Abstract
Ethnopharmacological relevance:
Yam (Dioscorea sp.) extracts have been shown to possess a vast array of medicinal properties such as antihypocholesterolemic, antiatherogenic and antihypertensive bioactivity. However, the compounds conferring its antihypertensive bioactivity have not been fully explored.
Aim of the study:
The objective of this study was to identify extractable bioactive fractions and associated compounds in Jamaican Renta Yam (Dioscorea alata) that contributes to its antihypertensive properties, using an activity driven chemoinformatic profiling method.
Materials and methods:
A diethyl ether extract (DR2) of Dioscorea alata was obtained by sequential Solid-Liquid extraction (SLE) coupled to SPE-HPLC fractionation and its chemical composition was analyzed by GC-MS analysis. Its influence on hypertension was evaluated through a combination in vivo and in vitro ACE-Inhibitory activity assays and by molecular docking of the identified compounds to the ACE enzyme.
Results:
SLE revealed the presence of potent antihypertensive activity (ACE IC50 41.99 μg/mL) in the diethyl ether extract (DR2). GC-MS analysis of DR2 indicated the presence of small organic compounds (95.1 g/mol to 200 g/mol) with 2-Phenyl-1,3-oxazol-2-ine (2PO) being the most predominant small organic compound present in the bioactive extract. The binding affinity of 2PO was assessed using molecular docking of 2PO to the ACE enzyme and showed strong binding affinities forming two (2) hydrogen bonds with Tyr135 and Trp220 in the active site of the enzyme. The in vivo effect of DR2 using human umbilical vein endothelial cell lines (HUVECs) revealed; a significant dose-dependent ACE-Inhibitory activity, a stimulating of Nitric Oxide (NO) release and no toxicity towards HUVECs.
Conclusion:
Overall, this study identified Jamaican Renta Yam (Dioscorea alata) as an alternative source of antihypertensive compounds which may address the toxicity seen with known synthetic antihypertensives agents.
Keywords: Dioscorea alata, Jamaican Renta Yam, antihypertensive bioactivity, ACE-Inhibitory activity
Graphic Abstract:

1. Introduction
Hypertension is one of the world’s most important public health challenges due to its high prevalence and concomitant risks of cardiovascular and kidney disease (Chockalingam, Campbell, & Fodor, 2006; Kearney et al., 2005). Recent analysis estimated that approximately 1 billion persons worldwide are living with hypertension (Kumar, 2013); this value is predicted to increase to 1.5 billion by 2025 (Chockalingam, 2007; Chockalingam et al., 2006; Logan et al., 2020). Globally hypertension is responsible for at least 45% of deaths due to heart disease and 51% of deaths due to stroke (World Health Organization, 2013). Research further reports that hypertensive individuals in developing countries (Latin America and the Caribbean) outweigh that of developed countries by almost two-fold. This high cost of hypertension has resulted in treatment modes ranging from lifestyle changes (diet and exercise) to pharmacological therapy (hypertensive drugs) and natural medicines (dietary compounds).
The effective use of natural compounds as therapeutic agents against cardiovascular diseases such as hypertension has been steadily increasing for decades. These compounds possess bioactive phytochemicals with properties which reduces the effects and risk of hypertension (Al Disi et al., 2016). Research has extensively revealed the antihypertensive bioactivity of natural compounds derived from plants and common culinary ingredients. Allium sativum (Garlic) has been shown to contain natural products that possess hypotensive properties. These properties may be due to the presence garlic’s organo-sulfur constituents: such as Allicin, S- allylcysteine (SAC), diallyl disulfides (DADS), diallyl trisulfides (DATS), and methyl thiosulfonate (Qidwai & Ashfaq, 2013). Zingiber officinale (Ginger), has also been reported to possess hypotensive properties clinically. The observable activity has been attributed to the presence of two bioactive constituents of ginger, namely (6)-gingerol and (6)-shogoal (Liu et al., 2013). In another study an aqueous extract of fresh ginger was shown to lower Blood Pressure by engaging both endothelium-dependent (cholinergic) and endothelium-independent vasodilator pathways (Ghayur et al., 2005). Other known hypotensive natural products include: Andrographis paniculate (King of Bitter) (Awang et al., 2012), Apium graveolens (Celery) (Moghadam et al., 2013), Cymbopogon citratus (Lemongrass) (Devi et al., 2012), Hibiscus sabdariffa (Hibiscus) (Ojeda et al., 2010), Camelia synensis (green tea) and Vaccinium ashei reade (Blueberry leaf extract) (Sakaida et al., 2007).
The usage of natural products such as Dioscorea sp. (Yams) has risen because of their utilization by a large global population. These natural products (mainly in developing countries) have been used as primary health treatment in conditions such as hypertension and hypercholesterolemia. Yams are monocotyledonous plants belonging to the family of Dioscoreacea within the order Dioscoreales and belonging to the genus Dioscorea. The medicinal properties of yam varieties present in Jamaica have been evaluated extensively in the literature. Jamaican Bitter Yam (D. Polygonoides) have been implicated in possessing potent hypocholesterolemic properties (McKoy et al., 2014; Stennett et al., 2014). Round Leaf Yellow Yam (Dioscorea cayenensis) and Jamaican Bitter Yam (Dioscorea polygonoides) displays significant hypoglycaemic effects (Grindley, 2001; McAnuff et al., 2005). Other biological applications of Yams include: Anticancer effects and antifungal effects (Sautour et al., 2004), antioxidant and free radical scavenging activity (Roy et al., 2011) and their use as effective binders in tablet and capsule formation (Riley et al., 2006, 2008). Extracts of Dioscorea alata and Dioscorea oppsita Thunb has been shown to exhibit effective antihypertensive properties (Amat et al., 2014; Hsu et al., 2002; Nagai & Nagashima, 2006). Dioscorea batatas has also been shown to possess significant hypotensive activity via ACE inhibition (Lee et al., 2003). The Yam storage protein Dioscorin has also been implicated in having significant antihypertensive properties (Hsu et al., 2002). The natural compounds present in Yams possess activity ranging from antioxidant to antimicrobial and anticancer, however, their identity is not fully characterized. Thus, identification of antihypertensive bioactivity in Yams provides a framework for isolation, characterization and application of these compounds in hypertension treatment.
To the best of our knowledge, the effects of antihypertensive bioactivity in Yams and identification of the specific phenolic compounds from Dioscorea alata has not yet been fully studied. The aim of this study was to identify bioactive fractions and compounds of fresh Dioscorea alata extracted by a chemoinformatic method; reveals that the antihypertensive mechanisms occurring at the level of ACE activity; uses cultured cells human umbilical vein endothelial cell lines (HUVECs) to demonstrates its antihypertensive; and to determine the impact of the binding of these antihypertensive compounds to the ACE enzyme.
2.0. Material and Methods
2.1. Sampling of Yam Tubers
Dioscorea alata (White Yam) and (Renta Yam) was obtained from St Catherine & Trelawny (yam country) in Jamaica respectively. Dioscorea polygonoides (Bitter Yam) was obtained from St Andrew. The Jamaican yam samples were washed with 70% ethanol and dice into small cubes and left to dry at 55°C for 48 hours to constant weight. The dried pieces of yam were then milled to fine powdery consistency. These samples were sealed and stored in sterile containers in a dry area at room temperature until experimentation.
2.2. Solid-Liquid Extraction of Different Yam Samples Using Solvents of Different Polarities.
Solid-Liquid Extraction was performed according to Fontenot, Naragoni, Claville, and Gray (2007) with some modifications. Dried powdered samples of yam (315 g of White Yam; 277 g of Renta Yam or 193 g of Bitter Yam) were packed in separate thimbles and sequentially extracted in a 120 cm × 500 cm Soxhlet apparatus. The samples were sequentially extracted using 800 mL of 100% hexane, diethyl ether, acetone, methanol and distilled water at their respective boiling point for 24 – 48 hrs. Following extraction, particulate matter was removed by filtering the samples through a 0.45 μm fritted glass filter. The hexane, diethyl ether, acetone and methanol solvent were removed using rotary evaporator at 120 rpm at 40°C, 45 °C, 45 °C and 60 °C respectively. Distillation was used in the case of the water extract. Hexane and diethyl ether extracts were placed under the fume hood to allow complete evaporation to dryness. The dried samples were then analyzed gravimetrically and by UV-VIS chromatography. The bioactive extracts were then stored at −20 °C for further purification and biochemical analysis.
2.3. Screening of Yam for Angiotensin I Converting (ACE-I) Enzyme Inhibitory Activity
ACE1 inhibition activity in the extracts were performed using a modified Fluorometric ACE1 Inhibitor Screening Kit (Biovison Catalog # K228–100) according to the manufacturer’s instructions. Ten microliters (10 μL) of Yam extracts were added to a cocktail containing assay buffer (83.5 μL) and 10 unit of ACE1 enzyme (5 μL). For ACE1 inhibitory analysis, the hexane and diethyl ether extracts were dissolved in 10.0 % DMSO while the acetone, methanol and water samples were dissolved in 10% ethanol. To initiate the reaction, fluorometric substrate (1.5 μL) was then added to the sample in a final assay volume 100 μL. The relative fluorescence (Ex/Em = 330/430 nm) was measured in a kinetic mode for 1 hour at 37 °C using a 96-well BioTek fluorescent microplate reader. ACE activity was calculated using the formula ; where RFU- Relative Fluorescent Unit and NT- No treatment group assay blank). Captopril (100 nM) was used as a positive control for ACE inhibition.
2.4. Growth and induction of Human Umbilical Cord Endothelial cell lines (HUVEC)
All cell lines used in this study were obtained from The American Type Culture Collection (ATCC; Rockville, MD, USA). The Human Umbilical Cord Endothelial cell lines (HUVECs), endothelial media and growth supplements were purchased from ATCC (passage number 1, batch # 70014297). HUVECs were cultured in Vascular Cell Basal Medium (VCBM) containing Bovine Brain Extract (BBE)1.0 mL, rh EGF (0.5 mL), L-glutamine (25.0 mL), Heparin sulfate (0.5 mL), Hydrocortisone hemisuccinate (0.5 mL), Fetal Bovine Serum (10.0 mL), Ascorbic acid (0.5 mL) adjusted to contain 10% fetal bovine serum and 100 U/mL penicillin-streptomycin. The cells were incubated at 37°C in a 95% air, 5% CO2 atmosphere until they approached 80% confluence. The cell density was monitored daily, and growth media was removed and replaced after two days. HUVECs were treated with different Yam extracts (0 −7200 μg/mL) then an assessment of cell viability, ACE-I activity, and Nitric Oxide production was done following 24 hours exposure.
2.5. Assessment of Nitric Oxide production in HUVECs treated with DR2-S yam extract.
Extracellular nitric oxide (NO) release (nitrate/nitrite) from HUVECs was quantified by photometrical detection of nitrate (NO3−) and nitrite (NO2−) with a Griess reagent kit (Cayman’s Nitrate/Nitrite Colorimetric Assay Kit) according to the manufacturer’s instruction. 96 well plates were seeded with HUVECs at a cell density of 105 then treated with varying concentrations (0–7200 μg/μL) of Renta Yam extract overnight at 37 °C and 5% CO2 atmosphere. Following induction, the culture media (100 μL) was then collected and added to new 96-well plates contain 100 uL of Griess Reagent mix. In the case of total nitrate measurement, the enzyme Nitrate Reductase was added. The sample was incubated for 10 minutes at room temperature and the optical density (OD) of each sample was measured at 548 nm spectrophotometrically (BioTek 2000). The NO3− and NO2− concentrations were determined from a standard curve (0–10 μM) prepared using 200 μM stock solution. Nitric Oxide production in the sample were calculated using the following equations:
For each sample, the absorbance was normalized to the total cell count and the absorbance values for the no treatment control were set at 100%. All samples were run in triplicates.
2.6. High Performance Liquid Chromatography (HPLC) of Yam Extract
The bioactivity identified in Renta Yam ether extract (DR2) was fractionated using an Agilent HPLC 1200 series and Chemstation for LC systems Rev. B.04.03 SP2 equipped with a diode array detector. The method developed for this fractionation used a Kinetex 5 μm Biphenyl 100Å, LC Column 250×4.6 mm. The DR2 extract (109 mg) was precleaned by passing through a Strat C18-E SPE column (55 μm, 70A) 8B-S001-LEG) that was conditioned using 25% methanol. An aliquot (50 μL of 100 μg/mL) of the SPE flow-through, which contain the bioactivity, was injected into the HPLC at a flow rate of 1.0 mL/min and a separation buffer condition of 0.10 formic acid Buffer A and 90% methanol/0.1% formic acid, Buffer B. A 30-minute separation was performed: 0–2.5 min at 20% B and 1.0 μL/min, 2.6–5 min at 40 % B and 1.0 μL/min, 5.5–10 min at 80% B and 1.0 μL/min, 10.5–25 min at 90% B and 1.0 μL/min then 20.5–30 min at 90% B and 2.0 μL/min. The chromatography was monitored at 210 nm, 230 nm, 254 nm and 280 nm and 1.0 min fractions collected. A sample of each fraction were combined into six different group as assayed for bioactivity.
2.7. Gas Chromatography Mass Spectrometry (GC-MS) analysis of DR2-S1
GC-MS analysis was carried out at the Louisiana State University (LSU) Mass Spectrometry Facility, Department of Chemistry, 232 Choppin Hall, Baton Rouge, LA 70803, USA. DR2-S1(fraction 1 of DR2-S) was analyzed using an Agilent 5977A MSD (Mass Selective Detector) with Agilent 7890B Gas Chromatograph. The starting temperature was 80 °C and held for 4 minutes, while increasing at a rate of 6°/min to 300 °C and held for 15 minutes. The injector was run in splitless mode at a temperature of 250 °C. Carrier gas was helium at a constant flow rate of 1.5 mL/min. The column was a DB5-MS Ultra Inert (Agilent) 30 m long with a film thickness of 0.25 μm and an inner diameter of 0.25 μm. DB5 is a nonpolar phenyl arylene polymer, virtually equivalent to (5%-phenyl)-methylpolysiloxane. The MS parameters were as follows: Transfer temperature = 250 °C, source temperature = 230°C, Quad temperature = 150°C and solvent delay of 4 minutes.
2.8. Molecular Docking Simulations in the ACE Binding Site
The molecular docking analysis was carried out as described by (Zhang et al., 2015). Crystal structure of human ACE (PDB: 1O8A) was obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (http://www.rcsb.org). The structure of the ACE-inhibitory ligands Captopril (PubChem CID 44093) and Lisinopril (PubChem CID 5362119) were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and used as comparative references in the docking process. The structure of the top seven (7) most abundant compounds in DR2-S1 obtained from NIST database from the GC-MS analysis were obtained from PubChem. For the molecular docking simulation, the ACE enzyme was prepared by the removal of all water molecules and the cofactors zinc and chloride atoms were retained in the active site of ACE model. Docking was then carried out using the AutoDock 4.2 Vina software (TSRI, USA) with free-energy scoring function to determine the possible protein–ligand complex conformation. The grid box (50 _ 70 _ 50 Å), coordinates (x: 40.79, y: 33.61 and z: 43.38) was defined to cover all active residues around the Zn (II) prosthetic group. The best-ranked docking pose of the ligand in relation to ACE was determined based on the scores and binding-energy values.
3.0. Results
3.1. Chemoinformatic profile of Dioscorea sp. for Antihypertensive Bioactivity Activity
Several Yam (Dioscorea sp.) species have been show to possess bioactive properties which reduces the effects and risk of hypertension (Al Disi, Anwar, & Eid, 2016) (Amat et al., 2014; Hsu et al., 2002; Nagai & Nagashima, 2006). However, the nature and identity of the compounds responsible for this effect are largely unknown. As an initial step in identifying the compounds present in Dioscorea sp. that may be responsible for its biological effect on hypertension, a chemoinformatic profile was generated using solvents of different polarity. Five unique bioactive extracts derived from Jamaican Renta (DR), White (DP) or Bitter (DL) Yams suspected of having antihypertensive properties was generated using sequential solid-liquid extraction. A sample of each yam was extracted sequentially with hexane, Extract 1; diethyl ether, Extract 2; acetone, Extract 3; methanol, Extract 4 and water, Extract 5. The chemoinformatic profiles were generated using the solubility of the analytes, pigmental analysis (interaction of the analytes with the solvent) and UV-VIS spectrum of analytes present in the five (5) Yam Extracts (Figure 1). Our extraction procedure resulted in a multi-array of compounds (represented by the differences in pigment) depending on the yam and the solvent used to extract the analytes. The Renta Yam extracts (DR1-DR5) resulted in pigmentation ranging from pale yellow to light brown to dark brown depending on the solvent used. Differences in solubility and pigment coloration was seen among the three yams examined (Figure 1). As predicted, solvents of varying polarities (non-polar to polar) extracted identifiable compounds present in our Yam samples (Figure 1A–C, top panel).
Figure 1. Chemoinformatic Profiling of Dioscorea sp. (Yam) using Solvents of Different Polarity.

Dried powdered Dioscorea sp. was subjected to sequential solid-liquid extraction using solvents ranging from non-polar to polar; Hexane (1), Ether (2), Acetone (3), Methanol(4), and Water (5). Extractives were analysed for their UV-VIS electronic absorbance spectral characteristics. (A) RentaYam (D. alata), (B) Bitter Yam (D. polygonoides), (C) White Yam (D. alata). Top panel: Bioactive extracts of Dioscorea sp. prepared by sequential solid-liquid extraction revealing their pigments and Bottom panel: UV-VIS spectra for each yam extract.
To corroborate the solubility data (pigmentation analysis), a UV-VIS spectrum analysis was performed to determine the absorbance ranges of the different analytes in each sample. The electronic absorption spectra of each Yam extracts were measured from 190 nm to 700 nm. The UV-VIS spectra for the hexane, diethyl ether and acetone extracts were performed in 0.1% DMSO while the methanol and water extracts were obtained in 10% MeOH. The UV-VIS spectra of each Yam extract resulted in a unique spectrum signature (Figures 1A, 1B and 1C, bottom panel). Overall majority of the absorption was observed within 190 nm and 400 nm and each spectrum contained between three to five different absorptions maxima. The water extract for Renta Yam (DR5) had two (2) major peaks at 221 & 268 nm. Its’s hexane extract, DR1 and methanolic extract DR4 contain major peaks 233 nm, 273 nm 227 nm & 265 nm respectively. We observed similar unique UV-VIS spectra for the DL, (D. alata -White Yam) and DP (D. polygonoides - Bitter Yam) (Figure 1B and 1C). The variations in absorption spectrum among Yam extracts coupled to variable solubility revealed by the pigmentation analysis alludes to the fact that we have generated specific bioactive extracts with compounds distinctive to individual yam samples.
3.2. Screening of Yam Extract for Antihypertensive Activity
To determine if any of the Yam extracts possess putative hypertensive properties, we screened each extract for Angiotensin Converting Enzyme (ACE) inhibitory activity. Angiotensin converting enzyme (ACE), plays an important role in hypertension by cleaving the carboxy terminal His-Leu dipeptide from the inactive decapeptide angiotensin I to produce the potent vasopressor octapeptide angiotensin II (Natesh, Schwager, Sturrock, & Acharya, 2003). A decrease in the vasopressor octapeptide angiotensin II is associated with a decrease in high blood pressure. Therefore, the ACE inhibitory activity of each extract was determined by utilizing their ability to inhibit ACE hydrolysis of the synthetic substrate, amiobenzoyl peptide (Abz) (i.e., an analogue of angiotensin I) (Figure 2). The released Abz fluorophore is quantified using a fluorescence microplate reader. Different concentration of extracts was incubated with either non-fluorescent or fluorescent label Hippuryl-histidyl-leucine (HHL) or amiobenzoyl peptide (Abz) ACE substrate with or without ten (10) units of ACE enzyme. The relative intensity of the released Histidyl-leucine (HL) fluorophore or amiobenzoyl peptide (Abz) was measured in a kinetic mode for 1 hour at 37°C (Figure 2A). ACE activity was calculated from the ratio of Δ RFU/ extract /Δ RFU/no extract) for each sample. Captopril, a known ACE inhibitor was used as a positive control for the assay (Figure 2A, insert) and resulted in a decrease in ACE activity to 22.02 ± 0.251 %. Screening of the extracts obtained from the three Yams; Renta Yam (DR), White Yam (DL), Bitter Yam (DP) revealed that only Renta Yam (DR), and Bitter Yam (DP) possessed significant antihypertensive activity as measured by ACE inhibition (Figure 2B and Table 1). The percent inhibition of ACE enzyme activity by the extract was determined by the Δ RFU/ extract divided by Δ RFU/without extract. We observed a significant decrease in ACE activity in the Bitter Yam hexane extract (DP1) 52.51 ± 0.45 and Renta Yam diethyl ether extract (DR2) 50.99 ± 0.10 (48%−50% ACE Inhibition respectively) when compared to the control. There was a significant stimulation in ACE activity in selective extracts obtained from the White Yam (DL), and Bitter Yam (DP). The methanolic extract of White Yam (DL), resulted in over 200% increase in ACE activity (Table 1). The relative stimulation of ACE activity for Bitter Yam was 129.33 ± 1.33 %, 168.51 ± 5.28 %, 157.28 ± 0.81 % for DP2, DP3 and DP4 respectively. Using Cola Nut as a positive control for our natural extracts, we observe significant reduction in ACE activity in the Biz’s methanolic (11%) and water (30%) extracts. The relative potency of the ACE inhibitory activity of the Bitter Yam DP1 and Renta Yam DR2 extract was only 1.5 less potent than Captopril control (Table 1).
Figure 2: Identification of Angiotensin Converting Enzyme (ACE) Activity in Jamaica Renta Yam Extracts.
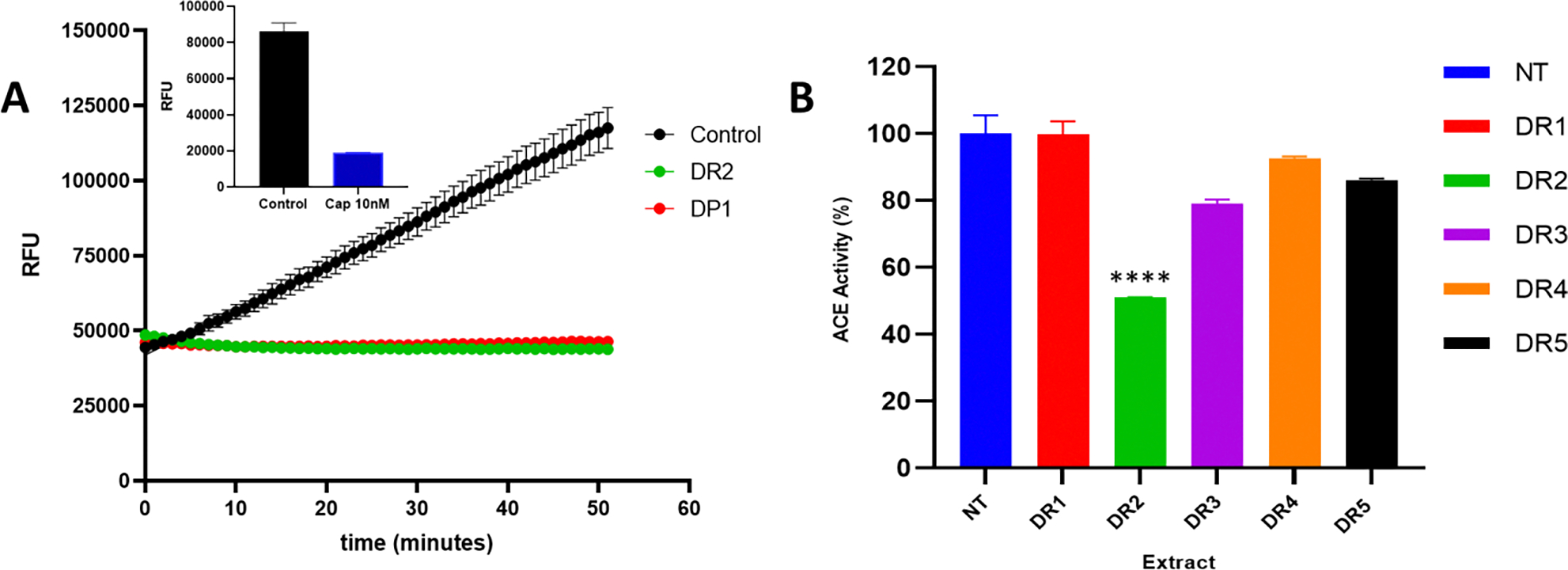
An Angiotensin Converting Enzyme (ACE) assay was performed with fluorogenic substrate and different Yam extracts and the change in fluorescence (Ex/Em = 330/430 nm) was measured in a kinetic mode for 1 hr at 37°C. A. The effect of different Yam extracts on Relative Fluorescence (RFU) from the kinetic ACE inhibitory assay. Figure 2A insert represents the RFU of captopril comparative to control (no inhibitor). B. ACE activity of different Renta Yam extracts (DR). ACE activity was calculated using the formula . The Results in Figure 2A present the Relative Fluorescence Units (RFU) means ± SEM. Control, no inhibitor; DR2, Ether extract of Renta Yam; DP1, Hexane extract of Bitter Yam; Cap 10 nM, Captopril. Each value in Figure 2B is presented as means ± SEM (triplicates) where ****- p < 0.0001. NT, No extract group (control); DR1, Hexane extract of DR; DR2, Ether extract of DR; DR3, Acetone extract of DR; DR4, Methanol extract of DR; DR5, Water extract of DR.
Table 1:
The ACE Activity of Jamaica’s Dioscorea sp. Yams
| Extract | Extract 1 (Hexane) | Extract 2 (Ether) | Extract 3 (Acetone) | Extract 4 (Methanol) | Extract 5 (Water) |
|---|---|---|---|---|---|
| DR Renta Yam | 99.83 ± 3.80 | 50.99 ± 0.10 | 78.94 ± 1.28 | 92.661 ± 0.46 | 86.19 ± 0.41 |
| DP White Yam | 52.51 ± 0.45 | 129.33 ± 1.33 | 168.51 ± 5.28 | 157.28 ± 0.81 | 92.11 ± 1.51 |
| DL Bitter Yam | 98.97 ± 1.66 | 104.01 ± 1.86 | 113.43 ± 0.29 | 225.34 ± 16.29 | 121.54 ± 9.68 |
| Biz | 61.23 ± 0.73 | 60.72 ± 0.22 | 150.32 ± 3.73 | 11.62 ± 0.44 | 30.34 ± 3.36 |
The ACE-inhibitory assay was performed as described in Material and Methods. The crude extract of each Yam was utilized for the study. Captopril was used as a positive control at concentration 10nM resulted in a decrease in ACE activity to 22.02 ± 0.251 %.
3.3. Enrichment of the Antihypertensive Activity Present in Renta Yam’s Diethyl Ether Extract
The antihypertensive bioactivity present in DR2 was purified using a coupled SPE-HPLC protocol (see material and method). The compounds present in DR2 were absorbed to a SPE-C18-E column, eluted with 50% methanol, 100% methanol and 100% acetonitrile and was then assayed for ACE inhibitory activity. Screening of the SPE elutes for ACE inhibitory activity revealed that 64.71± 3.25 % of the inhibitory activity did not bind to the SPE and was present in flow through (FT). The SPE wash and eluates collectively contained less than 24% of the ACE inhibitory activity. The SPE clean-up of the sample resulted in a 72% recovery of the bioactivity present in DR2. As an initial step in identifying the antihypertensive compounds present in DR2 and studying their biological activity, the SPE-C18-E -FT (DR2-S), was fractionated using a Kinetex 5um Biphenyl 100Å, LC Column 250 × 4.6 mm preparative high pressure liquid chromatography (HPLC) column. The chromatography was carried out using a gradient of 10% to 90% MeOH at a flow rate of 1.000 mL/min, for 30 minutes at a temperature of 25 °C. The spectral signal was monitored at a wavelength of 254 nm and 280 nm. (Figure 3A). Forty-five (45) peaks were detected through-out the chromatography spectrum with approximately nine (9) major peaks being identified. All major peaks had a relative absorbance above 500 AU with peaks at approximately four (4) minutes and twelve (12) minutes constituting the highest absorbance (>2000) and relative abundance. Peaks within the retention time range of 7–11 minutes had relative absorbance <2000.
Figure 3: HPLC Purification and Characterization of Anti-ACE Activity from Renta Yam.

DR2 (Ether extract) samples were subjected to a gradient HPLC partition using Kinetex Biphenyl LC column as described in Material and Methods. A. Chromatogram of DR2 showing three (3) independent injections. B. DR2 Chromatogram showing associated peaks responsibly for reduction in ACE activity. The DR2 chromatogram was partitioned into six (6) pooled fractions and assayed for ACE-I activity. C. A chemoinformatic fingerprint of DR2-S (DR2-S1). Fraction one from DR2 HPLC fractionation (DR2-S1) which showed the most significant reduction in ACE activity was collected and re-fractionated by HPLC. D. The UV-VIS spectrum of the fractionated DR2-S1 for each peak identified in the sample.
To identify the bioactivity (ACE inhibitory activity), the chromatogram was dived into six regions and assayed for ACE inhibitory activity (Figure 3B). Region 1 with a retention time from 2.49–6.6 minutes contained 79.39 % of the ACE inhibitory activity; region 2, 6.61–7.72 minutes, 44.0% activity, region 3, 7.73–10.44 minutes, 28.57 %; region 4, 10.45–11.77 minutes, 36.51 %; region 5, 11.78–14.75, 14.68 %; and region 6, 14.76–24.45 minutes with 6.35 % of the activity. The cluster of analytes present in region one was determine by reinjecting a pool of this region in the HPLC (Figure 3C). The chemoinformatic fingerprinting of region 1 revealed the presence of ten (10) identifiable peaks within retentions times 3–15 minutes (Figure 3C and 3D peaks labelled a-j). The ultraviolet scan of each peak revealed multiple peaks with different absorption maxima and electronic transitions. Each of the UV-VIS spectrum of the peaks identified contained two to three different electronic transitions between 220 and 290 suggesting that at least twenty to twenty-two molecules are present in region 1. UV-VIS scans of each peak at different times during the chromatography revealed the present of a single transition in the identified peak except for peaks a and c, suggesting that these peaks may contain a single compound (Figure 3D).
To confer the identity of the analytes present in DR2-S1 (region one of the HPLC chromatogram), this sample was subject to GC-MS analysis. The total ion Chromatograph of DR2-S1 is shown in (Figure 4 ). The result of the GC-MS analysis confirmed the presence of various compounds in DR2-S1. GC-MS identified over fifty distinct peaks by their retention time and were identified through the NIST12 database search. The MS data for the major identifiable compounds are shown in Table 2. Among the major compounds identified were 2-Phenyl-1,3-oxazol-2-ine at a RT of 10.8 minutes and abundance of 20.34 % respectively, Glyceric Acid, Butanedioic acid and 3-pyridinol.
Figure 4. Gas Chromatogram spectrum of the bioactive species present in DR2-S1 and their relative abundance.

DR2-S1 was analyzed using an Agilent 5977A MSD (Mass Selective Detector) with Agilent 7890B Gas Chromatograph. Presented is the total ion Chromatograph of DR2-S1. The M/Z was determined and used to search the NIST database for compound identification. Fifty compounds of interest were identified along with their relative abundance in the DR2-S1 extract.
Table 2:
The Relative abundance of major identifiable compounds in GC-MS analysis of DR2-S1
| Peak # | Retention Time (mins) | Abundance (%) | Compound Name |
|---|---|---|---|
| 2 | 5.9 | 4.68 | Lactic Acid |
| 4 | 7.3 | 1.87 | 3-Pyridinol |
| 10 | 9.5 | 1.37 | Glycerol |
| 12 | 10.1 | 7.47 | Butanedioic acid |
| 13 | 10.4 | 17.40 | Glyceric acid |
| 14 | 10.8 | 20.34 | 2-Phenyl-1,3-oxazol-2-ine |
| 17 | 11.4 | 4.75 | 2-Butene-1,4-diol |
| 25 | 13.6 | 17.43 | L-(+)- Threose, tris (trimethylsilyl)ether, trimethylsilyloxime (isomer 2); L-(+)- Threose, tris (trimethylsilyl)ether, trimethylsilyloxime (isomer 1), L-(+)- Threose, tris (trimethylsilyl)ether, ethyloxime (isomer 2) |
3.4. In Vitro Characterization of the ACE inhibitory of DR2-S1
To further characterize the antihypertensive activity associated with our DR2-S1, we conducted binding analysis of the analytes present in DR2-S1 sample. This was performed using a purified ACE enzyme, endogenous ACE enzyme present in HUVECs and molecular docking of selective analytes to the ACE’s ligand binding pocket (Figures 5–7). The IC50 of our extract was carried out using competitive binding assay with Captopril as a reference. The potency of DR2 extract as an anti-hypertensive agent, was determined using the five-dose assay method at a concentration of 0–200 μg/mL of DR2-S for the purified ACE and 0–7200 μg/mL in the HUVECs. A plot of ACE activity as a function of concentration is shown in Figure 5. The IC50 of DR2-S was determined by plotting the normalized activity data to a downhill dose–response curve with a variable slope. A comparison of the IC50 of Captopril to the IC50 of DR2-S showed that our extract was only 3-fold less potent in inhibiting ACE activity (Figure 5A and 5B).
Figure 5. ACE-I Inhibitory Effect of DR2-S on purified ACE Enzyme and in HUVECs.

(A) Dose-Response of DR2 on ACE activity using pure ACE enzyme. (B) Dose-Response of Captopril on ACE activity using pure ACE enzyme. (C) Dose-Response of DR2-S on ACE activity using HUVECs. A colorimetric ACE assay was performed using pure ACE and varying concentrations of Captopril and DR2/DR2-S respectively (triplicates). could then be calculated where B is the absorbance with ACE and HHL without the ACE inhibitor components. A is the absorbance with ACE, HHL and ACE inhibitor components. C is the absorbance with HHL without ACE and ACE inhibitor components. Results are presented as means ± SEM where * p < 0.05, ** p < 0.005 versus Control.
Figure 7: Models showing the potential pose of 2-Phenyl-1,3-oxazol-2-ine (2PO) in the active site of ACE.

(A) Green molecule is representative of the enzyme ACE while the blue molecules represent the ligands, the yellow sphere represents Zn atom. (B) The binding mode of ACE residues with 2PO (black dots with purple connecting lines) after docking at the ACE active site. Green dotted lines signify the formation of molecular forces (strong hydrogen bonds).
Maximal inhibition of ACE enzyme activity by DR2-S was achieved at 100–200 μg/mL using the purified enzyme and 700 to 750 μg/mL using the HUVEC cells. We observed a time dependent effect of DR2-S in HUVECs since it took a 72-hour exposure of these cell to DR2-S for maximum inhibition of ACE activity. The ACE enzyme was more sensitive toward DR2-S extract with an IC50 value of 41.99± 2.3 μg/mL (CI 34.48 to 48.64 μg/mL), compared to that of HUVECs with an IC50 value of 59.69 μg/mL (CI 23.82 to 153.86 μg/mL).
To collaborate the ACE binding data, we examined how selective small ligands identified by GC-MS behave in the binding/active site of ACE proteins (Meng et al., 2011). The protein-ligand interaction screen was performed using AutoDock 4.2 Vina software (TSRI, USA) with free-energy scoring function to determine the possible protein–ligand complex conformation.
The crystal structure of human ACE (PDB: 1O8A) was obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (http://www.rcsb.org). The structure of the ACE-inhibitory ligands Captopril (PubChem CID 44093) and Lisinopril (PubChem CID 5362119) were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and used as comparative references in the docking process. The predicted structures of top seven (7) most abundant compounds retrieved from GC-MS analysis; 2-Phenyl-1,3-oxazol-2-ine, 2-Butene-1,4-diol, Butanedioic acid, Glyceric acid, Glycerol, Lactic Acid and 3-Pyridinol were obtained from PubChem. The docking analysis was then carried out using the AutoDock 4.2 Vina software (TSRI, USA) with free-energy scoring function to determine the possible protein–ligand complex conformation. The best-ranked docking pose of the ligand in relation to ACE was determined based on the scores and binding-energy values. The binding affinities and potential ACE inhibitory capabilities of known ACE inhibitors compared to the bioactive molecules identified in DR2-S1 is shown in Table 3. The docking simulation of 2-Phenyl-1,3-oxazol-2-ine (2PO) at the active site of ACE revealed the best docking pose and affinity energy of −6.0 kcal/mol compared to −5.5 kcal/mol for captopril (cap), a well chartered ACE inhibitor (Figure 6, Figure 7 and Table 3). Although 2PO and captopril, showed similar binding affinity, molecular interaction revealed a different conformational pose for 2-Phenyl-1,3-oxazol-2-ine (2PO) in comparison to the reference molecules lisinopril and captopril. Captopril had a docking conformation within the sphere of the Zn (II) ion in ACE and was shown to form three (3) hydrogens bonds with Asn277, Thr282 and Gln281 residues (Figure 6b). However, the best conformational pose for 2PO showed no direct interaction with the Zn (II) ion in the active site but form two (2) hydrogen bonds with Tyr135 and Trp220 in the ACE active site (Figure 7b). These results suggest that antihypertensive property of the compounds in Yam may be using a different mechanism in regulation ACE activity.
Table 3:
Comparison of the top scoring affinity values for lisinopril, captopril, and molecules from DR2-S1 in ACE.
| Ligand | Affinity (kcal/mol) |
|---|---|
| Lisinopril | −7.5 |
| Captopril | −5.5 |
| CSCZ | −8.3 |
| 2-Phenyl-1,3-oxazol-2-ine (2PO) | −6.0 |
| 2-Butene-1,4-diol | −4.3 |
| Butanedioic acid | −5.3 |
| Glyceric acid | −4.4 |
| Glycerol | −4.1 |
| Lactic Acid | −4.2 |
| 3-Pyridinol | −4.6 |
Figure 6: Models showing the potential pose of captopril (cap) in the active site of ACE.

(A) Green molecule is representative of the enzyme ACE while the blue molecules represent the ligands, the yellow sphere represents Zn atom. (B) The binding mode of ACE residues with captopril (black dots with purple connecting lines) after docking at the ACE active site. Green dotted lines signify the formation of molecular forces (strong hydrogen bonds).
3.5. Biological Activity of DR2-S on Hypertensive parameters in HUVECs
In order to further assess the potential antihypertensive effects of DR2-S in vitro we measured its Angiotensin Converting Enzyme Inhibitory (ACE-I) activity using HUVECs. These cells contain functional ACE enzyme and has been used to study the effect of antihypertensive compounds in a number of system (Berköz, Yıldırım, Yalın, İlhan, & Yunusoğlu, 2020).
First, we examined the toxicity of DR2-S in HUVECs using standard cell proliferation methods. The growth inhibitory effects of DR2-S on HUVECs proliferation were assessed using standard MTT cell viability assay and cell count. HUVECs were treated with varying concentrations of DR2-S at (0.0072–7200 μg/mL) for 24 hours then assessed for toxicity. We observed over 25% decrease in cell viability as compared to control. However, there were no dose-dependent changes in cell viability in the presence of DR2-S (Figure 8A) suggesting that DR2-S is not toxic to HUVECs. Examination of the in vitro ACE inhibitory effect on the endogenous ACE present in HUVECs showed a significant dose-dependent inhibition in enzyme activity. Cells were treated with increasing concentration of DR2-S or a single dose of 4 μM of Captopril as a positive control for ACE inhibitory activity. Figure 8A shows the ACE- inhibitory activity as a function of concentration. The ACE-inhibitory activity of DR2-S at 7200 μg/mL was similar to that of the Captopril control (The % ACE-I of Captopril at 4 μM is 61.10% ± 4.26 verses 77.92% ± 4.42 for DR2-S at 7200 μg/mL). To determine the IC50 (potency) of DR2-S, we plotted the percent ACE inhibitory activity verses log of the concentration and applied a nonlinear regression analysis. The calculated IC50 of DR2-S in the HUVECs cell was 59.69 μg/mL (CI 23.82 to 153.86 μg/mL). This IC50 was similar to what was observed using the purified ACE enzyme (see Figure 2).
Figure 8: The cytotoxicity of DR2-S in HUVECs.

Cells were seeded at a density 104 per well and were treated with varying concentrations of DR2-S for 24 hours. (A) Cell viability was measured using Cell Titer 96® Non-Radioactive Cell Proliferation Assay (MTS). (B) A colorimetric ACE assay was set up using HUVECs treated for 24 hours with varying concentrations of DR2. ACE inhibition (%) was calculated using the formula . Results are presented as means ± SEM where * p < 0.05, ** p < 0.005 versus Control.
To collaborate DR2-S effect on the hypertensive pathway, we examined Nitric Oxide production in HUVECs. Nitric oxide (NO) production and its availability plays an important role in hypertension and a modulation of its level is a target in hypertension treatment. To determine DR2-S effect on NO production, we analyzed total Nitrite (NO2−) and Nitrate (NO3−) levels due to the short half-life of NO and its ability to undergo several changes in biological fluids (Hakim, Sugimori, Camporesi, & Anderson, 1996). HUVECs were treated with DR2-S and the concentration of Nitrate and Nitrite in HUVECs in response to DR2-S was extrapolated from standard curve for Nitrate and Nitrite (Figure 9A insert). The total Nitrate + Nitrite concentration significantly increased as concentration of DR2-S increased (Figure 9A and 9B). A dose-dependent increase was also noted for Total Nitrite concentration for cells treated with DR2-S. The Total Nitrate + Nitrite concentration at 7.2 μg/mL of DR2-S was 3.35 ± 0.050 μg/mL, Nitrate concentration was 2.29 μg/mL while Nitrite concentration was 1.06 ± 0.028 μg/mL. The Total Nitrate + Nitrite concentration at concentration 7200 μg/mL of DR2-S was 21.65 ± 0.250 μg/mL, Nitrate concentration was 19.81 μg/mL while Nitrite concentration was 1.84 ± 0.028 μg/mL.
Figure 9: Effect of different concentrations of DR2-S on the production of nitric oxide (NO).

Cells were seeded at a density 105 per well and were treated with varying concentrations of DR2-S for 24 hours. (A) Total Nitrate/Nitrite production was measured by assaying the cell supernatant. Nitrate Reductase and Cofactor were followed by a 2-hour incubated at room temperature. Griess Reagent was then added, and absorbance was measured at 540 nm. Total Nitrate/Nitrite concentration was obtain using the formula: . (B) Total Nitrite production was measure by adding Griess Reagent followed by incubation and measured at 540nm. Concentration of nitrite was obtained using the formula: . Total Nitrate concentration was calculated suing the formula: . The results are presented as mean ± SEM. Comparisons of mean values were made using a one-way ANOVA followed by Tukey’s test, **** p < 0.0001 versus Control. Insert in Figure 9A represents the standard curve for both Nitrate and Nitrite standards.
4.0. Discussion
Hypertension has become a great social and economic burden worldwide. Thus, effective treatment of this disease is very important in maintaining good socio-economic values as well reducing mortality and morbidity. Yam, a staple food product of most developing nations has been proposed as a potential strategy for managing hypertension since it has been shown to possess antihypertensive activity under certain conditions. Here, we have applied chemoinformatic profiling, biochemical analysis and molecular docking analysis to characterize the antihypertensive activity associated with the Jamaica Renta Yam. The antihypertensive activity of Renta Yam was found to be associated with small organic compounds present in the Yam which can be isolated using organic solvent such as diethyl ether. The compounds which are present in the diethyl ether extract can inhibit the activity of ACE both in vitro and in vivo. The inhibitory compounds present in the extract can bind to the active site of the ACE enzyme; however, they induce a different conformation as compared to standard antihypertensive compounds such as captopril and lisinopril. In addition, these compounds can modulate biochemical pathways involving nitric oxide that contributes to the controlling of hypertension. Taken together these findings support the idea of using natural product such as Yam in the control of hypertension.
Yams are monocotyledonous plants belonging to the family of Dioscoreacea that produces edible tubers, bulbil or rhizomes and are known to be of significant economic importance (Ayensu & Coursey, 1972; Wallace et al, 2022). Several studies have showed that they exhibit effective antihypertensive properties (Amat et al., 2014; Hsu et al., 2002; Nagai & Nagashima, 2006). This antihypertensive activity was proposed to be associated with the Yam storage protein Dioscorin (Hsu et al., 2002). Dioscorin (Mr 31,000) is the major soluble protein present in tubers such as yam (Dioscorea spp.). Due to its relatively high abundance in yams, it is considered to play a role as a storage protein (Conlan et al, 1998). Although Dioscorin contains antioxidant activity, it does not account totally for the antihypertensive effects associated with the Yams. Therefore, we applied a chemoinformatic approach which divided the components of the yam into specific chemical groups. The use of solid-liquid extraction with solvents of different polarity made it possible to solubilize potent bioactive compounds from D. alata (Renta Yam) using diethyl ether. The diethyl ether extract of Renta Yam showed significant ACE-inhibitory activity having an IC50 of 41.99 ± 2.3 μg/mL (CI 34.48 to 48.64) which represented a threefold less potency when compared to the ACE- inhibitory drug captopril (used as a control). The IC50 values for our extract were also consistent with the IC50 of known plant extracts with potent ACE-Inhibitory activity such as: Hibiscus sabdariffa (Hibiscus) 91 μg/mL (Ojeda et al., 2010), Vaccinium ashei reade (Blueberry leaf extract) 46 μg/mL (Sakaida et al., 2007) and Dioscorea opposita Thunb at 41.1 μg/ml (Nagai & Nagashima, 2006). This shows the potential use of our extract as an ACE Inhibitor.
The compounds identified from our extract using GC-MS were small organic molecules, not proteins such as Dioscorin. The significant ACE-Inhibitory activity of these compounds however stands in stark contrast to already existing knowledge of ACE-Inhibitory polyphenolic compounds (Balasuriya & Rupasinghe, 2011; Oboh et al., 2012) and the ACE-Inhibitory activity observed in the Yam storage protein Dioscorin (Hsu et al., 2002). These observations centralize the antihypertensive potential of compounds extracted from Yam using more non-polar organic solvents such as diethyl ether. A major compound from the GC-MS analysis such as 2-Phenyl-1,3-oxazol-2-ine (2PO) shares similarities with other known antihypertensive compounds such as captopril. However, our antihypertensive Yam extract contained upward of ten unique chemical compounds as reveal by GC-MS. Therefore, we cannot rule out the possibility that these compounds may be working synergistically in modulating the hypertensive parameters we examined (Roswiem et al, 2012). However, we have confirmed using molecular docking that at least two of our identifiable compounds possesses the ability to bind to the active site of ACE. The compound 2PO showed a greater binding affinity (−6.0 kcal/mol) to ACE when compared to captopril (−5.5 kcal/mol). This presents the strong binding capabilities of 2PO to the active site of ACE. The binding affinity of 2PO to ACE falls within a −1.5 kcal/mol range to that observed for the effective ACE-I peptide from rice bran (Tyr-Ser-Lys) at −7.9 kcal/mol (Wang et al., 2017). The binding affinity of 2PO to ACE when compared to the peptide WPMGF, derived from enzymatic hydrolysates of Cyclina sinensis was however significantly lower with WPMGF showing a binding affinity at −29.97 kJ/mol (Yu et al., 2018). ACE is known to possess three main active site pockets (S1, S2 and S1`). The S1 pocket contains Ala354, Glu384 and Tyr523 residues, S2 pocket contains Gln281, His353, Lys511, His513 and Tyr520 residues, while the S1` pocket contains Glu162 residue (Rohit et al., 2012). The docking confirmation with 2PO showed the molecular forces: electrostatic (not shown), hydrogen bonds and extensive hydrophobic interactions. Although most of the interactions were mediated by hydrophobic interactions, 2PO was found to interact with Tyr135 and Trp220 via strong hydrogen bonds (Figure 7B). The binding interaction of 2PO was dissimilar to the binding of captopril in the binding pocket of ACE and were also dissimilar to that observed for the peptide WPMGF which formed hydrogen bonds with ACE residues S1 pocket (Ala315, Glu345), S2 pocket (His314, His474), Arg483, and His348 (Yu et al., 2018). The results were also different to that observed in Tyr-Ser-Lys which established hydrogen bonds with the S2 pocket (Ala354) and the S2 pocket (Gln281 and His353) (Wang et al., 2017). This binding mode of 2PO could therefore suggest strong hydrophobic and hydrogen binding within the S1` pocket.
In vivo screening methods (usually performed in animal models) have been widely used when studying hypertension since these methods often share features common to that of human hypertension. Angiotensin I-converting enzyme (ACE) plays a pivotal role in blood pressure regulation in humans and is a key component in the renin-angiotensin system (RAS), which is well known for its role in the regulation of blood pressure, electrolyte balance, and vascular remodelling (Balasuriya & Rupasinghe, 2011). The ACE inhibitory screen used in this study was design to be biologically relevant. Angiotensin converting enzyme inhibitors (ACE I) have become premier choices for high risk hypertensive patients (Elisseeva & Kugaevskaya, 2009; Sridevi et al., 2011). In comparing the in vitro anti-ACE activity of our extract to that of the in vivo anti-ACE activity of our extract in HUVECs we noted that there may be some bioavailability or metabolism of the compound taking place in the HUVECs. Not only did we observe a significant dose-dependent decrease in ACE activity, a significant increase in nitric oxide (NO) was observed which may be due to a stimulation of eNOS which subsequently increases the bioavailability of NO in the endothelium (Mount et al., 2007). Additionally, the reduction in ACE activity may be resulting in a reduction of a breakdown of bradykinin (a very potent vasodilator) which applies its vasodilatory activity by causing endothelial cells to release nitric oxide, prostacyclin and/or a hyperpolarising factor [endothelium-derived hyperpolarising factor (EDHF)]. A reduction in the breakdown of bradykinin may be responsible for the increased levels of released nitric (Hornig & Drexler, 1997). However, it should be noted that our extract could also be targeting other hypertensive pathway such as functioning as angiotensin receptor blockers (ARBs). Therefore, analysis of the antihypertensive activity of our extract in other models of hypertension will provide information concerning extract’s specific mechanism of action.
5.0. Conclusion
Our research has shown that small organic molecules from natural sources such as Dioscrea sp. (Yam) possesses significant bioactivity that can reduce the danger of cardiovascular diseases such as hypertension. This study revealed the findings of the positive therapeutic effects of compounds from natural sources (Yam) on hypertension in vivo and in vitro. The work provides a good framework for the potential use of Jamaica Renta Yam as an alternative antihypertensive supplement which could have better acceptability in the body and less presentable side effects. In addition, it provided a starting point of infusing value-added produce into the Yam’s economic market of developing countries.
Highlight from this Study.
A diethyl ether extract (DR2) of contain Dioscorea alata possess antihypertensive bioactivity.
The DR2 extract of Dioscorea alata is inhibitory to the Angiotensin I Converting enzyme ACE
2-Phenyl-1,3-oxazol-2-ine (2PO) a component of DR2 bind to the ACE with high affinity
DR2 showed no cytotoxicity towards HUVECs but is able to stimulate Nitric Oxide (NO) release.
Jamaican Renta Yam (Dioscorea alata) may serves ab a natural preventative remedy for hypertension.
Abbreviations:
- DR2
diethyl ether extract
- SLE
sequential Solid-Liquid extraction
- ACE
Angiotensin I Converting
- HUVEC
human umbilical vein endothelial cell
- NO
Nitric Oxide
- ATCC
American Type Culture Collection
- VCBM
Vascular Cell Basal Medium
- BBE
Bovine Brain Extract
- DR
Jamaican Renta
- DP
DL, D. alata -White Yam
- DP
D. polygonoides - Bitter Yam
- DR2-S1
bioactive fraction of Renta yam
References
- Al Disi SS, Anwar MA, & Eid AH (2016). Anti-hypertensive herbs and their mechanisms of action: part I. Front. Pharmacol, 6, 323. 10.3389/fphar.2015.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat N, Amat R, Abdureyim S, Hoxur P, Osman Z, Mamut D, & Kijjoa A (2014). Aqueous extract of Dioscorea opposita thunb. normalizes the hypertension in 2K1C hypertensive rats. BMC Complement Altern Med, 14(1), 36. 10.1186/1472-6882-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awang K, Abdullah NH, Hadi AHA, & Su Fong Y (2012). Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J. Biotechno. Biomed, 2012. 10.1155/2012/876458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasuriya BN, & Rupasinghe HV (2011). Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. J. Funct. Foods in Health Dis., 1(5), 172–188. 10.31989/ffhd.v1i5.132 [DOI] [Google Scholar]
- Berköz M, Yıldırım M, Yalın S, İlhan M, & Yunusoğlu O (2020). Myricetin inhibits angiotensin converting enzyme and induces nitric oxide production in HUVEC cell line. Gen. Physiol. Biophys, 39, 249–258. 10.4149/gpb_2020007. [DOI] [PubMed] [Google Scholar]
- Chockalingam A (2007). Impact of world hypertension day. Can J Cardiol, 23(7), 517–519. 10.1016/S0828-282X(07)70795-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockalingam A, Campbell NR, & Fodor JG (2006). Worldwide epidemic of hypertension. Can J Cardiol, 22(7), 553–555. 10.1016/S0828-282X(06)70275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan S, Griffiths LA, Turner M, Fido R, Tatham A, Ainsworth C, & Shewry P (1998). Characterization of the yam tuber storage protein dioscorin. J. Plant Physiol, 153(1–2), 25–31. [Google Scholar]
- Devi RC, Sim SM, & Ismail R (2012). Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid.-Based Complementary Altern. Med, 2012. 10.1155/2012/539475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisseeva YE, & Kugaevskaya E (2009). Structure and physiological importance of angiotensin converting enzyme domains. Biochem, (MOSCOW) Suppl. B: Biomed. Chem, 3(3), 237. 10.1134/S1990750809030032. [DOI] [Google Scholar]
- Fontenot K, Naragoni S, Claville M, & Gray W (2007). Characterization of bizzy nut extracts in estrogen-responsive MCF-7 breast cancer cells. Toxicol. Appl. Pharmacol, 220(1), 25–32. 10.1016/j.taap.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Ghayur MN, Gilani AH, Afridi MB, & Houghton PJ (2005). Cardiovascular effects of ginger aqueous extract and its phenolic constituents are mediated through multiple pathways. Vascul. Pharmacol, 43(4), 234–241. 10.1016/j.vph.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Grindley FOO, Asemota Helen N., Morrison Errol Y. St. A., Phillip. (2001). Effect of yam (Dioscorea cayenensis) and dasheen (Colocassia esculenta) extracts on the kidney of streptozotocin-induced diabetic rats. Int J Food Sci Nutr., 52(5), 429–433. 10.1080/09637480120078311. [DOI] [PubMed] [Google Scholar]
- Hakim T, Sugimori K, Camporesi E, & Anderson G (1996). Half-life of nitric oxide in aqueous solutions with and without haemoglobin. Physiol. Meas, 17(4), 267. 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- Hornig B, & Drexler H (1997). Endothelial function and bradykinin in humans. Drugs, 54(5), 42–47. 10.2165/00003495-199700545-00007. [DOI] [PubMed] [Google Scholar]
- Hsu F-L, Lin Y-H, Lee M-H, Lin C-L, & Hou W-C (2002). Both dioscorin, the tuber storage protein of yam (Dioscorea alata cv. Tainong No. 1), and its peptic hydrolysates exhibited angiotensin converting enzyme inhibitory activities. J. Agric. Food Chem, 50(21), 6109–6113. 10.1021/jf0203287. [DOI] [PubMed] [Google Scholar]
- Kearney P, Whelton M, Reynolds K, Muntner P, Whelton PK, & He J (2005). Global burden of hypertension: analysis of worldwide data. Lancet, 365(9455), 217–223. 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kumar. (2013). Epidemiology of hypertension. Clin. Nephrol, 2(2), 56–61. 10.1016/j.cqn.2013.04.005. [DOI] [Google Scholar]
- Lee M-H, Lin Y-S, Lin Y-H, Hsu F-L, & Hou W-C (2003). The mucilage of yam (Dioscorea batatas Decne) tuber exhibited angiotensin converting enzyme inhibitory activities. Bot. Bull. Acad, Sin, 44. https://ejournal.sinica.edu.tw/bbas/content/2003/4/bot444-02.html. [Google Scholar]
- Liu Q, Liu J, Guo H, Sun S, Wang S, Zhang Y, . . . Qiao Y (2013). [6]-Gingerol: a novel AT1 antagonist for the treatment of cardiovascular disease. Planta Med, 79(05), 322–326. 10.1055/s-0032-1328262 . [DOI] [PubMed] [Google Scholar]
- Logan KA, Asemota H, Nwokocha C, Lawrence MA, Thompson R, Nwokocha M, & Bakir M (2020). The Effects of Synthesized Semicarbazone Copper Complex on Blood Pressure in Normotensive and L-NAME Induced Hypertensive Rats. J, Biotechnol. Biomed, 3(2), 67–77. 10.26502/jbb.2642-91280028. [DOI] [Google Scholar]
- McAnuff MA, Harding WW, Omoruyi FO, Jacobs H, Morrison EY, & Asemota HN (2005). Hypoglycemic effects of steroidal sapogenins isolated from Jamaican bitter yam, Dioscorea polygonoides. Food Chem. Toxicol, 43(11), 1667–1672. 10.1016/j.fct.2005.05.008. [DOI] [PubMed] [Google Scholar]
- McKoy M-L, Thomas P-G, Asemota H, Omoruyi F, & Simon O (2014). Effects of Jamaican bitter yam (Dioscorea polygonoides) and diosgenin on blood and fecal cholesterol in rats. J. Med. Food, 17(11), 1183–1188. 10.1089/jmf.2013.0140. [DOI] [PubMed] [Google Scholar]
- Meng X-Y, Zhang H-X, Mezei M, & Cui M (2011). Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput-Aided Drug Des, 7(2), 146–157. 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam MH, Imenshahidi M, & Mohajeri SA (2013). Antihypertensive effect of celery seed on rat blood pressure in chronic administration. J. Med. Food, 16(6), 558–563. 10.1089/jmf.2012.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount PF, Kemp BE, & Power DA (2007). Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J. Mol. Cell. Cardiol, 42(2), 271–279. 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Nagai T, & Nagashima T (2006). Functional properties of dioscorin, a soluble viscous protein from Japanese yam (Dioscorea opposita thunb.) tuber mucilage Tororo. Z. Naturforsch., C, J. Biosci, 61(11–12), 792–798. 10.1515/znc-2006-11-1204. [DOI] [PubMed] [Google Scholar]
- Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, & Alvarez L (2010). Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin-and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol, 127(1), 7–10. 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- Qidwai W, & Ashfaq T (2013). Role of garlic usage in cardiovascular disease prevention: an evidence-based approach. Evidence-Based Complementary and Alternative Medicine, 2013. 10.1155/2013/125649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley CK, Adebayo SA, Wheatley AO, & Asemota HN (2006). Fundamental and derived properties of yam (Dioscorea spp.) starch powders and implications in tablet and capsule formulation. Starch-Stärke, 58(8), 418–424. 10.1002/star.200600491. [DOI] [Google Scholar]
- Riley CK, Adebayo SA, Wheatley AO, & Asemota HN (2008). Surface properties of yam (Dioscorea sp.) starch powders and potential for use as binders and disintegrants in drug formulations. Powder Technology, 185(3), 280–285. 10.1016/j.powtec.2007.10.028. [DOI] [Google Scholar]
- Rohit A, Sathisha K, & Aparna HS (2012). A variant peptide of buffalo colostrum β-lactoglobulin inhibits angiotensin I-converting enzyme activity. Eur. J. Med. Chem, 53, 211–219. 10.1016/j.ejmech.2012.03.057. [DOI] [PubMed] [Google Scholar]
- Roswiem AP, Kiranadi B, Bachtiar TSP, & Ranasasmita R (2012). Antihypertensive Effect of Brucea javanica (L.)(Merr.) Fruit Extract. Makara J. Science, 71(76), 76. 10.7454/mss.v16i2.1400. [DOI] [Google Scholar]
- Roy A, Sitalakshmi T, Geetha R, Lakshmi T, & Priya VV (2011). In vitro antioxidant and free radical scavenging activity of the ethanolic extract of Dioscorea villosa (Wild Yam) Tubers. Drug Invention Today, 3(9). https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=56fdafbf4dd413104eb0f0da9fa75c3b89209484. [Google Scholar]
- Sakaida H, Nagao K, Higa K, Shirouchi B, Inoue N, Hidaka F, . . . Yanagita T (2007). Effect of Vaccinium ashei reade leaves on angiotensin converting enzyme activity in vitro and on systolic blood pressure of spontaneously hypertensive rats in vivo. Biosci. Biotechnol. Biochem, 71(9), 2335–2337. 10.1271/bbb.70277. [DOI] [PubMed] [Google Scholar]
- Sautour M, Mitaine-Offer A-C, Miyamoto T, Dongmo A, & Lacaille-Dubois M-A (2004). Antifungal steroid saponins from Dioscorea cayenensis. Planta Medica, 70(01), 90–92. 10.1055/s-2004-815467. [DOI] [PubMed] [Google Scholar]
- Stennett D, Oladeinde F, Wheatley A, Bryant J, Dilworth L, & Asemota H (2014). Effects of Dioscorea Polygonoides (Jamaican bitter yam) supplementation in normocholesterolemic and genetically modified hypercholesterolemic mice species. J. Food Biochem, 38(1), 28–37. 10.1111/jfbc.12022. [DOI] [Google Scholar]
- Wallace K, Asemota H and Gray W (2021) Acetone Extract of Dioscorea alata Inhibits Cell Proliferation in Cancer Cells. Am. J.Plant Sci, 12, 300–314. 10.4236/ajps.2021.123019. [DOI] [Google Scholar]
- Wang X, Chen H, Fu X, Li S, & Wei J (2017). A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT, 75, 93–99. 10.1016/j.lwt.2016.08.047. [DOI] [Google Scholar]
- World Health Organization. (2013). A global brief on hypertension : silent killer, global public health crisis: World Health Day 2013. https://apps.who.int/iris/handle/10665/79059. [Google Scholar]
- Yu F, Zhang Z, Luo L, Zhu J, Huang F, Yang Z, . . . Ding G (2018). Identification and molecular docking study of a novel angiotensin-I converting enzyme inhibitory peptide derived from enzymatic hydrolysates of Cyclina sinensis. Marine Drugs, 16(11), 411. 10.3390/md1611041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Unal H, Desnoyer R, Han GW, Patel N, Katritch V, . . . Stevens RC (2015). Structural Basis for Ligand Recognition and Functional Selectivity at Angiotensin Receptor. J. Biol. Chem,, 290(49), 29127–29139. 10.1074/jbc.M115.689000. [DOI] [PMC free article] [PubMed] [Google Scholar]


