Abstract
Repair of damaged cartilage usually requires replacement tissue or substitute material. Tissue engineering is a promising means to produce replacement cartilage from autologous or allogeneic cell sources. Scaffolds provide a three-dimensional (3D) structure that is essential for chondrocyte function and synthesis of cartilage-specific matrix proteins (collagen type II, aggrecan) and sulfated proteoglycans. In this study, we assessed porous, 3D collagen sponges for in vitro engineering of cartilage in both standard and serum-free culture conditions. Bovine articular chondrocytes (bACs) cultured in 3D sponges accumulated and maintained cartilage matrix over 4 weeks, as assessed by quantitative measures of matrix content, synthesis, and gene expression. Chondrogenesis by bACs cultured with Nutridoma as a serum replacement was equivalent or better than control cultures in serum. In contrast, chondrogenesis in insulin-transferrinselenium (ITS+3) serum replacement cultures was poor, apparently due to decreased cell survival. These data indicate that porous 3D collagen sponges maintain chondrocyte viability, shape, and synthetic activity by providing an environment favorable for high-density chondrogenesis. With quantitative assays for cartilage-specific gene expression and biochemical measures of chondrogenesis in these studies, we conclude that the collagen sponges have potential as a scaffold for cartilage tissue engineering.
Keywords: 3D scaffolds, Chondrocytes, ITS, Nutridoma, Serum substitutes, Tissue engineering
Abbreviations: 2D – two-dimensional, 3D – three-dimensional, AGG – aggrecan, bACs – bovine articular chondrocytes, COL I – type I collagen, COL II – type II collagen, FBS – fetal bovine serum, G3PDH – glyceraldehyde-3-phosphate dehydrogenase, HEPES – 4-(2-hydroxyethyl) piperazine-1-ethansulfonic acid, ITS – insulin-transferrin-sodium selenite, Nut – nutridoma, OD – optical density, RT-PCR – reverse transcriptase-polymerase chain reaction, s-GAG – sulfated glycosaminoglycan
Introduction
Cartilage has a poor capacity for healing and regeneration, and therefore most defects require replacement tissue or substitute material. In mosaicplasty, cores of healthy tissues from non-load-bearing surfaces of the joint are transplanted into the defect (Hangody and Fules 2003). In another procedure, cells are isolated from healthy cartilage, expanded in vitro, and transplanted as a suspension into the defect under a periosteal flap (Brittberg et al. 1994, 2003).
Tissue engineering holds promise as an alternative for cartilage repair. Central issues for this approach are the type and quantity of cells needed to generate sufficient tissue. Both chondrocytes and cells with chondrogenic potential, such as bone marrow mesenchymal cells, have been used for experimentally engineered cartilage. One hypothesis is that articular chondrocytes will be superior for tissue engineering because the appropriate genetic program has been fully activated. Because of the small number of chondrocytes in cartilage, however, isolated cells must be expanded in vitro. The chondrocyte phenotype in vitro is cell shape-dependent (Glowacki et al. 1983). Growth in monolayers results in dedifferentiation, i.e. decreased synthesis of cartilage-specific matrix components such as collagen type II, aggrecan, and proteoglycans (Horwitz and Dorfman 1970; Hering et al. 1994) and increased synthesis of non-cartilage-specific collagen type I (Benya et al. 1978). Chondrocytes recover their ability to synthesize cartilage matrix if they are returned to a three-dimensional (3D) environment, such as agarose gels (Benya and Shaffer 1982), alginate beads (Hauselmann et al. 1994), or high-density culture (Hering et al. 1994).
Scaffolds are used in tissue engineering as a carrier to deliver cells to a defect, as a framework to support in vitro histogenesis, or as both. Previously, we developed porous 3D collagen (type I) sponges that support in vitro histogenesis (Mizuno and Glowacki 1996). The highly porous collagen sponges are comprised of a web or lattice of interconnected fibers that support cell distribution and matrix accumulation (Mizuno and Glowacki 1996). This structure differs from that of woven polymer lattices (Li et al. 2003; Woodfield et al. 2004) or collagen hydrogels. Our previous work demonstrated that these constructs meet several criteria that are desirable for tissue engineering of cartilage: they are biologically compatible (demonstrated in mice, with osteoblasts) (Gerstenfeld et al. 1996); they are adherent to cartilage (Zaleske et al. 2003); and they support chondrogenesis in vitro (Mizuno and Glowacki 1996) and in vivo (Warden et al. 2004).
The purpose of this study was to more fully evaluate the porous, 3D collagen sponges for engineering of cartilage. One objective was to compare maintenance of the chondrocyte pheno-type during time in 2D monolayer and 3D culture. A current limitation of tissue engineering is that fetal bovine serum (FBS) is used commonly. Therefore, the second objective of this study was to assess the chondrocyte phenotype in defined media.
Materials and methods
Collagen sponge fabrication
Collagen sponges were prepared according to the protocol of Mizuno et al. (Mizuno and Glowacki 1996). In brief, a 0.5% (w/v) bovine pepsin-digested, acid-soluble type I collagen (CellagenTM solution PC-5, ICN Biomedicals, CostaMesa, CA) was neutralized with 1/100 volumes of both 1 M HEPES buffer (pH 7.4) and 1 M NaHCO3 (pH 7.4). A 250 μl volume of the solution was cast in an 8 mm diameter mold and was frozen at −20 °C. After lyophilization, the sponges were removed from the mold and were exposed to UV-irradiation in a laminar-flow safety cabinet for 3 h on each side. Scanning electron microscopy showed that the pores were interconnected through the whole sponge and the collagen fibers resembled a 3D web or lattice (Mizuno and Glowacki 1996). The diameter of the pores ranged between 120 and 200 μm (Mizuno and Glowacki 1996).
Cells and culture conditions
Bovine articular chondrocytes (bACs) were prepared from fresh shoulders of neonatal calves from a local abattoir as previously described (Mizuno and Glowacki 1996). The joints were exposed under aseptic conditions and the cartilage was sliced and minced with a scalpel blade. The fragments were rinsed with cold PBS three times and digested with 0.15% collagenase CI (Worthington Biochemical Corp, Lakewood, NJ) in Ham’s F12 medium (Invitrogen, Carlsbad, CA) at 37 °C overnight on a rocking platform. Following filtration with 70 μm cell strainers (Becton Dickinson Labware, Franklin Lakes, NJ), the cell suspensions were centrifuged for 10 min at 1500 × g. The cells were washed and suspended in medium consisting of Ham’s F12, 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Irvine Scientific, Santa Ana, CA).
High-density monolayer cultures were established by seeding 1 × 106 cells/cm2 in 60 mm dishes. On day 7, some dishes were harvested for DNA, s-GAG, or 35S-sulfate incorporation assays; the remaining cells were replated in dishes at a density of 2 × 105 cells/cm2. Medium was changed every 2–3 days and the cells were replated 2–4 days before each additional timepoint. Sponge cultures were established by injecting cell suspensions (5 × 106 cells/50 μl medium per sponge) onto dry sponges that were positioned in seeding chambers (Mizuno and Glowacki 1996). After 1 h in a humidified incubator at 37 °C and 5% CO2 in air, 100 μl of complete medium was deposited onto the sponges. After an additional 3 h of incubation, each seeding device was transferred to 8 ml of medium and turned sideways in deep sixwell plates (Corning Inc., Corning, NY) for efficient nutrient exchange. After 18 h, each sponge was transferred to 35 mm wells with 4 ml medium. For experiments that used defined media, bACs were precultured in collagen sponges in medium containing 5% FBS for 7 days. The culture dishes were rinsed three times in PBS before addition of medium containing 2% Nutridoma-CS media supplement (Roche Applied Science, Indianapolis, IN) or 1% insulin-transferrin-sodium selenite liquid media supplement with linoleic and oleic acids (ITS+3, Sigma-Aldrich Co., St. Louis, MO). Culture medium was changed every 2–3 days. Samples were collected for biochemical and molecular analyses on 1, 2, or 4 weeks after seeding.
Histology
After designated times in culture, sponges (n = 3 per group) were fixed in 2% paraformaldehyde, 0.1 M cacodylate buffer (pH 7.4) for 24 h at 4 °C. After rinses in 0.1 M cacodylate buffer (pH 7.4), specimens were infiltrated with glycolmethacrylate catalyst (JB-4, Polysciences Inc., Warrington, PA) with vacuum for 48 h. Samples were embedded in glycolmethacrylate. The polymerized blocks were cut in cross-section (12.5 μm thick) and were stained with 0.5% toluidine blue-O at pH 4.0 (Fisher Scientific, Pittsburgh, PA).
Sulfated glycosaminoglycan content
Each sponge or monolayer culture was digested with 1 ml of 125 mg/ml papain (Sigma-Aldrich) for 16–18 h at 60 °C (Farndale et al. 1982). Twenty microliters of each digested extract was added to 150 μl of assay solution in a 96-well titer plate at room temperature. The difference between optical density of the sample at 595 and 540 nm was immediately determined with a microtiter plate reader. Sulfated GAG content was calculated with a standard curve for shark chondroitin sulfate (Sigma-Aldrich) and was expressed on the basis of DNA content (Mizuno and Glowacki 1996).
DNA content
DNA content was measured by the fluorescent dye method (Kim et al. 1988; Rymaszewski et al. 1990) as described (Mizuno et al. 2002). Twenty microliters of papain extracts was used for determination of DNA content in each sponge or monolayer culture. Each sample was added to 2 ml of 0.1 μg/ml Hoechst 33258 (Polysciences, Warrington, PA) in 0.1 M Tris–NaCl, 10 mM EDTA, pH 7.4 (Kim et al. 1988). The mixture was incubated in the dark for 10 min at room temperature. Fluorescence was measured with a fluorometer TKO 100 (Hoffer Scientific Instruments, San Francisco, CA). Calf thymus DNA (Sigma-Aldrich) was used as a standard.
35S-sulfate incorporation
Twenty-five μCi of 35S sodium sulfate (NEN Life Science Products Inc., Boston, MA) in 5 ml medium was added per sponge (Mizuno et al. 2002) or dish during the last 18 h of the 1, 2, or 4 week culture period (Robbins et al. 1997). Papain extracts were heat-treated at 95 °C for 45 min and desalted with Sephadex G-25 (PD-10TM, Amersham Pharmacia Biotech Inc., Piscataway, NJ). 35S sulfate was determined by liquid scintillation counting.
RNA extraction
Total cellular RNA was extracted from monolayers by lysis in 1 ml Trizol reagent (Invitrogen). Sponges were homogenized in 1 ml Trizol reagent with a Power Gen 125 Tissue Homogenizer fitted with a 7 mm saw-tooth generator (Fisher Scientific). RNA quality was assessed by the ratio of absorbance at 260 and 280 nm. The yields of RNA were calculated based on the absorbance at 260 nm. Total yields of RNA were 6–14 μg per dish and per sponge. The difference in yield from each group was not statistically significant.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Five μg of total RNA was treated with 1 μl ribonuclease- free DNase I (Roche Applied Science) and cDNA synthesis was performed with and without reverse transcriptase to confirm the absence of genomic DNA. The cDNA synthesis reactions were performed in an 80 μl volume containing 4 μg DNase treated RNA, 8 μl random hexamers (Invitrogen), and 800 units of reverse transcriptase (Superscript II, Invitrogen). In a reaction volume of 50 μl, 2 μl of cDNA was amplified by 1 U Platinum Taq Polymerase (Invitrogen) with primers specific for bovine type II collagen (COL II), forward 5′-CTGGATGCCATGAAGGTTTT-3′ and reverse 5′-TAGTCTTGCCCCACTTACCG-3′; bovine aggrecan (AGG), forward 5′-CACTGTTACCGCCACTTCCC-3′ and reverse 5′-GACATCGTTCCACTCGCCCT-3′; bovine type I collagen (COL I), forward 5′-TGCTGGCCAACTATGCCTCT-3′ and reverse (5′-TTGCACAATGCTCTGATC-3′; and mammalian glyceraldehyde-3-phosphate dehydrogenase (G3PDH), forward 5′-ACCA CAGTCCATGCCATCAC-3′ and reverse 5′-T CCACCACCCTGTTGCTGTA-3′. Amplification conditions consisted of an initial denaturation step at 94 °C for 10 min; 35 cycles of 94 °C for 1 min, 53 °C for 2 min, 72 °C for 2 min; and a final 6 min extension at 72 °C.
Quantitative, competitive RT-PCR
COL II, AGG, and COL I mRNA transcripts were measured by specific, quantitative competitive RT-PCR assays. Competitive DNA templates were produced with the competitive DNA Construction Kit (TaKaRa Biomedicals, Madison, WI). The competitor sizes for COL II and AGG were 290 and 190 bp, respectively. The competitor sizes for COL I and G3PDH were both 340 bp. For PCRs, 1 μl of target cDNA was added to each of a series of 10-fold dilutions of competitor (1 fmol to 0.001 amol). This was followed by PCRs with a 1:2 dilution series to measure the number of transcripts in each sample. The amount of mRNA transcripts for each gene was normalized to the amount of G3PDH mRNA in the cDNA samples.
In experiments that compared 2D monolayers to 3D sponges, gene expression of COL II and AGG were expressed as ratios to COL I. A ratio of <1.0 indicates a phenotype that is more fibroblastic than chondroblastic. In experiments that compared serum-free to serum-containing media, COL I mRNA levels were not measured because the histology results did not suggest dedifferentiation of bACs in either Nutridoma or ITS3+. Therefore, COL II and AGG expression levels (normalized to G3PDH) were evaluated directly.
Statistical analysis
Sample size for biochemical and gene expression assays in the collagen sponge system was determined from empirical data (Mizuno et al. 2001). Five replicate dishes or sponges were used for measurements of DNA content, total s-GAG content, and sulfate incorporation. Pooled samples (2 dishes or sponges per group) were used for comparisons of gene expression levels in 2D and 3D conditions. Gene expression levels were measured in individual sponges (n = 3) for experiments that used Nutridoma and ITS+3.
Statistical analyses were performed with Sigma-Stat 2.0 and SigmaPlot 5.0 software (SSPS Inc., Chicago, IL) or InStat software (Advanced Graphics Software, Encinitas, CA). One-way ANOVA with Bonferroni correction for multiple comparisons (α = 0.05) was used to compare total s-GAG contents and 35S-sulfate incorporation in monolayers and sponges. The Kruskal–Wallis test was used to compare levels of mRNA expression for COL II and AGG.
Results
Comparison of bovine chondrocytes cultured in 3D collagen sponges and 2D monolayers
The chondrocyte phenotype was evaluated in monolayer and sponge cultures by quantitative biochemical and gene expression assays for cartilage matrix components. DNA content was used to make comparisons on the basis of cellularity in the two types of cultures. In 2D monolayers, DNA content was similar at 1 and 2 w. Thereafter, DNA content increased 2.6-fold between 2 w (109 ng/dish ±7) and 4 w (285 ng/dish ±34) (p = 0.008). In 3D sponges, DNA content was ~2-fold greater than in monolayers and did not change significantly over time (235 ng/sponge ±12.8 at 1 w). Similar results were obtained in a second experiment.
In 2D cultures, there was accumulation and endurance of matrix sulfated glycosaminoglycans (s-GAG) during 2 w of culture (Figure 1a). Thereafter, between 2 and 4 w, s-GAG decreased 53% (p < 0.001). In 3D sponges, there was also accumulation and endurance of s-GAG during 2 w of culture. Thereafter, between 2 and 4 w, s- GAG decreased 28% (p = 0.001). Sponges contained significantly more s-GAG than monolayer cultures (p < 0.001) at 2 w (47% greater in sponges) and at 4 w (125% greater).
Figure 1.
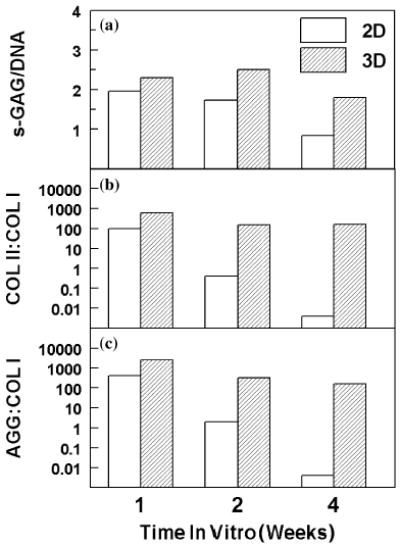
Effect of 2D (monolayer) or 3D (porous collagen sponge) conditions on phenotype of bovine articular chondrocytes cultured for 1, 2, and 4 weeks. (a) Total sulfated glycosaminoglycan content (s-GAG) was measured by DMB assay and normalized to DNA content in each dish or sponge (n = 5). (b) Ratio of collagen type II (COL II) to collagen type I (COL I) was calculated from levels of each RNA as measured by quantitative, competitive RT-PCR assays and normalized to glyceraldehyde 3-phosphate dehydrogenase (G3PDH). (c) Ratio of aggrecan (AGG) to collagen type I (COL I) was calculated from levels of each RNA as measured by quantitative, competitive RT-PCR and normalized to G3PDH.
Synthesis of s-GAG was measured by 35S-sulfate incorporation (Table 1). In 2D cultures, sulfate incorporation was not significantly changed between 1 and 2 w or between 2 and 4 w (p > 0.05). In 3D cultures, sulfate incorporation was similar at 1 and 2 w. Thereafter, there was a decline in sulfate incorporation (53% less) from 2 to 4 w (p < 0.001). At all time points, sulfate incorporation was significantly greater in sponges than in monolayer cultures (Table 1).
Table 1.
Comparison of 35S-sulfate incorporation into glycosaminoglycans by bovine articular chondrocytes cultured in monolayer or in 3D sponges.
| Culture period (weeks) | Monolayer (2D) | Sponge (3D) | p |
|---|---|---|---|
| 1 | 10.2 ± 1.9 | 96.0 ± 24.4 | <0.001 |
| 2 | 14.5 ± 2.1 | 117.6 ± 11.3 | <0.001 |
| 4 | 4.8 ± 1.0 | 55.2 ± 6.7 | <0.001 |
Mean ± SD (cpm × 10−3/μg DNA); (n = 5).
Expression of signature cartilage genes was used as an indicator of changes in the chondrocyte phenotype in vitro. Gene expression of type II collagen (COL II) and aggrecan (AGG) transcripts were expressed as ratios to type I collagen (COL I). Baseline ratios of COL II: COL I and AGG:COL I transcripts in freshly isolated bACs were 5759 and 2833, respectively. After chondrocytes were cultured in monolayer for 1 w, the COL II ratio was 1.7% of baseline and decreased (to 0.4% of the ratio at 1 w) between 1 to 2 w (Figure 1B). Between 2 and 4 w in culture, COL II decreased further (0.5%). Similarly, the AGG ratio was 14.1% of baseline at 1 w, decreased between 1 and 2 w (0.5%), and decreased further between 2 and 4 w (0.02%) (Figure 1C). Both the increased COL I expression and the decreased COL II expression contributed to the change in gene expression ratios in monolayer cultures with time in culture. Expression of COL I was similar between 1 and 2 w, but increased 2000-fold between 2 and 4 w. COL II expression decreased between 1 and 2 w (2%).
In contrast to the monolayer cultures, greater COL II and AGG ratios were maintained in 3D sponges. After chondrocytes were cultured in sponges for 1 w, the COL II ratio was 10.9% of baseline. The COL II ratio decreased between 1 and 2 w (25.0%), and was maintained at 4 w (Figure 1B). The AGG ratio was 88.2% of baseline at 1 w, decreased between 1 and 2 w (12.5%), and again between 2 and 4 w (51.1%) (Figure 1C). COL I expression levels were low in 3D sponges at all timepoints: at 1 and 2 w, COL I mRNA level was 2 orders of magnitude lower than monolayers, and increased only 3-fold between 2 and 4 w.
Effects of serum substitutes on bovine chondrocytes cultured in 3D collagen sponges
The effect of serum substitutes on the chondrocyte phenotype was evaluated in 3D collagen sponges. Sponges were precultured for 1 week in medium containing 5% FBS to allow bACs to attach to the scaffold and synthesize matrix. Thereupon (day 0), the sponges were transferred to defined media containing Nutridoma (Nut) or ITS+3 or control medium containing 5% FBS.
As expected, histologic evaluation showed that sponges maintained in FBS were populated with round cells that were surrounded by abundant metachromatic matrix on day 0 (Figure 2a), and more so on day 7 (Figure 2b). The majority of cells within the lattice were associated with cartilage matrix production, and there was no histological evidence of dedifferentiation of cells adjacent to the collagen fibers of the lattice. Chondrocytes at the surface of the porous sponges (not shown) tended to have a spindle shape without metachromatic extracellular matrix. Sponges that were cultured for 7 days in medium containing Nutridoma showed the most extensive metachromatic matrix (Figure 2c). Individual cells in lacunae were frequently seen. In contrast, sponges cultured in medium containing ITS+3 for 7 days showed little matrix (Figure 2d). Small clusters of cells were surrounded by weakly metachromatic matrix. The amount of the matrix was less than after the preculture period. The presence of pycnotic cells was notable only in the ITS+3 group.
Figure 2.
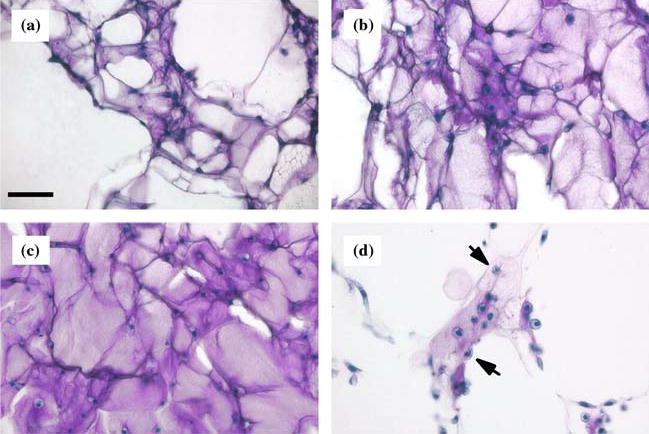
Photomicrographs of bovine articular chondrocytes cultured in collagen sponges (Toluidine blue stain). Matrix that contains sulfated glycosaminoglycans appears pink/purple and cell nuclei are blue. (a) Collagen sponge was precultured for 1 week in medium containing FBS. Scale bar is 50 μm. (b) Collagen sponge was cultured in FBS for an additional week. (c) After preculture, collagen sponge was cultured in media containing 2% Nutridoma for 7 days. (d) After preculture, collagen sponge was cultured in media containing ITS+3 for 7 days. Arrows indicate pycnotic cells.
Expression of cartilage signature genes was also measured in 3D sponges after 7 days culture with different supplements (Figure 3). Although p values did not achieve significance, trends in gene expression were clear. Compared with FBS control, the COL II mRNA levels were 170% greater in Nutridoma and 110% greater in ITS+3. AGG mRNA levels were also elevated in Nutridoma (300% greater than control) and ITS (360% of control).
Figure 3.
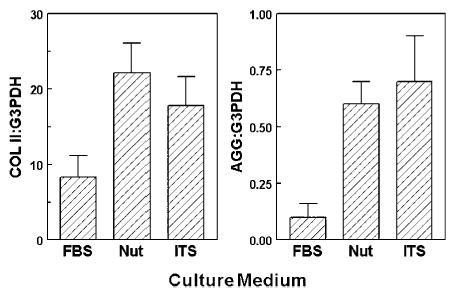
Effect of culture media on expression levels of cartilage signature genes in bovine articular chondrocytes in collagen sponges. Media contained 5% fetal bovine serum (FBS), 2% Nutridoma (Nut) or 1% ITS+3. Gene expression levels of collagen type II (COL) and aggrecan (AGG) were measured by competitive RT-PCR and normalized to G3PDH.
Discussion
Potential sources for replacement cartilage include allograft, autograft, and engineered tissue. Tissue banking of allograft cartilage is limited by the detrimental effects of cryopreservation on chondrocyte viability (Ohlendorf et al. 1996; Jomha et al. 2002) and biomechanical properties (Kubo et al. 2001). Extended cold storage (>2 weeks) also reduces chondrocyte viability (Williams et al. 2003). Therefore, fresh tissue is recommended for osteochondral and cartilage allografts (Sammarco et al. 1997). Inspired by studies on the pathophysiology of the cauliflower ear, Skoog championed the use of autogenous perichondrial grafts for cartilage repair (Skoog et al. 1972), but results are often highly variable (Upton et al. 1981).
Successful, cell-based tissue engineering of cartilage requires the maintenance of the chondrocyte’s highly differentiated phenotype and its ability to produce cartilage-specific matrix. In this study, we evaluated the chondrocyte signature of cells cultured in a biocompatible porous collagen sponge scaffold. Articular chondrocytes isolated from shoulders of young calves (bovine articular chondrocytes; bACs) were used for these experiments because cells could be obtained from many samples at similar ages. Chondrogenesis by bACs was maintained significantly better in 3D sponges than in monolayers, as assessed by sulfated GAG content, 35S-sulfate incorporation, and expression of chondrocyte phenotypic genes. The maximum magnitude of cartilage matrix synthesis occurred in 3D sponges during the first 2 w of culture. In experiments to compare serum-free culture conditions with serum-containing medium, a preculture period was performed in serum-containing media so that effects of serum replacement on chondrogenesis could be specifically measured with sponges of equal cell density. Chondrogenesis in medium containing Nutridoma was as good or better than in medium containing serum, as assessed by histologic analysis and chondrocyte phenotypic gene expression. In this system, ITS+3 is a poor serum substitute (Glowacki et al. 2005). There was marked loss of viable cells and of matrix that had accumulated during the preculture period.
Several investigators have used different forms of type I collagen-based matrices for tissue engineering of cartilage. Human chondrocytes produce cartilage matrix when cultured on collagen microcarrier beads (Frondoza et al. 1996), and periosteal and bone marrow-derived cells differentiate into chondrocytes when cultured in a collagen hydrogel (Wakitani et al. 1994). It is difficult to directly compare results with the porous collagen sponges used herein, however, because the microarchitecture of some matrices is not well described, with some possibly being hydrogels. We directly compared collagen hydrogels with the porous collagen fiber lattices used in this study, and found that fibroblasts failed to migrate into and thrive in the hydrogel (Mizuno and Glowacki 1996). The polymerized collagen fiber lattice that provides a scaffold for cell adhesion and migration is not present in amorphous collagen hydrogels.
Woven lattices of synthetic polymers, such as poly(lactide-co-glycolide), poly(lactic acid) (Vacanti et al. 1991), poly(ɛ-caprolactone), and polyactide (Grande et al. 1997) have been used as scaffolds for cartilage tissue engineering. Biological materials used for scaffolds include alginate beads (Lee et al. 2003) and collagen hydrogels (Wakitani et al. 1994; Chaipinyo et al. 2002, 2004). Our results with 35S-sulfate incorporation by bACs cultured in porous 3D collagen scaffolds were consistent with matrix accumulation in other 3D scaffolds. Polylactic porous scaffolds allow cell migration and cartilage-specific matrix accumulation (Vacanti et al. 1991). Further, resorbable synthetic polymers such as polyglycolic acid enhance proteoglycan synthesis by chondrocytes (Grande et al. 1997). A drawback of polymer constructs, however, is the potentially large cell number and extended culture time necessary to seed the scaffolds in vitro (Nuttelman et al. 2001; Lee et al. 2004; Woodfield et al. 2004).
Our analyses did not show significant changes in DNA content in 3D cultures. That result is consistent with the understanding that chondrocytes are engaged either in cell cycle/proliferation processes or in activities related to differentiated function (Solursh and Meier 1974). Morphology of chondrocytes reflects to a great extent cellular activity. We previously reported that chondrocytes that were constrained to have a round shape were more active in matrix synthesis and less active in DNA synthesis (Glowacki et al. 1983). A scaffold’s characteristics can determine whether cells will maintain their native morphology (Boyan et al. 1996). Culture systems that use hydrogels such as collagen (Chaipinyo et al. 2002, 2004) and alginate beads (Lee et al. 2003) may permit proliferation of chondrocytes. Chondrocytes may retain their original spherical morphology within porous collagen sponges because of the high volumetric cell density and cell aggregation possible with in the pores of the lattice.
We found contrasting effects on chondrogenesis with different formulations of serum substitutes. ITS+3 supplement with linoleic and oleic acids negatively affected viability and chondrogenesis by bovine chondrocytes in porous 3D collagen sponges. Loss of matrix that had accumulated during the preculture period was striking. The apparently high levels of COL II and AGG expression in ITS+3 cultures indicated that the few remaining viable cells continued to exhibit the chondrocyte signature. Increased expression of cartilage phenotypic genes, without a concomitant increase in cell proliferation, was also found in fetal bovine chondrocytes that were cultured in ITS+ supplement with linoleic acid in fibrous poly(ɛ-caprolactone) scaffolds (Li et al. 2003). In contrast, both proliferation and matrix synthesis by porcine or equine chondrocytes cultured in alginate beads were stimulated by the same ITS formulation (Loredo et al. 1996). We found that induced chondrogenesis of human bone marrow mesenchymal cells is supported in medium containing ITS+1, which contains linoleic but not oleic acid (Zhou et al. 2004).
Repair methods that use autologous chondrocytes aim to transplant cells at high density. If cells are injected at a density of 30 million per ml (Brittberg et al. 2003), approximately 9 million chondrocytes are needed to treat an average defect of 0.3 cc (Chaipinyo et al. 2004). In this study, the density of bACs seeded onto porous collagen sponges was ~30 million/ml (5 million cells seeded onto a 150 mm3 sponge). Our results with Nutridoma serum replacement demonstrate the feasibility of manipulating culture conditions in vitro to enhance chondrogenesis and possibly decrease the number of cells needed to achieve repair.
The porous 3D collagen sponges maintain chondrocyte viability, shape, and synthetic activity by providing an environment favorable for high density chondrogenesis. Scaffolds that are adherent to cartilage would be advantageous when the cartilage defect lacks a peripheral margin or has other anatomical features that complicate injection of cells (Brittberg et al. 2003). With quantitative assays for cartilage-specific gene expression and biochemical measures of chondrogenesis in these studies, we conclude that the porous collagen sponges have potential as a scaffold for cartilage tissue engineering. Our data show that certain defined supplements can be effective replacement for serum. Enhanced maintenance of highly differentiated chondrocytes and their production of matrix may lead to improved cartilage constructs for potential tissue engineering applications.
Acknowledgments
The authors thank Shuichi Mizuno and Rebecca MacLean for assistance with experiments. This research was supported by NIH Grants AR 44873 and AR 45870 and by a fellowship to F.A. from Swiss National Science Foundation. This work was presented in part at the 22nd Annual Meeting of the American Society for Bone and Mineral Research.
References
- Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85A(Suppl 3):109–115. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- Chaipinyo K, Oakes BW, van Damme MP. Effects of growth factors on cell proliferation and matrix synthesis of low-density, primary bovine chondrocytes cultured in collagen i gels. J Orthop Res. 2002;20:1070–1078. doi: 10.1016/S0736-0266(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Chaipinyo K, Oakes BW, Van Damme MP. The use of debrided human articular cartilage for autologous chondrocyte implantation: Maintenance of chondrocyte differentiation and proliferation in type i collagen gels. J Orthop Res. 2004;22:446–455. doi: 10.1016/j.orthres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Frondoza C, Sohrabi A, Hungerford D. Human chondrocytes proliferate and produce matrix components in microcarrier suspension culture. Biomaterials. 1996;17:879–888. doi: 10.1016/0142-9612(96)83283-2. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Uporova T, Schmidt J, Strauss PG, Shih SD, Huang LF, Gundberg C, Mizuno S, Glowacki J. Osteogenic potential of murine osteosarcoma cells: comparison of bone-specific gene expression in in vitro and in vivo conditions. Lab Invest. 1996;74:895–906. [PubMed] [Google Scholar]
- Glowacki J, Trepman E, Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med. 1983;172:93–98. doi: 10.3181/00379727-172-41533. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Yates K.E., MacLean R. and Mizuno S. 2005. In vitro engineering of cartilage: Effects of serum substitutes, TGF-β, and IL-1α. Orthodont. Craniofac. Res. (In press). [DOI] [PubMed]
- Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85A(Suppl 2):25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- Hauselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, Kuettner KE, Thonar EJ. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107(Pt 1):17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- Hering TM, Kollar J, Huynh TD, Varelas JB, Sandell LJ. Modulation of extracellular matrix gene expression in bovine high-density chondrocyte cultures by ascorbic acid and enzymatic resuspension. Arch Biochem Biophys. 1994;314:90–98. doi: 10.1006/abbi.1994.1415. [DOI] [PubMed] [Google Scholar]
- Horwitz AL, Dorfman A. The growth of cartilage cells in soft agar and liquid suspension. J Cell Biol. 1970;45:434–438. doi: 10.1083/jcb.45.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomha NM, Lavoie G, Muldrew K, Schachar NS, McGann LE. Cryopreservation of intact human articular cartilage. J Orthop Res. 2002;20:1253–1255. doi: 10.1016/S0736-0266(02)00061-X. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Kubo T, Arai Y, Namie K, Takahashi K, Hojo T, Inoue S, Ueshima K, Shiga T, Yutani Y, Hirasawa Y. Time-sequential changes in biomechanical and morphological properties of articular cartilage in cryopreserved osteochondral allografting. J Orthop Sci. 2001;6:276–281. doi: 10.1007/s007760100047. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim YH, Park KD, Jee KS, Shin JW, Hahn SB. Importance of integrin beta1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials. 2004;25:1901–1909. doi: 10.1016/j.biomaterials.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Lee DA, Reisler T, Bader DL. Expansion of chondrocytes for tissue engineering in alginate beads enhances chondrocytic phenotype compared to conventional monolayer techniques. Acta Orthop Scand. 2003;74:6–15. doi: 10.1080/00016470310013581. [DOI] [PubMed] [Google Scholar]
- Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in threedimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- Loredo GA, MacDonald MH, Benton HP. Regulation of glycosaminoglycan metabolism by bone morphogenetic protein-2 in equine cartilage explant cultures. Am J Vet Res. 1996;57:554–559. [PubMed] [Google Scholar]
- Mizuno S, Allemann F, Glowacki J. Effects of medium perfusion on matrix production by bovine chondrocytes in three-dimensional collagen sponges. J Biomed Mater Res. 2001;56:368–375. doi: 10.1002/1097-4636(20010905)56:3<368::aid-jbm1105>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Glowacki J. Three-dimensional composite of demineralized bone powder and collagen for in vitro analysis of chondroinduction of human dermal fibroblasts. Biomaterials. 1996;17:1819–1825. doi: 10.1016/0142-9612(96)00041-5. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Tateishi T, Ushida T, Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193:319–327. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes nih3t3 cell adhesion, proliferation, and migration. J Biomed Mater Res. 2001;57:217–223. doi: 10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ohlendorf C, Tomford WW, Mankin HJ. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res. 1996;14:413–416. doi: 10.1002/jor.1100140311. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Evanko SP, Vogel KG. Mechanical loading and tgf-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–211. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Rymaszewski Z, Abplanalp WA, Cohen RM, Chomczynski P. Estimation of cellular DNA content in cell lysates suitable for rna isolation. Anal Biochem. 1990;188:91–96. doi: 10.1016/0003-2697(90)90532-e. [DOI] [PubMed] [Google Scholar]
- Sammarco VJ, Gorab R, Miller R, Brooks PJ. Human articular cartilage storage in cell culture medium: Guidelines for storage of fresh osteochondral allografts. Orthopedics. 1997;20:497–500. doi: 10.3928/0147-7447-19970601-04. [DOI] [PubMed] [Google Scholar]
- Skoog T, Ohlsen L, Sohn SA. Perichondrial potential for cartilagenous regeneration. Scand J Plast Reconstr Surg. 1972;6:123–125. doi: 10.3109/02844317209036711. [DOI] [PubMed] [Google Scholar]
- Solursh M, Meier S. Effects of cell density on the expression of differentiation by chick embryo chondrocytes. J Exp Zool. 1974;187:311–322. doi: 10.1002/jez.1401870302. [DOI] [PubMed] [Google Scholar]
- Upton J, Sohn SA, Glowacki J. Neocartilage derived from transplanted perichondrium: What is it? Plast Reconstr Surg. 1981;68:166–174. doi: 10.1097/00006534-198108000-00007. [DOI] [PubMed] [Google Scholar]
- Vacanti CA, Langer R, Schloo B, Vacanti JP. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plast Reconstr Surg. 1991;88:753–759. doi: 10.1097/00006534-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cellbased repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- Warden S, Zaleske DJ, Glowacki J. Fate of a chimeric joint construct in an ectopic site in scid mice. Cell Transplant. 2004;13:161–168. doi: 10.3727/000000004773301843. [DOI] [PubMed] [Google Scholar]
- Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- Woodfield TB, Malda J, De Wijn J, Peters F, Riesle J, Van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiberdeposition technique. Biomaterials. 2004;25:4149–4161. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Zaleske D, Peretti G, Allemann F, Strongin D, MacLean R, Yates KE, Glowacki J. Engineering a joint: A chimeric construct with bovine chondrocytes in a devitalized chick knee. Tissue Eng. 2003;9:949–956. doi: 10.1089/107632703322495592. [DOI] [PubMed] [Google Scholar]
- Zhou S, Yates KE, Glowacki J. Demineralized bone promotes chondrocyte or osteoblast differentiation of human marrow stromal cells cultured in collagen sponges. Cell and Tissue Banking. 2004;6:33–44. doi: 10.1007/s10561-005-4253-y. (This issue). [DOI] [PMC free article] [PubMed] [Google Scholar]


