Abstract
Ostracism (i.e., being ignored/excluded) can cause intense emotional reactions that detrimentally impact mental and physical health. Adolescents may be particularly susceptible to these negative consequences due to brain maturation and changing social priorities. To better understand how neural mechanisms of ostracism vary across development (i.e., age, puberty), the current study employed a pictorial adaptation of Hudac’s (2019) Lunchroom electroencephalography (EEG) task in a sample of 84 adolescents (aged 10–14 years). Results indicated unique effects across event-related potential amplitudes, including a reversed pattern (greater sensitivity to inclusion) for the P1, the “classic” ostracism effect (greater sensitivity to exclusion) for the N2, and classic effects when modulated by puberty for the P3. Source estimation identified different neural networks that were likely driving sensitivity to exclusion (e.g., amygdala, SCG, and IFG) or inclusion (e.g., ACC, cingulate, fusiform, insula, SPL, STG). Further, sensitivity to exclusion increased over pubertal development for P3 amplitude but over age for amygdala and IFG. Sensitivity to inclusion decreased over age for P1 amplitude and inclusion sensitive regions. The current study emphasizes the utility of using paradigms that isolate neural processes associated with ostracism while controlling for participant involvement.
Keywords: Ostracism, Social exclusion and inclusion, Affective processes, Adolescence, Electroencephalography (EEG), Puberty
Graphical Abstract
Highlights
-
•
Broadly, social primes increased ERP amplitudes and neural sources activation.
-
•
P1 amplitude was increased to inclusion events but decreased with age.
-
•
N2 amplitude was increased to exclusion relative to inclusion events.
-
•
Exclusion effects increased with maturation for P3 and with age for AMY and IFG.
-
•
Inclusion effects decreased with maturation for ACC, fusiform, insula, MFG, and STG.
1. Introduction
Ostracism (i.e. being ignored and/or excluded) is a negative social experience that influences a variety of affective, cognitive, and behavioral responses. For instance, ostracism can reduce self-esteem, threaten meaningful existence, and lead to harmful psychological consequences (Rudert et al., 2020, Williams and Nida, 2011). The temporal need-threat model of ostracism (Williams, 2009) posits that reactions to ostracism occur in three stages, including an immediate reflexive pain response, an attempt to understand and cope, and resignation in instances of persistent ostracism. While ostracism is a universal experience (Nezlek et al., 2012) and reactions to ostracism are adaptive in that they alert individuals to potential social danger (Hales et al., 2016), heightened sensitivity to ostracism is related to worse mental health outcomes (Lei et al., 2024, Lobmaier et al., 2019) and affective disorders that may perpetuate ostracism (Reinhard et al., 2020).
Adolescence is marked by increased sensitivity to social exclusion (Masten et al., 2009, Sebastian et al., 2010) and a greater desire to be accepted, especially by peers (Altikulaç et al., 2019, Gunther Moor et al., 2010). Since these developmental processes help adolescents pursue and navigate new social connections outside their family, it is critical to characterize and differentiate normative sensitivity to exclusion from a heightened sensitivity that could create or worsen mental health issues. Using event-related potentials (ERP) via electroencephalography (EEG) provides an opportunity to distinguish between individual and developmental differences by examining individuals’ reflexive, immediate response to ostracism at the millisecond level.
The neural bases of reflexive responses to ostracism are often evaluated using Williams et al., (2000) Cyberball task, which is an online ball-tossing game where participants believe they are playing with other people, although the frequency of ostracism is manipulated experimentally. Results consistently find that enduring two to three minutes of ostracism in this context produces strong negative emotions, especially sadness and anger (Williams, 2009). Functional magnetic resonance imaging (fMRI) studies have shown that social pain, the painful feelings that follow social rejection, exclusion, or loss, relies on some of the same neural regions that process physical pain (e.g., anterior cingulate cortex [ACC]) as well as emotional distress (e.g., ventrolateral prefrontal cortex, insula; see Eisenberger, 2015a; Masten et al., 2009).
ERP work using the Cyberball paradigm in adults and adolescents indicates an early N2 component over frontal electrodes between 100 and 250 ms post-stimulus onset that is sensitive to exclusion (Themanson et al., 2013). Additionally, a mid-latency P3 component elicited between 250 and 600 ms post-stimulus onset is increased during exclusion periods (Crowley et al., 2010). These components have been associated with the “neural alarm” that is thought to be set off by the ACC when conflict occurs (Botvinick et al., 2001, Themanson et al., 2013, Yeung et al., 2004). Thus, it is clear that the brain is sensitive to exclusion in adolescence. As adolescence is defined broadly as a transition period from childhood to adulthood marked by the onset of puberty (Sawyer et al., 2018), adolescent samples may include individuals anywhere from 10 to 24 years of age. Despite this broad categorization, very few studies have investigated developmental differences across different age ranges or developmental stages within adolescence. This is surprising given the large-scale social and maturational changes occurring during this developmental period. Indeed, a recent systematic review (see Mills et al., 2024) found only four studies (Crowley et al., 2010, McPartland et al., 2011, Sreekrishnan et al., 2014, White et al., 2012) that examined ERP correlates in early adolescence (e.g., 10–14 years) in which they either controlled for age or did not find age to be a significant predictor of neural responses to ostracism.
The lack of early adolescent developmental differences to exclusion during Cyberball could be due to task characteristics, including minimal participant involvement during the exclusion condition. In fact, research has critiqued the ability to make comparisons between the exclusion and inclusion conditions (Hudac, 2019, Themanson et al., 2015). Additionally, while the inclusion condition may be an effective control comparison, it does not necessarily evoke specific feelings related to social inclusion (Simard and Dandeneau, 2018). To account for this, some researchers utilizing Cyberball have incorporated an overinclusion condition (i.e., participants receiving a majority of the throws) to better delineate between responses to social exclusion and inclusion. This research has found that P3 amplitude (Niedeggen et al., 2014) and activity in the dorsal ACC and posterior insula (Cheng et al., 2020) decrease in response to overinclusion. While these findings begin to capture nuanced differences in neural responses to social exclusion and inclusion, the Cyberball exclusion condition remains a relatively passive task for participants. Thus, it is unclear if differences in neural responses to social inclusion (or overinclusion) and exclusion are modulated by the ostracism/affective experiences or if these responses are influenced by the amount of participant involvement between the conditions. As such, Hudac (2019) created a paradigm known as the Lunchroom task to address the inequity of participant involvement across conditions within Cyberball by controlling the amount of active involvement preceding the ostracism event.
The Lunchroom task is set in a social context (a lunchroom table) where exclusion or inclusion feedback is presented as a discrete event following the participant’s decision, rather than as part of an ongoing interaction. In each trial, the participant chooses between written descriptions of two object options. After the participant makes their decision, their “friends” either choose to sit at the table with the participant’s avatar (included) or sit at a different table (excluded). ERP outcomes are time-locked to the moment participants learn where their “friends” choose to sit. Importantly, the choices presented to participants vary based upon context, such that participants choose between pairs of social stimuli or nonsocial stimuli. Thus, the exclusion or inclusion feedback is primed by either a social or nonsocial decision and participants are required to put in identical amounts of effort prior to the feedback phase. Results from this task in an adult sample suggest that the N2 component was sensitive to social priming, while the P3 component discriminated between ostracism and inclusion (Hudac, 2019). In addition, source estimation indicated three critical neural sources of ostracism (ACC, amygdala, and superior temporal gyrus [STG]), consistent with prior fMRI findings (for review see Vijayakumar et al., 2017). Additionally, the superior parietal lobule (SPL) was sensitive to nonsocial priming. As Lunchroom has yet to be used in an adolescent sample and it remains unclear how individual developmental differences within adolescence may affect sensitivity to ostracism, the current study used an adapted Lunchroom paradigm designed for adolescents to examine neural responses to social exclusion and inclusion.
1.1. Present study
Our goal was to evaluate EEG/ERP correlates of ostracism in the context of developmental factors across early adolescence (age 10–14 years). To eliminate potential reading-related challenges for youth, we created a pictorial version of the Lunchroom task where participants had to choose between pictures of people (social priming) or pictures of landscapes (nonsocial priming). First, we investigated feedback and priming differences in average amplitude and latency in the N2 and P3 components. We predicted that the N2 and P3 components would be sensitive to exclusion such that the N2 and P3 amplitude would be more negative and more positive respectively during exclusion trials compared to inclusion trials. This is in line with previous research investigating neural responses to ostracism in adolescents (Crowley et al., 2010) and adults (Hudac, 2019, Kawamoto et al., 2013, Themanson et al., 2013) and might be related to neural processes implicated in expectancy violation (Bartholow et al., 2001, Dickter and Gyurovski, 2012) and emotional regulation (Yang et al., 2021). Additionally, we expected that both the N2 and the P3 would be sensitive to differences in primes (e.g., social versus nonsocial). Here, we predicted that the N2 and P3 would indicate sensitivity to social primes by having more negative and more positive amplitudes respectively during social primed trials compared to nonsocial primed trials. This might further support that these components may be modulated by reward systems (Hudac, 2019; Weschke and Niedeggen, 2015) that are more sensitive to social stimuli valence evaluation (Rossi et al., 2017).
Previous behavioral and EEG neuroscience research using the Cyberball task has not found distinct developmental differences in responses to ostracism within early adolescence (Crowley et al., 2010, Sebastian et al., 2010; Sreekrishnan et al., 2014; Wölfer and Scheithauer, 2013). This is surprising given that as children transition to early adolescence, changes in social demands (e.g., formation of social cliques and hierarchies) can increase the likelihood of experiencing ostracism from peers (Björkqvist et al., 1992, Brown, 2004). Additionally, pubertal changes that begin during early adolescence are believed to correspond with increased biological sensitivity to social input (Andrews et al., 2021). To better understand developmental differences, we critically explored how ostracism and social priming effects in the N2 and P3 components varied across development during the Lunchroom task. EEG research investigating neural sensitivity to ostracism in early adolescence has commonly controlled for age or has not found age to be a significant predictor of neural sensitivity to ostracism (Crowley et al., 2010, McPartland et al., 2011, Sreekrishnan et al., 2014, White et al., 2012). Brain development, however, has been linked to pubertal development (Herting and Sowell, 2017). Thus, we opted to investigate both age and pubertal status as developmental predictors. Considering early adolescence is marked by the onset of puberty (Sawyer et al., 2018) and changes in social cognition during adolescence that might influence more immediate sensitivity to ostracism is linked to pubertal biological changes (Ernst et al., 2009, Nelson et al., 2005), we predicted that pubertal status would likely modulate N2 and P3 sensitivity to ostracism, particularly following social primes.
Third, as in the original paper (Hudac, 2019), we opted to use source estimates to aid interpretability across ERP/EEG and fMRI studies of ostracism. The same a priori candidate brain regions were selected due to their noted role in ostracism (ACC; cingulate gyrus; medial frontal gyrus, MFG; insula; per Eisenberger, 2012) and social information processing (amygdala, STG, SPL, and fusiform gyrus; per Sebastian et al., 2010). We expected similar results to the adults, such that ACC, amygdala, and STG would be more active during ostracism and that SPL would be sensitive to social primes. Recent meta-analysis reports suggest that ventrolateral prefrontal cortex and ventral striatum are two regions with more activation during earlier adolescent development (Vijayakumar et al., 2017); thus, we also included the inferior frontal gyrus (IFG; contains ventrolateral prefrontal cortex) and subcallosal cingulate gyrus (SCG; contains ventral striatum) with predictions that ostracism effects in these regions will increase across development, reflective of increased activation of these regions during social exclusion.
2. Methods
2.1. Participants
Adolescent participants were recruited from communities in the Southeastern United States. Participants were recruited via flyers distributed electronically and at community events and local community centers. In addition, 35 adolescents participated in one of four “research summer camp” weeks sponsored by the research lab, where participants socialized with other adolescents, completed activities with the research team (e.g., campus scavenger hunt), and completed research procedures. All participants had normal or corrected-to-normal vision. Table 1 reports the final sample1 that consisted of 84 adolescents (aged 10–14 years; 38.1 % non-White and/or Hispanic or Latine). Participants were only excluded from the current analyses if they were not 10–14 years-old at time of data collection. Based upon self-report via the Pubertal Development Scale (PDS; Petersen et al., 1988), most adolescents were prepubertal (n = 37, 44.05 %) or early pubertal (n = 21, 25.00 %), with eight mid-pubertal (9.52 %), four late pubertal (4.76 %), and two post-pubertal (2.38 %).2 The local ethical review board approved this project and procedures. All legal guardians of participants gave written informed consent, and assent was obtained from participants prior to beginning the study.
Table 1.
Demographic information for sample. PDS = Pubertal Development Scale.
| Age (M, SD) | 12.2 (1.40) |
| PDS Score(M, SD) | 1.36 (0.75) |
| Pubertal Status (n, %) | |
| Prepubertal Early Puberty Midpubertal Late Puberty Post-Puberty Unknown/Not Reported |
37 (44.05) 21 (25.00) 8 (9.52) 4 (4.76) 2 (2.38) 12 (14.28) |
| Gender (n, %) | |
| Female Male Other |
37 (44.05) 46 (54.76) 1 (1.19) |
| Ethnicity (n, %) | |
| Hispanic/Latine Not Hispanic/Latine Unknown/Not Reported |
7 (8.33) 76 (90.48) 1 (1.19) |
| Race (n, %) | |
| Asian Native Hawaiian/Pacific Islander Black/African American White More than one race |
3 (3.57) 1 (1.19) 15 (17.86) 57 (67.86) 8 (9.52) |
| Psychiatric Conditions (n, %) | |
| Autism Anxiety Developmental Delay Attention Deficit Hyperactivity Disorder (ADHD) Other |
4 (4.76) 3 (3.57) 1 (1.19) 14 (16.67) 12 (14.28) |
Note. Psychiatric conditions were reported by legal guardians and were not confirmed diagnostically. Other psychiatric conditions reported included depression, social anxiety, dysgraphia, sensory processing disorders, speech impairments, Tourette’s syndrome, dyslexia, obsessive compulsive disorder, tic disorders, and hypermobility spectrum disorder.
2.2. Stimulus design and procedures
2.2.1. Lunchroom task
In the Lunchroom task, participants are asked to select an avatar to represent themselves (Fig. 1). Two other avatars are introduced as representatives of their best friends. Unlike Cyberball, participants are simply told that these avatars are their friends without emphasizing that they are real people. Each trial, participants choose between two picture options (primes) and are told that based upon their decision, their best friends would choose whether they want to sit with them at the lunchroom table (feedback phase). In comparison to the original task that presented written descriptions of interpersonal or physical words as primes (see Hudac, 2019), this version used photo options that varied based on social and nonsocial stimuli (see Fig. 1). The nonsocial prime condition asked participants to make decisions between pairs of various landscape images, while the social prime condition asked participants to make decisions between pairs of social scene images of 2–3 children smiling. Stimuli were non-licensed freely available images and were selected in pairs using the “similar photos” options on Google images. Images were selected for content similarities (e.g., children playing soccer, a lake in front of a mountain) and prime pairs did not differ in perceptual properties (p > 0.48). Although out of scope of the current study, perceptual similarity analysis of images indicated that social stimuli (versus nonsocial stimuli) were more luminant (i.e., brighter), less blue-yellow and more red-green chrominant (i.e., higher saturation), and had more similar structural vectors (i.e., shapes and edges), p < .0002 (see SM2).
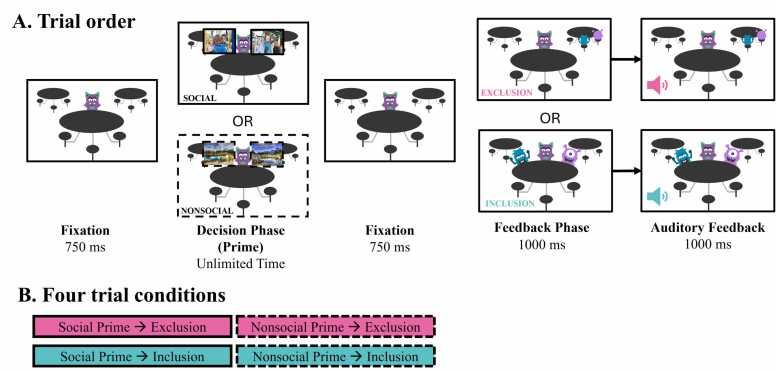
Fig. 1.
Lunchroom task schematic.Panel A represents the order in which trials proceeded. Panel B represents the four different conditions participants experienced that varied by social (solid border) and nonsocial (dashed border) primes and exclusion (pink) or inclusion (turquoise) feedback.
Fig. 1 indicates how trials proceeded. All participants saw the same trials (order randomized across participants), such that decision pairs were fixed and led to predetermined feedback (inclusion, exclusion). First, participants’ chosen avatars were presented alone for 750 ms as a fixation before social and nonsocial choices (i.e., primes) were then presented. Participants had unlimited time to choose via a button press, though responses were not accepted by the computer until options were viewed for 500 ms. Upon selection, the options disappeared, and the avatar remained sitting alone on the screen for 750 ms before the ostracism outcome feedback was revealed. The avatar was either sitting alone with their friends at a nearby table (excluded) or the avatar was surrounded by best friends (included). The feedback was visually presented for 1000 ms. To increase the salience of the outcome, after the initial 1000 ms, an auditory token was presented for 500 ms while the image remained on the screen for another 1000 ms. A social/biological sound of a male voice exclaiming “Yeah!” was presented during inclusion trials, and a nonsocial/non-biological sound of three descending tones (e.g. “sad trombone”) was presented during exclusion trials.3 Perceptual similarity analysis of images indicated that inclusion stimuli were more luminant and had less structural similarity than exclusion stimuli, p < .0002 (see Supplementary Materials [SM]2).
Of the 104 total trials, participants saw 26 trials for each of the four conditions based upon prime and feedback: social exclusion, social inclusion, nonsocial exclusion, and nonsocial inclusion. Stimuli were presented using E-Prime 3.0 software (Psychological Software Tools, Inc., Pittsburgh, PA) on a separate computer that integrated ongoing EEG data collection to mark events.
2.2.2. Pubertal Development Scale (PDS)
The PDS was given to participants to complete as a self-report measure of pubertal status based upon physical development (Petersen et al., 1988). All participants are asked about hair and height development, male participants report on vocal and facial hair development, and female participants are asked about breast development and menarche. Mean PDS scores are calculated by taking the average of responses items (range 1–4), such that one indicates that development has yet to begin and four indicates development seems completed.
2.3. EEG acquisition and processing
Continuous EEG was recorded from high-density 128-channel geodesic sensor nets using Net Station 5.3 software integrated with an EEG high-impedance 400-series amplifier (Magstim-EGI, Eugene OR USA). During acquisition, EEG signals were referenced to the vertex electrode, analog filtered (0.1 Hz high-pass, 100 Hz elliptical low-pass), amplified, and digitized with a sampling rate of 250 Hz. Standard post-processing procedures included bandpass filtering between 0.1 Hz – 40 Hz and automated artifact rejection using the clean_rawdata plugin in EEGLAB (Delorme and Makeig, 2004). In line with Delorme’s (2023) examination of preprocessing standards, channels were rejected using a rejection threshold of 0.9. Large artifacts were then removed via spectrum thresholding using the pop_rejcont function of EEGLAB (frequency range: 20–40 Hz, threshold: 10 dB). All removed channels were interpolated, and data was re-referenced to average. Data was then epoched from −200–700 ms time-locked to the moment of inclusion or exclusion and baseline corrected from −200–0 ms.
Per a priori hypotheses, we first confirmed time windows with visual confirmation of the grand-averaged waveform (Supplementary Materials[SM] Fig. 1) across electrode regional clusters (frontal, central, posterior) and hemisphere (left, medial, right). Unlike our prior adult paper (see Hudac, 2019), the negative N2 component and positive P3 component were elicited across posterior electrodes with inverse counterparts elicited across frontal electrodes. Thus, analyses focused on posterior electrodes of the Magstim-EGI 128-channel geodesic net electrodes, including left [58 (P7), 59, 64 (P9), 65 (PO7)], medial [61, 62 (Pz), 67 (PO3), 72 (POz), 77 (PO4), 78], and right [90 (PO8), 91, 95 (P10), 96 (P8)]. Peak N2 amplitudes and latencies were extracted from 150 to 325 ms and peak P3 amplitudes and latencies were extracted from 250 to 500 ms for each trial across posterior electrodes (left, medial, and right). Lastly, a positive P1 posterior component was identified from the grand-averaged waveform; thus, peak P1 amplitudes and latency were extracted from 75 to 200 ms across posterior electrodes (left, medial, and right). Trials with amplitudes ± 40 µV were deemed artificial and excluded. Average number of trials across the four conditions included in the analyses (see SM Table 7) did not significantly differ, F(3, 332) = 0.104, p = 0.958.
Utilizing the same procedures in Hudac (2019), we generated source waveforms for candidate brain regions using finite difference models in GeoSource software program (v3.0, Magstim-EGI, Eugene OR). The finite difference model applied estimations across a total of 2447 source dipole triplets (three orthogonal orientations: x, y, and z orientations) parceled across 7-mm voxels. Conductivity values used in the finite difference model included 0.25 S/m for brain, 1.8 S/m for cerebral spinal fluid, 0.018 S/m for skull, and 0.44 S/m for scalp (Ferree et al., 2000). Weighting was placed equally across locations with regularization carried out via Tikhonov (1 ×10−2) using standardized low-resolution brain electromagnetic tomography (sLORETA) as a constraint. Here, we estimated the same eight a priori candidate brain source regions from the adult study (Hudac, 2019: amygdala, ACC, cingulate gyrus, fusiform gyrus, insular cortex, medial frontal gyrus, superior parietal lobule [SPL], and superior temporal gyrus [STG]) and two additional regions implicated in adolescent ostracism (IFG, SCG). For each region, source activation (i.e., estimated strength of source current density, in nanoamperes) was extracted from both left and right hemispheres using a moving window approach where 100 ms bins offset were offset by a 50 ms moving window (e.g., 0–100 ms, 50–150 ms, and so on).
2.4. Analytic plan
All analyses were conducted in R (version 4.3.3). For all analyses requiring posthoc testing, estimated marginal means (EMM) and standard errors (SE) are reported with false-discovery rate (FDR) correction (Benjamini and Hochberg, 1995) applied for multiple comparisons. Multilevel mixed effects models for each EEG/ERP outcome were built stepwise. First, as a base (SM 3.1), all models were fitted with a random intercept for each person to account for repeated measures and fixed effects for prime (2: social, nonsocial), feedback (2: exclusion, inclusion), hemisphere (3: left, medial, right) and interactions between all fixed effects. Upon initial review of results, there was only one significant condition effect for latency: P3 latency was slower to exclusion (EMM = 202 ms, SE = 11.5) than inclusion (EMM = 197 ms, SE = 11.5), p = 0.0041. Thus, all remaining analyses focused on amplitude outcomes only. For ERP analyses, hemisphere was only included if significant in the base model; otherwise, hemisphere was removed as a fixed and interacting factor per model building recommendations to avoid overfitting the model (Bates, 2016, Matuscheck et al., 2017, Hoffman, 2015). For source estimation analyses, if hemisphere was significant, source estimation was analyzed separately for left or right hemisphere. Otherwise, analyses were collapsed across hemisphere. Finally, developmental factors of age and PDS average score were added as continuous fixed effects (SM 3.2). To interpret developmental interactions, model estimates were generated at discrete levels. In this way, we interpret differences at each age of the developmental sample (10, 11, 12, 13 and 14 years) and at PDS average scores corresponding to developmental stages (1 = pre-pubertal, 2 = early pubertal, 3 = mid-pubertal, 4 = late/post-pubertal).
3. Results
3.1. Effects of ostracism, prime, and development on ERP component amplitudes
Hemisphere did not interact with feedback nor prime effects in base models for P1 or N2 and was removed from subsequent analyses, p > 0.061 (see SM Table 1). The base model estimating effects on P3 amplitude revealed significant effects of hemisphere, including a main effect and interactions with prime and feedback, p < .044. As such, we included hemisphere in the full model (see SM 3). Omnibus results for the final models by component are reported in Table 2.
Table 2.
Amplitude Model Results by ERP Component. Bolded p-values indicate significant predictors of amplitude values. PDS = Pubertal Development Score.
|
Interaction With: |
|||||||
|---|---|---|---|---|---|---|---|
|
Fixed Effects |
Age |
PDS |
|||||
| P1 Amplitude | DF | F-value | p-value | F-value | p-value | F-value | p-value |
| Intercept | 1, 28398 | 853.311 | < 0.0001 | ||||
| Prime | 1, 28398 | 0.194 | 0.6595 | 8.286 | 0.0040 | 0.159 | 0.6901 |
| Feedback | 1, 28398 | 129.086 | < 0.0001 | 17.272 | < 0.0001 | 0.004 | 0.9487 |
| Prime*Feedback | 1, 28398 | 1.038 | 0.3083 | 0.618 | 0.4318 | 4.151 | 0.0416 |
| Age | 1, 74 | 6.384 | 0.0137 | ||||
| PDS | 1, 74 | 0.091 | 0.7635 | ||||
| N2 Amplitude | DF | F-value | p-value | F-value | p-value | F-value | p-value |
| Intercept | 1, 28599 | 481.434 | < 0.0001 | ||||
| Prime | 1, 28599 | 1.300 | 0.2542 | 11.549 | 0.0007 | 1.555 | 0.2123 |
| Feedback | 1, 28599 | 183.149 | < 0.0001 | 0.885 | 0.3469 | 3.328 | 0.0681 |
| Prime*Feedback | 1, 28599 | 2.638 | 0.1043 | 1.842 | 0.1747 | 2.942 | 0.0863 |
| Age | 1, 74 | 8.309 | 0.0052 | ||||
| PDS | 1, 74 | 0.008 | 0.9280 | ||||
| P3 Amplitude | DF | F-value | p-value | F-value | p-value | F-value | p-value |
| Intercept | 1, 27054 | 1121.574 | < 0.0001 | ||||
| Prime | 1, 27054 | 8.014 | 0.0046 | 2.185 | 0.1394 | 3.411 | 0.0648 |
| Feedback | 1, 27054 | 0.036 | 0.8495 | 0.723 | 0.3951 | 9.835 | 0.0017 |
| Hemisphere | 1, 27054 | 4.354 | 0.013 | 4.368 | 0.0127 | 12.464 | < 0.0001 |
| Prime*Feedback | 1, 27054 | 0.261 | 0.6092 | 0.744 | 0.3884 | 0.005 | 0.9421 |
| Prime*Hemisphere | 1, 27054 | 3.122 | 0.0441 | 2.973 | 0.0512 | 3.648 | 0.0260 |
| Feedback*Hemisphere | 1, 27054 | 2.336 | 0.0967 | 6.813 | 0.0011 | 0.018 | 0.9824 |
| Prime*Feedback*Hemisphere | 2, 27054 | 0.408 | 0.6647 | 0.309 | 0.7344 | 2.519 | 0.0805 |
| Age | 1, 74 | 4.758 | 0.0323 | ||||
| PDS | 1, 74 | 0.003 | 0.9595 | ||||
3.1.1. P1 amplitude
There was a significant reversed effect of feedback, such that inclusion (EMM = 11.4 μV, SE = 0.37) elicited a more positive P1 amplitude than exclusion (EMM = 10.1 μV, SE = 0.37; p < 0.0001; see Fig. 2). While there was a significant main effect of age in the full model, posthoc evaluation revealed that P1 amplitude did not statistically change over age (slope = −0.57, p = 0.068. Fig. 3A illustrates interactions between prime, feedback, and age. Although the model indicated an interaction between prime and age, p = 0.004, posthoc comparison tests (see SM Table 2) revealed that within age groups, there were no significant differences between social and nonsocial primes (slope = 0.25, p=0.062). A significant interaction between feedback and age revealed that inclusion sensitivity decreased with age (slope = −0.34, p = 0.011 i.e., becoming more sensitive to exclusion, see SM Table 2). Finally, there was a significant three-way interaction between prime, feedback, and pubertal development (slope = 0.73, p = 0.042; see Fig. 3B). Posthoc predictions of puberty as a categorical factor indicate that at each stage of pubertal development P1 amplitude was more positive to inclusion when following social primes (p < 0.0008; see SM Table 3). This effect, however, did not significantly differ between pubertal developmental stages (p = 0.163). In contrast, only adolescents in early stages of puberty demonstrated a more positive P1 amplitude to inclusion following nonsocial primes (i.e., PDS = 1 & 2, p < 0.0002; PDS 3 & 4, p > 0.236). This effect, however, did not differ between early pubertal developmental stages (i.e., PDS 1 vs. 2, p = 0.163).
Fig. 2.
P1, N2, and P3 modulation based on hemisphere and prime.Top Panel: Grand-average waveforms for exclusion (pink) and inclusion (turquoise) collapsed across social primes for left, medial, and right hemispheres. Bottom Panel: Grand-average waveforms for exclusion (pink) and inclusion (turquoise) collapsed across nonsocial primes for left, medial, and right hemispheres.
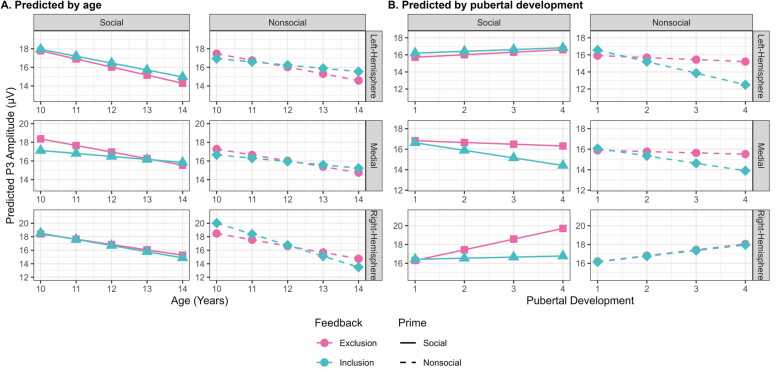
Fig. 3.
Model-based ERP prediction (in microvolts) for P1, N2, and P3 across developmental factors. Feedback conditions are depicted by color (exclusion = pink, inclusion = turquoise). Social primes are depicted by line type (solid lines = social, dashed lines = nonsocial). Panel A presents amplitude changes relative to age. Panel B represents amplitude changes relative to Pubertal Development Score (PDS) where larger values reflect greater maturation.
3.1.2. N2 amplitude
There was a significant “classic” effect of ostracism, such that exclusion elicited more negative amplitudes (EMM μV = −8.62, SE = 0.36) than inclusion trials (EMM μV = −6.91, SE =.36; p < 0.0001; see Fig. 2). While there was a significant main effect of age in the full model, posthoc evaluation revealed that N2 amplitude did not statistically change over age (slope = 0.58, p = 0.056. Additionally, a significant interaction between prime and age collapsed across feedback conditions (Fig. 3A) indicated that N2 sensitivity to primes changed across age (slope = 0.32, p = 0.027). Posthoc evaluation of age as a categorical variable revealed an interesting pattern: N2 amplitude was more negative to social primes for younger adolescents (at age 10: p = 0.013; see SM Table 4), was not significantly different for slightly older adolescents (at age 11: p = 0.083; at age 12: p = 0.734), and was more negative to nonsocial primes for older adolescents (at age 13: p = 0.012; at age 14: p = 0.002).
3.1.3. P3 amplitude
There was a significant main effect of prime that suggested P3 amplitude was more positive for social primes (EMM = 16.40 μV, SE = 0.49) than nonsocial primes (EMM = 16.10 μV, SE = 0.49; p = 0.006; see Fig. 2). There was also a significant main effect of hemisphere that suggested P3 amplitude was more positive across right (EMM = 16.50 μV, SE = 0.49) electrodes compared to left (EMM = 16.00 μV, SE = 0.49; p = 0.009). Although there was a significant main effect of age, posthoc evaluation of age at discrete levels indicated no significant change in P3 amplitudes across age (slope = −0.72, p = 0.089).
Four significant two-way interactions were present in the model. First, a significant prime by hemisphere interaction revealed that P3 amplitude was more positive to social primes (EMM = 16.60, SE = 0.50) compared to nonsocial primes (EMM = 15.90, SE = 0.50; p = 0.0003) only at medial electrodes. Second, there was a significant hemisphere by age interaction indicating that as adolescents got older P3 amplitude decreased but only across right electrodes (slope = −1.07; p = 0.008). Third, while there was no significant main effect of feedback (p = 0.850), there was a significant interaction between feedback and pubertal development such that P3 amplitudes in adolescents further along in puberty were more positive to exclusion (slope = −1.12; p = 0.013; see Fig. 3B). Indeed, posthoc evaluation of puberty at discrete levels indicated that adolescents who had yet to start pubertal development (PDS = 1) did not demonstrate a significant ostracism effect (i.e., inclusion = exclusion; p = 0.125). However, adolescents in early to late stages of pubertal development demonstrated significantly more positive P3 amplitude for exclusion compared to inclusion (PDS = 2, 3, & 4; p < 0.035; see SM Table 5). Finally, while there was a significant interaction between hemisphere and puberty in the full model, posthoc evaluation of pubertal development trends across hemispheres revealed no statistically significant differences (p > 0.409).
Lastly, the model indicated two significant three-way interactions (see Fig. 4A). A significant interaction between feedback, hemisphere, and age indicated that P3 sensitivity to exclusion (slope = −0.86) and inclusion (slope = −1.27) decreased with age only across right electrodes (p < 0.036). To better understand ostracism condition effects, posthoc evaluation of age at discrete levels indicated that there were reverse ostracism effects (inclusion > exclusion) in left electrodes for older adolescents (i.e., 13 &14 year-olds; p < 0.022). There was also evidence of classic ostracism effects (exclusion > inclusion) in the medial electrode cluster for younger adolescents (i.e., 10 & 11 year-olds; p < 0.039) and right electrodes for 14 year-olds (p = 0.022). Another significant three-way interaction between prime, hemisphere, and pubertal development was indicated in the full model; however, posthoc evaluation indicated slopes were stable for both prime conditions and all hemispheres (p > 0.303; see Fig. 4B). To better understand P3 amplitude condition differences across hemisphere and puberty, posthoc comparisons between social and nonsocial primes at each electrode cluster and discrete stages of puberty were evaluated. This indicated more positive P3 amplitudes to social primes compared to nonsocial primes for adolescents who had yet to begin or were in early stages of puberty (i.e., PDS = 1 & 2; p < 0.017) but only at medial electrodes. Adolescents in early to late stages of puberty (i.e., PDS = 2, 3, & 4) demonstrated greater sensitivity to social primes compared to nonsocial primes in the left hemisphere (p < 0.011).
Fig. 4.
Model based P3 amplitude prediction (in microvolts) across developmental factors and hemisphere. Feedback conditions are depicted by color (exclusion = pink, inclusion = turquoise). Social primes are depicted by line type (solid lines = social, dashed lines = nonsocial). Panel A presents P3 amplitude changes relative to age. Panel B represents amplitude changes relative to Pubertal Development Score (PDS) where larger values reflect greater maturation.
3.2. Source estimation results
Source activation was strongest to amygdala (EMM = 10.54), SCG (EMM = 8.44), fusiform (EMM = 7.66), and ACC (EMM = 3.91), with all other regions exhibiting less than 3 nA. Extracted source estimation is plotted per condition in Fig. 5 for each region across epoch (time-locked to ostracism outcome reveal) like Hudac (2019), although temporal dynamics are not a focus of the current paper. All omnibus results are presented in SM Table 6. To simplify interpretations of condition effects, EMMs were extracted for all conditions and posthoc pairwise FDR-corrected comparisons of feedback and prime effects (Table 3). Condition differences reported below are all p < 0.05 (FDR-corrected).
Fig. 5.
Source estimation (in nanoamperes) for each candidate brain region across epoch (i.e., time-locked to ostracism outcome reveal). Feedback condition is depicted by color (exclusion = turquoise; inclusion = pink) and prime condition is depicted by line type (social = solid line; nonsocial = dotted line). The time window of the P3 component is shaded grey. Abbreviations: ACC = anterior cingulate cortex, IFG = inferior frontal gyrus, MFG = medial frontal gyrus, SCG = subcallosal gyrus, SPL = superior parietal lobule, STG = superior temporal gyrus.
Table 3.
Model-derived descriptive statistics for each condition for each candidate source region. For post-hoc pairwise comparisons, color indicates direction of effect: turquoise (inclusion > exclusion), pink (exclusion > inclusions), yellow (social > nonsocial). Abbreviations: ACC = anterior cingulate cortex, MFG = medial frontal gyrus, SCG = subcallosal gyrus, IFG = inferior frontal gyrus, SPL = superior parietal lobule, STG = superior temporal gyrus.
 |
3.2.1. Hemisphere interactions in the base model
In the base model, hemisphere did not interact with feedback nor prime effects (p > 0.07) for ACC, cingulate gyrus, fusiform gyrus, insula, MFG, or SCG, and thus, was removed from subsequent models. Separate models were generated for left and right hemispheres of these regions: (1) amygdala, IFG, and SPL the right hemisphere were estimated to be more active (by 2.28 nA,.068 nA, and.31 nA, respectively) than the left hemisphere, p < 0.0001; (2) left STG hemisphere was estimated to be 0.17 nA more active than the right hemisphere, p < 0.0001 (see SM Figure 2).
3.2.2. Primary effects of ostracism, prime, and their interaction
Three regions exhibited “classic” ostracism effects (exclusion > inclusion): bilateral amygdala, IFG, and SCG. Another set of regions exhibited a reversed pattern with sensitivity to inclusion (inclusion > exclusion), including ACC, cingulate gyrus, fusiform, insula, SPL, and right STG. All candidate brain regions except the left amygdala, IFG (both hemispheres), and SCG exhibited a main effect of prime, such that activation was estimated to be increased following social relative to nonsocial primes.
Six regions indicated feedback by prime interactions (see Table 3 for EMM and pairwise statistics). First, classic ostracism effects (exclusion > inclusion) were stronger following (a) social than nonsocial primes in the right amygdala, right IFG, and SCG, and (b) nonsocial than social primes in the left IFG. Second, the left SPL and right STG feedback effects (inclusion > exclusion) were observed following nonsocial primes, p < 0.016, but not social primes, p > 0.28. In addition, both the left SPL and the right STG were more active during exclusion outcomes following social than nonsocial primes, p<0.0001.
3.2.3. Interactions with age
Fig. 6A illustrates classic ostracism effects increased with age for right amygdala and bilateral IFG. In addition, prime effects (social > nonsocial) increased with age for right IFG. Broadly, for regions that were more sensitive to inclusion (per effects reported in Section 3.2.2), ostracism effects also increased with age such that sensitivity decreased to inclusion and increased to exclusion. This was identified by several patterns: (1) younger adolescents had greater sensitivity to exclusion within the fusiform and insula (following nonsocial primes) and left STG (following social primes); and (2) older adolescents had greater sensitivity to exclusion within the right SPL (following social primes). In addition, prime effects (social > nonsocial) decreased with age for ACC, cingulate gyrus, fusiform gyrus, insula, and left STG.
Fig. 6.
Model-based source estimation prediction (in nanoamperes) for example regions across developmental factors. Feedback condition is depicted by color (exclusion = turquoise; inclusion = pink) and prime condition is depicted by line type (social = solid line; nonsocial = dotted line). Example regions were chosen based upon classic ostracism main effects (exclusion > inclusion
: right amygdala, AMY; right inferior frontal gyrus, IFG) and unexpected ostracism effects (inclusion > exclusion; anterior cingulate cortex, ACC; insula). Increased Pubertal Development Score (PDS) reflects increased maturation.
3.2.4. Interactions with pubertal development
With pubertal maturation, there was greater sensitivity to exclusion within ACC, fusiform gyrus, insula, MFG, and left STG (see Fig. 6B). In addition, sensitivity to social primes increased with pubertal maturation within ACC, right IFG, and right STG. During mid-to late pubertal maturation, left amygdala and left SPL exhibited more sensitivity to nonsocial primes.
4. Discussion
To assess affective responses to social exclusion in adolescence, the present study adapted the Lunchroom task (Hudac, 2019) to better understand ERP and neural source correlates of ostracism. Unlike the frontal-oriented adult response, we found a posterior-oriented adolescent response in P1, N2, and P3 components. While latency was largely insensitive to conditions, we found unique amplitude effects across components by feedback, prime, age, and pubertal development. In addition, a network of regions was estimated to be the strongest neural sources to exclusion, including right amygdala, bilateral IFG, and SCG. Most other regions (ACC, fusiform, insula, MFG, left STG) elicited stronger activation to inclusion, though these effects were predicted to shift towards being more sensitive to exclusion during mid- to late-pubertal maturation.
4.1. Sensitivity to social primes
The Lunchroom task relies on a strategy in which each trial “resets” and two choices are presented so that participants feel that the feedback is related to their current choice or action. Importantly, these choices differed in content (i.e., both social, both nonsocial; see Fig. 1) to better understand how social priming influences neural responses to ostracism. This strategy allows for more accurate comparisons across inclusion and exclusion trials than Cyberball because participant involvement remains consistent. Across nearly all regions, neural sources were more engaged following social than nonsocial primes, demonstrating clear efficacy of using a priming strategy (Higgins and Eitam, 2014, Barsalou, 2016). P3 was the only ERP component that was more sensitive to social primes than nonsocial primes. While this is somewhat in-line with previous research that suggests the P3 is sensitive to social exclusion (Themanson et al., 2013, Weschke and Niedeggen, 2015), the sensitivity to social primes in the current study was a main effect (i.e., collapsed across feedback conditions). This, taken together with the lack of sensitivity to social priming in N2, was surprising given our previous work showcasing a priming effect for the N2 amplitude in adults (Hudac, 2019). It may be the case that pictures are a less effective prime than words. However, nearly all source regions except for left amygdala and left IFG indicated increased activation after social primes relative to nonsocial primes, suggesting that the effect of social priming in this context should continue to be explored.
4.2. “Classic” and unexpected ostracism effects
Our analyses indicated different feedback effects for each component. In summary, the P1 amplitude was larger to inclusion, N2 amplitude was larger to exclusion (“classic” response), and the P3 amplitude was larger to exclusion but only for more pubertally mature adolescents. The N2 results support previous research that has found increased N2 sensitivity to exclusion (Sreekrishnan et al., 2014, Themanson et al., 2013, Weschke and Niedeggen, 2015). However, increased sensitivity to inclusion in the P1 and no effect of ostracism except when including developmental trajectories in the P3 was unexpected.
P1 is not consistently extracted in ostracism literature but it is often associated with attentional mechanisms (Holmes et al., 2003) and early perception of low-level visual cues related to faces (Rossion and Caharel, 2011). As the Lunchroom task is one of the first ostracism paradigms that controls for participant involvement across inclusion and exclusion conditions, this task may be better equipped to capture nuanced differences between neural correlates of social inclusion and exclusion. For instance, increases in P1 sensitivity to inclusion may suggest that adolescents divert attentional mechanisms toward more positive stimuli as a way of suppressing negative emotions associated with ostracism. This is consistent with one imaging study that found the dorsomedial prefrontal cortex was less engaged for experimentally excluded adults viewing negative social scenes compared to adults who were included; however, for the excluded group, this region became as active as the included group when viewing positive social cues suggesting an increased effort to suppress negative feelings (Powers et al., 2013). Alternatively, and in line with face perception work (Rossion and Caharel, 2011), adolescents may be more attentive during inclusion events because the other avatars’ faces are more salient in the inclusion trials compared to the exclusion trials.
Importantly, although we confirmed physical visual similarity between the two feedback images, the P1 is sensitive to luminance and other structural features (e.g., Craddock et al., 2015, Schettino et al., 2016). Thus, we caution that P1 effects may be driven by low-level physical differences or visual scanning patterns that may not be tethered to social interpretations. For instance, there may be short saccades in the inclusion feedback between the three avatar faces or long saccades in the exclusion feedback between the central self-avatar and the friend avatars in the periphery. Taken together, these interpretations should be further examined in the future by utilizing both EEG and eye tracking. Continued use of this task would benefit from redesigning the stimuli to reduce the perceptual differences, perhaps by simplifying the visual feedback (e.g., using characters like a checkmark or an “X” to indicate outcome) while maintaining the developmental relevance.
Due to design limitations in other ostracism paradigms that limit participant involvement, it has been difficult to understand how neural and cognitive mechanisms are differentially influenced by social exclusion and inclusion. For instance, in direct contradiction to the “neural alarm” theory but aligned with the current results, P3 sensitivity is argued to be modulated by subjective expectations of social involvement not just exclusion (Niedeggen et al., 2014). Likewise, research that has found increased P1 sensitivity to faces with negative affect following exclusion contradicts the idea that attentional mechanisms are suppressed as a way of thwarting social pain (Kawamoto et al., 2014). Incorporation of the Lunchroom task or others that more clearly isolate neural processes associated with ostracism while controlling for participant involvement in future developmental studies may help delineate conflicting theories and explicate developmental processes.
Our findings implicate a neural network of regions (amygdala, SCG, and IFG) that were more active to exclusion events, as aligned with evidence from fMRI using the Cyberball paradigm (Eisenberger, 2015a, Masten et al., 2009). We highlight the P3 window in Fig. 5 to emphasize the period of maximal activation for social exclusion, specifically. Importantly, SCG and IFG were included in this study as regions containing the ventral striatum and dorsolateral prefrontal cortex, respectively, given their role in adolescent ostracism (Vijayakumar et al., 2017). Greater activation in these regions has been linked to socio-affective processing, including reward learning and motivational processes (Masten et al., 2009, Perino et al., 2019), perhaps serving a dual purpose of sensitivity to social threat and sensitivity to social connection (Telzer, 2016). Critically, our study also identified a network of regions (ACC, cingulate, fusiform, insula, SPL, STG) that were more sensitive to inclusion events, further supporting the notion of dual processes in the adolescent brain. For instance, our findings suggest that the ACC is one of the most active neural sources, aligned with imaging evidence that the ACC is implicated by social violations (Bolling et al., 2011) and ACC activation is connected to self-reported distress (Eisenberger, 2015b, Rotge et al., 2015). Although the temporal dynamics of source estimation was not a focus of this study, it is evident in Fig. 5 that many of the inclusion-sensitive regions have early and rapid increases in activation, which may correspond with increased P1 amplitude to inclusion. Thus, it may be possible that our results highlight initial and rapid responses to inclusion that are otherwise masked due to temporal resolution in imaging.
4.3. Developmental individual differences in prime and ostracism effects
The interesting developmental patterns across age and pubertal development in the current study may better contextualize conflicting findings among existing literature. For instance, P1 was more sensitive to feedback effects and N2 was more sensitive to priming effects in younger adolescence, potentially driven by overall age effects that are in line with other cognitive and maturational changes that occur during adolescence (Frodl et al., 2001, Van Dinteren et al., 2014). For instance, this aligns with a study that found adolescents relative to adults demonstrated an increased sensitivity in early attentional components (e.g., N1) when they viewed people in naturalistic settings, similar to the stimuli used in the current study (diFilipo and Grose-Fifer, 2016); however, this study did not examine developmental differences within the adolescent sample. Another study that focused solely on 12-year-olds reported that the N1 was greater when experiencing social rejection versus social acceptance (Kujawa et al., 2017). Apart from these studies, most EEG studies in this area have examined clinical and/or adult samples. This suggests that future studies may replicate our findings, which potentially demonstrate developmental shifts, if adolescent age is examined continuously in samples without clinical concerns.
While the P1 is not reported in many adult studies, we found that the P1 becomes less sensitive to inclusion in older adolescents, which may implicate developmental shifts in attending to inclusion versus exclusion. Additionally, we found that within all stages of pubertal development, P1 was more sensitive to social-primed inclusion than social-primed exclusion. Yet, greater P1 sensitivity to nonsocial-primed inclusion was only apparent for adolescents in earlier pubertal developmental stages. This may suggest that the P1 early in development is sensitive to inclusion in general but as adolescents mature, they become more sensitive to inclusion when related to social decision-making. This idea aligns with studies that demonstrate significant peer influence on adolescents’ decision-making in risky (e.g., substance use) and positive (e.g., prosocial behaviors) contexts (van Hoorn et al., 2016, Watts et al., 2024) as well as neural sensitivity to peer feedback (Irani et al., 2024).
Additionally, we found that adolescents who had yet to begin or were very early on in pubertal development did not demonstrate P3 sensitivity to ostracism. Adolescents who were more mature, however, were predicted to have the largest P3 ostracism effect. As our sample was largely early to mid-pubertal, this may explain why there was no main effect of ostracism. Research also suggests that prior experiences, individual characteristics (e.g., cognitive flexibility, trait anxiety) and personality factors (e.g., optimism, neuroticism) can all influence expectancy violation (Pinquart et al., 2021). Given that the P3 is linked to expectancy violation (Bartholow et al., 2001), the current results might be influenced by corresponding development of such individual differences. Despite this, age did not seem to influence P3 sensitivity to ostracism in the same way, which was surprising given that pubertal development coincides with age. These findings may emphasize separable roles between brain maturation and socio-emotional development. Indeed, previous research has failed to identify different affective responses to exclusion across younger and older adolescents (Sebastian et al., 2010). Further, more recent literature has suggested that physical and neural changes occurring during pubertal development influences adolescents’ perception of self and others (Pfeifer and Allen, 2021). Therefore, it may be that puberty is a more sensitive developmental outcome in predicting and characterizing neural mechanisms related to ostracism.
4.4. Limitations and considerations for future work
For simplicity and to remain consistent with the findings from the other ERP components in the current study, our interpretation of P3 effects focused solely on those not involving hemispheric interactions. Given that P3 demonstrated significant hemispheric differences, future work would benefit from a closer inspection of topography and hemisphere effects. For instance, we found greater P3 amplitude across right electrodes (overall) that decreased with age, and greater P3 amplitude to social relative to nonsocial primes at medial electrodes. Additionally, there were two different developmental and hemispheric patterns for the feedback and prime conditions. First, P3 sensitivity to both exclusion and inclusion decreased with age only at right electrodes where classic ostracism effects (exclusion > inclusion) were only observed for older adolescents. Second, P3 sensitivity to social and nonsocial primes were stable for all hemispheres across pubertal development. However, deeper evaluation revealed a topographic shift in greater P3 sensitivity to social over nonsocial primes such that adolescents earlier in pubertal development demonstrated greater sensitivity at medial electrodes while adolescents later in pubertal development demonstrated greater sensitivity at left electrodes. While it was out of scope for the current study to further examine these effects, it remains important to investigate shifts in topography particularly when investigating how neural sensitivity to social exclusion develops. The topographic shift of the N2 and P3 components from frontal electrodes in the original adult Lunchroom findings (Hudac, 2019) to posterior electrodes in the current adolescent sample deserves further investigation. For instance, this shift may be due to differences in stimuli used (i.e., words with adults versus pictures with adolescents) or may reflect developmental differences. Similarly, the social stimuli used in the current study included children who appear younger than the adolescent sample, so future work would benefit from better age-matched social stimuli.
Another limitation is the lack of subjective measurement of adolescents’ feelings of exclusion/inclusion during the task, though integration of other physiological measurements (e.g., heart-rate, eye tracking) during the task would help clarify real-time attention and mood/affect. The Lunchroom task also does not specifically instruct participants that the avatars are real people or their real friends. Thus, this task may not evoke a response that is as salient or ecologically valid as Cyberball. While we believe that telling the participants that the avatars are representative of their friends, allowing them to choose their own avatar, and requiring them to make a decision on each trial that is later associated with feedback helps with saliency, future research might benefit from comparing neural responses to Cyberball and the Lunchroom task across participants. Further, this study demonstrated neurodevelopmental trends but did not focus on other individual differences that may influence sensitivity to exclusion and inclusion and subsequent coping strategies (e.g., sex/gender, clinical characteristics, psychological maturity, social motivation, need to belong, life experiences of ostracism, general social preferences). We opted to focus on developmental and maturational differences; however, in a larger sample, it would be possible to better interrogate how other factors potentially modulate responses to ostracism. These factors are likely relevant for understanding the relationships between sensitivity to ostracism, coping with experiences of ostracism, and risk for mental health and affective disorders, particularly during developmentally critical periods. For instance, in daily life, there is evidence that women interpret ostracism in different ways then men (Nezlek et al., 2015), yet there is a limited understanding of how neural correlates vary by sex or gender. Additionally, the current study was limited in that we only examined development with a cross-sectional design. Examining within-person longitudinal changes in sensitivity to ostracism while also broadly examining other individual differences might better inform how sensitivity to ostracism develops in adolescence.
Lastly, while we would have liked to map neural correlates of ostracism to the temporal need-threat model of ostracism (Williams, 2009), the Lunchroom task was not designed to distinguish between these stages and would likely need to be adapted further. However, additional analyses on habituation responses throughout the task (for instance, Revilla et al., 2024) may map onto the reflective or resignation stages during which individuals’ reactions change with persistent ostracism.
4.5. Implications and conclusions
The current study was the first to use an ostracism task that effectively controls participant involvement across social inclusion and exclusion (i.e., the Lunchroom task; Hudac, 2019) in a sample of adolescents. Our results suggest that increased neural responsivity to exclusion and inclusion vary across age and pubertal development in early adolescence. Given that very little ostracism research has found developmental differences within adolescence, these findings emphasize the utility of the Lunchroom task in investigating the development of ostracism neural underpinnings. Additionally, the effects of age and puberty on ostracism neural correlates were inconsistent which emphasizes the importance of using multiple developmental measures to better understand individual differences in adolescence. Finally, greater neural sensitivity to social-primed inclusion in our more mature adolescents provides implications for understanding how differences in adolescents’ psychophysiological responses to social reward could help them remain resilient in the face of ostracism. Ostracism is a universal experience that often produces automatic negative emotional responses but does not always lead to worsened mental health outcomes (Niu et al., 2016). Indeed, research suggests that individual factors (e.g., personality, rejection sensitivity, attribution bias related to existing mental health difficulties) can protect against or exacerbate maladaptive effects (Büttner and Greifeneder, 2024, Lei et al., 2024, Waldeck et al., 2023). By highlighting different neural mechanisms and networks underlying evolving sensitivity to social exclusion, this study may help future research begin to delineate how the neural underpinnings of ostracism unfold across critical developmental periods and vary across individuals. This is particularly relevant for identifying when intervention may be needed to ensure negative consequences of persistent ostracism are mitigated in adolescence. Additionally, it can be the basis for developing systems-level interventions that increase opportunities for adolescents to experience more social inclusion and acceptance.
CRediT authorship contribution statement
Cailee M. Nelson: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation. Rebecca Revilla: Writing – review & editing, Writing – original draft. Nicole R. Friedman: Writing – review & editing, Writing – original draft, Investigation. Mengya Xia: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Caitlin M. Hudac: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding was supported by the National Institutes of Health [R15MH124041, R01HD107593].
Bias Statement
We have no biases to report.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2025.101607.
Excluded six participants that were younger than 10 (n = 2) and older than 14 (n = 4).
Self-report categories were not available for 12 participants.
A minority of participants (n = 18) completed an earlier version of the experiment with the following timing differences: (1) selections were accepted after 400 ms of receiving choices, (2) post-selection fixation was 500 ms, and (3) outcome was presented for 750 ms before salient auditory feedback presented.
Contributor Information
Cailee M. Nelson, Email: caileen@mailbox.sc.edu.
Caitlin M. Hudac, Email: chudac@mailbox.sc.edu.
Appendix A. Supplementary material
Supplementary material
Data availability
Data will be made available on request.
References
- Altikulaç S., Bos M.G.N., Foulkes L., Crone E.A., van Hoorn J. Age and gender effects in sensitivity to social rewards in adolescents and young adults. Front. Behav. Neurosci. 2019;13:171. doi: 10.3389/fnbeh.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J.L., Ahmed S.P., Blakemore S.J. Navigating the social environment in adolescence: the role of social brain development. Biol. Psychiatry. 2021;89(2):109–118. doi: 10.1016/j.biopsych.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Barsalou L.W. Situated conceptualization offers a theoretical account of social priming. Curr. Opin. Psychol. 2016;12:6–11. doi: 10.1016/j.copsyc.2016.04.009. [DOI] [Google Scholar]
- Bartholow B.D., Fabiani M., Gratton G., Bettencourt B.A. A psychophysiological examination of cognitive processing of and affective responses to social expectancy violations. Psychol. Sci. 2001;12(3):197–204. doi: 10.1111/1467-9280.00336. [DOI] [PubMed] [Google Scholar]
- Bates D. lme4: linear mixed-effects models using eigen and S4. R. Package Version. 2016;1(1) [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Björkqvist K., Österman K., Kaukiainen A. In: Of Mice and Women: Aspects of Female Aggression. Björkqvist K., Niemela P., editors. Academic; San Diego, CA: 1992. The development of direct and indirect aggressive strategies in males and females; pp. 51–64. [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., Mayes L.C., Pelphrey K.A. Development of neural systems for processing social exclusion from childhood to adolescence. Dev. Sci. 2011;14(6):1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108(3):624–652. doi: 10.1037//0033-295X.I08.3.624. [DOI] [PubMed] [Google Scholar]
- Brown B.B. In: Handbook of Adolescent Psychology. Lerner R.M., Steinberg L., editors. John Wiley & Sons, Inc; 2004. Adolescents' relationships with peers; pp. 363–394. [DOI] [Google Scholar]
- Büttner C.M., Greifeneder R. Everyday ostracism experiences of depressed individuals: uncovering the role of attributions using experience sampling. J. Affect. Disord. Rep. 2024;17 doi: 10.1016/j.jadr.2024.100804. [DOI] [Google Scholar]
- Cheng T.W., Vijayakumar N., Flournoy J.C., Op de Macks Z., Peake S.J., Flannery J.E., Mobasser A., Alberti S.L., Fisher P.A., Pfeifer J.H. Feeling left out or just surprised? Neural correlateds of social exclusion and overinclusion in adolescence. Cogn. Affect. Behav. Neurosci. 2020;20:340–355. doi: 10.3758/s13415-020-00772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock M., Martinovic J., Müller M.M. Early and late effects of objecthood and spatial frequency on event-related potentials and gamma band activity. BMC Neurosci. 2015;16:6. doi: 10.1186/s12868-015-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.J., Wu J., Molfese P.J., Mayes L.C. Social exclusion in middle childhood: rejection events, slow-wave neural activity, and ostracism distress. Soc. Neurosci. 2010;5(5-6):483–495. doi: 10.1080/17470919.2010.500169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A. EEG is better left alone. Sci. Rep. 2023;13:2372. doi: 10.1038/s41598-023-27528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dickter C., Gyurovski I. The effects of expectancy violations on early attention to race in an impression-formation paradigm. Soc. Neurosci. 2012;7(3):240–251. doi: 10.1080/17470919.2011.609906. [DOI] [PubMed] [Google Scholar]
- diFilipo D., Grose-Fifer J. An event-related potential study of social information processing in adolescents. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. Social pain and the brain: controversies, questions, and where to go from here. Annu. Rev. Psychol. 2015;66:601–629. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. Meta-analytic evidence for the role of the anterior cingulate cortex in social pain. Soc. Cogn. Affect. Neurosci. 2015;10(1):1–2. doi: 10.1093/scan/nsu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Romeo R.D., Andersen S.L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol. Biochem. Behav. 2009;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ferree T.C., Eriksen K.J., Tucker D.M. Regional head tissue conductivity estimation for improved EEG analysis. IEEE Trans. Biomed. Eng. 2000;47(12):1584–1592. doi: 10.1109/10.887939. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Müller D., Leinsinger G., Juckel G., Hahn K., Mӧller H.-J., Hegerl U. The effect of the skull on event-related P300. Clin. Neurophysiol. 2001;112(9):1773–1776. doi: 10.1016/S1388-2457(01)00587-9. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B., van Leijenhorst L., Rombouts S.A.R.B., Crone E.A., Van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5-6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Hales A.H., Ren D., Williams K.D. In: The Oxford Handbook of Social Influence. Harkins S.J., Burger J.M., Williams K.D., editors. Oxford University Press; New York: 2016. Protect, correct, and eject: ostracism as a social tool. [Google Scholar]
- Herting M.M., Sowell E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins E.T., Eitam B. Priming…shmiming: It’s about knowing when and why stimulated memory representations become active. Soc. Cogn. 2014;32:225–242. doi: 10.1521/soco.2014.32.supp.225. [DOI] [Google Scholar]
- Hoffman L. Routledge/Taylor & Francis Group; 2015. Longitudinal analysis: modeling within-person fluctuation and change. [Google Scholar]
- Holmes A., Vuilleumier P., Eimer M. The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Cogn. Brain Res. 2003;16(2):174–184. doi: 10.1016/S0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- van Hoorn J., van Dijk E., Meuwese R., Rieffe C., Crone E.A. Peer influence on prosocial behavior in adolescence. J. Res. Adolesc. 2016;26(1):90–100. doi: 10.1111/jora.12173. [DOI] [Google Scholar]
- Hudac C.M. Social priming modulates the neural response to ostracism: a new exploratory approach. Soc. Neurosci. 2019;14(3):313–327. doi: 10.1080/17470919.2018.1463926. [DOI] [PubMed] [Google Scholar]
- Irani F., Muotka J., Lyyra P., Parviainen T., Monto S. Social influence in adolescence: behavioral and neural responses to peer and expert opinion. Soc. Neurosci. 2024;19(1):25–36. doi: 10.1080/17470919.2024.2323745. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Nittono H., Ura M. Cognitive, affective, and motivational changes during ostracism: an ERP, EMG, and EEG study using a computerized cyberball task. Neurosci. J. 2013;2013 doi: 10.1155/2013/304674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Nittono H., Ura M. Social exclusion induces early-stage perceptual and behavioral changes in response to social cues. Soc. Neurosci. 2014;9(2):174–185. doi: 10.1080/17470919.2014.883325. [DOI] [PubMed] [Google Scholar]
- Kujawa A., Kessel E.M., Carroll A., Arfer K.B., Klein D.N. Social processing in early adolescence: associations between neurophysiological, self-report, and behavioral measures. Biol. Psychol. 2017;128:55–62. doi: 10.1016/j.biopsycho.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Li M., Lin C., Zhang C., Yu Z. The effect of ostracism on social withdrawal behavior: the mediating role of self-esteem and the moderating role of rejection sensitivity. Front. Psychol. 2024;15 doi: 10.3389/fpsyg.2024.1411697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier J.S., Probst F., Lory V., Meyer A.H., Meinlschmidt G. Increased sensitivity to social exclusion during the luteal phase: progesterone as resilience factor buffering against ostracism. Psychoneuroendocrinology. 2019;107:217–224. doi: 10.1016/j.psyneuen.2019.05.019. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J.C., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuscheck H., Kliegl R., Vasishth S., Baayen H., Bates D. Balancing type I error and power in linear mixed models. J. Mem. Lang. 2017;94:305–315. doi: 10.1016/j.jml.2017.01.001. [DOI] [Google Scholar]
- McPartland J.C., Crowley M.J., Perszyk D.R., Naples A.J., Mukerji C.E., Wu J., Molfese P., Bolling D.Z., Pelphrey K.A., Mayes L.C. Temporal dynamics reveal atypical brain response to social exclusion in autism. Dev. Cogn. Neurosci. 2011;1(3):271–279. doi: 10.1016/j.dcn.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills L., Driver C., McLoughlin L.T., Anijärv T.E., Mitchell J., Lagopoulos J., Hermens D.F. A systematic review and meta-analysis of electrophysiological studies of online social exclusion: evidence for the neurobiological impacts of cyberbullying. Adolesc. Res. Rev. 2024;9:135–163. doi: 10.1007/s40894-023-00212-0. [DOI] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nezlek J.B., Wesselmann E.D., Wheeler L., Williams K.D. Ostracism in everyday life. Group Dyn. Theory Res. Pract. 2012;16(2):91–104. doi: 10.1037/a0028029. [DOI] [Google Scholar]
- Nezlek J.B., Wesselmann E.D., Wheeler L., Williams K.D. Ostracism in everyday life: the effects of ostracism on those who ostracize. J. Soc. Psychol. 2015;155(5):432–451. doi: 10.1080/00224545.2015.1062351. [DOI] [PubMed] [Google Scholar]
- Niedeggen M., Sarauli N., Cacciola S., Weschke S. Are there benefits of social overinclusion? Behavioral and ERP effects in the cyberball paradigm. Front. Hum. Neurosci. 2014;8:935. doi: 10.3389/fnhum.2014.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G.-F., Sun X.J., Tian Y., Fan C.-Y., Zhou Z.-K. Resilience moderates the relationship between ostracism and depression among Chinese adolescents. Personal. Individ. Differ. 2016;99:77–80. doi: 10.1016/j.paid.2016.04.059. [DOI] [Google Scholar]
- Perino M.T., Moreira J.F.G., Telzer E.H. Links between adolescent bullying and neural activation to viewing social exclusion. Cogn. Affect. Behav. Neurosci. 2019;19(6):1467–1478. doi: 10.3758/s13415-019-00739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biol. Psychiatry. 2021;89(2):99–108. doi: 10.1016/j.biopsych.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M., Endres D., Teige-Mocigemba S., Panitz C., Schütz A.C. Why expectations do or do not change after expectation violation: a comparison of seven models. Conscious. Cogn. 2021;89 doi: 10.1016/j.concog.2021.103086. [DOI] [PubMed] [Google Scholar]
- Powers K.E., Wagner D.D., Norris C.J., Heatherton T.F. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Soc. Cogn. Affect. Neurosci. 2013;8(2):151–157. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M.A., Dewald-Kaufmann J., Wüstenberg T., Musil R., Barton B.B., Jobst A., Padberg F. The vicious circle of social exclusion and psychopathology: a systematic review of experimental ostracism research in psychiatric disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2020;270:521–532. doi: 10.1007/s00406-019-01074-1. [DOI] [PubMed] [Google Scholar]
- Revilla R., Nelson C.M., Friedman N.R., Braun S.S., Hudac C.M. Frontal alpha asymmetry predicts subsequent social decision-making: a dynamic multilevel and developmental perspective. Dev. Cogn. Neurosci. 2024;69 doi: 10.1016/j.dcn.2024.101434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi V., Vanlessen N., Bayer M., Grass A., Pourtois G., Schacht A. Motivational salience modulates early visual cortex responses across task sets. J. Cogn. Neurosci. 2017;29(6):968–979. doi: 10.1162/jocn_a_01093. [DOI] [PubMed] [Google Scholar]
- Rossion B., Caharel S. ERP evidence for the speed of face categorization in the human brain: disentangling the contribution of low-level visual cues from face perception. Vis. Res. 2011;51(12):1297–1311. doi: 10.1016/j.visres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rotge J.-Y., Lemogne C., Hinfray S., Huguet P., Grynszpan O., Tartour E., George N., Fossati P. A meta-analysis of the anterior cingulate contribution to social pain. Soc. Cogn. Affect. Neurosci. 2015;10(1):19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudert S.C., Janke S., Greifeneder R. The experience of ostracism over the adult life span. Dev. Psychol. 2020;56(10):1999–2012. doi: 10.1037/dev0001096. [DOI] [PubMed] [Google Scholar]
- Sawyer S.M., Azzopardi P.S., Wickremarathne D., Patton G.C. The age of adolescence. Lancet Child Adolesc. Health. 2018;2(3):223–228. doi: 10.1016/S2352-4642(18)30022-1. [DOI] [PubMed] [Google Scholar]
- Schettino A., Keil A., Porcu E., Müller M., M.M Shedding light on emotional perception: interaction of brightness and semantic content in extrastriate visual cortex. Neuroimage. 2016;133:341–353. doi: 10.1016/j.neuroimage.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Viding E., Williams K.D., Blakemore S. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Simard V., Dandeneau S. Revisiting the cyberball inclusion condition: fortifying fundamental needs by making participants the target of specific inclusion. J. Exp. Soc. Psychol. 2018;74:38–42. doi: 10.1016/j.jesp.2017.08.002. [DOI] [Google Scholar]
- Sreekrishnan A., Herrera T.A., Wu J., Borelli J.L., White L.O., Rutherford H.J., Mayes L.C., Crowley M.J. Kin rejection: social signals, neural response and perceived distress during social exclusion. Dev. Sci. 2014;17(6):1029–1041. doi: 10.1111/desc.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H. Dopaminergic reward sensitivity can promote adolescent health: a new perspective on the mechanism of ventral striatum activation. Dev. Cogn. Neurosci. 2016;17:57–67. doi: 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themanson J.R., Khatcherian S.M., Ball A.B., Rosen P.J. An event-related examination of neural activity during social interactions. Soc. Cogn. Affect. Neurosci. 2013;8(6):727–733. doi: 10.1093/scan/nss058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themanson J.R., Schreiber J.A., Larsen A.D., Dunn K.R., Ball A.B., Khatcherian S.M. The ongoing cognitive processing of exclusionary social events: evidence from event-related potentials. Soc. Neurosci. 2015;10(1):55–69. doi: 10.1080/17470919.2014.956899. [DOI] [PubMed] [Google Scholar]
- Van Dinteren R., Arns M., Jongsma M.L.A., Kessels R.P.C. P300 development across the lifespan: a systematic review and meta-analysis. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0087347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Cheng T.W., Pfeifer J.H. Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. Neuroimage. 2017;153:359–368. doi: 10.1016/j.neuroimage.2017.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck D., Smyth C., Riva P., Adie J., Holliman A.J., Tyndall I. Who is more likely to feel ostracized? A latent class analysis of personality traits. Personal. Individ. Differ. 2023;213 doi: 10.1016/j.paid.2023.112325. [DOI] [Google Scholar]
- Watts L.L., Hamza E.A., Bedewy D.A., Moustafa A.A. A meta-analysis study on peer influence and adolescent substance use. Curr. Psychol. 2024;43(5):3866–3881. doi: 10.1007/s12144-023-04944-z. [DOI] [Google Scholar]
- Weschke S., Niedeggen M. ERP effects and perceived exclusion in the cyberball paradigm: correlates of expectancy violation? Brain Res. 2015;1624:265–274. doi: 10.1016/j.brainres.2015.07.038. [DOI] [PubMed] [Google Scholar]
- White L.O., Wu J., Borelli J.L., Rutherford H.J.V., David D.H., Kim-Cohen J., Mayes L.C., Crowley M.J. Attachment dismissal predicts frontal slow-wave ERPs during rejection by unfamiliar peers. Emotion. 2012;12(4):690–700. doi: 10.1037/a0026750. [DOI] [PubMed] [Google Scholar]
- Williams K.D. Vol. 41. Academic Press; 2009. Ostracism: a temporal Need-Threat model; pp. 275–314. (Advances in Experimental Social Psychology). [DOI] [Google Scholar]
- Williams K.D., Cheung C.K., Choi W. Cyberostracism: effects of being ignored over the Internet. J. Personal. Soc. Psychol. 2000;79(5):748–762. doi: 10.1037/0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Nida S.A. Ostracism: consequences and coping. Curr. Dir. Psychol. Sci. 2011;20(2):71–75. doi: 10.1177/0963721411402480. [DOI] [Google Scholar]
- Wölfer R., Scheithauer H. Ostracism in childhood and adolescence: emotional, cognitive, and behavioral effects of social exclusion. Soc. Influ. 2013;8(4):217–236. doi: 10.1080/15534510.2012.706233. [DOI] [Google Scholar]
- Yang M., Deng X., An S. The immediate and lasting effect of emotion regulation in adolescents: an ERP study. Int. J. Environ. Res. Public Health. 2021;18:10242. doi: 10.3390/ijerph181910242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111(4):931–959. doi: 10.1037/0033-295X.111.4.931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.