Abstract
Mycoplasma pneumonia, a primary aetiological agent of atypical pneumonia, necessitates the implementation of rapid point-of-care diagnostics. Lateral flow immunoassays (LFIAs) hold promise for point-of-care testing (POCT), yet their sensitivity levels are frequently constrained by probe affinity and matrix interference. We introduce an orientational labelling strategy that employs magnetic nanoparticles (MNPs) functionalized with staphylococcal protein A (SPA) to simultaneously enhance antibody orientation and facilitate magnetic enrichment. The SPA–MNPs were synthesized via aggregation‒precipitation crosslinking, utilizing the Fc-binding specificity of SPA to enable oriented antibody conjugation and reduce steric hindrance. The oriented probe demonstrated robust stability, exceptional specificity, and enhanced binding affinity, which were considered crucial for the detection of low-abundance targets. In concentration gradient tests, the orientational labelling method resulted in the lowest visible colorimetric signals at 105 CFU·mL−1, whereas conventional random probes (BSA–MNPs) resulted in detectable coloration at concentrations as low as 106 CFU·mL−1. The color sensitivity and LOD of the orientational labelling approach were further increased to 104 CFU/mL and 0.668 × 104 CFU/mL, respectively, through magnetic enrichment treatment. The capture binding kinetics analysis indicated that the enrichment efficiency depended on the binding effect of the antibody and the antigen concentration, showing a clear correlation with the dissociation constant (KD). A clinical evaluation of 87 patient samples showed 88.5% agreement with the results of the quantitative fluorescence qPCR, which validated the diagnostic accuracy of the method. This study presents an orientational labelling platform for LFIA that combines Fc-directed antibody alignment with magnetic nanoparticle enrichment to overcome sensitivity barriers in the detection of low-abundance pathogens. This strategy comprises a universal design principle for achieving high-performance point-of-care testing (POCT) for various infectious diseases.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s00604-025-07392-7.
Keywords: Orientational labelling, SPA-functionalized magnetic nanoparticles, Lateral flow assay, Mycoplasma pneumonia, Magnetic enrichment, Capture-binding kinetics
Introduction
The development of advanced functional materials with precisely engineered interfaces has emerged as a pivotal strategy for addressing critical limitations in biosensing technologies [1]. Lateral flow immunoassays (LFIAs) are cornerstones of rapid diagnostics, but their sensitivity levels are limited by probe design and complex biological matrices [2]. The detection process in dual-antibody sandwich lateral flow immunoassays (LFIAs) is fundamentally governed by two sequential reactions: (1) the interaction between the capture antibody and the target antigen and (2) the subsequent binding of the detection antibody to the antigen‒antibody complex.
The binding of the detection antibody to the antigen‒antibody complex [3] is governed by both kinetic parameters (e.g., association/dissociation rates, ka/kd) and thermodynamic properties (e.g., binding affinity, KD). The interactions between the detection antibody and the antigen‒antibody complex are governed by both the association rate and the thermodynamics. While the antibody affinity is inherent and difficult to modulate, the association kinetics are adjusted by optimizing the membrane conditions [4] (e.g., pore size, surface chemistry, and flow dynamics).
In the capturing–binding process, the sensitivity is influenced primarily by the antigen‒antibody reaction [5], which arises from antigen‒antibody affinity, reaction kinetics [6], and matrix effects [7]. Although significant progress has been made in this area through antibody engineering for optimized affinity [8], signal amplification via advanced labels, such as quantum dots [9], fluorescent microspheres [10], and aggregation-induced emission (AIE) materials [11], preenrichment strategies for antigen‒probe prereactions [12], enhanced detection systems [13], and multilabelling or synergistic signalling approaches [14], among others, the interface engineering of nanomaterials remains underexplored. The crux of this challenge lies in the precise immobilization of biomolecules onto nanoparticle surfaces to maximize target recognition efficiency, a critical factor for ensuring the functionality of nanoparticles.
Magnetic nanoparticles (MNPs) have gained importance in target enrichment because of their rapid magnetic separation and localized concentration effects [15]. However, the mechanisms by which magnetic probes enhance the sensitivity of LFIAs remain undefined. Specifically, the quantitative contributions of magnetic enrichment to signal amplification are unclear . For example, the relationships between the enrichment efficiencies of magnetic probes and the quantities of antibodies they carry and between the enrichment efficiencies of magnetic probes and the densities of antibodies remain unknown. This knowledge gap impedes the rational design of next-generation LFIAs [16].
Central to this challenge is the interface engineering of nanomaterials, specifically regarding the controlled immobilization of biomolecules on nanoparticle surfaces to maximize target recognition efficiency [17]. Currently, the surface modification of magnetic nanomaterials is achieved primarily by coating them with a layer of polymer material [18]. This surface modification technique typically employs physical adsorption [19] or chemical bonding [20] to form a stable polymer protective layer on the surfaces of nanoparticles. Commonly used coating materials include polyethylene glycol (PEG) [21], polyethyleneimine (PEI) [22], chitosan [23], and other biocompatible polymers [24]. This method not only effectively prevents the oxidation and aggregation of nanoparticles [25], thereby increasing their stability [26], but also endows nanoparticles with specific functional properties through the selection of different polymers [27]. Moreover, the polymer coating layer can provide active groups for subsequent functional modifications, creating favorable conditions for further bioconjugation or surface modification [27]. This approach is an important technique in the surface engineering of magnetic nanomaterials, with broad application prospects in biomedicine [28], catalysis [29], environmental remediation [30], and other areas [31].
Conventional antibody conjugation strategies, such as carbodiimide EDC/NHS coupling or maleimide–thiol chemistry [33], generally result in random antibody orientations on nanoparticle surfaces. This random orientation can lead to steric hindrance and a reduction in the antigen-binding capacity of the probes [34]. This limitation underscores the urgent need for innovative approaches to achieve orientational antibody immobilization, thereby optimizing both probe performance and assay sensitivity [32].
Recent advances in protein-assisted surface functionalization provide promising solutions [35]. Staphylococcal protein A (SPA) [36], renowned for its Fc-specific binding to antibodies, has been employed to orient antibodies on gold nanoparticles, thereby increasing affinity by up to tenfold in lateral flow immunoassays (LFIAs) [37]. Nevertheless, the utilization of this strategy into magnetic nanoparticles (MNPs) is challenging because of the inefficient adsorption of MNPs coupled with SPAs, which can compromise the stability and reproducibility of the probe. To overcome these barriers, we propose a novel interfacial engineering strategy: aggregation‒precipitation crosslinking of SPA on MNPs, followed by oriented antibody conjugation. This approach ensures precise control over antibody orientation and, by maintaining a reduced thickness, preserves magnetic responsiveness. This phenomenon enables the synergistic integration of both target enrichment and signal generation.
In this work, we demonstrate that the optimized SPA–MNPs significantly increase the affinity of the probe and the detection sensitivity of LFIAs for Mycoplasma pneumoniae (MP). Compared with conventional random conjugation methods, orientational labelling of mAbs on MNPs via SPA significantly improves antigen-binding efficiency while preserving magnetic responsiveness for target preconcentration. Furthermore, we establish a generalizable framework for the surface modification of probes. The modular architecture of this system facilitates facile adaptation to various diagnostic targets via antibody substitution, rendering it a versatile solution for precise detection across clinical, environmental, and food safety applications.
Experimental procedures
Chemicals, materials, and equipment
The following materials were used in this study: goat polyclonal anti-mouse IgG (H + L) antibodies and Mycoplasma pneumoniae (MP) polyclonal antibody AB53600 from Sigma‒Aldrich (St. Louis, MO, USA); mouse monoclonal antibodies (MP-5 and MP-19), murine SP20 ascites, and inactivated rabbit serum produced in our laboratory; a qPCR-based MP nucleic acid detection kit from XuanYa Biotechnology (Shanghai, China); standard proteins of Mycoplasma pneumoniae (MP), Chlamydia trachomatis (CT), Chlamydophila pneumoniae (CP), adenovirus (ADV), respiratory syncytial virus (RSV), influenza A virus (IFA), influenza B virus (IFB), Staphylococcus aureus, and Streptococcus pneumoniae from Envirologix (Maine, USA); staphylococcal protein A from ProSpec (East Brunswick, NJ, USA); analytical grade chemicals, including FeCl₂, FeCl₃, CTAB, Tween-20, BSA, PEG6000, and other reagents, from Sigma‒Aldrich; nitrocellulose membranes and ELISA plates from Kinbio Tech (Shanghai, China); a Milli-Q water purification system (Bedford, USA); a Thermo Fisher microplate reader (Waltham, MA, USA); a Bio-Dot Quanti 3000 Biojet dispenser (Irvine, CA, USA); and a constant-temperature oven from NingBo HongLing (China).
All buffer solutions were prepared with ultrapure water (18.2 MΩ·cm). Carbonate buffer (CBS; 50 mM, pH 9.6) and phosphate buffer (PBS; 50 mM, pH 7.4) served as the primary buffer systems. The blocking buffer (BPBS) contained 1% (w/v) bovine serum albumin (BSA) in PBS, while the washing buffer (PBST) consisted of 0.5% (v/v) Tween-20 in PBS. The MP stock solutions (1 mg·mL−1 in PBS) were stored at − 20 °C, and the working solutions were prepared via dilution with ultrapure water and stored at 4 °C. The lyophilization buffer (LB) was prepared with 1% (w/v) BSA, 10% (w/v) sucrose, 5% (w/v) trehalose, 1% (v/v) Tween-20, and 0.05% (w/v) PEG6000 in PBS. The substrate solution for detection was prepared by combining phosphate–citrate buffer (pH 5.0) with tetramethylbenzidine (TMB; 10 mg·mL−1 in DMSO) and hydrogen peroxide (0.65% v/v).
Preparation and surface modification of magnetic nanoparticles
Synthesis of magnetic nanoparticles
The MNPs were synthesized via the microemulsion method [38] after slightly changing the process. Then, 12 g of cetyltrimethylammonium bromide (CTAB), 7 mL of water, 10 mL of butanol, and 30 mL of octane were dissolved in a 100-mL two-neck flask. The solution was stirred at room temperature until it became homogeneous. The mixture was stirred for an additional hour under a nitrogen atmosphere. Subsequently, 0.834 g of FeSO₄·7H₂O was added, and the mixture was stirred until it fully dissolved. A solution of 3.2 g of NaOH in 4 mL of water was then added, and the mixture was stirred until it turned brown. The product was washed three times with 95% ethanol, resuspended in 50 mL of ultrapure water, stirred for 15 min, and allowed to settle. The washing step was repeated three times to remove residual impurities. The nanoparticles were dried and stored in a desiccator. Then, 1 mg of the dried product was dispersed in 1 mL of ultrapure water and ultrasonicated to ensure homogeneity. This solution was used for magnetic property measurements, particle size analyses, and subsequent experiments.
SPA-functionalized magnetic nanoparticles
SPA was immobilized on the MNPs via the adsorption and precipitation method, after which it was chemically cross-linked with EDC [40]. Varying masses of SPA protein and MNPs were dissolved in 1.5-mL EP tubes. The volume was expanded to 50 µL by adding water, ensuring that the adsorption reaction occurred for 1 h in the cradle at 200 rpm at room temperature. Then, 350 µL of saturated ammonium sulfate (SAS) and 2.3 µmol of EDC were added. The reaction volume was 500 µL after adding water. The EP tubes were incubated in a cradle at 200 rpm for 2 h at room temperature. Then, 100 µL of BPBS was added for blocking, and the mixture was incubated at 200 rpm for another 2 h. The mixture was centrifuged to obtain a precipitate that was washed at least three times with PBS. The collected precipitate consisted of SPA-functionalized magnetic nanoparticles (SPA–MNPs), which were resuspended and stored in PBS with an MNP content of 1 mg·mL−1. The SPA–MNPs were utilized for detection and subsequent experiments.
Preparation of orientation-specific labelled probes
SPA-functionalized magnetic nanoparticles (1 mL, 1 mg·mL−1 MNPs in PBS) were precisely aliquoted via a calibrated micropipette [39]. To initiate conjugation, 200 μg of MP-5 monoclonal antibody was introduced under vortex mixing (2000 rpm, 30 s). The reaction mixture was then subjected to end-over-end rotation (15 rpm) at 25 °C for 1 h to achieve oriented antibody immobilization. Subsequent blocking of residual protein-binding sites was accomplished by adding 100 μL of filtered murine SP20 ascites (10% w/v in TBS), followed by static incubation (30 min, 25 °C). The mixture was subsequently centrifuged at 2000 rpm at 4 °C for 10 min, after which the supernatants were removed. The precipitated pellets were then resuspended in 1 mL of phosphate buffer (1 mmol·L−1, pH 7.2) containing 1% BSA, 2% sucrose, and 0.05% NaN3. The MNPs, which were orientationally labelled with antibodies (Ab–SPA–MNPs), were resuspended and stored in PBS with an MNP content of 1 mg·mL−1 for future use. Ab–SPA–MNPs were utilized for detection and subsequent experiments.
Preparation of randomly labelled probes
To evaluate the impact of oriented labelling, a control group was prepared via random covalent conjugation. First, the MNPs were surface modified with bovine serum albumin (BSA) via the same aggregation‒precipitation crosslinking method. Subsequently, the antibodies were randomly conjugated to the BSA–MNPs via the EDC/NHS method. Briefly, the carboxyl groups on the BSA–MNPs were first activated with 10 mM EDC in MES buffer (pH 6.0) for 10 min, followed by the addition of 5 mM NHS for another 10 min. Then, the samples were magnetically separated and washed three times with borate buffer (pH 8.0), and the monoclonal antibody (0.2 mg of MP-19 per 1 mg of MNP) was conjugated to the activated BSA–MNPs via incubation at 37 °C for 2 h. Unbound reagents and excess antibodies were subsequently removed by magnetic washing. The resulting Ab–BSA–MNP conjugates were stored in PBS containing 1% BSA at 4 °C.
Measurement of the relative affinity of the probe for the antigen
The dosage‒response curve method was employed to systematically evaluate the relative affinity of oriented probes versus randomly labelled probes to the antigen via ELISA [40]. The experimental process was as follows. First, the antigen saturation coating concentration was established through optimization. Second, probe dilutions were prepared in a serial gradient and incubated with antigen-coated wells at 80% saturation to maintain optimal signal linearity within the dynamic range of the assay.
The binding interactions were quantitatively assessed by measuring the optical density at 450 nm (OD450) via microplate spectrophotometry. The dosage‒response curves were generated by plotting the OD450 values against the probe concentration. The half-maximal effective concentration (EC50), defined as the probe concentration yielding 50% of the maximal OD450 response, was derived through sigmoidal curve fitting analysis. The half-maximal effective dilution (ED50) was defined as the ratio of the probe starting concentration (1 µg/mL) to the EC50. This ED50 parameter served as an indicator of binding affinity, where higher ED50 values (corresponding to the lower probe concentrations needed for half-maximal binding) indicated stronger probe–antigen interactions.
Under standardized assay conditions, the comparative evaluation of ED50 values between labelled probes enabled precise quantification of their relative binding performance, with higher ED50 values indicating a stronger antigen recognition capability.
Characterization of the probes
During the preparation of the oriented probe, a variety of analytical methods were employed to ensure that the probe was modified precisely according to our design.
Scanning electron microscopy (SEM) could be used to provide high-resolution surface morphology information about the particles. With SEM analysis, we could characterize the surface morphology of the probe and evaluate whether the protein coating had already been wrapped on the magnetic nanoparticles.
Vibrating sample magnetometer (VSM) detection allowed us to understand and quantify the magnetic characteristics of nanoparticles in detail. The magnetic characteristics of probes (MNPs, SPA–MNPs, and Ab–SPA–MNPs) at different stages could be investigated via VSM detection, which would help us investigate whether probes performed as designed.
In addition, we conducted systematic dynamic light scattering (DLS) measurements of MNPs, SPA–MNPs, and Ab–SPA–MNPs to characterize their hydrodynamic diameter and colloidal stability during the surface modification process.
Moreover, we systematically analyzed the ζ-potentials of MNPs, SPA–MNPs, and Ab–SPA–MNPs to evaluate their changes and colloidal stabilities during functionalization.
In summary, the labelling modification process of MNPs was monitored by comprehensive characterization through SEM, VSM, DLS, and ζ-potential.
Magnetic probe-based lateral flow system preparation
This system of magnetic nanoparticles as a label for lateral flow immunoassays consists of two parts: a probe and a test strip.
Lateral flow probe optimization
Various quantities of magnetic nanoparticle probes (Ab–SPA–MNPs) were accurately measured, and each sample was diluted with lysis buffer to achieve a final volume of 100 µL. The diluted samples were distributed into individual wells of a 96-well plate. The wells were subsequently lyophilized to prepare them for subsequent reactions; these wells were designated probe reaction wells. All the experiments were conducted under consistent conditions to maintain reproducibility. The performance of each probes was evaluated on the basis of two key criteria: (1) the ability to increase the detection limit of the lateral flow strip and (2) the clarity and intensity of the colorimetric signal displayed on the strip. The optimal probe dose was selected on the basis of its ability to meet these criteria effectively.
Antibody coating on the NC membrane and strip preparation
The test strips were prepared with a nitrocellulose (NC) membrane. The coating reagents, the antibody against Mycoplasma pneumoniae (MP-19), and the polyclonal antibody of G-M (pAb), were diluted to a concentration of 1 mg·mL⁻1 in PBS and sprayed onto the test (T) and control (C) lines at a rate of 1 µL·cm⁻1, utilizing a Quanti 3000 Biojet system (Bio-Dot, Irvine, CA, USA). The T and C lines were positioned 4 mm apart on the NC membrane. After drying at 37 °C for 3 h, the membrane was sealed in a plastic bag with desiccated bags and preserved at room temperature.
The test strip consisted of a sample pad, an NC membrane, and an absorbent pad. The absorbent pad, which had dimensions of 31 mm × 300 mm, and the sample pad, which had dimensions of 25 mm × 300 mm, were prepared from absorbent paper and fibrous membranes, respectively. The sample pad was pretreated with PBS (1 mmol·L⁻1, pH 7.2), which contained 1% BSA, 0.5% Tween-20, 0.5% PEG-6000, and 0.05% NaN₃. The components were methodically assembled on a semirigid polyethylene sheet in the following sequence: an NC membrane, an absorbent pad, which overlapped the NC membrane by 1 mm, and a sample pad, which overlapped the NC membrane by 1 mm. The assembled sheet was cut into strips measuring 4 mm in width, which were then sealed within desiccated bags for storage.
Detection protocol and test strip result interpretation
For each assay, the lyophilized probe was added to the EP tube containing the sample, mixed thoroughly, and allowed to react with the antigen for 15 min. The tube was subsequently placed on a magnetic separator for 10 min, during which the magnetic probe, along with the antigen, was attracted to the magnet, effectively removing the matrix. After removing the tube from the magnetic separator, 100 µL of PBS was added, and the mixture was mixed thoroughly. The liquid, which included probe‒antigen complexes, was applied to the sample pad of the test strip and allowed to develop for 15 min. The results were visually observed and interpreted on the basis of the presence or absence of color development in the test (T) and control (C) lines.
During the testing of positive MP samples under standard detection protocols, the antigens of Mycoplasma pneumoniae first interacted with the capture antibodies (MP-5), which were conjugated to magnetic nanoparticles, forming antigen‒antibody‒nanoparticle complexes (MP‒Ab‒MNPs). The mixture was then applied to the test strip, where MP bound to the detect antibody (MP-19), which was immobilized on the test zone of the NC membrane, thus forming a brown complex (Ab–MP–Ab–MNPs) at the T line. Subsequently, any unbound Ab–MNPs or MP–Ab–MNPs bound to the goat anti-mouse polyclonal antibody (G-M) in the control zone, forming a brown complex (G–M–Ab–MNPs or G–M–(MP)–Ab–MNPs) at the C line. Thus, positive samples displayed two brown lines (T and C), whereas negative samples, lacking MPs, only formed a brown line at the C line. In the absence of a C line, the result was invalid. An illustration of the results (positive, negative, and invalid) is provided in Figure a.
Sensitivity, specificity, and stability analysis
The sensitivity, specificity, and stability levels of the strips were determined. For the sensitivity assessment, a series of MP concentrations of 1 mL in PBS (2 × 106, 1 × 106, 2 × 105, 1 × 105, 1 × 104, and 0 CFU·mL−1) was tested to determine the visible limit of detection of this test strip. The limit of detection (LOD) was calculated on the basis of the assay signal and converted to colony-forming units per milliliter (CFU/mL) via a conversion factor of 10 ng of MP standard protein per 104 CFU, as established by the quantitative relationship between protein concentration and CFU (data provided in the supporting materials). Specificity was assessed by investigating the cross-reactivity of the test strips with Chlamydia trachomatis (CT), Chlamydophila pneumoniae (CP), adenovirus (ADV), respiratory syncytial virus (RSV), influenza A virus (IFA), influenza B virus (IFB), Staphylococcus aureus, and Streptococcus pneumoniae standard solutions at concentrations of 105 CFU·mL−1. To evaluate the long-term stabilities of the test strips, we periodically measured their responses to standard MP solutions at various concentrations. The test strips were stored under controlled conditions—room temperature (RT, 25 ± 2 °C) in a desiccator (relative humidity < 30%)—and tested at 2-month intervals over a 12-month period.
Performance evaluation
A total of 87 throat swab samples from respiratory patients were obtained from Shaanxi Provincial People’s Hospital (Xi’an, China). Each sample was analyzed by using the strips established in this study and a commercial MP fluorescence quantification PCR kit for performance evaluation. The study was conducted in accordance with the Declaration of Helsinki (S2024-YF-YBSF-1055). Written informed consent was obtained from all participants before they took part in this study.
Results and discussion
Orientational probe labelling
The process of immobilizing antibodies on magnetic nanoparticles involves two steps. First, SPA was used to aggregate cross-linking precipitates on the surface of the MNPs, forming SPA-functionalized MNPs (SPA–MNPs). Second, the Fc of the antibody was attached to the SPA, and antibody-oriented labelling was performed on the surfaces of the particles (Ab–SPA–MNPs). A schematic diagram of the antibody immobilization process is shown in Fig. 1.
Fig. 1.
Schematic of orientational antibody immobilization on magnetic nanoparticles (MNPs)
Although SPA crosslinking could reduce the number of active binding sites of SPA, the antibody adsorption capacity remained SPA dependent. Therefore, the amount of adsorbed antibody depended on the SPA coated on the magnetic bead. Therefore, optimizing the SPA coating density was essential for increasing the probe’s detection sensitivity.
SPA-mediated functionalization of magnetic nanoparticles
Consistent with previous findings on protein‒nanoparticle interactions, systematic variation in the SPA:MNP mass ratio (0.3:1 to 1:1) under controlled conjugation conditions (0.5 mL reaction volume, 200 rpm agitation, 70% saturated ammonium sulfate, and 3 mg.mL−1 EDC) revealed a nonlinear relationship between the immobilization efficiency and surface functionalization degree. The mass ratio of the probe (SPA:MNP = 0.7:1) at the ED50 point was 326.8, whereas those of the probes (0.3:1, 0.5:1, and 1:1) were 15.3, 38.6, and 39.4, respectively (Fig. 2a). This finding suggested that the probe mass ratio (SPA:MNP = 0.3:1) at 15.3-fold dilution, the probe mass ratio (SPA:MNP = 0.5:1) at 38.6-fold dilution, the probe mass ratio (SPA:MNP = 0.7:1) at 326.8-fold dilution, and the probe mass ratio (SPA:MNP = 1:1) at 39.4-fold dilution were identical to the results of the antigen reaction. This finding indicated that when the amount of MNPs was fixed, adding more SPA would not enhance the performance, and excessive SPA could lead to specific steric effects.
Fig. 2.
Effects of SPA:MNP mass ratios on probe immobilization yield and relative affinity
Notably, while the antibody immobilization rate monotonically increased with increasing SPA loading (Fig. 2b), the antigen recognition efficiency exhibited a distinct maximum value at 0.7:1. This performance superiority stemmed from two synergistic effects: (1) sufficient SPA surface density for antibody capture and (2) minimized steric hindrance. This threshold behavior underscored the critical balance between SPA density and the binding affinity of nanoparticles. On the basis of these findings, all subsequent experiments utilized 0.7:1 (SPA:MNP) conjugated antibodies.
Characterization of the probes
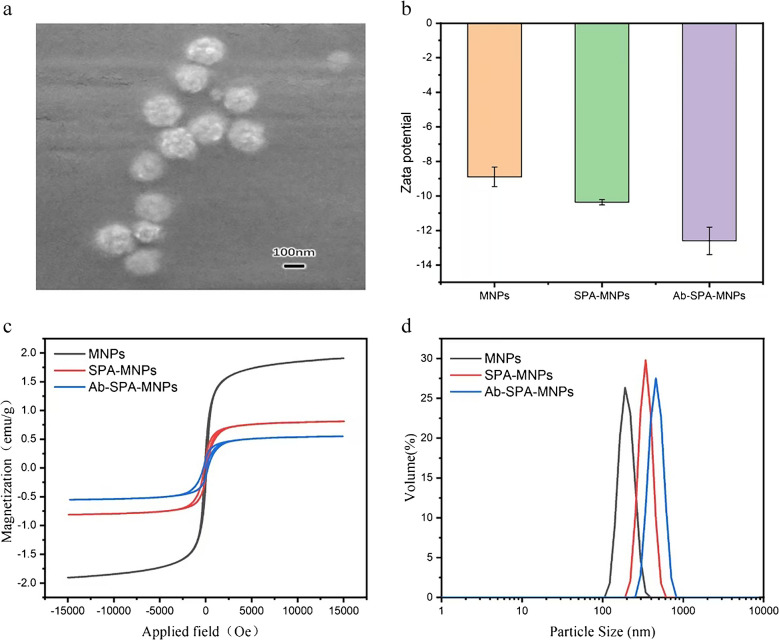
A representative scanning electron microscope (SEM) image is displayed in Fig. 3a. Owing to the low electron density of the proteinaceous components after functionalization, conventional SEM imaging failed to resolve clear morphological details of the antibody-conjugated magnetic nanoparticles (MNPs). Nevertheless, the SEM observations collectively confirmed the successful anchoring of protein-based antibodies onto the MNP surfaces, which was consistent with the anticipated surface modification outcomes. Additionally, the protein layer was observed to coat the surface of the magnetic nanoparticles, confirming successful functionalization.
Fig. 3.
Probe characterization. a SEM image. b Zeta potential. c Magnetization curves. d Hydrodynamic size
Zeta potential measurements revealed distinct surface charges across the nanoparticles (MNPs, SPA–MNPs, Ab–SPA–MNPs). Unmodified magnetic nanoparticles (MNPs) exhibited a zeta potential of − 8.90 mV in aqueous suspensions. Following functionalization with SPA, the zeta potential of the SPA–MNPs shifted to − 10.37 mV, whereas further conjugation with antibodies resulted in a more pronounced negative charge of − 12.60 mV. These results confirmed the successful preparation of SPA–MNPs and Ab–SPA–MNPs, as the increasingly negative zeta potential directly corresponded to the sequential deposition of charged biomolecules (SPA and antibodies) on the nanoparticle surface shown in Fig. 3b.
To validate whether the protein was labelled as per our design, the magnetization curves were measured for the MNPs, SPA–MNPs, and Ab–SPA–MNPs, and the results are shown in Fig. 3c. The saturation magnetization (Ms) values of the MNPs, SPA–MNPs, and Ab–SPA–MNPs were 476 emu·g−1, 114 emu·g−1, and 92 emu·g−1, respectively. The significant decrease in Ms from the MNPs to the SPA–MNPs and Ab–SPA–MNPs was indicative of the effective functionalization of the magnetic nanoparticles. The core magnetic properties of nanoparticles were invariable, but different protein layers on the surfaces of the particles reduced the Ms. This reduction in Ms could be attributed to the nonmagnetic protein coating, which limited the magnetic response. These results were consistent with our expectations, confirming that functionalization with SPA and antibody labelling were successful.
The hydrodynamic sizes of the MNPs, SPA–MNPs, and Ab–SPA–MNPs were measured via dynamic light scattering (DLS) and found to be 269.7, 492.7, and 502.7 nm, respectively. The increase in particle size with each step of protein labelling was consistent with the addition of the SPA protein and antibody layers to the surfaces of the nanoparticles. This stepwise increase in size further supported the conclusion that the labelling process was carried out as expected. The particle size distribution analysis is shown in Fig. 3d.
Together, these results—magnetization curves, zeta potential, and hydrodynamic size—comprehensively validated the functionalization and labelling processes. The decrease in Ms and increase in hydrodynamic size with each labelling step were consistent with the expected modifications to the nanoparticles, and they confirmed the successful coating with SPA and the antibody. The SEM and Ms values of the Ab–SPA–MNPs characterized the morphological and magnetic responsiveness characteristics of the labelled magnetic nanoparticles.
SPA-functionalized magnetic nanoparticles demonstrating enhanced antigen-binding affinity
A comparative analysis of the antigen-binding affinity was conducted between oriented antibody probes (Ab-SPA–MNPs) and randomly labelled probes (Ab–BSA–MNPs). The results revealed a substantial increase in the binding performance of the oriented probe.
Specifically, the ED50 value of the oriented probe was 332, whereas that of the random probe was 16. This finding indicated that the oriented probe significantly enhanced the binding affinity of the probe by 21-fold. The oriented probes, which leveraged the Fc-binding specificity of SPA to achieve oriented antibody immobilization, demonstrated a 21-fold higher ED50 than did their randomly labelled counterparts.
This marked improvement was attributed to the optimal spatial orientation of antibodies on the nanoparticle surface, which minimized steric hindrance and maximized the antigen-binding valency. These findings suggested that targeted antibody coupling offered superior performance compared with random labelling in immunoassays. The detailed results are illustrated in Fig. 4.
Fig. 4.
Comparison of antigen-binding affinity: oriented antibody probes (SPA‒MNPs) vs. randomly labelled probes (BSA‒MNPs)
Development of a lateral flow assay system with integrated magnetic probes
Optimization of Ab–SPA–MNP dosage for antigen capture
To determine the optimal probe dosage, a standard calibration curve was established using MP antigen concentrations ranging from 0.001 to 2 μg·mL⁻1 (R2 = 0.998) with a commercial ELISA system. Lyophilized Ab–SPA-MNP probes (5–45 μg) were incubated with 2 μg/mL antigen solution (n = 3) for 15 min, followed by magnetic separation. Quantitative analysis of residual antigens through ELISA revealed a sigmoidal binding efficiency curve, with nonlinear regression analysis identifying the half-maximal effective probe dose as 9.83 μg. To ensure operational robustness in practical applications, we selected 25 μg of probes that achieved near-saturation capture efficiency (97.8% ± 0.9%), effectively reducing the antigen concentration to 0.04 μg·mL⁻1. Subsequent lateral flow strip tests employing this optimized dosage demonstrated distinct visual detection gradients across MP antigen concentrations spanning three orders of magnitude (0–106 CFU·mL⁻1), confirming preserved analytical responsiveness (Fig. 5).
Fig. 5.
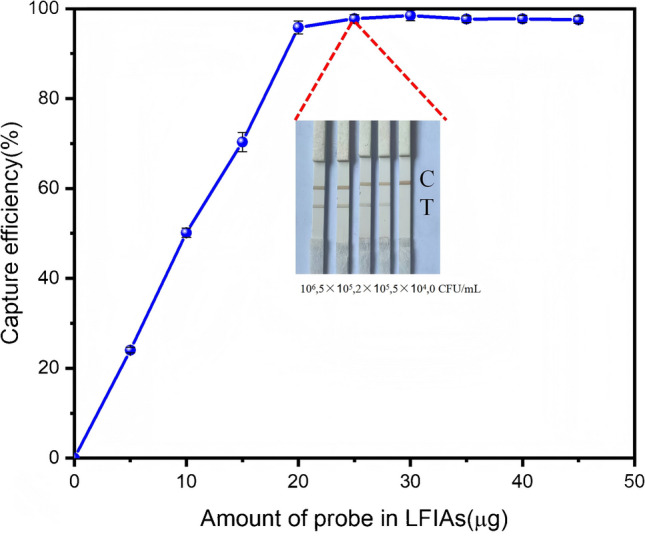
Optimal dosage of oriented probes for maximal antigen capture efficiency
For matrix interference verification, in lateral flow assays with 50 clinical swabs, 25 μg of probes eliminated nonspecific reactions in negative controls (0/25 false positives) while maintaining clear test lines in positive samples (100% concordance with PCR).
Optimizing nitrocellulose membrane conditions for immunobinding
In this detection system, the interaction between the capture antibody and antigen in the liquid phase had to be fully ensured. Membrane conditions were another critical factor influencing the lateral flow of the sandwich immunoassay as they affected the reaction between the immobilized capture antibody and the antigen‒probe complex. The pore size of the membrane directly impacted the chromatographic flow rate, the migration path of the complex, and the contact time between the antibody and antigen. Sartorius membranes, a cost-effective and commercially available nitrocellulose (NC) membrane option, were tested with the following specifications. The Sartorius CN 95 membrane (pore size: 15 μm) exhibited a wicking time of 96.9 s. The wicking time of the Sartorius CN 140 membrane (pore size: 10 μm) was 134.3 s. Test strips were prepared via the protocol outlined in antibody coating and strip preparation under identical conditions. When the same antigen concentration gradient was used, the results are shown in Fig. 6.
Fig. 6.
Impact of nitrocellulose (NC) membrane pore size and manufacturer variants on lateral flow immunoassay detection sensitivity
Among the tested Sartorius nitrocellulose membranes, the CN 140 membrane showed superior sensitivity compared with the CN 95 membrane. This finding indicated that small-pore membranes could improve sensitivity by balancing flow dynamics and interaction efficiency. This phenomenon could occur because the pore size of the membrane affected the binding kinetics of the immobilized antibody to the antigen. A good lateral flow assay process would need to balance the affinity of the immobilized antibody for the antigen and binding kinetics. High affinity with slow kinetics could result in insufficient binding under a limited reaction time, causing reduced sensitivity and false negatives. Conversely, fast kinetics with low affinity could lead to incomplete interactions and false negatives. The results showed that the CN 140 membrane effectively balanced flow dynamics and interaction efficiency, demonstrating the importance of selecting a membrane that optimized both the flow rate and antigen‒antibody interaction efficiency. Therefore, the CN 140 membrane was chosen for enhanced detection performance in subsequent experiments.
Magnetic enrichment and oriented labelling to synergistically increase detection sensitivity
To systematically evaluate the effects of magnetic enrichment and oriented antibody labelling on detection sensitivity, we designed four experimental test strip groups: (a) direct application of freeze-dried random probes without magnetic enrichment, (b) direct application of freeze-dried oriented probes without magnetic enrichment, (c) application of freeze-dried random probes with magnetic enrichment, and (d) application of freeze-dried oriented probes without magnetic enrichment. Antigen concentrations of 0‒2 × 107 CFU/mL were used to test all strips in the four groups.
As shown in Fig. 7, the detection performance of different test strips was evaluated through concentration gradient analysis. The test strip with oriented probes produced a visible colorimetric signal at 105 CFU/mL, whereas the conventional random probe test strip (BSA‒MNPs) only demonstrated detectable coloration at 10⁶ CFU/mL. When integrated with magnetic enrichment, the oriented probe system achieved observable coloration at 104 CFU/mL, representing a tenfold improvement in detection sensitivity compared with that of the nonenriched oriented probes.
Fig. 7.
Evaluation of detection sensitivity in lateral flow immunoassays with different probe formulations. a Oriented probe. b Oriented probe with magnetic enrichment. c Random probe. d Random probe with magnetic enrichment
Both probe types (random/oriented) showed higher minimum detectable concentrations in nonenriched systems than their magnetically enriched counterparts did, confirming the critical role of magnetic enrichment in sensitivity enhancement. Notably, across all the experimental groups, oriented probes presented higher minimum detectable concentrations than random probes did. This dual enhancement pattern (orientation-dependent labelling + magnetic enrichment) established both strategies as essential components for optimizing test strip performance.
Capturing‒binding kinetics
The interactions of antibodies and Mycoplasma pneumoniae on magnetic nanoparticles were assumed to be transient in the sample, and the diffusion effect was ignored.
The chemical equation for the antigen‒antibody binding reaction was as follows:
| 1 |
Where [Ag], [Ab], and [AgAb] are the concentrations of the antigen, the antibody in the MNPs, and the antigen‒antibody complex in the MNPs, respectively; [Ag]0, [Ab]0, and [AgAb]0 are the initial concentrations of the antigen, the antibody in the MNPs, and the antigen − antibody complex in the MNPs ([AgAb]0 = 0), respectively; and [Ag]e, [Ab]e, and [AgAb]e are the equilibrium concentrations of the antigen, the antibody in the MNPs, and the antigen − antibody complex in the MNPs at capture equilibrium, respectively.
The number fraction (%) of Ab that captured MP (η) at equilibrium could then be calculated by the following expression:
| 2 |
For [AgAb]e, the rate equation for Eq. (1) was expressed as follows:
| 3 |
Where ka and kd are the association and dissociation rate constants, respectively. If [Ab]0 ≫ [Ag]0, [Ab] could be regarded as a constant, and thus, [Ab] ≅ [Ab]0. Therefore, when the pseudo-first-order approximation was used, Eq. (3) could be rewritten as follows:
| 4 |
At equilibrium, the following relation was maintained because [Ag]e + [AgAb]e = [Ag]0; Eq. (4) could be rewritten as follows:
| 5 |
Under these conditions, Eq. (2) could be rewritten as follows:
| 6 |
where KD = kd/ka is the dissociation constant.
The enrichment factor was defined as φ = Vs/Ve, where Vs and Ve were the volumes of the sample and eluent required to recover the trapped magnetically active materials, respectively.
| 7 |
where NA is the Avogadro constant; NAb is the number of antibodies added at the beginning of the experiment; and V is the volume of the liquid before capture. The volume of the liquid before capture was equal to the volume of the sample plus the volume of the probe. For magnetic enrichment operations, the volume of the added antibody probe was very small. Therefore, V Vs. NAb was fixed in lateral flow. For the lateral flow strip, Ve = 0.1 mL (as experimentally determined in this study); thus, Vs = φ/10.
Equation (6) could be rewritten as follows:
| 8 |
When the binding condition was optimal and the antigen concentration was at the limit of detection (LOD), the efficiency of the antibody reaction was η ≈ 100%; then, Eq. (8) could be rewritten as follows:
| 9 |
Where m represents the number of antibodies labelled on each probe, e represents the efficiency of the antibodies labelled on each probe, and Nprobe represents the number of probes used. Equation (9) could be rewritten as follows:
| 10 |
Where m × Nprobe/NA represents the antibody loading quantity utilized for functionalizing the SPA–MNPs in this detection system, which was equal to [Ab]0.
In our orientation-specific labelling and magnetic enrichment-based detection system, the antigen exhibited a minimum detectable coloration of 10 ng·mL−1 for the MP standard protein, which was equivalent to 1.538 × 10⁻1⁰ M (calculated using the molecular weight of the MP standard: 65 kDa). The antigen‒antibody complex had a dissociation constant (KD) of 1.333 × 10⁻1⁰ M (data provided in the supporting materials).
For functionalization, approximately 140 μg of active antibody (with a molecular weight of 150 kDa) was required to label 1 mg of SPA–MNPs, corresponding to a molar quantity of 0.933 × 10⁻1⁰ M. In individual assays, 25 μg of nanoprobes was used, resulting in an effective antibody concentration of 0.2333 × 1010 M.
Under the specified conditions of SPA–MNPs, the antibody efficiency (e) approached unity. However, owing to the stochastic orientation effects intrinsically associated with the coupling process, the efficiency was inherently suboptimal. Consequently, an empirically determined value of 0.9 was adopted for subsequent calculations to account for these stochastic variations. By substituting these values into Eq. (9), we obtained a phase accumulation factor (φ) of 10.485, which could be approximated to 10.
These calculation results confirmed that the magnetic nanoprobe enrichment system achieved a tenfold increase in detection sensitivity, which was in agreement with the experimental observations in lateral flow immunoassays. This finding indicated that our kinetic analysis was correct.
Evaluation of the lateral flow assay system with integrated magnetic probes
Sensitivity analysis
The optimized test strips were systematically evaluated using antigen solutions of graded concentrations ranging from 0 to 106 CFU·mL⁻1, as shown in Fig. 8b. Quantitative analysis of colorimetric responses was conducted by measuring grayscale intensity via BrandScan software (version 5.2, BioImaging Systems). As depicted in Fig. 8a, a robust linear correlation (Y = 0.0925X + 7321.98632, R2 = 0.96763) was observed between the grayscale values and bacterial cell number within the range of 0‒106 CFU·mL⁻1, demonstrating the quantitative detection capability of the optimized system. The limit of detection (LOD), calculated as the mean blank signal plus three standard deviations (3σ method, with σ = 202.996), was determined to be 6.58 × 103 CFU·mL⁻1.
Fig. 8.
Performance evaluation of test strips. a Relationship curve between the grayness of the test strip and the antigen concentration. b Color response of the test strips under a gradient of Mycoplasma pneumoniae antigen concentrations. c Specificity assessment. Cross-reactivity screening against common respiratory pathogens: (1) Chlamydia trachomatis (CT), (2) Chlamydophila pneumoniae (CP), (3) adenovirus (ADV), (4) respiratory syncytial virus (RSV), (5) influenza A virus (IFA), (6) influenza B virus (IFB), (7) Staphylococcus aureus, (8) Streptococcus pneumoniae, and (9) Mycoplasma pneumoniae (MP). d Stability analysis. Accelerated storage stability at room temperature/sealed desiccator equipped with anhydrous silica gel (relative humidity < 30%) for 12 months
The visible coloration in lateral flow detection increased by only ten times, whereas the affinity of the probes increased by 21-fold, which could be attributed to the following factors. Our detection method relied on the visual observation of the lateral flow test strips. Although magnetic nanoparticles enhanced the binding affinity, they contributed limited optical signals that could be discerned by the naked eye. Even if the amount of antigen‒antibody binding increased, the difference in color intensity would not be sufficiently distinguishable. While improving the affinity of capture probes could enhance the detection line sensitivity, in lateral flow assays, it was closely related to the reaction efficiency between detection antibodies and antigen‒antibody complexes. As a result, the actual effective detection range did not expand in proportion to the increase in affinity.
Test strip specificity
The specificity of the Ab‒SPA‒MNP strips was evaluated via cross-reactivity with Chlamydia trachomatis (CT), Chlamydophila pneumoniae (CP), adenovirus (ADV), respiratory syncytial virus (RSV), influenza A virus (IFA), influenza B virus (IFB), Staphylococcus aureus, Streptococcus pneumoniae, and Mycoplasma pneumoniae (MP) at 105 CFU·mL⁻1 in PBS buffer. Only the MP-positive samples produced distinct brown bands in both the test (T) and control (C) zones, whereas all the other pathogens exhibited a single band in the C zone. These results confirmed the high specificity of the strip for MP detection.
The excellent specificity of the test strip arose due to the intrinsic binding selectivity of the antibody and the optimized probe preparation scheme. During probe synthesis, SPA was immobilized onto MNPs via an aggregation‒precipitation cross-linking method. BSA was used to block nonspecific binding sites, ensuring minimal interference. Subsequently, monoclonal antibodies against MP-5 were orientationally conjugated to the SPA–MNPs through Fc-specific interactions, followed by blocking with inactivated murine SP20 ascites. This orientational labelling strategy preserved antibody reactivity while eliminating cross-reactivity, as evidenced by Fig. 8c.
Storage stability of the strip
To assess long-term stability, test strips were stored in desiccated containers at room temperature (RT) for 12 months. At bimonthly intervals, strips were tested against MP antigen standards at high (106 CFU·mL⁻1), medium (5 × 105 CFU·mL⁻1), and low (5 × 104 CFU·mL⁻1) concentrations. The grayscale intensity of the T line was quantified via Bandscan 5.0 software to evaluate signal consistency.
The results demonstrated excellent storage stability, with no significant degradation in signal intensity even after 12 months (Fig. 8d). This stability was attributed to the lyophilized formulation of reagents on the strip substrate, which minimized moisture-induced degradation and preserved antibody functionality under ambient storage conditions [41].
Assay validation
A prospective evaluation of 87 pediatric throat swab samples from early-stage Mycoplasma pneumoniae pneumonia patients (symptom onset ≤ 3 days) at Shaanxi Provincial People’s Hospital demonstrated the clinical utility of this novel magnetic nanoparticle-enhanced lateral flow immunoassay (MNE‒LFIA LOD: 6.58 × 104 CFU·mL−1) for rapid MP detection. Compared with P1 gene-targeted quantitative fluorescence PCR (qPCR) (LOD: 35 copies·mL−1), the point-of-care assay achieved comparable diagnostic performance, with a sensitivity of 88.2% (15/17 PCR-confirmed cases detected) and specificity of 88.6% (62/70 PCR-negative samples correctly identified), showing moderate concordance (κ = 0.53; McNemar’s P = 0.11).
Notably, the strip assay demonstrated a higher positivity rate (26.4% vs. 19.5%), suggesting a reduced false-negative risk through enhanced antigen capture capability during the acute infection phases. While an 11.4% false-positive rate (8/70) necessitated clinical correlation with patient symptoms, this rapid test (within 30 min) had three critical advantages: (1) operational simplicity requiring minimal training, (2) comparable accuracy to nucleic acid amplification methods under practical application conditions, and (3) enhanced early detection potential that could decrease diagnostic omissions. In an environment with limited resources or in emergency clinical situations, this test strip was particularly valuable as an effective primary screening tool, and positive results could be further confirmed with clinical symptoms and qPCR to guide treatment. The results are shown in Fig. 9b.
Fig. 9.
Assay evaluation of 87 pediatric throat swab samples. a Schematic of experimental outcomes. b Comparison of lateral flow strips and quantitative fluorescence PCR (qPCR)
Conclusions
We developed a highly sensitive and specific lateral flow immunoassay for Mycoplasma pneumoniae detection by using orientationally labelled magnetic nanoparticles. This method addressed key limitations of conventional LFIAs, such as nonspecific binding and low sensitivity at low analyte concentrations. By functionalizing SPA on magnetic nanoparticles, we effectively minimized steric hindrance while preserving antibody functionality. The probe was integrated into the lateral flow detection system, significantly increasing the detection sensitivity and enabling the detection line to reach a limit of detection (LOD) of 6.58 × 104 CFU·mL−1. The application verification demonstrated that LFIAs were in excellent agreement with qPCR, confirming the reliability and accuracy of the assay. Additionally, we established a capture-binding kinetic model to calculate antigen capture dynamics, with experimental results aligning closely with theoretical predictions. This model not only demonstrated that oriented labels could enhance sensitivity but also provided a framework for the optimization of magnetic nanoparticle-based probes and their applications in separation and concentration. Overall, our assay offered a rapid, cost-effective, and high-performance platform with potential for broader applications in diagnostic testing.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Penghua Zhao: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing original draft, Writing review & editing, Visualization. Yaping Li:Methodology, Investigation, Writing review & editing. Qing Feng: Methodology, Validation, Data curation Xueping Huo: Methodology, Investigation, Resources, Writing review & editing. JIngying Sun: Investigation, Resources, Writing – review & editing, Supervision. Zifan Lu: Conceptualization, Investigation, Supervision, Funding acquisition. Zhangjun Song:Writing review & editing,Investigation, Resources All authors reviewed the manuscript.
Funding
This work was supported by the Shaanxi Provincial People’s Hospital Science and Technology Development Incubation Fund (2023YJY-22) and the Key Research and Development Program of Shaanxi Province (2024SF-YBXM-081).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zifan Lu, Email: pharmluzifan@163.com.
Zhangjun Song, Email: DoctorSong051107@126.com.
References
- 1.Kabay G, DeCastro J, Altay A, Smith K, Lu HW, Capossela AM, Moarefian M, Aran K, Dincer C (2022) Emerging biosensing technologies for the diagnostics of viral infectious diseases. Adv Mater 34. https://onlinelibrary.wiley.com/doi/10.1002/adma.202201085. https://onlinelibrary.wiley.com/doi/pdf/10.1002/adma.202201085
- 2.Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y (2016) Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron 75:166–180, https://go.exlibris.link/hjbC3nqp
- 3.Park J, Paek S, Kim D, Seo S, Lim G, Kang J, Paek S, Cho I, Paek S (2016) Conformation-sensitive antibody-based point-of-care immunosensor for serum Ca2+ using two-dimensional sequential binding reactions. Biosensors Bioelectronics 85:611–617, https://linkinghub.elsevier.com/retrieve/pii/S0956566316304912https://api.elsevier.com/content/article/PII:S0956566316304912?httpAccept=text/xml
- 4.Sykes DA, Jain P, Charlton SJ (2019) Investigating the influence of tracer kinetics on competition-kinetic association binding assays: identifying the optimal conditions for assessing the kinetics of low-affinity compounds. Mol Pharmacol 96:378–392. https://linkinghub.elsevier.com/retrieve/pii/S0026895X24009404https://syndication.highwire.org/content/doi/10.1124/mol.119.116764
- 5.Qi Liu A, X C A J, Hangming Dong C YLAB (2025) Mechanism of antibody-antigen reaction. Biosensors and Bioelectronics 271:1–9
- 6.Seibert E, Tracy TS (2021) In: Nagar S, Argikar UA, Tweedie D (eds) vol 2342, Springer US
- 7.Drake AW, Tang ML, Papalia GA, Landes G, Haak-Frendscho M, Klakamp SL (2012) Biacore surface matrix effects on the binding kinetics and affinity of an antigen/antibody complex. Anal Biochem 429:58–69. https://linkinghub.elsevier.com/retrieve/pii/S0003269712003375https://api.elsevier.com/content/article/PII:S0003269712003375?httpAccept=text/xml
- 8.FengY, Lee J, Yang L, Hilton MB, Morris K, Seaman S, Edupuganti VVSR, Hsu K, Dower C, Yu G, So D, Bajgain P, Zhu Z, Dimitrov DS, Patel NL, Robinson CM, Difilippantonio S, Dyba M, Corbel A, Basuli F, Swenson RE, Kalen JD, Suthe SR, Hussain M, Italia JS, Souders CA, Gao L, Schnermann MJ, St Croix B (2023) Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability. Cell reports (Cambridge) 42:113503. https://go.exlibris.link/pWsSpBLB
- 9.Jianghua Jia A, B. L. A. C. & Chenxing Jiang A, J. W. A. J (2022) Quantum dots assembly enhanced and dual-antigen sandwich structured lateral flow immunoassay of SARS-CoV-2 antibody with simultaneously high sensitivity and specificity. Biosensors and Bioelectronics
- 10.Chen X, Huang X, Kanwal S, Wang J, Wen J, Zhang D (2024)A portable fluorescent lateral flow immunoassay platform for rapid detection of FluA. Biosensors-Basel 14. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=38920567&query_hl=1
- 11.Bin Zuo HSWL (2021)Magnetic mesoporous nanomaterials with AIE properties for selective detection and removal of CN- from water under magnetic conditions. Analyst
- 12.Zhang Y, Liu X, Wang L, Yang H, Zhang X, Zhu C, Wang W, Yan L, Li B (2020) Improvement in detection limit for lateral flow assay of biomacromolecules by test-zone pre-enrichment. Sci Rep 10:9604, https://go.exlibris.link/jcyrZhQY
- 13.Deng Y, Jiang H, Li X, Lv X (2021) Recent advances in sensitivity enhancement for lateral flow assay. Mikrochim. Acta 188. https://link.springer.com/10.1007/s00604-021-05037-zhttps://link.springer.com/content/pdf/10.1007/s00604-021-05037-z.pdf
- 14.Wang Y, Feng Q, Yan H, Sun R, Cao Y, Wu H, Xi J, Xuan C, Xia J, Sun B, Wang L (2024) Trifunctional nanocomposites with colorimetric magnetic catalytic activities labels in sandwich immunochromatographic detection of Escherichia coli o157:h7. Anal Chem 96:1232–1240. https://pubs.acs.org/doi/10.1021/acs.analchem.3c04476https://pubs.acs.org/doi/pdf/10.1021/acs.analchem.3c04476
- 15.Rezaei B, Yari P, Sanders SM, Wang H, Chugh VK, Liang S, Mostufa S, Xu K, Wang JP, Gómez Pastora J, Wu K (2024) Magnetic nanoparticles: a review on synthesis, characterization, functionalization, and biomedical applications. Small 20. https://onlinelibrary.wiley.com/doi/10.1002/smll.202304848. https://onlinelibrary.wiley.com/doi/pdf/10.1002/smll.202304848
- 16.Omidfar K, Riahi F, Kashanian S (2023) Lateral flow assay: a summary of recent progress for improving assay performance. Biosensors 13:837. https://www.mdpi.com/2079-6374/13/9/837. https://www.mdpi.com/2079-6374/13/9/837/pdf
- 17.Moyano DF, Liu Y, Peer D, Rotello VM (2016) Modulation of immune response using engineered nanoparticle surfaces. Small (Weinheim an der Bergstrasse, Germany) 12:76–82, https://go.exlibris.link/1LqWs5GL
- 18.Mourdikoudis S, Kostopoulou A, LaGrow AP (2021) Magnetic nanoparticle composites: synergistic effects and applications. Adv Sci 8. https://onlinelibrary.wiley.com/doi/10.1002/advs.202004951. https://onlinelibrary.wiley.com/doi/pdf/10.1002/advs.202004951
- 19.Tee GT, Gok XY, Yong WF (2022) Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: a review. Environ. Res 212. 113248. https://linkinghub.elsevier.com/retrieve/pii/S0013935122005758https://api.elsevier.com/content/article/PII:S0013935122005758?httpAccept=text/xml
- 20.Akhtar N, Mohammed HA, Yusuf M, Al-Subaiyel A, Sulaiman GM, Khan RA (2022) SPIONs conjugate supported anticancer drug doxorubicin’s delivery: current status, challenges, and prospects. Nanomaterials 12, 3686. https://www.mdpi.com/2079-4991/12/20/3686https://www.mdpi.com/2079-4991/12/20/3686/pdf
- 21.Mannu R, Karthikeyan V, Velu N, Arumugam C, Roy VAL, Gopalan A, Saianand G, Sonar P, Lee K, Kim W, Lee D, Kannan V (2021) Polyethylene glycol coated magnetic nanoparticles: hybrid nanofluid formulation, properties and drug delivery prospects. Nanomaterials 11:440, https://www.mdpi.com/2079-4991/11/2/440https://www.mdpi.com/2079-4991/11/2/440/pdf
- 22.Gerulová K, Kucmanová A, Sanny Z, Garaiová Z, Seiler E, Čaplovičová M, Čaplovič Ľ, Palcut M (2022) Fe3O4-PEI nanocomposites for magnetic harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials 12:1786, https://www.mdpi.com/2079-4991/12/11/1786https://www.mdpi.com/2079-4991/12/11/1786/pdf
- 23.Assa F, Jafarizadeh-Malmiri H, Ajamein H, Vaghari H, Anarjan N, Ahmadi O, Berenjian A (2017) Chitosan magnetic nanoparticles for drug delivery systems. Crit Rev Biotechnol 37:492–509. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=27248312&query_hl=1
- 24.Wang W, Mattoussi H (2020) Engineering the bio–nano interface using a multifunctional coordinating polymer coating. Acc Chem Res 53:1124–1138, https://pubs.acs.org/doi/10.1021/acs.accounts.9b00641https://pubs.acs.org/doi/pdf/10.1021/acs.accounts.9b00641
- 25.Araújo EV, Carneiro SV, Neto DMA, Freire TM, Costa VM, Freire RM, Fechine LMUD, Clemente CS, Denardin JC, Dos Santos JCS, Santos-Oliveira R, Rocha JS, Fechine PBA (2024) Advances in surface design and biomedical applications of magnetic nanoparticles. Adv Colloid Interface Sci 328:103166. https://linkinghub.elsevier.com/retrieve/pii/S0001868624000897https://api.elsevier.com/content/article/PII:S0001868624000897?httpAccept=text/xml
- 26.Quan K, Mao Z, Lu Y, Qin Y, Wang S, Yu C, Bi X, Tang H, Ren X, Chen D, Cheng Y, Wang Y, Zheng Y, Xia D (2024) Composited silk fibroins ensured adhesion stability and magnetic controllability of Fe3O4 -nanoparticle coating on implant for biofilm treatment. Mater Horiz 11:3157–3165. https://xlink.rsc.org/?DOI=D4MH00097Hhttp://pubs.rsc.org/en/content/articlepdf/2024/MH/D4MH00097H
- 27.Wang W, Jing Y, He S, Wang J, Zhai J (2014) Surface modification and bioconjugation of FeCo magnetic nanoparticles with proteins. Colloids and Surfaces B: Biointerfaces 117:449–456. https://linkinghub.elsevier.com/retrieve/pii/S0927776513007480https://api.elsevier.com/content/article/PII:S0927776513007480?httpAccept=text/xml
- 28.Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knüchel R, Kiessling F, Lammers T (2019) Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev 138:302–325. https://linkinghub.elsevier.com/retrieve/pii/S0169409X19300055https://api.elsevier.com/content/article/PII:S0169409X19300055?httpAccept=text/xml
- 29.Ye M, Zhu Y, Lu Y, Gan L, Zhang Y, Zhao Y (2021) Magnetic nanomaterials with unique nanozymes-like characteristics for colorimetric sensors: a review. Talanta 230:122299, https://linkinghub.elsevier.com/retrieve/pii/S0039914021002204https://api.elsevier.com/content/article/PII:S0039914021002204?httpAccept=text/xml
- 30.Ali N, Hassan Riead MM, Bilal M, Yang Y, Khan A, Ali F, Karim S, Zhou C, Wenjie Y, Sher F, Iqbal HMN (2021) Adsorptive remediation of environmental pollutants using magnetic hybrid materials as platform adsorbents. Chemosphere 284:131279. https://linkinghub.elsevier.com/retrieve/pii/S0045653521017513https://api.elsevier.com/content/article/PII:S0045653521017513?httpAccept=text/xml
- 31.Brunauer A, Verboket RD, Kainz DM, von Stetten F, Früh SM (2021) Rapid detection of pathogens in wound exudate via nucleic acid lateral flow immunoassay. Biosensors 11:74, https://www.mdpi.com/2079-6374/11/3/74https://www.mdpi.com/2079-6374/11/3/74/pdf
- 32.Cao Y, Wu J, Zheng X, Lu Y, Piper JA, Lu Y, Packer NH (2022) Assessing the activity of antibodies conjugated to upconversion nanoparticles for immunolabeling. Anal Chim Acta 1209:339863. https://linkinghub.elsevier.com/retrieve/pii/S0003267022004342https://api.elsevier.com/content/article/PII:S0003267022004342?httpAccept=text/xml
- 33.Matsushita T, Toda N, Koyama T, Hatano K, Matsuoka K (2022) Dendritic maleimide-thiol adducts carrying pendant glycosides as high-affinity ligands. Bioorganic Chem 128:106061. https://linkinghub.elsevier.com/retrieve/pii/S0045206822004679https://api.elsevier.com/content/article/PII:S0045206822004679?httpAccept=text/xml
- 34.Jinchuan Yang KWHX, Cui AD (2019) Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: a review. Talanta 054
- 35.Tang J, Yang H, Gao X, Zeng X, Wang F (2021) Directional immobilization of antibody onto magnetic nanoparticles by Fc-binding protein-assisted photo-conjugation for high sensitivity detection of antigen. Anal Chim Acta 1184:339054. https://linkinghub.elsevier.com/retrieve/pii/S0003267021008801https://api.elsevier.com/content/article/PII:S0003267021008801?httpAccept=text/xml
- 36.Rigi G, Ghaedmohammadi S, Ahmadian G (2019) A comprehensive review on staphylococcal protein a (SPA): its production and applications. Biotechnol Appl Biochem 66:454–464. https://iubmb.onlinelibrary.wiley.com/doi/10.1002/bab.1742. https://iubmb.onlinelibrary.wiley.com/doi/pdf/10.1002/bab.1742
- 37.Zhao P, Huang X, Tao H, Li Y, Sun L, Hu J (2022) Antibody orientational labeling via staphylococcus a protein to improve the sensitivity of gold immunochromatography assays. Anal Biochem 641:114403. https://linkinghub.elsevier.com/retrieve/pii/S0003269721003043https://api.elsevier.com/content/article/PII:S0003269721003043?httpAccept=text/xml
- 38.Morán D, Gutiérrez G, Mendoza R, Rayner M, Blanco-López C, Matos M (2023) Synthesis of controlled-size starch nanoparticles and superparamagnetic starch nanocomposites by microemulsion method. Carbohydr Polym 299:120223. https://linkinghub.elsevier.com/retrieve/pii/S0144861722011286https://api.elsevier.com/content/article/PII:S0144861722011286?httpAccept=text/xml
- 39.Ujwal Patil MCBV, Willson ARC (2020) Continuous Fc detection for protein A capture process control. Biosensors Bioelectronics 1–11
- 40.Bobrovnik SA (2000) ELISA-based method for determining the affinity of bivalent antibodies of two specificities in a mixture. Ukrains'kyi biokhimichnyi zhurnal (1999 ) 72:133. https://go.exlibris.link/QL592pWR
- 41.Groël S, Menzen T, Winter G (2023) Possibilities and limitations of α-relaxation data of amorphous freeze-dried cakes to predict long term IgG1 antibody stability. Int J Pharm 646:123445. https://linkinghub.elsevier.com/retrieve/pii/S0378517323008669https://api.elsevier.com/content/article/PII:S0378517323008669?httpAccept=text/xml
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.