Abstract
Ciliary and flagellar motility is regulated by changes in intraflagellar calcium. However, the molecular mechanism by which calcium controls motility is unknown. We tested the hypothesis that calcium regulates motility by controlling dynein-driven microtubule sliding and that the central pair and radial spokes are involved in this regulation. We isolated axonemes from Chlamydomonas mutants and measured microtubule sliding velocity in buffers containing 1 mM ATP and various concentrations of calcium. In buffers with pCa > 8, microtubule sliding velocity in axonemes lacking the central apparatus (pf18 and pf15) was reduced compared with that of wild-type axonemes. In contrast, at pCa4, dynein activity in pf18 and pf15 axonemes was restored to wild-type level. The calcium-induced increase in dynein activity in pf18 axonemes was inhibited by antagonists of calmodulin and calmodulin-dependent kinase II. Axonemes lacking the C1 central tubule (pf16) or lacking radial spoke components (pf14 and pf17) do not exhibit calcium-induced increase in dynein activity in pCa4 buffer. We conclude that calcium regulation of flagellar motility involves regulation of dynein-driven microtubule sliding, that calmodulin and calmodulin-dependent kinase II may mediate the calcium signal, and that the central apparatus and radial spokes are key components of the calcium signaling pathway.
INTRODUCTION
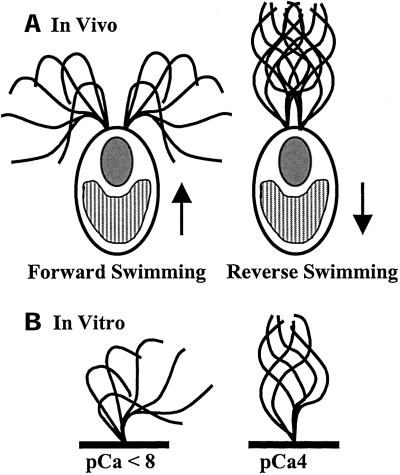
Our goal is to understand the molecular mechanism by which calcium regulates the size and shape of ciliary and flagellar bends to modulate motility. For many organisms or cell types, external stimuli trigger changes in cytosolic free calcium concentration, which in turn produce altered ciliary and flagellar motility. Much of what we know about calcium modulation of ciliary and flagellar motility comes from studies of isolated axonemes or demembranated cell models reactivated to beat in vitro. For example, in the biflagellate green alga Chlamydomonas reinhardtii, calcium is required for phototaxis as well as the photophobic response (Figure 1) (reviewed in Witman, 1993). In vitro studies using reactivated cell models indicate that small increases in calcium (pCa9 − pCa7) differentially activate one flagellum or the other (Kamiya and Witman, 1984). A larger increase in calcium (pCa5 − pCa4) causes a momentary cessation of motility followed by a complete switch from an asymmetric to a symmetric waveform (Figure 1; Hyams and Borisy, 1978; Bessen et al., 1980; Omoto and Brokaw, 1985). Axonemes isolated from sea urchin sperm and reactivated in vitro under low calcium conditions beat with a symmetric waveform. Upon increasing calcium in the buffer, the axonemes beat with increasing asymmetry (Brokaw et al., 1974; Brokaw, 1979); at extremely high calcium concentrations, quiescence is induced (Gibbons and Gibbons, 1980; Sale, 1986). For reactivated cell models of Paramecium and Tetrahymena, an increase in calcium induces reversal of swimming direction by changing the direction of the ciliary effective stroke (Naitoh and Kaneko, 1972; Izumi and Miki-Noumura, 1985; Hamasaki et al., 1989; Bonini et al., 1991). Although the axonemal response to changes in calcium concentration has been well described, we still do not know the precise molecular mechanism by which motility is modulated by fluctuations in the concentration of cytosolic free calcium.
Figure 1.
(a) In vivo, Chlamydomonas cells normally swim forward, toward the light, with an asymmetric, ciliary waveform. During the photophobic response, bright light induces a shift from an asymmetric waveform to a symmetric, flagellar waveform, and the cells swim in reverse. The arrows indicate swimming direction (for example, see Ringo, 1967 and Ruffer and Nultsch, 1985). (b) This change in waveform can be induced in vitro. Isolated axonemes lacking membranes and soluble flagellar matrix components beat with an asymmetric waveform in buffers of pCa < 8 and beat with a symmetric waveform in buffers of pCa4. (Waveform traces adapted from Brokaw and Luck, 1985.)
The in vitro reactivation experiments described above clearly demonstrate that all of the regulatory proteins required for modulating motility, including key calcium sensors, are structural components of the axoneme. Several highly conserved calcium-binding proteins are associated with the axoneme. Calmodulin has been identified as a component of ciliary and flagellar axonemes of Chlamydomonas, Paramecium, Tetrahymena, Elliptio, and sperm cells from mammals and echinoderms (reviewed in Otter, 1989; Brokaw, 1991; Plattner and Klauke, 2001). In Chlamydomonas, a subset of axonemal calmodulin is associated with the radial spokes (Yang et al., 2001). In addition, cilia and flagella also contain the calcium-binding protein centrin/caltractin (Huang et al., 1988a; Salisbury et al., 1988), which is a component of a subset of inner dynein arms (Piperno et al., 1992, Yanagisawa and Kamiya, 2001). And, King and Patel-King (1995) have determined that the 18-kDa light chain of the outer dynein arm in Chlamydomonas is a calcium-binding protein with homology to both calmodulin as well as centrin. Therefore, cilia and flagella contain at least three different classes of calcium-binding proteins that predictably mediate calcium control of motility.
In addition to sensing changes in calcium, the axoneme must also possess a mechanism for converting the calcium signal into altered axonemal bends, presumably resulting from localized modulation of dynein-driven microtubule sliding (reviewed in Satir, 1985). The relationship between changes in intraflagellar free calcium concentration and predicted changes in dynein activity has not yet been determined. To test the hypothesis that calcium regulates axonemal dynein, our strategy was to assess dynein activity in axonemes isolated from mutant and wild-type cells using an in vitro assay to measure dynein-driven microtubule sliding velocity (Summers and Gibbons, 1971; Okagaki and Kamiya, 1986). This assay has two key advantages. First, measurement of microtubule sliding in isolated axonemes assesses dynein activity in situ with most or all of the endogenous regulatory components intact. Second, the availability of Chlamydomonas mutants with axonemes lacking particular structures provides an opportunity to detect regulatory mechanisms not easily revealed in wild-type axonemes. For example, although axonemes isolated from radial spoke and central apparatus defective mutants cannot be reactivated in vitro in buffers containing 1 mM ATP, dynein activity in these mutants can still be assessed using the microtubule sliding assay (Witman et al., 1978; Okagaki and Kamiya, 1986; Smith and Sale, 1992; Habermacher and Sale, 1997; Smith, 2002). Studies using this assay have provided crucial information toward the development of a model in which axonemal dynein is regulated by the coordinate action of several kinases and phosphatases anchored to the axoneme (Yang et al., 2000, 2001; reviewed in Porter and Sale, 2000).
To define the role of calcium in regulating dynein, and hence flagellar waveform, we used the microtubule sliding assay to measure dynein activity in axonemes isolated from wild-type and mutant Chlamydomonas strains in response to calcium. In low calcium conditions, dynein activity is reduced in axonemes lacking the radial spokes and central apparatus. However, in high calcium conditions, dynein activity is restored to nearly wild-type levels in mutant axonemes lacking the entire central apparatus. Furthermore, the increase in dynein activity is inhibited by the addition of either calmodulin or calmodulin-dependent kinase II antagonists. These studies provide evidence that dynein activity is regulated by calcium, that this regulation involves a signaling pathway that includes an axonemal calmodulin and calmodulin-dependent kinase, and that the calcium control system includes the radial spokes and central apparatus.
MATERIALS AND METHODS
Cell Strains and Growth Conditions
Strain A54-e18 (nit1-1, ac17, sr1, mt+) is the “wild-type” strain used in motility assays and is the strain used in transformation experiments to obtain the insertional pf16 allele, pf16C (Smith and Lefebvre, 1996). The central pair–defective strains, pf18, and pf15, and the radial spoke–defective strains pf14 and pf17 were obtained from the Chlamydomonas Genetics Center (Duke University). All cells were grown in constant light in TAP media (Gorman and Levine, 1965).
Isolation of Axonemes and the Microtubule Sliding Assay
Flagella were severed from cell bodies by the dibucaine method (Witman, 1986) and isolated by differential centrifugation in buffer A (10 mM HEPES, pH 7.4, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, and 50 mM potassium acetate). Axonemes were isolated by adding NP-40 (Calbiochem, La Jolla, CA) to flagella for a final concentration of 0.5% (wt/vol) to remove flagellar membranes.
Measurement of sliding velocity between doublet microtubules was based on the methods of Okagaki and Kamiya (1986). Approximately 8 μl of axonemes were applied to a perfusion chamber (Smith and Sale, 1992); the chamber was perfused with wash buffer (buffer A containing 1 mM ATP) to remove nonadherent axonemes. To initiate microtubule sliding, the chamber was perfused with motility buffer (buffer A containing 1 mM ATP (Roche Molecular Biochemicals, Indianapolis, IN) and 2 mg/ml Nagarse (Type XXVII Protease; Sigma Chemical Co., St. Louis, MO). Although all of the experiments in this report were performed using Nagarse, it should be noted that this protease is no longer available. The supplier recommended replacement is Type VIII protease (catalogue number P-5380; Sigma). We have recently used Type VIII protease in microtubule sliding assays and detected no qualitative or quantitative differences in microtubule sliding. For experiments involving buffers with different concentrations of free calcium, all buffers were made as described in Wakabayashi et al. (1997) minus polyethylene glycol, creatine kinase, and phosphocreatine. For pharmacological treatments, inhibitors were added to isolated axonemes followed by a 20-min incubation at room temperature. Inhibitors were maintained in both the wash and motility buffers when appropriate. Microtubule sliding was observed using an Axioskope 2 microscope (Zeiss Inc., Thornwood, NY) equipped for dark-field optics including a Plan-Apochromate 40× oil immersion objective with iris and ultra dark-field oil immersion condenser. Images were recorded by a silicon-intensified target camera (VE-1000 SIT; Dage-MTI, Inc., Michigan City, IN) through a time-date generator, on videotape by a videocassette recorder (AG-1980; Panasonic, Secaucus, NJ). Microtubule sliding velocity was measured manually from calibrated video screens using the jog/shuttle device to measure displacement versus time. All data are presented as mean ± SD. The Student's t test was used to determine the significance of differences between means.
Kinase, Phosphatase, and Calmodulin Inhibitors
All inhibitors were purchased from Calbiochem. Microcycstin-L-R was prepared as a 500 μM stock in methanol. DRB (5, 6-dichloro-1-b-d-ribofuranosylbenzimidazole) was prepared as a 10 mM stock solution in ethanol. For microtubule sliding assays, the final concentrations of these inhibitors were: microcystin, 1 μM; DRB, 100 μM.
KN-92 and KN-93 were prepared as 1.0 mM stocks in water and were used at a final concentration of 1.0 μM (Sumi et al., 1991). The calmodulin binding domain peptide (Leu-Lys-Lys-Phe-Asn-Ala-Arg-Arg-Lys-Leu-Lys-Gly-Ala-Ile-Leu-Thr-Thr-Met-Leu-Ala), calmodulin inhibitory peptide (Arg-Arg-Lys-Trp-Gln-Lys-Thr-Gly-His-Ala-Val-Arg-Ala-Ile-Gly-Arg-Leu), and calmodulin inhibitory peptide control peptide (Arg-Arg-Lys-Glu-Gln-Lys-Thr-Gly-His-Ala-Val-Arg-Ala-Ile-Gly-Arg-Glu) were prepared as 6.0 mM stocks in water and used at a final concentration of 60 μM or as indicated in figures (Payne et al., 1988; Torok and Trentham, 1994; James et al., 1995). The autocamtide-2–related inhibitory peptide (Lys-Lys-Ala-leu-Arg-Arg-Gln-Glu-Ala-Val-Asp-Ala-Leu) was stored as a 300 μM stock and used at a concentration of 3 μM or as indicated (Ishida et al., 1995). All stock solutions were stored at −20°C.
RESULTS
Calcium Regulation of Microtubule Sliding
Many studies have indicated that the central apparatus and radial spokes are involved in a signal transduction pathway that includes several axoneme-associated enzymes that regulate dynein activity to produce the bending motion characteristic of eukaryotic ciliary and flagellar motility. It is also well established that changes in intraflagellar calcium modulate the size and shape of ciliary and flagellar bends (see Figure 1). Based on these observations, we postulated that changes in free calcium concentration would differentially affect dynein activity in Chlamydomonas mutants lacking the radial spokes or central apparatus. To test this, we used a microtubule-sliding assay to measure dynein-driven microtubule sliding velocity in axonemes isolated from radial spoke and central apparatus defective Chlamydomonas mutants (Table 1) and as a function of calcium.
Table 1.
Chlamydomonas mutants for this study
| Mutant | Structural defect | Gene product identity, localization |
|---|---|---|
| pf15 | Lacks entire central apparatus | p80 subunit of katanin, axonemal (Smith and Lefebvre, in preparation) |
| pf16 | Central apparatus, lacks C1 | 57-kDa armadillo repeat protein, C1 localization (Smith and Lefebvre, 1996) |
| pf18 | Lacks entire central apparatus | Unknown |
| pf14 | Lacks radial spokes | rsp3, A-kinase anchoring protein, base of radial spoke (Curry and Rosenbaum, 1993; Deiner et al., 1993; Gaillard et al., 2001) |
| pf17 | Lacks radial spoke heads | Unknown |
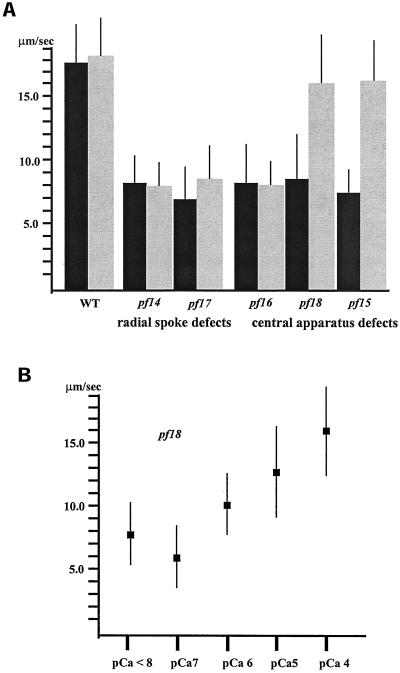
We first compared microtubule sliding velocity in motility buffer containing 10−4 M calcium (pCa4) with that in buffer containing <10−8 M calcium (Figure 2a). Microtubule sliding velocity in wild-type axonemes was not affected by changes in the concentration of free calcium (17.0–19.0 μm/s, Figure 2a). Similarly, microtubule sliding velocity in axonemes isolated from the radial spoke defective mutants pf14 and pf17 and the C1 central microtubule defective strain pf16 was not affected by changes in calcium concentration. Axonemes isolated from these mutants have the same slow microtubule sliding velocity in buffers of either low or high calcium (between 7.5 and 8.5 μm/s, Figure 2a). In striking contrast, the velocity of microtubule sliding in mutant axonemes lacking the entire central apparatus, pf18 and pf15, was calcium sensitive. The velocity of microtubule sliding was slow in low calcium buffer (between 7.5 and 8.5 μm/s, Figure 2a), yet increased to nearly wild-type velocity in high calcium buffer (16.0–17.0 μm/s, Figure 2a). Evidently, the inhibition of dynein activity caused by the lack of the central apparatus is bypassed by the presence of high calcium. However, the calcium-induced rescue of dynein activity fails if the C2 microtubule of the central apparatus is present (pf16) or if radial spoke components are lacking (pf14 and pf17).
Figure 2.
(a) Microtubule sliding velocities in wild-type and mutant axonemes in low calcium (pCa > 8, black bars) and high calcium (pCa4, gray bars) buffers. Sliding velocities in wild-type, radial spoke–defective (pf14 and pf17), and C1 central tubule–defective (pf16) axonemes in low calcium buffer are not significantly different from those in high calcium buffer. In contrast, sliding velocities in axonemes completely lacking the central apparatus (pf15 and pf18) in high calcium buffer are significantly increased from those in low calcium buffer (p < 0.001; Student's t test). All bars represent the mean of >60 measurements ± SD from a minimum of three experiments. (b) Microtubule sliding velocity in pf18 axonemes in microtubule sliding buffer with varying concentrations of free calcium. As the concentration of free calcium increases, microtubule sliding velocity in pf18 axonemes increases to nearly wild-type level. Each point represents the mean of >40 measurements ± SD from two experiments.
The sliding velocity of axonemes isolated from pf18 increased in a linear manner with increasing free calcium concentration (Figure 2b). The half-maximal velocity occurs between pCa5 and pCa6, the same concentration of free calcium that induces the switch from ciliary to flagellar waveforms (Hyams and Borisy, 1978). These results provide evidence that dynein activity is modulated by calcium and indicate that dynein activity is regulated by the response of a particular enzyme to increasing concentrations of free calcium.
The Calcium Signaling Pathway Acts Independently of Casein Kinase 1 and Protein Phosphatase-2A Activity
Previous studies have indicated that in conditions of low calcium, the central apparatus and radial spokes form a signaling pathway that regulates dynein activity, at least in part, through the action of casein kinase 1 (CK1) and protein phosphatase-2A (PP2A) located in the axoneme (Yang and Sale, 2000; Smith, 2002). This conclusion is based on the observation that upon the addition of DRB, a CK1 inhibitor, dynein activity is restored in mutant axonemes lacking the radial spokes or central apparatus (Yang and Sale, 2000; Smith, 2002). Moreover, in low calcium buffer, the DRB induced increase in sliding velocity observed for both radial spoke and central apparatus defective axonemes requires the presence of an active phosphatase, most likely PP2A (Yang and Sale, 2000; Smith, 2002). The addition of microcystin, an inhibitor of PP1 and PP2A, blocks the DRB-induced increase in dynein activity in these mutants (Yang and Sale, 2000; Smith, 2002). To investigate the relationship between the calcium-mediated increase in dynein activity and the CK1/PP2A-mediated regulation of dynein activity, we compared the effect of CK1 and PP2A inhibitors on microtubule sliding velocity in low versus high calcium buffer conditions.
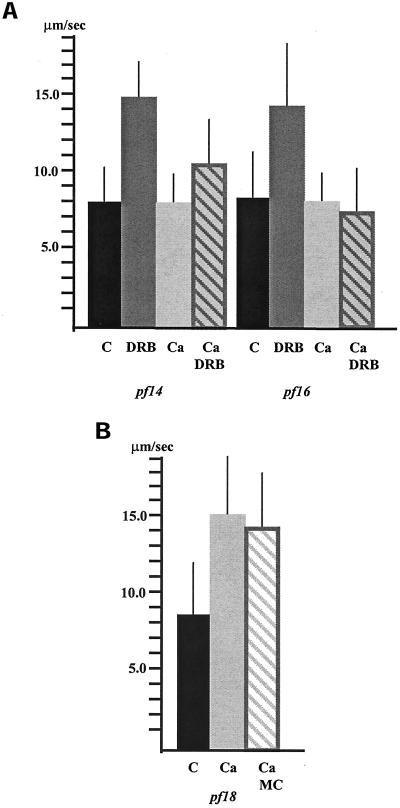
First, we determined whether the DRB-induced increase in dynein activity for pf16 and pf14 axonemes is calcium sensitive by testing whether DRB restores dynein activity to axonemes isolated from these mutants in pCa4 buffer. As previously reported, we found that DRB increases the microtubule sliding velocity of axonemes isolated from pf14 in low calcium buffer (Figure 3a; Yang and Sale, 2000). However, the sliding velocity in pf14 axonemes in pCa4 buffer with DRB is significantly different from sliding velocity in pf14 axonemes in both pCa4 buffer alone (p < 0.001) as well as low calcium buffer with DRB (p < 0.001; Figure 3a). Therefore, the DRB-induced increase in dynein activity for pf14 axonemes is to some degree calcium sensitive. Even more pronounced effects of calcium were observed for pf16 axonemes. The addition of DRB to pf16 axonemes in low calcium buffer increased dynein activity to nearly wild-type levels (Smith, 2002). However, the sliding velocity in pf16 axonemes in pCa4 buffer with DRB is not significantly different from that observed in either high or low calcium buffer alone (Figure 3a). Evidently, in the presence of high calcium, the inhibition of CK1 fails to restore dynein activity to pf16 axonemes.
Figure 3.
(a) Microtubule sliding velocities in pf14 and pf16 axonemes. Dynein-driven microtubule sliding velocities in axonemes isolated from the radial spoke defective mutant pf14 and central apparatus defective mutant pf16 significantly increase (p < 0.001; Student's t test) upon the addition of DRB compared with control, untreated axonemes in low calcium buffer (C = pCa > 8). In high calcium buffer, (Ca = pCa4) DRB is less effective at increasing microtubule sliding velocity in pf14 axonemes and is completely ineffective at increasing sliding velocity in pf16 axonemes. (b) The increase in sliding velocity observed for pf18 axonemes in high calcium buffer is not inhibited by the addition of microcystin (MC). All bars represent the mean ± SD. N ≥ 60 from a minimum of three experiments.
Second, we used specific inhibitors to investigate the involvement of axonemal phosphatases in regulating dynein activity. As noted, the addition of microcystin, a potent inhibitor of phosphatases PP1 and PP2A, blocks the DRB induced increase in sliding velocity in both radial spoke and central apparatus defective mutants (Yang and Sale, 2000; Smith, 2002). To determine whether the calcium-mediated increase in dynein activity for pf18 axonemes also requires the presence of PP1 or PP2A, axonemes were incubated with microcystin before and during induction of microtubule sliding in pCa4 buffer. The addition of microcystin does not inhibit the calcium-induced increase in sliding velocity in pf18 axonemes (Figure 3b). Therefore, neither PP1 nor PP2A activity is required for the calcium-induced increase in dynein activity. These combined results indicate that the calcium-induced increase in dynein activity in pf18 axonemes occurs by a mechanism not directly dependent on the activity of CK1 or PP2A.
Calmodulin Plays a Role in the Calcium-dependent Regulatory Pathway
As demonstrated above, high calcium does not increase sliding velocities in axonemes isolated from either pf14 or pf17 mutants. Therefore, we predicted that an important component of the calcium-mediated signaling pathway must be either missing or inactivated in pf14 and pf17 axonemes. Several calcium-binding proteins reside within the axoneme, including calmodulin (Gitelman and Witman, 1980; Van Eldik et al., 1980). Yang et al. (2001) have recently shown that a fraction of axonemal calmodulin is associated with the radial spokes; axonemes isolated from pf14 lack this fraction of calmodulin. One possibility is that calcium-induced increase in dynein activity in pf18 axonemes involves a calmodulin-mediated regulatory pathway. If this were the case, we predicted that the addition of calmodulin antagonists would inhibit dynein activity in pf18 axonemes in high calcium buffers. A significant advantage of using the microtubule sliding assay to measure dynein activity is that we have complete experimental access to the regulatory machinery associated with isolated axonemes. Therefore, the use of pharmacological agents, such as peptide inhibitors, has been particularly effective in elucidating signal transduction components important for dynein regulation.
To test whether calmodulin is involved in calcium-induced dynein regulation, we tested two peptide inhibitors of calmodulin for their ability to reduce dynein activity in pf18 axonemes in pCa4 buffer. These peptide inhibitors represent the calmodulin binding sites for two different enzymes, myosin light chain kinase, and CaM-kinase II; when bound to calmodulin, they prevent interaction with calmodulin-binding proteins (Torok and Trentham, 1994; James et al., 1995). Both the calmodulin-binding domain (CBD) peptide of CaM-kinase II as well as the calmodulin inhibitory peptide (CIP, calmodulin-binding domain of myosin light-chain kinase) inhibit the high calcium-induced increase in dynein activity in pf18 axonemes (Figure 4a). The velocity of microtubule sliding in pf18 axonemes incubated in pCa4 buffer with either of these calmodulin inhibitors is not significantly different from that in pf18 axonemes incubated in low calcium buffer. Importantly, sliding velocity in pf18 axonemes incubated in pCa4 buffer with the control peptide for the calmodulin inhibitory peptide (CIPc) was not significantly different from that in pf18 axonemes in pCa4 buffer alone. The velocity of microtubule sliding in wild-type axonemes in pCa4 buffer was unaffected by the addition of the CBD peptide.
Figure 4.
(a) The addition of calmodulin inhibitors blocks the calcium-induced increase in sliding velocity observed for pf18 axonemes in pCa4 buffer (Ca). The sliding velocity in pf18 axonemes incubated in pCa4 buffer with either the calmodulin-binding domain peptide (CBD, 60.0 μM) or the calmodulin inhibitory peptide (CIP, 60.0 μM) is not significantly different from that in pf18 axonemes incubated in low calcium buffer. Sliding velocity in pf18 axonemes in pCa4 buffer incubated with the control peptide (CIPc, 60.0 μM) for the calmodulin inhibitory peptide is not significantly different from that in pf18 axonemes in pCa4 buffer alone. All bars represent the mean of >60 measurements ± SD from a minimum of three experiments. (b) Microtubule sliding velocity in pf18 axonemes in microtubule sliding buffer with varying concentrations of the calmodulin binding domain peptide (CBD) inhibitor. Increasing concentration of CBD decreases sliding velocity in pf18 axonemes in high calcium buffer. Each point represents the mean of >40 measurements ± SD from two experiments.
To investigate the effective concentration range of calmodulin inhibitor necessary for reducing dynein activity, pf18 axonemes were incubated in various concentrations of the CBD peptide, and the microtubule sliding assay was performed. The CBD peptide decreased microtubule sliding velocity with half-maximal inhibition at ∼6.0 × 10−7 M (Figure 4b). These results provide evidence that the increase in sliding velocity of pf18 axonemes in high calcium buffers is mediated by an axonemal calmodulin.
The Calmodulin-dependent Increase in Sliding Velocity is Mediated by a Calmodulin-dependent Kinase
Our results indicate that the increase in microtubule sliding velocity in pf18 axonemes in high calcium buffer is mediated by calmodulin. Calmodulin is known to bind to a variety of enzymes including calmodulin-dependent kinases (CaM-kinase), calcineurin or protein phosphatase 2B (PP2B), and cyclic nucleotide phosphodiesterases (reviewed in Chin and Means, 2000). To investigate whether any of these enzymes are possible targets of the calmodulin-mediated increase in microtubule sliding velocity, we tested available inhibitors in our sliding assay for their ability to block the calcium-induced increase in dynein activity in pf18 axonemes in high calcium buffer. An inhibitor (KN-93) of calmodulin-dependent kinase II (CaM-KII) significantly reduced dynein activity in pf18 axonemes in high calcium buffer (Figure 5a). The microtubule sliding velocity in pf18 axonemes incubated with KN-93 in the presence of high calcium is not significantly different from that in pf18 axonemes incubated in low calcium buffer alone. Importantly, the control compound KN-92 did not reduce microtubule sliding velocity in pf18 axonemes in pCa4 buffer. The sliding velocity in pf18 axonemes incubated with KN-92 in the presence of high calcium is not significantly different from that in pf18 axonemes incubated in high calcium buffer alone.
Figure 5.
(a) The addition of CaM-KII inhibitors blocks the calcium-induced increase in sliding velocity observed for pf18 axonemes in pCa4 buffer (Ca). The sliding velocity in pf18 axonemes incubated in pCa4 buffer with either the compound KN-93 (1.0 μM) or the autocamtide-2 related inhibitory peptide (AIP, 3.0 μM) is not significantly different from that in pf18 axonemes incubated in low calcium buffer. Sliding velocity in pf18 axonemes in pCa4 buffer incubated with the control compound KN-92 (1.0 μM) is not significantly different from that in pf18 axonemes in pCa4 buffer alone. All bars represent the mean of >60 measurements ± SD from a minimum of three experiments. (b) Microtubule sliding velocity in pf18 axonemes in pCa4 microtubule sliding buffer with varying concentrations of the autocamtide-2 related inhibitory peptide (AIP). Increasing concentration of AIP decreases sliding velocity in pf18 axonemes in high calcium buffer. Each point represents the mean of >40 measurements ± SD from two experiments.
We also investigated whether a specific peptide inhibitor of CaM-KII reduced dynein activity in pf18 axonemes in high calcium buffer. The autocamtide-2–related inhibitory peptide (AIP) is a potent and specific inhibitor of CaM-KII with a reported Ki of 2–8 × 10−9 M (Ishida et al. 1994, 1995; Ishida and Fujisawa, 1995). On the addition of AIP, dynein activity in pf18 axonemes in pCa4 buffer is significantly reduced (Figure 5a); the velocity of microtubule sliding in pf18 axonemes incubated with AIP in pCa4 buffer was not significantly different from that in low calcium buffer alone. In experiments using varying concentrations of AIP, half-maximal inhibition was achieved at AIP concentrations of 1.6 × 10−7 M (Figure 5b). These results indicate that a signaling pathway that includes calmodulin and a calmodulin-dependent kinase controls dynein-driven microtubule sliding in response to calcium.
DISCUSSION
In the work described here, we present data demonstrating that calcium regulation of flagellar motility involves regulation of dynein-driven microtubule sliding. In addition, our results suggest that calmodulin is a key axonemal calcium sensor and that a calmodulin-dependent kinase may mediate the calcium signal. Finally, these studies reveal that the calcium control system is regulated by the central apparatus and radial spokes. We propose that in wild-type axonemes, the central apparatus locally controls the calcium sensor to locally regulate microtubule sliding and to modulate the size and shape of flagellar bends. These conclusions are consistent with genetic analyses implicating the radial spokes and central apparatus in control of flagellar waveform (Brokaw et al., 1982; Huang et al., 1982).
Calcium Modulates Dynein Activity
Our analysis of dynein-driven microtubule sliding velocity in central apparatus–defective mutants has revealed a role for calcium in modulating dynein activity. This conclusion is founded on the observation that buffers with high calcium concentration restored dynein activity to nearly wild-type levels in mutant axonemes completely lacking the central apparatus. In addition, dynein activity increased in a linear manner with increasing concentrations of calcium. This result was surprising because the calcium-induced switch from asymmetric to symmetric waveform occurs somewhat abruptly with increasing calcium. One explanation for this result is that increasing calcium concentration affects the concentration of MgATP in the buffer, which in turn, directly affects dynein activity. However, this explanation is unlikely because increased dynein activity is not observed in all mutants and is abolished upon the addition of specific inhibitors. Omoto and Brokaw (1985) report the concentration of CaATP2- at pCa4 to be ∼10−5 M in buffer virtually identical to that used in our assay.
A second explanation is that the effect of calcium on dynein activity is mediated by the response of a particular enzyme to increasing concentrations of free calcium. Based on their studies of isolated axonemes reactivated in vitro, Omoto and Brokaw (1985) concluded that the flagellar response to calcium is a multicomponent process involving subtle quantitative changes in flagellar movement that ultimately affect beat frequency and waveform. The microtubule sliding assay used here to assess dynein activity in particular mutants has provided us with a unique opportunity to resolve subtle details of dynein regulation that would not easily be detected in wild-type axonemes. Our results using mutant axonemes and inhibitors of specific enzymes are most consistent with a model in which calcium acts directly through an enzyme-driven mechanism to ultimately affect dynein activity.
The question is, what is the molecular mechanism by which calcium modulates dynein activity? One possibility is that the direct binding of calcium to dynein arm components modulates the activity of the dynein heavy chains. For example, centrin is component of the inner dynein arms (Piperno et al., 1992), and a calcium-binding light chain is a component of the outer dynein arms (King and Patel-King, 1995). A second possibility is that calcium activates a signal transduction cascade that ultimately regulates dynein activity by posttranslational modification of axonemal components, possibly including the dynein arms. Although these two possibilities are not mutually exclusive, our data provides greater support for the hypothesis that calcium modulates dynein activity by activating a signal transduction pathway that includes calmodulin and a calmodulin-dependent kinase.
Several studies have demonstrated that changes in calcium concentration result in the altered phosphorylation of particular axonemal components (Tash and Means, 1982; Segal and Luck, 1985; Hamasaki et al., 1989). More recent studies have confirmed that axonemal dynein is regulated in part by a network of kinases and phosphatases that are structural components of the axoneme (reviewed in Porter and Sale, 2000). In particular, the central apparatus–radial spoke system has been implicated in a signal transduction cascade that controls the activity of an axonemal CK1 and PP2A to regulate the I1 inner dynein arm subform (Smith and Sale, 1992, 1994; Howard et al., 1994; Habermacher and Sale, 1996, 1997; Yang and Sale, 2000; Smith, 2002). Also, analyses of phototaxis-defective mutants have suggested that changes in the phosphorylation state of inner dynein arm I1 may play a role in regulating motility during phototaxis, which is a calcium-dependent response (King and Dutcher, 1997). Therefore, one hypothesis is that calcium regulates dynein through a pathway that directly alters the activity of axonemal CK1 and PP2A. For example, high calcium may inhibit CK1 either directly or indirectly, to increase dynein activity in axonemes lacking the central apparatus. Alternatively, calcium alters dynein-driven motility through a separate signaling pathway.
Our data supports the hypothesis that calcium does not modulate dynein activity in pf18 and pf15 axonemes by inhibiting CK1. The sliding velocity in pf15 axonemes did not increase upon the addition of DRB but did increase in high calcium. In contrast, dynein activity in both pf14 and pf16 axonemes was restored after the addition of DRB in low calcium buffer but not in high calcium buffer. That sliding velocities do not increase in pf16 axonemes in high calcium in either the presence or absence of DRB suggests that the C2 central microtubule may be sufficient to maintain dynein inhibition in the presence of high calcium regardless of whether CK1 is inhibited.
Our data also support the hypothesis that neither PP1 nor PP2A is required for the calcium-mediated regulation of dynein. First, the presence of high calcium restores wild-type sliding velocity to pf18 axonemes in the presence of microcystin, an inhibitor of PP1 and PP2A. Second, pf15 axonemes lack PP2A (Yang et al., 2000) and yet, dynein activity in pf15 axonemes increases in the presence of high calcium. Therefore, the calcium-mediated increase in dynein activity for these central apparatus defective axonemes does not require either PP1 or PP2A. These results do not, however, rule out the possibility that additional phosphatases such as calcineurin are involved in the calcium-mediated signaling pathway.
The Calcium-signaling Pathway Includes Calmodulin and a Calmodulin-dependent Kinase
Our pharmacological data suggest that the calcium-induced increase in dynein activity in central pairless axonemes is mediated by calmodulin and CaM-KII. The addition of either of two specific peptide inhibitors of calmodulin blocks the calcium-mediated increase in dynein activity in central pairless axonemes. It is possible that the calmodulin peptide inhibitors bind to centrin and/or the 18-kDa calcium-binding light chain. However, these proteins share only 45% amino acid identity with calmodulin, whereas Chlamydomonas calmodulin shares 85% amino acid identity with vertebrate calmodulin (Huang et al., 1988b; Zimmer et al., 1988; King and Patel-King, 1995). Based on the high degree of specificity of these inhibitors, the simplest interpretation is that they bind to and block the function of an axonemal calmodulin.
This interpretation is also supported by our results using inhibitors to CaM-KII. KN-93, a specific inhibitor of CaM-KII (Sumi et al., 1991), and AIP, a highly specific peptide inhibitor of CaM-KII (Ishida et al., 1995) also inhibit dynein activity in central pairless axonemes in high calcium buffer. Importantly, both the control calmodulin inhibitory peptide as well as the control compound for the CaM-KII inhibitor (KN-92) fail to inhibit the calcium-induced increase in dynein activity. Based on these results, and given the concentration at which half-maximal inhibition is achieved, we believe that the affect of these inhibitors is specific.
The Radial Spokes and Central Apparatus Are Key Components of the Calcium-signaling Pathway
The results using axonemes from pf18 imply that the calmodulin-dependent mechanism that mediates calcium-induced change in dynein activity is not located in the central apparatus. Our observation that high calcium does not increase sliding velocity in pf14 axonemes suggests that an important component of the calcium-signaling pathway is either missing or inactivated in radial spoke defective axonemes. One prediction is that the calmodulin and calmodulin-dependent kinase that bind to the CBD and AIP peptides, respectively, are components of the radial spokes. As noted, one component of the radial spokes is calmodulin (Yang et al., 2001). If the radial spoke associated calmodulin is located in the spoke stalk and is necessary and sufficient for calcium-induced rescue of wild-type sliding velocity, we predicted that the pf17 mutant (lacking the radial spoke heads; Piperno et al., 1977, 1981) would also have restored dynein activity in high calcium. However, the velocity of microtubule sliding in pf17 axonemes was not significantly different from that in pf14 in high calcium buffer. In addition, microtubule sliding in axonemes isolated from pf16 was not restored in high calcium buffer, even although pf16 axonemes contain wild-type radial spokes. Evidently, the assembly of wild-type radial spokes alone is not sufficient for restoring dynein activity in high calcium buffer. Therefore, either the radial spoke–associated calmodulin is not the target of the inhibitors used in these studies, or, in the absence of the C1 central microtubule or radial spoke heads, the radial spoke calmodulin is unable to respond to increases in intraflagellar calcium. The latter hypothesis is intriguing given that the central apparatus rotates during flagellar beating (reviewed in Omoto et al., 1999) and the central apparatus projections make transient contact with the radial spoke heads in active regions of microtubule sliding (Warner and Satir, 1974). Moreover, in recent functional analyses of reactivated axonemes isolated from sea urchin sperm, Bannai et al. (2000) demonstrate that calcium-induced changes in microtubule sliding are mediated by a rotatable component and suggest that this component is most likely the central apparatus.
Based on our observation that changes in calcium concentration differentially affect dynein activity in radial spoke mutants compared with central apparatus defective mutants, we propose that these structures are part of a control system that modulates dynein-driven microtubule sliding to regulate the size and shape of flagellar bends in response to calcium. In contrast, Wakabayashi et al. (1997), Frey et al. (1997), and Yagi and Kamiya (2000) have proposed that the radial spokes and central apparatus are not essential for calcium-induced waveform conversion. Their conclusion is based on the observation that isolated axonemes from central pairless and radial spokeless mutants reactivated at low ATP concentration (Omoto et al., 1996) or in the presence of certain organic compounds undergo waveform conversion in response to changes in calcium concentration. Apparently, at low ATP concentration or in organic compounds dynein is activated and this activation bypasses the requirement for intact radial spokes and central apparatus complex. However, for wild-type axonemes in 1 mM ATP, calcium-mediated change in waveform requires the presence of the central apparatus (Hosokawa and Miki-Noumura, 1987). Wakabayashi et al. (1997) propose that key calcium sensors may be localized to the axoneme in positions other than the radial spokes or central apparatus. Our results support the hypothesis that the calcium sensor is not exclusively localized to the central apparatus. However, our results do not rule out the possible involvement of the central apparatus in regulating the calcium sensor to modulate dynein-driven microtubule sliding during calcium-dependent waveform conversion.
What Are the Targets of Calcium-mediated Regulation?
The biggest challenge to understanding the mechanism of flagellar motility is determining how asymmetric regulation of dynein activity is achieved. At any particular moment during flagellar beating, all of the dynein arms are not simultaneously active. The experiments described here do not address the possibility that under specific conditions, the dynein arms on different subsets of doublet microtubules are differentially affected by calcium. The only structure within the flagellum that is asymmetric in both structure and composition is the central apparatus. There are two possibilities to explain how calcium may produce asymmetric regulation of dynein activity to modulate waveform. The key components may be uniformly distributed within the axoneme, and the central apparatus modulates their activity in the presence of calcium. Alternatively, the key components may be asymmetrically organized onto axoneme structures such as the radial spokes or specific doublet microtubules. In either case, it is crucial to determine the location of these components and to identify the dynein arm subforms involved.
To determine which dynein arms are the targets of the calmodulin-mediated increase in sliding velocity observed for pf18 axonemes in pCa4 buffer, we are currently constructing double mutants that lack the central apparatus as well as specific dynein arm subforms. Mitchell and Rosenbaum have reported that although the switch from asymmetric to symmetric waveform can be ellicited in outer dyenin armless mutants, the response of outer dyenin armless mutants is abnormal (Mitchell and Rosenbaum, 1985). Unfortunately, double mutants lacking the central apparatus and outer dynein arms have very short flagella. Because of these technical limitations, we have been unable to obtain interpretable results using axonemes isolated from these mutants in our microtubule sliding assay. Therefore, we must develop alternative methods to investigate the involvement of the outer dynein arms in regulating sliding velocity in high calcium conditions. The construction of central pairless mutants lacking subforms of inner dynein arms is underway.
We are also investigating whether CaM-KII is a structural component of the axoneme. The Chlamydomonas EST database contains several cDNAs that show a high degree of amino acid identity with subunits of human CaM-KII. However, positive identification of an axonemal CaM-KII will require further biochemical and functional analyses as well as definitive localization. Experiments are underway to identify an axonemal calmodulin-dependent kinase as well as additional calmodulin-binding proteins within the axoneme.
ACKNOWLEDGMENTS
I thank Dr. Duane Compton, Dr. Roger Sloboda, and Dr. Winfield Sale for helpful discussion of the manuscript. This work was supported by National Institutes of Health grant GM51379 as a consortium agreement with Dr. Paul Lefebvre, University of Minnesota, and was also supported in part, by research grant 5-FY99–766 (to E.F.S.) from the March of Dimes Birth Defects Foundation.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0185. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0185.
REFERENCES
- Bannai H, Yoshimura M, Takahashi K, Shingyoji C. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J Cell Sci. 2000;113:831–839. doi: 10.1242/jcs.113.5.831. [DOI] [PubMed] [Google Scholar]
- Bessen M, Fay RB, Witman GB. Calcium control of waveform in isolated flagellar axonemes of. Chlamydomonas. J Cell Biol. 1980;86:446–455. doi: 10.1083/jcb.86.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini NM, Evans TC, Miglietta LA, Nelson DL. The regulation of ciliary motility in Paramecium by Ca2+ and cyclic nucleotides. Adv Second Messenger Phosphoprot Res. 1991;23:227–272. [PubMed] [Google Scholar]
- Brokaw CJ. Calcium-induced asymmetrical beating of Triton-demembranated sea urchin sperm flagella. J Cell Biol. 1979;82:401–411. doi: 10.1083/jcb.82.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ. Calcium sensors in sea urchin sperm flagella. Cell Motil Cytoskelet. 1991;18:123–130. doi: 10.1002/cm.970180207. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ, Josslin R, Bobrow L. Calcium ion regulation of flagellar beat symmetry in reactivated sea urchin spermatozoa. Biochem Biophys Res Commun. 1974;58:795–800. doi: 10.1016/s0006-291x(74)80487-0. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ, Luck J, Luck DJL, Huang B. Analysis of the movement of Chlamydomonas flagella: the function of the radial spoke system is revealed by comparison of wild type and mutant flagella. J Cell Biol. 1982;92:722–732. doi: 10.1083/jcb.92.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ, Luck DJL. Bending patterns of Chlamydomonas flagella: III. A radial spoke and head deficient mutant and a central pair deficient mutant. Cell Motil. 1985;5:195–208. doi: 10.1002/cm.970050303. [DOI] [PubMed] [Google Scholar]
- Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- Curry AM, Rosenbaum JL. Flagellar radial spoke: a model molecular genetic system for studying organelle assembly. Cell Motil Cytoskelet. 1993;24:224–232. doi: 10.1002/cm.970240403. [DOI] [PubMed] [Google Scholar]
- Diener DR, Ang LH, Rosenbaum JL. Assembly of flagellar radial spoke proteins in Chlamydomonas: identification of the axoneme binding domain of radial spoke protein 3. J Cell Biol. 1993;123:183–190. doi: 10.1083/jcb.123.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey E, Brokaw CJ, Omoto CK. Reactivation at low ATP distinguishes among classes of paralyzed flagella mutants. Cell Motil Cytoskelet. 1997;38:91–99. doi: 10.1002/(SICI)1097-0169(1997)38:1<91::AID-CM8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gaillard AR, Diener DR, Rosenbaum JL, Sale WS. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP) J Cell Biol. 2001;153:443–448. doi: 10.1083/jcb.153.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons BH, Gibbons IR. Calcium-induced quiescence in reactivated sea urchin sperm. J Cell Biol. 1980;84:13–27. doi: 10.1083/jcb.84.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman SE, Witman GB. Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J Cell Biol. 1980;87:764–770. doi: 10.1083/jcb.87.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, Sale W. Regulation of a flagellar dynein by axonemal type-1 phosphatase in Chlamydomonas. J Cell Sci. 1996;109:1899–1907. doi: 10.1242/jcs.109.7.1899. [DOI] [PubMed] [Google Scholar]
- Habermacher G, Sale W. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J Cell Biol. 1997;136:167–176. doi: 10.1083/jcb.136.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Murtaugh TJ, Satir BH, Satir P. In vitro phosphorylation of Paramecium axonemes and permeabilized cells. Cell Motil Cytoskelet. 1989;12:1–11. doi: 10.1002/cm.970120102. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Miki-Noumura T. Bending motion of Chlamydomonas axonemes after extrusion of central-pair microtubules. J Cell Biol. 1987;105:297–301. doi: 10.1083/jcb.105.3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DR, Habermacher G, Glass DB, Smith EF, Sale WS. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J Cell Biol. 1994;127:1683–1692. doi: 10.1083/jcb.127.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Ramanis Z, Luck DJL. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Huang B, Watterson DM, Lee VD, Schibler MJ. Purification and characterization of a basal-body-associated Ca-binding protein. J Cell Biol. 1988a;107:121–131. doi: 10.1083/jcb.107.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Mengersen A, Lee VD. Molecular cloning of cDNA for caltractin, a basal body associated Ca2+-binding protein: homology in its protein sequence with calmodulin and the yeast CDC31 gene product. J Cell Biol. 1988b;107:133–140. doi: 10.1083/jcb.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams JS, Borisy G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J Cell Sci. 1978;33:235–253. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kitani T, Okuno S, Fujisawa H. Inactivation of Ca2+/calmodulin-dependent protein kinase II by Ca2+/calmodulin. J Biochem (Tokyo) 1994;115:1075–82. doi: 10.1093/oxfordjournals.jbchem.a124460. [DOI] [PubMed] [Google Scholar]
- Ishida A, Fujisawa H. Stabilization of calmodulin-dependent protein kinase II through the autoinhibitory domain. J Biol Chem. 1995;270:2163–2170. doi: 10.1074/jbc.270.5.2163. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Izumi A, Miki-Noumura T. Tetrahymena cell model exhibit Ca-dependent behavior. Cell Motil. 1985;5:323–331. [Google Scholar]
- James P, Vorherr T, Carafoli E. Calmodulin-binding domains: just two faced or multi-faceted? Trends Biochem Sci. 1995;20:38–42. doi: 10.1016/s0968-0004(00)88949-5. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of. Chlamydomonas. J Cell Biol. 1984;98:97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Dutcher SK. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J Cell Biol. 1997;136:177–191. doi: 10.1083/jcb.136.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Patel-King RS. Identification of a Ca2+-binding light chain within Chlamydomonas outer arm dynein. J Cell Sci. 1995;108:3757–3764. doi: 10.1242/jcs.108.12.3757. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Rosenbaum JL. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. Cell Motil Cytoskeleton. 1985;6:510–520. doi: 10.1002/cm.970060510. [DOI] [PubMed] [Google Scholar]
- Naitoh Y, Kaneko H. Reactivated triton extracted models of Paramecium: modification of ciliary movement by calcium ions. Science. 1972;176:523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Okagaki T, Kamiya R. Microtubule sliding in mutant Chlamydomonas axonemes devoid of outer or inner dynein arms. J Cell Biol. 1986;103:1895–1902. doi: 10.1083/jcb.103.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto CK, Brokaw CJ. Bending patterns of Chlamydomonas flagella: II. Calcium effects on reactivated Chlamydomonas flagella. Cell Motil. 1985;5:53–60. doi: 10.1002/cm.970050105. [DOI] [PubMed] [Google Scholar]
- Omoto CK, Yagi T, Kurimoto E, Kamiya R. Ability of paralyzed flagella mutants of Chlamydomonas to move. Cell Motil Cytoskelet. 1996;33:88–94. doi: 10.1002/(SICI)1097-0169(1996)33:2<88::AID-CM2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell. 1999;10:1–4. doi: 10.1091/mbc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter T. Calmodulin and the control of flagellar movement. In: Warner FD, Satir P, Gibbons IR, editors. Cell Movement. Vol. 1. New York: Alan R. Liss; 1989. pp. 281–298. [Google Scholar]
- Payne ME, Fong YL, Ono T, Colbran RJ, Kemp BE, Soderling TR, Means AR. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988;263:7190–7195. [PubMed] [Google Scholar]
- Piperno G, Huang B, Luck DJL. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1977;74:1600–1614. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Huang B, Ramanis Z, Luck DJL. Radial spokes of Chlamydomonas flagella: polypeptide composition and phosphorylation of stalk components. J Cell Biol. 1981;88:73–79. doi: 10.1083/jcb.88.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H, Klauke N. Calcium in ciliated protozoa: sources, regulation, and calcium-regulated cell functions. Int Rev Cytol. 2001;201:115–208. doi: 10.1016/s0074-7696(01)01003-8. [DOI] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axonemal scaffold anchors multiple dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol. 1967;33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffer U, Nultsch W. High-speed cinematographic analysis of the movement of Chlamydomonas reinhardtii. Cell Motil. 1985;5:251–263. [Google Scholar]
- Sale WS. The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol. 1986;102:2042–2052. doi: 10.1083/jcb.102.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury JL, Baron AT, Sanders MA. The centrin based cytoskeleton of Chlamydomonas reinhardtii: distribution in interphase and mitotic cells. J Cell Biol. 1988;107:635–641. doi: 10.1083/jcb.107.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P. Switching mechanisms in the control of ciliary motility. In: Satir P, editor. Modern Cell Biology. New York: Alan R. Liss, Inc.; 1985. pp. 1–46. [Google Scholar]
- Segal RA, Luck DJ. Phosphorylation in isolated Chlamydomonas axonemes: a phosphoprotein may mediate the Ca2+-dependent photophobic response. J Cell Biol. 1985;101:1702–1712. doi: 10.1083/jcb.101.5.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskelet. 2002;52:33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol. 1996;132:359–370. doi: 10.1083/jcb.132.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science. 1992;257:1557–1559. doi: 10.1126/science.1387971. [DOI] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Microtubules, J.S. Hyams, C.W. Lloyd. New York: John Wiley & Sons, Inc.; 1994. Mechanisms of flagellar movement: functional interactions between dynein arms and the radial spoke-central apparatus complex; pp. 381–392. [Google Scholar]
- Sumi, M., Kiuchi, K., Tshikawa, T., Ishii, A., Hagiwara, M., Nagatsu, T., and Hidaka, H. (1991). The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine content in PC12h cells. Biochem. Biophys. Res. Commun. 968–975. [DOI] [PubMed]
- Summers KE, Gibbons IR. Adenosine triphosphate-induced sliding of tubules in trypsin treated flagella of sea urchin sperm. Proc Natl Acad Sci USA. 1971;68:3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tash JS, Means AR. Regulation of protein phosphorylation and motility of sperm by cyclic adenosine monophosphate and calcium. Biol Reprod. 1982;26:745–763. doi: 10.1095/biolreprod26.4.745. [DOI] [PubMed] [Google Scholar]
- Torok K, Trentham DR. Mechanism of 2-chloro-(epsilon-amino-Lys75)-[6-[4-(N,N-diethylamino)phenyl]-1,3,5-triazin-4-yl]calmodulin interactions with smooth muscle myosin light chain kinase and derived peptides. Biochemistry. 1994;43:12807–12820. doi: 10.1021/bi00209a012. [DOI] [PubMed] [Google Scholar]
- Van Eldik LJ, Piperno G, Watterson DM. Similarities and dissimilarities between calmodulin and a Chlamydomonas flagellar protein. Proc Natl Acad Sci USA. 1980;77:4779–4783. doi: 10.1073/pnas.77.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Yagi T, Kamiya R. Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central-pair/radial spoke system. Cell Motil Cytoskelet. 1997;38:22–28. doi: 10.1002/(SICI)1097-0169(1997)38:1<22::AID-CM3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Warner FD, Satir P. The structural basis of ciliary bend formation: radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974;63:35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134:280–290. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]
- Witman GB. Chlamydomonas phototaxis. Trends Cell Biol. 1993;3:403–408. doi: 10.1016/0962-8924(93)90091-e. [DOI] [PubMed] [Google Scholar]
- Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. J Cell Biol. 1978;76:729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Kamiya R. Vigorous beating of Chlamydomonas axonemes lacking central pair/radial spoke structures in the presence of salts and organic compounds. Cell Motil Cytoskelet. 2000;46:190–199. doi: 10.1002/1097-0169(200007)46:3<190::AID-CM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Yanagisawa HA, Kamiya R. Association between actin and light chains in Chlamydomonas flagellar inner-arm dyneins. Biochem Biophys Res Commun. 2001;288:443–447. doi: 10.1006/bbrc.2001.5776. [DOI] [PubMed] [Google Scholar]
- Yang P, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275:18905–18912. doi: 10.1074/jbc.M002134200. [DOI] [PubMed] [Google Scholar]
- Yang P, Fox L, Colbran RJ, Sale WS. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci. 2000;113:91–102. doi: 10.1242/jcs.113.1.91. [DOI] [PubMed] [Google Scholar]
- Yang P, Diener DR, Rosenbaum JL, Sale WS. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J Cell Biol. 2001;153:1315–1325. doi: 10.1083/jcb.153.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer WE, Scloss JA, Silflow CD, Yongblum J, Watterson DM. Structural organization, DNA sequence, and expression of the calmodulin gene. J Biol Chem. 1988;263:19370–19383. [PubMed] [Google Scholar]