Abstract
Calcineurin is a Ca2+-calmodulin–dependent serine/threonine protein phosphatase that has been implicated in various signaling pathways. Here we report the identification and characterization of calcineurin genes in Caenorhabditis elegans (cna-1 and cnb-1), which share high homology with Drosophila and mammalian calcineurin genes. C. elegans calcineurin binds calcium and functions as a heterodimeric protein phosphatase establishing its biochemical conservation in the nematode. Calcineurin is expressed in hypodermal seam cells, body-wall muscle, vulva muscle, neuronal cells, and in sperm and the spermatheca. cnb-1 mutants showed pleiotropic defects including lethargic movement and delayed egg-laying. Interestingly, these characteristic defects resembled phenotypes observed in gain-of-function mutants of unc-43/Ca2+-calmodulin–dependent protein kinase II (CaMKII) and goa-1/Go-protein α-subunit. Double mutants of cnb-1 and unc-43(gf) displayed an apparent synergistic severity of movement and egg-laying defects, suggesting that calcineurin may have an antagonistic role in CaMKII-regulated phosphorylation signaling pathways in C. elegans.
INTRODUCTION
Calcineurin (CaN), a protein phosphatase 2B (PP2B), is a serine/threonine phosphatase under the control of Ca2+/calmodulin (Klee et al., 1979; Stewart et al., 1982; Klee et al., 1998). Although CaN is a member of a family of protein phosphatases, it is structurally and functionally distinct from alkaline and acid phosphatases (Cohen, 1989; Guerini and Klee, 1991). Calcineurin is a heterodimer of an ∼60-kDa catalytic subunit, calcineurin A (CNA), and a 19-kDa regulatory subunit, calcineurin B (CNB; Klee et al., 1988), and the two-subunit structure is well conserved from yeast to human. CaN is abundantly expressed in the brain and broadly distributed in nonneural tissues as well (Kincaid, 1993). Among its several functions in controlling intracellular Ca2+ signaling, CaN participates in gene regulation and external signal-mediated biological responses in many organisms and in many cell types (Crabtree, 1999).
CaN functions have been extensively studied in the yeast, Saccharomyces cerevisiae. To investigate the biological role of CaN, immunosuppressant drugs Cyclosporin A (CsA) and FK506 were used to inhibit CaN function (Cyert, 1993). Calcineurin has been shown to regulate Ca2+ pumps and exchangers to maintain Ca2+ homeostasis (Stark, 1996). Calcineurin is also known to regulate adaptation to high salt stress (Hirata et al., 1995). However, in higher animals, it is better known to regulate the transcription of the T-cell growth factor, interleukin-2 (Schreiber and Crabtree, 1992). Dephosphorylation of the transcription factor NF-ATp (nuclear factor–activated T cells) by CaN is required for the translocation of NF-AT from the cytoplasm to the nucleus, in response to an increased intracellular Ca2+ level. Calcineurin also plays a role in programmed cell death (Shibasaki and McKeon, 1995) and in hippocampal long-term depression (Mulkey et al., 1994). Furthermore, studies reveal that CaN plays a critical role in the pathogenesis of hypertrophic cardiomyopathy (Molkentin, 1998). Thus, calcineurin as a key signaling molecule has been shown to be involved in diverse types of physiological processes.
Caenorhabditis elegans has been an ideal model to study gene functions especially at the organism level using a genetic approach. Moreover, C. elegans has been useful for identifying interactions between molecules in biochemical signaling pathways that are associated with a certain behavior or phenotype. In this study, we have identified and characterized the C. elegans homologue of calcineurin B, which binds calcium and functions together with calcineurin A as a heterodimeric protein phosphatase. Null mutants of calcineurin B showed multiple adverse phenotypes including defects in locomotion and egg laying. Interestingly, these phenotypes are quite similar to those observed in gain-of-function mutants of unc-43, which encodes the Ca2+-calmodulin–dependent protein kinase CaMKII. Recently, a G-protein signaling pathway regulated by unc-43 has been found to be involved in locomotory and egg-laying functions (Robatzek and Thomas, 2000). Our results describing the relationship between the cnb-1 null mutant and mutants of unc-43 indicate that calcineurin and CaMKII may have opposing and complementary functions in this G-protein signaling pathway in C. elegans.
MATERIALS AND METHODS
C. elegans Strains
The following strains were obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota, Duluth, MS: Bristol N2, CB224 dpy-11(e224)V, CB1482 sma-6(e1482)II, MT1092 unc-43(n498)IV, unc-43(n1186)IV, and CB1282 dpy-20(e1282) IV. KJ300 cnb-1(jh103) was isolated by reverse genetics method (Park et al., 2001b). cnb-1(ok276) was isolated by the C. elegans Knockout Consortium (G. Molder, Oklahoma). Worm breeding and handling were conducted as described (Brenner, 1974).
Cloning of C. elegans Calcineurin A and Calcineurin B cDNAs and Northern Analysis
To obtain full-length cDNA clones of C. elegans calcineurin A (cna-1) or calcineurin B (cnb-1), cDNA library screening was conducted following plaque hybridization procedure (Sambrook et al., 1989). A mixed-stage worm cDNA library (kindly provided by P. Okkema and A. Fire) was probed with the partial cDNA clones, yk375h10 or yk496e12 (obtained from Y. Kohara). The largest inserts of 2869 base pairs and 943 base pairs for cna-1 and cnb-1, respectively, were sequenced and confirmed by Northern analysis. For Northern blotting, total RNA was prepared from staged animals and transferred to a Zeta probe membrane (Bio-Rad, Hercules, CA) as described (Krause, 1995; Cho et al., 2000). The membrane was hybridized in hybridization buffer (0.25 M Na2HPO4, pH 7.2), 0.25 M NaCl, 1 mM EDTA, 7% SDS, 50% Formamide, 5% dextran sulfate, and 100 μg/ml denatured salmon sperm DNA at 42°C with random-primed 32P-labeled probes. Exposure of the blots after high stringent washing in 0.5× SSC and 0.1% SDS was performed on x-ray films (Fuji) overnight at −80°C.
Expression and Purification of GST-CNA-1 and GST-CNB-1, Ca2+-binding, and Yeast Two-hybrid Assays
Complementary DNAs encoding the entire open reading frames of cna-1 or cnb-1 were subcloned in pGEX4T-3 or pGEX4T-1 (Pharmacia, Piscataway, NJ), encoding GST-CNA-1 or GST-CNB-1, respectively. Syntheses of GST-fused proteins were carried in Escherichia coli strains (BL21) at 30°C for CNA-1 and at 37°C for CNB-1 in the presence of 1 mM IPTG. Cells were harvested and sonicated according to the methods described (Zhao et al., 1993).
Ca2+-binding assay for CNB-1 was performed as described earlier (Maruyama et al., 1984). Purified GST-CNB-1 (see above) was resolved by 12% SDS-PAGE, transferred to PVDF membrane, and then probed with 45Ca2+ (Cho et al., 2000). Purified GST, bovine calmodulin (CaM; Sigma), and C. elegans calsequestrin (CSQ-1) were used as controls.
Wild-type cna-1 and cnb-1 cDNAs, covering the entire open reading frames, were fused in-frame to the GAL4 DNA-binding domain and GAL4 activation domain of the yeast vectors pAS2–1 and pACT2 (Clontech, Palo Alto, CA) to produce the plasmids CJ1 and CJ2, respectively. Plasmids were transformed into the yeast strain AH109 according to the manufacturer's protocol (Clontech). Transformants were plated on synthetic dropout (SD) media lacking Trp and Leu. Interaction assays were conducted on plates containing 5 mM 3-aminotriazole (3-AT) in SD without Trp, Leu, His, and Ade in absence or presence of 2 mM CaCl2.
In Vitro Phosphatase Assay
Equimolar concentrations (0.016 nmoles each) of purified GST-CNA-1 and GST-CNB-1 (see above) were used to conduct phosphatase assay (Promega) using a phosphopeptide (100 μM) as a substrate. To test the Ca2+ dependency of calcineurin, 0.2 mM EGTA (Ca2+ chelator) was used in the reactions. Inhibition of phosphatase activity by the immunosuppressant drug, cyclosporin A, CsA (Calbiochem, La Jolla, CA), was tested by preincubating GST-CNA-1 and GST-CNB-1 together with 1 μM each of CsA and bovine cyclophilin (Sigma, St. Louis, MO) at 4°C for 1 h. The dephosphorylation of the phosphopeptide was determined spectrophotometrically at 595 nm. The optical density was further converted to pmol phosphate release/min/μg protein using appropriate standards supplied with the kit.
Construction and Expression of gfp Fusion Constructs
Two cosmid clones C02F4 and F55C10 were obtained from A. Coulson (The Sanger Center, UK). To check the reporter gene (green fluorescent protein, gfp) expression, promoterless GFP vectors, pPD95.70 containing the nuclear localization signal (NLS), pPD95.79 and pPD95.75 (vectors provided by A. Fire) were used in the present study. All constructs (pJJ001–pJJ004) were generated as translational fusions with gfp. Microinjection using pRF4 (dominant rol-6) as a transformation marker was performed as described by Mello and Fire (1995).
Immunofluorescence and Immunogold Microscopy
Wild-type C. elegans was immunostained as described (Miller and Shakes, 1995; Cho et al., 2000). For gonad immunostaining, gonads were extruded by decapitating adult C. elegans and fixed with 3% formaldehyde, 0.1 M K2HPO4 (pH 7.2), for 1 h and postfixed with cold (−20°C) 100% methanol for 5 min. Antibody incubations and washes were performed as described (Jones et al., 1996). Anti–CNA-1 mouse monoclonal and anti–CNB-1 rabbit polyclonal antibodies were used as primary antibodies. TRITC-conjugated anti-mouse (Sigma) and FITC-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) were used as secondary antibodies. Sperm immunostaining was conducted according to the methods described previously (Arduengo et al., 1998).
Immunogold staining with anti–CNB-1 antibody for N2 worms was carried out as described previously (Park et al., 2001a). Samples were examined under a transmission electron microscope (Jeol 1200 EXII, Peabody, MA).
Isolation of a cnb-1 Deletion Mutant from a Mutagenized DNA Library
TMP (Trimethylpsoralen)/UV method was used to generate C. elegans deletion mutant. Screening of mutants from the mutagenized DNA library was carried out by a nested PCR-based method and subsequent sib selections as described (Barstead, 1999). Primers were designed based on the predicted sequences spanning the full genomic DNA of cnb-1: outer upstream primer (5′-ACA TTC TAC TAC ATT CTG GCT GTG TGA TCC-3′) and downstream primer (5′-ATG AGC ATC ATT TAT TTG GCG GAC C-3′), inner upstream primer (5′-AAG CCC TCT GCT GGA CTG CTG TCC ACC-3′), and downstream primer (5′-AAT GCG AGG AAA CGC TTC CCA ATT GGC-3′). A homozygous line of animals with a 950-base pair deletion relative to the wild-type was isolated. This animal was outcrossed six times to wild-type animals to establish the strain KJ300 cnb-1(jh103) and was used in subsequent analysis. Deletion region for the cnb-1 hermaphrodites was determined by nested PCR followed by sequencing the PCR products.
Construction of Double Mutant Strain and Phenotype Analysis
Double mutants of unc-43(n498);cnb-1(jh103), unc-43(n1186);cnb-1(jh103), and goa-1(n1134);cnb-1(jh103) were constructed by standard genetic methods. dpy-20(e1282) was used as a genetic marker. PCR was used to detect the cnb-1 mutant.
The brood size of N2, cnb-1, unc-43, and unc-43(n498);cnb-1(jh103) hermaphrodites was determined by placing individual worms on seeded plates and allowing self-fertilization at the indicated temperature. The P0 mother was then transferred to a fresh plate at 24-h intervals for each of the next 4 days. Total F1 progeny on the plates were counted. The brood size of crossed progeny was determined by placing a single N2 or cnb-1 hermaphrodite with three wild-type males on seeded plates and allowing crossing at 20°C for 2 d. Total F1 progeny and the number of male progeny on the plates were counted.
Body length, body width, and uterine embryos were carefully examined under a dissecting microscope. L4-stage larvae were transferred to a new plate and allowed to grow for 36 h. The resulting 1-d adult worms were then examined for phenotypes. Movement of animals was examined by placing five adult hermaphrodites on a bacterial lawn, and after 5 min the tracks made by the worms were photographed. Serotonin-mediated egg-laying phenotypes were examined as previously described (Trent et al., 1983). Levamisole-mediated egg-laying phenotypes were examined in the same way at concentrations ranging from 25 to 100 μm.
Sperm morphology was analyzed in hermaphrodites as previously described (L'Hernault and Roberts, 1995) by hand-dissecting hermaphrodite spermatheca in sperm medium (50 mM NaCl, 25 mM KCl, 5 mM CaCl2, 1 mM MgSO4, 5 mM HEPES, 10 mM dextrose, 4 mM levamisole, pH 7.8). The spermatheca was observed with a Zeiss Universal microscope equipped with Nomarski optics.
Transformation Rescue
To test for rescue of cnb-1(jh103) homozygotes, cnb-1 genomic DNA cloned in pGEM-T Easy vector (Promega) and csq-1::gfp fusion construct as a transformation marker (Cho et al., 2000) at final concentrations of 50 and 50 μg/ml, respectively, were coinjected into cnb-1 mutant animals using standard methods (Mello and Fire, 1995). The injected parents were allowed to self-fertilize. Individual GFP-expressed progeny were then picked and examined for phenotypes.
RESULTS
Characterization of the C. elegans Calcineurin B (cnb-1) Gene
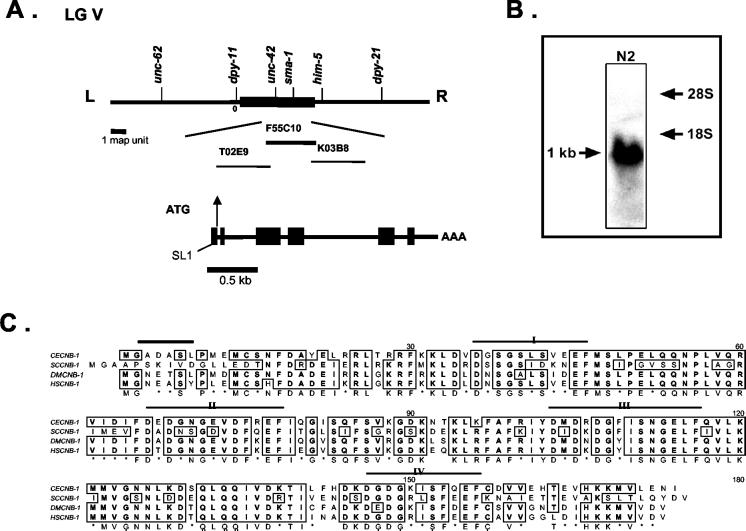
C. elegans calcineurin B-like genes have been physically mapped to the cosmids F55C10 and F30A10, respectively. However, alignment studies and molecular cloning (this study) have revealed that F55C10.1 is the putative calcineurin B (cnb-1), the regulatory subunit of the protein phosphatase 2B with four EF-hand motifs for Ca2+ binding (see below). The cnb-1 locus is physically mapped to the gene cluster region of chromosome V (LGV) on the cosmid F55C10 that corresponds to the region between unc-42 and sma-1 loci on the genetic map (Figure 1A). Northern blot analysis confirmed a single 1.0-kb mRNA transcript of cnb-1 (Figure 1B).
Figure 1.
Genomic organization of calcineurin genes in C. elegans. (A) Genetic and physical maps of the cnb-1 region. The cnb-1 locus relative to the nearby genetic markers on the gene cluster region of LG V is shown. cnb-1, which is mapped to the cosmid F55C10 (Accession No. Z74036), and the neighboring cosmids are indicated. The predicted F55C10.1 gene (cnb-1) structure has been revised and is presently shown to contain six exons (black boxes). (B) Northern blot analysis detects a 1-kb band in RNA samples of wild-type N2 worms. (C) Amino acid sequence alignment for calcineurin B of C. elegans (CECNB-1), Drosophila (DMCNB-1, Accession No. P48451), human (HSCNB-1, Accession No. NP000936), and yeast (SCCNB-1, Accession No. NP_012731). Regions of identity among calcineurin B homologues are boxed. Four Ca2+-binding sites are shown (I–IV). The myristoylation signature is indicated by a black bar.
C. elegans calcineurin B, as in other species, is also a small protein of 171 amino acids exhibiting high sequence homology with other organisms (80% identity with human and Drosophila calcineurin B and 58% with the yeast; Figure 1C). A putative initiation Met (ATG) codon is located at nucleotide position 51, which is surrounded by a relatively good consensus sequence for translation (Kozak, 1991) also found in other C. elegans genes (Krause, 1995). Additionally, the cDNA appears to contain a partial SL1 leader sequence at the 5′ end. The C. elegans calcineurin A (cna-1) gene was recently identified by Kuhara et al. (2002). The tax-6 locus was shown to encode calcineurin A, and our results confirmed the cna-1/tax-6 sequence and gene structure from previously obtained results (Kuhara et al., 2002).
CNB-1 Is a Ca2+-binding Protein and Enhances CNA-1 Phosphatase Activity
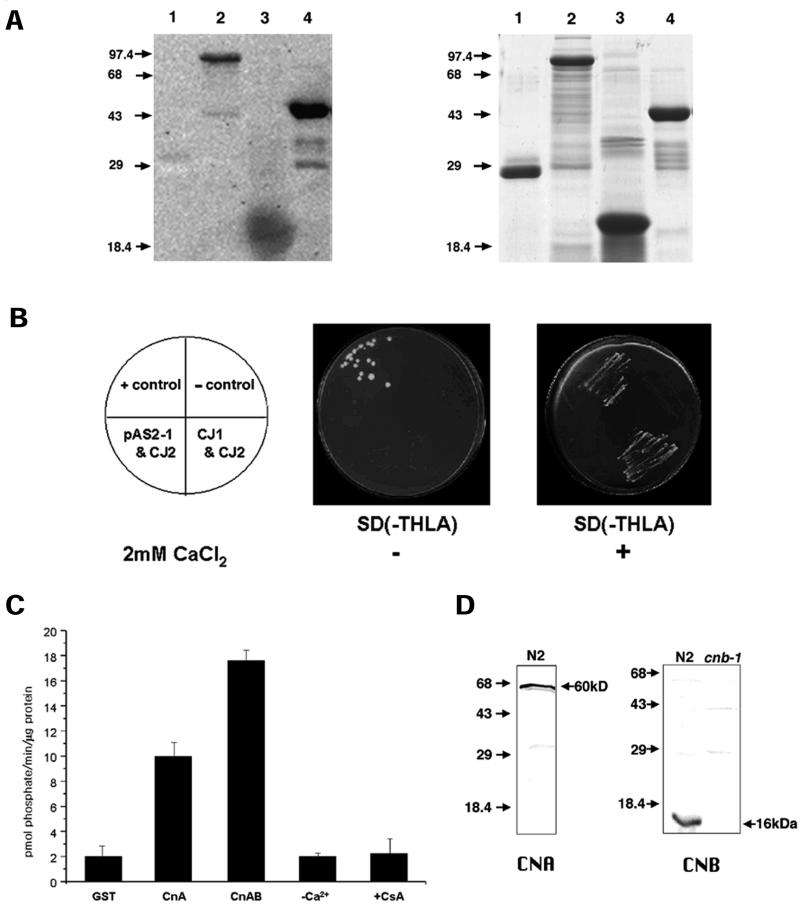
It is well known that CaN binds Ca2+ and calmodulin (CaM), as originally shown by Klee et al. (1979). Calcineurin B, the regulatory subunit of CaN binds 4 molecules of Ca2+ with high affinity (Kd ≤ 10−6 M), and has sequence homology with CaM and troponin C, two other calcineurin binding proteins (Aitken et al., 1982). Recombinant GST-CNB-1 had strong Ca2+-binding activity (Figure 2A, lane 4) whereas GST alone showed no Ca2+-binding activity (Figure 2A, lane 1). Thus, Ca2+ overlay experiments confirmed that CNB-1 has high affinity for Ca2+ as in other Ca2+-binding proteins such as C. elegans calsequestrin CSQ-1 (Cho et al., 2000) and bovine CaM (Figure 2A, lane 2 and lane 3, respectively).
Figure 2.
Biochemical characterization of C. elegans calcineurin. (A) cnb-1 is a Ca2+-binding protein. Ca2+-binding assay was performed with GST (glutathione-S-transferase) (lane 1), C. elegans calsequestrin, GST-CSQ-1 (lane 2), bovine CaM (lane 3), and GST-CNB-1 (lane 4). 45Ca2+-labeled proteins are shown by the dark signals on the x-ray film. GST (lane 1) is used as a negative control. A corresponding gel shown in the right panel is stained with Coomassie Blue. Molecular weight markers (in kDa) are denoted by arrows. (B) Interaction between CNA-1 and CNB-1 in the yeast two-hybrid system. Growth of yeast transformants on SD −Trp −His −Leu −Ade with 5 mM 3-AT (panel 2) and 5 mM 3-AT + 2 mM CaCl2 (panel 3) are shown. Plates supplemented with 5 mM 3-AT + 2 mM CaCl2 select for a positive interaction between the fusion proteins. Positive interaction between AD/T-antigen and DNA-BD/p53 fusion proteins and the negative or no interaction between AD/T-antigen and DNA-BD/lamin C were used as positive (+ control), and negative controls (− control), respectively. The array of the yeasts containing the different constructs is indicated in the schemes shown in panel 1. (C) Serine/threonine phosphatase activity of CNA-1. Dephosphorylation of the phosphopeptide by GST, GST-CNA-1 (CnA), and GST-CNA-1 + GST-CNB-1 (CnAB) are shown. Ca2+-dependence of the phosphatase activity (−Ca2+) was determined by adding 0.2 mM EGTA (Ca2+-chelator) during the CnAB reaction. Inhibition of phosphatase activity was determined by adding 1 μM each of CsA and bovine cyclophilin (+CsA) in the CnAB reaction mixture. Assays were performed in triplicate with equimolar concentrations of CNA-1 and CNB-1. (D) Western blot analysis with anti–CNA-1 and anti–CNB-1 antibodies. Worm protein extracts prepared from N2 wild-type worms or cnb-1(jh103) mutants (CNB, lane 2). The band is absent in the cnb-1(jh103) deletion worms indicating they are functionally null mutants.
Calcineurin is a tight heterodimer composed of the catalytic A subunit and the regulatory B subunit (Klee et al., 1988; Perrino et al., 1995). This interaction is necessary for the function and stabilization of the enzyme. We utilized the yeast two-hybrid system to check if CNA-1 interacted with CNB-1. As shown in Figure 2B, the subunits interacted with each other in the presence of Ca2+ as indicated by the growth of colonies in the selective media (compare panels 2 and 3).
To further test if C. elegans calcineurin exhibits phosphatase activity in vitro, a serine/threonine phosphatase assay was conducted with purified full-length GST-CNA-1 and GST-CNB-1 expressed in E. coli. It was previously demonstrated that GST does not interfere with or possess phosphatase activity (Chin-Sang and Spence, 1996). We carried out experiments with or without GST-CNB-1 to check whether the rate of dephosphorylation can be regulated in the absence or presence of the regulatory subunit, and at the same time tested whether there was basal activity of GST-CNA-1. As shown in Figure 2C, GST-CNA-1 alone showed a dephosphorylation activity (∼10 pmol phosphate/min/μg protein), whereas addition of GST-CNB-1 elevated the activity by ∼2-fold indicating that the full activity of the enzyme requires both subunits of calcineurin. Addition of EGTA (a Ca2+-chelator) abolished the phosphatase activity to control levels indicating that the phosphatase required Ca2+ for its activity (Figure 2C). Furthermore, we tested if the immunosuppressant, cyclosporin A (CsA), affects the phosphatase activity. Cyclophilin-CsA complex has long been known to block the phosphatase activity of calcineurin (Liu et al., 1991). Previously, several cyclophilin (cyp) genes had also been cloned and characterized in C. elegans indicating once again a well-conserved role(s) for cyclophilin in signal transduction and protein folding (Page et al., 1996). In the present study, we used bovine cyclophilin with CsA to test if C. elegans calcineurin activity is affected. As expected, the phosphatase activity of CNA-1 was completely inhibited (Figure 2C) in the presence of cyclophilin-CsA implicating a conserved phenomenon of phosphatase inhibition by the immunosuppressant drug.
Expression and Localization of Calcineurin in C. elegans
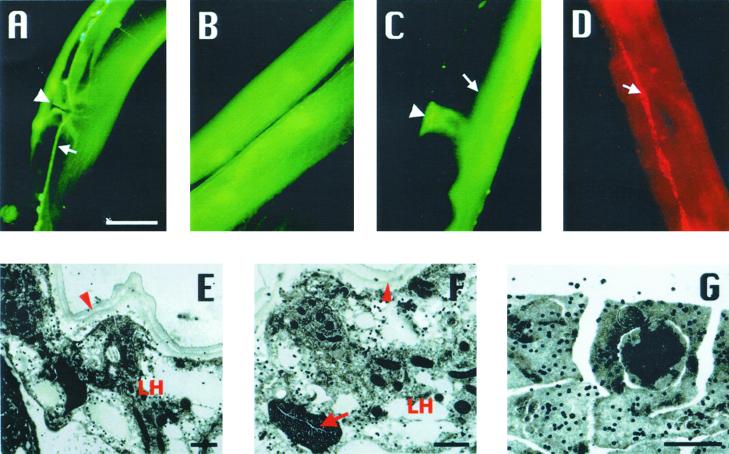
We examined the temporal and spatial pattern of cna-1 and cnb-1 expression using the gfp reporter driven by cna-1 or cnb-1 5′-upstream regulatory sequences. Both cna-1 and cnb-1 reporter transgenes expressed in diverse tissues. Expression was detected at all stages of development starting from early comma stage embryos to adult stages. Calcineurin is expressed in vulval muscle, body-wall muscle, spermatheca (Figure 3, A–C), and in a majority of neuronal cell bodies in the head and tail similar to previously obtained results (Kuhara et al., 2002)
Figure 3.
Expression and localization of calcineurin. Images show only representative expression. CNA-1::GFP expressed in (A) ventral nerve cord (arrow) and vulva muscles (arrowhead) and (B) body-wall muscles of the midbody region. CNB-1::GFP expresses in spermatheca (C; arrowhead) and intestine (C; arrow). Nerve cord expression can also be observed in this image. (D) Immunostaining of wild-type worms with anti–CNB-1 antibodies detected CNB-1 in hypodermal seam cells (arrow). Bar, 20 μm. (E–G) Immunogold electron micrograph (EM) showing the subcellular localization of CNB-1. (E) Transverse section through the midbody of a hermaphrodite showing CNB-1 localization as indicated by the signals of gold particles in the seam cells of the lateral hypodermis (LH) closely apposed to the cuticle (arrowhead). (F) Another region of seam cell showing signals. Lateral hypodermis (LH), cuticle (arrowhead), and excretory canal (arrow) are labeled. (G) Localization of CNB-1 in a wild-type male gonad. Distinct and scattered signals of CNB-1 are observed mainly in the cytoplasmic regions of the cellularized spermatids. Bar, 1 μm.
Polyclonal antibodies against CNA-1 and CNB-1 were generated, and western blots with total protein extracts from wild-type worms detected a single band around 60 kDa for CNA-1 and 16 kDa for CNB-1, respectively (Figure 2D). Immunostaining performed with these antibodies for both subunits of calcineurin showed similar localization to GFP expression patterns and additionally showed localization in hypodermal seam cells (Figure 3D). We localized CNB-1 at the subcellular level in specific tissues of wild-type animals by immunogold electron microscopy (EM). Signals of CNB-1 were observed in the seam cells of the lateral hypodermis of wild-type hermaphrodites consistent with our immunostaining data (Figure 3, D–F). Additionally, the male gonad also expressed CNB-1. This was evident from the scattered and distinct cytoplasmic signals of CNB-1 surrounding the cellularized spermatids (Figure 3G).
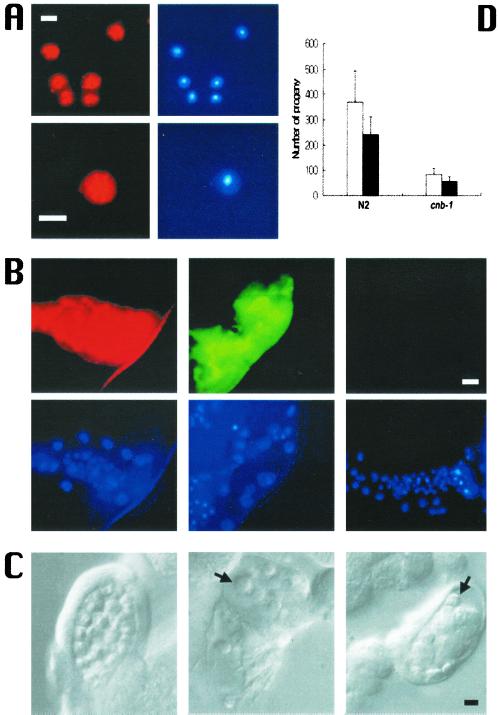
Based on these observations we further examined wild-type male sperm and immunostained with anti-CNA-1 or anti–CNB-1 antibodies. As expected, we observed robust staining in the wild-type sperm for both proteins and the staining was distinctly cytoplasmic (Figure 4A). We also confirmed CNA-1 and CNB-1 localization in the spermatheca by immunostaining isolated gonads (Figure 4B). Hence, electron microscopy and immunostaining data reveal that calcineurin is expressed in the C. elegans male germline, and therefore may have possible roles in germline development.
Figure 4.
Calcineurin in sperm and the spermatheca. (A) Immunostaining of wild-type sperm with anti-CNA-1 (top left panel) and anti–CNB-1 (bottom left panel) antibodies and the respective nuclei are shown by DAPI staining (right panels). Bar, 2 μm. (B) Immunostaining of wild-type and cnb-1 mutant spermatheca with calcineurin antibodies. Anti-CNA-1 (top left panel) and anti–CNB-1 (top middle panel) antibodies stain wild-type spermatheca. Spermatheca of cnb-1 mutant worms do not show any anti–CNB-1 staining (top right panel). DAPI staining of spermatheca is shown in the bottom panels. Bar, 20 μm. (C) Sperm and spermatheca defects. Compared with wild-type (left panel) cnb-1 mutant worms display defects in sperm and the spermatheca. cnb-1 sperm (middle and right panel). Arrows point to cnb-1 sperm, which are fewer in number and show a smoother appearance compared with N2 sperm (left panel). The surrounding cnb-1 spermatheca appears to contain debris. (D) Mating of N2 and cnb-1 hermaphrodites with N2 males. Three N2 males were mated with one N2 or one cnb-1 hermaphrodite for 2 days. Total progeny and outcrossed progeny are indicated by white and black bars, respectively.
cnb-1 Is Involved in Normal Cuticle Formation, Sperm Morphology, and Brood Size
We have isolated cnb-1(jh103) deletion mutants by PCR-based TMP/UV mutagenesis method. The cnb-1(jh103) null mutants are viable but exhibit multiple phenotypes. A second deletion mutant strain, cnb-1(ok276), was also isolated (kindly donated by the C. elegans Knockout Consortium, Oklahoma) and exhibited identical phenotypes to cnb-1(jh103). We further checked the protein profile of the cnb-1(jh103) deletion mutants and found no protein band on western blots confirming that the deletion mutants are functionally null (Figure 2D, lane 2).
The cnb-1(jh103) mutants were examined for phenotypic defects compared with the wild-type worms. At the surface of the worm, the cuticle of cnb-1 mutants appears to thin out, resulting in the animals having a more transparent appearance. This phenotype seems consistent with a loss of calcineurin in hypodermal tissue in cnb-1 mutant worms. cnb-1 mutants also have a significantly decreased brood size compared with wild-type (Table 1). This observation taken together with the immunostaining data led us to believe that there may be defects in cnb-1 sperm. Indeed, under close inspection, cnb-1 sperm morphology was different from wild-type sperm. The defective sperm were smaller and smoother than wild-type sperm (Figure 4C) and displayed smaller pseudopods. Moreover, sperm, which normally gather in high numbers in the spermatheca, were scarcely found in mutant worms. In addition, these cnb-1–deficient spermatheca were filled with oocyte debris indicative of a possible endomitotic oocyte (emo)-like defect caused by spermatheca defects. To distinguish whether the small brood size phenotype observed in the mutant was a direct cause of defective sperm or spermatheca defects, we mated N2 male worms with cnb-1 hermaphrodites. As Figure 4D shows, wild-type sperm only partially rescues the low brood size, and the percentage of outcrossed progeny of cnb-1 hermaphrodites is almost the same as outcrossed of N2 hermaphrodites. This suggests that altered sperm alone cannot account for the decreased fertility in cnb-1 mutants, and that defective spermatheca and/or oocytes may also play a role in this phenotype.
Table 1.
Brood size, body size, and egg-laying phenotypes
| Brood size (self progeny at 20°C) | Body length (mm) | Body width (μm) | Late stage embryos in uterus
|
||
|---|---|---|---|---|---|
| < commaa | ≥ commab | ||||
| N2 (WT) | 270 ± 45 (10) | 1.12 ± 0.05 (17) | 78.53 ± 2.35 (17) | 13 ± 1.8 | 0 (17) |
| cnb-1 | 106 ± 17 (15) | 0.79 ± 0.03 (20) | 55.13 ± 2.22 (20) | 6 ± 0.9 | 2 ± 0.9 (20) |
| cnb-1;Ex[cnb-1] | 146 ± 27 (8) | 1.04 ± 0.04 (17) | 68.38 ± 3.05 (17) | 13 ± 3.3 | 0 (17) |
| sma-6 | 123 ± 17 (10) | 0.80 ± 0.04 (20) | 69.88 ± 2.75 (20) | 10 ± 2.1 | 0 (20) |
| unc-43(gf) | 61 ± 12 (15) | 0.96 ± 0.04 (19) | 71.18 ± 2.81 (19) | 10 ± 1.7 | 12 ± 1.9 (19) |
| unc-43(null) | 173 ± 29 (15) | 1.01 ± 0.05 (19) | 70.53 ± 2.29 (19) | 6 ± 2.8 | 0 (19) |
| unc-43(gf);cnb-1 | 8 ± 7 (16) | 0.62 ± 0.03 (16) | 56.41 ± 3.16 (16) | 4 ± 1.3 | 3 ± 1.6 (16) |
Values are expressed as means ± SD; number in parentheses indicates the sample sizes for worms.
Number of embryos prior to comma stage inside a 1-d-old adult.
Number of embryos from comma stage onwards (comma–3-fold stage) inside a 1-d-old adult.
Defects in cnb-1 Mutants Resemble Defects Observed in unc-43 Mutants
Along with its small brood size, cnb-1 shows several other characteristic phenotypes. Firstly, mutant worms have small and slender bodies compared with wild-type animals. cnb-1 worms also retained embryos in the uterus varying from early to very late in development, such as threefold stage embryos, which was not seen in the wild-type worms. cnb-1 mutations also cause the worms to be uncoordinated (unc), or lethargic in their movement. Generally wild-type worms move rapidly in a sinusoidal pattern as evidenced from the tracks made by the worms (Figure 5A), but cnb-1 mutants moved slowly and with decreased amplitude of tracks. (Figure 5B). Each of these phenotypes was not constant over the lifetime of the worm, but rather all became more severe as the worm grew older. Brood size, body size, and embryo retention phenotypes are quantified and summarized in Table 1. We then sought to determine whether cnb-1 genomic DNA could rescue the cnb-1(jh103) phenotypes by microinjection. Movement of these transgenic animals appeared to be fully rescued (Figure 5C) and there was a significant recovery in brood size (146 ± 27 progeny; Table 1). The small body size and retention of late-stage embryos in the uteri were almost completely restored to normal state (Table 1) indicating that these pleiotropic phenotypes observed in the mutant were specifically caused by the loss of cnb-1 gene.
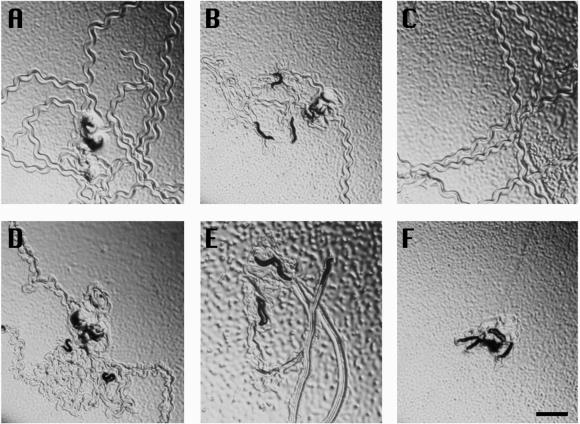
Figure 5.
Locomotion defects in mutants of cnb-1 and unc-43. Five animals were placed in the center of a bacterial lawn and photographed 5 min later. (A) Tracks of movement by wild-type animals; (B) cnb-1 mutants; (C) rescued cnb-1 mutants; (D) unc-43(lf) mutants; (E) unc-43(gf) mutants; (F) cnb-1; unc-43(gf) double mutants. Bar, 1 mm.
Although the phenotypes of cnb-1 mutants appeared to be pleiotropic, it is interesting to note that similar phenotypes were observed in other particular mutants. Mutants of the unc-43 gene, which encodes the Ca2+/calmodulin-dependent protein kinase CaMKII also show defects in brood size and progressive defects in body size, egg laying, and movement (Reiner et al., 1999). We compared phenotypes of gain-of-function and loss-of-function mutants of unc-43 with those of the cnb-1 null mutant and observed some interesting relationships. cnb-1 mutants and unc-43(n498)[gain-of-function (gf)] mutants were quite similar in phenotype, with both mutants showing much lower brood sizes, delayed egg laying, and smaller body size (Table 1). In addition, both worms displayed severe uncoordinated movement (Unc) phenotypes (Figure 5, B and E). Conversely, unc-43(n1186) [loss-of-function (lf)] mutants showed phenotypes mostly opposite to that of unc-43(gf) and cnb-1 mutants, displaying hyperactive movement (Figure 5D), a significantly higher brood size, and earlier egg-laying compared with that of cnb-1. On the other hand, sma-6 mutants, which also have a small body size phenotype, do not show other defects present in cnb-1 mutants suggesting that small body size alone does not directly affect these other phenotypes and that sma-6 and cnb-1 likely function in different pathways.
Phenotypes Related to G-protein–coupled Phosphorylation Signaling Pathways in C. elegans
It was shown that UNC-43/CaMKII regulates a G-protein pathway involving the Go-protein α-subunit, goa-1, in locomotory and egg-laying behavior (Robatzek and Thomas, 2000). A transgenic gain-of-function mutant of goa-1, syIs9[goa-1(gf)], displayed lethargic movement and egg retention behavior similar to phenotypes seen in unc-43(gf) mutants (Mendel et al., 1995). Conversely, loss-of-function mutants of goa-1 showed hyperactive movement and premature egg laying (Mendel et al., 1995). Thus, mutations in goa-1 result in similar phenotypes to those of unc-43 mutants, and, consequently, opposite phenotypes to that of cnb-1 mutants. We decided to further test whether cnb-1 could be operating in similar G-protein pathways. Along with the phenotypes tested in Table 1, goa-1 also had defects in serotonin-regulated egg laying (Mendel et al., 1995). Thus, we tested whether cnb-1 and unc-43 mutants had defects in this egg-laying behavior as well. Exogenous serotonin and imipramine were shown to stimulate egg laying in wild-type worms (Trent et al., 1983). However, both cnb-1 and unc-43(gf) mutants failed to respond to exogenous serotonin, whereas unc-43(lf) mutants were hypersensitive to serotonin treatment by laying even more eggs than wild-type (Table 2). cnb-1 and unc-43(gf) mutants also show a decreased sensitivity to imipramine, an agent that induces endogenous release of serotonin from stores in the presynaptic HSN neuron, compared with wild-type animals. When compared with the egg-laying defects observed in serotonin-treated syIs9[goa-1(gf)] mutants reported elsewhere (Mendel et al., 1995) cnb-1 and unc-43(gf) mutants show slightly more severe phenotypes in response to the treatment.
Table 2.
Characterization of serotonin-mediated egg-laying phenotypes
| Genotype | No. of animals laying the indicated number of eggs after treatment with:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M9 buffer
|
5-HT
|
Imipramine
|
||||||||||
| >7 | 4–7 | 1–3 | 0 | >7 | 4–7 | 1–3 | 0 | >7 | 4–7 | 1–3 | 0 | |
| N2 (WT) | 0 | 0 | 2 | 46 | 19 | 6 | 20 | 3 | 20 | 18 | 6 | 4 |
| cnb-1 | 0 | 0 | 1 | 47 | 0 | 0 | 8 | 40 | 0 | 5 | 21 | 22 |
| cnb-1;Ex[cnb-1] | 0 | 0 | 3 | 45 | 6 | 4 | 21 | 17 | 22 | 19 | 7 | 0 |
| unc-43(null) | 0 | 0 | 2 | 46 | 26 | 18 | 4 | 0 | 3 | 15 | 26 | 4 |
| unc-43(gf) | 0 | 1 | 11 | 36 | 1 | 6 | 19 | 3 | 4 | 18 | 18 | 8 |
| unc-43(gf);cnb-1 | 0 | 0 | 10 | 38 | 0 | 0 | 12 | 36 | 0 | 0 | 14 | 34 |
Worms were treated with either exogenous 5-HT, imipramine, or control M9 buffer for 90 min after which laid eggs were counted.
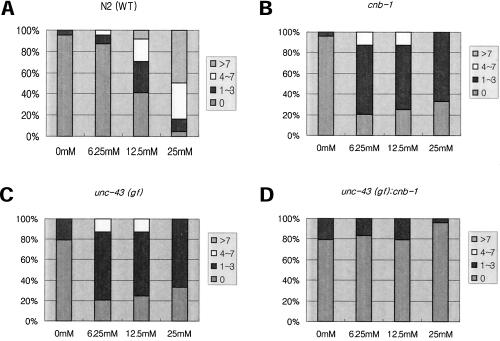
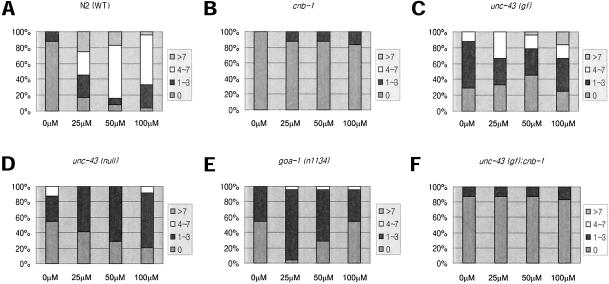
We next tested whether the serotonin-mediated egg-laying phenotypes observed in cnb-1 and unc-43 mutants were concentration-dependent. Unlike wild-type animals, which show increased egg laying in response to elevated concentrations of serotonin (Figure 6A), all three mutants displayed resistance to serotonin even at high concentrations (Figure 6, B and C). We also tested the egg-laying response to another exogenous agent, levamisole. Levamisole is an agonist of the UNC-29 nicotinic acetylcholine receptor localized in postsynaptic muscle, and levamisole treatment to wild-type C. elegans results in muscle hyper-contraction and subsequent egg laying (Figure 7A). In contrast, cnb-1 mutants and both unc-43(gf) and unc-43(lf) mutants are resistant to levamisole at all concentrations (Figure 7, B, C, and D). Finally, goa-1(n1134) loss-of-function mutants also show concentration-dependent resistance to levamisole (Figure 7E). This further suggests that cnb-1, like unc-43 and goa-1, are involved in similar aspects in the regulation of egg laying.
Figure 6.
Dose-dependent serotonin-mediated egg laying. Color-coded area in bars represent percentage of worms from the total number tested. Respective number of eggs were laid after serotonin treatment at the given concentrations. In each case N = 24. (A) N2 worms show serotonin-mediated egg laying in a dose-dependent manner, whereas (B) cnb-1 and (C) unc-43(gf) mutants and (D) unc-43(gf);cnb-1 double mutants were mostly resistant even at high concentration of serotonin.
Figure 7.
Dose-dependent levamisole-mediated egg laying. Color-coded area in bars represent percentage of worms from the total number tested. Respective number of eggs were laid after levamisole treatment at the given concentrations. In each case N = 24. (A) N2 worms show levamisole-mediated egg laying in a dose-dependent manner, whereas (B) cnb-1, (C) unc-43(gf), (D) unc-43(lf), (E) goa-1(n1134) mutants, and (F) unc-43; cnb-1 double mutants were mostly resistant even at high concentrations of levamisole.
To further validate the possibility that calcineurin, UNC-43, and GOA-1 may be functioning together to regulate these various functions, we attempted to generate double mutants between cnb-1 and unc-43 and goa-1 mutants and assess possible genetic relationships. Although we generated both unc-43(lf);cnb-1 and goa-1(n1134);cnb-1 double mutants, the resulting animals were arrested in early larval stages with severe morphological defects. Thus, epistatic phenotypes could not be assessed in these worms. On the other hand, unc-43(gf);cnb-1 double mutants were viable and developed to adulthood. Since unc-43(gf) encodes a protein kinase, mutants should have hyperactive phosphorylation activity in pathways where unc-43 may be involved in. Likewise, cnb-1, which encodes a protein phosphatase that was shown to have enzyme activity, should produce null mutants that have a loss of dephosphorylation activity in pathways which calcineurin may function in. If these two proteins function within a G-protein-coupled phosphorylation pathway in an opposing manner, then a double mutant of cnb-1 and unc-43(gf) would have phosphorylation pathways that are hyperpolarized without any opposing dephosphorylation activity, and, thus, should display more severe phenotypes than animals with single mutations alone. As we had predicted, this double mutant had even more severe defects than the individual mutants by themselves. The worms displayed extremely lethargic movement (Figure 5F), an average body size of less than half that of normal worms, synergistically small brood sizes with most worms being sterile, and severe egg retention leading to internally hatched young and eventual death of the parent worm (Table 1). unc-43(gf);cnb-1 double mutants also result in increased resistance to serotonin, imipramine, and levamisole treatment compared with the single mutants alone (Table 2; Figures 6D and 7F). In comparison to single mutants, synergistic effects in the double mutants can be clearly seen in fertility and movement phenotypes, and possibly egg-laying phenotypes as well. Therefore, our results suggest that calcineurin can regulate multiple functions in C. elegans that both unc-43 and goa-1 are known to be involved in, and a role for calcineurin phosphatase activity in a CaMKII-dependent G-protein coupled phosphorylation signaling pathway in C. elegans is a distinct possibility.
DISCUSSION
Calcineurin, a serine/threonine protein phosphatase, plays important roles in the transduction of Ca2+ signals to regulate various cellular processes (Klee et al., 1979; Stewart et al., 1982; Klee et al., 1988). In this study, we identified and characterized calcineurin from C. elegans, both at its molecular and cellular levels. In contrast to higher animals where calcineurin subunits are encoded by more than one gene, C. elegans calcineurin subunits are each encoded by a single gene. Results reported here also show that the homologues of the mammalian and Drosophila calcineurin subunits exist in C. elegans, and thus, they represent a conserved branch of the PP2B family of protein phosphatases having important roles in normal physiology (Crabtree, 2001).
The subunits of C. elegans calcineurin were shown to interact with one another, and this interaction conferred strong phosphatase activity to the heterodimer, which is consistent with studies on other conserved forms of calcineurin (Klee et al., 1979; Stewart et al., 1982; Klee et al., 1988). In addition, Ca2+-dependent phosphatase activity of CNA-1 was potently inhibited by the immunosuppressant CsA (Figure 2C) in agreement with previous reports (Liu et al., 1991). Sequence homology and Ca2+ overlay experiments verified that CNB-1 has strong calcium binding affinity. Thus, we believe that the biochemical function of calcineurin as a calcium-binding heterodimeric protein phosphatase is conserved in C. elegans.
A deletion null mutant of cnb-1 was isolated by target-selected mutagenesis. cnb-1(jh103) mutants are viable although loss of calcineurin activity leads to pleiotropic defects for the worms. It was shown that transgenic mice expressing a mutated form of CnB that cannot bind the CnA subunit will die early in embryo development (Graef et al., 2001). These mice exhibit vascular developmental abnormalities that stem from its inability to dephosphorylate and subsequently translocate the transcription factor NFAT from the cytoplasm into the nucleus. Thus, the dephosphorylation activity of calcineurin is dependent on both CnA and CnB subunits in vivo. This is also observed in S. cerevisiae as mutations in either CnA or CnB lead to a complete loss of calcineurin phosphatase activity and function in yeast (Cyert and Thorner, 1992). Finally, a recent study showed that a chemotaxis-defective mutant tax-6(p675), which displays thermotaxis, body size, and growth defects as well, carries a mutation in the cna-1 locus (Kuhara et al., 2002). Thus, a viable mutant of cnb-1 in a multicellular organism like C. elegans, although quite surprising, is important for further studies in the delineation of calcineurin's diverse functions in vivo.
Mutants of cnb-1 showed pleiotropic phenotypes including cuticle defects, small body size, decreased brood size, and locomotory and egg-laying defects. These defects were consistent with the loss of calcineurin function in tissues that normally express the protein phosphatase; these include hypodermal tissue, spermatheca, sperm, body-wall muscle, and vulva muscle. Most of the phenotypes that we observed in cnb-1 mutants appeared to be very similar to and characteristic of those observed in unc-43(gf) yet opposite to those in unc-43(lf) mutants. A double mutant between this cnb-1 null mutant and an unc-43(gf) mutant causes a synergistic effect of movement, fertility, and possibly egg-laying phenotypes as well, which may suggest a complementary relationship between the Ca2+-calmodulin–dependent protein kinase CaMKII and the Ca2+-calmodulin–dependent protein phosphatase calcineurin. Opposing functions of calcineurin and CaMKII have also been observed biochemically in integrin signaling, in the receptor associated protein RAP, in skeletal muscle dystrophin, and in T-cell signaling (Ngheim et al., 1994; Walsh et al., 1995; Peterson et al., 1996; Bouvard et al., 1998). Thus, this kind of biochemical role for calcineurin in C. elegans is a reasonable and plausible function.
It was previously shown that mutations in goa-1, the homologue of the Goα-subunit of the Go protein, can suppress locomotory defects associated with unc-43(n498) gain-of-function mutants (Robatzek and Thomas, 2000). Besides an uncoordinated movement phenotype, loss-of-function mutants of goa-1 also exhibited defects in egg laying and reduced brood size (Mendel et al., 1995) similar to those seen in unc-43(lf) mutants and opposite to those observed in our cnb-1 mutant. We tested to see if cnb-1 was also defective in another goa-1 related phenotype, serotonin-mediated egg laying (Mendel et al., 1995). cnb-1 mutants exhibited defects in serotonin-induced egg-laying similar to those seen in unc-43(gf) mutant and a transgenic goa-1 gain-of-function mutant syIs9[goa-1(gf)]. In addition, the dose-dependent serotonin-mediated and levamisole-mediated egg-laying curves confirmed that calcineurin is involved in similar aspects of C. elegans egg laying as unc-43 and goa-1 are involved in. From these data we suggest that calcineurin may be involved in G-protein–coupled phosphorylation pathways in locomotion, egg laying, and brood size in C. elegans.
The involvement of calcineurin in G-protein-mediated signaling has been observed in many different pathways including regulation of NFAT in cardiac myocytes, T helper cell immunity, and cardiac hypertrophy (Mende et al., 1998; Bikah et al., 2000; Ichida and Finkel, 2001). In addition, phosphatase activity as an inhibitor of G-protein phosphorylation signaling is a common method of pathway regulation (Xiao et al., 1999) so it is likely that calcineurin may be fulfilling this role in C. elegans. Finally, calcium oscillations evoked by G-protein coupled receptors and stimulated by regulators of G-protein signaling (RGS) proteins could initiate Ca2+/calmodulin-dependent calcineurin activity in C. elegans which has two homologous RGS proteins, EGL-10 and EAT-16, directly involved in G-protein pathways (Wilkie, 2000).
Although movement and egg-laying phenotypes are known to be involved in a G-protein-mediated pathway, other phenotypes observed in cnb-1 mutants such as body size and fertility were not yet determined to be involved in this specific pathway. Body-size phenotypes are not likely regulated by this specific pathway since small body size in unc-43 mutants is caused by tonically contracted body-wall muscle (Reiner et al., 1999), which we did not observe in cnb-1 mutants. On the other hand, the relationship of the brood size phenotype to the unc-43-regulated G-protein signaling pathway may be more difficult to determine. We showed that calcineurin expresses in the spermatheca and sperm, and loss of calcineurin function results in burst oocytes derived from defects in the spermatheca and sperm morphology defects. Attempted rescue of this phenotype by mating cnb-1 hermaphrodites with wild-type males (Figure 4) showed that the cause of this defect may be from multiple factors. To assess whether this phenotype is involved in this same pathway similar experiments need to be performed on mutant goa-1 and unc-43 worms.
Just as calcineurin is involved in many types of signaling, CaMKII and Goα are also general signaling molecules and have diverse functions. unc-43 mutants are not only involved in defective locomotion, egg laying, and brood size, but also show abnormal defecation behavior (Reiner et al., 1999). We also assessed the defecation cycle in cnb-1 mutants but worms showed normal defecation behavior. In the same way, goa-1 mutants also show other defective behaviors. Among these behaviors, goa-1 males have problems executing the “turning” behavior of male mating, which may be a result of defective diagonal muscles (Loer and Kenyon, 1993; Mendel et al., 1995). We also tested whether calcineurin RNAi-affected male worms show defects in turning behavior, but no significant defects nor any expression of calcineurin in diagonal muscle tissue could be detected. The common defects observed in all cnb-1, unc-43, and goa-1 mutants suggest that locomotion and egg-laying defects may be specific to a CNB-1/UNC-43 G-protein–coupled phosphorylation pathway in C. elegans.
Calcineurin may function upstream or downstream of unc-43, which is known specifically to regulate locomotion via the Go/Gq signaling network (Robatzek and Thomas, 2000). If a double mutant of cnb-1 and either unc-43(lf) or goa-1(lf) was generated, epistatic phenotypes between these functionally antagonistic proteins would distinguish where calcineurin may function in relation to UNC-43 or GOA-1. Unfortunately, both unc-43(lf);cnb-1 and goa-1(n1134);cnb-1 resulted in worms that were arrested in the L1 larval stage with severe morphology defects, indicating that both UNC-43 and GOA-1 in association with calcineurin are essential for developmental signaling pathways distinct from the G-protein pathway described above. Although egg-laying phenotypes could not be assessed in these double mutants, we attempted to observe locomotory behavior. Although goa-1(n1134);cnb-1 movement phenotypes were not assessed, cnb-1;unc-43(lf) double mutants exhibited hyperactive movement similar to unc-43(lf) mutants, although it was difficult to quantify this behavior due to the small size of the worm and its severe defects. Nevertheless, our observations indicate that unc-43 might be epistatic to cnb-1, which could further verify the role of calcineurin in a G-protein-mediated signaling pathway.
ACKNOWLEDGMENTS
We thank A. Coulson for the cosmid clones, Y. Kohara for the cDNA clones, G. Molder for the second allele of cnb-1 (ok276), and Drs. B. Grant, Y. Shim, and G. Seydoux for critical reading of the manuscript. We also thank the CGC for providing the strains used in this study, which was funded by the National Institutes of Health, National Center for Research Resources. This work was supported by grants from BK21 (to J. B.), Life Phenomena and Function Research (to D. H. K.), 00-J-LF-01-B-83 (to H-S K), and Frontier 21 (CFAHG to J. A.).
Footnotes
DOI: 10.1091/mbc.E02–01–0005.
REFERENCES
- Aitken A, Cohen P, Santikarn S, Williams DH, Calder AG, Smith A, Klee CB. Identification of the NH2-terminal locking group of calcineurin B as myristic acid. FEBS Lett. 1982;150:314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Arduengo PM, Appleberry OK, Chuang P, L'Hernault SW. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J Cell Sci. 1998;111:3645–3654. doi: 10.1242/jcs.111.24.3645. [DOI] [PubMed] [Google Scholar]
- Barstead RJ. Reverse genetics. In: Hope IA, editor. C. elegans: A Practical Approach. Oxford, UK: Oxford University Press Inc.; 1999. pp. 97–118. [Google Scholar]
- Bikah G, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Regulating T helper cell immunity through antigen responsiveness and calcium entry. Nat Immunol. 2000;1:402–412. doi: 10.1038/80841. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Molla A, Block MR. Calcium/calmodulin-dependent protein kinase II controls α5β1 integrin-mediated inside-out signaling. J Cell Sci. 1998;111:657–665. doi: 10.1242/jcs.111.5.657. [DOI] [PubMed] [Google Scholar]
- Chin-Sang ID, Spence AM. Caenorhabditis elegans sex-determining protein FEM-2 is a protein phosphatase that promotes male development and interacts directly with FEM-3. Genes Dev. 1996;10:2314–2325. doi: 10.1101/gad.10.18.2314. [DOI] [PubMed] [Google Scholar]
- Cho JH, et al. Calsequestrin, a calcium sequestering protein localized at the sarcoplasmic reticulum, is not essential for body-wall muscle function in Caenorhabditis elegans. J Cell Sci. 2000;113:3947–3958. doi: 10.1242/jcs.113.22.3947. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Calcium, calcineurin and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- Cyert MS. The function of Ca2+/calmodulin-regulated phosphatase in yeast. Adv Protein Phosphatases. 1993;7:429–443. [Google Scholar]
- Cyert MS, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Guerini D, Klee CB. Structural diversity of calcineurin, a Ca2+ and calmodulin-stimulated protein phosphatase. Adv Protein Phosphatases. 1991;6:391–410. [Google Scholar]
- Hirata D, Harada S-I, Namba H, Miyakawa T. Adaptation to high salt stress in Saccharomyces cerevisiae is regulated by Ca2+/calmodulin-dependent phosphoprotein phosphatase (calcineurin) and cAMP-dependent protein kinase. Mol Gen Genet. 1995;249:257–264. doi: 10.1007/BF00290525. [DOI] [PubMed] [Google Scholar]
- Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes. J Biol Chem. 2001;276:3524–3530. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- Jones AR, Francis R, Schedl T. GLD-1, a cytoplasmic protein essential for oocyte differentiation shows stage-and sex-specific expression during Caenorhabditis elegans germline development. Dev Biol. 1996;180:165–183. doi: 10.1006/dbio.1996.0293. [DOI] [PubMed] [Google Scholar]
- Kincaid RL. Calmodulin-dependent protein phosphatases from microorganisms to man: a study in structural convertism and biological diversity. Adv Second Messenger Phosphoprotein Res. 1993;27:1–23. [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci USA. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Klee CB, Draetta GF, Hubbard MJ. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- Krause M. Transcription and translation. In: Epstein HF, Shakes DC, editors. Methods in Cell Biology. Vol. 48. San Diego, CA: Academic Press; 1995. pp. 513–529. [DOI] [PubMed] [Google Scholar]
- Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–763. doi: 10.1016/s0896-6273(02)00607-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Loer CM, Kenyon CJ. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci. 1993;13:5407–5417. doi: 10.1523/JNEUROSCI.13-12-05407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault SW, Roberts TM. Cell biology of nematode sperm. In: Epstein HF, Shakes DC, editors. Methods in Cell Biology. Vol. 48. San Diego, CA: Academic Press; 1995. pp. 273–301. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Mikawa T, Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984;95:511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods in cell biology. In: Epstein HF, Shakes DC, editors. Methods in Cell Biology. Vol. 48. San Diego, CA: Academic Press; 1995. pp. 452–480. [PubMed] [Google Scholar]
- Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Gαq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA. 1998;95:13893–13898. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel JE, Korswagen HC, Liu KS, Hajdu-Cronin YM, Simon MI, Plasterk RHA, Sternberg PW. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science. 1995;267:1652–1655. doi: 10.1126/science.7886455. [DOI] [PubMed] [Google Scholar]
- Miller DM, Shakes DC. Immunofluorescence microscopy. In: Epstein HF, Shakes DC, editors. Methods in Cell Biology. Vol. 48. San Diego, CA: Academic Press; 1995. pp. 365–394. [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin inhibition and cardiac hypertrophy. Science. 1998;282:1007a. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Dorn GW. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Ngheim P, Ollick T, Gardner P, Schulman H. Interleukin-2 transcriptional block by multifunctional Ca2+/calmodulin kinase. Nature. 1994;371:347–350. doi: 10.1038/371347a0. [DOI] [PubMed] [Google Scholar]
- Page AP, MacNiven K, Hengartner MO. Cloning and biochemical characterization of the cyclophilin homologues from the free-living nematode Caenorhabditis elegans. Biochem J. 1996;317:179–185. doi: 10.1042/bj3170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, et al. Calreticulin, a calcium-binding molecular chaperone, is required for stress response and fertility in C. elegans. Mol Biol Cell. 2001a;12:2835–2845. doi: 10.1091/mbc.12.9.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Lee JI, Lee J, Kim S, Choi KY, Park CS, Ahnn J. Isolation of deletion mutants by reverse genetics in Caenorhabditis elegans. Korean J Biol Sci. 2001b;5:65–69. [Google Scholar]
- Perrino BA, Ng LY, Soderling TR. Calcium regulation of calcineurin phosphatase activity by its B subunit and calmodulin. J Biol Chem. 1995;270:340–346. doi: 10.1074/jbc.270.1.340. [DOI] [PubMed] [Google Scholar]
- Peterson CM, et al. The receptor-associated protein (RAP) binds calmodulin and is phosphorylated by calmodulin-dependent kinase II. EMBO J. 1996;15:4165–4173. [PMC free article] [PubMed] [Google Scholar]
- Robatzek M, Thomas JH. Calcium/calmodulin-dependent protein kinase II regulates C. elegans in concert with a Go/Gq signaling network. Genetics. 2000;156:1069–1082. doi: 10.1093/genetics/156.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Newton EM, Tian H, Thomas JH. Diverse behavioral defects caused by mutations in Caenorhabditis elegans unc-43 CaM Kinase II. Nature. 1999;402:199–203. doi: 10.1038/46072. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, McKeon F. Calcineurin functions in Ca2+ activated cell death in mammalian cells. J Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MJR. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80) FEBS Lett. 1982;137:80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode C. elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Busaan JL, Fraser ED, Fu SY, Pato MD, Michalak M. Characterization of the recombinant C-terminal domain of dystrophin: phosphorylation by calmodulin-dependent protein kinase II and dephosphorylation by type 2B protein phosphatase. Biochemistry. 1995;34:5561–5568. doi: 10.1021/bi00016a030. [DOI] [PubMed] [Google Scholar]
- Wilkie TM. G-protein signaling: satisfying the basic necessities of life. Curr Biol. 2000;10:853–856. doi: 10.1016/s0960-9822(00)00823-x. [DOI] [PubMed] [Google Scholar]
- Xiao R, Cheng H, Zhou Y, Kuschel M, Lakatta EG. Recent advances in cardiac β2-adrenergic signal transduction. Circ Res. 1999;85:1092–1100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- Zhao S, Bouchard P, Diltz CD, Shen SH, Fischer EH. Purification and characterization of a protein tyrosine phosphatase containing SH2 domains. J Biol Chem. 1993;268:2816–2820. [PubMed] [Google Scholar]