Abstract
Ypt1p regulates vesicle tethering and fusion events from the ER to the Golgi and through the early Golgi. Genetic studies have suggested a functional relationship between Ypt1p and Ypt31p/Ypt32p. Ypt31p and Ypt32p are a pair of functionally redundant GTPases that act after Ypt1p to mediate intra-Golgi traffic or the budding of post-Golgi vesicles from the trans-Golgi. Here we report that a novel Ypt32p exchange factor is a putative effector of Ypt1p. These findings implicate small GTP-binding proteins of the Ypt/Rab family in a signal cascade that directs membrane traffic through the secretory pathway.
INTRODUCTION
Newly synthesized secretory proteins are translocated into the ER and then transported to the plasma membrane via the Golgi apparatus by carrier vesicles. Intracellular vesicle traffic requires an efficient mechanism to direct vesicles to their appropriate target compartment. The Ypt/Rab family of Ras-related small GTP-binding proteins are involved in the regulation of protein transport through the different steps of the exocytic pathway (Pfeffer, 2001; Zerial and McBride, 2001). The Saccharomyces cerevisiae genome encodes 11 Ypt/Rab proteins (Lazar et al., 1997). Ypt1p, the ortholog of the mammalian small GTP-binding protein Rab1, acts in both ER-to-Golgi and intra-Golgi traffic (Bacon et al., 1989; Baker et al., 1990; Plutner et al., 1990; Tisdale et al., 1992; Jedd et al., 1995), whereas Ypt31p and its functional homologue Ypt32p have been implicated in traffic through and from the Golgi (Benli et al., 1996; Jedd et al., 1997).
Ypt/Rab proteins function as molecular switches by cycling between an inactive GDP-bound and active GTP-bound conformation (Novick and Zerial, 1997). This cycle of activation and inactivation is regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). GEFs promote GDP dissociation and GTP uptake, which converts Ypt/Rab proteins to their active form. A nucleotide exchange activity for Ypt1p located on Golgi membranes (Jones et al., 1998) is essential for Ypt1p mediated fusion events (Jones et al., 1995). Recently, we have demonstrated that this Ypt1p exchange factor is TRAPP, a highly conserved multiprotein complex that peripherally associates with the Golgi (Sacher et al., 1998; Barrowman et al., 2000; Wang et al., 2000).
There are two forms of the TRAPP complex, TRAPP I and TRAPP II. The two TRAPP complexes share seven subunits (Bet5p, Trs20p, Bet3p, Trs23p, Trs31p, Trs33p, and Trs85p), whereas three subunits (Trs65p, Trs120p, and Trs130p) are unique to TRAPP II. Although both complexes localize to the same early Golgi compartment, mutational analysis and in vitro transport studies have revealed they mediate different transport steps. TRAPP I is required for the tethering of ER-derived COP II vesicles to the Golgi, whereas TRAPP II has been implicated in Golgi traffic (Sacher et al., 2001). Both forms of TRAPP, TRAPP I and TRAPP II, can exchange nucleotide on Ypt1p. The finding that Ypt1p is activated by two distinct but related exchange factors explains how this small GTP-binding protein acts in two different transport events (Wang et al., 2000; Sacher et al., 2001). In addition to Ypt1p, TRAPP was also reported to act as an exchange factor for Ypt31p/32p (Jones et al., 2000).
In their active GTP-bound state, Ypt/Rab proteins interact with downstream effectors that control the targeting, docking and fusion of transport intermediates with their appropriate acceptor compartments (Guo et al., 2000; Somsel and Wandinger-Ness, 2000). Here we report that a novel Ypt32p exchange activity is a putative effector of Ypt1p. Furthermore, we show that TRAPP is the major exchange factor for Ypt1p, but not Ypt32p. Our findings imply that small GTP-binding proteins of the Ypt/Rab family may act in a signal cascade to direct membrane traffic.
MATERIALS AND METHODS
Purification of TRAPP
TRAPP was purified from a strain in which the sole copy of Trs33p was TAP tagged (Sacher et al., 2001). Approximately 10,000 OD599 units of cells were washed and lysed with a bead beater in 70 ml of buffer I (20 mM HEPES, pH 7.2, 150 mM NaCl). The salt concentration was adjusted to 300 mM before centrifugation at 25,000 × g for 20 min, and the supernatant was incubated with 0.4 ml of IgG-Sepharose beads (Amersham Biosciences, Piscataway, NJ) for 2 h. The beads were first washed with IPP150 buffer (10 mM Tris, pH 8.0, 150 mM NaCl) and then TEV buffer (10 mM Tris, pH 8.0, 150 mM NaCl, 0.5 mM EDTA, 1 mM DTT) and incubated with 700 U of the TEV protease (GIBCO BRL Product, Rockville MD) at room temperature for 2 h. The released TRAPP was bound to 0.3 ml of calmodulin affinity resin (Stratagene, La Jolla, CA) in binding buffer (10 mM Tris, pH 8.0, 150 mM NaCl, 1 mM Mg(OAc)2, 1 mM imidazole, 2 mM CaCl2, 10 mM 2-mercaptoethanol). The beads were washed with binding buffer and buffer II (50 mM Tris, pH 8.0, 5 mM MgCl2, 2 mM CaCl2, 1 mM ATP, 1 mM DTT, 1 mg/ml BSA) and then resuspended in uptake buffer (50 mM Tris, pH 8.0, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, 1 mM DTT, 1 mg/ml BSA) and aliquoted into six equal portions for the nucleotide exchange assay.
Protein A (PrA)-tagged TRAPP was purified as described before (Wang et al., 2000).
Preparation of TRAPP-depleted Cytosol
TRAPP was depleted from cytosol prepared from a strain in which the DSS4 gene was disrupted. DSS4 was disrupted by replacing the ORF with the HIS3 gene. Briefly, a hybrid sequence containing the HIS3 gene, flanked by part of DSS4, was amplified by PCR from plasmid pFA6a-His3MX6 (Longtine et al., 1998). This product was then transformed into SFNY1086 (MATα ura3-52 BET3Δ::URA3 leu2-3,112, BET3-protein A::LEU2, his3-Δ200) and Ura+ Leu+ His+ colonies were selected and purified. The disruption of DSS4 was confirmed by PCR, and one of the transformants was named SFNY1088 (MATα ura3-52 BET3Δ::URA3 leu2-3,112 BET3-protein A::LEU2, his3-Δ200, DSS4Δ::HIS3).
To deplete TRAPP from cytosol prepared from SFNY1088, 1000 OD599 units of cells were converted to spheroplasts as described before (Sacher et al., 2000) and lysed with a Wheaton dounce homogenizer in 5 ml of buffer containing 20 mM HEPES, pH 7.2, 150 mM NaCl, 1 mM DTT, and 1× protease inhibitor cocktail (PIC; Waters and Blobel, 1986). The lysate was centrifuged at 60,000 × g for 30 min, and 15.5 mg of the supernatant was incubated overnight with 0.1 ml of packed IgG-Sepharose beads. The beads were spun, and the supernatant was incubated two more times with 0.1 ml of fresh beads for 4 h. The same amount of supernatant was mock treated with Sepharose 4B (Sigma, St. Louis, MO) in the same way. The presence of TRAPP subunits in cytosol was detected by Western blot analysis using the ECL method.
Nucleotide Exchange Assays
The GTP uptake and GDP dissociation assays were performed as described in Wang et al. (2000). In the GTP uptake assay, 5 pmol of recombinant (His6)-Ypt1p or (His6)-Ypt32p, produced in Escherichia coli, was incubated at room temperature in uptake buffer with an aliquot (50 μl) of calmodulin-coated agarose beads ± TRAPP. Assays were performed in the presence of 5 pmol of [35S]GTPγS (NEN Life Science Products, Boston, MA) for the indicated times (see Figure 1). The exchange reaction was stopped by adding 1 ml of ice cold stop buffer (20 mM Tris, pH 8.0, 25 mM MgCl2) and filtered through a nitrocellulose filter (Millipore, Bedford, MA). The filter was washed three times with 3 ml of the stop buffer and then dried. The radioactivity bound to the filter was counted in a scintillation counter.
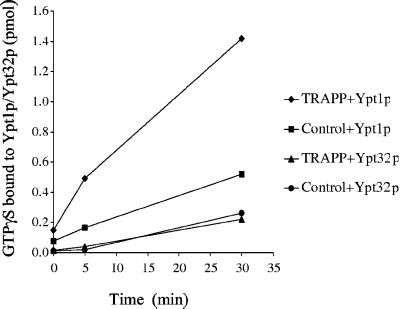
Figure 1.
Chemically pure TRAPP stimulates GTPγS uptake onto Ypt1p but not Ypt32p. TRAPP was purified by affinity purification from a strain in which Trs33p was TAP tagged. Calmodulin agarose beads with or without TRAPP were incubated with 5 pmol of Ypt1p or Ypt32p at room temperature in the presence of [35S]GTPγS. At the time intervals indicated, radioactivity that bound to protein was measured by the filter-binding assay described in MATERIALS AND METHODS. The data are expressed as picomoles of GTPγS retained on the filter.
The GDP dissociation assay was performed in the same way with the following modifications. Ypt1p (2 mM) or Ypt32p (2 mM) was preloaded with 12 mM of [8′-3H]GDP (Amersham Biosciences) at 30°C for 15 min in preloading buffer (20 mM HEPES, pH 7.2, 5 mM EDTA, 1 mM DTT). At the end of the incubation, 10 mM MgCl2 was added to each assay. [3H]GDP-Ypt1p (5 pmol) or [3H]GDP-Ypt32p (5 pmol) was incubated at 30°C in release buffer (20 mM HEPES, pH 7.2, 5 mM MgCl2, 1 mM DTT, 0.75 mM GTP, 0.75 mM GDP, 1 mg/ml BSA) with 0.61 pmol of PrA TRAPP, or 14 mg/ml lysate, or an aliquot of glutathione agarose (80 μl) coated with GST-Ypt1p or GST for varying periods of time.
In Vitro Binding to Ypt1p-GTPγS
Recombinant GST-Ypt1p and GST-Ypt51p were purified from 1 liter of E. coli (BL21). Expression was induced during a 15-h incubation at 20°C by the addition of IPTG (1 mM). The fusion protein was bound to 0.6 ml of glutathione Sepharose (Amersham Biosciences) according to the manufacturer's protocol and stored in phosphate-buffered saline (PBS) with 5 mM MgCl2. Beads bound with 4 mg of GST-Ypt1p or GST-Ypt51p were washed with buffer A (PBS plus 0.5 mM MgCl2, 1 mM DTT, 1 mg/ml BSA, and 1× PIC) containing 10 μM GTPγS or with buffer B (PBS plus 5 mM MgCl2, 10 mM EDTA, 1 mM DTT, 1 mg/ml BSA, and 1× PIC) containing 10 μM GDP. The beads were then incubated with buffer A in the presence of 1.5 mM GTPγS or buffer B in the presence of 1.5 mM GDP for 30 min at room temperature. The washes and incubations were repeated once more before the beads were washed with buffer C (PBS plus 10 mM MgCl2, 1 mM DTT, 1 mg/ml BSA) containing 0.2 mM GTPγS or GDP.
A wild-type yeast lysate (SFNY26-3a, MATa ura3-52) was prepared by converting 15,000 OD599 units of cells to spheroplasts and lysing the spheroplasts in 100 ml of lysis buffer (20 mM HEPES, pH 7.2, 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 mM DTT, and 1× PIC) with a Wheaton dounce homogenizer. The lysate was centrifuged at 18,000 × g for 15 min, and 1 g of the supernatant was incubated with GST-Ypt1p or GST-Ypt51p, prepared as described above, for 2 h at 4°C in the presence of 0.2 mM GTPγS or GDP. The beads were then washed sequentially with buffer C containing 10 μM GTPγS or 10 μM GDP and buffer D (20 mM HEPES, pH 7.2, 5 mM MgCl2, 1 mM DTT, 1 mg/ml BSA) containing 0.75 mM GTP or GDP, resuspended in release buffer and aliquoted into five equal portions for nucleotide exchange assays.
Cell Fractionation and Elution of Ypt32p Exchange Activity
Approximately 1000 OD599 units of SFNY1088 or SFNY26-3a cells were converted to spheroplasts as described by Sacher et al. (2000) and lysed in 10 ml of lysis buffer (20 mM HEPES, pH 7.2, 5 mM MgCl2, 1 mM DTT, and 1× PIC). The unbroken cells were removed during a 5-min spin at 500 × g, and the supernatant (lysate) was centrifuged at 12,000 × g for 10 min. The pellet (P12) was resuspended in lysis buffer (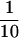 of the initial volume), and the supernatant (S12) was centrifuged at 100,000 × g for 1 h to obtain S100 and P100 fractions. The P100 fraction was resuspended in lysis buffer (
of the initial volume), and the supernatant (S12) was centrifuged at 100,000 × g for 1 h to obtain S100 and P100 fractions. The P100 fraction was resuspended in lysis buffer (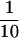 of the initial volume) and incubated for 1 h on ice with an equal volume of lysis buffer with or without 1 M NaCl. The mixture was centrifuged at 100,000 × g for 1 h, and the supernatant was assayed for exchange activity.
of the initial volume) and incubated for 1 h on ice with an equal volume of lysis buffer with or without 1 M NaCl. The mixture was centrifuged at 100,000 × g for 1 h, and the supernatant was assayed for exchange activity.
Purification of Uso1p
Uso1p was purified from SFNY779 (MAT a ura3-52 pUSO1-myc [2 μm, URA3]) as described before (Barlowe, 1997) with the following minor modifications. Cytosol was loaded onto a Mono Q HR10/10 column (Amersham Biosciences), and the column was eluted with a 20-ml linear salt gradient (0.5–2 M KOAc). Fractions of 0.5 ml were collected, and the presence of Uso1p in each fraction was detected by Western blot analysis with 9E10 mAb. The peak of Uso1p from the Mono Q column was applied to a Superdex-200 gel filtration column, and the fractions containing Uso1p were pooled and concentrated.
RESULTS
Chemically Pure TRAPP Stimulates Guanine Nucleotide Exchange on Ypt1p but not Ypt32p
We previously reported that TRAPP is an exchange factor for Ypt1p (Wang et al., 2000). Subsequently, TRAPP was also reported to be an exchange factor for Ypt32p (Jones et al., 2000). In these earlier studies, partially purified preparations of TRAPP were assayed for exchange activity. Thus, it is formally possible that one or both of these activities is the consequence of a contaminant in these preparations. To directly compare the exchange activity of TRAPP on Ypt1p and Ypt32p, we purified TRAPP by tandem affinity purification from a yeast strain containing TAP-tagged Trs33p. Briefly, the two forms of TRAPP were recovered from extracts by affinity absorption onto IgG-Sepharose beads. TEV protease was then added to release the bound material, and the eluate was incubated with calmodulin-coated agarose beads. This second affinity step removed the TEV protease as well as other contaminating proteins. TAP-tagged Trs33p yielded chemically pure TRAPP that was previously shown on a silver-stained gel to only contain TRAPP subunits (Sacher et al., 2001).
To determine the nucleotide exchange activity of this highly purified preparation of TRAPP, a GTPγS uptake assay was performed. TRAPP, immobilized on calmodulin agarose beads, was incubated with GTPγS in the presence of recombinant Ypt1p or Ypt32p, and the amount of GTPγS that bound to protein was measured. Although TRAPP stimulated the uptake of GTPγS onto Ypt1p, it did not appreciably stimulate the uptake of GTPγS onto Ypt32p (Figure 1). These results, together with previously published results showing that TRAPP does not stimulate nucleotide exchange on Sec4p (Wang et al., 2000), imply that TRAPP is a specific guanine nucleotide exchange factor for Ypt1p.
The Depletion of TRAPP from Cytosol Abolishes the Ypt1p but not Ypt32p GDP Release Activity
To determine if TRAPP is the only exchange factor for Ypt1p, we depleted TRAPP from cytosol and then assayed for GDP release activity. For these studies, cytosol was prepared from a strain (SFNY 1088) in which the DSS4 gene was disrupted, and the sole copy of Bet3p was tagged with Protein A (PrA). Dss4p, a putative chaperonin for the nucleotide-free form of Rabs, stimulates the dissociation of GDP from both Ypt1p and Sec4p (Moya et al., 1993; Collins et al., 1997).
TRAPP was depleted from cytosol by affinity absorption onto IgG-Sepharose beads. As Bet3p is found in TRAPP I and TRAPP II (Sacher et al., 2001), both forms of the complex were precipitated onto the beads. To completely deplete cytosol of TRAPP, we found it necessary to treat cytosol three times with IgG-Sepharose beads. Each incubation was done with a fresh batch of beads. The amount of TRAPP remaining in the cytosol was detected by Western blot analysis using anti-Trs33p antibody (Figure 2C). Quantification of the data indicated that ∼99% of the TRAPP was removed from the cytosol. Because Trs33p is present in both forms of TRAPP, this result confirms that TRAPP I and TRAPP II were depleted from the cytosol that we used to assay exchange activity.
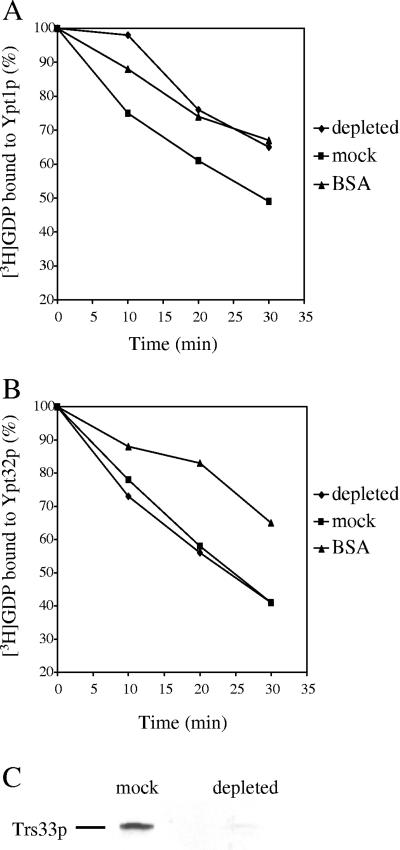
Figure 2.
Depletion of TRAPP abolishes the Ypt1p exchange activity from cytosol. TRAPP was depleted by incubating IgG-Sepharose beads with cytosol prepared from a strain in which Bet3p is Protein A tagged and the DSS4 gene is deleted. Samples containing 0.2 mg of mock-treated or 0.5 mg of IgG-Sepharose–treated cytosol were resolved by SDS-PAGE and analyzed by Western blot analysis with α-Trs33p serum (C). TRAPP-depleted or mock-treated cytosol was incubated with 5 pmol of Ypt1p (A) or Ypt32p (B) preloaded with [3H]GDP. At the time intervals indicated, radioactivity that bound to protein was determined by filter-binding, and the data are expressed as the percentage of label bound to Ypt1p or Ypt32p. The intrinsic rate of [3H]GDP release from Ypt1p or Ypt32p was measured in the presence of BSA.
TRAPP-depleted or mock-treated cytosol (treated with Sepharose beads) was then assayed for its ability to displace GDP from Ypt1p and Ypt32p. Recombinant Ypt1p, or Ypt32p, preloaded with [3H]GDP was incubated with the depleted cytosol, and the radioactivity that remained bound to protein was counted. The intrinsic rate of [3H]GDP release was measured in the presence of BSA. Although TRAPP stimulated dissociation on Ypt1p is concentration dependent, the affinity depletion of TRAPP abolished this activity (Figure 2A). In contrast, the GDP release activity on Ypt32p remained unchanged (Figure 2B). We conclude that TRAPP is the major exchange factor for Ypt1p, but not Ypt32p.
The Ypt32p Exchange Factor Is Associated with the P100 Fraction
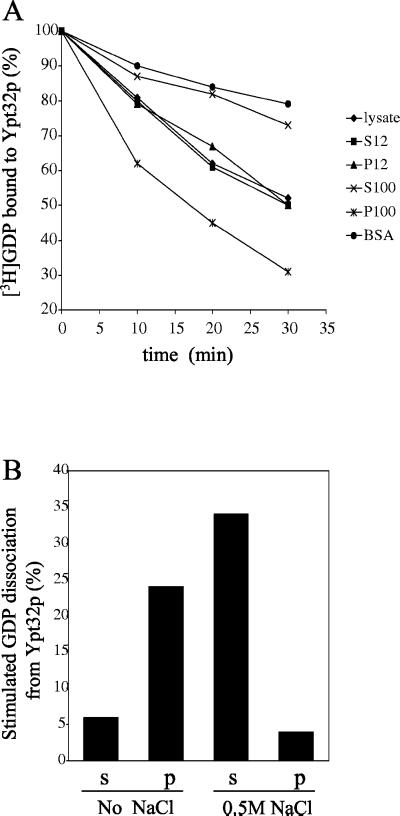
To begin to characterize the Ypt32p exchange factor, differential fractionation experiments were performed. Cell lysates were centrifuged at 12,000 × g to generate supernatant (S12) and pellet (P12) fractions. The S12 was centrifuged at 100,000 × g, and the GDP release activity of the S100 (supernatant) and P100 (pellet) fractions were compared with that of the lysate, S12 and P12 fractions. When equivalent amounts of protein from each of these fractions were assayed, the P100 fraction was found to be enriched in Ypt32p GDP release activity (Figure 3A). Treatment of the P100 fraction, prepared from SFNY 1088, with 0.5 M NaCl released the activity from membranes (Figure 3B). This release factor had exchange activity as it stimulated the uptake of GTPγS onto Ypt32p (Figure 4A).
Figure 3.
The Ypt32p exchange factor is associated with the P100 fraction. (A) A cell lysate was prepared from SFNY26-3a and fractionated as described in MATERIALS AND METHODS. Cell fractions or BSA (1 mg/ml) were incubated with [3H]GDP-Ypt32p for various times at 30°C. For each time point, the data are expressed as the percentage of label bound to Ypt32p. (B) The P100 fraction, prepared from SFNY 1088, was treated with 0.5 M NaCl or no salt and centrifuged at 100,000 × g for 1 h. The supernatant (s) and pellet (p) were assayed for their ability to stimulate the release of [3H]GDP from Ypt32p. The intrinsic rate of [3H]GDP release from Ypt32p was measured in the presence of BSA, and the value obtained was subtracted as background.
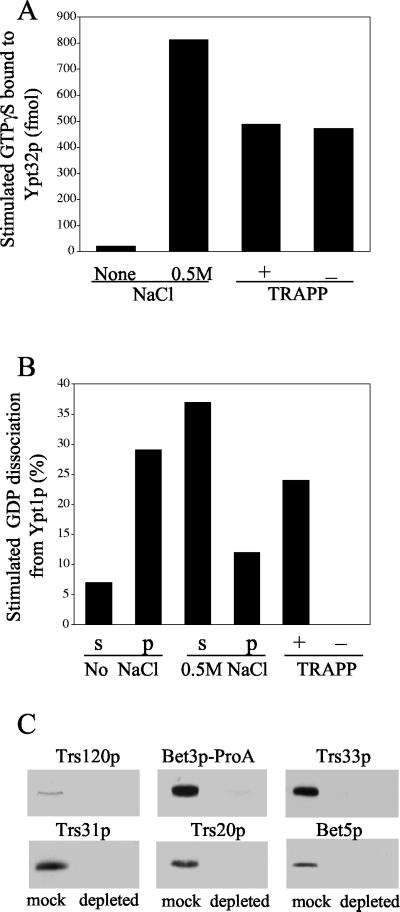
Figure 4.
Depletion of TRAPP does not affect the Ypt32p exchange activity. (A) The P100 fraction of SFNY1088 was treated with buffer (control) or 0.5 M NaCl, centrifuged, and assayed as described below. To deplete TRAPP, the salt-treated supernatant (2.4 mg) was incubated with 20 μl of packed IgG-Sepharose or mock-treated with Sepharose 4B as described in MATERIALS AND METHODS. The TRAPP-depleted or mock-treated sample was incubated with [35S]GTPγS for 30 min at room temperature in the presence or absence of (His6)-Ypt32p. Samples were then incubated with 20 μl of packed Nickel-nitrilotriacetic acid-agarose (Ni-NTA) beads for 1 h at 4°C, and GTPγS uptake was measured. The intrinsic rate of GTPγS uptake onto Ypt32p measured in the presence of BSA, and the value obtained in the absence of (His6)-Ypt32p was subtracted as background. (B) A P100 fraction, prepared from SFNY1088, was treated with buffer (control) or 0.5 M NaCl and centrifuged. The supernatant was incubated with IgG-Sepharose or mock treated with Sepharose 4B. The supernatants (s) and pellets (p) as well as the TRAPP-depleted and mock-treated samples were assayed at 30°C for 30 min for their ability to stimulate the release of [3H]GDP from Ypt1p. The intrinsic rate of [3H]GDP release from Ypt1p was measured in the presence of BSA, and the value obtained was subtracted as background. (C) TRAPP-depleted (0.5 mg) and mock-treated (0.3 mg) samples were resolved by SDS-PAGE and analyzed by Western blot analysis using antibodies directed against Trs120p, Bet3p, Trs33p, Trs31p, Trs20p, and Bet5p.
TRAPP is also efficiently extracted from membranes with 0.5 M NaCl (Sacher et al., 2000 and Figure 4B). To determine if the Ypt32p exchange factor we are characterizing is TRAPP, the salt extract of the P100 fraction was depleted of this complex by treatment with IgG-Sepharose beads. These fractions were then assayed for Ypt1p or Ypt32p exchange activity and blotted for the presence of TRAPP subunits. The IgG-Sepharose–treated fraction was devoid of Ypt1p exchange activity (Figure 4B), whereas the Ypt32p exchange activity was unchanged (Figure 4A). Furthermore, antibodies directed against Trs120p, Bet3p-PrA, Trs33p, Trs31p, Trs20p, and Bet5p detected each of these subunits in mock-treated but not IgG Sepharose–treated fractions (Figure 4C). Trs120p is only present in the TRAPP II complex, whereas Bet3p, Trs33p, Trs31p, Trs20p, and Bet5p are present in both forms of the complex (Sacher et al., 2001). Antibodies recognizing the remaining TRAPP components were not of sufficient titer to detect subunits in the mock-treated sample. These findings demonstrate that the Ypt32p GDP release factor we are characterizing is an exchange factor for Ypt32p. Furthermore, this exchange factor is not TRAPP.
The Ypt32p Exchange Factor Is a Putative Effector of Ypt1p
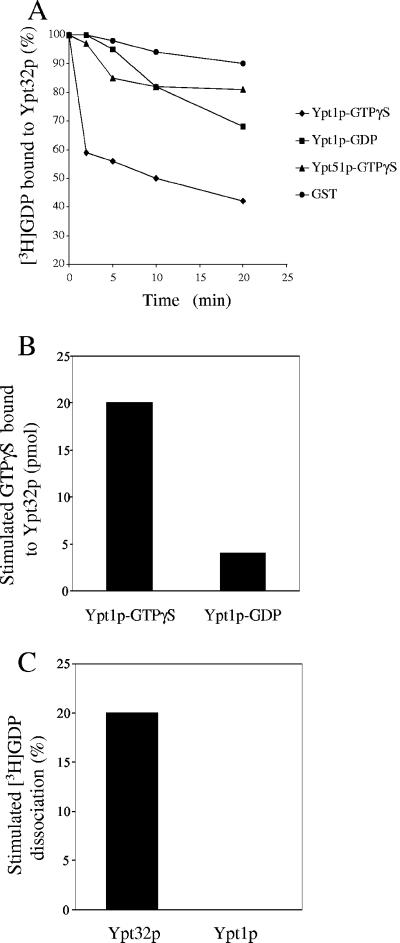
Genetic studies have shown that the overexpression of YPT31 or YPT32 suppresses the dominant YPT1-D124N mutation, which fails to bind guanine nucleotides (Jedd et al., 1995, 1997). Additionally, we found that the overexpression of YPT32 suppresses the trs130ts2 mutant (our unpublished data). As cells defective in trs130 have decreased amounts of active Ypt1p (Wang et al., 2000), we reasoned that the exchange factor for Ypt32p might be an effector of Ypt1p. To test this possibility, we purified Ypt1p as a recombinant GST fusion protein on glutathione Sepharose beads. The beads containing Ypt1p were loaded with either GDP or GTPγS and incubated with a yeast lysate. As controls, immobilized GST-Ypt51p or GST were preloaded with GTPγS and incubated with lysate. Ypt51p is a small GTP-binding protein that regulates membrane traffic on the prevacuolar/endosomal pathway (Gerrard et al., 2000). The beads were washed and assayed for Ypt32p exchange activity. The Ypt1p-GTPγS coated beads accelerated the release of GDP from Ypt32p (Figure 5A) and the uptake of GTPγS onto Ypt32p (Figure 5B). This activity was not dependent on TRAPP, as the Ypt32p GDP release activity was unaffected when TRAPP-depleted cytosol was incubated with the Ypt1p-GTPγS coated beads (our unpublished data). These results imply that the Ypt32p exchange factor is an effector of Ypt1p. This factor preferentially binds to the GTP-bound form of Ypt1p and is specifically recruited by Ypt1p, but not Ypt51p. Furthermore, this exchange factor appears to be specific because it does not stimulate the release of GDP from Ypt1p (Figure 5C).
Figure 5.
The Ypt32p exchange factor is a putative effector of Ypt1p. (A) Lysate, prepared from a wild-type yeast strain, was incubated with beads that contain GST-Ypt1p, GST-Ypt51p, or GST. The beads were either preloaded with GTPγS or GDP. The treated beads were incubated with [3H]GDP-Ypt32p at 30°C for various periods of time. [3H]GDP that bound to protein was measured by by filter-binding, and the data are expressed as the percentage of label bound to Ypt32p. (B) Beads containing 0.8 mg of GST-Ypt1p-GTPγS, GST-Ypt1p-GDP, or GST were incubated with a yeast lysate (800 mg) and then assayed for 30 min at room temperature in the presence of 1.6 nmol of (His6)-Ypt32p and 32 nmol of [35S]GTPγS. The reaction was stopped by the addition of 1 ml of ice-cold stop buffer. The beads were spun, and the supernatant was incubated with 25 μl of packed Ni-NTA agarose beads for 1 h at 4°C. The Ni-NTA beads were washed three times with 1 ml of stop buffer, and the amount of GTPγS that bound to the beads was measured by filter-binding. The intrinsic uptake of GTPγS onto Ypt32p was measured in the presence of immobilized GST, and the value obtained was subtracted as background. (C) TRAPP-depleted cytosol was incubated with GST-Ypt1p-GTPγS immobilized on beads. The beads were then incubated with [3H]GDP-Ypt32p or [3H]GDP-Ypt1p for 20 min at 30°C. The intrinsic rate of [3H]GDP release from Ypt32p or Ypt1p was measured in the presence of immobilized GST, and the value obtained was subtracted as background.
Uso1p Is not an Exchange Factor for Ypt32p
In general, small GTP-binding proteins have many effectors (Horiuchi et al., 1997; Christoforidis et al., 1999; Allan et al., 2000; Moyer et al., 2001). To date, only Uso1p has been implicated as a putative effector of Ypt1p. Uso1p, a large protein that contains coiled coil and globular domains, plays a key role in tethering COP II vesicles to the Golgi complex (Sapperstein et al., 1996; Barlowe, 1997). The proposal that Uso1p is an effector of Ypt1p is based on two observations. First, its orthologue p115 was recently shown to be an effector of Rab1 (Allan et al., 2000). Second, the extraction of Ypt1p from membranes by GDI was found to decrease the association of membrane-bound Uso1p (Cao et al., 1998). These findings prompted us to test the possibility that Uso1p is a Ypt1p effector with exchange activity on Ypt32p.
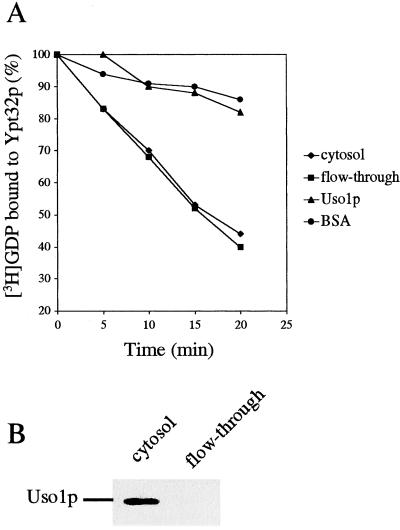
Uso1p was purified from a strain harboring a 2-μm plasmid that contains a myc-tagged version of Uso1p. Cytosol prepared from this strain was loaded onto a Mono Q column and eluted with a linear salt gradient. The peak of Uso1p was further purified on a Superdex-200 gel filtration column, and the fractions containing Uso1p were pooled and concentrated. The cytosol, Mono Q column flow-through and purified Uso1p were assayed for exchange activity on Ypt32p. Ypt32p preloaded with [3H]GDP was incubated with these fractions, and the radioactivity that bound to protein was measured using a filter binding assay. Although Uso1p was retained on the Mono Q column and none could be detected in the flow-through (Figure 6B), the flow-through and cytosol had the same Ypt32p GDP release activity (Figure 6A). Consistent with this observation, purified Uso1p did not display any exchange activity on Ypt32p (Figure 6A). These findings clearly demonstrate that Uso1p is not an exchange factor for Ypt32p.
Figure 6.
Uso1p is not a nucleotide exchange factor for Ypt32p. Uso1p was purified from a yeast cytosol using Mono Q ion exchange and Superdex-200 gel filtration columns. [3H]GDP dissociation from Ypt32p was measured by filter-binding for the indicated times in the presence of either 2 mg/ml cytosol, Mono Q column flow-through, purified Uso1p or BSA. Data are expressed as the percentage of label bound to Ypt32p (A). Samples containing 0.25 mg of cytosol or Mono Q column flow-through were resolved by SDS-PAGE and analyzed by Western blot analysis with 9E10 antibody (B).
DISCUSSION
Previous studies have shown that preparations of partially purified TRAPP stimulate guanine nucleotide exchange on two different small GTP-binding proteins, Ypt1p and Ypt32p. Although partially purified TRAPP stimulated Ypt1p nucleotide exchange activity robustly, very little Ypt32p exchange activity was observed (Wang et al., 2000). These findings suggested that the reported Ypt32p exchange activity was either insignificant or was due to a contaminant. To resolve this issue and to definitively prove that TRAPP is an exchange factor for Ypt1p, we assayed chemically pure amounts of TRAPP for Ypt1p and Ypt32p exchange activity. Earlier work demonstrated that both forms of the TRAPP complex (TRAPP I and TRAPP II) are exchange factors for Ypt1p (Sacher et al., 2001). Chemically pure amounts of TRAPP were prepared from a strain in which Trs33p, a subunit that is present in both forms of the complex, was TAP tagged. Purified TRAPP was found to have Ypt1p, but not Ypt32p, exchange activity.
If TRAPP is not an exchange factor for Ypt32p, lysates depleted of TRAPP I and TRAPP II should still retain Ypt32p exchange activity. Approximately 99% of the TRAPP was depleted from lysates prepared from a strain in which the sole copy of Bet3p is fused to Protein A. Although these lysates no longer had Ypt1p exchange activity, their Ypt32p exchange activity was unaffected. These results imply that TRAPP I and TRAPP II may be the only exchange factors for Ypt1p. Furthermore, they clearly demonstrate that TRAPP is not a major exchange factor for Ypt32p.
In an earlier study from another group, TRAPP was reported to have comparable Ypt32p and Ypt1p exchange activities (Jones et al., 2000). Jones et al. (2000) purified TRAPP from a strain in which Bet3p was overproduced and fused to GST. However, when we assayed purified, GST-tagged TRAPP in the presence of 5 pmol of [3H]GDP-Ypt1p, the specific exchange activity (504 pmol/min/mg) was found to be approximately twofold less than PrA-tagged TRAPP (962 pmol/min/mg). In our hands, very little Ypt32p exchange activity was observed with either preparation of TRAPP and was the same as previously reported (Wang et al., 2000).
Genetic experiments have suggested a functional relationship between Ypt1p and Ypt32p. The overexpression of either YPT31 or YPT32 was found to suppress the growth defect of the dominant YPT1-D124N mutation (Jedd et al., 1997). Furthermore, we isolated YPT31 and YPT32 as high copy suppressors of the trs130ts2 mutant, which is defective in the activation of Ypt1p. These suppression studies suggested that the Ypt32p exchange factor may be an effector of Ypt1p. The finding that a factor that stimulates nucleotide exchange on Ypt32 will specifically bind to Ypt1p in its GTP-bound form supports this hypothesis. This Ypt1p effector is not Uso1p and appears to be novel.
Our findings imply that the activated form of Ypt1p can influence the activity of Ypt32p by recruiting the Ypt32p exchange factor. Furthermore, our data suggest that Ypt1p and Ypt31p/Ypt32p may interact in a signal cascade that directs traffic to and through the Golgi complex. Interestingly, in a parallel study it was shown that activated Ypt32p recruits Sec2p, the exchange factor for Sec4p. Sec4p is the small GTP-binding protein that regulates membrane traffic from the Golgi to the plasma membrane (Ortiz et al., 2002). Thus, one may speculate that the activated form of each Rab may recruit the exchange factor that activates the Rab that functions at the next stage of membrane traffic. It will be important to see if the activated forms of other small GTP-binding proteins in the Rab family do the same.
ACKNOWLEDGMENTS
We thank Michael Sacher for guidance in the construction of SFNY1088 and Elaine Downie and Divya Srivastava for technical assistance. We also thank Jemima Barrowman, Eric Grote, and Douglas Gregory for a critical reading of the manuscript. W.W. was supported as an Associate of the Howard Hughes Medical Institute.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0577. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0577.
REFERENCES
- Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COP II vesicles for fusion. Science. 2000;289:444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- Bacon RA, Salminen A, Ruohola H, Novick P, Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989;109:1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Wuestehube L, Schekman R, Botstein D, Segev N. GTP-binding Ypt1 protein and Ca2+ function independently in a cell-free protein transport reaction. Proc Natl Acad Sci USA. 1990;87:355–359. doi: 10.1073/pnas.87.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J Cell Biol. 1997;139:1097–1108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman J, Sacher M, Ferro-Novick S. TRAPP stably associates with the Golgi and is required for vesicle docking. EMBO J. 2000;19:862–869. doi: 10.1093/emboj/19.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli M, Doring F, Robinson DG, Yang X, Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HR, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Collins NR, Brennwald P, Garrett M, Lauring A, Novick P. Interactions of nucleotide release factor Dss4 with Sec4 in the post-Golgi secretory pathway of yeast. J Biol Chem. 1997;272:18281–18289. doi: 10.1074/jbc.272.29.18281. [DOI] [PubMed] [Google Scholar]
- Gerrard S, Bryant NJ, Stevens T. VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol Biol Cell. 2000;11:613–626. doi: 10.1091/mbc.11.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Jedd G, Richardson CJ, Litt RJ, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPase are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Litt RJ, Richardson CJ, Segev N. Requirement of nucleotide exchange for Ypt1 GTPase mediated protein transport. J Cell Biol. 1995;130:1051–1061. doi: 10.1083/jcb.130.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Richardson CJ, Litt RJ, Segev N. Identification of regulators for Ypt1 GTPase nucleotide cycling. Mol Biol Cell. 1998;9:2819–2837. doi: 10.1091/mbc.9.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1p and Ypt31/32. Mol Biol Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar T, Gotte M, Gallwitz D. Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem Sci. 1997;22:468–472. doi: 10.1016/s0968-0004(97)01150-x. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Mckenzie III A, Demarini DJ, Shan NG, Wach A, Brachat A, Philippsen P, Pringle J. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Moya M, Roberts D, Novick P. DSS4–1 is a dominant suppressor of sec4-8 that encodes a nucleotide exchange protein that aids Sec4p function. Nature. 1993;361:460–463. doi: 10.1038/361460a0. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Allan BB, Balch W. Rab1 interaction with a GM130 effector complex regulates COP II vesicle cis-Golgi tethering. Traffic. 2001;2:268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Soilmena C, Novick P. Ypt32p recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles: evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Plutner EJ, Schwaninger R, Pind S, Balch WE. Synthetic peptides of the Rab effector domain inhibit vesicular transport through the secretory pathway. EMBO J. 1990;9:2375–2383. doi: 10.1002/j.1460-2075.1990.tb07412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston L, Zhang L, Schieltz JR, Yates III JR, Abeliovich H, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;9:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Barrowman J, Schieltz D, Yates III JR, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur J Cell Biol. 2000;79:71–80. doi: 10.1078/S0171-9335(04)70009-6. [DOI] [PubMed] [Google Scholar]
- Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, Ferro-Novick S. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell. 2001;7:433–442. doi: 10.1016/s1097-2765(01)00190-3. [DOI] [PubMed] [Google Scholar]
- Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsel RJ, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far RCJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–295. doi: 10.1083/jcb.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986;102:1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]