Abstract
After permeabilization with the pore-forming toxin streptolysin-O mast cells can be triggered to secrete by addition of both calcium and a GTP analogue. If stimulation is delayed after permeabilization, there is a progressive decrease in the extent of secretion upon stimulation, eventually leading to a complete loss of the secretory response. This loss of secretory response can be retarded by the addition of cytosol from other secretory tissues, demonstrating that the response is dependent on a number of cytosolic proteins. We have used this as the basis of a bioassay to purify Secernin 1, a novel 50-kDa cytosolic protein that appears to be involved in the regulation of exocytosis from peritoneal mast cells. Secernin 1 increases both the extent of secretion and increases the sensitivity of mast cells to stimulation with calcium.
INTRODUCTION
Mast cells are secretory cells found on the mucosal and serosal surfaces of tissues throughout the body where they are involved in the allergic response (Wedemeyer and Galli, 2000). The cells can be activated by the cross-linking of high-affinity IgE receptors by antigen-specific IgE that leads to activation of phospholipase C, generating IP3 that subsequently causes a release of calcium from cytosolic stores and thus triggers secretion (Kinet, 1999). The mast cells secrete a variety of inflammatory mediators, including histamine, from granules that contain many lysosomal markers (Griffiths, 1996). The presence of lysosomal markers in the secretory granules of mast cells and other secretory cells of hemopoietic lineage (such as basophils, cytotoxic T cells, natural killer cells, neutrophils, eosinophils, and macrophages) has led to the suggestion that these secretory granules are not derived from the classical secretory pathway, but are derived from the lysosomal pathway (Stinchcombe and Griffiths, 2001).
Permeabilization of secretory cells with detergents such as digitonin, bacterial toxins such as Streptolysin-O (SLO) or by mechanical disruption allows exocytosis to be triggered by the addition of buffered calcium solutions, bypassing the need for activation of a triggering receptor and associated signal transduction events (Gomperts and Tatham, 1992). In mast cells an absolute requirement for guanine nucleotides has been reported, unlike neuroendocrine cells where guanine nucleotides appear to have a modulatory role (Lillie and Gomperts, 1992). This has led to the identification of a number of GTP binding proteins including Giα3 (Aridor et al., 1993), βγ subunits (Pinxteren et al., 1998), rac (O'Sullivan et al., 1996), rho (Price et al., 1995), and cdc42 (Brown et al., 1998), which all appear to regulate secretion in mast cells. Although the final fusion event is mediated by the SNAP/NSF/SNARE system in both mast cells (Guo et al., 1998; Paumet et al., 2000, Baram et al., 2001), neurons and neuroendocrine cells (Jahn and Südhof, 1999), it is possible that the origin of these secretory granules accounts for the differences observed in the regulation of secretion in these cells when compared with the more commonly studied neuroendocrine systems.
After permeabilization secretory cells leak cytosolic proteins, leading to a progressive loss of responsiveness to Ca2+ and nucleotides. This loss of response or rundown can be slowed by the provision of exogenous cytosol and has been used as the basis of a bioassay in mast cells (O'Sullivan et al., 1996), chromaffin cells (Morgan and Burgoyne, 1992), and PC12 and GH3 cells (Walent et al., 1992) to purify cytosolic proteins that regulate secretion from these cells. We have previously shown that a rac/RhoGDI complex isolated from bovine brain cytosol partially restores secretory responsiveness in Streptolysin-O–permeabilized mast cells (O'Sullivan et al., 1996). A number of other activities were partially purified during the purification of the rac/rhoGDI complex. Here we report the full purification and identification of a second brain cytosolic protein, Secernin 1 (Secern is an archaic English term for secrete), which is also capable of regulating exocytosis in permeabilized mast cells.
MATERIALS AND METHODS
Frozen bovine brains were purchased from First Link UK (Brierley Hill, West Midlands, UK). Male Sprague Dawley rats were purchased from B&K Universal Ltd. (Hull, UK). GTP-γ-S and bovine serum albumin was purchased from Roche Diagnostics Ltd. (Lewes, East Sussex, UK). Streptolysin-O (Murex formulation) was purchased from Corgenix Biotech Limited (Temple Hill, Dartford, Kent, UK). Ceramic hydroxyapatite column was purchased from Bio-Rad (Hemel Hempstead, UK), and all other chromatography columns were purchased from Amersham-Pharmacia (Amersham, UK). Donkey anti-rabbit horseradish peroxidase (HRP) antibody was purchased from Amersham-Pharmacia. The GATEWAY cloning system and ThermalAce DNA polymerase kit was obtained from Invitrogen, Life Technologies (Paisley, UK). The human cDNA clone of the gene KIAA0193 was obtained from the Kazusa DNA Research Institute (Kisararazu, Chiba, Japan) and inserted in the pBluescript SK+ vector. All other chemicals used were of the highest quality available from standard commercial sources.
Experimental Procedures
Secretion Measurements.
Cells were obtained by peritoneal lavage of male Sprague Dawley rats (>300 g), and mast cells were purified to >98% purity by centrifugation through Percoll as previously described (Tatham and Gomperts, 1990). Cells, suspended in assay buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 20 mM piperazine-N,N′-bis(2-ethanesulfonic acid), pH 6.8) supplemented with 1 mg/ml bovine serum albumin (BSA) were incubated with metabolic inhibitors (0.6 mM 2-deoxyglucose and 10 μM antimycin A) for 5 min at 37°C and then cooled to ice temperature and added to SLO (1.6 IU/ml) in the presence of 0.1 mM EGTA. After 5 min, cells were washed free of unbound SLO and contaminating impurities (Larbi and Gomperts, 1996) by dilution and centrifugation at 4°C. Permeabilization and hence rundown of the secretory response was initiated by transferring the cells to prewarmed (37°C) assay buffer containing 1 mg/ml BSA, 0.3 mM Ca/EGTA buffer (10 nM Ca2+), 100 μM Mg·ATP, and proteins under test in 96-well microtiter plates. After allowing predetermined times for rundown (generally between 5 and 20 min), the cells were stimulated to secrete by addition of solutions containing Ca/EGTA buffers formulated to regulate 10 μM Ca2+(or 100 nM Ca2+ for controls) to a final concentration of 3 mM and GTP-γ-S to a final concentration of 100 μM (or zero for controls) with sufficient Mg·ATP to maintain the concentration at 100 μM. After 20 min the reactions were quenched by addition of ice-cold buffer supplemented with EGTA (10 mM), and the cells were sedimented by centrifugation. The supernatants were sampled for measurement of secreted hexosaminidase as previously described (Tatham and Gomperts, 1990).
Calcium/EGTA buffers were prepared by mixing solutions of EGTA and end-point–titrated Ca·EGTA made up at identical concentrations and adjusted to pH 6.8, according to a computer program, as previously described (Tatham and Gomperts, 1990).
Secretion is expressed as the percent of total cellular hexosaminidase released, calibrated by reference to appropriate reagent blanks and the total cell content released by 0.1% Triton X-100. Stimulated secretion is calculated as the difference in the amount of hexosaminidase released in response to 100 nM Ca2+ or 10 μM Ca2+ + 100 μM GTPγS. All determinations were carried out in quadruplicate unless otherwise stated.
Purification of Secernin 1.
All chromatography was carried out on a Bio-Rad Biologic liquid chromatography system at 4°C. Frozen bovine brains, 500 g, were thawed at 4°C before homogenization in a Waring blender in 1 liter homogenization buffer (137 mM NaCl, 2.3 mM KCl, 1 mM MgCl2, 1 mM EGTA, 1 μM Pepstatin, 1 μM Leupeptin, 0.1 mM PMSF, 0.02% NaN3, 20 mM Pipes, pH 6.8). The homogenate was then centrifuged for 10 h at 10,000 × g at 4°C in a fixed angle rotor. This cytosol extract was subjected to ammonium sulfate precipitation and an active fraction between 60 and 90% (NH4)2SO4 was resuspended in 40 ml homogenization buffer. The active material was subjected to chromatography on Octyl Sepharose FF as previously described (O'Sullivan et al., 1996), and the active fractions were combined. All column fractions under test were buffer exchanged into assay buffer using NAP-5 columns before assay.
DEAE Chromatography.
The active fractions from the Octyl Sepharose column were combined and desalted into buffer A (20 mM diethanolamine, 0.02% NaN3, pH 8.7) in aliquots of 5 ml on a HiPrep 26/10 desalting column (Pharmacia). The desalted material was loaded, using on line dilution via the pump, at 20% protein with 80% buffer A, onto a DEAE Sepharose column (XK26/50, 100 ml, Pharmacia) that had been equilibrated with buffer A. The column was then washed with 36 ml 20 mM diethanolamine, pH 8.7, before elution with a linear gradient of 0–40% buffer B (1 M NaCl, 20 mM diethanolamine, 0.02% NaN3, pH 8.7) over 372 ml followed by a final elution in 100% buffer B over 120 ml. The column was run at 5 ml/min, and 8-ml fractions were collected. The fractions were assayed for activity, and the active fractions (45–52) on the third peak were combined.
Hydroxyapatite Chromatography.
Active fractions (45–52) from the third peak of the DEAE chromatography were concentrated to 5 ml on a 50-ml Amicon pressure concentrator (43-mm YM10 membrane) and then desalted into buffer C (50 mM MES, 0.02% NaN3, pH 6.0) on a HiPrep 26/10 desalting column. This was then loaded onto a ceramic hydroxyapatite column (Econo-Pac CHT-II, 1 ml, Bio-Rad) preequilibrated with buffer C. The column was then washed with 2 ml buffer C before elution with a linear gradient of 0–100% buffer D (500 mM NaCl, 50 mM MES, 0.02% NaN3, pH 6.0) over 16 ml. The column was then washed with an additional 2 ml buffer D, before elution with a 0–100% linear gradient of buffer E (500 mM KH2PO4, 50 mM MES, 0.02% NaN3, pH 6.0) over 16 ml and finally washed with an additional 4 ml of buffer E. The column was run at 1 ml/min, and 1-ml fractions were collected. The fractions were assayed for activity, and the active fractions (35–38) in the second peak were combined.
Phenyl Superose Chromatography.
The combined fractions from peak 2 of the hydroxyapatite column were diluted with 3.4 M (NH4)2SO4, buffered with 50 mM NaH2PO4, 0.02% NaN3, pH 7.5, to produce a final concentration of 2 M (NH4)2SO4. The protein was then applied to a Phenyl Superose column (Pharmacia, HR5/5, 1 ml) equilibrated in buffer F ((NH4)2SO4, 50 mM NaH2PO4, 0.02% NaN3, pH 7.5) and the column washed with 5 ml 100% buffer F before eluting with a 30–80% gradient of buffer G (50 mM NaH2PO4, 0.02% NaN3, pH 7.5) over 30 ml, followed by a final 5 ml of 100% buffer G. The column was run at 0.4 ml/min, and 1-ml fractions were collected.
Superose 12 Chromatography.
Active fractions from the Phenyl Superose (21–23) were concentrated to 240 μl on a 10K Microsep centrifugal concentrator (Filtron, Northborough, MA) at 4°C and injected onto a Superose 12 column (Pharmacia, HR10/30, 24 ml) equilibrated in homogenization buffer. The column was run at 0.2/ml min, and 0.5-ml fractions were collected. The active fractions were combined and concentrated before use in secretion experiments.
Leakage of Secernin.
Purified mast cells were treated with diisopropyl fluorophosphate (2 mM) for 10 min at 4°C. The cells were treated with SLO at ice temperature as described above, resuspended at ∼1 × 106cells/ml, and then permeabilized by bringing the temperature to 37°C. Samples of cells, 100 μl, were removed at intervals and sedimented by centrifugation at 14,000 × g, and the supernatants were harvested. Ice-cold acetone was added to the supernatants to a final concentration of 80%, and the mixture was maintained at −20°C for 2 h after which the aggregated proteins were sedimented by centrifugation at 14,000 × g. These were taken up in Laemmli sample buffer and separated on 12% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose using a wet blot method and probed for Secernin using the polyclonal anti-Secernin antibodies, SK1147 and SK1148, at a dilution of 1:4000. Antibody binding was detected using a donkey anti-rabbit HRP-linked secondary antibody at 1:2000 dilution and an ECL detection kit.
Plasmid Construction.
Oligonucleotide primers for the amplification of the KIAA0193 gene were designed with attB1 or attB2 sites for the insertion into the GATEWAY donor vector pDONR201 (Life Technologies) by homologous recombination. Primers with the following sequences were synthesized by MWG, Inc.: KIAA0193 (forward), 5′- GGGGACAAGTTTGTACAAAAAAGCAGGCTTCG A A GGAGATAGAACCATGATAAGCAGACCCGCCTGGCTCT-3′; KIAA 0193 (reverse), 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTC-CTATCACTTAAAGAACTTAATCTCCGTG-3′. The primers were used to generate the attB PCR product using a ThermalAce DNA polymerase kit (Invitrogen) from the pBluescript SK+ vector containing KIAA0193. The PCR products were cloned into pDONR201, and the resulting plasmid pENTR KIAA0193 were used to transfer the gene sequences into pDEST15 (N terminal GST fusion) or pDEST17 (N terminal His fusion) via homologous recombination. The corresponding plasmids, pEXP15 KIAA0193 and pEXP17 KIAA0193, were used for overexpression of the fusion proteins in Escherichia coli BL21-SI.
Recombinant Protein Purification.
Cells carrying either pEXP15 KIAA0193 or pEXP17 KIAA0193 were grown overnight in LB broth without NaCl in the presence of 100 μg/ml ampicillin at 30°C. The cells were then diluted 1 in 10 in prewarmed LB broth without NaCl, in the presence of 100 μg/ml ampicillin at 30°C, and grown to an optical density of 0.6 at 600 nm. Protein expression was induced by the addition of NaCl to 0.3 M, and the cells were grown for a further 3 h. Cells were harvested by centrifugation, and inclusion bodies were purified by the method of Marston et al. (1984).
Affinity Purification of Antibody SK1147.
Inclusion bodies of the recombinant GST-tagged KIAA 0193 (human Secernin 1) were solubilized in SDS-sample buffer and separated on 10% SDS-PAGE. After blotting onto nitrocellulose paper the recombinant protein was visualized with Ponceau S staining, and the bands were excised. The antibody SK1148 was affinity purified against this protein by the method of Smith and Fisher (1984). The antibody was dialyzed overnight against homogenization buffer before use in neutralization experiments.
Immunoneutralization of Secernin.
Polyclonal anti-Secernin antibody SK1147 or preimmune serum was diluted 1:50 into assay buffer and added an equal volume of either purified 60 μg/ml Secernin or 6 mg/ml freshly prepared rat brain cytosol in assay buffer. The antibody and proteins were incubated at 4°C for 30 min before addition to permeabilized mast cells at a final concentration of 10 μg/ml Secernin and 1 mg/ml cytosol as described above. Varying concentrations of affinity-purified SK1147 was incubated with 3 mg/ml cytosol in the presence or absence of 5 mg/ml inclusion body containing recombinant human Secernin 1-His fusion protein. After incubation for 60 min at 4°C, the cytosol was centrifuged at 14,000 × g to remove the inclusion body before addition to permeabilized mast cells at a final concentration of 1 mg/ml cytosol and antibody as indicated.
Protein Assay.
Protein concentration was assayed by the method of Bradford (1976) using BSA as a standard.
Protein Analysis.
Purity of protein samples was assessed by electrophoretic separation on 12% SDS-polyacrylamide gels (Laemmli, 1970) and detection by silver staining (Morrissey, 1981).
Production of Antisera.
Two polyclonal rabbit antisera (SK1147 and SK1148) were raised against purified Secernin 1 by Abcam Ltd. (Cambridge, UK), using a 30-μg initial injection followed by three booster injections of 30 μg.
Mass Spectrometric Analysis.
p50 was alkylated with iodoacetamide in sample buffer (Novex, Encinitas, CA) and run on a 4–12% SDS-PAGE gel with a MOPS running buffer system (Novex). The gel was stained with Sypro Orange (Molecular Probes, Eugene, OR), and the p50 band was excised and digested with 12.5 μg/ml modified trypsin (Roche) in 20 mM NH4CO3. A proportion of the sample was analyzed by MALDI-TOF MS, and the tryptic peptide ions were searched against NCBI and Swiss Prot databases using the MS-FIT search algorithm from Protein Prospector (UCSF, San Francisco, CA).
The remainder of the sample was chromatographed on a 150 × 0.075-mm Pepmap C18 capillary column coupled to an LC Packings Ultima HPLC system (Dionex, Camberley, UK). The column was equilibrated with 2% acetonitrile/0.1% formic acid in water at 0.2 μl/min, and developed with a gradient of acetonitrile/0.1% formic acid. The outlet of the column was connected to a Micromass Q-TOF2 mass spectrometer, equipped with a nanoflow source, and peptide ions were automatically submitted for ms/ms fragmentation. Spectra from ms/ms experiments were interpreted, and the sequences were searched against NCBI nr and dbEST databases using the BLAST search algorithm. Spectral data was also searched against the same databases using the Sonar ms/ms search algorithm (http://service.proteometrics.com/prowl/sonar.html).
RESULTS
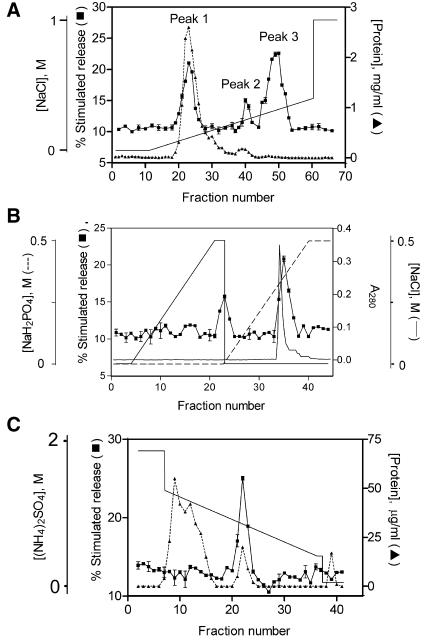
Bovine brain cytosol was prepared and fractionated by ammonium sulfate precipitation followed by Octyl Sepharose chromatography as previously described (O'Sullivan et al., 1996). The purification of Secernin 1 described in MATERIALS AND METHODS is summarized in Table 1. Activity from cytosol prepared from frozen bovine brains is only detectable after the Octyl Sepharose column, unlike cytosol from freshly isolated rat brains (O'Sullivan et al., 1996). The purification is therefore calculated from the pooled activity from this column. Figure 1A shows that the activity eluted from the Octyl Sepharose can be separated by a DEAE Sepharose column into three distinct peaks of activity by a gradient of NaCl. Peak 1 was found to contain the previously purified rac and rhoGDI, as assessed by Western blotting, so further purification of this peak was not undertaken. Peak 3 appeared to have the highest activity and was therefore subjected to further purification.
Table 1.
Purification of secernin 1
| Step | Total protein (mg) | Specific activity (% Stimulation/ mg protein)a | Fold enrichment |
|---|---|---|---|
| Ammonium sulphate pellet | 1550 | ND | — |
| Octyl Sepharose pool | 228 | 0.097 | 1 |
| DEAE peak 3 pool | 1.45 | 5.10 | 52.6 |
| Hydroxyapatite peak 2 pool | 0.307 | 19.87 | 205 |
| Phenyl Superose pool | 0.034 | 208.82 | 2,153 |
| Superose 12 pool | 0.0044 | 1454.5 | 14,995 |
Specific activity of each of the pooled peaks is calculated as average increase in stimulated release per mg protein for a typical purification.
Figure 1.
Purification of secernin. (A) DEAE chromatography. Active fractions from Octyl Sepharose were desalted and loaded onto a DEAE Sepharose column, the activity was eluted with a rising gradient of NaCl. (B) Hydroxyapatite chromatography. Pooled fractions from peak 3 on the DEAE column were desalted and applied to a ceramic hydroxyapatite column. The activity was initially eluted with a rising gradient of NaCl followed by a second gradient of KH2PO4. (C) Phenyl Superose chromatography. Pooled fractions from peak 2 on the hydroxyapatite column were adjusted to 2 M (NH4)2SO4 and applied to a Phenyl Superose column. The activity was eluted with a falling gradient of (NH4)2SO4. All column activities were assayed for protein concentration and stimulated secretion as described in the MATERIALS AND METHODS. Data shown are mean ± SEM (n = 4); similar results were obtained on at least 10 occasions. Some error bars are smaller than symbols used.
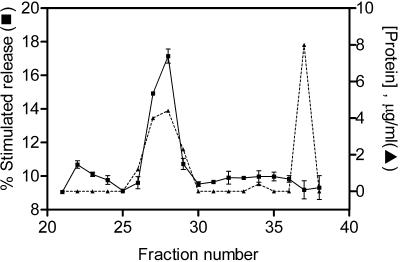
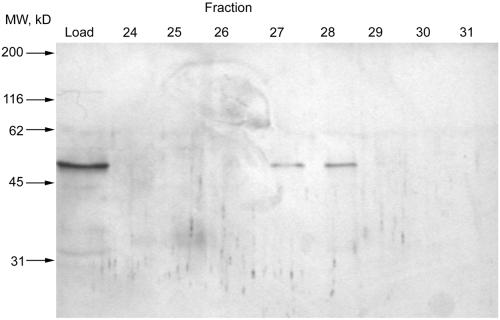
The combined fractions of peak 3 from the DEAE column were applied to a hydroxyapatite column and eluted with a rising gradient of NaCl, followed by a rising gradient of KH2PO4 as shown in Figure 1B. A small peak of activity elutes with NaCl and a larger second activity elutes with KH2PO4. This second activity peak was pooled, applied to a Phenyl Superose column and eluted by a decreasing gradient of (NH4)2SO4 as shown in Figure 1C. Fractions from the column were then subjected to SDS-PAGE analysis, a single protein of 50 kDa appears to correlate with the activity from the phenyl superose column. To confirm the correlation, the pooled fractions from the Phenyl Superose column were concentrated and applied to a Superose 12 gel filtration column. The fractions were assayed for their ability to retard the rundown of exocytosis in mast cells and subjected to SDS-PAGE, and again a single 50-kDa protein was found to correlate with the activity, as shown in Figure 2.
Figure 2.
Superose 12 chromatography. (A) Active fractions from the Phenyl Superose column were concentrated and loaded onto a superose 12 gel filtration column and assayed for protein concentration and stimulated secretion as described in the MATERIALS AND METHODS. Data shown are mean ± SEM (n = 4); similar results were obtained on at least 10 occasions. Some error bars are smaller than symbols used. (B) Active fractions were analyzed by silver-stained 12% SDS-PAGE.
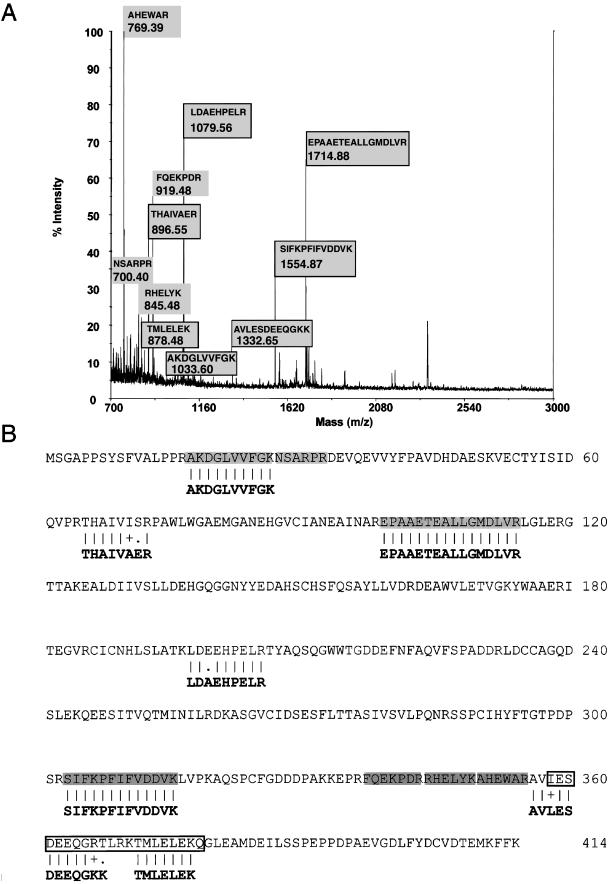
To identify the 50-kDa protein, the purified material was subjected to SDS-PAGE and in-gel trypsinization. The peptide mixture was recovered and subjected to MALDI-TOF mass spectrometry, and the resultant mass fingerprint used to interrogate sequence databases. This analysis identified p50 as a protein corresponding to a previously cloned mouse cDNA (GenBank accession no. AK012765) of unknown function (Figure 3). To confirm the identity of p50, the balance of the peptide mixture was chromatographed by reverse-phase HPLC, and individual peptides were delivered to an online Q-TOF mass spectrometer for ms/ms fragmentation and consequent peptide sequence identification. A total of seven peptides were sequenced (Figure 3B, bold). Of these, three corresponded to peptides previously identified by mass fingerprint, and each showed sequence identity with the putative protein encoded by AK012765. One peptide, not identified in the initial mass fingerprint also was identical to the mouse sequence, whereas three additional peptides were closely related, showing >75% homology with it. We conclude that p50 is a novel protein, which we have named Secernin 1, which is encoded by the bovine orthologue of mouse AK012765.
Figure 3.
Identification of p50 by MALDI-TOF and Q-TOF mass spectrometry. A tryptic digest of p50 excised from a SDS-PAGE gel was subjected either to fingerprint analysis by MALDI-TOF mass spectrometry or reverse-phase HPLC and online Q-TOF ms/ms fragmentation. (A) MALDI-TOF mass spectrum annotated with the sequence and molecular mass (m/z) of identified peptides (highlighted in gray). Peptides whose sequence was deduced by Q-TOF fragmentation analysis are boxed. (B) The predicted polypeptide sequence encoded by mouse cDNA AK012765. Peptide sequences identified by MALDI fingerprint analysis above are highlighted in gray. Peptides whose sequence was deduced by MS/MS fragmentation (in bold font) are aligned. ‖ indicates a match, + indicates a conserved substitution, and (.) indicates a mismatch between the sequences. The outlined area (□) represents the predicted coiled-coil region.
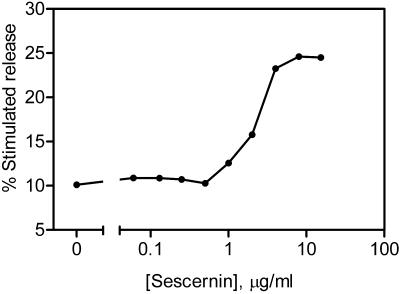
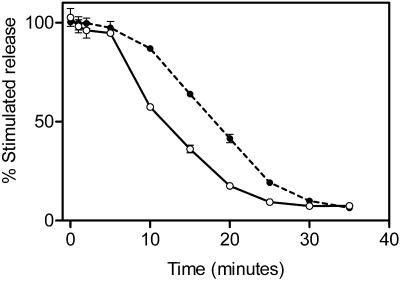
Figure 4 shows the effect of increasing concentrations of purified Secernin 1 on the secretion of hexosaminidase from permeabilized mast cells. The optimal concentration of Secernin 1 is 7.1 ± 0.9 μg/ml with an EC50 of 2.21 ± 0.2 μg/ml (mean ± SEM, n = 4), which is comparable to other proteins previously demonstrated to regulate secretion in these cells (O'Sullivan et al., 1996; Pinxteren et al., 2001). This effect on secretion is unlikely to be due to nonspecific protein effects because neither boiled Secernin 1 at 10 μg/ml nor BSA up to 1 mg/ml had any effect on secretion from these cells (O'Sullivan, unpublished observations). Figure 5 shows the effect of the protein at various times of rundown, the protein initially has no effect, but as the cytosol leaks from the cells, there is an increasing effect, which in turn declines as other proteins leak from the cell. However, Secernin 1 is incapable of completely preventing rundown.
Figure 4.
Secernin 1 dose response. Mast cells permeabilized with SLO in the presence of varying concentrations of Secernin 1. After 15 min the cells were stimulated by addition of solutions containing EGTA buffered to 10 μM Ca2+ plus GTPγS (final concentration, 100 μM) or 100 nM Ca2+. After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Stimulated secretion is calculated as the difference in the amount of hexosaminidase released in response to either 100 nM Ca2+ or 10 μM Ca2+ + 100 μM GTPγS. Data shown are mean ± SEM (n = 4); similar results were obtained on four occasions. Some error bars are smaller than symbols used.
Figure 5.
Effect of Secernin on rundown of secretory response. Mast cells permeabilized with SLO in the presence (●) and absence (○) of Secernin 1 at a final concentration of 3.5 μg/ml. At times indicated, samples were removed and stimulated by transfer to solutions containing EGTA buffered to 10 μM Ca2+ plus GTPγS (final concentration, 100 μM) or 100 nM Ca2+. After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Stimulated secretion is the difference in the amount of hexosaminidase released in response to either 100 nM Ca2+ or 10 μM Ca2+ + 100 μM GTPγS. Data shown are mean ± SEM (n = 4); similar results were obtained on four occasions. Some error bars are smaller than symbols used.
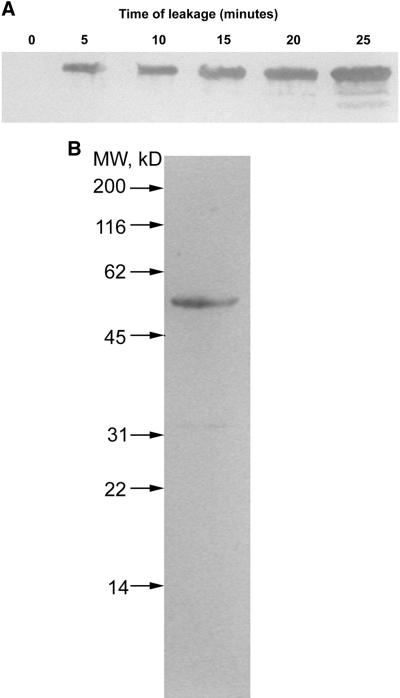
To determine that the protein is present in mast cells, a rabbit polyclonal antibody SK1147 was generated against the purified protein. Figure 6A shows a Western blot of whole mast cells demonstrating a single immunoreactive band at 50 kDa; a similar result was obtained with a second polyclonal antibody SK1148. The results shown in Figure 5 are consistent with a model in which Secernin 1 leaks slowly from the permeabilized cell and the exogenously added Secernin 1 replaces the lost protein. To test this hypothesis, supernatants were collected from cells permeabilized over increasing periods. The supernatants were precipitated by acetone at −20°C, separated by SDS-PAGE, and analyzed by Western blot. Figure 6B shows that the antibody detects an increasing amount of the 50-kDa protein in the supernatants with time after permeabilization, confirming that the protein is cytosolic and leaks from the cell after permeabilization with a similar time course to the rundown of the secretory response.
Figure 6.
Presence of Secernin in mast cell cytosol. (A) Western blot of intact mast cells. After transfer to nitrocellulose membranes, the proteins were detected by probing with the rabbit polyclonal antibody SK1147 and a donkey anti-rabbit HRP secondary antibody visualized by ECL. (B) The permeabilized mast cells were sampled at the times indicated and rapidly sedimented. Proteins present in the supernatants were precipitated and Western blotted. Proteins were detected with antibody SK1147.
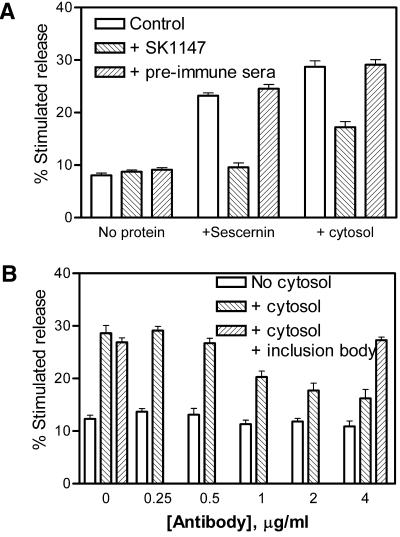
To confirm that that the effects of Secernin 1 were due to the 50-kDa protein the anti-Secernin antibody, SK1147 was used to immunoneutralize the purified protein. Figure 7A shows that the antibody blocks the effect of the optimal dose of Secernin 1, whereas same concentration of the preimmune sera has no significant effect. This concentration of antibody is also inhibits the effect of freshly prepared rat brain cytosol by 50%, indicating that Secernin is responsible for a significant amount of the activity found in whole cytosol. To confirm that this effect was due to the interaction of SK1147 with Secernin, the antibody was affinity purified against recombinant human Secernin 1. Figure 7B demonstrates that the affinity-purified antibody inhibits fresh rat brain cytosol and that this inhibition can be blocked by inactive recombinant human Secernin 1 in an inclusion body.
Figure 7.
Inhibition of Secernin in cytosol by anti-Secernin antibody. (A) Mast cells permeabilized with SLO in the presence of 10 μg/ml purified Secernin 1 or 1 mg/ml rat brain cytosol, which had been immunoneutralized with SK1147 antisera, preimmune serum, or sham neutralized with buffer. After 15 min the cells were stimulated by addition of solutions containing EGTA buffered to 10 μM Ca2+plus GTPγS (final concentration, 100 μM) or 100 nM Ca2+After a further 20 min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Stimulated secretion is the difference in the amount of hexosaminidase released in response to either 100 nM Ca2+ or 10 μM Ca2+ + 100 μM GTPγS. Data shown are mean ± SEM (n = 4); similar results were obtained on four occasions. (B) Mast cells permeabilized with SLO in the presence or absence of 1 mg/ml rat brain cytosol that had been immunoneutralized varying concentrations of affinity-purified SK1147 antisera, in the presence and absence of inclusion bodies containing recombinant human Sescernin 1. After 15 min the cells were stimulated by addition of solutions containing EGTA buffered to 10 μM Ca2+plus GTPγS (final concentration, 100 μM) or 100 nM Ca2+. After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Stimulated secretion is the difference in the amount of hexosaminidase released in response to either 100 nM Ca2+ or 10 μM Ca2+ + 100 μM GTPγS. Data shown are mean ± SEM (n = 4); similar results were obtained on four occasions.
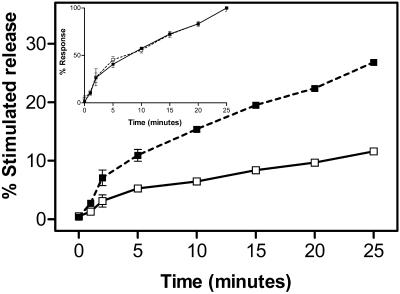
Two possible mechanisms for Secernin 1 to increase the extent of secretion would be to increase either the rate of secretion or to allow secretion to occur over a much longer period. Figure 8 clearly demonstrates that the addition of Secernin 1 to the cells during rundown, before stimulation, increases the rate of secretion from the permeabilized mast cells. If the data are normalized to the response at 25 min, as shown in the inset to Figure 8, it becomes clear that although the rate of secretion is increased by Secernin 1, the time course of the secretory response is unaffected by the presence of Secernin 1.
Figure 8.
Effect of Secernin on the time course of secretion. Mast cells permeabilized with SLO in the presence (▪) or absence (□) of 3 μg/ml purified Secernin 1. After 15 min the cells were stimulated by addition of solutions containing EGTA buffered to 10 μM Ca2+plus GTPγS (final concentration, 100 μM). After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Data shown are mean ± SEM (n = 3); similar results were obtained on four occasions. Some error bars are smaller than symbols used. The inset figure shows the same data normalized to 100% response for each of the conditions after 25 min secretion.
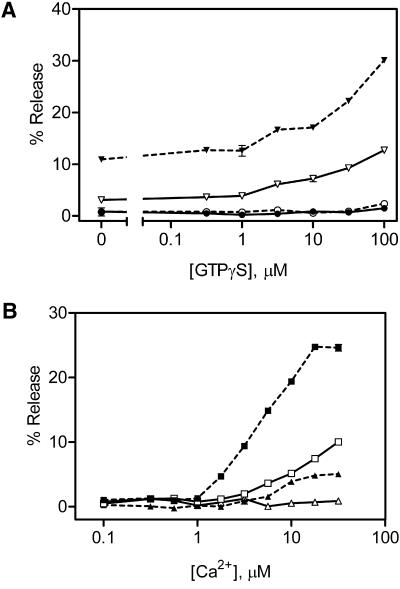
We have previously shown that the sensitivity of the mast cells to both Ca2+ and GTPγS declines as a consequence of the rundown of the secretory response (Brown et al., 1998). Therefore, we investigated the possibility that Secernin 1 not only increased the rate and extent of secretion, but also had an effect on the sensitivity of the mast cells to stimulation with both Ca2+ and GTPγS. Figure 9A shows the effect of Secernin on the GTPγS sensitivity of cells in the presence of 0.1 and 10 μM Ca2+. Under these rundown conditions, all concentrations of GTPγS in the presence of 0.1 μM Ca2+ are incapable of inducing secretion, whether Secernin 1 is present or not. In the presence of 10 μM Ca2+ Secernin 1 increases the extent of secretion by the same amount, independent of the concentration of GTPγS. Figure 9B clearly demonstrates that Secernin 1 partially restores the Ca2+ sensitivity of the rundown mast cells, with some Ca2+-dependent secretion even occurring in the absence of GTPγS.
Figure 9.
(A and B) Effect of Secernin on the sensitivity of mast cell secretion to Ca2+ and GTPγS. Mast cells permeabilized with SLO in the presence (filled symbols) or absence (open symbols) of 3 μg/ml Secernin 1. After 15 min the cells were stimulated by addition of solutions containing the following: (A) EGTA buffered to either 0.1 μM (●, ○) or 10 μM Ca2+(▾, ▿) plus varying concentrations of GTPγS or (B) EGTA buffered to varying concentrations of Ca2+ plus a final concentration of either 0 (▴, ▵) or 100 μM GTPγS (▪, □). After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Data shown are mean ± SEM (n = 4); similar results were obtained on four occasions. Some error bars are smaller than symbols used.
DISCUSSION
We have purified a novel cytosolic protein, termed Secernin 1, which regulates exocytosis in permeabilized rat peritoneal mast cells. Database analysis reveals that fragments of Secernin 1 identified by mass spectrometry show 93.5% identity and 95.7% homology with the predicted product of mouse cDNA AK012765, which has a predicted MW of 46296, which is consistent with our estimated molecular weight of 50 kDa from SDS-PAGE. Database searching indicates that Secernin 1 is encoded by one of a small family of related genes (Figure 10). The human genome contains three Secernin genes (termed Secernins 1 through 3), which are localized on chromosomes 7 (7p14.3-p14.1), 17 (17q21.3), and 2 (2p14-q14.3), respectively. Analysis of cDNAs corresponding to human Secernin 2 indicates the existence of at least two splice variants (2a and 2b), one of which generates a protein containing a truncated C terminus. Comparison of human and mouse Secernin 1 cDNAs suggests that the former has a truncated N-terminus lacking ∼60 amino acids. However, it is also possible that this is a splice variant of a larger protein, because inspection of the 5′ noncoding region of the existing cDNA as well as the genomic DNA sequence indicates the presence of a putative exon, which is highly homologous to the relevant 5′ coding sequence of mouse Secernin 1. We have also identified two partial cDNA sequences in the TIGR bovine gene index database. These correspond to a bovine homologue of human Secernin 2a (Figure 10). The mouse genes are ubiquitously expressed (Kawai et al., 2001), which would imply some common role in many cell types rather than a protein specifically involved in mast cell exocytosis.
Figure 10.
Identification of Secernin family members in mammalian species. The protein sequence for mouse Secernin 1 was used to interrogate the NCBI Human Genome and nonredundant databases using the BLAST search program. Sequences were aligned using the PIMA sequence alignment program and formatted using BOXSHADE. Amino acid identities are shown as black boxes. Conservative changes are shown as shaded boxes. The GenBank accession numbers for Secernins are as follows: hsSES1, AAD15417; hsSes2a, XP_054038.1; hsSes2b, XP_032208.1; hsSes3, XP_035198.1; mmSes1, AK012765; mmSes3, AK014701; BtSes2a, TC 80787, Tc80786.
The full-length sequence for Secernin 1 shows no significant degree of homology with any protein known to be involved in exocytosis, membrane fusion events, or intracellular signaling. The protein contains no known domain structures apart from a small 21 amino acid region between 358 and 378, which is predicted to form a coiled-coil domain by the COILS program (Lupas et al., 1991). It is therefore possible that the actions of the protein may be mediated by a direct interaction with another protein.
Two rabbit polyclonal antibodies raised against purified bovine Secernin 1 recognize a single immunoreactive band at 50 kDa in mast cells, confirming the presence of Secernin 1 in mast cells. After permeabilization this protein leaks from the mast cells and can be precipitated from the supernatant, confirming that the protein is cytosolic. Secernin 1 leaks from the cell at a rate similar to the decrease in secretory response of the mast cells during rundown, implying that the leakage of Secernin 1 is a major cause of the loss of secretory response. Addition of Secernin 1 to permeabilized cells followed by stimulation within the first 5 min has no effect on secretion, demonstrating a lack of effect of the protein until sufficient Secernin 1 has leaked from the cells to impair secretion. The data are consistent with the hypothesis that exogenously added Secernin 1 is replacing the endogenous protein leaking from the permeabilized cells, thus enhancing the secretory response under these conditions. The addition of Secernin 1 alone is not capable of blocking the loss of secretory response but slows the rate of loss of response.
When cells are permeabilized and incubated at 37°C before stimulation, there is a decline in sensitivity to both Ca2+ and guanine nucleotide. Secernin 1 partially restores Ca2+ sensitivity, but not sensitivity to guanine nucleotide. We have shown that although Secernin 1 increases the extent of secretion from mast cells, the time course of secretion remains the same. If we assume that any granule that fuses with the plasma membrane releases either all or a constant proportion of its hexosaminidase, then the simplest explanation of this data is that Secernin 1 causes the recruitment of additional secretory granules to the site of exocytosis in a calcium-dependent manner. Alternatively Secernin 1, in the presence of calcium, may be acting to increase the granule swelling, core expulsion and breakdown observed in fused granules (Zimmerberg et al. 1987; Monck et al., 1991), thus increasing the release of hexosaminidase from the cells.
The loss of the secretory response is due to a number of protein factors, including Secernin 1, Rac/RhoGDI, and the other partially purified activities shown in Figure 1, and it is likely that the full reconstitution of the secretory response will require a large number of different proteins. This is confirmed by the finding that inhibition of Secernin in cytosol by immunoneutralization blocks ∼50% of the recovery of secretion, indicating that although Secernin is not the only cytosolic component that regulates mast cell secretion, it appears to be an important component of the response.
Cytosolic proteins that are capable of regulating exocytosis in permeabilized cell assays appear to fall into four major categories: 1) proteins that directly interact with the fusion machinery, including α-SNAP (Chamberlain et al., 1995); 2) GTPases involved in intracellular signaling, such as Arf (Fensome et al., 1996, Caumont et al., 1998), rac (O'Sullivan et al., 1996), rho (Price et al., 1995), and cdc42 (Brown et al., 1998); 3) proteins involved in other intracellular signaling pathways, such as PKC (Ozawa et al., 1993) and 14–3-3 (Morgan and Burgoyne, 1992); and 4) proteins involved in the regulation of PIP2, such as PI kinases (Hay et al., 1995) and PITP (Hay et al., 1995; Fensome et al., 1996; Pinxteren et al., 2001). Our data clearly demonstrate that the cytosolic protein Secernin 1 has a major role in the regulation of exocytosis in mast cells, but further work is required to determine which or if any of these pathways is the site of action of Secernin 1.
ACKNOWLEDGMENTS
We thank Douglas Lamont for help with MALDI-TOF mass spectrometry. This work was supported by a grant from the Wellcome Trust to A.J.O'S. and a grant to C.S. from the Association for International Cancer Research.
Abbreviations used:
- BSA
bovine serum albumin
- GTP-γ-S
guanosine5′-3-O-(thio) triphosphate
- SLO
streptolysin-O
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E01–10–0094. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E01–10–0094.
REFERENCES
- Aridor M, et al. Activation of exocytosis by the heterotrimeric G-protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Baram D, Mekori YA, Sagi-Eisenberg R. Synaptotagmin regulates mast cell functions. Immunol Rev. 2001;179:25–34. doi: 10.1034/j.1600-065x.2001.790103.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown AM, O'Sullivan AJ, Gomperts BD. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol Biol Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumont AS, et al. Regulated exocytosis in chromaffin cells—translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J Biol Chem. 1998;273:1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, et al. Distinct effects of alpha-snap, 14–3-3-proteins, and calmodulin on priming and triggering of regulated exocytosis. J Cell Biol. 1995;130:1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensome A, et al. ARF and PITP restore GTP gamma S-stimulated protein secretion from cytosol-depleted HL60 cells by promoting PIP2 synthesis. Curr Biol. 1996;6:730–738. doi: 10.1016/s0960-9822(09)00454-0. [DOI] [PubMed] [Google Scholar]
- Gomperts BD, Tatham PER. Regulated exocytotic secretion from permeabilized cells. Methods Enzymol. 1992;219:178–189. doi: 10.1016/0076-6879(92)19020-7. [DOI] [PubMed] [Google Scholar]
- Griffiths GM. Secretory lysosomes—a special mechanism of regulated secretion in hemopoietic cells. Trends Cell Biol. 1996;6:329–332. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- Guo ZH, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Hay JC, et al. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Kawai J, et al. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685–690. doi: 10.1038/35055500. [DOI] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larbi KY, Gomperts BD. Practical considerations regarding the use of streptolysin-O as a permeabilizing agent for cells in the investigation of exocytosis. Biosci Rep. 1996;16:11–21. doi: 10.1007/BF01200997. [DOI] [PubMed] [Google Scholar]
- Lillie THW, Gomperts BD. Guanine-nucleotide is essential and Ca2+is a modulator in the exocytotic reaction of permeabilized rat mast cells. Biochem J. 1992;288:181–187. doi: 10.1042/bj2880181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, et al. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Marston FAO, et al. Purification of calf prochymosin (prorennin) synthesized in Escherichia-coli. Bio-technology. 1984;2:800–804. [Google Scholar]
- Monck JR, Oberhauser AF, DeToledo GA, Fernandez JM. Is swelling of the secretory granule matrix the force that dilates the exocytotic fusion pore. Biophys J. 1991;59:39–47. doi: 10.1016/S0006-3495(91)82196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A, Burgoyne RD. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992;355:833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels—a modified procedure with enhanced uniform sensitivity. Anal Biochem, 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ, et al. Purification and identification of FOAD-II, a cytosolic protein that regulates secretion in streptolysin-O permeabilized mast cells, as a Rac/RhoGDI complex. Mol Biol Cell. 1996;7:397–408. doi: 10.1091/mbc.7.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, et al. Ca2+-dependent and Ca2+-independent isozymes of protein-kinase-c mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells—reconstitution of secretory responses with Ca2+and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- Paumet F, et al. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells. Functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- Pinxteren JA, et al. Regulation of exocytosis from rat peritoneal mast cells by G protein beta gamma-subunits. EMBO J. 1998;17:6210–6218. doi: 10.1093/emboj/17.21.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinxteren JA, et al. Phosphatidylinositol transfer proteins and protein kinase C make separate but non-interacting contributions to the phosphorylation state necessary for secretory competence in rat mast cells. Biochem J. 2001;356:287–296. doi: 10.1042/0264-6021:3560287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LS, et al. The small GTPases Rac and Rho as regulators of secretion in mast-cells. Curr Biol. 1995;5:68–73. doi: 10.1016/s0960-9822(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Smith DE, Fisher PA. Identification, developmental regulation, and response to heat-shock of 2 antigenically related forms of a major nuclear-envelope protein in Drosophila embryos—application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Griffiths GM. Normal, and abnormal secretion by hemopoietic cells. Immunology. 2001;103:10–16. doi: 10.1046/j.1365-2567.2001.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham PER, Gomperts BD. In: Peptide Hormones: A Practical Approach. Siddle K, Hutton JC, editors. Oxford, United Kingdom: IRL Press; 1990. pp. 257–269. [Google Scholar]
- Walent JH, Porter BW, Martin TFJ. A novel 145-kd brain cytosolic protein reconstitutes Ca2+-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Wedemeyer J, Galli SJ. Mast cells, and basophils in acquired immunity. Br Med Bull. 2000;56:936–955. doi: 10.1258/0007142001903616. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Curran M, Cohen FS, Brodwick M. Simultaneous electrical and optical measurements show that membrane-fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci USA. 1987;84:1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]