Abstract
Introduction
Patients with advanced solid tumors may be considered for early phase clinical trials investigating the safety, tolerability, and dosing of experimental therapies. Optimizing participant selection is critical to maximize clinical benefit and meet trial endpoints with fewer participants. One in six participants does not meet routine life expectancy requirements (>3 months), highlighting the need for improved prognostication. Variant allele frequency (VAF) in circulating tumor DNA (ctDNA) correlates with overall survival (OS) in advanced solid tumors. We aimed to derive an optimal VAF threshold as a prognostic biomarker to enhance participant selection.
Methods
ctDNA testing was performed as part of the TARGET (NIHR Clinical Research Network CPMS ID 39172) and TARGET National (NCT04723316) prospective cohort studies, in patients with advanced solid tumors referred for early phase clinical trials. Maximum (maxVAF) and mean VAF (meanVAF) were compared in their association with OS and ability to delineate favorable and poor outcomes at set threshold points using hazard ratios (HRs). Optimal thresholds of VAF were explored using receiver operating characteristic curve analysis to predict 3-month landmark OS. Univariable and multivariable analysis was performed to determine whether VAF was an independent prognostic marker.
Results
Of 631 patients, 587 had evaluable ctDNA results. MeanVAF and maxVAF exhibited similar correlation with OS (rs = −0.32 vs −0.35, respectively) and similar prognostic utility at matched threshold points. A maxVAF value of 4% was selected as optimal for prognostic subgrouping (area under curve 0.77). OS was 5.9 versus 12.1 months (p < 0.0001) for patients with more than 4% and 4% or less maxVAF, respectively. Multivariable analysis confirmed more than 4% maxVAF as independently associated with reduced 3-month landmark OS (HR 2.17 [1.76–2.70], p < 0.001).
Conclusion
VAF is an independent prognostic marker in patients with advanced solid tumors, with 4% maxVAF deemed optimal for delineating favorable and poorer prognostic subgroups in this patient cohort. Further validation and integration into existing prognostic scores are warranted.
Keywords: circulating tumor DNA, variant allele frequency, prognostic biomarker, advanced solid tumors, early phase clinical trials
INTRODUCTION
Developments in circulating tumor DNA (ctDNA) assays afford testing advantages over tissue-based genomic analysis in turnaround time, representation of tumor heterogeneity, and reduced risk associated with tissue sampling.[1] ctDNA testing is increasingly used in routine care, particularly in lung cancer, where treatments can be prescribed according to actionable genomic variants identified by validated ctDNA assays. [2–4]
The coverage with comprehensive genomic profiling (CGP) assays has led to the formal exploration of ctDNA testing as a route to accessing targeted therapies, where undertaking sequential single-gene tests on biopsied tissue becomes inefficient.[5–8] In parallel, ctDNA has applications beyond treatment selection, including monitoring of minimal residual disease, identifying treatment resistance mechanisms, and prognostication in metastatic disease.[9]
An increasing body of evidence suggests that higher pretreatment variant allele frequency (VAF) of ctDNA variants is negatively prognostic across multiple disease types.[6,10–13] VAF as a prognostic marker has been examined in early phase clinical trial cohorts with heterogeneous advanced solid tumors, which have reported poorer overall survival (OS) with VAF in ctDNA above various thresholds.[14,15] However, there is inconsistency in how VAF levels are analyzed and reported, and whether prespecified thresholds or nonfixed points, such as the median or mean VAF, should be used for prognostication.
Improving prognostic accuracy would be of value in all advanced tumor indications, but specifically in early phase clinical trial recruitment, where most interventional studies require participants to have more than 3 months of life expectancy. Correct assessment of this remains elusive in a significant proportion of patients, despite the adoption of objective prognostic scoring criteria, and VAF-based scoring criteria may enhance this process.[16–18]
We aimed to investigate the prognostic value of VAF in ctDNA. We examined both the mean and maximum VAF and sought to derive the optimal VAF threshold for prognostic utility to aid the estimation of trial participants’ life expectancy. The study was conducted using baseline ctDNA findings from the TARGET and TARGET National study cohorts, comprising advanced solid tumor patients being considered for early phase clinical trials.[1,19]
METHODS
Patients
Patients were prospectively recruited to the TARGET study (NIHR Clinical Research Network CPMS ID 39172; single-site sample collection) and TARGET National study (NCT04723316; multi-site collection) between August 2016 and July 2022 at nine early phase oncology trial centers in the United Kingdom. Each study was approved by the North-West (Preston) National Research Ethics Service (reference 15/NW/0078) and North West – Greater Manchester West Research Ethics Committee (reference 21/NW/0010), respectively. Both studies adhered to principles derived from the Declaration of Helsinki and Good Clinical Practice. All patients provided fully informed written consent for blood sampling, data analysis, and sharing. The Reporting Recommendations for Tumor Marker Prognostic Studies guidelines have presented data and results from the report.[20] The manuscript has been drafted to adhere to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies.[21]
These studies included patients with any advanced solid tumor being considered for an early phase clinical trial (interventional studies in phase 1 or phase 2 settings).[1,19] Testing was conducted at baseline before commencing further systemic therapy. Blood samples were acquired for CGP by ctDNA using FoundationOne Liquid or, subsequently, the updated FoundationOne liquid CDx panel. The main inclusion criteria were 16 years of age or older, histologically confirmed advanced solid malignancy, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0 to 2, and being referred to an experimental cancer medicine center (a specialist early phase clinical research center) for consideration of early phase clinical trials. Exclusion criteria prohibited the concurrent use of cytotoxic systemic therapy (a 3-week washout period was required), and patients who were considered clinically unsuitable for early phase clinical trials. Participants were followed up every 3 months to obtain survival status.
Data Collection
Patient demographics were collected, including age, sex, and ECOG PS. Disease characteristics collected included primary site of disease, lines of prior systemic therapy, and blood parameters, such as hemoglobin (g/L), lactate dehydrogenase (LDH [U/L]), and albumin (g/L). Overall survival (OS) was determined from the date of ctDNA testing until the date of death or censored at the last date known to be alive. Patients were deemed evaluable for analysis if they had had a technically successful ctDNA analysis. Patients with insufficient DNA for testing or those whose tests experienced analytical failure during processing were deemed nonevaluable.
Genomics Testing and VAF Reporting
ctDNA profiling was performed by taking a single blood draw. CGP performed in the TARGET study used the FoundationOne Liquid assay (2019–2021), which captured selected coding regions across 70 cancer-related genes, as previously described in the literature.[19] CGP performed in the TARGET National study used the FoundationOne Liquid CDx assay (2021–2022; www.rochefoundationmedicine.com/) as previously described in the literature.[1] This assay is FDA approved and captures variants in the complete coding region of 309 cancer-related genes and a further 15 genes with select intronic or noncoding regions only. Foundation Medicine, Inc. generated all genomic data that supplied precise VAF levels for this analysis, based on the proportion of pathogenic or likely pathogenic variant (hereon termed pathogenic variant) reads compared with total cell-free DNA read depth for each locus. Evaluable patients were those with an analytically successful test, including those with no detectable DNA; tests that failed to generate results due to technical failure during sequencing were excluded.
Analyses were performed on all point mutations and indels captured from ctDNA testing due to the robust assay capability of quantifying VAF. Rearrangements, copy number variants, and microsatellite instability status were not included. Variants of uncertain significance were excluded, being deemed nonbiologically significant at the time of analysis.
To decrease the influence of germline variants on VAF, ctDNA reports from the TARGET National cohort were corroborated with prior germline testing to remove known germline variants. Patients from the TARGET study cohort, who underwent parallel germline testing, confirmed germline variants excluded from the analysis. In patients without known germline variants, variants with less than 40% VAF were treated as somatic variants and included in the analysis. Remaining ctDNA variants with VAF 40% or greater were reviewed by a multidisciplinary team of medical oncologists and clinical scientists to remove known or likely germline variants in the context of the primary cancer type and overall ctDNA fraction. In uncertain cases, variants with 40% or greater VAF and more than twice the frequency of the next most frequent pathogenic variant were considered likely germline and excluded.
Statistical Analysis
To determine whether using the calculated mean VAF of all pathogenic variants detected (meanVAF) in each ctDNA sample or the numerically highest somatic gene VAF from all pathogenic variants detected (maxVAF) in each ctDNA sample provided a more robust prognostic readout, Spearman’s rank correlation coefficient was calculated for each approach and compared. For both meanVAF and maxVAF approaches, all patients were dichotomized into groups above and below prespecified VAF thresholds of all integers from 0% (no pathogenic variants detectable) to 20% VAF. Hazard ratios (HR) were calculated for each threshold and compared to determine if the mean or maximum VAF (according to each specific threshold) held greater power to discriminate between prognostic groups. A Bonferroni correction was applied to all HRs to account for multiple hypothesis testing.
To select the optimal VAF threshold, time-dependent receiver operating characteristic (ROC) curve analysis was performed for each VAF threshold using survival time as a continuous variable and 3 months as a landmark threshold.[22] The ROC curve analysis was covariate adjusted to minimize skew on the area under the curve (AUC) values using a multivariable model on patient demographics and disease characteristics.[23] Complete case analysis (CCA) was used for the ROC analysis, where participants with missing data points were excluded from the analysis. The 3-month survival landmark was selected as this was the most clinically relevant timeframe for recruitment to early phase clinical trials.
Survival Analysis
To more comprehensively explore the relationship between VAF levels and OS across the cohort, an exploratory post-hoc analysis using Kaplan-Meier curves was conducted by partitioning patients into seven groups delineated by VAF levels. MaxVAF intervals of 4% were assessed (0%, >0– 4%, >4–8%, >8–12%, >12–16%, >16–20%, and >20%), with associated 3-month survival probabilities. Data points were censored when mortality status was unknown at the last follow-up. HRs in each group were presented with 95% CI, and compared with the reference group that had nil ctDNA detected after successful ctDNA testing (considered 0% VAF for the purpose of analysis). Log-rank testing was applied between survival curves to generate p-values for determining statistical significance. Statistical significance was reached if p ≤ 0.05. A further Kaplan-Meier curve was generated using the appropriately selected VAF threshold, using the same approach.
Univariable Cox proportional hazards regression was used to quantify the relationship between the following variables and OS: an appropriately selected VAF threshold based on the above analyses, biochemical parameters (including albumin, hemoglobin, LDH), the number of prior lines of therapy, performance status, and TP53 variant status (altered: yes/no). Statistically significant variables then underwent a multivariable Cox proportional hazards regression analysis to determine whether associations seen in univariable analysis were independent of one another. CCA was also used for univariable and multivariable analyses. Any parameter missing in a substantial number of evaluable patients would be excluded from the analyses.
To derive 80% power to detect a difference between prognostically favorable and poorer survival groups, with a magnitude of 1.5 difference in survival probability, at an alpha level of 0.05, we estimated the need to recruit 569 patients. The number of participants selected for analysis was prespecified to surpass 569, and a 10% (n = 57) buffer was added to compensate for assay failure while maintaining adequate sample size in the evaluable cohort. All statistical analyses were conducted on RStudio v4.4.2.
RESULTS
Patient Characteristics
The first 450 patients enrolled in the TARGET National study, and all patients enrolled in the TARGET study with FoundationOne Liquid testing (n = 181) were considered for analysis. Of 631 patients, 44 were nonevaluable due to insufficient DNA for testing or analytical failure (Fig. 1). The evaluable cohort consisted of 587 patients (FoundationOne Liquid n = 169 [28.8%], FoundationOne Liquid CDx n = 418 [71.2%]). Median age was 60 years; performance status was predominantly 0 to 1, and disease types were heterogeneous, with the most prevalent tumor types constituting 403 (68.7%) of evaluable patients, including non–small cell lung, colorectal, prostate, breast, upper gastrointestinal, hepatobiliary, and pancreatic cancers (Table 1). LDH levels were excluded from the subsequent analyses as 283 (48.2%) values were not recorded. Less prevalent tumor types (<5% frequency) were found in 184 of 587 (31.3%) patients (Supplementary Table S1, available online).
Figure 1.

Consort diagram.
Table 1.
Patient demographics in the evaluable cohort (N = 587 participants) and the 10 most frequently mutated genes
| Characteristics (N = 587) | n (%) |
|---|---|
| Median Age (range), yr | 60 (16–87) |
| Sex | |
| Female | 281 (47.9) |
| Male | 306 (52.1) |
| Prior lines of therapy: Median (range) | 3 (0–13) |
| 0 | 23 (3.9) |
| 1 | 79 (13.5) |
| 2 | 122 (20.8) |
| ≥3 | 362 (61.7) |
| Most Common Tumor Types (>5% Prevalence) | |
| Non–small cell lung cancer (NSCLC) | 98 (16.7) |
| Colorectal (CRC) | 98 (16.7) |
| Prostate | 61 (10.4) |
| Breast | 49 (8.3) |
| Upper GI | 33 (5.6) |
| Hepatobiliary | 32 (5.4) |
| Pancreatic | 32 (5.4) |
| Less frequent disease types (<5% prevalence)* | 184 (31.3) |
| ECOG Performance Status | |
| 0 | 250 (42.6) |
| 1 | 305 (52.0) |
| 2 | 19 (3.2) |
| Hemoglobin <LLN (%) | 298 (50.7) |
| Albumin <LLN (%) | 66 (11.2) |
| Most Frequent Gene Mutations | n variants** (%) | n participants (%) |
|---|---|---|
| TP53 | 452 (21.6) | 335 (57.1) |
| DNMT3A | 147 (7.0) | 113 (19.3) |
| KRAS | 136 (6.5) | 128 (21.8) |
| APC | 136 (6.5) | 98 (16.7) |
| PIK3CA | 87 (4.1) | 79 (13.5) |
| TET2 | 65 (3.1) | 53 (9.0) |
| ATM | 63 (3.0) | 45 (7.7) |
| RB1 | 35 (1.7) | 33 (5.6) |
| TERT | 34 (1.6) | 34 (5.8) |
| PTEN | 33 (1.6) | 33 (5.6) |
Less frequent disease types (<5% frequency) are displayed in Supplementary Table 1.
Listed genes harbored 1155 pathogenic variants (56.7% of all pathogenic variants [n = 2097]), and the frequency of each gene harboring a pathogenic variant amongst the cohort is also displayed.
GI: gastrointestinal; LLN: lower limit of normal.
At the time of analysis, 432 (73.6%) patients had died, and median OS for the evaluable cohort was 7.2 months (Supplementary Table S2). The median follow-up time was 16.2 months (range, 0.4–103.9 months). Mortality rates at 3-month, 6-month, and 1-year landmarks were 18.0%, 38.2%, and 62.2%, respectively (Supplementary Table S2).
Optimal Threshold of VAF for Prognostication
Of evaluable patients, 529 of 587 (90.1%) had detectable pathogenic variants, constituting 2097 unique short variants, and 58 (9.9%) patients had nil detectable ctDNA. Pathogenic variants were distributed among 174 unique genes, with the 10 most frequently mutated genes harboring 1155 (56.7%) of all variants (Table 1).
To evaluate the prognostic characteristics of the prespecified VAF thresholds (all integer values, 0–20%), HRs and 95% CIs were calculated for all evaluable patients using meanVAF and maxVAF concerning OS (Table 2). All thresholds showed statistical significance after Bonferroni correction, and HRs were similar when comparing matched thresholds of meanVAF and maxVAF. The correlations of VAF and OS were similar when comparing both approaches, indicated by Spearman’s rank correlation coefficients of −0.35 and −0.32, maxVAF and meanVAF, respectively. Owing to similarities across HRs in matched thresholds and similar correlation coefficients, the prognostic utility of maxVAF was deemed equivalent to meanVAF, and maxVAF was the metric used for subsequent analyses.
Table 2.
Hazard ratios with 95% CI calculated for threshold values for maximum and mean VAF with respect to overall survival
| VAF Threshold (%) | MaxVAF > Threshold |
MeanVAF > Threshold |
||||
|---|---|---|---|---|---|---|
| n (%) | HR (95% CI) | p–value | n (%) | HR (95% CI) | p–value | |
| 0 | 529 (90.1) | 1.96 (1.37–2.79) | < 0.001 | 529 (90.1) | 1.96 (1.37–2.79) | < 0.001 |
| 1 | 428 (72.9) | 2.11 (1.67–2.68) | < 0.001 | 388 (66.1) | 2.2 (1.77–2.73) | < 0.001 |
| 2 | 368 (62.7) | 2.15 (1.74–2.65) | < 0.001 | 309 (52.6) | 2.24 (1.84–2.73) | < 0.001 |
| 3 | 329 (56.0) | 2.21 (1.81–2.7) | < 0.001 | 272 (46.3) | 2.15 (1.77–2.6) | < 0.001 |
| 4 | 305 (52.0) | 2.36 (1.94–2.87) | < 0.001 | 245 (41.7) | 2.09 (1.73–2.53) | < 0.001 |
| 5 | 287 (48.9) | 2.29 (1.88–2.78) | < 0.001 | 231 (39.4) | 2.07 (1.71–2.5) | < 0.001 |
| 6 | 267 (45.5) | 2.25 (1.85–2.72) | < 0.001 | 217 (37.0) | 2.1 (1.73–2.54) | < 0.001 |
| 7 | 255 (43.4) | 2.3 (1.9–2.8) | < 0.001 | 199 (33.9) | 2.22 (1.83–2.69) | < 0.001 |
| 8 | 242 (41.2) | 2.23 (1.84–2.7) | < 0.001 | 181 (30.8) | 2.39 (1.96–2.92) | < 0.001 |
| 9 | 228 (38.8) | 2.34 (1.93–2.84) | < 0.001 | 166 (28.3) | 2.41 (1.97–2.94) | < 0.001 |
| 10 | 223 (38.0) | 2.32 (1.91–2.82) | < 0.001 | 153 (26.1) | 2.5 (2.04–3.07) | < 0.001 |
| 11 | 216 (36.8) | 2.3 (1.9–2.8) | < 0.001 | 149 (25.4) | 2.58 (2.1–3.17) | < 0.001 |
| 12 | 210 (35.8) | 2.3 (1.89–2.79) | < 0.001 | 138 (23.5) | 2.51 (2.04–3.09) | < 0.001 |
| 13 | 200 (34.1) | 2.35 (1.93–2.85) | < 0.001 | 134 (22.8) | 2.52 (2.04–3.11) | < 0.001 |
| 14 | 190 (32.4) | 2.46 (2.02–3) | < 0.001 | 125 (21.3) | 2.6 (2.1–3.22) | < 0.001 |
| 15 | 185 (31.5) | 2.51 (2.06–3.06) | < 0.001 | 121 (20.6) | 2.52 (2.03–3.13) | < 0.001 |
| 16 | 180 (30.7) | 2.62 (2.15–3.19) | < 0.001 | 114 (19.4) | 2.55 (2.05–3.18) | < 0.001 |
| 17 | 176 (30.0) | 2.68 (2.19–3.27) | < 0.001 | 108 (18.4) | 2.57 (2.05–3.21) | < 0.001 |
| 18 | 171 (29.1) | 2.64 (2.16–3.23) | < 0.001 | 101 (17.2) | 2.74 (2.17–3.45) | < 0.001 |
| 19 | 168 (28.6) | 2.67 (2.18–3.26) | < 0.001 | 95 (16.2) | 2.74 (2.16–3.48) | < 0.001 |
| 20 | 157 (26.7) | 2.73 (2.22–3.34) | < 0.001 | 86 (14.7) | 2.73 (2.13–3.49) | < 0.001 |
HR: hazard ratio; maxVAF: maximum variant allele frequency; minVAF: minimum variant allele frequency; VAF: variant allele frequency.
To further explore maxVAF threshold prognostic utility, time-dependent ROC curve analysis classifying 3-month mortality status was conducted using all maxVAF thresholds (0–20%) (Supplementary Figure S1). All patients with complete data (n = 573; 97.6%) were included to allow for covariate adjustment. The 20% maxVAF threshold displayed the greatest AUC, closely followed by 4% (0.78 and 0.77, respectively) (Supplementary Table S3). MaxVAF thresholds of 4% and 20% also held superior positive and negative likelihood ratios for classifying the 3-month mortality compared with other thresholds (Supplementary Table S3). The optimal threshold selected for subsequent analyses was 4% maxVAF due to its test performance and because its positioning in the cohort distribution of maxVAF fell near the median, limiting the skew from underlying subgroups.
MaxVAF thresholds were explored in subgroups representing the five most common disease types in the evaluable cohort, with reported HRs and 95%CI (Supplementary Tables S4a–e).
Survival Analyses
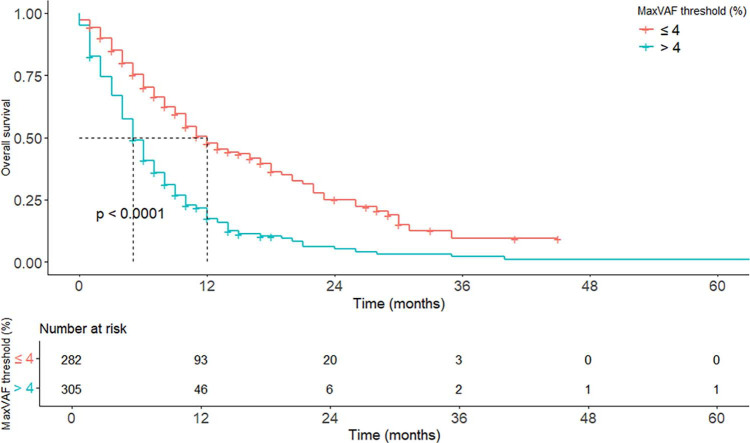
Survival analyses were undertaken for patients according to maxVAF level. Having more than 4% maxVAF was significantly associated with poorer survival compared with 4% or less maxVAF (HR 2.36, 95%CI, 1.94–2.87, p < 0.0001), with median survival times of 5.9 and 12.1 months, respectively (Fig. 2, Supplementary Table S5).
Figure 2.
Kaplan Meier curves plotting survival probability above and less than or equal to 4% of the maximum variant allele frequency threshold.
When compared with patients with 0% VAF, survival probability decreased with higher baseline levels of maxVAF (p < 0.0001) analyzed according to ranges of 4% (>0–4%, >4–8%, >8–12%, >12–16%, >16–20%, >20%) (Fig. 3, Supplementary Table S6). Presence of more than 0% but less than 4% maxVAF versus 0% maxVAF was associated with poorer survival (11.6 vs 16.0 months, respectively; HR, 1.27 0.871–1.86; p = 0.214), though this did not reach statistical significance (Fig. 3, Supplementary Table S6). In the four remaining groups with more than 4% maxVAF, maxVAF was associated with poorer survival compared with the 0% VAF group. HRs for maxVAF thresholds between 8% and 20% held broader 95% CIs (likely due to the small sample size) and are therefore less reliably interpreted; however, there is clear trend to worse survival with higher amounts of ctDNA with HR increased significantly in the more than 20% VAF group (HR 4.02, 95% CI, 2.75–5.88, p < 0.001) when compared with all 20% or less maxVAF groups. MaxVAF strata subgroups of 1% increments were also analyzed up to 5% maxVAF due to the large patient numbers in this cohort (Supplementary Fig. S2, Supplementary Table S7). Within this, only the more than 4–5% maxVAF was associated with poorer survival (HR 2.49, 95% CI, 1.32–4.68, p = 0.005).
Figure 3.
Kaplan Meier curves plotting survival probability at 4% intervals of the maximum variant allele frequency.
Univariable and Multivariable Analyses
To determine whether the 4% cutoff was significant independently of other clinical prognostic parameters, univariable and multivariable Cox proportional hazards regression analyses were conducted among evaluable patients with complete data (n = 573; 97.6%) (Table 3). In univariable analysis, male sex, poorer performance status, albumin and hemoglobin being below the lower limit of normal, TP53 variant positivity, and maxVAF greater than 4% were statistically significant (Table 3). Age as a continuous variable was not significant.
Table 3.
Univariable and multivariable cox proportional hazards regression analysis
| Parameter | Univariable |
Multivariable |
||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Age | 1 (0.99–1) | 0.4 | 1 (0.99–1) | 0.21 |
| Sex: male | 1.21 (1–1.47) | 0.049 | 1.21 (0.99–1.5) | 0.066 |
| Performance status: 1 vs 0* | 1.66 (1.36–2.03) | <0.001 | 1.52 (1.24–1.9) | <0.001 |
| Performance status: 2 vs 0* | 4.47 (2.69–7.42) | <0.001 | 3.5 (2.09–5.8) | <0.001 |
| Prior lines of treatment ≥ 3 vs < 3 | 1.2 (0.98–1.47) | 0.072 | 0.98 (0.94–1) | 0.396 |
| Albumin: < LLN vs ≥ LLN | 1.51 (1.19–1.92) | <0.001 | 1.20 (0.94–1.55) | 0.15 |
| Hemoglobin: < LLN vs ≥ LLN | 1.3 (1.07–1.58) | 0.007 | 1.10 (0.90–1.35) | 0.4 |
| MaxVAF: >4% threshold vs ≤ 4%* | 2.35 (1.92–2.87) | <0.001 | 2.17 (1.76–2.70) | <0.001 |
| TP53 variant: yes vs no* | 1.63 (1.34–1.99) | <0.001 | 1.32 (1.07–1.60) | 0.009 |
All analysis reaching the statistical significance threshold (p < 0.05) are bold.
Covariates that remained statistically significant after multivariable analysis.
LLN: lower limit of normal; maxVAF: maximum variant allele frequency.
In multivariable analysis, maxVAF greater than 4% (HR 2.17, 95% CI, 1.76–2.70, p < 0.001), poorer performance status, and TP53 variant positivity were independent factors associated with poorer OS at 3 months (Table 3). Blood-based parameters (hemoglobin and albumin) below the lower limit of normal range were associated with poorer survival but did not exhibit statistical significance. We repeated the univariable and multivariable analyses with maxVAF categorized as a continuous variable, which demonstrated poorer OS at 3 months with increasing maxVAF (HR 1.34, 95% CI, 1.25–1.40, p < 0.001), in addition to poorer performance status (Supplementary Table S8).
DISCUSSION
Stratifying survival outcomes holds strong relevance to the early phase trial population, who have high levels of 3-month mortality (∼18%).[16,24] Enhancing patient selection may reduce the number of participants required to meet trial endpoints, optimize recruitment to dose escalation cohorts for expedient drug development, and increase the likelihood of clinical benefit. Therefore, exploring novel methods of ctDNA interpretation may improve patient selection. Our findings suggest that an increase in the maxVAF is indicative of a poorer prognosis for patients with advanced solid tumors, in keeping with previous reports. Schwaederle et al[13] explored the association with maxVAF on OS in 168 advanced solid tumor patients, finding that more than 5% maxVAF was associated with poorer OS. Pairawan et al[25] added weight to this in a slightly larger cohort (n = 209), where more than 8.6% maxVAF (the median maxVAF) was associated with poorer OS. Hsiehchen et al[26] demonstrated poorer OS in increasing maxVAF quartiles, in 561 patients with mostly advanced solid tumors; however, cutoff values for VAF thresholds were not reported, and most patients had 1 or fewer lines of therapy, indicating a more treatment-naïve group. Zhang et al[15] found a significant correlation between maximum and median VAF and OS in 776 early phase trial patients. However, specific VAF thresholds were not reported.[15] The outcome of poorer OS with higher maxVAF has also been demonstrated in multiple metastatic tumor types.[6,10,12,27] While this evidence is supportive of VAF as a prognostic marker, varied or unreported thresholds limit the application of these findings in routine practice.
Our approach, using threshold-based HR analysis, was selected for simplicity, reproducibility, and to provide an actionable cutoff to stratify survival outcomes. Furthermore, we report that the mean and maximum VAFs are equivalent in their prognostic performance, which is a novel finding. A threshold of 4% maxVAF was selected for further survival analysis based on several statistical considerations. The distribution of maxVAF levels in our cohort followed an asymmetric distribution, and the spread of maxVAF levels was much broader when greater than 20% maxVAF (Supplementary Fig. S3). Therefore, selecting a 4% threshold, which dissected the cohort approximately in half, accounted for any potential skew in the data at higher maxVAF levels. This threshold was also favorable in ROC Analysis (AUC 0.77) and positive or negative predictive value parameters. Furthermore, 4% intervals of maxVAF (0%, >0–4%, and so on) were selected for survival analysis to balance sample size per group, while exploring groups more selectively in narrower ranges of maxVAF. Given this was a post-hoc analysis, these results should be interpreted as exploratory and require further validation. Notably, we chose to use CCA, which excluded participants with missing data from ROC, univariable, and multivariable analyses. No imputation or sensitivity analyses were performed for missing covariate data, though the proportion of excluded cases was small (<3%), hence a low likelihood of bias from this approach.
Our study has also demonstrated that progressive increases in maxVAF among patients with advanced solid tumors correlate with progressively worsening OS, particularly at the greater than 20% maxVAF threshold, exhibiting in a dose-dependent relationship. For maxVAF strata between 8% and 20%, given the relatively low sample size in this group, the reliability of the derived prognostic utility metrics is limited. However, the dose-dependency assumption of increasing maxVAF and decreasing OS is a plausible interpretation given the substantially poorer OS observed in the greater than 20% maxVAF subgroup and an underlying biological association between maxVAF and disease burden.[27–29] The multivariable analysis further supports this results with maxVAF categorized as a continuous variable, as this analysis captured all levels of maxVAF and was associated with poorer 3-month OS. Multivariable analysis confirmed a 4% maxVAF threshold, performance status, and TP53 variant status as independent prognostic indicators. TP53 variant positivity was included as a covariate before multivariable analyses due to its known prognostic effect on multiple tumor types and high prevalence in the evaluable cohort.[30] Hemoglobin was included in prognostic analyses given prior evidence demonstrating its prognostic utility in the advanced cancer setting, and multifactorial anemia being correlated to the advancement of disease.[31–35]
We intended to use maxVAF in conjunction with the Royal Marsden Hospital (RMH) score to develop a prognostic model in this study.[17] The incorporation of albumin and LDH into the RMH score justified their inclusion in our dataset. However, the absence of half of all LDH values and the absence of the number of sites of disease made direct comparison, or incorporation as an additional variable, unfeasible. Given that the 4% maxVAF threshold demonstrated independent prognostic value in our analysis, further work should explore its potential to enhance or complement existing prognostic models (such as the RMH score). However, formal integration should follow external validation of this threshold. LDH and the number of metastatic sites were not routinely collected at all hospitals, and this may also reflect a limitation in the RMH score’s ability to be used in routine practice.
When considering these findings in the wider setting of ctDNA-derived biomarkers, various parameters have been previously assessed for prognostication. For instance, studies have reported on the relationship between the detection of more pathogenic variants in ctDNA and poorer OS.[26,36,37] This is limited by heterogeneous and increasingly large panel sizes, which may bias the number of alterations detected toward larger panel sizes and/or increased reporting of variants of uncertain significance, which are not clinically relevant. MaxVAF does not possess this limitation as it is unaltered by CGP panel size. MaxVAF is also readily identified on ctDNA reports, which gives greater usability in routine practice, and criteria have been proposed for its use as a surrogate marker for radiological response in clinical trials.[38]
Prior literature has also confirmed the prognostic value of tumor fraction (TF) in ctDNA when using a 10% binary threshold.[37,39–43] When TF is 10% or greater then, the TF is typically derived from aneuploidy rates across the genome, and at the time of our study, TF less than 10% could not be quantified by our assays.[42] Furthermore, such calculations are inherently more variable than simply quantifying the number of variant reads per locus of sequenced DNA, as is done in calculating VAF and limiting precision when interpreting TF. Recent consensus guidelines in non–small cell lung cancer highlight the risk of false-negative and false-positive results in the setting of low TF, which may require confirmatory tissue-based testing.[44] It is also not routinely reported in some assays, which limits its applicability for prognostication. However, emerging improvements in assays such as the FoundationOne liquid CDx allow for routine reporting of TF in lower ranges (1–10%).[45,46] This creates the opportunity to prospectively explore and validate optimal threshold values of TF and conduct future comparative studies of maxVAF and TF to optimize prognostication with ctDNA test results.
A limitation in our methodology is the need to manually exclude suspected germline variants, which may falsely elevate maxVAF and is a process that lacks standardization. No standardized approach exists to remove variants of clonal hematopoiesis of indeterminate potential (CHIP), and we did not filter these from our dataset. This may skew meanVAF more severely than maxVAF as CHIP variants reportedly exhibit lower VAF levels and are ubiquitous in patients with advanced solid tumors, potentially accounting for the frequent presence of TET2 and DNMT3A variants in our sample.[47,48] Future studies should use methods to reliably exclude CHIP variants before analyses to limit their skew in the data, as they are considered less biologically relevant. Our analysis excluded more complex genomic changes, such as rearrangements and amplifications, which are not reported in VAF percentages, but may be relevant to prognosis. We did not collect subsequent treatment data after ctDNA testing, which may confound survival outcomes. However, regarding early phase trial enrolment, assessing 3-month survival probability should be undertaken independently of access to subsequent therapy, making subsequent therapy outcomes less relevant to our study aims.
Moreover, our findings should be interpreted in the context of an early phase trial population, which consistently includes a spectrum of disease types with inherently different survival rates and VAF levels in their disease course. We acknowledge and report the heterogeneity of baseline maxVAF levels between disease types, which implies that the prognostic performance of maxVAF may vary between different cancer types. However, these exploratory subgroup analyses are highly limited by sample size, so we did not attempt further disease-specific analyses. We intended to identify a VAF cutoff, which could be universally applied to the phase I trial candidate population. Furthermore, patients in our cohort were heavily pretreated, making results not applicable to early stage disease settings. Despite these limitations, improving prognostication in this unique patient population may optimize patient access to experimental therapies and enhance the ability of early phase trials to reach clinical endpoints more efficiently through enhanced selection of patients. Therefore, a more precise, biomarker-driven approach to the selection of early phase trial candidates may add value to the clinical care of this unique heterogeneous patient population.
CONCLUSION
Our data illustrate the prognostic value of ctDNA in patients with advanced cancer in the early phase trial population, extending beyond previous findings. We demonstrated 4% maxVAF as a suitable cutoff in this dataset, which is readily adoptable in clinical practice in early phase clinical trials. This finding should be further validated in prospective cohorts and its utility confirmed in the context of existing prognostic models, such as the RMH score. Optimizing the use of ctDNA testing in the early phase population has the potential to inform prognosis meaningfully. It could be conducted at the point of early phase trial referral to enhance patient selection, which may help expedite drug development.
Supplementary Material
Acknowledgment
The authors thank the patients and their families for contributing samples for these analyses. They also acknowledge the Manchester Experimental Cancer Medicine Centre and the Experimental Cancer Medicine Network in the UK for supporting recruitment to the study. They thank the digital Experimental Cancer Medicine Team at Cancer Research UK Manchester Institute for their support with the eTARGET platform. They acknowledge the participating sites and staff from the TARGET National Consortium who have supported the recruitment of patients and the provision of samples for parts of the included analyses.
CONFLICTS OF INTEREST
The following authors have disclosed potential conflicts of interest. Alastair Greystoke: consultant and speaker fees from AstraZeneca, Amgen, Boehringer–Ingelheim, Bristol–Myers Squibb (BMS), Janssen/J&J, MSD, Novartis, Pfizer, Lilly, Takeda, and Roche; institutional research funding from AstraZeneca; clinical lead for Cancer North East England Hull, and Yorkshire Genomic Laboratory Hub; and a member of the National Test Directory Evaluation Working Group. Louise Carter: consultant role/attended advisory boards for Bicycle Therapeutics; and institutional funding from Sierra Oncology, Athenex, Takeda, CellCentric, CytomX Therapeutics, Eli Lilly, Boehringer Ingelheim, Bicycle Therapeutics, Lupin Pharmaceuticals, Repare Therapeutics, ADC Therapeutics, Merck Serono, Moma Therapeutics, Loxo Oncology, and Nurix Therapeutics. Fiona Thistlethwaite: institutional research funding from GSK, Pfizer, GenMab, CytomX, Incyte, Janssen, Adaptimmune, BMS, Immunocore, Achilles Ltd, Crescendo, Oxford Vacmedix, RS Oncology LLC, Roche, AstraZeneca, Kymab Ltd/Sanofi, Chugai, T–Knife, Novalgen, Iovance, Nucana, Biontech, Moderna, Zymeworks, Seagen, and Grey Wolf Therapeutics; consultant roles for Guidepoint; honoraria from AstraZeneca; advisory board participation for CytomX, Grey Wolf Therapeutics, Oncobayes, Waypoint, T–Knife Therapeutics, Immatics, and Scenic Biotech; travel and meeting support from ESMO; unpaid leadership roles as a funding committee member for MRCP DPFS and GAP; scientific advisory roles for Sarcoma UK and Target Ovarian Cancer;and serves as a scientific committee member for ESMO Congress, TAT Congress, and ESMO IO. Natalie Cook: consulting fees from Roche Pharmaceuticals, Servier, and REDX Pharmaceuticals; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche Pharmaceuticals; support for attending meetings and/or travel from Roche Pharmaceuticals; research funding to research team from AstraZeneca, Orion, F. Hoffmann–La Roche Ltd, Taiho, Novartis, Starpharma, Bayer, Eisai, UCB, RedX Pharmaceuticals, LOXO–oncology, Avacta, Boehringer Ingelheim, Merck, and Tarveda Therapeutics; and participation in data safety monitoring board or advisory board at Roche Pharmaceuticals and Cancer Research UK. Donna M. Graham: honoraria from the Cancer Drug Development Forum; consultant roles for Clinigen Group; institutional research funding from Taiho Pharmaceutical, Merck Sharp & Dohme, Debiopharm Group, Incyte, Carrick Therapeutics, Bayer, AstraZeneca, Bristol–Myers Squibb, Synthon, BerGenBio, Genmab, Takeda, Roche, CytomX Therapeutics, Eisai, Janssen, Athenex, Novartis, Octimet, Redx Pharma, Sierra Oncology, 3–V Biosciences, Actuate Therapeutics, Blueprint Medicines, Boehringer Ingelheim, Ignyta, Macrogenics, Millenium Pharmaceuticals, Orion, Adaptimmune, GlaxoSmithKline, and MedImmune; and travel and accommodation support from Novartis. Matthew G. Krebs: advisory boards or consultancy roles for Astellas, Bayer, Guardant Health, Janssen, Roche, and Seattle Genetics; acts as scientific advisory board member for Zai Therapeutics; travel support from BerGenBio, BMS, Janssen, Roche, and Zai Lab; institutional research funding from Novartis and Rochel; and speaker fees from BMS, Eisai, Janssen, and Roche.
References
- 1.Ortega–Franco A, Darlington E, Greystoke A, Krebs MG. TARGET National: a UK–wide liquid–based molecular profiling programme: on behalf of the TARGET National Consortium. Clin Oncol. 2023;35:33–37. [DOI] [PubMed] [Google Scholar]
- 2.Visser E, de Kock R, Genet S, et al. Up-front mutation detection in circulating tumor DNA by droplet digital PCR has added diagnostic value in lung cancer. Transl Oncol. 2023;27:101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Olsen S, Ku BM, et al. High concordance of actionable genomic alterations identified between circulating tumor DNA-based and tissue-based next-generation sequencing testing in advanced non–small cell lung cancer: the Korean Lung Liquid Versus Invasive Biopsy Program. Cancer. 2021;127:3019–3028. [DOI] [PubMed] [Google Scholar]
- 4.List of cleared or approved companion diagnostic devices (in vitro and imaging tools) . Food and Drug Administration. Accessed June 23, 2025. www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools [Google Scholar]
- 5.Sehayek O, Kian W, Onn A, et al. Liquid first is “solid” in naïve non–small cell lung cancer patients: faster turnaround time with high concordance to solid next-generation sequencing. Front Oncol. 2022;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortolini Silveira A, Bidard FC, Tanguy ML, et al. Multimodal liquid biopsy for early monitoring and outcome prediction of chemotherapy in metastatic breast cancer. NPJ Breast Cancer. 2021;7:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26:1859–1864. [DOI] [PubMed] [Google Scholar]
- 8.Hahn AW, Nussenzveig RH, Maughan BL, Agarwal N. Cell-free circulating tumor DNA (ctDNA) in metastatic renal cell carcinoma (mRCC): current knowledge and potential uses. Kidney Cancer. 2019;3:7–13. [Google Scholar]
- 9.Krebs MG, Malapelle U, André F, et al. Practical considerations for the use of circulating tumor dna in the treatment of patients with cancer: a narrative review. JAMA Oncol. 2022;8(12):1830–1839. [DOI] [PubMed] [Google Scholar]
- 10.Manca P, Corallo S, Lonardi S, et al. Variant allele frequency in baseline circulating tumour DNA to measure tumour burden and to stratify outcomes in patients with RAS wild-type metastatic colorectal cancer: a translational objective of the Valentino study. Br J Cancer. 2022;126:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocaña A, Díez-González L, García-Olmo DC, et al. Circulating DNA and survival in solid tumors. Cancer Epidemiol Biomarkers Prev. 2016;25(2):399–406. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Zhang Y, Cheng Y, Zhang D, Zhu S, Ma X. Prognostic value of circulating cell-free DNA in patients with pancreatic cancer: a systemic review and meta-analysis. Gene. 2018;679:328–334. [DOI] [PubMed] [Google Scholar]
- 13.Schwaederle M, Husain H, Fanta PT, et al. Use of liquid biopsies in clinical oncology: pilot experience in 168 patients. Clin Cancer Res. 2016;22:5497–5505. [DOI] [PubMed] [Google Scholar]
- 14.Boscolo Bielo L, Trapani D, Repetto M, et al. Variant allele frequency: a decision-making tool in precision oncology? Trends Cancer. 2023;9:1058–1068. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Luo J, Wu S, et al. Prognostic and predictive impact of circulating tumor dna in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov. 2020;10:1842–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loh J, Wu J, Chieng J, et al. Clinical outcome and prognostic factors for Asian patients in Phase I clinical trials. Br J Cancer. 2023;128:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkenau HT, Olmos D, Ang JE, et al. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangro P, Magdalena AL, Lizundia TZ, et al. 28O Comparison of prognostic scores in oncology patients participating in phase I clinical trials with immunotherapy. ESMO Open. 2024;9:102356. [Google Scholar]
- 19.Rothwell DG, Ayub M, Cook N, et al. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat Med. 2019;25:738–743. [DOI] [PubMed] [Google Scholar]
- 20.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 22.Heagerty PJ, Lumley T, Pepe MS. Time-dependent roc curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 23.Janes H, Pepe MS. Adjusting for covariate effects on classification accuracy using the covariate–adjusted receiver operating characteristic curve. Biometrika. 2009;96:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chau NG, Florescu A, Chan KK, et al. Early mortality and overall survival in oncology phase I trial participants: can we improve patient selection? BMC Cancer. 2011;11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pairawan S, Hess KR, Janku F, et al. Cell-free circulating tumor DNA variant allele frequency associates with survival in metastatic cancer. Clin Cancer Res. 2020;26:1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiehchen D, Espinoza M, Gerber DE, Beg MS. Clinical and biological determinants of circulating tumor DNA detection and prognostication using a next-generation sequencing panel assay. Cancer Biol Ther. 2021;22:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haseltine JM, Offin M, Flynn JR, et al. Tumor volume as a predictor of cell free DNA mutation detection in advanced non–small cell lung cancer. Transl Lung Cancer Res. 2022;11:1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvoniemi A, Laine J, Aro K, et al. Circulating tumor DNA in head and neck squamous cell carcinoma: association with metabolic tumor burden determined with FDG-PET/CT. Cancers (Basel). 2023;15:3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong J, Jiang H, Liu X, et al. Variant allele frequency in circulating tumor DNA correlated with tumor disease burden and predicted outcomes in patients with advanced breast cancer. Breast Cancer Res Treat. 2024;204:617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li VD, Li KH, Li JT. TP53 mutations as potential prognostic markers for specific cancers: analysis of data from the Cancer Genome Atlas and the International Agency for Research on Cancer TP53 Database. J Cancer Res Clin Oncol. 2019;145:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gou M, Zhang Y, Liu T, et al. The prognostic value of pre–treatment hemoglobin (Hb) in patients with advanced or metastatic gastric cancer treated with immunotherapy. Front Oncol. 2021;11:655716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao X, Bao C, Song Q, et al. Effect of hemoglobin on the prognosis of patients with advanced cancer in palliative care settings. Palliat Med Reports. 2024;5:177–184. [Google Scholar]
- 33.Zhang Z, Zhang F, Yuan F, et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non–small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920970049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li WH, Zhang JY, Liu WH, Chen XX. Role of the initial degree of anaemia and treatment model in the prognosis of gastric cancer patients treated by chemotherapy: a retrospective analysis. BMC Cancer. 2020;20:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilreath JA, Rodgers GM. How I treat cancer-associated anemia. Blood. 2020;136:801–813. [DOI] [PubMed] [Google Scholar]
- 36.Muendlein A, Geiger K, Gaenger S, et al. Significant impact of circulating tumour DNA mutations on survival in metastatic breast cancer patients. Sci Rep. 2021;11:6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu P, Khagi Y, Riviere P, Goodman A, Kurzrock R. Total number of alterations in liquid biopsies is an independent predictor of survival in patients with advanced cancers. JCO Precis Oncol. 2020;4:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gouda MA, Janku F, Wahida A, et al. Liquid biopsy response evaluation criteria in solid tumors (LB-RECIST). Ann Oncol. 2024;35:267–275. [DOI] [PubMed] [Google Scholar]
- 39.Kohli M, Tan W, Zheng T, et al. Clinical and genomic insights into circulating tumor DNA–based alterations across the spectrum of metastatic hormone-sensitive and castrate–resistant prostate cancer. EBioMedicine. 2020;54:102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stover DG, Parsons HA, Ha G, et al. Association of cell–free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichert ZR, Morgan TM, Li G, et al. Prognostic value of plasma circulating tumor DNA fraction across four common cancer types: a real-world outcomes study. Ann Oncol Off J Eur Soc Med Oncol. 2023;34:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Husain H, Pavlick DC, Fendler BJ, et al. Tumor fraction correlates with detection of actionable variants across > 23,000 circulating tumor DNA samples. JCO Precis Oncol. 2022;6:e2200261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousin S, Belcaid L, Trin K, et al. Tumor fraction to improve patient selection for oncology early phase clinical trials: analysis of two precision medicine studies. J Clin Oncol. 2023;41(16_suppl):3005. [Google Scholar]
- 44.Malapelle U, Leighl N, Addeo A, et al. Recommendations for reporting tissue and circulating tumour (ct)DNA next-generation sequencing results in non–small cell lung cancer. Br J Cancer. 2024;131:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolfo CD, Madison RW, Pasquina LW, et al. Measurement of ctDNA tumor fraction identifies informative negative liquid biopsy results and informs value of tissue confirmation. Clin Cancer Res. 2024;30:2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo A, Lee JK, Pasquina LW, et al. Liquid biopsy of lung cancer before pathological diagnosis is associated with shorter time to treatment. JCO Precis Oncol. 2024;8:e230053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semenkovich NP, Szymanski JJ, Earland N, et al. Genomic approaches to cancer and minimal residual disease detection using circulating tumor DNA. J Immunother Cancer. 2023;11:e006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanton C, Venn O, Aravanis A, et al. Prevalence of clonal hematopoiesis of indeterminate potential (CHIP) measured by an ultra-sensitive sequencing assay: exploratory analysis of the Circulating Cancer Genome Atlas (CCGA) study. J Clin Oncol. 2018;36(15_suppl):12003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.