Abstract
Avian haemosporidian parasites are vector-borne apicomplexans that infect bird species globally and pose considerable challenges in detection due to frequent co-infections and morphological convergence. In the present study, we first used Oxford Nanopore Technologies (ONT) to resolve co-infections of haemosporidians in Swinhoe’s pheasant (Lophura swinhoii), an island-endemic galliform. Blood smears revealed two morphologically distinct gametocyte forms: roundish and circumnuclear, and molecular analyses identified three mitochondrial lineages: two novel Haemoproteus lineages (hLOPSWI01 and hLOPSWI02) and one Plasmodium lineage (pNILSUN01). Phylogenetic reconstruction of mitogenomes resolved hLOPSWI01 and hLOPSWI02 within the Parahaemoproteus clade, whereas pNILSUN01 clustered in the Giovannolaia-Haemamoeba clade. Overall, this study revealed the efficacy of ONT in resolving cryptic co-infections through unfragmented mitogenome assembly, overcoming ambiguities inherent to Sanger sequencing. Our findings establish baseline haemosporidian diversity in L. swinhoii and highlight the necessity of combining long-read genomics with morphological scrutiny for accurate parasite taxonomy, particularly in understudied avian hosts facing conservation threats.
Keywords: Avian haemosporidian parasites, Co-infection, Lophura swinhoii, Mitochondrial genome assembly, Nanopore sequencing, Phylogenetic reconstruction
Graphical abstract
Highlights
-
•
Nanopore sequencing of haemosporidian co-infections for species-level resolution.
-
•
Two novel Haemoproteus lineages (hLOPSWI01 and hLOPSWI02) identified in Lophura swinhoii.
-
•
Plasmodium lineage pNILSUN01 demonstrates cross-order host transmission.
-
•
Integration of morphology with long-read genomics advances parasite taxonomy.
1. Introduction
Avian haemosporidian parasites (Haemosporida: Apicomplexa), comprising four genera, Plasmodium, Fallisia, Haemoproteus, and Leucocytozoon (Valkiunas, 2004; Pacheco and Escalante, 2023), are globally distributed dipteran-vectored hematozoa that infect > 50 % of the avian species across diverse ecosystems in non-polar regions (Clark et al., 2014; Rivero and Gandon, 2018; Santiago-Alarcon and Marzal, 2020). These parasites have catalyzed advances in parasitological medicine as well as the understanding of evolutionary ecology and host-pathogen dynamics as proxies for human malarial studies (Valkiunas, 2004; Santiago-Alarcon and Marzal, 2020). Therefore, the taxonomy and systematics of these parasites must be studied to enhance our understanding of the evolution and ecology of these species.
Studies have increasingly included molecular methods to investigate the prevalence and genetic diversity of avian haemosporidians (Bensch and Hellgren, 2020). The cytochrome b (cytb) barcode is pivotal for identifying haemosporidians and has been used to assign genetic lineage names that are recorded in the MalAvi database (Bensch et al., 2009). The database contains nearly 5000 lineages as of October 2024, providing insights into the DNA sequence variation and diversification of avian haemosporidians. This variation indicates the presence of mixed infections and/or co-infections in an avian host (Ricklefs and Fallon, 2002; Valkiūnas et al., 2003; Hellgren et al., 2004). Individual wild birds are often infected with two or more different parasites (Valkiunas, 2004; Valkiūnas et al., 2006). However, traditional methods such as cytb amplification and direct Sanger sequencing are unable to separate the infections in co-infected individuals, often resulting in jumbled chromatograms and double base-calling (Marzal et al., 2008; Martínez et al., 2009; Silva-Iturriza et al., 2012). In addition, conventional polymerase chain reaction (PCR) amplifies DNA sequences for species with higher DNA concentrations or DNA sequences that more closely match the primers, potentially masking the presence of co-infections (Bernotienė et al., 2016). Even multiplex PCR primers cannot detect mixed infections with haemosporidians of the same genus (Ciloglu et al., 2019). An alternative approach for identifying co-infections in unknown or complex situations involves performing the tedious step of cloning barcode fragments and then separate sequencing (Pérez-Tris and Bensch, 2005). Next-generation sequencing-based barcoding methods could be applied to identify co-infections (Yeo et al., 2022). However, these methods are limited by the small size of the cytb gene fragment, which reduces the number of informative phylogenetic sites and affects phylogenetic reconstructions and species delimitation (Ellis and Bensch, 2018; Pacheco et al., 2018a).
Phylogenetic analyses of the order Haemosporida have increasingly relied on mitochondrial genomes (Pacheco et al., 2020; Lotta-Arévalo et al., 2023; Valkiūnas et al., 2024) owing to the availability of a comprehensive dataset, which includes mitochondrial genomes from > 100 species of haemosporidians. These mitochondrial genomes help in increasing the resolution of phylogenetic analyses, which in turn resolves polytomies in the haemosporidian radiation in phylogenetic trees (Pacheco et al., 2018b). Conventional multifragment overlapping PCR is recommended for amplifying the mitochondrial genome in single-infection samples to ensure specificity and mitigate chimeric sequence artefacts (Musa, 2023). Long-range PCR and cloning are required for co-infected samples (Vieira et al., 2023). The sequences of the cloned PCR products contain polymerase errors, thereby requiring analysis of multiple clones to verify the correct sequence (Bensch and Hellgren, 2020). Although next-generation sequencing can amplify mitochondrial genomes (Karadjian et al., 2016; Ciloglu et al., 2020), the lack of reference genomes increases the risk of chimeras (Pacheco and Escalante, 2023). Therefore, long-read sequencing technologies have been developed to address the limitations of conventional PCR-dependent or short-read methods as robust approaches for handling the mitochondrial genome complexity in the separation of haemosporidian co-infections (Pacheco et al., 2024). The utility of these approaches is verified using PacBio HiFi sequencing by generating high-fidelity mitochondrial genomes across samples of mixed infections, thereby achieving single-read coverage that minimizes chimera formation and detects lineages even at low parasitemia (Pacheco et al., 2024). Oxford Nanopore Technologies (ONT) has emerged as a transformative platform for both resolving intricate genomic architectures of parasitic protozoans and enabling portable, real-time pathogen diagnostics. Its long-read sequencing capabilities can decipher complex structural genomic features across diverse parasitic taxa (Díaz-Viraqué et al., 2019; Liechti et al., 2019; Lee et al., 2021; Namasivayam et al., 2021; Menon et al., 2022; Higuera et al., 2023; Tetzlaff et al., 2024; Zhang et al., 2025). Beyond genome assembly, ONT shows clinical utility through its capacity to rapidly identify polyparasitic co-infections in animal reservoirs (Huggins et al., 2024a, 2024b; Jiménez et al., 2024; Matoute et al., 2024), while simultaneously supporting cost-effective molecular surveillance of antimalarial resistance. This is exemplified by targeted amplicon sequencing of Plasmodium falciparum drug-resistance loci in endemic regions, where platform portability and real-time data analysis enable timely public health interventions (Runtuwene et al., 2018; Girgis et al., 2023; De Cesare et al., 2024; Holzschuh et al., 2024).
Swinhoe’s pheasant (Lophura swinhoii) (family Phasianidae; order Galliformes) is a near-threatened species on the IUCN Red List and indigenous to Taiwan (Bird Life International, 2019) that exhibits the genetic island adaptation characteristics as an endemic island bird species (Xu et al., 2024). This phenomenon, referred to as island syndrome, leads to the reduced parasite resistance of these island hosts (Baeckens and Van Damme, 2020), potentially facilitating haemosporidian co-infection cascades. This study aimed to use Nanopore sequencing technology to separate complex co-infections of avian haemosporidians to detect and analyze their mitochondrial genomes at the species level. In addition, we aimed to fill the knowledge gap regarding avian haemosporidian infections in Swinhoe’s pheasant.
2. Materials and methods
2.1. Sample collection and blood film examination
The blood sample was obtained from an adult male Swinhoe’s pheasant that was rescued from Yanping Township (22°56′5″N, 121°1′58″E), Taitung, Taiwan, in March 2024. The bird was promptly transferred to WildOne Wildlife Conservation Association (https://www.wildonetaiwan.org/) for subsequent examinations and treatment. Approximately 1 ml of blood was collected from the brachial wing vein and stored in a lithium heparin tube until hematological examination. The blood smears were stained with Wright-Giemsa stain (BaSO Biotech, New Taipei, Taiwan) and observed using a Zeiss Axioscope 5 light microscope (ZEISS Group, Oberkochen, Germany). Photomicrographs were captured using a Tekfar Digital Camera (TEKFAR SCIENCE, Taichung, Taiwan), and digital measurements were conducted using ImageJ software (Schneider et al., 2012). The remaining blood was stored in a refrigerator (4–8 °C) until DNA extraction and molecular analysis.
2.2. DNA extraction and PCR screening
The genomic DNA was extracted from 100 μl of whole blood using a QIAamp® DNA Mini Kit (Qiagen, Hilden, Germany) and a taco™ mini-Automatic Nucleic Acid Extraction System (GeneReach, Taichung, Taiwan) that use magnetic bead separation technology. The PCR screening of haemosporidian parasites was based on a partial cytb gene (Hellgren et al., 2004). Positive PCR products were subsequently subjected to bidirectional Sanger sequencing provided by Genomics BioSci & Tech (New Taipei, Taiwan). Raw chromatograms underwent systematic processing using Geneious Prime 2023.2.1 (Biomatters Limited, Auckland, New Zealand, available at https://www.geneious.com), wherein base-calling errors were corrected using manual trace inspection and low-quality termini (Q-score < 20) were algorithmically trimmed. Consensus sequences derived from forward-reverse read alignments were authenticated through comparative analysis against the GenBank NCBI reference database (https://www.ncbi.nlm.nih.gov/genbank/) using the Megablast algorithm in Geneious Prime.
2.3. Mitochondrial genome amplification and ONT sequencing
The almost complete mitochondrial genome was amplified using KAPA HiFi HotStart ReadyMix (Roche Molecular Systems, Pleasanton, USA) with the primer sets AE170 and AE171. These primers are effective in amplifying the mitochondrial genomes (approximately 6 kb) of various haemosporidian species from vertebrate hosts (Pacheco et al., 2024). The sample DNA was diluted 100-fold for PCR amplification to minimize the influence of host’s DNA on subsequent high-throughput sequencing. PCR reactions were conducted in 20 μl volumes with the following conditions: initial denaturation at 95 °C for 3 min; followed by 35 cycles of 98 °C for 30 s, 69 °C for 30 s, and 72 °C for 3 min; and a final extension of 6 min at 72 °C. The PCR products were evaluated for subsequent sequencing using agarose gel electrophoresis. The resulting products were purified using AMPure XP Beads (Beckman Coulter, Indianapolis, USA) for further ONT sequencing. The total DNA concentration was measured using a Qubit 4.0 fluorometer (Thermo Fisher Scientific, Waltham, USA) and diluted to approximately 350 ng for library construction. First, end repair and dA-tailing were performed using a KAPA Hyper Prep kit (Roche Molecular Systems, Pleasanton, USA), and subsequent steps were conducted following the manufacturer’s protocol for a Native Barcoding Kit 24 V14 (SQK-NBD114.24, Oxford Nanopore Technologies, Oxford, UK). The sequencing library was loaded onto MinION Spot-on flow cell (FLO-MIN114 version R10.4.1). Real-time super-accuracy base-calling was conducted, and the trimming sequencing barcodes was automatic after base-calling in MinKNOW v24.06.8.
2.4. De novo assembly and sequence analysis
The raw data were primarily analyzed using Geneious Prime 2023.2.1. All reads underwent systematic processing commencing with quality filtration through BBDuk v38.84 (Q20 threshold) to eliminate low-confidence bases. The qualified reads were taxonomically contextualized through alignment against a curated mitochondrial reference database comprising 181 haemosporidian genomes (Pacheco et al., 2024) using Minimap2 v2.24 (Li, 2018) with a minimum divergence of 95 %, enabling genus-level classification based on conserved signature sequences. Genomic segments exceeding 2000 bp showing congruence with known haemosporidian genera were isolated, followed by removal of duplicates using Dedupe v38.84. De novo assembly was executed via Flye v2.9.1 (minimum overlap: 2000 bp) (Lin et al., 2016; Kolmogorov et al., 2019), with resultant contigs cross-validated against Sanger-derived sequences through MAFFT v7.490 (Katoh and Standley, 2013) implemented in Geneious Prime 2023.2.1 to distinguish potential multiple sequences.
2.5. Phylogenetic analysis
The mitochondrial genomes obtained were compared with the sequences available in the GenBank and MalAvi databases (Bensch et al., 2009) using BLAST implemented in Geneious Prime 2023.2.1. The genome was annotated using P. falciparum (M76611) as the reference genome (Feagin et al., 2012). The fragmented ribosomal RNAs and three protein-coding genes (cytochrome c oxidase 3 (cox3), cytochrome c oxidase 1 (cox1), and cytb) were identified.
A total of 110 avian haemosporidian mitochondrial genomes were retrieved from GenBank, encompassing three genera (Plasmodium, Haemoproteus, and Leucocytozoon). The non-protein coding region and the protein-coding genes exhibit a phylogenetic signal consistent with the previous mitogenomic phylogeny (Pacheco et al., 2018b, 2020; Lotta-Arévalo et al., 2023). Therefore, the alignment was performed using Clustal Omega v1.2.2 (Sievers et al., 2011) implemented in Geneious Prime 2023.2.1, which included these sequences and the three newly obtained mitochondrial sequences. The phylogenetic relationships were inferred from the alignment of partial mitochondrial genomes (5358 bp excluding gaps), which maintained their original order in the genome. Both Maximum Likelihood (ML) and Bayesian Inference (BI) methods were used to construct the phylogenetic tree. The substitution model was estimated for each method using ModelFinder v2.2.0 (Kalyaanamoorthy et al., 2017) implemented in PhyloSuite v1.2.3 (Zhang et al., 2020; Xiang et al., 2023). GTR+F+I+G4 was the best-fitting model for both methods based on the corrected Akaike information criterion (AIC) score. The BI phylogenies were inferred using MrBayes v3.2.7a (Ronquist et al., 2012) implemented in PhyloSuite v1.2.3. The Markov Chain Monte Carlo algorithm was run for 50 million generations, with sampling every 1000 generations, and the initial 25 % of the sampled data were discarded as “burn-in”. The ML phylogenies were executed with 1000 ultrafast (Minh et al., 2013) bootstraps using IQ-TREE v2.2.0 (Nguyen et al., 2015) implemented in PhyloSuite v1.2.3.
3. Results
3.1. Morphological characteristics
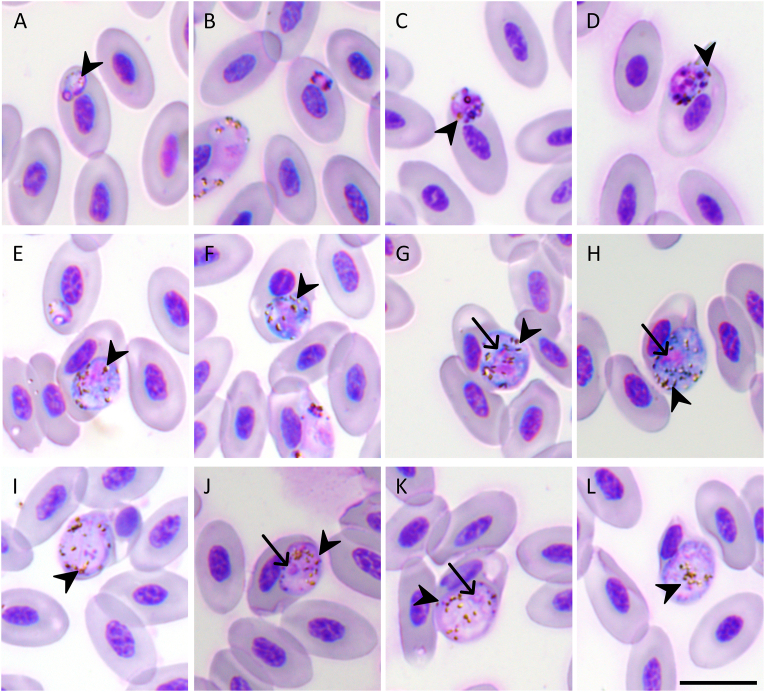
The gametocytes and meronts were identified based on standardized morphological criteria (Valkiūnas and Iezhova, 2018, 2022). Two distinct morphological forms of fully grown gametocytes were discernible: a roundish and a circumnuclear (Fig. 1, Fig. 2). The roundish, fully grown gametocytes induced substantial deformation of the infected erythrocytes, with pronounced displacement of the host cell nuclei (Fig. 1E–L). The cytoplasmic staining of these round-type gametocytes was darker than that of the circumnuclear forms. The circumnuclear gametocytes displayed a large vacuole (approximately 1.3 μm in diameter) (Fig. 2B and C) and morphologically altered the host erythrocytes, including the development of rounded nuclear conformations in some infected cells (Fig. 2D and H). In addition, microhalteridial-type gametocytes with an intensely stained vacuole-free cytoplasm were observed (Fig. 2I and J); however, their developmental status could not be determined due to the morphological variations that occur during gametocyte maturation. Putative macrogametes and/or zygotes were observed in the blood film (Fig. 2K and L), possibly because of the blood being collected and stored prior to being sent to the laboratory. In vitro development may have occurred during this period (Valkiūnas et al., 2021).
Fig. 1.
Haemosporidian parasites from the blood of Swinhoe’s pheasant (Lophura swinhoii). A Trophozoites. B-D Erythrocytic meronts. E-H Roundish macrogametocytes. I-L Roundish microgametocytes. Long arrows: parasite nuclei; arrowheads: pigment granules. Wright-Giemsa-stained thin blood films. Scale bar: 10 μm.
Fig. 2.
Haemosporidian parasites from the blood of Swinhoe’s pheasant (Lophura swinhoii). A-D Circumnuclear macrogametocytes. E-H Circumnuclear microgametocytes. I, J Microhalteridial gametocytes. K, L Putative macrogametes and/or zygotes. Long arrows: parasite nuclei; short arrows: vacuoles; arrowheads: pigment granules. Wright-Giemsa-stained thin blood films. Scale bar: 10 μm.
3.2. Molecular analyses and lineage identification
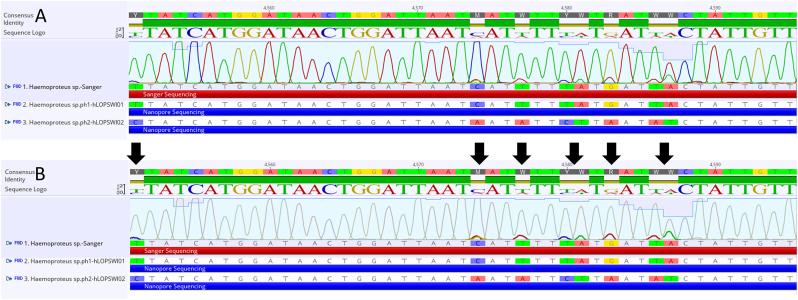
A 539 bp sequence that aligned with Haemoproteus sp. lineage hOTULEM01 (98.12 % similarity) in the MalAvi database was produced using Sanger sequencing. The ambiguous chromatographic peaks (such as positions 4583G/A and 4588A/T; Fig. 3) reveal possible mixed infections, thereby necessitating ONT sequencing for resolving the haplotypes.
Fig. 3.
Alignment of sequences from Sanger sequencing and Nanopore sequencing. A Chormatogram of Sanger sequencing demonstrated complete concordance between Sanger sequencing reads and lineage hLOPSWI01. B Highlight of the nine positions of low-abundance double base-calling (black arrows), which are consistent with lineage hLOPSWI02.
Sequencing on the MinION platform yielded 7343 reads (11 Mb total), of which 6744 and 54 were specifically mapped to Haemoproteus and Plasmodium, respectively, using Minimap2 v2.24. The long-read assemblies generated by Flye v2.9.1 showed genus-specific genomic signatures: three contigs were recovered, including two Haemoproteus spp. (pairwise identity 93.87 %; GenBank: LC867942, LC867943) and one Plasmodium sp. (GenBank: LC867944) contig. The results of BLASTn analysis against the MalAvi database (Bensch et al., 2009) identified the Plasmodium sequence as genetic lineage pNILSUN01 (GenBank: DQ659586). Both Haemoproteus lineages (hLOPSWI01 and hLOPSWI02) considerably diverged from the existing entries; LOP denoted host genus Lophura (Phasianidae) and SWI represented swinhoii based on the MalAvi guidelines, qualifying as novel genetic lineages. The results of cross-validation with the Sanger sequencing results revealed complete agreement between the conventional sequencing reads and hLOPSWI01, whereas the ONT reads resolved ambiguous base calls as a low-abundance hLOPSWI02 lineage (Fig. 3), thereby confirming a cryptic mixed infection.
3.3. Phylogenetic analysis of the avian haemosporidian mitochondrial genomes
The phylogenetic reconstruction of the avian haemosporidian mitogenomes showed congruent topologies between the BI and ML analyses (Fig. 4). The genus Leucocytozoon exhibited polyphyly, with its subgenus Leucocytozoon forming a sister clade with other avian haemosporidians. The subgenus Akiba was basally located with subgenus Parahaemoproteus (BI posterior probability/ML bootstrap: 0.71/68). The genus Haemoproteus showed polyphyly; however, the monophyly of its two subgenera, Haemoproteus and Parahaemoproteus, was strongly supported, consistent with prior studies (Pacheco et al., 2018b; Lotta-Arévalo et al., 2023). The clade containing H. multivacuolatus and H. nisi (infecting accipitrid raptors in France) (Harl et al., 2024) basally diverged to the Haemoproteus-Plasmodium complex, with maximum statistical support (1/100). The genetic lineage hBAREGI09 (Haemoproteus sp. ex Balearica regulorum), a sister taxon to H. antigonis (Shen et al., 2024), shared a common ancestor with the subgenus Haemoproteus (0.98/89). Both of the newly identified genetic lineages fell within the Parahaemoproteus clade: lineage hLOPSWI01 weakly aligned with the H. caprimulgi lineage hNYCTALB02 (ex Chordeiles acutipennis, Colombia; topology support: 0.46/40; patristic distance Δ = 0.032). Lineage hLOPSWI02 formed a strongly-supported clade with the H. major lineage hWW2 (ex Phylloscopus trochilus, Sweden; topology support: 1/99; patristic distance Δ = 0.038). Avian Plasmodium spp. resolved as a monophyletic group with distinct subclades, consistent with previously published taxonomic frameworks (Pacheco and Escalante, 2023). The identified Plasmodium sp. lineage pNILSUN01 clustered within the Haemamoeba-Giovannolaia clade, sharing ancestry with the P. (Giovannolaia) circumflexum lineage pTURDUS1 and Plasmodium sp. lineage pBT7 (topology support: 1/100; patristic distance Δ = 0.028).
Fig. 4.
Phylogenetic reconstructions of avian haemosporidian using mitochondrial genomes. Bayesian Inference (BI) and Maximum Likelihood (ML) phylogenies were inferred using 110 partial mitochondrial genomes (5358 bp excluding gaps) belonging to three genera. The values near branches are BI posterior probabilities/ML bootstrap values, respectively. The new mitochondrial genomes obtained in this study are indicated in bold.
4. Discussion
We successfully used ONT sequencing to resolve co-infections with avian haemosporidian parasites in Swinhoe’s pheasant, L. swinhoii, and identify three mitochondrial genomes. The integration of long-read sequencing overcame the limitations of traditional Sanger sequencing methods, which fail to separate co-infections in samples due to ambiguous chromatograms (Bernotienė et al., 2016). The capacity of ONT to generate mitochondrial contigs enabled species-level resolution, confirming cryptic co-infections of Plasmodium spp. lineage pNILSUN01 and two undescribed Haemoproteus lineages, named hLOPSWI01 and hLOPSWI02. This methodological advance aligns with efforts to leverage long-read sequencing for resolving complex haemosporidian assemblages (Pacheco et al., 2024).
The lineage pNILSUN01 (Plasmodium sp.), initially described in Niltava sundara (Passeriformes) from Myanmar (Beadell et al., 2006), has subsequently been detected across diverse passerine hosts in Asia (Ishtiaq et al., 2017; Menzies et al., 2021; Huang et al., 2022). These prior studies did not morphologically characterize this lineage. Our study documents the occurrence of the lineage pNILSUN01 in a galliform host (L. swinhoii), supporting the hypothesis that the host specificity of avian Plasmodium is low, showing cross-order transmission (Valkiūnas and Iezhova, 2018; Ciloglu et al., 2020). This characteristic sharply contrasts that of Haemoproteus spp., which typically reveal high host specificity and limited inter-order transmission (Valkiunas, 2004). Only six Haemoproteus spp. (H. lophortyx, H. ammoperdix, H. rileyi, H. mansoni, H. pratasi, and H. stableri) infecting galliform birds have been morphologically described in phasianid hosts (Valkiūnas and Iezhova, 2022). Haemoproteus rileyi was reported in the Taiwan bamboo partridge (Bambusicola sonorivox), another endemic Taiwanese species (Bennett and Peirce, 1989). However, the gametocyte morphology observed in our study diverges from previous descriptions of gametocyte morphology of Haemoproteus spp. described in phasianid hosts (Bennett and Peirce, 1989). In addition, to the best of our knowledge, no published study has used the associated genetic data to determine morphological and molecular taxonomy.
Molecular surveys of haemosporidian genetic lineages in hosts of the Phasianidae have been comprehensively investigated, with most studies concentrating on the domestic chicken (Gallus gallus). This host species has been documented to harbor one Haemoproteus lineage, 15 Plasmodium lineages, and 46 Leucocytozoon lineages (Bensch et al., 2009; Swangneat et al., 2025). However, sporadic molecular reports have been published for non-domestic phasianid hosts, predominantly involving Plasmodium spp. (Table 1). Haemosporidian studies on Phasianidae in Taiwan have exclusively relied on morphological methods (Manwell, 1962; Pan, 1963; Morii et al., 1986), leaving a gap regarding the molecular characterization of the parasites. We addressed this by obtaining molecular evidence of co-infections in the Swinhoe’s pheasant (L. swinhoii), thus highlighting the need to apply integrative taxonomic approaches in understudied avian hosts.
Table 1.
Genetic lineages of haemosporidian parasites in non-domestic phasianid hosts.
| Host species | Host subfamily | Parasite lineage | Location | Reference |
|---|---|---|---|---|
| Crossoptilon crossoptilon | Phasianinae | Plasmodium juxtancleare pGALLUS02 | Japan (captive) | Murata et al. (2008) |
| Pavo cristatus | Phasianinae | Plasmodium elongatum pGRW06 | Brazil (captive) | Chagas et al. (2017) |
| Plasmodium sp. pDENVID01 | ||||
| Pavo muticus | Plasmodium sp. pDENVID01 | |||
| Lophura swinhoii | Phasianinae | Haemoproteus sp. hLOPSWI01&02 | Taiwan | This study |
| Plasmodium sp. pNILSUN01 | ||||
| Peliperdix sephaena | Perdicinae | Plasmodium sp. pPELSEP01-04 | South Africa | Ndlovu et al. (2024) |
| Alaskan grouse and ptarmigan | Tetraoninae | Haemoproteus sp. hAKGPH01-05 | USA | Smith et al. (2016) |
| Plasmodium sp. pAKGPP01-03 | ||||
| Leucocytozoon sp. lAKGPL01-14 | ||||
| Alaskan grouse and ptarmigan | Tetraoninae | Haemoproteus sp. hTETURO01-02 | USA | De Amaral et al. (2023) |
| Plasmodium sp. pBT7 | ||||
| Leucocytozoon sp. lCOLBF22, lCOLBF24, lAKGPL09, lAKGPL14, lLAGLAG02-04, lGALLUS25, lCYASTE01&02, lSPISEN05 | ||||
| Meleagris gallopavo | Meleagridinae | Leucocytozoon sp. lGHA146 | Ghana | Agbemelo-Tsomafo et al. (2023) |
| Meleagris gallopavo | Meleagridinae | Plasmodium gallinaceum pGALLUS01, pMELGAL | Thailand | Chatan et al. (2024) |
| Plasmodium juxtancleare pGALLUS02 | ||||
| Plasmodium sp. pACCBAD01 |
We identified two distinct gametocyte morphotypes in blood smears: roundish and circumnuclear types (Fig. 1, Fig. 2). Although three mitochondrial lineages were detected molecularly, the morphology of the third lineage (microhalteridial-type gametocytes) was not assigned (Fig. 2I and J). These microhalteridial forms may represent immature developmental stages of the observed roundish or circumnuclear gametocytes, as the morphology of immature gametocytes often differs from that of fully mature gametocytes (Valkiunas, 2004). Differentiating Haemoproteus and Plasmodium spp. based on gametocyte morphology is inherently challenging, as both genera exclusively develop within erythrocytes and produce haemozoin pigment granules (Valkiūnas and Iezhova, 2018, 2022).
Roundish gametocytes are rare among Haemoproteus spp., with fully mature forms definitively documented only in H. ortalidum (parasitizing Neotropical Galliformes) (Valkiunas, 2004) and H. parus (Passeriformes) (Bennett, 1989). The roundish gametocytes studied here differed morphologically from the macrogametocytes of H. ortalidum, which characteristically display a large, clear vacuole (Valkiunas, 2004). The genetic divergence (96 % identity) between the lineages we identified and H. ortalidum (GenBank: MW899346) further supports their distinct taxonomic status. The roundish gametocytes share morphological similarities with H. parus, originally described in Parus bicolor (Passeriformes) (Bennett, 1989), including deep cytoplasmic staining, scattered pigment granules, larger gametocyte dimensions, and erythrocyte deformation. However, H. parus had not been molecularly characterized. The subsequent morphological analyses indicated that this species belongs to Plasmodium (Haemamoeba) owing to overlapping features (Valkiunas, 2004; Valkiūnas and Iezhova, 2022). The roundish morphotype may correspond to Plasmodium lineage pNILSUN01, which clustered within the Giovannolaia-Haemamoeba clade in the phylogenetic tree in our study. These findings align with prior hypotheses positing misclassification of certain “roundish Haemoproteus” morphotypes as Plasmodium spp., particularly because of the association of the lineage with hosts of the Passeriformes (Valkiunas, 2004). Circumnuclear-type gametocytes positioning further complicates diagnostics, as this morphology occurs in Plasmodium (predominantly subgenus Giovannolaia) and Haemoproteus (subgenus Parahaemoproteus) (Valkiūnas and Iezhova, 2018, 2022). The molecular lineages phylogenetically corresponded to the Giovannolaia-Haemamoeba and Parahaemoproteus clades in our study, agreeing with the observed circumnuclear morphology. However, resolving the precise morpho-molecular linkages requires further sampling across multiple host individuals to account for potential developmental polymorphism and interspecific variation.
Long-read sequencing technologies can resolve complex haemosporidian co-infections, addressing the limitations of traditional Sanger sequencing and short-read approaches (Pacheco et al., 2024). These technologies generate unfragmented mitochondrial genomes, enabling precise haplotype phasing and accurate detection of co-infecting lineages, unlike short-read methods, which require fragment assembly and cannot resolve haplotypes in mixed infections (Pacheco and Escalante, 2023; Pacheco et al., 2024). Although PacBio HiFi sequencing has been validated for this purpose (Pacheco et al., 2024), the present study represents the first application of ONT in avian haemosporidian co-infection resolution. The real-time data streaming and adaptive sampling capabilities of ONT are advantageous in co-infection resolution compared with PacBio HiFi. The ability of ONT to dynamically adjust sequencing parameters during runs without prior library modification allows the targeted enrichment of low-abundance lineages, which is critical for comprehensive detection of the pathogens (Petersen et al., 2019; Shafin et al., 2020; Wang et al., 2021; De Meulenaere et al., 2024). In contrast, the circular consensus sequencing mode in PacBio lacks this adaptability, requiring fixed run durations and post-sequencing bioinformatic corrections (Wenger et al., 2019). Furthermore, the portability of ONT devices facilitates field-deployable sequencing in remote avian habitats, and the flexible run configurations and reusable flow cells of ONT substantially reduce the operational costs compared with those of PacBio (Wang et al., 2021; Girgis et al., 2023; Matoute et al., 2024; De Cesare et al., 2024; Holzschuh et al., 2024). However, we identified limitations in the primer affinity biases during mitochondrial genome amplification. The disproportionate read counts of Haemoproteus (6744 reads) versus Plasmodium (54 reads) indicate the preferential amplification of certain lineages. Such biases are similar to the challenges reported in conventional PCR assays, where lineage-specific primer affinities skew detection sensitivity (Bernotienė et al., 2016; Ciloglu et al., 2019). As such, future studies should prioritize optimizing multiplex PCR primer sets to achieve balanced amplification across genera or implementing direct nanopore adaptive sequencing to reduce host DNA interference and preferentially enrich parasite genomes. A combinatorial strategy using phased primer panels (De Cesare et al., 2024; Killander et al., 2025), validated through in silico binding affinity analysis and experimental co-infection models, may prevent or reduce amplification bias. Alternatively, nanopore adaptive sequencing protocols that selectively sequence haemosporidian DNA during real-time runs (De Meulenaere et al., 2024) could enhance mitochondrial genome recovery from mixed infections by avoiding PCR competition. Finally, a standardized multigene reference database across mitochondrial, apicoplast, and nuclear loci is required to reconcile morphological descriptions with molecular lineage diversity in understudied avian hosts.
5. Conclusions
This study revealed that ONT effectively resolves complex avian haemosporidian co-infections in Swinhoe’s pheasant (L. swinhoii), identifying two novel Haemoproteus lineages (hLOPSWI01 and hLOPSWI02) and a Plasmodium lineage (pNILSUN01) through mitochondrial genome assembly and phylogenetic reconstruction. By generating unfragmented mitochondrial genomes, ONT overcame the limitations of traditional Sanger sequencing that obscure mixed infections, whereas phylogenetic analyses validated cryptic lineage differentiation within morphologically ambiguous gametocytes. These findings emphasize the necessity of integrating long-read genomics with morphological validation to advance taxonomic accuracy in avian haemosporidians, particularly for conservation-priority species threatened by co-infection-driven pathogenicity. Overall, this study provides a methodological framework for resolving cryptic parasite diversity in island-endemic birds, informing both evolutionary ecology and wildlife disease management strategies.
Ethical approval
Not applicable.
CRediT authorship contribution statement
Peihang Hong: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Sijia Yu: Data curation, Methodology, Writing – original draft. Hau-You Tzeng: Conceptualization, Methodology, Writing – review & editing. Yu-Hsuan Lin: Methodology, Investigation. Chao-Min Wang: Conceptualization, Validation, Resources. Chung-Hung Lai: Resources, Supervision, Writing – review & editing. Shyun Chou: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors sincerely thank Professor Wei-Li Hsu for providing access to laboratory equipment essential for this study. We also thank Editage (www.editage.com.tw) for English language editing assistance. This work would not have been possible without their invaluable support.
Data availability
The nucleotide sequences obtained in this study were deposited in the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp) with the following accession numbers: LC867942, LC867943 and LC867944.
References
- Agbemelo-Tsomafo C., Adjei S., Kusi K.A., Deitsch K.W., Amoah D., Obeng-Kyeremeh, et al. Prevalence of Leucocytozoon infection in domestic birds in Ghana. PLoS One. 2023;18 doi: 10.1371/journal.pone.0294066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeckens S., Van Damme R. The island syndrome. Curr. Biol. 2020;30:R338–R339. doi: 10.1016/j.cub.2020.03.029. [DOI] [PubMed] [Google Scholar]
- Beadell J.S., Ishtiaq F., Covas R., Melo M., Warren B.H., Atkinson C.T., et al. Global phylogeographic limits of Hawaii's avian malaria. Proc. R. Soc. B Biol. Sci. 2006;273:2935–2944. doi: 10.1098/rspb.2006.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G.F. New species of haemoproteids from the avian families Paridae and Sittidae. Can. J. Zool. 1989;67:2685–2688. doi: 10.1139/z89-379. [DOI] [Google Scholar]
- Bennett G.F., Peirce M.A. The haemoproteids of the avian family Phasianidae. Can. J. Zool. 1989;67:1557–1565. doi: 10.1139/z89-221. [DOI] [Google Scholar]
- Bensch S., Hellgren O. In: Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics. Santiago-Alarcon D., Marzal A., editors. Springer International Publishing; Cham: 2020. The use of molecular methods in studies of avian haemosporidians; pp. 113–135. [DOI] [Google Scholar]
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Bernotienė R., Palinauskas V., Iezhova T., Murauskaitė D., Valkiūnas G. Avian haemosporidian parasites (Haemosporida): A comparative analysis of different polymerase chain reaction assays in detection of mixed infections. Exp. Parasitol. 2016;163:31–37. doi: 10.1016/j.exppara.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Bird Life International . IUCN Red List of Threatened Species. 2019. Lophura swinhoii. [DOI] [Google Scholar]
- Chagas C.R.F., Valkiūnas G., De Oliveira Guimarães L., Monteiro E.F., Guida F.J.V., Simões R.F., et al. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar. J. 2017;16:83. doi: 10.1186/s12936-017-1729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatan W., Khemthong K., Akkharaphichet K., Suwarach P., Seerintra T., Piratae S. Molecular survey and genetic diversity of Plasmodium sp. infesting domestic poultry in northeastern Thailand. J. Vet. Res. 2024;68:101–108. doi: 10.2478/jvetres-2024-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciloglu A., Ellis V.A., Bernotienė R., Valkiūnas G., Bensch S. A new one-step multiplex PCR assay for simultaneous detection and identification of avian haemosporidian parasites. Parasitol. Res. 2019;118:191–201. doi: 10.1007/s00436-018-6153-7. [DOI] [PubMed] [Google Scholar]
- Ciloglu A., Ellis V.A., Duc M., Downing P.A., Inci A., Bensch S. Evolution of vector-transmitted parasites by host switching revealed through sequencing of Haemoproteus parasite mitochondrial genomes. Mol. Phylogenet. Evol. 2020;153 doi: 10.1016/j.ympev.2020.106947. [DOI] [PubMed] [Google Scholar]
- Clark N.J., Clegg S.M., Lima M.R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New insights from molecular data. Int. J. Parasitol. 2014;44:329–338. doi: 10.1016/j.ijpara.2014.01.004. [DOI] [PubMed] [Google Scholar]
- De Amaral F., Wilson R.E., Sonsthagen S.A., Sehgal R. Diversity, distribution, and methodological considerations of haemosporidian infections among Galliformes in Alaska. Int. J. Parasitol. Parasites Wildl. 2023;20:122–132. doi: 10.1016/j.ijppaw.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesare M., Mwenda M., Jeffreys A.E., Chirwa J., Drakeley C., Schneider K., et al. Flexible and cost-effective genomic surveillance of P. falciparum malaria with targeted nanopore sequencing. Nat. Commun. 2024;15:1413. doi: 10.1038/s41467-024-45688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meulenaere K., Cuypers W.L., Gauglitz J.M., Guetens P., Rosanas-Urgell A., Laukens K., Cuypers B. Selective whole-genome sequencing of Plasmodium parasites directly from blood samples by nanopore adaptive sampling. mBio. 2024;15 doi: 10.1128/mbio.01967-23. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Viraqué F., Pita S., Greif G., De Souza R.D.C.M., Iraola G., Robello C. Nanopore sequencing significantly improves genome assembly of the protozoan parasite Trypanosoma cruzi. Genome Biol. Evol. 2019;11:1952–1957. doi: 10.1093/gbe/evz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V.A., Bensch S. Host specificity of avian haemosporidian parasites is unrelated among sister lineages but shows phylogenetic signal across larger clades. Int. J. Parasitol. 2018;48:897–902. doi: 10.1016/j.ijpara.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Feagin J.E., Harrell M.I., Lee J.C., Coe K.J., Sands B.H., Cannone J.J., et al. The fragmented mitochondrial ribosomal RNAs of Plasmodium falciparum. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis S.T., Adika E., Nenyewodey F.E., Senoo Jnr D.K., Ngoi J.M., Bandoh K., et al. Drug resistance and vaccine target surveillance of Plasmodium falciparum using nanopore sequencing in Ghana. Nat. Microbiol. 2023;8:2365–2377. doi: 10.1038/s41564-023-01516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harl J., Fauchois A., Puech M.-P., Gey D., Ariey F., Izac B., et al. Novel phylogenetic clade of avian Haemoproteus parasites (Haemosporida, Haemoproteidae) from accipitridae raptors, with description of a new Haemoproteus species. Parasite. 2024;31:5. doi: 10.1051/parasite/2023066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren O., Waldenström J., Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- Higuera A., Salas-Leiva D.E., Curtis B., Patiño L.H., Zhao D., Jerlström-Hultqvist J., et al. Draft genomes of Blastocystis subtypes from human samples of Colombia. Parasites Vectors. 2023;16:52. doi: 10.1186/s13071-022-05619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh A., Lerch A., Fakih B.S., Aliy S.M., Ali M.H., Ali M.A., et al. Using a mobile nanopore sequencing lab for end-to-end genomic surveillance of Plasmodium falciparum: A feasibility study. PLoS Glob. Public Health. 2024;4 doi: 10.1371/journal.pgph.0002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Chen Z., Yang G., Xia C., Luo Q., Gao X., Dong L. Assemblages of Plasmodium and related parasites in birds with different migration statuses. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms231810277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins L.G., Colella V., Young N.D., Traub R.J. Metabarcoding using nanopore long‐read sequencing for the unbiased characterization of apicomplexan haemoparasites. Mol. Ecol. Resour. 2024;24 doi: 10.1111/1755-0998.13878. [DOI] [PubMed] [Google Scholar]
- Huggins L.G., Namgyel U., Wangchuk P., Atapattu U., Traub R., Colella V. Metabarcoding using nanopore sequencing enables identification of diverse and zoonotic vector-borne pathogens from neglected regions: a case study investigating dogs from Bhutan. One Health. 2024;19 doi: 10.1016/j.onehlt.2024.100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishtiaq F., Bowden C.G.R., Jhala Y.V. Seasonal dynamics in mosquito abundance and temperature do not influence avian malaria prevalence in the Himalayan foothills. Ecol. Evol. 2017;7:8040–8057. doi: 10.1002/ece3.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez P., Muñoz M., Cruz-Saavedra L., Camargo A., Ramírez J.D. Blastocystis genetic diversity in animal and human samples from different departments of Colombia using complete sequencing of the 18S rRNA gene (SSU rRNA) by Oxford Nanopore Technologies (ONT) Acta Trop. 2024;249 doi: 10.1016/j.actatropica.2023.107090. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadjian G., Hassanin A., Saintpierre B., Gembu Tungaluna G.-C., Ariey F., Ayala F.J., et al. Highly rearranged mitochondrial genome in Nycteria parasites (Haemosporidia) from bats. Proc. Natl. Acad. Sci. USA. 2016;113:9834–9839. doi: 10.1073/pnas.1610643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killander G., Isak G., Linde A.-M., Advani A., Rönnberg C., Bujila I. Duplex PCR-nanopore sequencing assay for Cryptosporidium species and subtype determination. Infect. Genet. Evol. 2025;129 doi: 10.1016/j.meegid.2025.105727. [DOI] [PubMed] [Google Scholar]
- Kolmogorov M., Yuan J., Lin Y., Pevzner P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Lee V.V., Judd L.M., Jex A.R., Holt K.E., Tonkin C.J., Ralph S.A. Direct nanopore sequencing of mRNA reveals landscape of transcript isoforms in apicomplexan parasites. mSystems. 2021;6 doi: 10.1128/msystems.01081-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti N., Schürch N., Bruggmann R., Wittwer M. Nanopore sequencing improves the draft genome of the human pathogenic amoeba Naegleria fowleri. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-52572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Yuan J., Kolmogorov M., Shen M.W., Chaisson M., Pevzner P.A. Assembly of long error-prone reads using de Bruijn graphs. Proc. Natl. Acad. Sci. USA. 2016;113:E8396–E8405. doi: 10.1073/pnas.1604560113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta-Arévalo I.A., González A.D., Gamboa-Suárez B.A., Pacheco M.A., Escalante A.A., Moreno C., et al. Haemosporidians in non-passerine birds of Colombia: An overview of the last 20 years of research. Diversity. 2023;15:57. doi: 10.3390/d15010057. [DOI] [Google Scholar]
- Manwell R.D. A new species of avian Plasmodium. J. Protozool. 1962;9:401–403. doi: 10.1111/j.1550-7408.1962.tb02642.x. [DOI] [Google Scholar]
- Martínez J., Martínez-De La Puente J., Herrero J., Del Cerro S., Lobato E., Rivero-De Aguilar J., et al. A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: On the inefficiency of general primers for detection of mixed infections. Parasitology. 2009;136:713–722. doi: 10.1017/S0031182009006118. [DOI] [PubMed] [Google Scholar]
- Marzal A., Bensch S., Reviriego M., Balbontin J., De Lope F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008;21:979–987. doi: 10.1111/j.1420-9101.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- Matoute A., Maestri S., Saout M., Laghoe L., Simon S., Blanquart H., et al. Meat-borne-parasite: A nanopore-based meta-barcoding workflow for parasitic microbiodiversity assessment in the wild fauna of French Guiana. Curr. Issues Mol. Biol. 2024;46:3810–3821. doi: 10.3390/cimb46050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V.K., Okhuysen P.C., Chappell C.L., Mahmoud M., Mahmoud M., Meng Q., et al. Fully resolved assembly of Cryptosporidium parvum. GigaScience. 2022;11 doi: 10.1093/gigascience/giac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies R.K., Borah J.R., Srinivasan U., Ishtiaq F. The effect of habitat quality on the blood parasite assemblage in understorey avian insectivores in the Eastern Himalaya, India. Ibis. 2021;163:962–976. doi: 10.1111/ibi.12927. [DOI] [Google Scholar]
- Minh B.Q., Nguyen M.A.T., von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii T., Nakamura K., Lee Y.C., Iijima T., Hoji K. Observations on the Taiwanese strain of Leucocytozoon caulleryi (Haemosporina) in chickens. J. Protozool. 1986;33:231–234. doi: 10.1111/j.1550-7408.1986.tb05597.x. [DOI] [PubMed] [Google Scholar]
- Murata K., Nii R., Sasaki E., Ishikawa S., Sato Y., Sawabe K., Tsuda Y., Matsumoto R., Suda A., Ueda M. Plasmodium (Bennettinia) juxtanucleare infection in a captive white eared-pheasant (Crossoptilon crossoptilon) at a Japanese zoo. J. Vet. Med. Sci. 2008;70:203–205. doi: 10.1292/jvms.70.203. [DOI] [PubMed] [Google Scholar]
- Musa S. Mitochondrial genome amplification of avian haemosporidian parasites from single-infected wildlife samples using a novel nested PCR approach. Parasitol. Res. 2023;122:2967–2975. doi: 10.1007/s00436-023-07986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namasivayam S., Baptista R.P., Xiao W., Hall E.M., Doggett J.S., Troell K., Kissinger J.C. A novel fragmented mitochondrial genome in the protist pathogen Toxoplasma gondii and related tissue coccidia. Genome Res. 2021;31:852–865. doi: 10.1101/gr.266403.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndlovu M., Wardjomto M.B., Pori T., Nangammbi T.C. Diversity and host specificity of avian haemosporidians in an Afrotropical conservation region. Animals. 2024;14:2906. doi: 10.3390/ani14192906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco M.A., Cepeda A.S., Bernotienė R., Lotta I.A., Matta N.E., Valkiūnas G., Escalante A.A. Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. Int. J. Parasitol. 2018;48:657–670. doi: 10.1016/j.ijpara.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco M.A., Cepeda A.S., Miller E.A., Beckerman S., Oswald M., London E., et al. A new long-read mitochondrial-genome protocol (PacBio HiFi) for haemosporidian parasites: A tool for population and biodiversity studies. Malar. J. 2024;23:134. doi: 10.1186/s12936-024-04961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco M.A., Ceríaco L.M.P., Matta N.E., Vargas-Ramírez M., Bauer A.M., Escalante A.A. A phylogenetic study of Haemocystidium parasites and other haemosporida using complete mitochondrial genome sequences. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104576. [DOI] [PubMed] [Google Scholar]
- Pacheco M.A., Escalante A.A. Origin and diversity of malaria parasites and other Haemosporida. Trends Parasitol. 2023;39:501–516. doi: 10.1016/j.pt.2023.04.004. [DOI] [PubMed] [Google Scholar]
- Pacheco M.A., Matta N.E., Valkiūnas G., Parker P.G., Mello B., Stanley C.E., et al. Mode and rate of evolution of haemosporidian mitochondrial genomes: timing the radiation of avian parasites. Mol. Biol. Evol. 2018;35:383–403. doi: 10.1093/molbev/msx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan I.C. A new interpretation of the gametogony of Leucocytozoon caulleryi in chickens. Avian Dis. 1963;7:361. doi: 10.2307/1587871. [DOI] [PubMed] [Google Scholar]
- Pérez-Tris J., Bensch S. Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitology. 2005;131:15–23. doi: 10.1017/S003118200500733X. [DOI] [PubMed] [Google Scholar]
- Petersen L.M., Martin I.W., Moschetti W.E., Kershaw C.M., Tsongalis G.J. Third-generation sequencing in the clinical laboratory: Exploring the advantages and challenges of nanopore sequencing. J. Clin. Microbiol. 2019;58 doi: 10.1128/jcm.01315-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E., Fallon S.M. Diversification and host switching in avian malaria parasites. Proc. R. Soc. B Biol. Sci. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero A., Gandon S. Evolutionary ecology of avian malaria: Past to present. Trends Parasitol. 2018;34:712–726. doi: 10.1016/j.pt.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runtuwene L.R., Tuda J.S.B., Mongan A.E., Makalowski W., Frith M.C., Imwong M., et al. Nanopore sequencing of drug-resistance-associated genes in malaria parasites, Plasmodium falciparum. Sci. Rep. 2018;8:8286. doi: 10.1038/s41598-018-26334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Alarcon D., Marzal A. In: Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics. Santiago-Alarcon D., Marzal A., editors. Springer International Publishing; Cham: 2020. Research on avian haemosporidian parasites in the tropics before the year 2000; pp. 1–44. [DOI] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafin K., Pesout T., Lorig-Roach R., Haukness M., Olsen H.E., Bosworth C., et al. Nanopore sequencing and the Shasta toolkit enable efficient de novo assembly of eleven human genomes. Nat. Biotechnol. 2020;38:1044–1053. doi: 10.1038/s41587-020-0503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Zhai J., Li Y., Gan Y., Liang X., Yu H., et al. Identification of Haemoproteus infection in an imported grey crowned crane (Balearica regulorum) in China. Parasitol. Res. 2024;123:349. doi: 10.1007/s00436-024-08373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using clustal omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Iturriza A., Ketmaier V., Tiedemann R. Prevalence of avian haemosporidian parasites and their host fidelity in the central Philippine islands. Parasitol. Int. 2012;61:650–657. doi: 10.1016/j.parint.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Smith M.M., Van Hemert C., Merizon R. Haemosporidian parasite infections in grouse and ptarmigan: Prevalence and genetic diversity of blood parasites in resident Alaskan birds. Int. J. Parasitol. Parasites Wildl. 2016;5:229–239. doi: 10.1016/j.ijppaw.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swangneat K., Srikacha N., Soulinthone N., Paudel S., Srisanyong W., Stott C.J., et al. Molecular prevalence of avian haemosporidian parasites in Southeast Asia: Systematic review and meta-analysis. Animals. 2025;15:636. doi: 10.3390/ani15050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff S., Hillebrand A., Drakoulis N., Gluhic Z., Maschmann S., Lyko P., et al. Small RNAs from mitochondrial genome recombination sites are incorporated into T. gondii mitoribosomes. eLife. 2024;13 doi: 10.7554/eLife.95407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiunas G. CRC Press; Boca Raton: 2004. Avian Malaria Parasites and Other Haemosporidia. [DOI] [Google Scholar]
- Valkiūnas G., Bensch S., Iezhova T.A., Križanauskienė A., Hellgren O., Bolshakov C.V. Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: Microscopy is still essential. J. Parasitol. 2006;92:418–422. doi: 10.1645/GE-3547RN.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G., Iezhova T.A. Keys to the avian malaria parasites. Malar. J. 2018;17:212. doi: 10.1186/s12936-018-2359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiūnas G., Iezhova T.A. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae) Malar. J. 2022;21:269. doi: 10.1186/s12936-022-04235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiūnas G., Iezhova T.A., Duc M., Dunn J.C., Bensch S. A new blood parasite of the accentor birds: Description, molecular characterization, phylogenetic relationships and distribution. Parasitology. 2024;151:1163–1173. doi: 10.1017/S0031182024000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiūnas G., Iezhova T.A., Shapoval A.P. High prevalence of blood parasites in hawfinch Coccothraustes coccothraustes. J. Nat. Hist. 2003;37:2647–2652. doi: 10.1080/002229302100001033221. [DOI] [Google Scholar]
- Valkiūnas G., Ilgūnas M., Bukauskaitė D., Duc M., Iezhova T.A. Description of Haemoproteus asymmetricus n. sp. (Haemoproteidae), with remarks on predictability of the DNA haplotype networks in haemosporidian parasite taxonomy research. Acta Trop. 2021;218 doi: 10.1016/j.actatropica.2021.105905. [DOI] [PubMed] [Google Scholar]
- Vieira L.M. de C., Pereira P.H.O., Vilela D.A. da R., Landau I., Pacheco M.A., Escalante A.A., et al. Leucocytozoon cariamae n. sp. and Haemoproteus pulcher coinfection in Cariama cristata (Aves: Cariamiformes): First mitochondrial genome analysis and morphological description of a leucocytozoid in Brazil. Parasitology. 2023;150:1296–1306. doi: 10.1017/S0031182023000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yunhao, Zhao Y., Bollas A., Wang Yuru, Au K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021;39:1348–1365. doi: 10.1038/s41587-021-01108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger A.M., Peluso P., Rowell W.J., Chang P.-C., Hall R.J., Concepcion G.T., et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat. Biotechnol. 2019;37:1155–1162. doi: 10.1038/s41587-019-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C.-Y., Gao F., Jakovlić I., Lei H.-P., Hu Y., Zhang H., et al. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta. 2023;2:e87. doi: 10.1002/imt2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wang C., Xu C., Yuan J., Wang G., Wu Y., et al. Genomic evolution of island birds from the view of the Swinhoe's pheasant (Lophura swinhoii) Mol. Ecol. Resour. 2024;24 doi: 10.1111/1755-0998.13896. [DOI] [PubMed] [Google Scholar]
- Yeo H., Harjoko D.N., Rheindt F.E. Double trouble: Untangling mixed sequence signals in bird samples with avian haemosporidian co-infections. Parasitology. 2022;149:799–810. doi: 10.1017/S0031182022000245. [DOI] [PubMed] [Google Scholar]
- Zhang D., Gao F., Jakovlić I., Zou H., Zhang J., Li W.X., Wang G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020;20:348–355. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
- Zhang K., Cai Y., Chen Y., Fu Y., Zhu Z., Huang J., et al. Chromosome-level genome assembly of Eimeria tenella at the single-oocyst level. BMC Genom. 2025;26:257. doi: 10.1186/s12864-025-11423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nucleotide sequences obtained in this study were deposited in the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp) with the following accession numbers: LC867942, LC867943 and LC867944.