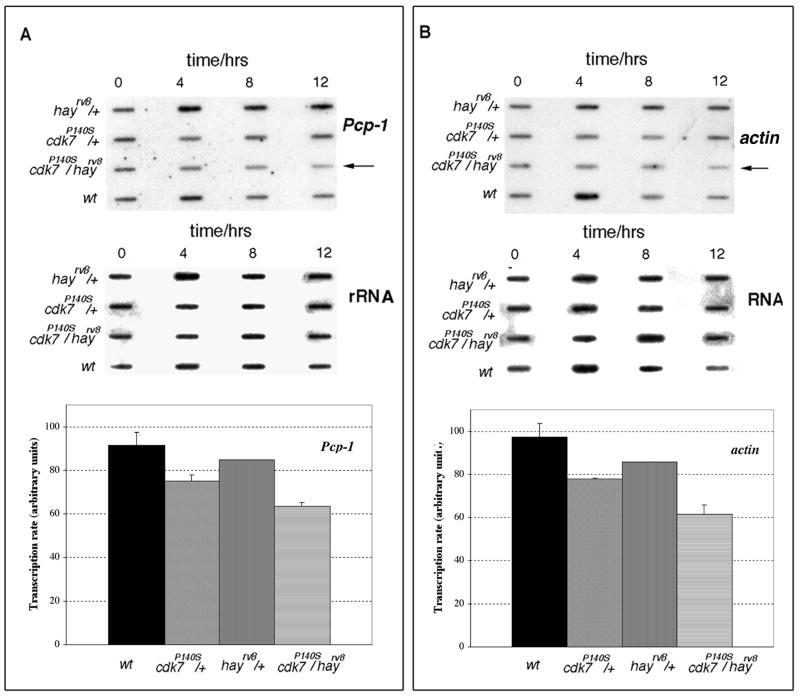
To complement the genetic interaction data between cdk7 and hay, mRNA levels of cuticular protein Pcp-1 and Actin (Rother et al., 1985; Vigoreaux and Tobin, 1987), which should be related to the observed phenotypes, were analyzed in haync2rv8/cdk7P140S flies and compared with heterozygous and wild-type flies. haync2rv8 flies were crossed with cdk7P140S organisms and the obtained transheterozygous adults were incubated at the restrictive temperature (29°C) at different times (0, 4, 8, and 12 h). Then total RNA from three different experiments was purified, and similar amounts of for each sample were loaded in a slot blot and hybridized against a Pcp-1 and Actin cDNA probes. Because mutations in the TFIIH components should affect a large number of genes transcribed by RNA polymerase II we used as control the levels of rRNA. Figure 3 shows that RNA levels of Pcp-1 and Actin were reduced in the haync2rv8/cdk7P140S flies incubated at 29°C compared with hay and cdk7 heterozygous flies incubated at a similar temperature. Quantification of the Pcp-1 and actin mRNA levels in haync2rv8/cdk7P140S flies, of three independent blots, indicates that the reduction was of ∼35 and 40%, respectively, if compared with the wild-type at 12 h of incubation at the restrictive temperature (Figure 3, A and B). The reduction of both mRNA levels occurs at 29°C, confirming that this is due to the cdk7P140S conditional mutant. These results support that the interaction between cdk7 and hay mutants has an effect on transcription that may result in the observed phenotypes.
Figure 3.
RNA levels of the Pcp-1 cuticular and Actin proteins in cdk7P140S and hay double mutant flies. Slot blot hybridization of the Pcp-1 (A) and Actin probes (B) against total RNA purified at different times from adult flies incubated at 29°C. The quality of the RNA was confirmed in typical formamide-agarose gels. Independent membranes were hybridized for each probe. The different genotypes are indicated in the figure. The same blots were washed and hybridized against rRNA as loading control. Note that in the cdk7P140S/hayrv8 flies (indicated with an arrow) the Pcp-1 and Actin transcript levels are reduced with the incubation time. The graphs show for each genotype, the average (n = 3) percentage of reduction in specific transcript levels after 12 h of incubation at the restrictive temperature (29°C) compared with rRNA levels.
Flies Affected in Hay Have a High Rate of Apoptosis in Imaginal Discs and in CNS
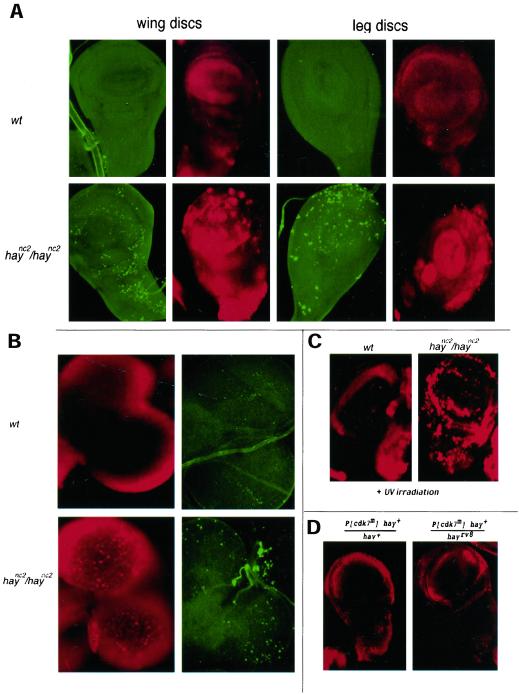
It has recently been shown that human cells with defects in XPB are not able to repair oxidative DNA damage by BER during transcription (transcription-coupled repair or TCR) (Le Page et al., 2000). These defects in TCR by XPB have been related to neurodegenerative problems found in CS patients, suggesting that deficient TCR may induce apoptosis (Hanawalt, 2000; Le Page et al., 2000; Vermeulen and Hoeijmakers, 2000). To know whether apoptosis was increased in hay flies, we analyzed the presence of apoptotic bodies in larval CNS and in the imaginal discs. Interestingly, an increase of apoptotic bodies in both kinds of tissues in hay larvae was found (Figure 4, A and B). The amount of apoptotic cells is increased in hay discs after UV irradiation compared with irradiated wild-type discs (Figure 4C). In contrast, we could not detect abnormal apoptotic bodies in cdk7P140S imaginal discs and in the CNS, even in cdk7P140S/haync2rv8 transheterozygous flies (Figure 4D). Because cdk7 only participates in transcription and in cell cycle control, but not in DNA repair, we suggest that apoptosis in haync2 mutants is due to DNA repair defects. These results also show that DNA repair by TFIIH is required during development and constitutes the first in vivo evidence that defects in the hay gene may induce apoptosis. cdk7P140S and hay flies present different wing phenotypes. For cdk7, the allele-specific interaction with haync2rv8 suggests that wing phenotype is related to deficient transcription, although a deficiency in cell cycle cannot be ruled out. With the evidence presented herein, we propose that hay wing phenotype is probably a consequence of an abnormal high apoptosis found in wing imaginal discs of mutant hay flies, caused by a deficient DNA repair during development.
Figure 4.
Apoptosis in hay imaginal discs and brains. (A) Wing and leg disc preparations from wild-type and haync2/haync2 flies stained with acridine orange (red) and TUNEL assay (green) for the presence of apoptotic cells. (B) CNS preparations from wild-type and haync2/haync2 flies stained with acridine orange (red) and TUNEL (green). Note the high number of apoptotic bodies in the hay larval tissues. (C) Wild-type and haync2/haync2 wing discs stained for apoptosis 24 h after UV irradiation (half LD for the wild-type, 164 J/m2). (D) cdk7P140S hay+/hay+ and cdk7P140S hay+/haync2rv8 discs. Note that only background is detected as in the wild-type discs. In all cases cell death is also detected with Nile blue (our unpublished data). Relevant genotypes of these flies are as in Figure 1 and MATERIALS AND METHODS. Acridine orange-treated samples were observed under a fluorescence microscope. The TUNEL assay was visualized using confocal microscopy. For each tissue sample maximum projections of the whole set of images are shown.
Dmp53/hay Genetic Interaction
In mammalian cells, apoptosis induced by DNA damage is mediated by p53 (Ford and Hanawalt, 1995). A physical interaction between p53 and TFIIH has been demonstrated (Wang et al., 1995), and p53-mediated apoptosis seems to be defective in XPB and XPD mutant cells (Wang et al., 1996). The Drosophila p53 homolog gene (Dmp53) can activate apoptosis in response to DNA damage by γ-irradiation (Brodsky et al., 2000; Ollman et al., 2000). Wild-type Dmp53 overexpression in the eye and wing discs induces apoptosis and severe deformations in both adult organs (Brodsky et al., 2000; Ollman et al., 2000; Figure 5B). Wing deformations are characterized by abnormal shape and a dramatic reduction of the wing blade as a consequence of massive cell death (Figure 5B). To know whether there is an interaction between Dmp53 and TFIIH in Drosophila, we used transgenic flies with either wild-type p53 (Dmp53) gene or with several dominant negative alleles affected in the DNA binding domain (Dmp53R155H, Dmp53H159N, Dmp53K259H, and Dmp53C) (Brodsky et al., 2000; Ollman et al., 2000). Both wild-type and mutant p53 alleles were overexpressed under GAL4-UAS system in the third instar larval wing discs of hay mutant individuals, by using the GAL4 wing driver MS1069 (see MATERIALS AND METHODS).
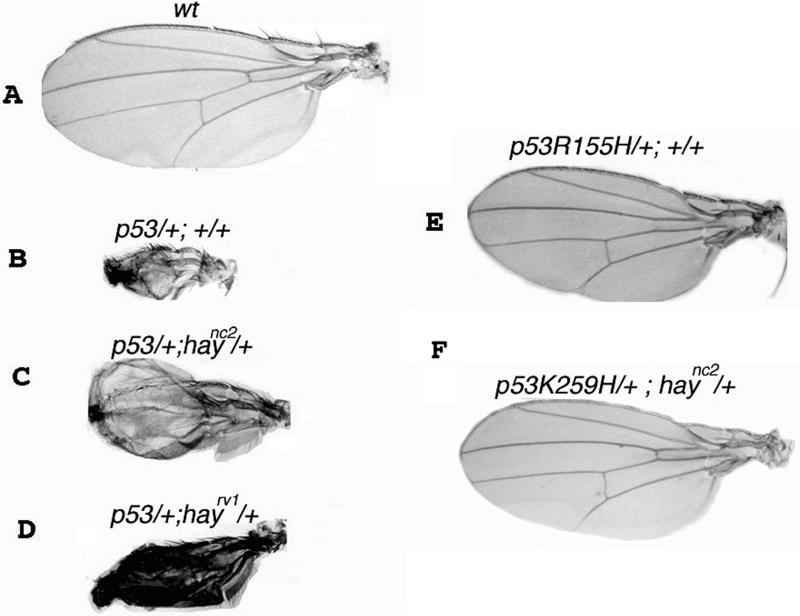
Figure 5.
hay and Dmp53 genetic interaction. Wild-type Dmp53 and a dominant negative Dmp53R155H, Dmp53H159N, Dmp53K259H, and Dmp53C+ alleles were overexpressed in the wing disc by using the MS1096 GAL4 driver in the presence or the absence of hay alleles. (A) Wild-type OreR wing. (B) Overexpression of the wild-type Dmp53 produces aberrant wings. (C and D) Wing overexpression of the wild-type Dmp53 in haync2 and haync2rv1 heterozygous backgrounds. Note that the apoptotic phenotype is reduced. (E) Overexpression of the dominant negative Dmp53R155H allele does not produces defects in the wing. (F) Example that shows that no genetic interaction occurs between Dmp53 negative dominant mutants and hay alleles.
We found that wing defects produced by the Dmp53 overexpression were suppressed in the presence of a hay mutant allele. Although this suppression was partial, the penetrance was 100%, even in the presence of a single hay mutant allele (haync2/+ and haync2rv1/+ flies; Table 4 and Figure 5, C and D). These results showed that Dmp53 needs an intact TFIIH to induce apoptosis because it occurs in human cells derived from patients affected in XPB and XPD (Wang et al., 1996).
Table 4.
Genetic interaction between Dmp53 and hay Data represent percentages from at least 50 individuals of each genotype. MS1096 is a wing imaginal disc GAL4-driver. Dmp53R155H is a p53 negative dominant mutation that is not able to induce apoptosis (Ollman et al., 2000).
| Genotype | Apoptotic phenotypea | Phenotype suppressionb |
|---|---|---|
| MS1096 | 0 | - |
| Dmp53 | 0 | - |
| MS1096; Dmp53 | 100 | - |
| MS1096; Dmp53; haync2 | - | 100 |
| MS1096; Dmp53; haync2rv1 | - | 100 |
| MS1096; Dmp53R155H | 0 | - |
| MS1096; Dmp53R155H; haync2 | 0 | - |
Apoptotic wing phenotype is identically to phenotype presented in Figure 4 in all flies with the same genetic background.
The suppressed wing phenotype is identical to the phenotype presented in the figure with the same genetic background in each case. The suppression is fully penetrant.
DISCUSSION
Phenotypic defects produced by some of the components of TFIIH have been difficult to characterize in mammalian systems. Analyzing combinations of mutant alleles of genes encoding two TFIIH subunits (hay and cdk7), we have been able to dissect phenotypes associated with transcription and with DNA repair.
Brittle Bristles and Cuticular Phenotypes Are Associated with TFIIH Transcriptional Deficiencies
Reduction of the Hay activity in different heteroallelic combinations produces analogous defects to the ones observed in humans affected in the XPB gene. Besides the increase in sensitivity to UV irradiation and sterility that were described by Mounkes et al. (1992), the most obvious defects in adult hay heteroallelic flies are severe cuticular deformations in the abdomen, brittle bristles, and aberrant wings. These defects can be rescued by overexpression of the Hay wild-type form in all the heteroallelic combinations. From the data reported herein, we propose that both the cuticular abdominal deformations and the brittle bristles are associated with deficiencies in transcription caused by defective TFIIH. For the adult cuticles, electron microscopy studies showed that this phenotype is due to a reduction of the deeper lamellate procuticle layers. As in the cuticle, construction of hair-like structures of bristle requires the abundant transcription of genes that encode bristle structural components (Frinstrom and Frinstrom, 1993). Bristles in hay flies are very fragile and they have severe deformations in their structure and texture. These defects resemble defects observed in TTD patients' hair caused by reduced transcription of genes encoding structural components such as the sulfur-rich proteins of the hair (Lehmann, 2001). This phenotype is also caused by a mutation in cdk7 producing a defective TFIIH. We found a clear genetic interaction between hay and cdk7 for the brittle bristle and the abdominal cuticular phenotypes. Because these two TFIIH subunits are involved in transcription, but Cdk7 is not involved in DNA repair, we concluded that lower levels of transcription cause brittle bristles and the abdominal cuticular phenotypes. This conclusion was supported by the fact the double hay/cdk7 mutants can affect transcript levels of genes related to the cuticular and bristle defects (Figure 3). In addition, we have observed similar defects by the genetic interaction of hay and mutant alleles of other basal transcription factors genes (our unpublished data).
The general transcription machinery is affected in hay mutants used in this work. Nevertheless, some hay individuals, although defective in many senses, are able to have a proper cell differentiation and to develop until adults. However, we believe that in hay cells that require the overexpression of specific genes, the TFIIH machinery is probably exhausted before the transcriptional program is completed, resulting in defects such as cuticle and bristles phenotypes. These defects could be the result of a partial reduction in the transcriptional rate of highly expressed genes. This could be similar to the proposed explanations for the brittle hair and ichthyosis manifestations observed in TTD patients (Lehmann, 2001).
The penetrance of the defects associated with hay is enhanced in the presence of hayXPCS and the haync2 alleles (Figure 2). The XPCS patient carrying the mutation similar to the hayXPCS allele is heterozygous, and it has been speculated that this mutation could be a dominant allele (Weeda et al., 1990). However, the presence of a second mutation in the control region of the other XPB allele (paternal) or in other gene has not yet been ruled out, in particular, because it was observed that the transcript of the paternal allele is not detectable in the patient (Weeda et al., 1990). Our results show that in Drosophila, hayXPCS allele is not dominant, and it only produces a hay phenotype when it is in combination with other hay mutant alleles. It is important to note that, although the nature of the two mutations (TTD and XP/CS) is different, in Drosophila they both produce a truncated Hay form that could affect the function of TFIIH. Interestingly, Mounkes and Fuller (1999) reported that haync2 mutant also accumulates a truncated product of similar size to the one found in this work, suggesting that in the fly some hay mutations produce a nonstable product. It has not been tested whether this truncated protein retains some function or whether it could be assembled into TFIIH complexes.
Wing Defects in hay Flies Can Be Associated with Defects in DNA Repair during Development
Both cdk7P140S and hay flies have aberrant wings, but these defects are different in each case. Results presented herein show that the wing phenotype present in cdk7P140S (phenotype B) is not enhanced by most of the hay alleles, with the exception of haync2rv8 allele (see below). This suggests that wing defects observed in both mutants are due to deficiencies in different processes. Wing defects in cdk7 mutant can be due to a deficiency in the cell cycle control and/or in transcription (Larochelle et al., 1998; Leclerc et al., 2000). However, an allele-specific interaction with haync2rv8 is observed also for wing defects. haync2rv8 allele has a modest effect in the bristles in combination with haync2, but it does not cause any wing defects in this situation (Table 1). In contrast, a single copy of this allele in the presence of a wild-type hay gene strongly enhances cdk7P140S wing phenotype (Table 3, in bold). We propose that the nature of these two mutations produces an interaction that has a strong effect on TFIIH activity, most likely affecting transcription. Conversely, aberrant wings observed in hay heteroallelic combinations (phenotype A) seem not to be related to transcription. This hypothesis is supported by the fact that most of the hay alleles do not increase wing phenotype B observed in cdk7P140S flies. Taking these results plus the fact that apoptosis is not detected in cdk7P140S/haync2rv8 flies as it occurs in the haync2/haync2 background, we suggest that aberrant wings observed in hay mutant flies are not due to transcription but to DNA repair.
Defects in hay Increase Apoptosis during Fly Development: Interaction with Dmp53
A higher rate of apoptotic bodies is detected in hay heteroallelic combinations and in haync2 homozygous flies. Apoptosis is also dramatically increased in hay imaginal discs after UV irradiation but not in wild-type discs. As stated above, apoptosis is not observed in cdk7 mutant flies, suggesting that the presence of a larger number of apoptotic cells in hay flies is due to abnormal DNA repair. These results suggest that DNA repair function of TFIIH is required during development even without the challenge of external physical or chemical agents that damage DNA. This is the first in vivo evidence that defective hay, an XPB homolog, may induce cell death during development. The TFIIH function in DNA repair could be either in NER or in BER. TFIIH is required for TCR of 8-oxo-guanine and thymine glycol caused by oxidative damage of DNA (Le Page et al., 2000). It has been proposed that in CS patients the accumulation of 8-oxo-guanine and/or thymine glycol caused by defective TCR may induce apoptosis during development of the nervous system (Hanawalt, 2000). We also propose that apoptosis observed in hay imaginal discs and in CNS is mostly due to an accumulation of oxidative damage that normally occurs during development. This accumulation of oxidative damage may be caused by defective TFIIH.
Interestingly, the presence of a hay mutant allele suppresses the penetrance of the wing defects due to the overexpression of Dmp53 in wing disc. It is remarkable that this suppression is fully penetrant (Table 4), showing a clear genetic interaction between Dmp53 and hay. This information is of particular relevance because it has been reported that primary cultured fibroblast derived from individuals with xeroderma pigmentosum, which are deficient in DNA repair by mutations in XPD or XPB, are not able to undergo p53-induced apoptosis (Wang et al., 1996). This deficiency can be rescued by transferring the wild-type XPD or XPB genes in the corresponding mutant cells, suggesting that XPB and XPD are components of the p53-mediated apoptosis pathway (Wang et al., 1996). The results presented in this work are in agreement with this information and confirm that the fly is an excellent animal model to study the role of TFIIH during development. However, a paradox emerges from these results. Mutations in hay induce apoptosis during development, but the same mutations suppress the apoptotic effect of the Dmp53 overexpression. Dmp53 apparently needs an intact TFIIH to induce apoptosis as in cultured human cells from XP patients. Then how is the apoptosis produced by hay mutations activated? It is possible that the apoptosis seen in hay mutants could be triggered by a Dmp53-independent mechanism. Alternatively, it cannot be ruled out that Dmp53 may still respond to other molecules that may activate it as a response to DNA damage produced by a defect in TFIIH during development. TFIIH is able to phosphorylate p53 in vitro, and the phosphorylated p53 is able to bind more efficiently to its target DNA sequences (Lu et al., 1997). There is evidence that p53 can modulate the TFIIH-associated nucleotide excision repair activity (Wang et al., 1995). Thus, there is an intricate cross talk between p53 and TFIIH.
In conclusion, mutations in TFIIH components produce as diverse phenotypes in Drosophila as they do in humans. Some of these defects are caused by faulty transcription, such as the bristle and cuticular phenotypes. Other defects, such as aberrant wings in hay flies are related to problems in DNA repair, which could increase cell death during development. A genetic interaction between hay and the fly homolog of p53 seems to be similar in some aspects to what has been found with human alleles in the same genes, confirming the value of the fly as a model for human diseases produced by mutations in TFIIH. Studies of humans affected in TFIIH have proposed that some mutant alleles of XPB and XPD genes may impair only DNA repair or transcription and others may affect both. Most of the hay (XPB homolog) alleles analyzed in this work seem to affect both mechanisms. This work shows that by using Drosophila genetics, the developmental role of the different components of the TFIIH complex can be analyzed in detail and that this information is relevant for the analysis of the syndrome manifestations in humans.
ACKNOWLEDGMENTS
We thank Dr. M.A. West and J. Sepúlveda for scanning microscopy; Biol. L. López for histological sample preparation and electron microscope analysis; Dr. Patricia León, Dr. Veronica Narvaez, M.C. Miranda Gonzalez, and Dr. Diana Escalante for commenting on the manuscript; V. Barajas for technical support; Dr. Margaret Fuller for providing the different hay alleles used in this work; and the Bloomington stock center. We also thank Drs. Gerald Rubin and Casey Kopczynski for providing the different Dmp53 stocks and Dr. Juan Riesgo-Escovar for the GAL-4 driver. This work was supported by the Consejo Nacional de Ciencia y Tecnologia Grant 31786, DGPA Grant IN-200799, and the Howard Hughes Medical Institute Grant 55003712.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0087. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0087.
REFERENCES
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Buratowski S. DNA repair and transcription: the helicase connection. Science. 1993;260:37–38. doi: 10.1126/science.8465198. [DOI] [PubMed] [Google Scholar]
- Burnette W. Electrophoretic transfer of proteins from SDS-polyacrilamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. Target expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–3451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- Cleaver JE. Common pathways for ultraviolet skin carcinogenesis in the repair and replication defective groups xeroderma pigmentosum. J Dermatol Sci. 2000;23:1–11. doi: 10.1016/s0923-1811(99)00088-2. [DOI] [PubMed] [Google Scholar]
- Conaway JW, Conaway RC. Transcription elongation and human disease. Annu Rev Biochem. 1999;68:301–319. doi: 10.1146/annurev.biochem.68.1.301. [DOI] [PubMed] [Google Scholar]
- de Boer J, Donker I, de Wit J, Hoeijmakers JHJ, Weeda G. A mouse model for the basal transcription/DNA repair syndrome trichothiodystrophy. Mol Cell. 1998a;1:981–990. doi: 10.1016/s1097-2765(00)80098-2. [DOI] [PubMed] [Google Scholar]
- de Boer J, Donker I, de Wit J, Hoeijmakers JHJ, Weeda G. Disruption of the mouse xeroderma pigmentosum group D DNA repair/basal transcription gene results in preimplantation lethality. Cancer Res. 1998b;58:89–94. [PubMed] [Google Scholar]
- de Boer J, Hoeijmakers JHJ. Cancer from outside, aging from the inside: mouse models to study the consequences of defective nucleotide excision repair. Biochimie. 1999;81:127–137. doi: 10.1016/s0300-9084(99)80045-5. [DOI] [PubMed] [Google Scholar]
- de Boer J, Hoeijmakers JHJ. Nucleotide excision repair and human syndromes. Carcinogenesis. 2000;21:453–460. doi: 10.1093/carcin/21.3.453. [DOI] [PubMed] [Google Scholar]
- Feaver WJ, Svejstrup JQ, Henry NL, Kornberg RD. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79:1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Friedberg ER. Xeroderma pigmentosum, Cockayne's syndrome, helicases, and DNA repair: what's the relationship? Cell. 1992;71:887–889. doi: 10.1016/0092-8674(92)90384-o. [DOI] [PubMed] [Google Scholar]
- Friedberg ER. Relations between DNA-repair and transcription. Annu Rev Biochem. 1996;65:15–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- Frinstrom D, Frinstrom JW. The Development of Drosophila melanogaster. II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. The metamorphic development of the adult epidermis; pp. 843–898. [Google Scholar]
- Frinstrom D, Liebrich W. The hormonal coordination of cuticulin deposition and morphogenesis in Drosophila imaginal disc in vivo and in vitro. Dev Biol. 1986;114:1–11. doi: 10.1016/0012-1606(86)90378-7. [DOI] [PubMed] [Google Scholar]
- Ford JM, Hanawalt PC. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-couple repair and enhance UV resistance. Proc Natl Acad Sci USA. 1995;92:8876–8880. doi: 10.1073/pnas.92.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H, Hagmann M, Seipel K, Georgiev O, West MAL, Litingtung Y, Schaffner W, Corden JL. RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature. 1995;374:660–662. doi: 10.1038/374660a0. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC. The bases for Cockayne's syndrome. Nature. 2000;405:415–416. doi: 10.1038/35013197. [DOI] [PubMed] [Google Scholar]
- Itin PH, Pittelkow MR. Tricothiodystrophy: review of sulfur-deficient brittle hair syndromes and association with the ectodermal dysplasias. J Am Acad Dermatol. 1990;20:705–717. doi: 10.1016/0190-9622(90)70096-z. [DOI] [PubMed] [Google Scholar]
- Koken MHM, Vreeken C, Bol SAM, Cheng NC, Jaspers-Dekker I, Hoeijmakers HJH, Eeken JCJ, Weeda G, Pastink A. Cloning and characterization of the Drosophila homologue of the xeroderma pigmentosum complementation-group B correcting gene, ERCC3. Nucleic Acids Res. 1992;20:5541–5548. doi: 10.1093/nar/20.21.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle S, Pandur J, Fisher RP, Salz HK, Suter B. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev. 1998;12:370–381. doi: 10.1101/gad.12.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V, Raisin S, Léopold P. Dominant-negative mutants reveal a role for the Cdk7 kinase at the mid-blastula transition in Drosophila embryos. EMBO J. 2000;19:1567–1575. doi: 10.1093/emboj/19.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AR. Dual functions of DNA repair genes: molecular, cellular and clinical implications. BioEssays. 1998;20:146–155. doi: 10.1002/(SICI)1521-1878(199802)20:2<146::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, tree diseases. Genes Dev. 2001;15:15–23. doi: 10.1101/gad.859501. [DOI] [PubMed] [Google Scholar]
- Le Page F, Kwoh E, Avrutskaya A, Gentil A, Leadon S, Sarasin A, Cooper P. Transcription-coupled repair of 8-oxoGuanine: requirement for XPG, TFIIH, and CSB and implications for Cockayne syndrome. Cell. 2000;101:159–171. doi: 10.1016/s0092-8674(00)80827-2. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego: Academic Press; 1990. [Google Scholar]
- Ljungman M, Fenfen Z. Blockage of RNA polymerase as possible trigger for UV light-induced apoptosis. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- Lu H, Fisher RP, Bailey P, Levine AJ. The CDK7-CycH-p36 complex of transcription factor IIH phosphorylates p53, enhancing its sequence-specific DNA binding activity in vitro. Mol Cell Biol. 1997;17:5923–5934. doi: 10.1128/mcb.17.10.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino E, Osuna J, Bolívar F, Soberón X. A general, PCR-based method for single or combinatorial oligonucleotide-directed mutagenesis on pUC/M13 vectors. Biotechniques. 1992;12:508–510. [PubMed] [Google Scholar]
- Mounkes LC, Jones RS, Bee-Choo L, Gelbart W, Fuller MT. A Drosophila model for xeroderma pigmentosum and Cockayne's syndrome: haywire encodes the fly homologue of ERCC3, a human excision repair gene. Cell. 1992;71:925–937. doi: 10.1016/0092-8674(92)90389-t. [DOI] [PubMed] [Google Scholar]
- Mounkes LC, Fuller MT. Molecular characterization of mutant alleles of the DNA repair/basal transcription factor haywire/ERCC3 in Drosophila. Genetics. 1999;152:291–297. doi: 10.1093/genetics/152.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance MA, Berry SA. Cockayne Syndrome: review of 140 cases. Am J Med Genet. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Ollman M, et al. Drosophila p53 is a structural and functional homologue of the tumor suppressor p53. Cell. 2000;101:91–101. doi: 10.1016/S0092-8674(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Reynaud E, Lomelí H, Vázquez M, Zurita M. The Drosophila melanogaster homologue of the xeroderma pigmentosum D gene product is located in euchromatic regions and has a dynamic response to UV light-induced lesions in polytene chromosomes. Mol Biol Cell. 1999;10:1191–1203. doi: 10.1091/mbc.10.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou L, Zeng L, Chevallier-Lagente O, Stary A, Nikaido O, Taieb A, Weeda G, Mezzina M, Sarasin A. The relative expression of mutated XPB genes results in xeroderma pigmentosum/Cockayne's syndrome or trichothiodystrophy cellular phenotypes. Hum Mol Genet. 1999;8:1125–1133. doi: 10.1093/hmg/8.6.1125. [DOI] [PubMed] [Google Scholar]
- Robles AI, Wang XW, Harris CC. Drug-induced apoptosis is delayed and reduced in XPD lymphoblastoid cell lines: possible role of TFIIH in p53-mediated apoptotic cell death. Oncogene. 1999;19:4681–4688. doi: 10.1038/sj.onc.1202862. [DOI] [PubMed] [Google Scholar]
- Rolig RL, McKinnon PJ. Linking DNA damage and neurodegeneration. T Neurobiol Sci. 2000;23:417–423. doi: 10.1016/s0166-2236(00)01625-8. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Londesborough A, Korsisaari N, Pihlak A, Lehtonen E, Henkemeyer M, Mäkelä TP. Inability to enter S phase and defective RNA polymerase II CTD phosphorylation in mice lacking Mat1. EMBO J. 2001;20:2844–2856. doi: 10.1093/emboj/20.11.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M, Kolb-Cheynel I, Egly JM. Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH. EMBO J. 1997;16:1628–1637. doi: 10.1093/emboj/16.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roter AH, Spofford JB, Swiff S. Synthesis of the major adult cuticle proteins of Drosophila melanogaster during hypoderm differentiation. Dev Biol. 1985;107:420–431. doi: 10.1016/0012-1606(85)90324-0. [DOI] [PubMed] [Google Scholar]
- Roy R, Adamczewski JP, Seroz T, Vermeulen W, Tassan J-P, Schaeffer L, Nigg EA, Hoeijmakers JHJ, Egly JM. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994;79:1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Roy R, Humbert S, Moncolin V, Vermeulen W, Hoeijmakers JHJ, Chambon P, Egly JM. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993;260:58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Serizawa H, Makela TP, Conaway JW, Conaway RC, Weinberg RA, Young RA. Association of Cdk-activation kinase subunits with transcription factor TFIIH. Nature. 1995;374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- Shiekhattar R, Mermelstein F, Fisher RP, Dynlacht B, Wesslilng HC, Morgan DO, Reinberg D. Cdk-activating kinase complex is a component in human transcription factor TFIIH. Nature. 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- Smith DB. Purification of glutathione-S-transferase fusion proteins. Methods Mol Cell Biol. 1993;4:220–229. [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P-elements into Drosophila germline chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Stathakis DG, Burton DY, McIvor WE, Krishnakumar S, Wright TR, O′Donell JM. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup JQ, Wang Z, Feaver WJ, Wu X, Bushnell DA, Donahue TF, Friedberg EC, Kornberg RD. Different forms of TFIIH for transcription and DNA repair: holo-TFIIH and a nucleotide excision repairosome. Cell. 1995;80:21–28. doi: 10.1016/0092-8674(95)90447-6. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Bernard CB, Botta E, Stefanini M, Sarasin A, Jaspers NGJ, Fawcett H, A, Colin FA, Lehmann AR. Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc Natl Acad Sci USA. 1997;94:8658–8663. doi: 10.1073/pnas.94.16.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Regulation of actin filament cross-linking and bundle shape in Drosophila bristles. J Cell Biol. 2000;148:87–99. doi: 10.1083/jcb.148.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen CE, Hoeijmakers JH. Transcriptional healing. Cell. 2000;101:447–450. doi: 10.1016/s0092-8674(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Vigoreaux J, O, Tobin SL. Stage-specific selection of alternative transcriptional initiation sites from the 5C actin gene of Drosophila melanogaster. Genes Dev. 1987;10:1161–1171. doi: 10.1101/gad.1.10.1161. ,. [DOI] [PubMed] [Google Scholar]
- Wang XW, et al. The XPB and XPD helicases are components of the p53-mediated apoptosis pathway. Genes Dev. 1996;10:1219–1232. doi: 10.1101/gad.10.10.1219. [DOI] [PubMed] [Google Scholar]
- Wang XW, Yeh H, Schaefer L, Roy R, Moncollin V, Egly JM, Wang Z, Friedberger EC, Evans MK, Taffe BG. p53 modulation of TFIIH-associated nucleotide excision-repair activity. Nat Genet. 1995;10:188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- Weeda G, Eveno E, Donker I, Vermeulen W, Chevallier-Lagente O, Taieb A, Stary A, Hoeijmakers JHJ, Mezzina M, Sarasin A. A mutation in the XPB/ERCC3 DNA repair transcription gene, associated with trichothiodystrophy. Am J Hum Genet. 1997;60:320–329. [PMC free article] [PubMed] [Google Scholar]
- Weeda G, van Ham RCA, Vermeulen W, Bootsma D, van der Eb AJ, Hoeijmakers JHJ. A presumed DNA helicase encoded by ERCC-3 is involved in repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990;62:777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Wood RD. DNA repair in eukaryotes. Annu Rev Biochem. 1996;65:135–167. doi: 10.1146/annurev.bi.65.070196.001031. [DOI] [PubMed] [Google Scholar]
- Yankulov YK, Bentley DL. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 1997;16:1638–1646. doi: 10.1093/emboj/16.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]