Abstract
We tested whether transfer of the gene coding for glutamic acid decarboxylase to dorsal root ganglion using a herpes simplex virus vector to achieve release of GABA in dorsal horn would attenuate nociception in this condition. Subcutaneous inoculation of a replication-defective herpes simplex virus vector expressing glutamic acid decarboxylase (vector QHGAD67) 7 days after selective L5 spinal nerve ligation reversed mechanical allodynia and thermal hyperalgesia; the antiallodynic effect lasted 6 weeks and was reestablished by reinoculation. QH-GAD67 inoculation also suppressed induction of c-Fos and phosphorylated extracellular signal–regulated kinase 1 and 2 in the spinal cord.
Peripheral neuropathic pain is a common and difficult to treat concomitant of polyneuropathy or structural nerve injury. Opioids are relatively ineffective, and their use is limited by side effects. Antidepressants and anticonvulsants have demonstrated efficacy in randomized controlled trials but provide only 50% relief in less than half of patients treated.1 Among the complex mechanisms underlying neuropathic pain, partial nerve injury results in a selective loss of GABAergic inhibitory synaptic currents in spinal cord2 that contribute to abnormal pain sensitivity and the phenotypic features of the neuropathic pain syndrome. GABAergic agents have not been widely used in the treatment of neuropathic pain because the therapeutic window of these agents is modest and the dose is limited by side effects.
Gene transfer represents a novel and useful means to target expression of peptides to focal sites within the nervous system. We have shown that transduction of sensory neurons of the dorsal root ganglion (DRG) by footpad inoculation with herpes simplex virus (HSV)-based vectors can be used to achieve a regional antinociceptive effect. HSV vectors coding for proenkephalin or the glial cell–derived neurotrophic factor produce an antihyperalgesic and antiallodynic effect in rodent models of inflammatory pain, neuropathic pain, and pain resulting from cancer in bone,3–6 and an HSV-vector expressing glutamic acid decarboxylase (GAD) provides an analgesic effect in the central neuropathic pain syndrome resulting from spinal cord injury.7 In this study, we examined the antinociceptive effect of GAD expressed from an HSV-based vector in the spinal nerve ligation (SNL) model of neuropathic pain in the rat.
Materials and Methods
The nonreplicating HSV vector QHGAD67, which is defective in expression of the HSV immediate early genes ICP4, ICP22, ICP27, and ICP47 and contains the human GAD67 gene under the control of the human cytomegalovirus immediate early promoter in the UL41 locus, has been described previously.7 The control vector QOZHG is defective in the same HSV genes but contains the green fluorescent protein and Escherichia coli lacZ reporter genes.7
Male Sprague–Dawley rats weighing 225 to 250gm underwent selective L5 SNL, as described previously,5 with the approval of the University Committee on Use and Care of Animals. One week after SNL, 30μl of vector (either QH-GAD67 or QOZHG, 4 × 108 plaque-forming units per milliliter) was injected subcutaneously in the plantar surface of the left hind paw, ipsilateral to the ligation. Mechanical allodynia induced by SNL was determined by assessing the response of paw withdrawal to von Frey hairs of graded tensile strength as described previously,5,8 with a tactile stimulus producing a 50% likelihood of withdrawal determined using the up-down method.9 Thermal hyperalgesia was determined using a Hargreaves apparatus,10 recording the time to withdrawal from a radiant thermal stimulus positioned directly under the hind paw.
Results
After L5 SNL, rats displayed a significant decrease in the magnitude of the mechanical stimulus necessary to evoke a brisk withdrawal response to von Frey hair stimulation (Fig 1A) and a significant reduction in latency to withdraw from a heat stimulus (thermal hyperalgesia; see Fig 1B). Rats inoculated with QH-GAD67 showed a statistically significant increase in mechanical threshold beginning 1 week after inoculation. The antiallodynic effect of QHGAD67-mediated GABA expression was sustained and continuous, lasting 5 to 6 weeks and peaking at 2 weeks after inoculation (see Fig 1A). The peak value of mechanical threshold, 8.6gm, was close to the preoperative value. By 7 weeks after inoculation, the antiallodynic effect of vector transduction disappeared, and the mechanical threshold of QHGAD67-injected rats was identical to that of control rats. Reinoculation into the same paw with the same dose of QHGAD67 reestablished the antiallodynic effect (see Fig 1A).
Fig 1.
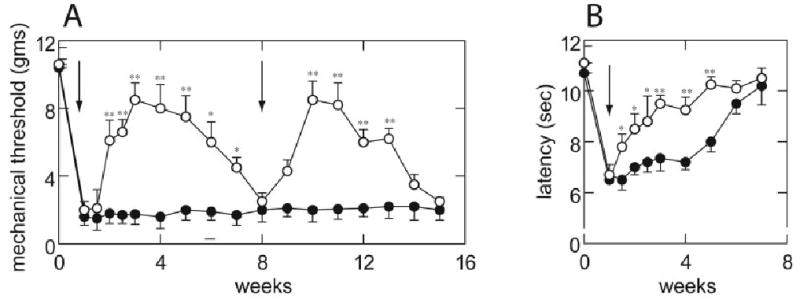
Antinociceptive effect of QOGAD67 in neuropathic pain. (A) L5 spinal nerve ligation (SNL) caused a significant decrease in the threshold to tactile stimulation, which persisted for more than 4 months. Subcutaneous inoculation of QHGAD67 (arrow) produced an antiallodynic effect reflected in an increase in the mechanical threshold. Reinoculation of QHGAD67 7 weeks after the initial inoculation (arrow) reestablished the antiallodynic effect. Results are expressed as mean ± standard error of the mean. (open circles) QHGAD67; (closed circles) QOZHG; *p < 0.05; **p < 0.01; n = 8 animals per group. (B) L5 SNL also caused a significant thermal hyperalgesia, which persisted for 6 weeks. Inoculation with QHGAD67 (arrow), but not QOZHG, reversed the thermal hyperalgesia induced by spinal nerve injury. *p < 0.05; **p < 0.01 versus QOZHG-inoculated; n = 8 animals per group. The statistical significances of the differences were determined by analysis of variance (StatView 5.2; SAS Institute, Cary, NC) corrected for the number of post hoc comparisons using Scheffé’s F test.
SNL induced a decrease in the thermal latency from 10.7 to 6.5 seconds, which lasted 3 weeks before gradually recovery. Rats inoculated with QHGAD67 showed a statistically significant increase in thermal latency in the ipsilateral paw beginning 1 week after inoculation (see Fig 1B), an effect that was sustained and continuous, lasting 3 to 4 weeks (see Fig 1B). Sham-operated animals had no change in mechanical threshold or thermal latency (data not shown).
Expression of c-Fos and phosphorylated extracellular signal–regulated kinase 1 and 2 (p-ERK1/2) induced by gentle touch is one indirect biological marker of nociceptive processes.11 Three weeks after SNL, gentle touch was applied once every 4 seconds for 10 minutes, with the flat surface of the experimenter’s thumb to the rat’s paw, and the number of immunoreactive cells (anti–c-Fos or anti–p-ERK1/2 antibodies; Santa Cruz Biotechnology, Santa Cruz, CA) detected avidinbiotin horseradish peroxidase followed by nickel-enhanced diaminobenzidine (Vector Laboratories, Burlingame, CA).12 The number of Fos-LI–positive neurons was substantially increased ipsilateral to SNL compared with sham-operated control rats (Fig 2), and inoculation of vector QHGAD67 significantly reduced the number Fos-LI–positive neurons in laminae I–VI (see Fig 2). p-ERK1/2 expression in laminae I and II was also increased in rats after gentle touch stimulation with SNL, and that increase was blocked in animals inoculated with QHGAD67 (Fig 3).
Fig 2.
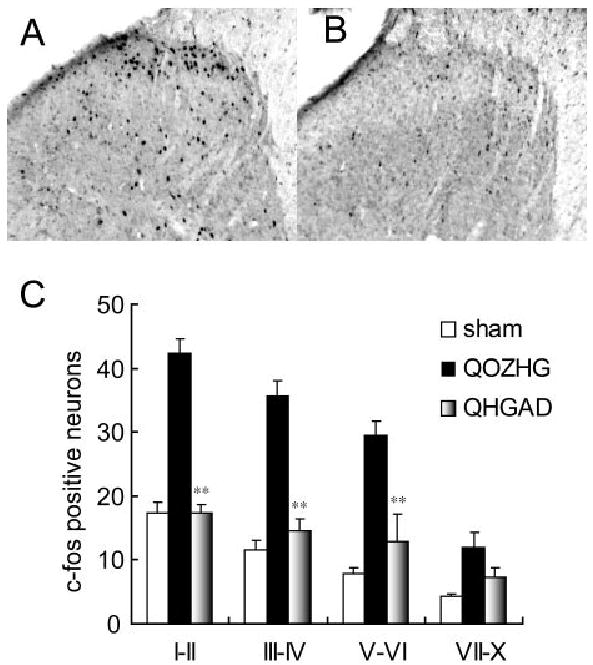
Effect of QHGAD67 on Fos-LI in dorsal horn. (A) Fos-LI in dorsal horn induced by 10 minutes of gentle tactile stimulation was markedly increased in rats inoculated with QOZHG 1 week after spinal nerve ligation (SNL) and tested 2 weeks later (3 weeks after SNL). (B) This increase was blocked in rats with SNL that had been inoculated with QHGAD67 1 week after SNL and tested 2 weeks later (3 weeks after SNL), and it was found in laminae I–VI of dorsal horn. Results are expressed as mean ± standard error of the mean. **p < 0.01; n = 5 animals per group. The difference between sham-operated and SNL animals inoculated with QOZHG was also statistically significant (p < 0.01).
Fig 3.
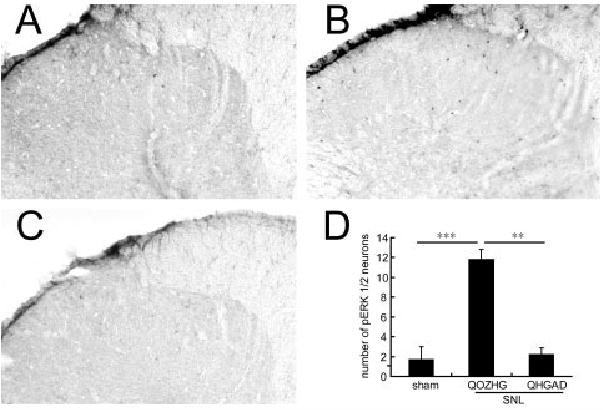
Effect of QHGAD67 on the phosphorylated extracellular signal–regulated kinase 1 and 2 (p-ERK1/2) expression in dorsal horn. p-ERK is not induced by 10 minutes of gentle tactile stimulation in sham-operated animals (A), but it is substantially induced after spinal nerve ligation (SNL) in animals inoculated with QOZHG (B). Touch-induced expression of p-ERK1/2 was suppressed in animals inoculated with QHGAD67 (C), confirmed by counts of p-ERK1/2–positive neurons in the dorsal horn (D). Results are expressed as mean ± standard error of the mean. **p < 0.01; ***p < 0.001; n = 5 animals per group.
Discussion
These results demonstrate that subcutaneous inoculation of an HSV vector expressing GAD to transduce DRG in vivo attenuated the behavioral manifestations of mechanical allodynia and thermal hyperalgesia in a model of peripheral neuropathic pain; the effect on behavior was confirmed by histological measures showing a block in the induction of expression of c-Fos and p-ERK1/2 in the ipsilateral spinal dorsal horn. We have demonstrated previously that transduction of primary sensory neurons with QHGAD67 results in the release of GABA from those cells in vitro and from sensory nerve terminals in spinal cord in vivo.7 Subcutaneous inoculation of the vector results in transduction of both large and small neurons in the DRG, as determined by immunocytochemical detection of GAD expression.7 GABA release from transduced cells is constitutive and is mediated by the GABA transporter type 1 (GAT-1). In the spinal cord hemisection model of central neuropathic pain, transduction of DRG by subcutaneous inoculation of QHGAD67 in both hind paws reduces below-level mechanical allodynia and thermal hyperalgesia.7 This study is the first application of this vector to peripheral neuropathic pain.
In the spinal cord, GABA is found primarily in interneurons of the superficial dorsal horn predominantly in laminae II and III that form axoaxonic synapses on primary afferent terminals and axodendritic synapses on projection neurons. Tonic GABAergic inhibition plays an important role in normal sensory processing, as indicated by increased behavior responsiveness13 and electrophysiological activity in spinal nociceptive neurons14 after spinal administration of the GABAA antagonist bicuculline to normal animals and by the development of mechanical allodynia after spinal administration of the GABAB receptor antagonist CGP35348.15
The mechanical allodynia that results from selective SNL can be reversed by the GABAB agonist baclofen16 or the GABAA agonist muscimol,17 and both mechanical allodynia and thermal hyperalgesia after spinal nerve injury are reversed by the GABAA agonist 4,5,6,7-tetrahydroisoxazolo [5,4-c] pyridin-3-ol (isoguvacine) and baclofen.18 These findings are consistent with observations of loss of GABA activity in dorsal horn after chronic constriction injury of sciatic nerve,19 a decrease in GABAA receptor–mediated inhibitory postsynaptic potentials, reduction of GAD65, and evidence of apoptosis in dorsal horn in both the chronic constriction and spared nerve injury models of neuropathic pain.2 Transgene-mediated GABA released in the dorsal horn acts at both GABAA and GABAB receptors. As we have demonstrated previously, the antinociceptive effect produced by QHGAD67-mediated GABA release can be partially blocked by the GABAA antagonist bicuculline or by the GABAB antagonist phaclofen.7
GABAergic therapy for neuropathic pain has had limited success. Gene transfer offers the possibility of targeted alteration in the neurotransmitter phenotype to produce the local release of selected neurotransmitters. Among the many viral and nonviral vectors that may be used for gene transfer, HSV, because of its natural neurotropism and high-affinity retrograde transport in sensory neurons, is uniquely suited for modifying neurotransmitter release in dorsal horn after subcutaneous inoculation.20 We have found previously that HSV-mediated transfer of proenkephalin produces a sustained antiallodynic effect in the SNL model of neuropathic pain,5 and that similar results can be achieved with a vector expressing glial cell–derived neurotrophic factor.6 The magnitude of the antiallodynic effect achieved with the GAD67-expressing vector is substantially greater than that produced by either the proenkephalin- or glial cell–derived neurotrophic factor–expressing vectors. We propose that this differential effect reflects the role of reduced spinal GABAergic tone in the pathogenesis of neuropathic pain and the selective ability of QHGAD67 to correct that deficit locally. The results suggest that this novel approach may prove useful in the treatment of neuropathic pain.
Acknowledgments
This study was supported by grants from the NIH (National Institute of Neurological Disorders and Stroke, NS44507, NS38850, D.J.F., NS43247, M.M.) and the Department of Veterans Affairs (M.M., D.J.F.).
Dr. Mata's work was supported by R01 NS43247 (M Mata, PI), Gene Transfer for Spinal Root Trauma.
We thank V. Thakur and S. Liu for their excellent technical assistance.
References
- 1.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 2.Moore KA, Kohno T, Karchewski LA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson SP, Yeomans DC, Bender MA, et al. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braz J, Beaufour C, Coutaux A, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao S, Mata M, Goins W, et al. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect. Pain. 2003;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 6.Hao S, Mata M, Wolfe D, et al. HSV-mediated gene transfer of the glial cell derived neurotrophic factor (GDNF) provides an anti-allodynic effect in neuropathic pain. Mol Ther. 2003;8:367–375. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wolfe D, Hao S, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 10.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 11.Catheline G, Le Guen S, Besson JM. Intravenous morphine does not modify dorsal horn touch-evoked allodynia in the mononeuropathic rat: a Fos study. Pain. 2001;92:389–398. doi: 10.1016/S0304-3959(01)00283-4. [DOI] [PubMed] [Google Scholar]
- 12.Hao S, Takahata O, Mamiya K, Iwasaki H. Sevoflurane suppresses noxious stimulus-evoked expression of Fos-like immunoreactivity in the rat spinal cord via activation of endogenous opioid systems. Life Sci. 2002;71:571–580. doi: 10.1016/s0024-3205(02)01704-6. [DOI] [PubMed] [Google Scholar]
- 13.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 14.Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77:181–190. doi: 10.1016/S0304-3959(98)00094-3. [DOI] [PubMed] [Google Scholar]
- 15.Hao JX, Xu XJ, Wiesenfeld-Hallin Z. Intrathecal gamma-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett. 1994;182:299–302. doi: 10.1016/0304-3940(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 16.Smith GD, Harrison SM, Birch PJ, et al. Increased sensitivity to the antinociceptive activity of (+/−)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994;33:1103–1108. doi: 10.1016/0028-3908(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 18.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Ibuki T, Hama AT, Wang XT, et al. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 20.Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004;58:253–271. doi: 10.1146/annurev.micro.58.030603.123709. [DOI] [PubMed] [Google Scholar]


