Abstract
Aziridines, structurally related to epoxides, are among the most challenging and fascinating heterocycles in organic chemistry due to their increasing applications in asymmetric synthesis, medicinal chemistry, and materials science. These three-membered nitrogen-containing rings serve as key intermediates in the synthesis of chiral amines, complex molecules, and pharmaceutically relevant compounds. This review provides an overview of recent progress in catalytic asymmetric aziridination, focusing on novel methodologies, an analysis of the scope and limitations of each approach, and mechanistic insights.
Keywords: Aza-Darzens, 2H-azirines, Chiral aziridines, Enantioselective aziridination, Kinetic resolution
Introduction
Aziridines are a class of three-membered saturated nitrogen-containing heterocycles that, like other strained systems such as epoxides and cyclopropanes, exhibit significant ring strain due to their small bond angles (~ 60°) [1]. This inherent strain, combined with the electronegativity of the nitrogen atom, renders aziridines highly reactive toward regio- and stereoselective ring-opening reactions under mild conditions [2–5]. Such reactivity facilitates the generation of nitrogen-containing compounds, which are particularly valuable for the synthesis of amines with diverse stereochemical and structural properties. Additionally, they benefit asymmetric synthesis by employing chiral aziridines as versatile chiral substrates, auxiliaries, and reagents, thereby enhancing the available methods for producing chiral amines with high stereoselectivity [6, 7]. As a result, aziridines have become valuable intermediates in synthetic chemistry, enabling the introduction of diverse functional groups and the construction of more complex molecular architectures.
Despite being structurally related to epoxides, aziridines are considerably less explored, and methods for their synthesis and functionalization have developed at a slower pace. However, the past few decades have witnessed significant advances in aziridine chemistry [8–12], leading to their increased application in asymmetric synthesis, medicinal chemistry, and materials science. These compounds serve as key intermediates in the synthesis of chiral amines, and the development of pharmaceutically relevant compounds [13, 14]. Furthermore, their intrinsic reactivity makes the isolation of aziridine-containing natural products particularly challenging. These compounds are found in a wide range of biologically active agents, natural products, and related molecules. The antitumor and antibiotic properties of some of these compounds, including azinomycin B [15, 16], mitomycin C [17, 18], maduropeptin [19], and FR-900482 [20–22], a close relative of mitomycin C due to their structural similarity, are well known (Fig. 1). N,N′,N″-Triethylenethiophosphoramide (thiotepa) is a trifunctional alkylating agent that it has regained interest as one of the most effective anticancer drugs in high-dose treatment regimens [23]. The therapeutic applications of others are more diverse [24]. For instance, ficellomycin [25, 26] exhibits high in vivo activity against Gram-positive bacteria, as well as, multidrug resistant strains of Staphylococcus aureus. In contrast, azicemicin A [27, 28] demonstrates inhibitory activity against Gram-negative bacteria and mycobacteria (Fig. 1).
Fig. 1.
Representative examples of aziridine-bearing covalent drugs. Aziridine ring is highlighted
The aziridine ring present in these chemotherapeutics appears to induce DNA monoalkylation via acid-activation, leading to the formation of a protonated aziridine that undergoes ring opening, thereby relieving the strain associated with the three-membered ring [29, 30]. Therefore, aziridines are potent alkylating agents that can function as covalent drugs due to their ability to act as DNA cross-linking agents via nucleophilic ring opening of the three-membered heterocycle [31].
Conversely, the ease of aziridine chemical transformations has gained significant attention for the generation of compound libraries, particularly for their role in ring-expansion reactions, which serve as a crucial step in the total synthesis of active drugs. For instance, Michida et al. [32] reported a well-designed illustration describing the asymmetric synthesis of the renin inhibitor DS-8108b, where an aziridine ring-opening reaction served as the key step. DS-8108b has entered human clinical trials as a landmark drug, demonstrating superior activity, efficiency, and safety compared to previous antihypertensive agents (Fig. 2). Lacosamide (Vimpat®) [33], an antiepileptic drug; MK-3281 [34], an inhibitor of the hepatitis C virus NS5B polymerase; sumanirole [35], a highly selective dopamine D2 receptor agonist developed for the treatment of Parkinson’s disease; oseltamivir (Tamiflu®) [36], a top-selling drug used for the treatment and prevention of the seasonal influenza caused by mutant viral strains; and BIRT-377 [37], a potent negative allosteric modulator of LFA-1 (lymphocyte function-associated antigen-1) used for the treatment of inflammatory and immune disorders, are representative examples in which the regio- and stereoselective aziridine ring-opening reaction was used as the key step in their synthesis (Fig. 2).
Fig. 2.
Selected pharmaceutical and bioactive compounds resulting from transformations of aziridines. The ethylamine structural unit derived from the aziridine moiety is highlighted
With ongoing advancements in synthetic methodologies, aziridines continue to expand their role as indispensable tools in modern organic chemistry. In recent decades, several methodologies have emerged or been improved and are now available for the highly diastereo- and enantioselective synthesis of aziridines. In this context, it might be observed that several reviews concerning the stereoselective synthesis of aziridines have appeared previously [7, 38–43]. Moreover, during the preparation of this review, a new publication on the synthesis of chiral aziridines was released [44].
This review highlights recent progress in the field of catalytic asymmetric aziridination, focusing on novel methodologies, analysis of the scope and limitations of each approach, and mechanistic insights. It is divided into four sections, covering asymmetric nucleophilic addition to 2H-azirines, aziridination of imines, aziridination of alkenes, and the kinetic resolution and desymmetrization of aziridines and 2H-azirines. The discussion will focus on those advances published in the last 7 years. This area was previously reviewed in 2018 [40], covering the literature until the end of 2017.
Catalytic Asymmetric Nucleophilic Addition to 2H-Azirines
Nucleophilic addition to 2H-azirines yields aziridine derivatives [45–51], and its asymmetric variant offers a promising approach to synthesizing chiral aziridines. The stereochemical outcome of these nucleophilic additions can be effectively controlled using chiral auxiliaries or, more efficiently, chiral catalysts. Nevertheless, enantioselective reactions of 2H-azirines with nucleophiles are uncommon.
Organocatalysis
The Nakamura group [52] reported in 2018 the first organocatalyzed enantioselective reaction of 2H-azirines with thiols as sulfur nucleophiles using N-heteroarenesulfonylated chinchona alkaloid amide catalysts (Scheme 1). The corresponding 2H-azirine 2, which was generated in situ by heating α-azidoacrylate 1 in dichloromethane at 150 °C in a sealed tube, reacted with different thiols in the presence of several cinchone-derived alkaloid catalyst. The best results were attained using 1 mol% loading of N-heteroarenesulfonylated chinchona alkaloid amide catalysts 3 and 4 affording aziridines 5 in good yields with high enantioselectivity (80–97% yield, 72–96% ee).
Scheme 1.
Organocatalytic enantioselective reaction of 2H-azirines with sulfur nucleophiles promoted by cinchona alkaloid sulfonamide catalysts
The reaction of azirine 2 with thiols, which contain electron-withdrawing groups such as bromo-, chloro-, fluorine-, and trifluoromethyl at the ortho-, meta-, or para-positions of the phenyl ring, resulted in the corresponding products 5 in good yields with high enantioselectivity (80–96% yield, 90–96% ee). Similarly, the reaction with benzenethiol or even electron-rich thiols, bearing methyl or methoxy groups, also produced products 5 with good enantioselectivity (90–93% ee). Several bulky thiols, including 2-naphthalenethiol and benzothiazolethiol, also proved to be effective nucleophiles in this transformation (Scheme 1). The selective use of catalysts 3 or 4 enabled the synthesis of enantiomer R or S, respectively.
The postulated transition state for the reaction of azirine 2 with thiols using catalyst 4 (as depicted in Scheme 2), occurs within the coordination sphere of the chiral catalyst 4. Consequently, the thiol approaches the Si face of the 2H-azirine to minimize steric hindrance, leading to the formation of the S-isomer of the final product.
Scheme 2.
Proposed catalytic cycle for the enantioselective reaction of 2H-azirines with thiols in the presence of cinchona alkaloid sulfonamide catalysts
Likewise, the authors suggested a catalytic cycle for the enantioselective addition of thiols to 2H-azirines. The acidic proton of catalyst 4 likely activates azirine 2 through hydrogen bonding to form intermediate I, while the quinuclidine moiety of catalyst 4 may simultaneously activate the thiol via hydrogen bonding. Subsequently, the activated thiol reacts with the azirine in intermediate II to form an adduct, which then undergoes protonation and decomplexation, producing aziridine 5 and regenerating catalyst 4. This reaction mechanism was further corroborated by ESI–MS analysis of the reaction mixture, which detected a signal corresponding to intermediate I (Scheme 2).
Despite the work of Nakamura et al. and a few other successful examples of asymmetric heteronucleophilic addition to 2H-azirines [53, 54] previously reported in the literature, asymmetric carbon nucleophilic addition remains uncommon due to significant steric hindrance or low nucleophilicity. In this context, the same group [55] reported the use of 8-quinolinesulfonylated 9-amino-9-deoxy-epi-cinchonine catalyst 4 (see Scheme 1) for the catalytic enantioselective reaction of 2H-azirines with oxazolones as carbon nucleophiles (Scheme 3). The in situ prepared azirine 2 reacted with various oxazolones 6 bearing electron-withdrawing or electron-rich groups or even oxazolones 6 with bulky naphthyl group organocatalyzed by cinchona alkaloid sulfonamide catalyst 4, yielding exclusively the C–2 addition products 7 as single regio- and diastereoisomers (dr > 95:5) in good to excellent yields (61–99% yield) and up to 98% ee (Scheme 3). However, the use of oxazolone 6 with an alkyl substituent (R1 = Bn) mediated by 4, afforded aziridine 7 in moderate yield and ee value (61% yield, 72% ee). This enantioselective approach enables the synthesis of aziridines 7 featuring vicinal tetrasubstituted stereocenters.
Scheme 3.
Enantioselective addition of oxazolones to 2H-azirines organocatalyzed by cinchona alkaloid sulfonamide catalyst
A similar catalytic mechanism, previously proposed for the addition of thiols to 2H-azirines (see Scheme 2), was reported for the reaction of azirines 2 with oxazolones 6, as shown in Scheme 4. The sulfonamide moiety in 4 forms intramolecular hydrogen bonds and interacts with the 2H-azirine 2, leading to the formation of intermediate III, activating the electrophilicity of the 2H-azirine through hydrogen-bonding. Simultaneously, since catalyst 4 function as a dual-activating organocatalyst, the quinuclidine moiety in 4 generates an enol of oxazolone 6. Next, the reaction of activated oxazolone 6 and 2H-azirine 2 in intermediate IV afford the corresponding adduct, which after protonation and decomplexation delivered aziridines 7 and catalyst 4 (Scheme 4).
Scheme 4.
Postulated mechanism for the reaction of 2H-azirines with oxazolones promoted by cinchona alkaloid sulfonamide catalyst
Using ring-strained 2H-azirines as substrates, Peng et al. [56] explored the potential of N-heterocyclic carbenes (NHC) as catalysts in the aza-benzoin reaction with aldehydes to build chiral aziridines. Thus, the aza-benzoin reaction of 2H-azirines 2 with aldehydes 8 catalyzed by l-phenylalanine-derived triazolium catalyst 9, in combination with Cs2CO3, provides the desired aziridines 10 in 57–97% yield with 70–98% ee (Scheme 5). Aromatic aldehydes bearing an electron-withdrawing group, electron-rich aldehydes, or aldehydes with halides (e.g. F, Cl, and Br) in ortho-, meta-, or para-position of the phenyl ring were well-tolerated and provided aziridines 10 in good yields with excellent ee values. Likewise, a number of heteroaromatic aldehydes successfully afforded the desired products 10 in good conversions and with excellent enantioselectivity. However, no conversion was observed when an aliphatic aldehyde such as butyraldehyde participate in the enantioselective aza-benzoin reaction, which is in accordance with the low reactivity of other aliphatic aldehydes in NHC-catalyzed aza-benzoin reactions.
Scheme 5.
Organocatalyzed asymmetric aza-benzoin reaction of aldehydes with 2H-azirines mediated by N-heterocyclic carbenes
Concerning the 2H-azirine scope, the steric and electronic influences on the aromatic ring of azirines 2 were assessed by systematically varying the substitution patterns (Scheme 5). Azirines containing either electron-withdrawing or electron-donating groups in ortho-, meta-, or para-position of the phenyl ring, or substrates having either a heteroaryl group or a naphthyl motif, yielded the chiral aziridine derivatives 10 in good to high yields and high enantioselectivity. Replacing the aromatic ring in the azirine 2 with a benzyl group still resulted in a high ee value. However, when the azirine featured an alkyl substituent, the reaction exhibited relatively low activity but maintained high enantioselectivity.
Metal Catalysis
To date, the construction of chiral aziridines with vicinal tetrasubstituted stereocenters remains a significant challenge. Importantly, vicinal tetrasubstituted stereocenters are key structural motifs in pharmaceutically active compounds, making the development of new catalytic methods for their construction a highly sought-after goal in organic chemistry. In addition, enolates rank among the most important and versatile carbon nucleophiles for enantioselective carbon–carbon bond formation [57–59]. Consequently, the direct enantioselective Mannich reaction of enolates with 2H-azirines serve as an atom-economic strategy for the preparation of chiral aziridines. In this context, Hu et al. [60] reported in 2018 the first asymmetric Cu-catalyzed tertiary carbon nucleophilic addition of β-keto amides 12 to 2H-azirines 11 mediated by a chiral N,N′-dioxide ligand L1/Cu(II) complex catalytic system for the preparation of chiral aziridines 13 with vicinal tetrasubstituted stereocenters (Scheme 6). L-Ramipril-derived ligand L-RaPr3 L1 was selected for the investigation of the substrate scope. First, inden-1-one-derived β-keto amides with different substitution (electron-withdrawing or electron-donating groups at C–4, C–5, C–6, or C–7 positions), β-keto amide incorporating a naphthyl group, β-keto amides derived from cyclopentanone or cyclohexanone, or even β-keto ester 12 are well-tolerated and participated in the reaction to afford the corresponding chiral aziridines 13 in 65–90% yields, moderate to high diastereoselectivity (45:55 to 95:5 dr), with moderate to excellent enantioselectivity (32–94% ee). Next, nucleophilic addition of 5-phenyl-substituted β-keto amide 12 (R2 = 5-Ph) to several 2H-azirines 11 was examined (Scheme 6). It seems that the electronic properties of the substituents at the 3- or 4-position of the phenyl ring (R) in azirine 11 had a negligible effect on both the yields and enantioselectivity. The preparation of disubstituted 2H-aziridine 13 (R = 3, 5-Me2C6H3) was also achieved in 80% chemical yield with 83% ee value. Fused rings and naphthyl substituents in R are also tolerated in the Cu-catalyzed nucleophilic addition of β-keto amides 12 to 2H-azirines 11. The effect of the substituent R1 in 2H-azirines 11 was also investigated, revealing that the electronic properties had minimal influence on the reaction, affording aziridines with excellent enantioselectivity (up to 92% ee) and good yields (up to 85%). Conversely, the use of 2H-azirines 11 (R1 = Bn, H) gave the poorest results, providing aziridines 13 with limited stereocontrol.
Scheme 6.
Copper-catalyzed asymmetric addition of tertiary carbon nucleophiles to 2H-azirines
The assumed transition state for the nucleophilic addition of β-keto amides 12 to 2H-azirines 11 using chiral N,N′-dioxide ligand L1/Cu (II) complex catalytic system is reported in Scheme 6. The enolate of β-keto amide 12 coordinated to Cu (II) in a bidentate manner via the oxygen atoms of the dicarbonyl groups, resulting in the formation of a rigid octahedral complex (TS–1). In all instances, β-keto amide 12 selectively attacks 2H-azirine 11 from the backside relative to the substituent at the 2-position of the 2H-azirine, due to reduced steric hindrance, giving to the formation of (1S,2S,3S)-aziridine 13.
As expected, the addition of the enolizable 1,3-dicarbonyl compound to the 2H-azirine produces an aziridine nitrogen anion intermediate. The direct deprotonation of the enolizable 1,3-dicarbonyl compound in the absence of a catalyst may be assisted by the strong basicity of the aziridine nitrogen anion in the product, severely compromising the stereoselectivity of the products. In order to mitigate the impact of the strong basicity of the aziridine nitrogen anion, Feng et al. used the additional free acidic amide N–H bond of the nucleophile. Less acidic nucleophiles, such as prochiral nonstabilized ketone enolates, might be more favorable, with the enantiodetermining step being the attack of the enolate on the imine. Treating ProPhenol with Et2Zn can produce dinuclear main group metal salts, which have been shown to be effective metal catalysts for the addition of nonstabilized enolates to activated imines. In this regard, Trost et al. [61–65] described the Zn-ProPhenol catalyzed enantioselective Mannich reactions of nonstabilized enolates with acyclic imines activated with electron-withdrawing groups. The same group has applied this concept to the Zn-ProPhenol catalyzed enantioselective Mannich reaction of 2H-azirines with alkynyl ketones [66], providing chiral aziridines with vicinal tetrasubstituted stereocenters in high yields with excellent enantioselectivity (Scheme 7). Thus, reaction of substituted alkynyl ketones 14 as nucleophiles with 2H-azirines 2 in the presence of Zn-ProPhenol catalyst L2 in tetrahydrofuran (THF) as solvent, afforded aziridine intermediates 15, which were converted into aziridines 16 after sequential quantitative N–H bond acetylation. Concerning the scope of alkynyl ketones 14 that can participate in this reaction, it seems that electron-neutral naphthalene and phenanthrene-substituted alkynyl ketones proved to be excellent substrates for this Zn-ProPhenol catalytic system. Alkynyl ketones with electron-withdrawing groups at the para-, meta-, and ortho-positions of the phenyl ring gave the desired aziridines 16 in high yield with high to excellent enantioselectivity. Likewise, alkynyl ketones with electron-donating methyl group at the phenyl ring, heteroaromatic groups or even alkylsilyl groups are well tolerated in the catalytic enantioselective Mannich reaction. It is worth mentioning that cyclobutyl, cyclopentyl, cyclopent-3-en-1-yl, and cycloheptyl substituents in alkynyl ketones 14 delivered adducts 16 in reasonable yield with up to 95% ee (Scheme 7). In relation to the scope of 2H-azirines 2, electron-withdrawing groups at different positions of the phenyl ring or electron-neutral 2-naphthyl and (1,1′-biphenyl)-4-yl substituted 2H-azirines 2, performed well providing the desired products 16 in high yields with high to excellent enantioselectivity (Scheme 7).
Scheme 7.
Zn-ProPhenol catalyzed asymmetric Mannich reaction of 2H-azirines with alkynyl ketones
Considering the configuration of the obtained N-acetyl-aziridine adduct 16, the researchers proposed the mechanism illustrated in Scheme 8, which starts with the formation of the dinuclear Zn-ProPhenol complex. The catalyst’s Brønsted basic site facilitates the coordination and deprotonation of the alkynyl ketone 14 generating zinc enolate V. Subsequently, the 2H-azirine 2 coordinates to the Lewis acidic site of V, forming complex VI, which directs the Re face attack of the alkynyl ketone on the 2H-azirine, leading to the formation of complex VII. Finally, protonation and decomplexation of VII by a second equivalent of alkynyl ketone 14 yield the Mannich adduct 15 while regenerating zinc enolate V.
Scheme 8.
Proposed mechanism for the Zn-ProPhenol catalyzed asymmetric Mannich reaction
More recently, an enantioselective copper-catalyzed synthesis of aziridines with consecutive tetrasubstituted stereocenters has been reported by Xie et al. [67]. This procedure entails the Cu-mediated coupling of cyclic imino esters 17 with 2H-azirines 11 under mild reaction conditions (Scheme 9). Therefore, the model reaction between 2H-azirine 11 (R = Ph, R1 = H) and imino ester 17 (X = CH2, R2 = Ph) in the presence of Cu (MeCN)4PF6 (5 mol%), with different chiral Phosferrox ligands (5.5 mol%), and a catalytic amount of a base was explored. The reaction proceeded more efficiently when toluene was used as the solvent, using K2CO3 as the base, and the highest enantioselectivity with similar yields and diastereoselectivity was obtained using (S,Sp)-Ph-Phosferrox L3 as the ligand (83– > 99% ee, 61–99% yield, > 95:5dr, Scheme 9). Concerning the scope of the cyclic imino esters 17, it seems that the electronic properties and the position of substituents on the phenyl ring of ketimine esters have minimal influence on reactivity and stereoselectivity, except in the case of ortho-methyl substitution, which has a slightly negative effect on the enantiocontrol, mainly due to the increased steric hindrance. Likewise, not only aromatic cyclic imino esters 17 but also serine derived ketimine ester 17 (X = O, R2 = Ph) could efficiently participate in the reaction to afford aziridines 18 in excellent yields with high diastereo- and enantioselectivity. Notably, a wide variety of azirine substrates 11, including 3-aryl or 3-alkyl-2H-azirines and 2,3-diphenyl-2H-azirine, were well-tolerated, yielding the corresponding aziridines 18 with vicinal tetrasubstituted stereocenters in good to high yields with excellent diastereo- and enantioselectivity (Scheme 9).
Scheme 9.
Copper-catalyzed coupling of cyclic imino esters with 2H-azirines
The authors suggest that the reaction should initiate with the formation of a chiral Cu/(S,Sp)-L3 catalyst VIII and subsequently converts imino ester 17 into the nucleophilic N-metalated azomethine ylide IX in the presence of a base (Scheme 10). According to the absolute stereochemistry of aziridines 18 and based on previous reports [68–70], azirine 19 would approach the Si face of IX to minimize steric repulsion with the phenyl ring of the chiral ligand (S,Sp)-L3. Furthermore, the nitrogen atom of 2H-azirine 19 coordinates to the Cu(II) center in complex IX, which promotes a favorable nucleophilic attack from the Re face of 2H-azirine 19, leading to the formation of (S,S)-18 (TS–2). In contrast, without coordination to the Cu complex, nucleophilic attack from the Si face of 2H-azirine 19 would yield the (S,R)-18 product (TS–3).
Scheme 10.
Proposed transition state for the coupling reaction of cyclic imino esters with 2H-azirines
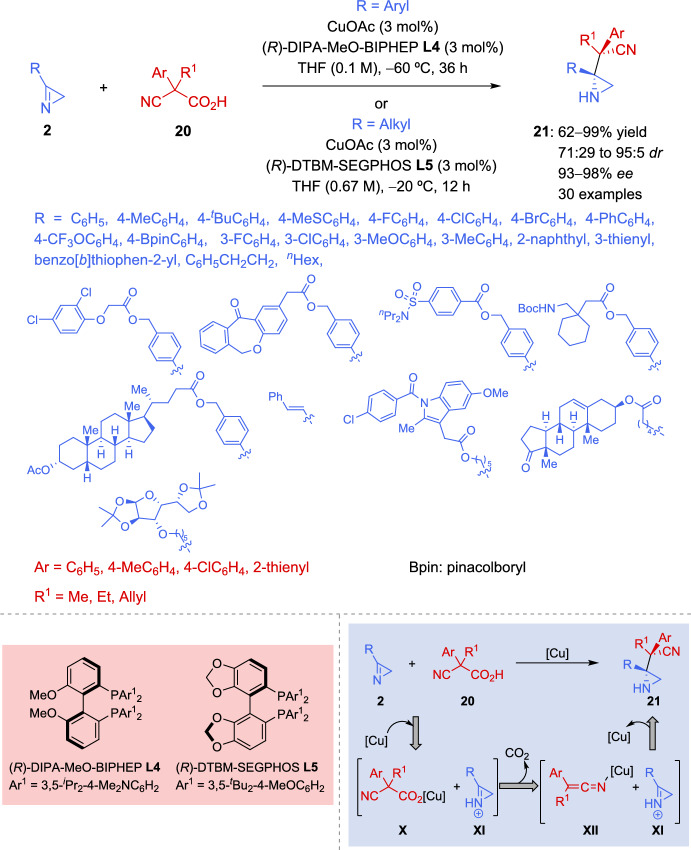
By combining nucleophilic generation through copper(I)-catalyzed decarboxylation and activation of poorly electrophilic 2H-azirines through protonation with carboxylic acids, Zhang et al. [71] reported an asymmetric decarboxylative Mannich-type reaction between α,α-disubstituted cyanoacetic acids 20 as pronucleophiles and 2H-azirines 2. This approach enables the formation of chiral aziridines 21 featuring vicinal tetrasubstituted and acyclic quaternary stereogenic carbon centers in good to excellent diastereo- and enantioselectivity (Scheme 11). The authors studied the substrate scope of aromatic 2H-azirines 2 with 3 mol% of CuOAc-(R)-DIPA-MeO-BIPHEP (Cu(I)/L4) complex. Electron-donating and electron-withdrawing groups were well tolerated at the para-position of the phenyl group. While diastereoselectivity was moderate in certain cases, the yield and enantioselectivity remained consistently high to excellent. 2H-Azirines 2 with a substituent at the meta-position of the phenyl ring performed well to afford aziridines 21 in good to excellent stereoselectivity. Conversely, ortho-substituted aromatic azirines 2 was not accepted largely due to the increased steric hindrance. As outlined in Scheme 11, aromatic 2H-azirines 2 containing complex moieties underwent the copper(I)-catalyzed decarboxylative Mannich reaction efficiently to afford aziridines 21 in excellent yield, high diastereoselectivity, and with excellent enantioselectivity. Additionally, aliphatic 2H-azirines 2 can also be used in this approach. However, since (R)-DTBM-SEGPHOS L5 provided better diastereoselectivity control than (R)-DIPA-MeO-BIPHEP L4 in the reaction with aliphatic 2H-azirine 2 (R = PhCH2CH2, 6:1 dr versus 4:1 dr), L5 was selected for substrate scope of aliphatic 2H-azirines. Although the diastereoselectivity was not satisfactory in some cases (71:29–86:14 dr) the yields and enantioselectivity were excellent in almost cases (Scheme 11). Furthermore, the aryl group of α,α-disubstituted cyanoacetic acid 20 was successfully broadened to include 4-MeC6H4, 4-ClC6H4, and 2-thienyl groups, achieving high to excellent stereoselectivity.
Scheme 11.
Catalytic asymmetric decarboxylative Mannich reaction of 2H-azirines
Taking into account the basicity and low electrophilicity of 2H-azirines, the authors proposed a strategy in which basic 2H-azirines may be activated via protonation by cyanoacetic acid 20, generating highly electrophilic iminium species XI [72, 73] (Scheme 11). Simultaneously, anion exchange results in the formation of copper(I) cyanoacetate X, which generates the nucleophilic copper(I) ketenimide XII through CO2 extrusion. Finally, the asymmetric addition of nucleophilic copper(I) ketenimide XII to the electrophilic iminium species XI enables the efficient formation of aziridines 21 featuring adjacent chiral tetrasubstituted and acyclic quaternary stereocenters.
Based on previous reports [74] on the development of copper-catalyzed enantioselective addition reactions to aldimines with silicon nucleophiles, Zhao et al. [75] described the uncovered enantioselective addition reactions of silicon nucleophiles to 2H-azirines. C-Silylated, N-unprotected aziridines with consistently high levels of enantioinduction were obtained by means of an asymmetric copper-catalyzed silylation of 2H-azirines 2 with a silyl boronic ester 22 as a silicon pronucleophile. The C-silylated aziridines 23 obtained as a result show high yields and excellent enantioselectivity (Scheme 12). Using McQuade’s catalyst [74, 76], 1.5 equiv of NaOMe, and 1.5 equiv of Me2PhSiBpin 22, C-silylated aziridines 23 were attained in 95% yield with only 81% ee. However, screening other catalyst systems successfully led with ligand (R,R)-Ph-BPE L6, which afforded 85% yield of C-silylated aziridine 23 with 95% ee in the presence of Cu(MeCN)4PF6 as the copper salt and LiOtBu as the alkoxide base (Scheme 12). This methodology demonstrated excellent functional group tolerance as it accommodated alkyl and aryl substituents at C–3 of the 2H-azirine 2 giving to the formation of adducts 23 in good yields and with high enantioselectivity. Conversely, a thienyl group as an example of a heteroaryl group afforded 23 with a low ee value of 77%.
Scheme 12.
Asymmetric copper-catalyzed silylation of 2H-azirines
Based on a well-established model for chiral copper(I) complexes with (R,R)-Ph-BPE L6 [77, 78], two reasonable transition states were proposed to outline the enantioinduction mechanism of the (R,R)-Ph-BPE-copper(I) catalyst (Scheme 12). Therefore, one of the available quadrants in the chiral catalyst’s pocket can host the substituent at C–3 of 2H-azirine 2 (left), whereas a less favorable transition state would experience steric hindrance between that substituent and a phenyl group of the ligand backbone (right). This supports the assignment of S as the induced absolute configuration.
Catalytic asymmetric hydrophosphination has been mainly limited to reactions with highly reactive Michael acceptors, and no catalytic methods have been described so far for the selective introduction of a phosphine moiety into the aziridine ring. However, a very recently report on the catalytic asymmetric nucleophilic addition of diarylphosphines to 2H-azirines assisted by Mn(I)-based systems has been reported by Ni et al. [79]. These Mn(I) catalysts exhibited high reactivity and excellent stereocontrol in hydrophosphination of Michael acceptors [80–82], which, in several instances, exceed catalytic systems based on noble metals. The author’s previous studies on the Clarke catalyst for the hydrophosphination of activated terminal alkenes [82] indicated that the NH moiety of the catalyst could play a crucial role in the stereoinduction step. This prompted the authors to propose that enantiodiscrimination could be improved by increasing steric bulk near the NH moiety. Then, Mn(I) complexes derived from the diastereoisomeric ligands (Rc,Sp,Sc)-L7 and (Rc,Sp,Rc)-L7, which differ from the Clarke catalyst’s ligand by the addition of a methyl group at the benzylic position adjacent to the pyridine moiety, could fulfill this role. Both Mn (I) complexes were tested in the enantioselective hydrophosphination of 2H-azirines 2. However, only (Rc, Sp, Rc)-L7/Mn yielded good results in terms of enantioselectivity (Scheme 13). Even though the base is crucial for catalyst and phosphine activation, studies on this reaction showed that it has a negligible impact on enantioselectivity. The aziridine phosphine sulfides 24 were obtained in yields ranging from 65 to 99% and with enantioselectivity of up to 98% ee, after protection of aziridine phosphine with elemental sulfur. 2H-Azirines 2 with different aryl and alkyl groups at the C–3 position demonstrated strong performance when reacted with diphenylphosphine. For instance, electron-donating or electron-withdrawing groups, acetoxyl and phenyl functional groups at the para-position of the phenyl ring, methyl and methoxy substituents at the meta-position of the phenyl ring were properly fitted in this protocol providing products 24 in high yields with excellent enantioselectivity. An increased catalyst loading (6 mol%) is needed when sterically hindered 2H-azirines 2, containing methyl or fluoro substituents at the ortho-position of the phenyl ring, were used. The (Rc,Sp,Rc)-L7/Mn catalytic system demonstrated broad compatibility, effectively accommodating 2H-azirines 2 bearing heteroaryl groups (Scheme 13). Concerning alkyl substitution at the C–3 position of the starting azirine 2, either branched or linear alkyl groups led to the formation of products with yields ranging from high to excellent, along with excellent enantiomeric excess. Other diarylphosphines were also evaluated in the catalytic asymmetric nucleophilic addition of diarylphosphines to 2H-azirines. Thus, using (4-MeC6H4)2PH and (4-MeOC6H4)2PH for hydrophosphination led to the formation of the target products with excellent enantiomeric excess, though the yields were moderate.
Scheme 13.
Asymmetric Mn (I)-catalyzed nucleophilic addition of diarylphosphines to 2H-azirines
As outlined in Scheme 14, the process starts with the formation of the Mn-phosphido complex intermediate XIII, which is generated by the interaction of the (Rc, Sp, Rc)-L7/Mn catalytic system with HPPh2 and the base. Next, the hydrogen bonding between the NH group of the ligand and the nitrogen atom of the 2H-azirine 19 promotes the reaction of species XIII with the 2H-azirine 19, resulting in the formation of species XIV. Once the aziridine phosphine is liberated, it produces species XV, which, through reaction with HPPh2, restores species XIII, allowing it to participate in the subsequent catalytic cycle.
Scheme 14.

Proposed mechanism for the asymmetric nucleophilic addition of diarylphosphines to 2H-azirines
Catalytic Asymmetric Aziridination of Imines
The enantioselective aziridination of imines has emerged as a powerful strategy in modern organic synthesis, offering an efficient route to chiral aziridines. Despite its potential, conventional approaches often rely on pre-existing chiral starting materials or the use of stoichiometric chiral auxiliaries [83–88], limiting their practicality. Therefore, the development of catalytic methods that enable aziridination from readily available achiral precursors remains a crucial challenge in the field.
Organocatalysis
The aza-Darzens reaction has become one of the most straightforward and efficient approaches for the construction of aziridine-containing molecules [40, 89–92]. Asymmetric variants of the acid-catalyzed aza-Darzens reaction were reported by Huang et al., Hu et al., and Antilla et al. [93–95] and Hashimoto et al. [96], providing cis- or trans-disubstituted aziridines with excellent stereoselectivity. Nevertheless, the asymmetric synthesis of trisubstituted aziridines has been less studied [97, 98]. In this context, in 2019, Pan et al. and Wu et al. [99, 100] reported the first enantioselective aza-Darzens reaction of cyclic imines 26 and α-halogenated ketones 25 using a dipeptide-based chiral phosphonium salt 27 as a phase-transfer promoter for the preparation of structurally dense and stereodefined trisubstituted 28 (Scheme 15) and tetrasubstituted aziridines 30 (Scheme 16). Therefore, the O-TBDPS-L-Thr-D-tert-Leu-based phosphonium salt 27 was discovered to be satisfactory in stereochemical control, and the combination of a wide range of cyclic ketimines 26 bearing electron-neutral, electron-donating, or electron-withdrawing groups on the phenyl ring and several α-bromo acetophenones 25 with different substitution in the ortho-, meta-, and para-positions of the phenyl ring, furnished di- (R = H) and trisubstituted aziridines 28 (R ≠ H) in high yields (up to 98%) and with excellent diastereo- (> 95:5) and enantioselectivity (83– > 99.9%) (Scheme 15).
Scheme 15.
Aza-Darzens reaction of cyclic imines with α-halogenated ketones under dipeptide-based chiral phosphonium salt phase-transfer catalytic conditions
Scheme 16.
Enantioselective synthesis of tetrasubstituted aziridines using a dipeptide-based chiral phosphonium salt phase-transfer catalyst
As an extension of this methodology, a more challenging synthesis of aziridine derivatives with all-carbon quaternary stereocenters has been reported for the same group (Scheme 16) [99, 100]. The catalyzed aza-Darzens reaction between cyclic ketimines 26 and cyclic α-bromoketones 29 using a 10 mol% of the dipeptide-based chiral phosphonium salt 27, proceeded smoothly to give the tetrasubstituted aziridines 30 having complex spiro-fused scaffolds in excellent yields and in a highly stereoselective way. As reported in Scheme 16, the reaction is suitable for various cyclic ketimines 26 bearing different aromatic rings and a wide variety of cyclic ortho-, meta-, and para-substituted six-membered α-bromoketones 29 (X = CH2, n = 1), seven-membered α-bromoketones 29 (X = CH2, n = 2), and α-bromobenzopyrones (X = O, n = 1).
Metal Catalysis
The “aza-Darzens-like” reaction, catalyzed by Brønsted or Lewis acids, involving diazocarbonyl compounds and activated imines, stands as a highly reliable and widely employed approach for synthesizing aziridines. This process involves the nucleophilic attack of the diazo carbon on the activated imine, followed by a 3-exo-tet cyclization of the resulting diazonium ion intermediate. Bao et al. have undoubtedly made groundbreaking contributions to the field of enantioselective aziridination of imines using diazo compounds, particularly through the development of vaulted chiral biaryl ligands such as biphenanthrol (VAPOL) and binaphthol (VANOL) [101]. Both catalysts exhibited exceptional enantioselectivity, which contrasts with other reactions where one catalyst typically outperforms the other. This field has been extensively studied [94, 102–104], focusing on the impact of N-substitution in the imine partner [93, 105], the influence of the diazocarbonyl compound [106], and the nature and structural design of catalysts [107]. Within this last scope, in an effort to discover new chiral VANOL ligands, Guan et al. [108] turned their attention to the nature of the substituents in the 3- and 3′-positions of the ligand. As shown in Scheme 17, the ligands were incorporated into boroxinate catalysts, which were used to screen the catalytic asymmetric aziridination of benzhydryl imines 31 with ethyl diazoacetate (32). The cis-aziridines 33 were attained with asymmetric inductions ranging from 64 to 96%. Imines 31 prepared from both benzaldehyde and cyclohexanecarboxaldehyde were used for screening of ligands L8 in the aziridination reaction. Modifying the 3- and 3′-positions of the VANOL ligand L8 with various substituents resulted in catalysts that afford aziridines 33 with slightly enhanced asymmetric inductions for both imines, compared to the original VANOL ligand with the standard phenyl group, with the exception of the para-cyano ligand and the thiophene substituent ligands. The best asymmetric induction of 96% ee was observed with the phenyl imine and a catalyst generated from ligand L8 (R1 = 3,5-Me2-4-MeOC6H2). The incorporation of alkyl groups in the 3- and 3′-positions of VANOL resulted in a dramatic loss of asymmetric induction in the resulting aziridines 33.
Scheme 17.
Catalytic asymmetric aziridination mediated by BOROX catalysts from 3,3′-disubstituted VANOL ligands
The synergetic effects of these ligands L8 and the electronic properties of the imines are also examined in the aziridination reaction. However, no significant improvement was observed in the asymmetric induction using the electron-rich imine derived from para-methoxybenzaldehyde and the electron-poor imine derived from para-nitrobenzaldehyde. The combination of substituents at both the 3,3′- and 7,7′-positions of the VANOL ligand L9 (Scheme 17) generally led to slightly improved results in the aziridination of the benzaldehyde-derived imine, compared to ligands L8 with substituents only at the 3,3′-positions. The authors also reported for the first time the X-ray structure of a boroxinate catalyst generated from a VANOL-derived ligand L8 (R1 = 3,5-Me2-4-MeOC6H2) and the imine 31 (R = Ph).
In another study of the same group [109], various alcohols and phenols are used for the generation of different boroxinate catalysts from either VANOL or VAPOL ligands. The aziridination reaction of benzhydryl imines 31 (R = Ph, C6H11) with ethyl diazoacetate (32) (see Scheme 17), using the generated catalysts, assume that the asymmetric induction in the aziridine product increases as the electron-donating ability of the alcohol or phenol becomes stronger. The authors support a mechanism where the boroxinate catalyst activates the imine through proton donation, with the resulting chiral anion organizing both the iminium and the diazo compound via hydrogen-bonding interactions. In the same study, the influence of electronic modulation in the VANOL ligand was investigated in the aziridination of imines 31 (R = Ph) with ethyl diazoacetate (32), using the boroxinate catalyst derived from a series of 5,5′-disubstituted VANOL ligands. However, after generating and evaluating all the catalysts, no correlation was observed between the asymmetric induction in the aziridines and the electronic properties of the substituents at the 5- and 5′-positions of the ligand.
A diastereo- and enantioselective Brønsted acid catalyzed aziridination of in situ formed aldimines and difluorodiazoethyl phenyl sulfone for the preparation of CF2-functionalized aziridines was described by the group of Tan et al. [110]. The multicomponent reaction involves 4-methoxyaniline (34), arylglyoxal monohydrate 35, and difluorodiazoethyl phenyl sulfone (38) (Scheme 18). Several chiral phosphoric acids were screened in the aziridination reaction; however, these efforts led to either a complete lack of conversion or an absence of enantioselectivity. Since arylboronic acids have been used to enhance Brønsted acidity in asymmetric organocatalysis when paired with chiral diols or chiral amino alcohols, the authors hypothesized that combining arylboronic acids with chiral Brønsted acids could establish a complementary catalytic platform. Indeed, a variety of BINOL-derived disulfonimides was employed as chiral additives in combination with 2-carboxyphenylboronic acid (36) for the multicomponent reaction. The best results in terms of both yields and enantioselectivity were observed by a combined strong Brønsted acid system consisting of chiral disulfonimide 37 and boronic acid 36 (Scheme 18). Given the limited solubility of aziridines 39, a dissolution–filtration process using isopropanol proved effective in enhancing the final dr (> 98:2), as well as the ee values (up to 99% ee). This procedure was not compatible with arylglyoxal monohydrates bearing strong electron-withdrawing groups.
Scheme 18.
Asymmetric aziridination of in situ imine formation catalyzed by disulfonimide-arylboronic acid combination
Supramolecular hosts are regarded as hybrid frameworks that emulate enzymes by achieving significant rate enhancements and selectivity through non-covalent interactions (NCIs), while retaining the simplicity and broad applicability of small-molecule catalysts [111–113]. Unlike enzymes and small-molecule systems, the importance of scaffold flexibility in promoting NCIs within supramolecular systems is often overlooked, and its impact on reaction rate and selectivity remains poorly understood. Supramolecular cage-mediated asymmetric catalysis offers an excellent opportunity to investigate the influence of cage flexibility on reaction outcomes; however, this area of supramolecular asymmetric catalysis remains largely unexplored [114, 115]. In addition, little progress has been made in the development of supramolecular host catalyst libraries. Recently, Bierschenk et al. [116] reported the preparation of a series of new chiral ligands to evaluate the impact of ligand architecture on reaction selectivity (Scheme 19). The diversification strategy for supramolecular host involved introducing various chiral amides at its apex. This was accomplished by constructing a library of chiral biscatecholate ligands through sequential amide bond formation reactions followed by boron tribromide-mediated deprotection of the catecholate oxygens. Enantiopure gallium-based supramolecular host serves as an effective catalyst for asymmetric aza-Darzens condensation between aromatic amines 40, aldehydes 41 and ethyl 2-diazopropanoate (42), delivering aziridine 43 (R1 = H, R2 = Et) with enantioselectivity of up to 98% ee along with excellent yield (Scheme 19). This level of selectivity is exceptional, especially given that this host lacks a high-performance chiral ligand, catalyst, or directing functional group, with enantioinduction solely arising from its supramolecular chirality. The study of different gallium-based hosts revealed that reactions with larger substrates exhibit varying reactivity, indicating that these hosts differ in their flexibility to accommodate the transition state. The selectivity observed in the aza-Darzens condensation was found to correlate with the exchange rate of a model non-reactive cationic salt, indicating that the most flexible hosts exhibit the highest selectivity for the condensation reaction. Aluminum-based host was less flexible and selective than the previous one, providing aziridine 43 (R1 = H, R2 = Et) in 47% yield and 77% ee. Whereas indium-based supramolecular host L10 (Scheme 19) appeared to be the most flexible and selective catalyst affording aziridines 43 in poor to high chemical yields (29–93%) with enantioselectivity ranging from 89 to 99% ee, except for aziridine 43 (R2 = H) derived from formaldehyde (5% ee).
Scheme 19.
Supramolecular host-promoted enantioselective aza-Darzens reaction of in situ imine formation
Catalytic Asymmetric Aziridination of Alkenes
Several methodologies for the synthesis of asymmetric aziridines use alkenes as starting materials. Some of them involved organocatalyzed reactions, while most rely on different metals for their synthesis. First, we review the organocatalyzed examples that have yielded enantioselective aziridines using different approaches.
Organocatalysis
In 2018, Mennie et al. described the diastereo- and enantioselective β-fluoroaziridination of cinnamyl tosylamide 44 derivatives, employing HF-pyridine as a nucleophilic fluoride source, together with mCPBA as a stoichiometric oxidant, and a chiral aryl iodide (R,R)-45 as the catalyst [117]. The ArI(III) intermediate 46 with an appropriately positioned nitrogen nucleophile, is crucial for the generation of the β-fluoroaziridine 47 as a single diastereoisomer with high enantioselectivity (80–97% ee). Electron-deficient substituents are required to undergo the reaction in a suitable manner, although hydroxyl derivatives can be obtained employing triflate-protected O-aryl substituents (Scheme 20). A considerable decrease in the reactivity and enantioselectivity was observed when a trisubstituted alkene was tested (48: 44% yield, 61% ee).
Scheme 20.
Catalytic diastereo- and enantioselective fluoroaziridination of alkenes
Chiral proline derivatives have been extensively used in catalytic asymmetric synthesis of a wide variety of organic compounds [118]. Monteiro et al. [119] used them for the asymmetric synthesis of 2-formylaziridines 52, which were subsequently employed to obtain reduced hydantoins. The reaction between protected amines 50 and α,β-unsaturated aldehydes 49 occurred under the catalysis of a chiral amine 51 via iminium and enamine activation, providing the related 2-formyl aziridines 52 with satisfactory yields and stereoselectivity when aliphatic enals were used (Scheme 21). Unfortunately, in the case of aromatic α,β-unsaturated aldehydes 49, aziridine ring opening was observed. A strong electron-withdrawing group and low temperatures (−20 ºC) were required to isolate the corresponding aziridine 52 in good yield, diastereoselectivity, and excellent enantioselectivity.
Scheme 21.
Synthesis of 2-formyl aziridines from α,β-unsaturated aldehydes
The same group took advantage of the developed aziridination reaction, followed by a one-pot Passerini multicomponent reaction, to deliver highly functionalized aziridine-α-acyloxycarboxamides 54 in a sustainable way, using environmentally benign solvents, a metal-free approach, and a step-economical protocol [120]. The procedure was slightly modified to make it greener; therefore, the organocatalyst was adapted to function in alcoholic media. Additionally, the base component was changed from sodium acetate to sodium carbonate to avoid the formation of acetic acid as by-product, which could compete with the acid component of the Passerini reaction. This adjustment enabled the reaction to be performed in a one-pot way (Scheme 22).
Scheme 22.
Sequential organocatalyzed aziridination and Passerini multicomponent reaction
The authors observed three diastereoisomers in the crude 1H-NMR spectrum, and considering the coupling constants and the selectivity of the aziridination reaction, deduced that the major ones were the trans-aziridines (54a and 54b). Single-crystal X-ray diffraction analysis was used to determine the stereochemistry of the major diastereoisomer, which was identified as 54a in all cases.
Another green method for the synthesis of aziridines was described in 2021 by Umeda et al. The aziridination of α,β-unsaturated carbonyl compounds was carried out in the presence of a nitrogen source that was generated in situ by treating tert-butyl carbamate with the oxidant sodium hypochlorite pentahydrate, using a quaternary ammonium salt as a phase transfer catalyst [121]. After optimization of the reaction conditions, the authors demonstrated that the process could be performed in an enantio- and diastereoselective fashion using an optically active phase-transfer catalyst and low temperatures (Scheme 23). In the enantioselective example, the dimethylpyrazole α,β-unsaturated carbonyl derivative 57 and cinchonine-derived antracenylmethylated ammonium salt (S)-56 were used, achieving the corresponding aziridine 58 in satisfactory yield and enantioselectivity (77%, 84% ee). Meanwhile, the diastereselective reaction was effectively carried out using L-mentholpyrazole derivative 59 as a chiral auxiliary and cinchonidine-derived (R)-56 as catalyst. After completion of the reaction, the chiral auxiliary was efficiently removed, obtaining the methyl ester derivative without epimerization in the asymmetric center and recovering the chiral auxiliary.
Scheme 23.
Enantio- and diastereoselective aziridination of α,β-unsaturated carbonyl compounds
Subsequently, the same reaction was described using a catalytic system developed by combining chiral amines with sterically demanding yet flexible phosphoric acids [122]. This catalytic system was suitable for several asymmetric organocatalyzed reaction of cyclic enones 61, including aziridination reaction, and allowed the achievement of both enantiomers simply by changing the configuration of the catalyst (Scheme 24). Cyclic enones 61 of different size were used in the reaction, including β-substituted enones (R ≠ H), which, after aziridination reaction, provided tetrasubstituted stereocenters with high enantioselectivity (88–89% ee). Additionally, the authors demonstrated that the reaction showed good tolerance not only toward cyclic enones 61 but also toward acyclic enones 62 although a slight detriment in enantioselectivity was observed in the latter case (64–72% ee).
Scheme 24.
Asymmetric aziridination of α,β-unsaturated carbonyl compounds
A new approach for the synthesis of chiral aziridines was reported by McLean et al. Chiral phosphoric acids were employed to catalyze the enantioselective protonation of catalytically generated prochiral chloroenamines. Treatment of the obtained asymmetric vicinal chloroamines 71 with an appropriate base yielded the corresponding aziridines in good yield and high enantioselectivity [123]. Different chloro vinyl N-aryl derivatives 68 and several anilines 69 were used as substrates, and both transformations could be performed in a one-pot process (Scheme 25). Density functional theory (DFT) calculations and experimental results were in agreement demonstrating that the best results regarding reactivity and selectivity were achieved when cyclohexyl-substituted catalyst 70 was employed.
Scheme 25.
Asymmetric protonation of a catalytically generated chloroenamines and subsequent aziridination
Metal Catalysis
For millions of years, nature has been designing highly efficient catalysts, refining a diverse range of proteins that facilitate most chemical reactions essential for life. Scientists have aimed to integrate nature’s rich toolkit of metalloproteins with the unique reactivity of transition metals in non-biological chemistry. These efforts have largely centered on heme-binding proteins, as heme and its analogues play a fundamental role in synthetic transition-metal chemistry and have been extensively explored as key cofactors [124]. Expanding the repertoire of metalloenzymes beyond heme-dependent systems by developing catalysts that are new to nature, could open up an entirely novel environment of biocatalysis driven by transition metals. In this sense, Goldberg et al. [125] reported a nitrene transfer reaction of styrene assisted by a non-heme iron enzyme. This non-native process is facilitated by the binding of non-native small-molecule ligands (Scheme 26).
Scheme 26.
Activation of a non-heme iron center by small molecules for nitrene transfer reaction of styrene. Structural model of PsEFE with mutated residues highlighted in orange. The coordinating residues H189, D191, and H268 are displayed as sticks, with the Mn ion (used for crystallization) shown as a purple sphere
A set of seven purified α-ketoglutarate (αKG)-dependent iron dioxygenase enzymes was screened for their activity in the intermolecular styrene aziridination reaction using p-toluenesulfonyl azide (73). However, only Pseudomonas savastanoi ethylene-forming enzyme (PsEFE 75) produced aziridine 76 at levels significantly above background. The native form of the enzyme shows a marked increase in aziridination activity when acetate L13 or N-oxalylglycine (NOG) L12 (a broad inhibitor of αKG-dependent enzymes) is introduced, in contrast to α-ketoglutarate L11. The authors’ strategy for enhancing PsEFE’s aziridination activity through directed evolution, focused on modifying the active-site residues using site-saturation mutagenesis, followed by screening to identify variants with improved catalytic performance. A variant with five mutations from the native enzyme was identified as capable of catalyzing the formation of 76, achieving a total turnover number (TTN) of 120 and an 88% ee, favoring the (R)-enantiomer.
Tan et al. [126] described a methodology for the asymmetric aminooxygenation of N-benzoyloxycarbamates of allylic alcohols using a “chiral-at-metal” ruthenium catalyst previously used for C-H amination reaction [127, 128]. When 1,2-disubstituted alkenes were used, the corresponding rearranged benzoylated cyclic carbamates were obtained. In contrast, the trisubstituted alkenes 77 lead to the formation of aziridines 79, which, unfortunately, were unstable and, consequently, difficult to isolate and purify. Therefore, the corresponding aziridines 79 were treated in situ with benzoic acid in the presence triphenylamine, yielding benzoylated cyclic carbamates 80 as single diastereoisomers with high enantioselectivity (Scheme 27). Only the geraniol-derived aziridine 81 proved to be stable and, therefore, could be isolated in good yield and with high stereoselectivity. The authors proposed that a ruthenium nitrenoid intermediate 82 is formed and adds to the alkene, producing intermediate 83. In the case of trisubstituted alkenes, the formation of aziridine 79 is favored over benzoate transfer (Scheme 28).
Scheme 27.
Ruthenium-catalyzed asymmetric aminooxygenation of trisubstituted allylic alcohols
Scheme 28.
Mechanistic proposal for the synthesis of aziridines through asymmetric aminooxygenation of allylic alcohols
An enantioselective radical aziridination reaction was developed in 2021 by Riart-Ferrer et al. [129]. 2,2,2-Trichloroethoxycarbonyl azide (85) was employed as nitrogen source, which, in contact with the appropriate Co(II)-metalloradical catalyst 86, generated a nitrogen radical capable of aziridinating aromatic and electron-deficient alkenes 84. The reaction tolerated a variety of substituents in the aromatic ring of styrenes, such as alkyl groups, halogens, electron-withdrawing groups, and even electron-donating substituents (51–99% yield, 82–94% ee) (Scheme 29). Interestingly, 1,1-disubstituted styrene derivative produced the corresponding aziridine 88, although in low yield and enantioselectivity (32% yield, 48% ee). Furthermore, the reaction was suitable for electron-deficient alkenes, such as alkyl acrylates; albeit, the addition of a catalytic amount of Pd(OAc)2 was required to achieve satisfactory results (42–43% yield, 52–82% ee). Unfortunately, the reactions was ineffective with heteroaromatic, aliphatic, and internal olefins.
Scheme 29.
Co(II)-catalyzed asymmetric aziridination of alkenes with carbonyl azide
A stepwise radical mechanism was proposed based on computational and experimental studies. DFT calculation suggested the formation of intermediate XVI, followed by the elimination of molecular nitrogen, producing the radical intermediate XVII. The next step, which had the highest activation barrier, was the addition of the radical to the styrene derivative. Finally, aziridine formation was exergonic and led to catalyst regeneration, thereby completing the catalytic cycle (Scheme 30).
Scheme 30.

Proposed catalytic cycle for the Co (II)-catalyzed asymmetric aziridination of alkenes with carbonyl azide
Very recently, the same group described the N-heterobicylization of N-sulfamoyl azides using a slightly modified Co(II)-based catalyst [130]. The metalloradical catalysis reaction allows the construction of chiral [3.1.0] bicyclic sulfamoyl aziridines in a stereoselective manner. After catalyst optimization, D2-symmetric chiral bridged amidoporphyrin 90 provided the best enantioselectivity for the homolitically activated intramolecular bicyclic formation. A wide variety of allylic sulfamoyl azides 89 were used in the radical reaction, including aliphatic and aromatic alkenes bearing electron-donating and electron-withdrawing substituents (Scheme 31). Additionally, the reaction also worked with Z-configured internal alkenes, generating the corresponding aziridines 91 in good yields (95–96%), excellent diastereoselectivity (> 99:1), and good enantioselectivity (62–71% ee).
Scheme 31.
Co(II)-catalyzed asymmetric intramolecular N-heterobicyclization of N-sulfamoyl azides
After experimental and DFT studies, a catalytic cyclic depicted in Scheme 31 was proposed by the authors. Initially, the aminyl radical XIX is generated. Subsequently, 6-endo-trig cyclization occurs producing the kinetically stable alkyl radical XX, which rapidly suffered intramolecular cyclization via 3-exo-tet radical reaction, generating the N-heterobicyclic 91 in a stereoselective fashion.
Kisetset al. synthesized and characterized new C3-symmetric tripodal tris(oxazoline) (TripTOX) ligand L14 in 2021 [131]. These ligands were successfully applied in the copper-catalyzed aziridination of chalcones 92, using of [N-(p-toluenesulfonyl)-imino]phenyliodinane (93) as the nitrogen source. After optimizing the reaction conditions, the process was effective with electron-neutral, weakly electron-withdrawing, and electron-donating groups (Scheme 32). However, heteroaromatic derivatives and strongly electron-withdrawing nitro group led to lower yields and enantioselectivity of the corresponding aziridines 94 (21–64% yield, 50–71% ee).
Scheme 32.
Cu(I)-catalyzed asymmetric aziridination of chalcones with [N-(p-toluenesulfonyl)-imino]phenyliodinane
The synthesis of trisubstituted enantioenriched aziridines has been an important milestone in alkene aziridination reactions. In 2022, Boquet et al. described a methodology for the asymmetric aziridination of diversely substituted alkenes, using aromatic sulfamates as the nitrogen source, iodine(III) as the oxidant, and a low catalyst loading of C4-symmetrical dirhodium(II) tetracarboxylates (Scheme 33) [132]. After optimizing the sulfamates and catalysts, p-tert-butylphenylsulfamate (96) and catalyst 97 were identified as the best reaction conditions. Subsequently, screening of iodine(III) oxidants, solvents, and temperature was carried out, selecting bis(tert-butylcarbonyloxy)iodobenzene, toluene, and –15 ºC, respectively, as the optimal conditions. Finally, the addition of a Brønsted acid improve the enantiocontrol of the reaction.
Scheme 33.
Rh(II)-catalyzed asymmetric aziridination of alkenes with p-tert-butylphenylsulfamate
Monosubstituted N-TBPhs-aziridines 98 (R1 = R2 = H) were successfully obtained from aromatic alkenes bearing either electron-donating or electron-withdrawing groups, as well as from aliphatic alkenes (51–91% yield, 74–90% ee). In the case of disubstituted N-TBPhs-aziridines, the reaction was demonstrated to be stereospecific when using trans- and cis-methylstyrene as precursor, yielding the trans-98 (67% yield, 90% ee) and cis-aziridine 98 (90% yield, 70% ee), respectively. Furthermore, outstanding results were provided when trisubstituted alkenes were used. Diversely substituted styrene derivatives demonstrated the reaction’s efficiency in producing trisubstituted N-TBPhs-aziridines 98 in very good yields and with excellent enantiocontrol (51–95% yield, 98–99% ee). Moreover, by changing the catalyst configuration, both enantiomers could be accessed. It is worth mentioning that the reaction is not only enantioselective but also chemoselective, as it does not provide the allylic or benzylic amination reaction in the case of alkenes bearing these susceptible groups. DFT studies performed with 2,2-dimethylstyrene suggested that the key enantioselective step of the reaction is the initial C-N bond formation, occurring at the less hindered and, therefore, more accessible benzylic position, with the Si face being the favored one (Scheme 34).
Scheme 34.
Si approach of p-tert-butylphenylsulfamate to the benzylic position of styrene derivative
Chiral aziridine derivatives have proven to be key intermediates in some enantioselective transformations. For instance, Sun et al. [133] described a chiral salen Mn(III)-catalyzed enantioselective intramolecular haloamination/cyclization reaction of alkenes 99. The formation and subsequent ring-opening of the chiral aziridinium ion intermediate 101 were crucial for the preparation of 2-halogenated pyrrolidine derivatives 102 (Scheme 35).
Scheme 35.
Enantioselective intramolecular haloamination of alkenes
Another interesting example in which asymmetric aziridine rings serve as key intermediates was developed by Akhtar et al. for the synthesis of 1H-benzo[c]azepines [134]. For this purpose, N-benzyl-N-cinnamyl amines 103 were treated with PhI = NNs in the presence of chiral Cu(II). Subsequently, the in situ generated aziridines 104 underwent a 7-endo-tet Friedel-Craft cyclization after the addition of an extra quantity of Cu(OTf)2 and an increase in temperature, providing the corresponding azepine derivatives 105 in very good yields, in almost all cases, with excellent stereoselectivity (Scheme 36). Both reaction conditions are suitable to carry out the transformation in a one-pot reaction. The authors suggested that the stereoselectivity of the reaction originates in the aziridination step. The reaction tolerates electron-withdrawing and electron-donating substituents in both aromatic rings.
Scheme 36.
One-pot catalytic asymmetric aminoarylation reaction of N-benzyl-N-cinammyl amines
An exhaustive study on the aziridination of different substituted alkenyl alcohols was performed in 2023 by Fanourakis et al. [135]. The reaction was catalyzed by a rhodium derived ion-paired chiral catalyst which provided a strong interaction with the primary alcohol of the substrate. The authors proposed that the chiral pocket formed by cinchona alkaloid-derived cations plays a key role in the stereoselectivity of the aziridination reaction. After optimization of the reaction conditions perfluorinated aminating agent 110 was selected as nitrogen source, and perfluoroiodosobenzene was used as the oxidant. Furthermore, the addition of C6F5I(OTFA)2 was beneficial, as it provided an easily measurable source of trifluoroacetic acid in solution. A broad scope of substrates was explored, incorporating different alcohol chains lengths and alkene substitution patterns. Successful results were achieved with homoallylic styrenyl alcohols 106 (Scheme 37a) and trishomoallylic styrenyl alcohols 107 (Scheme 37b). The reaction was also compatible with trans-108 (Scheme 37c) and exo-dialkyl alkenyl alcohols 109 (Scheme 37d).
Scheme 37.
Asymmetric aziridination of alkenyl alcohols
The authors proposed that the ion-paired rhodium catalyst 111 is able to discriminate between the prochiral faces of the alkene. For this purpose, the hydrogen-bonding interaction between the primary alcohol of the substrate, the chiral cation, and the sulfonate group is crucial. Simultaneously, the nitrogen source is coordinated to the rhodium, thereby ensuring that the aziridination reaction occurs in a chiral environment (Fig. 3).
Fig. 3.

Mechanistic proposal for the asymmetric aziridination of alkenyl alcohols
In the case of trisubstituted alkenes 116, although the corresponding aziridines 117 were identified by analysis of the reaction crudes, the aziridine acted as an intermediate that further progressed to the corresponding cyclized tetrahydrofuran or tetrahydropyran 118. Therefore, cyclization was promoted by increasing the reaction temperature, obtaining satisfactory results (Scheme 38).
Scheme 38.
Asymmetric aziridination of trisubstituted alkenyl alcohols and subsequent cyclization
Unfortunately, tetrasubstituted alkenes were not appropriate substrates for the reaction, leading to complex mixtures, while short-chain allylic alcohol derivatives resulted in aziridines with low enantioselectivity. Very recently, the same authors overcame the enantioselectivity limitations of allylic alcohols by slightly modifying the catalyst and reaction conditions [136]. On the one hand, they changed the geminal dialkyl groups adjacent to the carboxylate on the anionic component of complex 120, replacing the cyclobutyl group in 111 with two methyl groups in 120. On the other hand, the linker between the arene and the sulfonate group was replaced by a biaryl derivative, which improved the enantioselectivity. This effect was further enhanced by lowering the reaction temperature. Several trisubstituted allylic alcohols 119 were tested in the optimized aziridination reaction, yielding successful results (Scheme 39). Furthermore, the site selectivity of the reaction was demonstrated with geraniol and nerol derivatives, showing a preference for the aziridination of the alkene closest to the alcohol group.
Scheme 39.
Asymmetric aziridination of trisubstituted allylic alcohols
In recent years, the intramolecular aziridination of alkenes have been scarcely investigated. A very interesting intramolecular nitrene-transfer aziridination was recently developed by Trinh et al. [137]. The reaction was carried out with carbamimidates 122 bearing a bulky protecting group, in the presence of silver catalyst and bisoxazoline-derived ligand L16 (Scheme 40). The transformation was effective with a wide range of disubstituted alkenes 122, such as aliphatic alkenes with several alkyl chain lengths and bulky groups, protected alcohols, heteroarenes, and even complex molecules. The styrene derivative alkene provided moderate yield and enantioselectivity (37% yield, 62% ee). On the other hand, trisubstituted alkenes 122 also could serve as suitable substrates, producing interesting bicyclic aziridine derivatives 123 bearing a enantioenriched tetrasubstituted stereocenter. DFT studies conducted by the authors demonstrated that the indane arms and cyclopropyl backbone of the ligand are necessary to obtain a rigid structure required to adopt a completely planar conformation, which lead to an effective transition state that induces stereoselectivity.
Scheme 40.
Asymmetric intramolecular aziridination of carbamimidates
Although many examples of aziridination of alkenes have been described in recent years, most of them employ activated alkenes as starting materials. Gross et al. focused their attention in the development of a general methodology of asymmetric aziridination of unactivated alkenes [138]. For this aim, a planar chiral rhodium(III) indenyl catalyst was used in combination with a base and a silver halide scavenger to activate the catalyst. The transformation was applicable to a broad range of alkyl chains, including functionalized ones with several heteroatoms, and was also effective with complex substrates bearing heterocycles (Scheme 41). Chiral alkenes 84 with the stereocenter at the γ-position relative to the alkene provided successful results. However, when the stereocenter was shifted to the β-position, a detriment in yield was observed, although the stereocontrol was maintained. Disubstituted alkenes were also evaluated. While 1,1-disubstituted derivatives were not appropriate substrates, an interesting result was obtained with 1,2-disubstituted alkene, yielding the corresponding cis-aziridine 127 with good stereocontrol.
Scheme 41.
Rhodium-catalyzed asymmetric aziridination of unactivated alkenes
Next, the authors studied the regioselectivity of the transformation using terminal alkenes with an additional activated alkene, demonstrating the selectivity of the reaction favors the unactivated terminal alkene (Scheme 42).
Scheme 42.
Asymmetric aziridination of substrates bearing activated and unactivated alkenes
DFT studies conducted after evaluating different mechanistic proposals suggested that the formation of amide XXI, followed by the subsequent olefin insertion step, was crucial for the enantioselectivity of the reaction. A plausible catalytic cycle is depicted in Scheme 43.
Scheme 43.
Proposed catalytic cycle for the asymmetric aziridination of unactivated alkenes
Wang et al. published a nearly simultaneous report on the aziridination of unactivated alkenes [139]. In this case, the best results were achieved with the chiral cyclopentadienyl-rhodium(III) catalyst 132 (Scheme 44). The reaction showed broad applicability, converting a wide variety of aliphatic unactivated alkenes 84 into enantioenriched aziridines 133. The reaction tolerated several functional groups, such as halogen atoms, ether, ester, hydroxyl, O-tosyl, O-tert-buthyldimethylsilyl, nitro, amino, acyloxy, and phthalimide groups. In all cases, aziridines 133 were obtained in moderate to excellent yields with high enantioselectivity. Similarly, the regioselectivity of the reaction was confirmed by adding activated alkenes into the substrate and observing that the transformation selectivity occurred at the unactivated terminal alkene. Unfortunately, the aziridination reaction failed when aromatic alkenes, such as styrene, or disubstituted 1,1- or 1,2-alkenes were employed.
Scheme 44.
Catalytic asymmetric aziridination of unactivated alkenes
On the other hand, the authors also studied the scope of the nitrene source by varying the substituents on the aromatic ring of the N-pivalolyloxy sulfonamide 131. Successful yields and enantioselectivity were obtained with substituents of varying electronic properties (Scheme 44). The reaction also tolerated bulky groups and thiophene derivatives. Although a decrease in yields and enantioselectivity was observed with alkyl derivatives (77–84% ee), the expected aziridines 133 were successfully isolated.
Deuterium-labeling studies were performed to understand the reaction mechanism. The obtained results suggested that a multistep process was involved in the aziridination reaction. The authors proposed that the rhodium first coordinates with the amine group of sulfonamide 131, followed by the alkene migratory insertion in a stereocontrolled fashion, providing intermediate XXVI or XXVII. After N–O bond cleavage and C-N bond formation, the desired aziridine 133 is obtained, regenerating the catalyst and completing the catalytic cycle (Scheme 45).
Scheme 45.

Proposed catalytic cycle for the asymmetric aziridination of unactivated alkenes
Kinetic Resolution and Desymmetrization
Kinetic Resolution and Desymmetrization of Aziridines
Significant progress in catalytic asymmetric approaches was achieved in 2009 when Larson et al. pioneered the desymmetrization of meso-aziridines. This strategy was applied to functionalized aromatic thiols in combination with VAPOL phosphoric acid. The resulting α-thioamides were obtained in good to excellent yields with excellent enantioselectivity [140].
In 2021, Sun et al. [141] described the first organocatalytic kinetic resolution of unactivated aziridines using sulfur nucleophiles, achieving excellent enantioselectivity. A wide range of racemic aziridines 134 bearing different substituents proved to be suitable substrates for this ring-opening reaction, using 2-mercaptobenzothiazole 135 as the nucleophile. Additionally, various chiral phosphoric acids (CPAs) were examined as potential catalysts, with catalyst 136 providing the highest enantioselectivity. The corresponding β-amino thioether products 137 and the remaining aziridines (S)-134 were all obtained with good to high enantioselectivity (Scheme 46). Additionally, different substituted mercaptobenzothiazoles 135 were tested, with the 6-ethoxy-substituted (R1 = 6-EtO, 135) exhibiting excellent selectivity factor (s > 200). However, other sulfur nucleophiles, such as thiophenol, aliphatic thiols, and thioacids, did not react under the standard conditions. Remarkably, the results obtained in a large scale (2 mmol of rac-134) were similar in efficiency and stereoselectivity, highlighting the robustness of this methodology.
Scheme 46.
Organocatalytic kinetic resolution of unactivated racemic aziridines using 2-mercaptobenzothiazoles as nucleophiles
There are only a few asymmetric methods available for the synthesis of chiral heterocyclic systems containing fused aziridine rings, such as aziridinoquinoxaline. In 2023, Jhang et al. [142] described a highly selective parallel kinetic resolution, providing access to new chiral aziridinoquinoxalines. This resolution was achieved using racemic aziridinoquinoxaline 138 under transfer hydrogenation conditions with the standard tert-butyl-substituted Hantzsch esters, in the presence of 5 mol% (R)-TRIP 139 as the catalyst. The resolution was successfully accomplished for 16 different substrates, yielding highly enantioenriched diastereoisomers with the (R)-configuration of the newly formed stereocenter: 32–61% yield with 64–99% ee for the (R,R,R)-diastereoisomers 140, and 7–46% yield with 97–99% ee for the (S,S,R)-diastereoisomers 140 (Scheme 47). A detailed analysis of the product and starting material distribution suggests a complex interplay of the catalyst- and substrate-imposed factors effecting the enantio- and diastereoselectivity. In all cases, a highly enantioselective formation of the diastereoisomer (S,S,R)-140 was observed, as the enantiomer (R,R,S)-140 would result from a double-mismatched reaction. Similarly, high enantioselectivity for (R,R,R)-140 are attributed to the faster formation of the double-matched enantiomer (R,R,R)-140 in comparison to the mismatched enantiomer (S,S,S)-140, which suffers from opposing catalyst- and substrate-dictated selectivity (Scheme 47a).
Scheme 47.
a Enantioselective parallel kinetic resolution of aziridinoquinoxalines. b Addition of thiophenol to the reaction mixture at the end of the reduction of aziridinoquinoxalines
The reduction conditions could be used to produce p-nitrophenyl- and p-trifluoromethyl-substituted products, but the resulting mixtures of diastereoisomers (R,R,R)-140/(S,S,R)-140 could not be readily separated by flash chromatography. To address this challenge, a one-pot functionalization protocol was employed to selectively convert (S,S,R)-140 into a more easily separable compound 141. Notably, the aryl-substituted aziridines (S,S,R)-140 were found to be significantly more reactive toward thiophenol than their corresponding diastereoisomers (R,R,R)-140. Thus, the simple addition of thiophenol to the reaction mixture at the end of the reduction lead to quantitative and selective formation of the ring-opened product 141 in 99% ee. The resulting reaction mixture could be conveniently purified to separate (R,R,R)-140 from 141 (Scheme 47b).
The ring-opening of racemic aziridines via kinetic resolution with sulfur nucleophiles can lead to both enantiomerically enriched β-amino thioethers and aziridines. In 2019, Zhang et al. [143] developed an efficient kinetic resolution of racemic trans-2-acyl-3-aryl-N-tosylaziridines 142 using La(OTf)3 and chiral N,N-dioxide ligands L17 as catalyst, along with 2-mercaptobenzothiazoles 135 as active sulfur nucleophiles. A variety of enantioenriched β-amino thioethers 143 and chiral trans-aziridines 142 were obtained in good yields with high levels of stereocontrol (up to 96% ee). Furthermore, 4,5-dihydrothiazole-2-thiol 135, lacking the phenyl ring, also afford promising results in kinetic resolution. Other sulfur nucleophiles, such as 4-methylthiophenol and 2-thionaphtol, were tested but resulted in low yields (< 10%), while cyclohexanethiol showed no reaction under these conditions (Scheme 48).
Scheme 48.
Kinetic resolution of racemic trans-2-acyl-3-aryl-N-tosylaziridines via Lewis acid catalysis using sulfur nucleophiles
Zhang et al. reported an efficient catalytic kinetic resolution (KR) and dinamic kinetic asymmetric transformation (DyKAT) of racemic N-tosylaziridines 134 via [3 + 3] annulation with isatin-derived enals 144, by the cooperative catalysis with chiral NHCs and copper complexes. This approach led to highly enantioenriched spirooxindoles 146 and (R)-N-tosylaziridine derivatives 134. Through process optimization, the best results were obtained using chiral NHC precatalyst 145 and the chiral diphosphine ligand L18. The presence of a base significantly improved reaction efficiency. In the absence of either copper, the chiral diphosphine ligand, or NHC precatalyst, the reaction yielded less than 5% product, confirming their essential role (Scheme 49) [144].
Scheme 49.
Kinetic resolution of racemic N-tosylaziridines via NHC/Cu cooperative catalysis
The authors carried out a series of mechanistic studies, which suggested that the NHC catalyst 145 plays a dual role: acting both as a Lewis base organocatalyst and as a ligand coordinating with the copper-diphosphine complex to modulate the catalytic activity. The chemoselectivity between the KR and DyKAT could be switched by adjusting the dosage of the chiral NHC. Furthermore, the presence or absence of the Cu-diphosphine complex determines the reaction pathway.
A plausible catalytic cycle for the KR of aziridines 134 is depicted in Scheme 50. Initially, under basic conditions, the combination of Cu(MeCN)₄PF₆, ligand L18, and NHC precatalyst 145 leads to the formation of the active NHC/diphosphine Cu(I) complex catalyst, Cu(I)(L18)(145). Concurrently, the addition of isatin-derived enal 144 to this Cu (I) complex catalyst generates the Breslow intermediate XXVIII, which interconverts with its mesomeric azolium homoenolate species XXIX. During this process, partial dissociation of NHC 145 from the Cu (I) (L18)(145) complex releases catalytically active Cu(I)(L18) species, which participate in the catalytic cycle (left cycle, copper catalysis). The aziridine nitrogen and one oxygen atom of the tosyl group coordinate to the copper center, forming electrophilic intermediates XXX and XXXI. The diastereoisomeric complex (S)-134[Cu]* XXXI is more reactive and undergoes the transformation more rapidly than (R)-134[Cu]* XXX. As a result, intermediate XXXII is predominantly generated, while (R)-134 remains with high enantiopurity. Finally, N-acylation and subsequent cyclization of intermediate XXXII yield the final product 146, regenerating both the organocatalyst NHC 145 and the copper complex Cu(I)(L18) or Cu(I)(L18)(145) (Scheme 50).
Scheme 50.
Plausible catalytic cycle for the kinetic resolution of aziridines
To highlight the usefulness of meso compounds in catalytic asymmetric reactions, in 2021, Zheng et al. [145] developed the first example of a catalytic asymmetric reaction featuring the regiodivergent desymmetrization of meso-azabicycloheptene 147 via allylic oxidation. Using tert-butyl perbenzoate (148) as the oxidant in the presence of a single chiral copper catalyst, Cu (MeCN)4PF6 in combination with N,N-bidentate ligand L19, the reaction produced two different enantiomerically enriched structural isomers with high optical purity. A 1:1 mixture of (1S, 2S, 6S)-149 and (1R, 3R, 6S)-150 was obtained in 62% overall yield with 80% ee and 90% ee, respectively (Scheme 51). The absolute configurations of the products were determined by X-ray crystal structure analysis. The structural isomers 149 and 150 were separated using preparative HPLC. Compound 149 was recrystallized from a 1:4 mixture, improving its ee to 97%. Similarly, the ee of compound 150 was increased to > 99.5% by recrystallization from either toluene/hexane (1:2) mixture or ethyl acetate/hexane (1:4) mixture. In addition, the two isomers could also be separated without HPLC, as direct recrystallization of the 149:150 (1:1 mixture) yielded compound 150 with > 99.5% ee (Scheme 51).
Scheme 51.
Regiodivergent desymmetrization reaction of meso-aziridine with tert-butyl perbenzoate using a chiral copper catalyst
For the kinetic resolution, the authors have proposed a reaction mechanism (Scheme 52), that begins with the chiral catalyst species Cu (I) L19, consisting of a chiral N,N-bidentate ligand and a Cu(I) precursor. This species reacts with tert-butyl perbenzoate 148 to generate a peroxide complex, which subsequently interacts with meso-aziridine 147, leading to the formation of the diastereoisomeric Cu (III)-π-allyl intermediates XXXIII and XXXIV in a 1:1 ratio. From intermediate XXXIII, the σ-allyl-Cu (III) complex XXXV is formed, while from intermediate XXXIV, the σ-allyl-Cu (III) complex XXXVI is generated. In both cases, the sets of complexes XXXV and XXXVI exist in equilibrium. The nucleophilic attack of the benzoate carbonyl oxygen on the bottom face of XXXV (opposite to the aziridine moiety) results in the formation of compound 149. From intermediate XXXIV, the σ-allyl-Cu (III) complex XXXVI is regioselectively formed, yielding compound 150. Regarding the role of the chiral ligand on copper, the bulky tert-butyl group in the N, N-bidentate ligand is crucial for achieving high enantioselectivity in the formation of compounds 149 and 150.
Scheme 52.
Proposed reaction mechanism for the regiodivergent desymmetrization reaction of meso-aziridines mediated by chiral copper catalyst
In 2020, Liu et al. [146] confirmed the directing effect of the hydroxyl group in the Zn(OTf)2-catalyzed regioselective ring opening of 2,3-aziridinyl alcohols. More recently, in 2023 Chang et al. [147] extended the application of this methodology to develop, for the first time, an efficient kinetic resolution of racemic trans-2,3-aziridinyl alcohols 151 with various amines 152 as nucleophiles, using a dinuclear zinc cooperative catalyst (Zn2-L2). A range of enantioenriched vicinal diamines 153 and trans-2, 3-aziridinyl alcohols (2S, 3R)-151 were obtained in good yields with excellent levels of regio- and stereocontrol. The absolute configuration of the ring-opening product 153 was unambiguously confirmed to be (2R, 3R) by single-crystal X-ray diffraction analysis. Different aromatic amines were tested, including anilines bearing both electron-donating and electron-withdrawing groups on the aromatic ring, which led to products with good yields and high levels of enantioselectivity. The selectivity factors varied from 67 to > 200. When an aniline with an acetyl group reacted with racemic aziridines 151, the kinetic resolution efficiency was relatively low. A similar behavior was observed when secondary aromatic amines were employed as nucleophiles. This methodology was tested on a gram-scale using 4.0 mmol of racemic (3-phenyl-1-tosylaziridin-2-yl)methanol (151) (Ar = Ph) and 2.0 mmol of 3, 4-dimethoxyaniline 152 (Ar1 = 3,4-(MeO)2C6H3, R = H). The reaction maintained high levels of regio-and stereocontrol, giving to the formation of the ring-opening product 153 in 39% yield with 95% ee, along with (2S, 3R)-151 isomer in 38% yield with 97% ee. This reaction was applied to the functionalization of some natural products. The kinetic resolution was explored using estrone-derived aniline and geraniol-derived aniline, yielding the corresponding functionalized natural products (Scheme 53).
Scheme 53.
Dinuclear zinc-catalyzed kinetic resolution of trans-2, 3-aziridinyl alcohols with amines
The authors have proposed a catalytic mechanism for the kinetic resolution of trans-2, 3-aziridinyl alcohols 151. Initially, the dinuclear zinc complex distinguishes between the enantiomers of racemic trans-2,3-aziridinyl alcohols 151 via a deprotonation process involving one zinc atom, while the other zinc atom coordinates with the oxygen atom of the tosyl group. For the (2S, 3R)-151 isomer, the enantiodiscrimination process is slow (pathway XXXVII) due to the steric hindrance between the phenyl group of the aziridine ring and the catalyst framework. As a result, the ring-opening reaction of the (2S, 3R)-151 isomer is hindered, leading to its recovery. In the case of the (2R, 3S)-151 isomer, nucleophilic amines smoothly attack the aziridine ring via an SN2 pathway, affording the intermediate XXXIX. Finally, through a proton exchange process with another molecule of (2R, 3S)-151, the reaction yields the ring-opening product 153 and the intermediate XXXVIII (Scheme 54).
Scheme 54.
Rational catalytic mechanism for the kinetic resolution of trans-2,3-aziridinyl alcohols
Kinetic Resolution and Desymmetrization of 2H-Azirines
The kinetic resolution of 2H-azirines is a highly efficient strategy to simultaneously produce chiral 2H-azirines and functionalized aziridines through different approaches. Hu et al. [148] and An et al. [53] used nucleophilic addition to the C-N double bond, while Deng et al. [149] reported a highly efficient kinetic resolution of racemic 2H-azirines 11 using (S,Sp)-Ph-Phosferrox/Cu(MeCN)4BF4 complex L3 as the catalyst for the asymmetric 1,3-dipolar cycloaddition of azomethine ylides 154, achieving selectivity factors up to 845. A wide range of chiral 1, 3-diazabicyclo[3.1.0] hexane 155 and (S)-2H-azirines 11 were obtained with excellent enantioselectivity (both up to 99% ee) (Scheme 55).
Scheme 55.
Kinetic resolution of racemic 2H-azirines via Cu(I) catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides
In 2021, Pan et al. [150] reported the first kinetic resolution of racemic 2,3-diaryl-2H-azirines 11 via asymmetric allylations using allylboronates reagents 156. The reaction was catalyzed by a Bi (OAc)3/chiral phosphoric acid (CPA) 157 system under mild reaction conditions, providing a wide range of chiral 2H-azirines (S)-11 and aziridines 158 in excellent yields (Scheme 56). This methodology was compatible with a broad range of azirine substrates bearing electron-withdrawing and electron-donating groups on both aromatic rings. Additionally, the different boron reagents were successfully employed, providing good to excellent kinetic resolution performance. The absolute configuration of the aziridine products 158 were determined to be (2S, 3R) by X-ray crystallographic analysis. To explain the origin of enantioselectivity, the authors suggested that various weak interactions play a crucial role, based on computational studies.
Scheme 56.
Catalytic asymmetric kinetic resolution of 2H-azirine to produce chiral 2H-azirine and aziridines
The authors have proposed a possible catalytic cycle for the kinetic resolution of racemic 2H-azirines (Scheme 57). Initially, an anion exchange between Bi (OAc)3 and (S)-CPA 157 generates the chiral phosphate diacetate Bi(III) complex 159 as the active catalyst. The subsequent transmetalation with allylboronic acid pinacol ester 156 gives rise to the allyl-Bi(III) species 160, which then selectively transfers the allyl group to racemic 2,3-diphenyl-2H-azirine 161, proceeding at different reaction rates for each enantiomer. Since the difference in reaction rates is significant, the fast-reacting enantiomer (R)-161 undergoes allylation to form the enantioenriched product 158, while the slow-reacting enantiomer (S)-161 is preferentially recovered.
Scheme 57.
Possible catalytic cycle for the kinetic resolution of racemic 2H-azirine
Concluding Remarks
This review presents advances made over the past 7 years in the field of the enantioselective synthesis of aziridines. Several methodologies have been developed and improved for this purpose. Given the significant role of the aziridine ring in synthesis, having access to a diverse array of aziridination procedures represents a major advantage for synthetic chemists. This is particularly relevant when considering the potential to achieve stereocontrol in the process. Today, a variety of functionalized chiral aziridines can be attained in excellent yields with high enantiocontrol. Many outstanding and efficient methodologies have been developed and are highlighted in this review. New strategies based on the use of effective catalytic systems, including organocatalysis and metal catalysis, have enabled the preparation of enantioenriched and structurally complex aziridines from cost-effective and readily available starting materials. The design of new catalysts, inspired by biological systems, have led to the development of biomimetic systems based on heme-binding proteins and supramolecular hosts, offering innovative approaches for asymmetric catalysis. These systems combine the precision of enzymatic catalysis with the adaptability of synthetic design, opening new avenues for the synthesis of enantiomerically pure aziridines. As outlined in this review, in recent years, the most advanced methods for obtaining chiral aziridines using a chiral catalyst relied on nitrene transfer to alkenes, whereas protocols involving the transfer of a carbenoid to an imine have been less extensively explored. However, nucleophilic addition to 2H-azirines has emerged as a highly significant and resurging strategy for the synthesis of enantiomerically enriched aziridines. This approach takes advantage of the inherent reactivity of 2H-azirines, enabling regio- and stereoselective transformations under mild conditions. Recent advances in reaction optimization have further expanded the scope of this methodology, allowing for the efficient incorporation of diverse nucleophiles and the generation of structurally complex aziridines with high levels of enantiocontrol. Other catalytic asymmetric approaches for the preparation of chiral aziridines, such as kinetic resolution and desymmetrization, although less developed, have resurged and are gaining significant attention. As an example, the first organocatalytic kinetic resolution of unactivated aziridines using sulfur nucleophiles has recently been reported. Recent advancements in catalyst design have greatly enhanced the efficiency and selectivity of these approaches, making them promising tools for the synthesis of enantiomerically enriched aziridines. However, despite the remarkable progress achieved, several challenges remain that warrant further attention.
Future research in this field would benefit from a greater emphasis on broadening substrate scope, particularly with respect to unactivated and sterically hindered alkenes and imines. Improving diastereoselectivity and enantioselectivity across a wider array of substrates remains an essential goal to enhance the general applicability of current methodologies. Moreover, the development of greener and scalable catalytic protocols is crucial for the practical implementation of aziridination processes in industrial and pharmaceutical settings. This includes designing reactions that operate under milder conditions, use environmentally benign reagents, and minimize waste generation. Finally, a promising direction involves the application of enantioselective aziridination methods to the synthesis of complex, biologically active molecules and natural products. This would not only demonstrate the synthetic utility of current methodologies but also reveal new opportunities for late-stage functionalization and structural diversification.
This review highlights the significance of the asymmetric aziridination reaction in organic synthesis, which continues to draw considerable interest. The reaction’s ability to generate enantiopure aziridines makes it a valuable tool for the synthesis of complex molecules, while the diverse biological activities of aziridine-containing compounds, including key natural products, further enhance its appeal.
Acknowledgements
Financial support PID2021-122558OB-I00 funded by the Ministerio de Ciencia, Innovación y Universidades MICIU/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”, and by Gobierno Vasco (GV, IT1701-22; UPV-EHU) is gratefully acknowledged.
Author Contributions
Writing–original draft preparation: I. O., A. M. O., and J. M. S.; writing–review and editing: I. O., A. M. O., and J. M. S.; supervision: J. M. S. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Ministerio de Ciencia, Innovación y Universidades, PID2021-122558OB-I00, Eusko Jaurlaritza, GV, IT1701-22; UPV-EHU.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Proft F, Geerlings P (2014) Structure, bonding and reactivity of heterocyclic compounds in topics in heterocyclic chemistry, vol 38. Springer-Verlag, Berlin Heidelberg [Google Scholar]
- 2.Byeon H, Ha H-J, Yang JW (2025) Recent advances in the application of contiguously functionalized aziridines for the synthesis of pharmaceutical precursors via regioselective ring-opening reactions. Mol Catal 576:114943 [Google Scholar]
- 3.Stankovic S, Dhooghe M, Catak S, Eum H, Waroquier M, Van Speybroeck V, De Kimpe N, Ha H-J (2012) Regioselectivity in the ring opening of non-activated aziridines. Chem Soc Rev 41:643–665 [DOI] [PubMed] [Google Scholar]
- 4.Lu P (2010) Recent developments in regioselective ring opening of aziridines. Tetrahedron 66:2549–2560 [Google Scholar]
- 5.Florio S, Luisi R (2010) Aziridinyl anions: generation, reactivity, and use in modern synthetic chemistry. Chem Rev 110:5128–5157 [DOI] [PubMed] [Google Scholar]
- 6.Sun W-N, Mei G-J, Jia S-K (2024) Substrate directed regio- and enantioselective ring-opening of epoxides and aziridines. ChemCatChem 16:e202301431 [Google Scholar]
- 7.Chai Z (2020) Catalytic asymmetric transformations of racemic aziridines. Synthesis 52:1738–1750 [Google Scholar]
- 8.Dequina HJ, Jones CL, Schomaker JM (2023) Recent updates and future perspectives in aziridine synthesis and reactivity. Chem 9:1658–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalpozzo R, Lattanzi A, Pellissier H (2017) Applications of chiral three-membered rings for total synthesis: a review. Curr Org Chem 21:1143–1191 [Google Scholar]
- 10.Lapinsky DJ (2015) Three-membered ring systems. Prog Heterocycl Chem 27:61–85 [Google Scholar]
- 11.Singh GS, D’hooghe M, De Kimpe N (2007) Synthesis and reactivity of C-heteroatom-substituted aziridines. Chem Rev 107:2080–2135 [DOI] [PubMed] [Google Scholar]
- 12.Sweeney JB (2006). In: Yudin AK (ed) Aziridines and epoxides in organic synthesis. Wiley-VCH, Weinheim, p 117 [Google Scholar]
- 13.Ju X, Lee M, Leung JC, Lee J (2025) Synthesis and functionalization of aziridines: a perspective view from pharmaceutical industries. Eur J Org Chem 28:e202401414 [Google Scholar]
- 14.Uthumange SS, Liew AJH, Chee XW, Yeong KY (2024) Ringing medicinal chemistry: the importance of 3-membered rings in drug discovery. Bioorg Med Chem 116:117980 [DOI] [PubMed] [Google Scholar]
- 15.Armstrong RW, Salvati ME, Nguyen M (1992) Novel interstrand cross-links induced by the antitumor antibiotic carzinophilin/azinomycin B. J Am Chem Soc 114:3144–3145 [Google Scholar]
- 16.Nagaoka K, Matsumoto M, Ono J, Yokoi K, Ishizeki S, Nakashima T (1986) Azinomycins A and B, new antitumor antibiotics. I. producing organism, fermentation, isolation, and characterization. J Antibiot 39:1527–1532 [Google Scholar]
- 17.Tomasz M, Lipman R, Chowdary D, Pawlak J, Verdine GL, Nakanishi K (1987) Isolation and structure of a covalent cross-link adduct between Mitomycin C and DNA. Science 235:1204–1208 [DOI] [PubMed] [Google Scholar]
- 18.Wakaki S, Marumo H, Tomioka K, Shimizu G, Kato E, Kamada H, Kudo S, Fujimoto Y (1958) Isolation of new fractions of antitumor mitomycins. J Antibiot 8:228–235 [Google Scholar]
- 19.Hanada M, Ohkuma H, Yonemoto T, Tomita K, Ohbayashi M, Kamei H, Miyaki T, Konishi M, Kawaguchi H, Forenza S (1991) Maduropeptin, a complex of new macromolecular antitumor antibiotics. J Antibiot 44:403–414 [Google Scholar]
- 20.Suzuki M, Kambe M, Tokuyama H, Fukuyama T (2004) Enantioselective total synthesis of FR900482. J Org Chem 69:2831–2843 [DOI] [PubMed] [Google Scholar]
- 21.Katoh T, Itoh E, Yoshino T, Terashima S (1996) Total synthesis of natural (+)-FR900482. 1. synthetic and end-game strategies. Tetrahedron Lett 37:3471–3474 [Google Scholar]
- 22.Uchida I, Takase S, Kayakiri H, Kiyoto S, Hashimoto M, Tada T, Koda S, Morimoto Y (1987) Structure of FR 900482, a novel antitumor antibiotic from a Streptomyces. J Am Chem Soc 109:4108–4109 [Google Scholar]
- 23.van Maanen MJ, Smeets CJM, Beijnen JH (2000) Chemistry, pharmacology and pharmacokinetics of N,N′,N′′-triethylenethiophosphoramide (ThioTEPA). Cancer Treat Rev 26:257–268 [DOI] [PubMed] [Google Scholar]
- 24.Imail FMD, Levitsky DO, Dembitsky VM (2009) Aziridine alkaloids as potential therapeutic agents. Eur J Med Chem 44:3373–3387 [DOI] [PubMed] [Google Scholar]
- 25.He X, Li M, Song S, Wu X, Zhang J, Wu G, Yue R, Cui H, Song S, Ma C, Lu F, Zhang H (2018) Ficellomycin: an aziridine alkaloid antibiotic with potential therapeutic capacity. Appl Microbiol Biotechnol 102:4345–4354 [DOI] [PubMed] [Google Scholar]
- 26.Argoudelis AD, Reusser F, Whaley HA, Baczynskyj L, Mizsak SA, Wnuk RJ (1976) Antibiotics produced by Streptomyces ficellus I. Ficellomycin. J Antibiotics 29:1001–1006 [Google Scholar]
- 27.Tsuchida T, Iinuma H, Kinoshita N, Ikeda T, Sawa T, Hamada M, Takeuchi T (1995) Azicemicins A and B, a new antimicrobial agent produced by Amycolatopsis. I. Taxonomy, fermentation, isolation, characterization and biological activities. J Antibiot 48:217–221 [Google Scholar]
- 28.Kinoshita N, Ikeda T, Sawa R, Takahashi T, Naganawa H, Sawa T, Hamada M, Takeuchi T (1993) Azicemicin A, a new antimicrobial antibiotic from Amycolatopsis. J Antibiot 46:1772–1774 [Google Scholar]
- 29.Mallory CM, Carfi RP, Moon SP, Cornell KA, Warner DL (2015) Modification of cellular DNA by synthetic aziridinomitosenes. Bioorg Med Chem 23:7378–7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han I, Kohn H (1991) Aminoaziridinomitosenes: synthesis, structure, and chemistry. J Org Chem 56:4648–4653 [Google Scholar]
- 31.Vaidergorn MM, Carneiro ZA, Lopes CD, de Albuquerque S, Reis FCC, Mikolaou S, e Mello JFR, Genesi GL, Trossini GHG, Ganesan A, Emeri FS (2018) β-Amino alcohols and their respective 2-phenyl-N-alkyl aziridines as potential DNA minor groove binders. Eur J Med Chem 157:657–664 [DOI] [PubMed] [Google Scholar]
- 32.Michida M, Takayanagi Y, Imai M, Furuya Y, Kimura K, Kitawaki T, Tomori H, Kajino H (2013) Convergent asymmetric synthesis of a renin inhibitor: a highly efficient construction method of three stereogenic centers. Org Process Res Dev 17:1430–1439 [Google Scholar]
- 33.Jeong H, Yadav NN, Ha H-J (2017) Synthesis of Lacosamide (Vimpat) and its derivatives from aziridine-(2R)-carboxylate. Synthesis 49:1264–1272 [Google Scholar]
- 34.Colarusso S, Conte I, Di Filippo M, Ercolani C, Mackay AC, Palumbi MC, Rico Ferreira MR, Stansfield I, Zaramella S, Narjes F, Habermann J (2011) Development of a scalable chiral synthesis of MK-3281, an inhibitor of the hepatitis C virus NS5B polymerase. Synlett 2011:1527–1532 [Google Scholar]
- 35.Cantillo D (2022) Synthesis of active pharmaceutical ingredients using electrochemical methods: keys to improve sustainability. Chem Commun 58:619–628 [Google Scholar]
- 36.Chavan SP, Chavan PN, Khairnar LB (2014) A concise synthetic approach toward Tamiflu (oseltamivir phosphate): cis-aziridine as the key synthon and RCM. RSC Adv 4:11417–11419 [Google Scholar]
- 37.Patwardhan AP, Pulgam VR, Zhang Y, Wulff WD (2005) Highly diastereoselective alkylation of aziridine-2-carboxylate esters: enantioselective synthesis of LFA-1 antagonist BIRT-377. Angew Chem Int Ed 44:6169–6172 [Google Scholar]
- 38.Hao G-L, Wang J, Mou S-B, Luo M-P, Teng Q, Wang S-G (2024) Transition-metal catalyzed enantioselective aziridination reaction: recent development in catalysis and future perspectives. ChemCatChem 16:e202400791 [Google Scholar]
- 39.Roma E, Tosi E, Miceli M, Gasperi T (2018) Asymmetric organocatalytic aziridination: recent advances. Asian J Org Chem 7:2357–2367 [Google Scholar]
- 40.de los Santos JM, Ochoa de Retana AM, Martínez de Marigorta E, Vicario J, Palacios F (2018) Catalytic asymmetric Darzens and aza-Darzens reactions for the synthesis of chiral epoxides and aziridines. ChemCatChem 10:5092–5114 [Google Scholar]
- 41.Degennaro L, Trinchera P, Luisi R (2014) Recent advances in the stereoselective synthesis of aziridines. Chem Rev 114:7881–7929 [DOI] [PubMed] [Google Scholar]
- 42.Pellissier H (2014) Recent developments in asymmetric aziridination. Adv Synth Catal 356:1899–1935 [Google Scholar]
- 43.Müller P, Fruit C (2003) Enantioselective catalytic aziridinations and asymmetric nitrene insertions into CH bonds. Chem Rev 103:2905–2920 [DOI] [PubMed] [Google Scholar]
- 44.Liu Q-H, Liu J, Han Y-P, Liang Y-M, Peng L-Z (2025) Synthetic approaches for the construction of chiral aziridines. Eur J Org Chem 28:e202401048 [Google Scholar]
- 45.Allende J, Olaizola I, Ochoa de Retana AM, Palacios F, de los Santos JM (2024) Diastereoselective ZnCl2-mediated Joullié-Ugi three-component reaction for the preparation of phosphorylated N-acylaziridines from 2H-azirines. Molecules 29:1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carramiñana V, Ochoa de Retana AM, de los Santos JM, Palacios F (2020) First synthesis of merged hybrids phosphorylated azirino [2,1-b]benzo[e][1,3] oxazine derivatives as anticancer agents. Eur J Med Chem 185:111771 [DOI] [PubMed] [Google Scholar]
- 47.Carramiñana V, Ochoa de Retana AM, Vélez del Burgo A, de los Santos JM, Palacios F (2019) Synthesis and biological evaluation of cyanoaziridine phosphine oxides and phosphonates with antiproliferative activity. Eur J Med Chem 163:736–746 [DOI] [PubMed] [Google Scholar]
- 48.Vélez del Burgo A, Ochoa de Retana AM, de los Santos JM, Palacios F (2016) Reaction of 2H-azirine-phosphine oxides and -phosphonates with enolates derived from β-keto esters. J Org Chem 81:100–108 [DOI] [PubMed] [Google Scholar]
- 49.Palacios F, Ochoa de Retana AM, Vélez del Burgo A (2011) Selective synthesis of substituted pyrrole-2-phosphine oxides and -phosphonates from 2H-azirines and enolates from acetyl acetates and malonates. J Org Chem 76:9472–9477 [DOI] [PubMed] [Google Scholar]
- 50.Palacios F, Ochoa de Retana AM, Alonso JM (2006) Regioselective synthesis of fluoroalkylated, β-aminophosphorus derivatives and aziridines from phosphorylated oximes and nucleophilic reagents. J Org Chem 71:6141–6148 [DOI] [PubMed] [Google Scholar]
- 51.Palacios F, Ochoa de Retana AM, Alonso JM (2005) Reaction of 2H-azirine phosphine oxide and -phosphonates with nucleophiles. Stereoselective synthesis of functionalized aziridines and α- and β-aminophosphorus derivatives. J Org Chem 70:8895–8901 [DOI] [PubMed] [Google Scholar]
- 52.Nakamura S, Hayama D, Miura M, Hatanaka T, Funahashi Y (2018) Catalytic enantioselective reaction of 2H-azirines with thiols using Cinchona alkaloid sulfonamide catalysts. Org Lett 20:856–859 [DOI] [PubMed] [Google Scholar]
- 53.An D, Guan X, Guan R, Jin L, Zhang G, Zhang S (2016) Organocatalyzed nucleophilic addition of pyrazoles to 2H-azirines: asymmetric synthesis of 3,3-disubstituted aziridines and kinetic resolution of racemic 2H-azirines. Chem Commun 52:11211–11214 [Google Scholar]
- 54.Nakamura S, Hayama D (2017) Enantioselective reaction of 2H-azirines with phosphite using chiral bis(imidazoline)/Zinc(II) catalysts. Angew Chem Int Ed 56:8785–8789 [Google Scholar]
- 55.Fujita K, Miura M, Funahashi Y, Hatanaka T, Nakamura S (2021) Enantioselective reaction of 2H-azirines with oxazol-5-(4H)-ones catalyzed by cinchona alkaloid sulfonamide catalysts. Org Lett 23:2104–2108 [DOI] [PubMed] [Google Scholar]
- 56.Peng Q, Guo D, Bie J, Wang J (2018) Catalytic enantioselective aza-benzoin reactions of aldehydes with 2H-azirines. Angew Chem Int Ed 57:3767–3771 [Google Scholar]
- 57.Trost BM, Tracy JS (2020) Catalytically generated vanadium enolates formed via interruption of the Meyer–Schuster rearrangement as useful reactive intermediates. Acc Chem Res 53:1568–1579 [DOI] [PubMed] [Google Scholar]
- 58.Trost BM, Brindle CS (2010) The direct catalytic asymmetric aldol reaction. Chem Soc Rev 39:1600–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibasaki M, Kanai M, Matsunaga S, Kumagai N (2009) Recent progress in asymmetric bifunctional catalysis using multimetallic systems. Acc Chem Res 42:1117–1127 [DOI] [PubMed] [Google Scholar]
- 60.Hu H, Xu J, Liu W, Dong S, Lin L, Feng X (2018) Copper-catalyzed asymmetric addition of tertiary carbon nucleophiles to 2H-azirines: access to chiral aziridines with vicinal tetrasubstituted stereocenters. Org Lett 20:5601–5605 [DOI] [PubMed] [Google Scholar]
- 61.Trost BM, Hung C-IJ, Mata G (2020) Dinuclear metal-ProPhenol catalysts: development and synthetic applications. Angew Chem Int Ed 59:4240–4261 [Google Scholar]
- 62.Trost BM, Hung C-IJ, Mata G, Liu Y, Lu Y, Gnanamani E (2020) Direct enantio- and diastereoselective Zn-ProPhenol-catalyzed mannich reactions of CF3- and SCF3-substituted ketones. Org Lett 22:2437–2441 [DOI] [PubMed] [Google Scholar]
- 63.Trost BM, Tracy JS, Yusoontorn T, Hung C-IJ (2020) Acyclic branched α-fluoro ketones for the direct asymmetric mannich reaction leading to the synthesis of β-tetrasubstituted β-fluoro amines. Angew Chem Int Ed 59:2370–2374 [Google Scholar]
- 64.Trost BM, Hung C-IJ, Gnanamani E (2019) Tuning the reactivity of ketones through unsaturation: construction of cyclic and acyclic quaternary stereocenters via Zn-prophenol catalyzed Mannich reactions. ACS Catal 9:1549–1557 [Google Scholar]
- 65.Trost BM, Hung C-IJ, Saget T, Gnanamani E (2018) Branched aldehydes as linchpins for the enantioselective and stereodivergent synthesis of 1,3-aminoalcohols featuring a quaternary stereocentre. Nat Catal 1:523–530 [Google Scholar]
- 66.Trost BM, Zhu C (2020) Zn-ProPhenol catalyzed enantioselective Mannich reaction of 2H-azirines with alkynyl ketones. Org Lett 22:9683–9687 [DOI] [PubMed] [Google Scholar]
- 67.Xie F, Zhao J, Ren D, Xue J, Wang J, Zhao Q, Liu L, Liu X (2023) Enantio- and diastereoselective copper-catalyzed synthesis of chiral aziridines with vicinal tetrasubstituted stereocenters. Org Lett 25:8530–8534 [DOI] [PubMed] [Google Scholar]
- 68.Yu H, Zhang Q, Zi W (2022) Synergistic Pd/Cu-catalyzed enantioselective Csp2−F bond alkylation of fluoro-1,3-dienes with aldimine esters. Nat Commun 13:2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang X, Cheng X, Liu X-T, Fu C, Wang W-Y, Wang C-J (2022) Stereodivergent construction of 1,4-nonadjacent stereocenters via hydroalkylation of racemic allylic alcohols enabled by copper/ruthenium relay catalysis. Angew Chem Int Ed 61:e202206517 [Google Scholar]
- 70.Chen Z, Lin H, Han J, Fang D, Wang M, Liao J (2021) Enantioselective copper-catalyzed electrophilic sulfenylation of cyclic imino esters. Org Lett 23:9146–9150 [DOI] [PubMed] [Google Scholar]
- 71.Zhang H-J, Xie Y-C, Yin L (2019) Copper(I)-catalyzed asymmetric decarboxylative Mannich reaction enabled by acidic activation of 2H-azirines. Nat Commun 10:1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujita A, Kumazawa I, Yokoi J, Abe K, Takehara T, Suzuki T, Yasukawa N, Nakamura S (2024) Organocatalytic stereoselective decarboxylative addition of α-amido malonic acid half oxyesters to isatins. Adv Synth Catal 366:438–443 [Google Scholar]
- 73.Nakamura S (2014) Catalytic enantioselective decarboxylative reactions using organocatalysts. Org Biomol Chem 12:394–405 [DOI] [PubMed] [Google Scholar]
- 74.Hensel A, Nagura K, Delvos NL, Oestreich M (2014) Enantioselective addition of silicon nucleophiles to aldimines using a preformed NHC–copper(I) complex as the catalyst. Angew Chem Int Ed 53:4964–4967 [Google Scholar]
- 75.Zhao Z-Y, Cui M, Irran E, Oestreich M (2023) Copper-catalyzed highly enantioselective addition of a silicon nucleophile to 3-substituted 2H-azirines using an Si–B reagent. Angew Chem Int Ed 62:e202215032 [Google Scholar]
- 76.Delvos LB, Hensel A, Oestreich M (2014) McQuade’s six-membered NHC–copper(I) complexes for catalytic asymmetric silyl transfer. Synthesis 46:2957–2964 [Google Scholar]
- 77.Yoon WS, Han JT, Yun J (2021) Divergent access to benzocycles through copper-catalyzed borylative cyclizations. Adv Synth Catal 363:4953–4959 [Google Scholar]
- 78.Ye Y, Kevlishvili I, Feng S, Liu P, Buchwald SL (2020) Highly enantioselective synthesis of Indazoles with a C3-quaternary chiral center using CuH catalysis. J Am Chem Soc 142:10550–10556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ni C, Ramspoth T-F, Castiñeira Reis M, Harutyunyan SR (2024) Manganese(I)-catalyzed access to enantioenriched chiral aziridine phosphines. Angew Chem Int Ed 64:e202415623 [Google Scholar]
- 80.Sinnema EG, Ramspoth T-F, Bouma RH, Ge L, Harutyunyan SR (2024) Enantioselective hydrophosphination of terminal alkenyl aza-heteroarenes. Angew Chem Int Ed 63:e202316785 [Google Scholar]
- 81.Ge L, Harutyunyan SR (2022) Manganese(I)-catalyzed access to 1,2-bisphosphine ligands. Chem Sci 13:1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pérez JM, Postolache R, Reis MC, Sinnema EG, Vargová D, de Vries F, Otten E, Ge L, Harutyunyan SR (2021) Manganese(I)-Catalyzed H-P bond activation via metal-ligand cooperation. J Am Chem Soc 143:20071–20076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moragas T, Churcher I, Lewis W, Sockman RA (2014) Asymmetric synthesis of trisubstituted aziridines via aza-Darzens reaction of chiral sulfinimines. Org Lett 16:6290–6293 [DOI] [PubMed] [Google Scholar]
- 84.Colpaert F, Mangelinckx S, De Brabandere S, De Kimpe N (2011) Asymmetric synthesis of α-chloro-β-amino-N-sulfinyl imidates as chiral building blocks. J Org Chem 76:2204–2213 [DOI] [PubMed] [Google Scholar]
- 85.Arroyo Y, Meana A, Sanz-Tejedor MA, Alonso I, García-Ruano JL (2010) 2-(p-Tolylsulfinyl)benzyl halides as efficient precursors of optically pure trans-2,3-disubstituted aziridines. Chem Eur J 16:9874–9883 [DOI] [PubMed] [Google Scholar]
- 86.Hashimoto T, Nakatsu H, Watanabe S, Maruoka K (2010) Stereoselective synthesis of trisubstituted aziridines with N-α-diazoacyl camphorsultam. Org Lett 12:1668–1671 [DOI] [PubMed] [Google Scholar]
- 87.Midura WH (2007) Highly stereoselective aziridination of imines with (S)-dimethylsulfonium-(p-tolylsulfinyl)methylide. Tetrahedron Lett 48:3907–3910 [Google Scholar]
- 88.Davis FA, Wu Y, Yan H, McCoull W, Prasad KR (2003) Asymmetric synthesis of aziridine 2-phosphonates from enantiopure sulfinimines (N-sulfinyl imines). Synthesis of α-amino phosphonates. J Org Chem 68:2410–2419 [DOI] [PubMed] [Google Scholar]
- 89.Kodama Y, Imai S, Fujimoto J, Sato K, Mase N, Narumi T (2021) Stereoselective synthesis of highly functionalized (Z)-chloroalkene dipeptide isosteres containing an α,α-disubstituted amino acid. Chem Commun 57:6915–6918 [Google Scholar]
- 90.Chogii I, Das P, Delost MD, Crawford MN, Njardarson JT (2018) Asymmetric vinylogous aza-Darzens approach to vinyl aziridines. Org Lett 20:4942–4945 [DOI] [PubMed] [Google Scholar]
- 91.Larson SE, Li G, Rowland GB, Junge D, Huang R, Woodcock HL, Antilla JC (2011) Catalytic asymmetric aza-Darzens reaction with a vaulted biphenanthrol magnesium phosphate salt. Org Lett 13:2188–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akiyama T, Suzuki T, Mori K (2009) Enantioselective aza-Darzens reaction catalyzed by a chiral phosphoric acid. Org Lett 11:2445–2447 [DOI] [PubMed] [Google Scholar]
- 93.Huang L, Zhang Y, Staples RJ, Huang RH, Wulff WD (2012) Double stereodifferentiation in the catalytic asymmetric aziridination of imines prepared from α-chiral amines. Chem Eur J 18:5302–5313 [DOI] [PubMed] [Google Scholar]
- 94.Hu G, Huang L, Huang RH, Wulff WD (2009) Evidence for a boroxinate based brønsted acid derivative of VAPOL as the active catalyst in the catalytic asymmetric aziridination reaction. J Am Chem Soc 131:15615–15627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antilla JC, Wulff WD (1999) Catalytic asymmetric aziridination with a chiral VAPOL−boron Lewis acid. J Am Chem Soc 121:5099–5100 [Google Scholar]
- 96.Hashimoto T, Uchiyama N, Maruoka K (2008) trans-Selective asymmetric aziridination of diazoacetamides and N-Boc imines catalyzed by axially chiral dicarboxylic acid. J Am Chem Soc 130:14380–14381 [DOI] [PubMed] [Google Scholar]
- 97.Trost BM, Saget T, Hung C-I (2017) Efficient access to chiral trisubstituted aziridines via catalytic enantioselective aza-Darzens reactions. Angew Chem Int Ed 56:2440–2444 [Google Scholar]
- 98.Hashimoto T, Nakatsu H, Yamamoto K, Maruoka K (2011) Chiral Brønsted acid-catalyzed asymmetric trisubstituted aziridine synthesis using α-diazoacyl oxazolidinones. J Am Chem Soc 133:9730–9733 [DOI] [PubMed] [Google Scholar]
- 99.Pan J, Wu J-H, Zhang H, Ren X, Tan J-P, Zhu L, Zhang H-S, Jiang C, Wang T (2019) Highly enantioselective synthesis of fused tri- and tetrasubstituted aziridines: aza-Darzens reaction of cyclic imines with α-halogenated ketones catalyzed by bifunctional phosphonium salt. Angew Chem Int Ed 58:7425–7430 [Google Scholar]
- 100.Wu J-H, Pan J, Wang T (2019) Dipeptide-based phosphonium salt catalysis: application to enantioselective synthesis of fused tri- and tetrasubstituted aziridines. Synlett 30:2101–2106 [Google Scholar]
- 101.Bao J, Wulff WD, Rheingold AL (1993) Vaulted biaryls as chiral ligands for asymmetric catalytic Diels-Alder reactions. J Am Chem Soc 115:3814–3815 [Google Scholar]
- 102.Zhou Y, Gupta AK, Mukherjee M, Zheng L, Wulff WD (2017) Multicomponent catalytic asymmetric synthesis of trans-aziridines. J Org Chem 82:13121–13140 [DOI] [PubMed] [Google Scholar]
- 103.Lu Z, Zhang Y, Wulff WD (2007) Direct access to N-H-aziridines from asymmetric catalytic aziridination with borate catalysts derived from vaulted binaphthol and vaulted biphenanthrol ligands. J Am Chem Soc 129:7185–7194 [DOI] [PubMed] [Google Scholar]
- 104.Antilla JC, Wulff WD (2000) Catalytic asymmetric aziridination with arylborate catalysts derived from VAPOL and VANOL ligands. Angew Chem Int Ed 39:4518–4521 [Google Scholar]
- 105.Zhang Y, Lu Z, Desai A, Wulff WD (2008) Mapping the active site in a chemzyme: diversity in the N-substituent in the catalytic asymmetric aziridination of imines. Org Lett 10:5429–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren H, Wulff WD (2010) Trimethylsilyldiazomethane as a versatile stitching agent for the introduction of aziridines into functionalized organic molecules. Org Lett 12:4908–4911 [DOI] [PubMed] [Google Scholar]
- 107.Guan Y, Ding Z, Wulff WD (2013) Vaulted biaryls in catalysis: a structure-activity relationship guided tour of the immanent domain of the VANOL ligand. Chem Eur J 19:15565–15571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guan Y, Lu Z, Yin X, Mohammadlou A, Staples RJ, Wulff WD (2020) Catalytic asymmetric aziridination of benzhydryl imines and diazoacetate esters with BOROX catalysts from 3,3′-disubstituted VANOL ligands. Synthesis 52:2073–2091 [Google Scholar]
- 109.Osminski WEG, Lu Z, Zhao W, Mohammadlou A, Yin X, Matthews EC, Canestraight VM, Staples RJ, Allen CJ, Hirschi JS, Wulff WD (2021) Probing catalyst function − electronic modulation of chiral polyborate anionic catalysts. J Org Chem 86:17762–17773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan X-F, Zhang F-G, Ma J-A (2020) Asymmetric synthesis of CF2-functionalized aziridines by combined strong Brønsted acid catalysis. Beilstein J Org Chem 16:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olivo G, Capocasa G, Del Giudice D, Lanzalunga O, Di Stefano S (2021) New horizons for catalysis disclosed by supramolecular chemistry. Chem Soc Rev 50:7681–7724 [DOI] [PubMed] [Google Scholar]
- 112.Liu W, Stoddart JF (2021) Emergent behavior in nanoconfined molecular containers. Chem 7:919–947 [Google Scholar]
- 113.Morimoto M, Bierschenk SM, Xia KT, Bergman RG, Raymond KN, Toste FD (2020) Advances in supramolecular host-mediated reactivity. Nat Catal 3:969–984 [Google Scholar]
- 114.Dong J, Liu Y, Cui Y (2021) Supramolecular chirality in metal−organic complexes. Acc Chem Res 54:194–206 [DOI] [PubMed] [Google Scholar]
- 115.Tan C, Chu D, Tang X, Liu Y, Xuan W, Cui Y (2019) Supramolecular coordination cages for asymmetric catalysis. Chem Eur J 25:662–672 [DOI] [PubMed] [Google Scholar]
- 116.Bierschenk SM, Pan JY, Settineri NS, Warzok U, Bergman RG, Raymond KN, Toste FD (2022) Impact of host flexibility on selectivity in a supramolecular host-catalyzed enantioselective aza-Darzens reaction. J Am Chem Soc 144:11425–11433 [DOI] [PubMed] [Google Scholar]
- 117.Mennie KT, Banik SM, Reichert EC, Jacobsen EN (2018) Catalytic diastereo- and enantioselective fluoroamination of alkenes. J Am Chem Soc 140:4797–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Quintavalla A, Carboni D, Lombardo M (2023) Recent advances in asymmetric synthesis of pyrrolidine-based organocatalysts and their application: a 15-year update. Molecules 28:2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monteiro JL, Moreira NM, dos Santos DA, Paixȃo MW, Corrêa AG (2018) Step economy strategy for the synthesis of amphoteric aminoaldehydes, key intermediates for reduced hydantoins. Pure Appl Chem 90:121–132 [Google Scholar]
- 120.dos Santos DA, da Silva AR, Ellena J, da Silva CCP, Paixȃo MW, Corrêa AG (2020) Green one-pot asymmetric synthesis of peptidomimetics via sequential organocatalyzed aziridination and Passerini multicomponent reaction. Synthesis 52:1076–1086 [Google Scholar]
- 121.Umeda T, Minakata S (2021) A practical method for the aziridination of α,β-unsaturated carbonyl compounds with a simple carbamate utilizing sodium hypochlorite pentahydrate. RSC Adv 11:22120–22124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scharinger F, Pálvölgyi AM, Weisz M, Weil M, Stanetty C, Schnürch M, Bica-Schröder K (2022) Sterically demanding flexible phosphoric acids for constructing efficient and multi-purpose asymmetric organocatalysts. Angew Chem Int Ed 61:e202202189 [Google Scholar]
- 123.McLean LA, Ashford MW, Fyfe JWB, Slawin AMZ, Leach AG, Watson AJB (2022) Asymmetric synthesis of heterocyclic chloroamines and aziridines by enantioselective protonation of catalytically generated enamines. Chem Eur J 28:e202200060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brandenberg OF, Fasan R, Arnold FH (2017) Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr Opin Biotechnol 47:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goldberg NW, Knight AM, Zhang RK, Arnold FH (2019) Nitrene transfer catalyzed by a non-heme iron enzyme and enhanced by non-native small-molecule ligands. J Am Chem Soc 141:19585–19588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan Y, Han F, Hemming M, Wang J, Harms K, Xie X, Meggers E (2020) Asymmetric ring-closing aminooxygenation of alkenes en route to 2-amino-1,3-diols with vicinal stereocenters. Org Lett 22:6653–6656 [DOI] [PubMed] [Google Scholar]
- 127.Zhou Z, Chen S, Qin J, Nie X, Zheng X, Harms K, Riedel R, Houk KN, Meggers E (2019) Catalytic enantioselective intramolecular C(sp3)-H amination of 2-azidoacetamides. Angew Chem Int Ed 58:1088–1093 [Google Scholar]
- 128.Qin J, Zhou Z, Cui T, Hemming M, Meggers E (2019) Enantioselective intramolecular C−H amination of aliphatic azides by dual ruthenium and phosphine catalysis. Chem Sci 10:3202–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riart-Ferrer X, Sang P, Tao J, Xu H, Jin L-M, Lu H, Cui X, Wojtas L, Zhang XP (2021) Metalloradical activation of carbonyl azides for enantioselective radical aziridination. Chem 7:1120–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu H, Wang D-S, Zhu Z, Deb A, Zhang XP (2024) New mode of asymmetric induction for enantioselective radical N-heterobicyclization via kinetically stable chiral radical center. Chem 10:283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kisets I, Gelman D (2021) Synthesis of rigid chiral C3-symmetric triptycene-based ligands and their application in enantioselective Cu(I)-catalyzed aziridination of chalcones. J Organomet Chem 941:121804 [Google Scholar]
- 132.Boquet V, Nasrallah A, Dana AL, Brunard E, Di Chenna PH, Duran FJ, Retailleau P, Darses B, Sircoglou M, Dauban P (2022) Rhodium(II)-catalyzed enantioselective intermolecular aziridination of alkenes. J Am Chem Soc 144:17156–17164 [DOI] [PubMed] [Google Scholar]
- 133.Sun H, Shang H, Cui B (2022) (Salen)Mn(III)-catalyzed enantioselective intramolecular haloamination of alkenes through chiral aziridinium ion ring-opening sequence. ACS Catal 12:7046–7053 [Google Scholar]
- 134.Akhtar SMS, Hajra S (2023) Catalytic enantioselective synthesis of 4-amino-5-aryltetrahydro-1H-benzo[c]azepines by an aminoarylation reaction. Synlett 34:645–650 [Google Scholar]
- 135.Fanourakis A, Hodson NJ, Lit AR, Phipps RJ (2023) Substrate-directed enantioselective aziridination of alkenyl alcohols controlled by a chiral cation. J Am Chem Soc 145:7516–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodson NJ, Takano S, Fanourakis A, Phipps RJ (2024) Enantioselective nitrene transfer to hydrocinnamyl alcohols and allylic alcohols enabled by systematic exploration of the structure of ion-paired rhodium catalysts. J Am Chem Soc 146:22629–22641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Trinh TA, Fu Y, Hu DB, Zappia SA, Guzei IA, Liu P, Schromaker JM (2024) Chemo- and enantioselective intramolecular silver-catalyzed aziridinations of carbamimidates. Chem Commun 60:224–227 [Google Scholar]
- 138.Gross P, Im H, Laws D III, Park B, Baik M-H, Blakey SB (2024) Enantioselective aziridination of unactivated terminal alkenes using a planar chiral Rh(III) indenyl catalyst. J Am Chem Soc 146:1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang J, Luo M-P, Gu Y-J, Liu Y-Y, Wang S-G (2024) Chiral Cpxrhodium(III)-catalyzed enantioselective aziridination of unactivated terminal alkenes. Angew Chem Int Ed 63:e202400502 [Google Scholar]
- 140.Larson E, Baso J, Li G, Antilla J (2009) Chiral phosphoric acid-catalyzed desymmetrization of meso-aziridines with functionalized mercaptans. Org Lett 11:5186–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun S, Wang Z, Li S, Zhou C, Song L, Huang H, Sun J (2021) An organocatalytic kinetic resolution of aziridines by thiol nucleophiles. Org Lett 23:554–558 [DOI] [PubMed] [Google Scholar]
- 142.Jhang Y-J, Zhelavskyi O, Nagorny P (2023) Enantioselective parallel kinetic resolution of aziridine-containing quinoxalines via chiral phosphoric acid-catalyzed transfer hydrogenation. Org Lett 25:7721–7726 [DOI] [PubMed] [Google Scholar]
- 143.Zhang F, Zhang Y, Tan Q, Lin L, Liu X, Feng X (2019) Kinetic resolution of aziridines via catalytic asymmetric ring-opening reaction with mercaptobenzothiazoles. Org Lett 21:5928–5932 [DOI] [PubMed] [Google Scholar]
- 144.Zhang Z-J, Wen Y-H, Song J, Gong L-Z (2021) Kinetic resolution of aziridines enabled by N-heterocyclic carbene/copper cooperative catalysis: carbene dose-controlled chemo switchability. Angew Chem Int Ed 60:3268–3276 [Google Scholar]
- 145.Zheng Y, Huang Y, Gao P, Liu H, Murakami S, Matsubara R, Hayashi M (2021) Regiodivergent desymmetrization reaction of meso-azabicycloheptene providing two enantioenriched structural isomers. Org Lett 23:2411–2414 [DOI] [PubMed] [Google Scholar]
- 146.Liu J-W, Wang C (2020) Zinc-catalyzed hydroxyl-directed regioselective ring opening of aziridines in SN2 reaction pathway. ACS Catal 10:556–561 [Google Scholar]
- 147.Chang Z-R, Du S-S, Zhang C, Chen S-H, Hua Y-Z, Wang M-C, Mei G-J, Jia S-K (2023) Kinetic resolution of trans-2,3-aziridinyl alcohols via hydroxyl directed regio- and enantioselective ring opening reactions. ACS Catal 13:6873–6878 [Google Scholar]
- 148.Hu H, Liu Y, Lin L, Zhang Y, Liu X, Feng X (2016) Kinetic resolution of 2H-azirines by asymmetric imine amidation. Angew Chem Int Ed 55:10098–10101 [Google Scholar]
- 149.Deng H, Liu T-T, Ding Z-D, Yang W-L, Luo X, Deng W-P (2020) Kinetic resolution of 2H-azirines via Cu(I) catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Org Chem Front 7:3247–3252 [Google Scholar]
- 150.Pan Y-L, Shao Y-B, Wang J, Liu Z, Chen L, Li X (2021) Kinetic resolution of 2H-azirines by asymmetric allylation reactions. ACS Catal 11:13752–13760 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.