Abstract
Irreparable rotator cuff tears present significant challenges owing to tear size, tendon retraction, and poor tissue quality. This article describes a surgical approach integrating biologic tuberoplasty with rotator cuff repair, using an acellular human dermal allograft to re-establish the rotator cuff footprint and prevent bone-on-bone contact between the humeral head and acromion. Footprint reconstruction is defined as allograft coverage of the tuberosity combined with partial cuff repair that includes some contact of the native cuff over the allograft. Changing the nomenclature to “biologic footprint reconstruction” more accurately describes the procedure when combined with partial cuff repair in continuity with the allograft and avoids confusion with isolated biologic tuberoplasty. The graft alleviates pain and creates a biologic healing environment. This approach is designed to reduce surgical complexity and improve efficiency, ensuring reproducibility while restoring shoulder biomechanics and function.
Technique Video
Rotator cuff tears (RCTs) are a common source of shoulder pain and dysfunction.1 Although primary repair is effective for many cases, irreparable RCTs pose a significant challenge owing to factors such as large tear size, tendon retraction, muscle atrophy, and fatty infiltration, which may preclude successful conventional rotator cuff repair (RCR).2, 3, 4 In such cases, alternative surgical options, including tendon transfer, superior capsular reconstruction (SCR), balloon spacer implantation, or reverse total shoulder arthroplasty (rTSA), may be considered, although each presents technical complexities and variable clinical outcomes.5, 6, 7 Treatment should be tailored based on patient-specific factors, including age, activity level, symptom severity, and functional demands.8
This article presents a technique for RCR incorporating biologic tuberoplasty with an acellular human dermal allograft to reconstruct the native cuff footprint. The term “biologic tuberoplasty” was coined based on observations from a series of cases in which patients who underwent SCR achieved excellent clinical outcomes, even with a torn graft or incomplete healing, as long as the tuberosity remained covered.9 This approach is specifically designed for the management of irreparable RCTs in younger, low-demand patients who are suboptimal candidates for rTSA and as an alternative to SCR. One study has published a case report in which a similar technique was performed, showing excellent short-term results and a healed rotator cuff.10 The primary objectives include pain reduction, preservation and restoration of shoulder function and biomechanics, and prevention of humeral head–acromion contact.
Surgical Technique
Preoperative Workup
A detailed history and physical examination are conducted, focusing on shoulder pain, range of motion (ROM), functional deficits, and patient-specific factors such as activity level, comorbidities, and treatment expectations. Patients typically report pain predominantly along the lateral aspect of the shoulder, weakness, and limited active ROM. Imaging studies include plain radiographs to evaluate for glenohumeral osteoarthritis, bone quality, calcific tendinitis, and morphology of the tuberosity and acromion. Magnetic resonance imaging is used to determine the tear size, tendon retraction, muscle atrophy, and fatty infiltration.11 Careful patient selection for biologic footprint reconstruction is essential to optimize outcomes and minimize complications (Table 1).
Table 1.
Indications and Contraindications for Biologic Footprint Reconstruction
| Indications |
| Irreparable rotator cuff tears for which traditional repair is unfeasible because of tear size, tissue quality, tendon retraction, fatty infiltration, and/or muscle atrophy |
| Partial rotator cuff tear with intact or repairable subscapularis |
| Pain and loss of function in patients without severe glenohumeral joint degeneration |
| Poor candidates for reverse total shoulder arthroplasty—younger, low-demand patients |
| Contraindications |
| Full-thickness subscapularis tear |
| Pseudoparalysis |
| Poor surgical candidates owing to medical comorbidities, poor bone quality, and/or poor vascularity |
| No or limited potential for biologic healing |
| Inability to tolerate rehabilitation protocol |
Surgical Positioning
The patient is placed supine on the operating table, and general anesthesia is induced. This surgical procedure can be performed with the patient in either the beach-chair or lateral decubitus position. In this case, the patient is positioned in the beach-chair position. The operative extremity is prepared and draped in the usual standard sterile fashion, and preoperative antibiotics are administered.
Diagnostic Arthroscopy
A standard posterior portal is established, followed by an anterior mid-glenoid portal, using an outside-in technique. A diagnostic arthroscopy is performed to assess the rotator cuff for irreparability, tendon retraction, and overall tissue quality. A thorough evaluation of the glenohumeral joint is completed, with debridement as necessary. Attention is then directed to the subacromial space, where a lateral portal is established. A bursectomy is performed to ensure the preservation of the deltoid fascia. The undersurface of the acromion is identified, and if indicated, the coracoacromial ligament is released using electrocautery to fully expose the anterolateral corner of the acromion.
Greater Tuberosity Preparation
The greater tuberosity is prepared just lateral to the articular surface using a 4-mm arthroscopic burr for decortication, creating a well-vascularized bone bed that facilitates graft integration and healing. Care must be taken when treating individuals with osteoporosis because decorticating bone can affect anchor fixation. An arthroscopic ruler can be used to measure the anterior-posterior and medial-lateral dimensions of the tuberosity footprint for graft sizing. In this case, an SCR guide (Arthrex, Naples, FL) is used after medial anchor placement.
Medial Anchor Placement
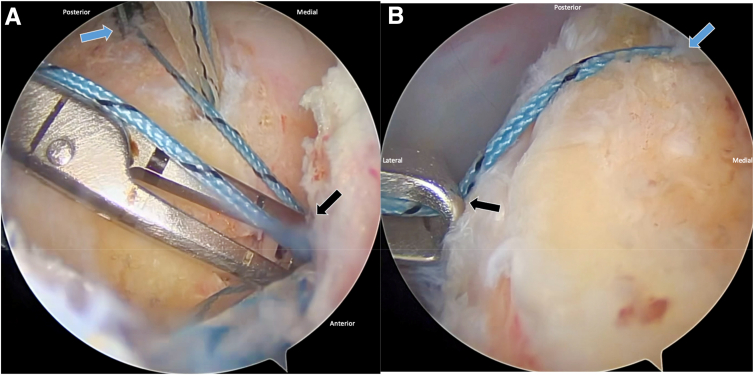
Three self-punching, knotless 2.6-mm FiberTak anchors (Arthrex) are inserted percutaneously along the medial aspect of the greater tuberosity footprint (Fig 1). An SCR guide is placed over the posterior anchor, capturing the suture through the eyelet. The suture is tensioned along the SCR guide, and a hemostat is placed on the suture at the zero mark on the guide. The distance between the anchor position and the suture from the anchor is used to estimate the anterior-posterior and medial-lateral dimensions of the tuberosity footprint for graft sizing (Fig 2).
Fig 1.
Arthroscopic image of a right shoulder with the patient in the beach-chair position, viewed from the lateral portal. The decorticated greater tuberosity footprint is shown with placement of 3 medial-row anchors: posterior (black arrow), middle (purple arrow), and anterior (green arrow). This configuration establishes the foundation for graft fixation in biologic tuberoplasty and shows appropriate medial-row spacing to optimize graft compression and coverage.
Fig 2.
Arthroscopic view of a right shoulder with the patient in the beach-chair position. (A) Anterior-posterior footprint length is measured (from blue arrow to black arrow) using a superior capsular reconstruction (SCR) guide. (B) Medial-lateral footprint width is similarly measured (from blue arrow to black arrow). These dimensions are critical for tailoring graft size to achieve adequate coverage of the decorticated tuberosity during biologic tuberoplasty.
The repair suture from each anchor is then passed through the rotator cuff tissue using a Scorpion suture passer (Arthrex). A traction suture can be placed into the rotator cuff in a luggage-tag fashion to aid in reduction. The repair suture and shuttle link looped suture from the anterior and posterior medial-row anchors are retrieved via a PassPort Button cannula (Arthrex) placed through the lateral portal. The anterior sutures should be separated more anteriorly within the PassPort device. Alternatively, a PassPort divider can be used for suture management.
Graft Preparation
On the back table, a 4-mm-thick ArthroFlex human dermal allograft (Arthrex) is trimmed to match the measured tuberosity dimensions—in this case, 16 mm anterior-posterior and 12 mm medial-lateral. Although the optimal graft thickness is currently unknown, ideally, a graft at least 3 mm thick—and perhaps up to 6 mm thick (or more)—should be used. To aid in proper orientation during graft passage, the medial border of the graft is marked with a sterile marking pen (Fig 3A).
Fig 3.
Intraoperative view of the back table. (A) The human dermal allograft (black arrow) is measured based on the anterior-posterior and medial-lateral dimensions of the tuberosity footprint. (B) The graft is trimmed, and sutures are placed in the 4 corners as follows: anterolateral, blue-and-white FiberLink suture tape in a luggage-tag configuration; posterolateral, black-and-white TigerLink suture tape in a luggage-tag configuration; anteromedial, blue-and-white FiberLink suture tape in a simple stitch configuration; and posteromedial, black-and-white TigerLink suture tape in a simple stitch configuration.
By use of a Scorpion suture passer, a 1.3-mm FiberLink (blue-and-white) suture tape (Arthrex) is placed through the anterolateral corner of the graft in a luggage-tag configuration. This step is repeated using a 1.3-mm TigerLink (black-and-white) suture tape (Arthrex) at the posterolateral corner of the graft. Next, another FiberLink suture is passed through the anteromedial corner of the graft in a simple stitch configuration so that the loop is positioned underneath the graft, which is critical for loop orientation later in the surgical procedure. This process is repeated at the posteromedial corner of the graft using a TigerLink suture, maintaining consistent orientation for efficient graft placement (Fig 3B).
Graft Passage
With the graft in the surgical field, the repair suture from the anterior anchor is passed through the FiberLink loop (oriented underneath the graft) at the anteromedial corner of the graft. The repair suture is then shuttled through the graft, replacing the FiberLink suture tape. This step is repeated for the posteromedial side using the TigerLink suture tape to shuttle the repair suture from the posterior anchor through the graft.
Once both the anterior and posterior medial-row anchor repair sutures have been passed through the graft, each repair suture is loaded into the loop of its respective shuttle link suture and folded at the purple mark. The free limb of the shuttle link at the percutaneous insertion site is then used to convert the knotless mechanism for each anchor so that the repair sutures replace the shuttle link through the percutaneous portals. With counter tension on the lateral luggage-tag sutures, the graft is shuttled via the PassPort cannula into the shoulder and positioned over the tuberosity by sequentially pulling each repair suture (Fig 4).
Fig 4.
Arthroscopic view of a right shoulder with the patient in the beach-chair position. (A) The suture anchor configuration is prepared on the greater tuberosity for graft fixation. (B) The dermal allograft is shuttled into the joint through a lateral PassPort cannula and positioned over the decorticated tuberosity. This technique facilitates controlled graft insertion and accurate placement for biologic tuberoplasty. Black arrow indicates the shuttle suture used for graft passage.
RCR and Lateral Anchor Placement
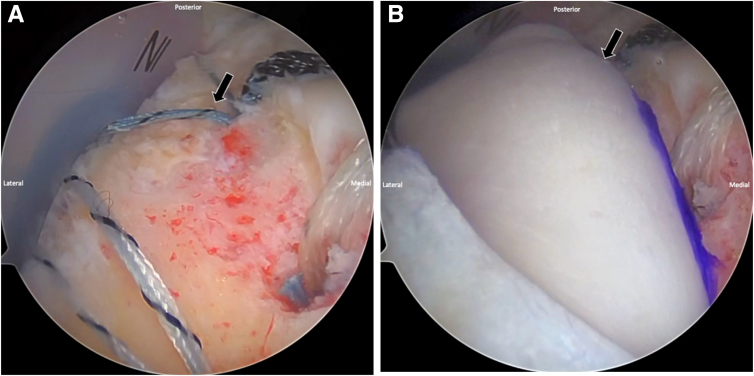
Attention is then turned to repairing the rotator cuff. The suture tapes that were originally passed through the rotator cuff are retrieved out of the lateral portal. The luggage-tag FiberLink suture tapes, along with the lateral corner sutures from the graft, are then loaded into the eyelets of 2 lateral-row self-punching 4.75-mm SwiveLock SP anchors (Arthrex), which are secured into the tuberosity anteriorly and posteriorly in standard fashion. The native cuff tissue is reduced to the medial footprint and over the medial aspect of the allograft, avoiding excessive tension on the repaired cuff tissue. Under arthroscopic visualization, the shoulder is taken through the ROM to confirm repair integrity. If necessary, the repair sutures are re-tensioned to achieve the desired repair (Fig 5, Video 1).
Fig 5.
Arthroscopic view of a right shoulder with the patient in the beach-chair position showing the final construct. The repaired rotator cuff (blue arrow) is augmented with a dermal allograft placed over the greater tuberosity (black arrow) as part of the biologic tuberoplasty. This configuration restores the superior shoulder contour and provides a biologic scaffold to protect and reinforce the repair.
Closure
The arthroscopic portals are closed in standard fashion, and a sterile dressing is applied. The patient is placed in a sling with an abduction pillow. Pearls and pitfalls of biologic tuberoplasty with RCR using the described technique are summarized in Table 2.
Table 2.
Pearls and Pitfalls of Rotator Cuff Repair Using Biologic Tuberoplasty
| Pearls |
| Use a traction suture placed into the rotator cuff to aid in reduction when passing each repair suture from the medial-row anchors. |
| Undersize the graft by 15%-20%. |
| Mark the medial border of the graft with a sterile marking pen to assist with medial and lateral orientation. |
| Use FiberLink (white-and-blue) and TigerLink (white-and-black) suture tapes to assist with anterior and posterior orientation. |
| Pitfalls |
| Failure to use a PassPort divider or maintain effective suture management can increase difficulty and surgical time. |
| Graft passage onto the greater tuberosity can be challenging without applying counter tension with the lateral sutures. |
| Poor suture tensioning or anchor placement may compromise graft fixation and healing. |
Rehabilitation Protocol
The rehabilitation protocol comprises 4 phases. Phase 1 (0-6 weeks) consists of immobilization to protect the repair, with initiation of passive ROM exercises to prevent stiffness. Phase 2 (6-12 weeks) involves the introduction of active-assisted ROM, with progression to active ROM as tolerated and initiation of isometric rotator cuff and scapular stabilizer strengthening. Phase 3 (≥12 weeks) comprises strengthening progression, incorporating dynamic stabilization and neuromuscular control, with a return to light functional activities. Phase 4 (6-12 months) incorporates full strength and endurance training with a return to sport and work.
Discussion
Biologic footprint reconstruction, when used as an adjunct to RCR, represents an approach aimed at preserving native shoulder anatomy. This technique shows promise in enhancing tendon healing and functional outcomes in cases of irreparable RCTs, potentially delaying the need for more invasive procedures such as rTSA. Additionally, it serves as a viable alternative to SCR in select patients.
This technique offers several advantages. The use of a dermal allograft serves as a protective barrier, mitigating direct bone-on-bone contact between the humeral head and the acromion, thereby reducing the risk of humeral abutment and acromial acetabularization.2 The combination of graft augmentation and tuberosity modification may further enhance the biologic healing potential of RCR.12 Additionally, the incorporation of self-punching, tensionable knotless anchor technology streamlines the procedure, potentially reducing technical demands, surgical risk, and operative time compared with alternative treatments such as SCR or tendon transfer.3 Unlike subacromial balloon spacers, which provide only transient symptomatic relief, dermal allografts may offer a more durable solution through integration with host tissue after implantation (Table 2).5
Early clinical outcomes after biologic tuberoplasty have shown significant improvements in pain relief and shoulder functionality in the short to medium term.13, 14, 15, 16 Although early integration is promising, long-term durability remains uncertain. Magnetic resonance imaging follow-ups have shown varied outcomes, ranging from intact grafts to partial or complete graft resorption over time.9
Despite its potential advantages, biologic footprint reconstruction has several limitations. Its long-term efficacy remains uncertain, necessitating further longitudinal studies to evaluate clinical outcomes and allograft survivorship. Furthermore, the cost-effectiveness of this technique requires assessment, particularly in comparison to more definitive surgical interventions. The graft-host interaction may also present risks, including inflammation, immune reaction, or infection, which warrant careful monitoring (Table 3). Continued research and clinical follow-up are essential to further define this technique’s safety, efficacy, and potential for widespread adoption.
Table 3.
Advantages and Disadvantages of Biologic Footprint Reconstruction
| Advantages |
| Dermal allograft provides a biologic cushion between the acromion and greater tuberosity. |
| Biologic tuberoplasty allograft retains growth factors and native collagen scaffold, which may enhance the healing potential when integrated into the rotator cuff repair. |
| This technique provides a durable dermal allograft that can remodel and integrate with host tissue. |
| Self-punching, tensionable knotless anchor technology allows for efficient, effective, and reproducible graft placement. |
| This technique provides pain relief and improved function, delaying the need for reverse total shoulder arthroplasty. |
| Disadvantages |
| Cost and availability of dermal allograft |
| Lack of long-term follow-up data |
| Potential risk of incomplete graft integration or resorption |
| Potential risk of inflammatory response from interaction between graft and host tissue |
| Unknown long-term durability compared with other surgical interventions |
Disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: K.A.B. reports a consulting or advisory relationship with Arthrex and Limacorporate; receives speaking and lecture fees from Arthrex; reports board membership with American Orthopaedic Society for Sports Medicine, American Shoulder and Elbow Surgeons, and Ruth Jackson Orthopaedic Society; and owns equity or stocks in Limacorporate. R.M.F. reports board membership with American Academy of Orthopaedic Surgeons, American Orthopaedic Society for Sports Medicine, American Shoulder and Elbow Surgeons, Arthroscopy Association of North America, International Cartilage Regeneration & Joint Preservation Society, International Society of Arthroscopy, Knee Surgery & Orthopaedic Sports Medicine, Journal of Shoulder and Elbow Surgery, and Orthopedics Today; reports a consulting or advisory relationship with AlloSource, Arthrex, and JRF Ortho; receives speaking and lecture fees from AlloSource, Arthrex, JRF Ortho, and Ossur; and receives funding grants from Arthrex. All other authors (E.H.R., D.J.S., P.A.S., D.R., P.B.M.) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Data
Biologic footprint reconstruction in a right shoulder with the patient in the beach-chair position, incorporating biologic tuberoplasty augmentation with an acellular human dermal allograft and partial rotator cuff repair. The technique highlights graft sizing, anchor placement, graft shuttling through a lateral portal, and final construct integration over the decorticated greater tuberosity to reinforce the repair and restore the superior shoulder contour.
References
- 1.Yamamoto A., Takagishi K., Osawa T., et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Mirzayan R., Bouz G. Biologic tuberoplasty with an acellular dermal allograft for massive rotator cuff tears. Arthrosc Tech. 2021;10:e1743–e1749. doi: 10.1016/j.eats.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suri M., Parry S., Dham M., Verma A. Arthroscopic biologic tuberoplasty for irreparable rotator cuff tears: An expedited technique. Arthrosc Tech. 2022;11:e2265–e2270. doi: 10.1016/j.eats.2022.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tashjian R.Z. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Davies A., Singh P., Reilly P., Sabharwal S., Malhas A. Superior capsule reconstruction, partial cuff repair, graft interposition, arthroscopic debridement or balloon spacers for large and massive irreparable rotator cuff tears: A systematic review and meta-analysis. J Orthop Surg Res. 2022;17:552. doi: 10.1186/s13018-022-03411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velasquez Garcia A., Nieboer M.J., de Marinis R., Morrey M.E., Valenti P., Sanchez-Sotelo J. Mid- to long-term outcomes of latissimus dorsi tendon transfer for massive irreparable posterosuperior rotator cuff tears: A systematic review and meta-analysis. J Shoulder Elbow Surg. 2024;33:959–974. doi: 10.1016/j.jse.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Mulieri P., Dunning P., Klein S., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 8.Kovacevic D., Suriani R.J., Jr., Grawe B.M., et al. Management of irreparable massive rotator cuff tears: A systematic review and meta-analysis of patient-reported outcomes, reoperation rates, and treatment response. J Shoulder Elbow Surg. 2020;29:2459–2475. doi: 10.1016/j.jse.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirzayan R., Stone M.A., Batech M., Acevedo D.C., Singh A. Failed dermal allograft procedures for irreparable rotator cuff tears can still improve pain and function: The “biologic tuberoplasty effect.”. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119863432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung J.W., Seo J.B., Lee J.Y., Yoo J.S. Arthroscopic medialization partial repair with biologic interposition tuberoplasty for large to massive irreparable rotator cuff tear. Medicina (Kaunas) 2024;60:484. doi: 10.3390/medicina60030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juhan T., Stone M., Jalali O., et al. Irreparable rotator cuff tears: Current treatment options. Orthop Rev (Pavia) 2019;11:8146. doi: 10.4081/or.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandalia K., Mousad A., Welborn B., et al. Scaffold- and graft-based biological augmentation of rotator cuff repair: An updated systematic review and meta-analysis of preclinical and clinical studies for 2010-2022. J Shoulder Elbow Surg. 2023;32:1784–1800. doi: 10.1016/j.jse.2023.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Mirzayan R. Preliminary outcomes of arthroscopic biologic tuberoplasty in the treatment of massive irreparable rotator cuff tears. Cureus. 2023;15 doi: 10.7759/cureus.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suri M., Verma A., Lim S.M., et al. Short-term outcomes of expedited arthroscopic tensionable knotless biologic tuberoplasty for massive irreparable rotator cuff tears. Ochsner J. 2023;23:277–283. doi: 10.31486/toj.23.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo J.B., Jung J.W., Yoo J.S. Combination of arthroscopic biologic tuberoplasty and bursal acromial reconstruction. J Orthop. 2024;51:1–6. doi: 10.1016/j.jor.2023.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuvoli N., Troiano E., Masini A., Colasanti G.B., Mondanelli N., Giannotti S. Biological patch in the repair of rotator cuff tears: Functional and clinical evaluation of twenty-three cases with a mean follow-up of six years. J Clin Med. 2024;13:5596. doi: 10.3390/jcm13185596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biologic footprint reconstruction in a right shoulder with the patient in the beach-chair position, incorporating biologic tuberoplasty augmentation with an acellular human dermal allograft and partial rotator cuff repair. The technique highlights graft sizing, anchor placement, graft shuttling through a lateral portal, and final construct integration over the decorticated greater tuberosity to reinforce the repair and restore the superior shoulder contour.