Abstract
Background and Purpose
Efgartigimod has demonstrated efficacy in generalized myasthenia gravis (gMG) in both clinical trials and real-world studies. However, factors influencing early response have been less reported. This study aimed to evaluate the efficacy of efgartigimod in a multicenter gMG cohort and to identify the clinical factors associated with early therapeutic response.
Methods
This multicenter, real-world, retrospective, observational study included 115 gMG patients administered efgartigimod across four myasthenia gravis (MG) centers. Responders were defined as patients with a ≥ 2-point Myasthenia Gravis Activities of Daily Living (MG-ADL) or ≥ 3-point Quantitative Myasthenia Gravis Score (QMG) score reduction, while early responders were those achieving score reductions after the first infusion. Subgroup analyses were conducted based on immunosuppressant (IST) use. Logistic regression analysis was performed to identify factors associated with response to first efgartigimod infusion according to MG-ADL or QMG scores reduction. Variables were compared between responders and non-responders to identify early response factors.
Results
After the first infusion, 72.5% of patients achieved improvement in MG-ADL and 60.5% in QMG, with these rates increasing to 93.3% and 87.5% respectively by the fourth infusion. Efgartigimod demonstrated the most significant improvement in bulbar, limb, and ocular symptoms; however, there was no statistically significant improvement in respiratory symptoms occurred during the initial 4-week treatment period. Multivariate logistic analysis showed that short disease duration and high MG-ADL bulbar score at baseline indicated early response. High QMG bulbar score at baseline also indicated early response. Efficacy was independent of IST use. No patients discontinued treatment due to severe adverse events; minor side effects were not recorded.
Conclusions
Efgartigimod demonstrated robust efficacy in gMG patients. Early response was linked to shorter disease duration and severe bulbar symptoms, which promotes the identification of patients who are likely to benefit quickly from efgartigimod.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-025-13367-8.
Keywords: Generalized myasthenia gravis, Efgartigimod, Treatment response, Predictors, Retrospective study
Introduction
Myasthenia gravis (MG) is a neuromuscular disorder caused by pathogenic immunoglobulin G (IgG) autoantibodies against the nicotinic acetylcholine receptor (AChR) and muscle-specific receptor tyrosine kinase (MuSK), both of which functionally interfere with normal synaptic transmission [1, 2]. Recent advances in immunotherapy have been made to improve the management of MG. The neonatal Fc receptor (FcRn) is a major histocompatibility complex class I–like molecule that recycles IgG, extending its half-life by approximately four-fold compared with that of other immunoglobulins not recycled by FcRn [3].
Efgartigimod, a human IgG1 antibody Fc fragment engineered for increased affinity to FcRn, acts as a natural ligand of FcRn [4]. By targeting FcRn, efgartigimod provides a novel therapeutic approach for various autoimmune disorders [1]. The efficacy of efgartigimod in gMG patients has been demonstrated in both randomized clinical trials and real-world studies [1, 5, 6]. In a phase 3 clinical trial (ADAPT) [5], 68% of gMG patients were MG-ADL responders during the first treatment cycle, with 57% achieving early response, defined as a ≥ 2-point improvement reduction in MG-ADL score by week 2. Long-term administration sustains therapeutic efficacy. Similar findings have been reported in observational studies [1, 6]. However, partial drug reimbursement and regional disparities in medical resources force many patients to travel repeatedly between cities for intravenous efgartigimod, placing a heavy burden on them and rendering long-term therapy impractical in China. Consequently, faster-acting, more affordable treatment options are urgently needed to lessen the financial burden on these patients. Factors associated with early response have not been identified in previous studies [1]. Therefore, this study analyzed the efficacy of efgartigimod, and factors associated with early response in a multicenter gMG cohort, aiming to provide valuable insights for predicting gMG patients likely to achieve a rapid response to efgartigimod treatment.
Methods
Study design
This study was a retrospective observational study. Patients with AChR- antibody-positive gMG (AChR-gMG) were administered an initial cycle of efgartigimod between September 2023 and June 2024 in Xuanwu Hospital, First Medical Center of PLA General Hospital, Qilu Hospital and People's Hospital of Shijiazhuang. Efgartigimod (10 mg/kg) was administered as four infusions (one infusion per week). Efficacy was assessed with MG-ADL and QMG scores weekly during consecutive infusions of efgartigimod and 1 week after the final infusion. Thus, the assessment time points included baseline, week 1, week 2, week 3 and week 4. Since the first cycle of efgartigimod treatment was effective in most patients, the onset of efficacy one week after the first infusion was used as the primary endpoint for exploring associated factors. MG-ADL and QMG responses were defined as ≥ 2-point and ≥ 3-point reductions in their respective scores by week 4 of the treatment cycle, respectively. Early responses were evaluated at one week post-first infusion to assess rapid treatment effects Minimal symptom expression (MSE) was defined by an MG-ADL score of 0 or 1.
MG-ADL and QMG subscores were also recorded. MG-ADL subscores included bulbar (speech/voice, swallowing, chewing), respiratory (breathing), limb (ability to brush teeth or comb hair and arise from a chair), diplopia and ptosis subdomains. QMG subscores included diplopia, ptosis, eye closure, respiratory (forced vital capacity), axial muscle (head-lift), limb (right and left arms outstretched, hand grip strength, right and left legs outstretched), and bulbar (swallowing and speech) Clinical data, including age at onset, age at baseline, sex, disease duration, presence of thymoma, Myasthenia Gravis Foundation of America (MGFA) classification at baseline, and IST used at baseline and during efgartigimod infusion, were also collected.
The patients receiving efgartigimod in this research were mainly divided into three categories:
Newly diagnosed gMG without immunotherapy, rapid disease progression, presenting with impending myasthenic crisis or documented "red flags" [7];
MG-ADL scores ≤ 1 but QMG scores ≥ 6 (with non-ocular symptoms ≥ 50%), with contraindications to or refusal of steroids/immunosuppressants due to comorbidities;
Meeting the definition of a highly active MG according to the 2023 German myasthenia management guidelines [8]: (1) Moderate/high MGFA status (≥ MGFA IIb) and/or at least two recurrent severe exacerbations/myasthenic crises with the need for rescue interventions (e.g. intravenous immunoglobulin [IVIG], plasma exchange, immunoadsorption) within 1 year after diagnosis despite adequate standard immunotherapy and symptomatic therapy, or (2) Persistent symptoms relevant to daily living (≥ MGFA IIa) and severe exacerbation/myasthenic crisis within the last calendar year despite adequate diseasemodifying and symptomatic therapy; or (3) Persistent symptoms relevant to daily living, even of the mild/moderate course type (≥ MGFA IIa), for more than 2 years despite adequate diseasemodifying and symptomatic therapy.
Statistical analysis
The normality of the data was assessed using the Shapiro–Wilk test. Continuous variables were displayed as median (interquartile range [IQR]), and categorical variables were expressed as number (percentage). Continuous variables were compared by the Mann–Whitney U test and categorical variables by the chi-square test. A logistic regression model was used to assess response to first efgartigimod infusion according to MG-ADL or QMG score. Variables with P < 0.05 in univariate analysis, along with clinically relevant variables, were included in multivariate analysis. Using Firth's method to handle the complete separation problem. Statistical significance was defined as two-tailed P values of < 0.05. For repeated measures, statistical analysis utilized a linear mixed model with Bonferroni correction. All analyses were performed with the R software version 4.2.1 (R foundation for Statistical Computing, Vienna, Austria).
Results
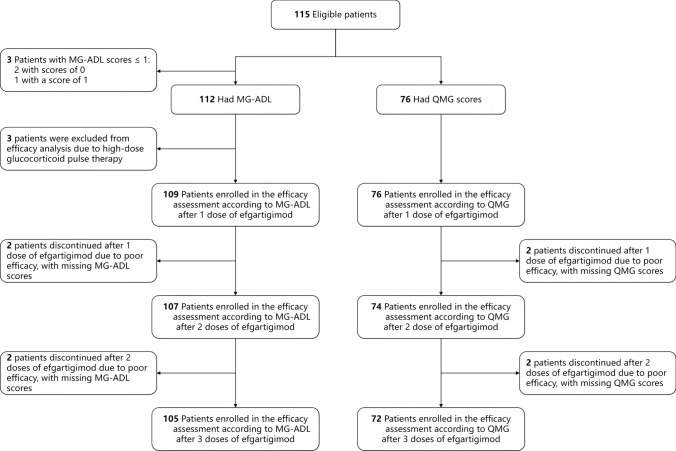
The workflow designed for the study is shown in Fig. 1. A total of 115 gMG patients started efgartigimod treatment between September 2023 and June 2024 in four centers. Among the 115 enrolled patients, 3 with MG-ADL scores of 0 or 1 at baseline were excluded from the efficacy analysis. Additionally, 3 patients who received high-dose glucocorticoid pulse therapy were also excluded because it could interfere with the evaluation of efgartigimod's efficacy. Consequently, 109 patients were evaluable for the MG-ADL response after the first efgartigimod infusion. Two patients discontinued treatment due to suboptimal response and lacked post-dose MG-ADL scores, leaving 107 patients for second-dose response assessment. Following the second infusion, two additional patients withdrew because of the inadequate response with missing MG-ADL data, resulting in 105 patients evaluable for third-dose response. A similar situation was observed in QMG assessments: 76 were initially evaluable for QMG response post-first infusion (including the 3 MG-ADL scores ≤ 1). Two discontinuations due to suboptimal response resulted in 74 assessable cases after the second dose, which further decreased to 72 following third-dose discontinuations for the same reason. Demographic and clinical characteristics are shown in Table 1. At baseline, the median patient age was 61 years, and 61 (53.0%) of the patients were male. The median disease duration was 16 months. Thymoma was present in 28 (24.3%) of patients. MGFA classification showed that around 96 (83.5%) cases were class II ~ III before infusion. Thirty-nine patients (33.9%) were IST-naive prior to receiving efgartigimod. During successive efgartigimod infusions, steroids and/or tacrolimus were added in 30 patients (26.1%), 13 of whom (43.3%) started this co-therapy during the first infusion. The median MG-ADL score at baseline was 7 (IQR 5 ~ 9). Seventy-six patients in Xuanwu Hospital and the First Medical Center of the Chinese People's Liberation Army General Hospital were assessed for QMG scores at baseline, and the median score was 14 (IQR 10 ~ 17).
Fig. 1.
Study flowchart
Table 1.
Patient Characteristics, total n = 115
| Characteristic | Median (IQR) or number (%) |
|---|---|
| Age at baseline, years | 61 [52,69] |
| Sex | |
| Female | 54 (47.0) |
| Male | 61 (53.0) |
| Age at onset, years | 60 [49,68] |
| Age at onset | |
| Early onset (≤ 50 years) | 38 (33.0) |
| Late onset (50 years) | 77 (67.0) |
| Disease duration, months | 16 [3,57] |
| Thymoma | 28 (24.3) |
| Thymectomy | 26 (22.6) |
| MGFA classification at baseline | |
| IIA | 23 (20.0) |
| IIB | 45 (39.1) |
| IIIA | 8 (7.0) |
| IIIB | 20 (17.4) |
| IVA | 3 (2.6) |
| IVB | 11 (9.6) |
| V | 5 (4.3) |
| MG-ADL score at baseline | 7 [5, 9] |
| QMG score at baseline | 14 [10, 17] |
| Treatment at baseline | |
| Without IST | 39 (33.9) |
| Steroid only | 31 (27.0) |
| Non-steroid IST only | 24 (20.9) |
| Combination therapy | 21 (18.3) |
| Baseline steroid dosage | 0 [0,20] |
| IST added during EFG infusion | 30 (26.1) |
| Steroid only | 16 (13.9) |
| Non-steroid IST only | 12 (10.4) |
| Combination therapy* | 1 (0.9) |
| With Telitacicept | 1 (0.9) |
| Oral steroids dosage during EFG infusion (patients numbers/dose in mg) | 17/ 0 [0,0] |
| At 1st infusion | 3/ 20 [20, 20] |
| At 2nd infusion | 4/ 20 [13, 24] |
| At 3rd infusion | 3/ 20 [20, 20] |
| At 4th infusion | 4/ 15 [6, 20] |
| Intravenous steroids maximum daily dose (patients numbers/dose in mg)# | 3/ 500 [325,750] |
| Timing of adding IST including steroids | |
| At 1st infusion | 13 (11.3) |
| At 2nd infusion | 7 (6.1) |
| At 3rd infusion | 4 (3.5) |
| At 4th infusion | 6 (5.2) |
*Combination therapy: Steroid and non-steroid IST therapy
#Intravenous steroids maximum dosage: Three patients received intravenous steroids, all during the first efgartigimod infusion
Figure 2 shows that among the 109 patients, 72.5% achieved a ≥ 2-point improvement in MG-ADL score one week after the first infusion, the rate increasing to 86.0% following the second infusion among the 107 patients and then increasing to 93.3% following the fourth infusion (Fig. 2A). Among the 76 patients assessed for QMG scores, 60.5% achieved a ≥ 3-point improvement after the first infusion, a rate that increased to 77.0% following the second infusion and then increased to 87.5% following the fourth infusion (Fig. 2B). MSE was detected in 11.9% of patients after the first infusion and in 44.8% after the fourth infusion (Fig. 2C). Figure 2D illustrates the changes in MG-ADL and QMG scores from baseline over time after infusion of efgartigimod. A significant improvement in scores was observed after each infusion (P < 0.0001).
Fig. 2.
Efficacy of efgartigimod in gMG patients according to changes in MG-ADL and QMG scores, n = 108. Panels A and B show the proportions of effective and ineffective responses following the infusion of efgartigimod. The efficacy criteria were: MG-ADL improvement ≥ 2 points for A and QMG improvement ≥ 3 points for B. Panel C shows the proportions of achieving MSE (MG-ADL ≤ 1) following the infusion of efgartigimod. Panel D shows the changes in MG-ADL and QMG scores during cycle 1. ****P < 0.0001
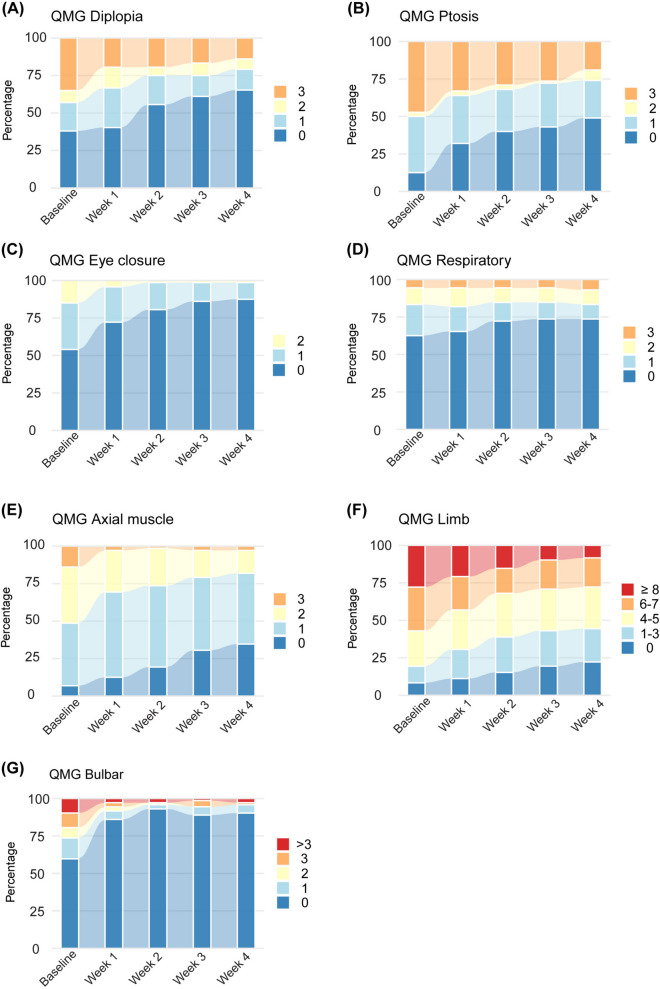
Figure 3 shows changes in MG-ADL subscores across various subdomains. Significant improvements from baseline were detected among patients in all MG-ADL subdomains after the first infusion (Supplementary Table 1 and 2, P < 0.001). Figure 4 shows significant changes occurred in all QMG subscores across various QMG subdomains except respiratory symptoms (Supplementary Table 3 and 4). Efgartigimod had the most pronounced effects on bulbar, axial muscle, eye closure and ptosis after the first infusion (Supplementary Table 3 and 4, P < 0.001), and secondary effects on limb and diplopia (Supplementary Table 3 and 4, P < 0.05). For respiratory symptoms, no significant improvement was observed after the whole treatment cycle.
Fig. 3.
Changes in MG-ADL subscores during the treatment for various MG-ADL subdomains. MG-ADL subscores included bulbar (speech/voice, swallowing, chewing), respiratory (breathing), limb (ability to brush teeth or comb hair and arise from a chair), diplopia and ptosis subdomains. The bulbar subscore ranges from 0 to 9 points. The respiratory subscore ranges from 0 to 3 points. The limb subscore ranges from 0 to 6 points. The diplopia subscore ranges from 0 to 3 points. The ptosis subscores range from 0 to 3 points
Fig. 4.
Changes in QMG subscores during the treatment for various QMG subdomains. QMG subscores included diplopia, ptosis, eye closure, respiratory (vital capacity), axial muscle (head-lifted), limb (right and left arms outstretched, hand grip strength, right and left legs outstretched), and bulbar (swallowing and speech). The diplopia subscore ranges from 0 to 3. The ptosis subscore ranges from 0 to 3. The eye closure subscore ranges from 0 to 3. The respiratory subscore ranges from 0 to 3. The axial muscle subscore ranges from 0 to 3. The limb subscore ranges from 0 to 18. The bulbar subscore ranges from 0 to 6
Patient characteristics, stratified by response to the first efgartigimod infusion, are shown in Supplementary Tables 5 and 6. A total of 79 of 109 (72.5%) patients were early MG-ADL responders and had shorter disease duration, had undergone thymectomy, more severe MG-ADL scores and more severe bulbar symptoms (P = 0.032, 0.036, 0.005 and 0.002 respectively) at baseline compared with non-responders. Totally 46 (60.5%) patients were QMG early responders and showed more severe facial and bulbar symptoms at baseline (P = 0.029 and 0.021 respectively).
Multivariate logistic analysis revealed that a short disease duration (OR = 0.989, 95% CI 0.978–0.999; P = 0.031), a high baseline MG-ADL bulbar score (OR = 1.335, 95% CI 1.040–1.762; P = 0.022) as well as a high baseline QMG bulbar score (OR = 1.698, 95% CI 1.044–2.761; P = 0.033) indicated an early response to efgartigimod, as evidenced by the MG-ADLand QMG score in Tables 2 and 3, respectively. The early efficacy of efgartigimod was not associated with age, sex, thymoma, MGFA classification at baseline, or IST use before and during infusions.
Table 2.
Multivariable logistic regression for factors of the first efgartigimod infusion responders according to MG-ADL scores ≥ 2
| OR | 95% CI | p | |
|---|---|---|---|
| Disease duration | 0.989 | 0.978–0.999 | 0.031 |
| Thymectomy | 0.455 | 0.159–1.305 | 0.141 |
| IST used at baseline | 0.819 | 0.263–2.389 | 0.718 |
| IST added during the first EFG infusion | 6.873 | 0.752–920.398* | 0.099 |
| MG-ADL bulbar scores at baseline | 1.335 | 1.040–1.762 | 0.022 |
Abbreviations: OR, odds ratio; CI, confidence interval.*The wide confidence interval reflects the uncertainty in estimation due to complete separation (zero event occurrence in the Non-responder group of IST added during the first EFG infusion)
Table 3.
Multivariable logistic regression for factors of the first efgartigimod infusion responders according to QMG scores ≥ 3
| OR | 95% CI | p | |
|---|---|---|---|
| Disease duration | 0.991 | 0.980–1.002 | 0.111 |
| Thymectomy | 0.944 | 0.243–3.668 | 0.933 |
| IST used at baseline | 1.505 | 0.415–5.457 | 0.534 |
| Baseline steroid dosage | 1.038 | 0.982–1.096 | 0.189 |
| QMG bulbar scores at baseline | 1.698 | 1.044–2.761 | 0.033 |
Abbreviations: OR, odds ratio; CI, confidence interval
To investigate whether IST use during efgartigimod treatment affected efficacy or not, responders were stratified by IST status, MG-ADL and QMG score changes were analyzed. Thirty patients were newly added IST during infusion. Interaction analysis indicated no significant difference in MG-ADL or QMG score changes between responders with and without IST newly addition during efgartigimod infusions (Supplementary Fig. 1A and 1B). To further analyze the impact of IST on the efficacy of efgartigimod, patients with newly added IST during treatment were excluded. The remaining patients were grouped based on baseline IST-naive status, and the trends of MG-ADL and QMG score changes were compared between the groups. Interaction analysis indicated no significant difference in the extent of score improvement between the baseline naive and non-naive responders (Supplementary Fig. 1C and 1D). No patients discontinued treatment due to severe adverse events; minor side effects were not recorded.
Discussion
This real-world retrospective observational study showed a significant, sustained drop in both MG-ADL and QMG scores from baseline following each efgartigimod infusion. MG-ADL and QMG responses were achieved in 72.5% and 60.5% of patients, respectively, after the first infusion of efgartigimod. Patients with shorter disease duration and more severe bulbar symptoms were more likely to exhibit an early response.
These results corroborate earlier reports of efgartigimod's favorable efficacy and safety profile [1, 5–10]. In particular, Chinese real-world studies have proved MG-ADL response rates of 60–70% after a single dose [10, 11] aligning closely with the present findings. However, early responses have not been reported by the ADAPT or real-world studies. This study was the first to evaluate the efficacy of efgartigimod based on early response. Additionally, the three patients with MG-ADL scores of ≤ 1 at baseline experienced a ≥ 3-point improvement in QMG score after their first infusion. This suggests a potential for widespread use of efgartigimod in clinical practice and indicates good efficacy not only for patients with myasthenic crisis or refractory disease, as evidenced by studies [12, 13], but also for those with mild to moderate diseases, as shown in studies [1, 6, 14].
Another key finding is the early response (by week 1) to efgartigimod observed in patients with shorter disease duration and more severe bulbar symptoms. Multivariable logistic regression analysis showed that shorter disease duration, high MG-ADL bulbar score at baseline, and high QMG bulbar score at baseline were associated with early response. In AChR-antibody–positive MG patients, pathogenic autoantibodies primarily induce postsynaptic membrane pathology or disrupt AChR clustering through three distinct molecular mechanisms: (1) direct blockade of cholinergic binding or interference with key protein interactions essential for neuromuscular junction formation (NMJ) and maintenance, (2) antigenic modulation, and (3) complement activation-mediated structural damage [15]. This destruction of the NMJ may result in irreversible damage, which could subsequently cause more severe weakness over time. Several studies showed favorable efficacy benefits of early treatment for MG with short disease duration [16–19]. Our results align with the hypothesis that early intervention in gMG may yield greater therapeutic benefits, possibly due to less extensive neuromuscular damage or preserved functional reserves [16]. When the definition of response was more restrictive, i.e., a reduction of at least 3 and 5 points in MG-ADL and QMG scores, respectively, 49.5% of patients achieved MG-ADL response and 40.8% achieved QMG response after first infusion of efgartigimod (Supplementary Table 7 and 8). Both analyses based on baseline differences and multivariable logistic regression analyses showed that high MG-ADL bulbar and QMG bulbar scores were associated with early response, which demonstrated good concordance of the study results (Supplementary Table 9 and 10). Analysis of the distribution of muscle weakness over time indicates that, despite a high degree of heterogeneity in the distribution, bulbar symptoms tend to disappear more frequently [20]. Several studies have shown the sensitivity of bulbar symptoms to treatment response [21–24] and the reason is not clear. The bulbar muscles, which are responsible for functions such as swallowing and articulation, may exhibit more noticeable and rapid functional improvement. Therefore, the observed faster treatment response in bulbar symptoms requires further investigation through basic research or larger prospective cohort studies for comprehensive interpretation.
Another multicenter real-world study from China [25], utilizing multivariate regression analysis, identified gender and baseline MG-ADL scores as significant predictors for distinguishing rapid and sustained responders to efgartigimod treatment. It should be noted that our study differs from these previous findings in both the definition of MG-ADL responders and the observation period, as we focused exclusively on early response during the initial treatment cycle. Future studies incorporating extended follow-up periods would enable more comprehensive evaluation of efgartigimod's therapeutic effects.
Nearly all MG-ADL and QMG subdomains demonstrated significant improvement after efgartigimod infusion (starting from Week 1) compared with those before treatment, and such improvement lasted for at least 4 weeks. But according to the assessment by the QMG scale, no statistically significant improvement in respiratory symptoms was observed during the treatment period (from baseline to week 4), and there were no significant changes between the weeks either. In ADAPT post-hoc analysis, differences between patients administered efgartigimod versus placebo were also smaller in the respiratory subdomain for MG-ADL and QMG than other subdomains [26]. However, another Chinese real-world study demonstrated that patients treated with efgartigimod showed significant improvement across all muscle groups, particularly in respiratory and bulbar muscles [13]. The present study demonstrated that subjective respiratory function (MG-ADL) significantly improved, whereas objective respiratory function (QMG) did not exhibit any notable improvement. In the authors' opinion, the limited cohort size, coupled with incomplete QMG data, potentially undermined the power of the response proportion analysis. The small sample size within the QMG respiratory subdomains reduced sensitivity in measuring changes in vital capacity, may have resulted in a lack of significant numerical improvement. The QMG respiratory score is based on specific objective test results, and changes in the score may require more significant alterations in physiological function to be achieved.
However, a prospective multicenter real-world study [27] in China enrolled 10 myasthenic crisis patients receiving efgartigimod as rescue therapy, all achieving successful liberation from mechanical ventilation within a mean duration of 10.44 ± 4.30 days. Additionally, another Chinese retrospective study [28] of 25 myasthenic crisis cases revealed that compared to 14 controls receiving conventional therapy (plasmapheresis), the efgartigimod-treated cohort (n = 11) demonstrated: shorter total hospital length of stay, shorter ICU length of stay, and shorter duration of mechanical ventilation. The findings above demonstrate significant therapeutic benefits of efgartigimod for respiratory musculature improvement in myasthenia crisis. Furthermore, additional studies are needed to separately assess the efficacy of efgartigimod for respiratory subscore improvement.
For patients with rapidly progressing, efgartigimod is used in conjunction with corticosteroid and/or IST, which are added based on the patient's clinical status and at the appropriate time. Consequently, by the conclusion of the efgartigimod treatment course, the corticosteroid or IST begin to take effect, leading to gradual stabilization of the condition. This allows for the discontinuation of efgartigimod maintenance therapy. In clinical practice, the efficacy of corticosteroid and/or IST used with efgartigimod may be the result of a combined effect. In this study, subgroup analysis was conducted to address this issue by stratifying patients based on whether immunosuppressive therapy was administered or not at baseline before efgartigimod treatment. The results demonstrated that efgartigimod was effective regardless of IST use at baseline or during the treatment period, suggesting that efgartigimod provides a consistent benefit independent of concomitant immunosuppressive therapy. This finding also supports the utility of efgartigimod as an effective treatment in a wide range of gMG cases, including those who are IST-naive or inadequately controlled by such therapies.
This study had some limitations. Firstly, the sample size was limited. Particularly considering MG-ADL and QMG subscores, caution is needed in data interpretation, as the number of patients with baseline scores > 0 for each symptom was relatively small. Secondly, only 66.1% of patients underwent QMG assessment at baseline, The assessment of early rapid response based on QMG scores was exclusively conducted in patients who actually had QMG measurements, necessitating future validation of this finding in a wider range of patients and clinical contexts. Thirdly, this study's design did not include long-term follow-up observations of patients, and thus did not obtain data on the sustained duration of the therapeutic effects. We hope future studies can provide data on efgartigimod's long-term treatment outcomes. Finally, four patients discontinued the treatment because of poor efficacy, so the actual effective rate may be lower than the available results. Moreover, the subsequent score changes were not collected for four patients due to treatment discontinuation and loss to follow-up, potentially preventing the capture of delayed response states.
This study contributes to the expanding evidence supporting efgartigimod as an effective and well-tolerated therapeutic option for gMG. Efficacy was not associated with the use of IST before and during treatment. These findings suggest that efgartigimod is a promising therapeutic option for gMG patients. Moreover, disease duration and bulbar symptoms may serve as potential clinical markers for identifying patients likely to achieve early efficacy, which may help physicians select individuals who may benefit most from the treatment after the first infusion.
Disclosure
The authors report no relevant disclosures.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Funding
This work was supported by the National Natural Science Foundation of China [62171299], and the Clinical Cohort Study of Myasthenia Gravis. National Key R&D Program of China, Precision Medicine Project [2017YFC0907700].
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary files.
Declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Footnotes
Wenjia Zhu, Yuping Chen, Cong Tian and Jingsi Wang have contributed equally to this work.
Change history
11/2/2025
Article note has been updated.
Contributor Information
Guoyan Qi, Email: zzjwlsys@163.com.
Yuwei Da, Email: dayuwei100@hotmail.com.
References
- 1.Suzuki S, Uzawa A, Nagane Y et al (2024) Therapeutic responses to efgartigimod for generalized myasthenia gravis in Japan. Neurol Clin Pract 14(3):e200-276. 10.1212/cpj.0000000000200276 [Google Scholar]
- 2.Li J, Wu X, Chu T et al (2024) The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: a systematic review and meta-analysis. J Neurol 271(5):2298–2308. 10.1007/s00415-024-12247-x [DOI] [PubMed] [Google Scholar]
- 3.Pyzik M, Kozicky LK, Gandhi AK, Blumberg RS (2023) The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol 23(7):415–432. 10.1038/s41577-022-00821-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulrichts P, Guglietta A, Dreier T et al (2018) Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest 128(10):4372–4386. 10.1172/jci97911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard JF Jr, Bril V, Vu T et al (2021) Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 20(7):526–536. 10.1016/s1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 6.Fuchs L, Shelly S, Vigiser I et al (2024) Real-world experience with efgartigimod in patients with myasthenia gravis. J Neurol 271(6):3462–3470. 10.1007/s00415-024-12293-5 [DOI] [PubMed] [Google Scholar]
- 7.Stetefeld H, Schroeter M (2019) SOP myasthenic crisis. Neurol Res Pract 1:19. 10.1186/s42466-019-0023-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiendl H, Abicht A, Chan A et al (2023) Guideline for the management of myasthenic syndromes. Ther Adv Neurol Disord 16:17562864231213240. 10.1177/17562864231213240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo S, Jiang Q, Zeng W et al (2024) Efgartigimod for generalized myasthenia gravis: a multicenter real-world cohort study in China. Ann Clin Transl Neurol 11(8):2212–2221. 10.1002/acn3.52142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Zhang B, Yin J et al (2024) Prospective cohort study evaluating efficacy and safety of efgartigimod in Chinese generalized myasthenia gravis patients. Front Neurol 15:1407418. 10.3389/fneur.2024.1407418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu G, Zhou H, Wang W et al (2025) Application of efgartigimod in Chinese patients with myasthenia gravis: a single-center real-world prospective study. Ther Adv Neurol Disord 18:17562864241311128. 10.1177/17562864241311127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remijn-Nelissen L, Tannemaat MR, Ruiter AM, Campman YJM, Verschuuren J (2024) Efgartigimod in refractory autoimmune myasthenia gravis. Muscle Nerve 70(3):325–332. 10.1002/mus.28184 [DOI] [PubMed] [Google Scholar]
- 13.Song J, Wang H, Huan X et al (2024) Efgartigimod as a promising add-on therapy for myasthenic crisis: a prospective case series. Front Immunol 15:1418503. 10.3389/fimmu.2024.1418503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frangiamore R, Rinaldi E, Vanoli F et al (2024) Efgartigimod in generalized myasthenia gravis: a real-life experience at a national reference center. Eur J Neurol 31(4):e16189. 10.1111/ene.16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S (2016) Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol 12(5):259–268. 10.1038/nrneurol.2016.44 [DOI] [PubMed] [Google Scholar]
- 16.Uzawa A, Suzuki S, Kuwabara S et al (2023) Effectiveness of early cycles of fast-acting treatment in generalised myasthenia gravis. J Neurol Neurosurg Psychiatry 94(6):467–473. 10.1136/jnnp-2022-330519 [DOI] [PubMed] [Google Scholar]
- 17.Zhen L, Zhao X, Li W et al (2023) Effectiveness of early glucocorticoids in myasthenia gravis: a retrospective cohort study. Front Neurol 14:1259484. 10.3389/fneur.2023.1259484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uzawa A, Suzuki S, Kuwabara S et al (2023) Impact of early treatment with intravenous high-dose methylprednisolone for ocular myasthenia gravis. Neurotherapeutics 20(2):518–523. 10.1007/s13311-022-01335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piehl F, Eriksson-Dufva A, Budzianowska A et al (2022) Efficacy and safety of Rituximab for new-onset generalized Myasthenia Gravis: the RINOMAX randomized clinical trial. JAMA Neurol 79(11):1105–1112. 10.1001/jamaneurol.2022.2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Meel RHP, Tannemaat MR, Verschuuren J (2019) Heterogeneity and shifts in distribution of muscle weakness in myasthenia gravis. Neuromuscul Disord 29(9):664–670. 10.1016/j.nmd.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 21.Fan Z, Li Z, Shen F et al (2020) Favorable effects of Tacrolimus monotherapy on Myasthenia Gravis patients. Front Neurol 11:594152. 10.3389/fneur.2020.594152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakata N, Saito T, Tanaka S, Hirano T, Oka K (2003) Tacrolimus hydrate (FK506): therapeutic effects and selection of responders in the treatment of myasthenia gravis. Clin Neurol Neurosurg 106(1):5–8. 10.1016/s0303-8467(03)00046-5 [DOI] [PubMed] [Google Scholar]
- 23.Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G (1987) Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med 316(12):719–724. 10.1056/nejm198703193161205 [DOI] [PubMed] [Google Scholar]
- 24.Tindall RS, Phillips JT, Rollins JA, Wells L, Hall K (1993) A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci 681:539–551. 10.1111/j.1749-6632.1993.tb22937.x [DOI] [PubMed] [Google Scholar]
- 25.Jin L, Zou Z, Wang Q et al (2025) Patterns and predictors of therapeutic response to efgartigimod in acetylcholine receptor-antibody generalized myasthenia gravis subtypes. Ther Adv Neurol Disord 18:17562864251319656. 10.1177/17562864251319656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bril V, Howard JF Jr., Karam C et al (2024) Effect of efgartigimod on muscle group subdomains in participants with generalized myasthenia gravis: post hoc analyses of the phase 3 pivotal ADAPT study. Eur J Neurol 31(1):e16098. 10.1111/ene.16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi F, Lai R, Feng L et al (2025) Fast-acting treatment of myasthenic crisis with efgartigimod from the perspective of the neonatal intensive care unit. BMC Neurol 25(1):79. 10.1186/s12883-025-04063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Zhu X, Zhou H et al (2025) Efficacy of multi-cycle Efgartigimod in achieving minimal symptom expression in myasthenia gravis: a comparative multi-center study. Int Immunopharmacol 154:114603. 10.1016/j.intimp.2025.114603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary files.