Abstract
The development of the embryonic mammary gland involves communication between the epidermis and mesenchyme and is coordinated temporally and spatially by various signaling pathways. Although many more genes are likely to control mammary gland development, functional roles have been identified for Wnt, fibroblast growth factor, and parathyroid hormone-related protein signaling. This review describes what is known about the molecular mechanisms that regulate embryonic mammary gland development.
Introduction
Over the past several years excellent progress has been made in beginning to define the signaling pathways that are involved in the very earliest stages of mammary development. In this review we describe embryonic morphogenesis in general terms and review recent developments regarding the molecular signaling involved at each stage of fetal mammary development. Our discussion is limited to the mouse, which has become the experimental model of choice.
Overview of morphogenesis
For the purposes of discussion, embryonic mammary gland development can be divided into a series of specific developmental stages [1-3]. The initiating event is the formation of bilateral milk lines running between the fore and hind limbs on embryonic day (E) 10.5 in the mouse. Epidermal cells within the milk line become columnar and multilayered, defining a ridge that protrudes above and below the plane of the single-layered primitive epidermis or periderm.
The second stage occurs by E11.5, when five pairs of lens-shaped placodes form at specific locations along the mammary line. Placodes are thought to arise from the migration of cells within the mammary line, although this has yet to be formally documented. Individual placodes form in a characteristic sequence; pair 3 is first, followed by pairs 4, 1 and 5, and finally by pair 2.
The third stage involves the invagination of cells within the placode into the underlying mesenchyme to form the typical bulb-shaped mammary buds and occurs between E11.5 and E12.5 (Fig. 1). Part of this process involves the condensation and differentiation of the underlying mesenchyme into specialized, dense mammary mesenchyme arrayed radially around the epithelial bud. Differentiation of the dense mesenchyme is associated with expression of the androgen receptor, and in many mouse strains fetal androgens lead to the destruction of the mammary anlage in male embryos [2] (Fig. 1c). In female embryos the buds remain morphologically quiescent until the final stages of embryonic development begin at E15.5–E16.5. At this point, the mammary epithelial cells begin to proliferate, and the bud sprouts down out of the dense mesenchyme and into the developing mammary fat pad located within the dermis. Concurrent with this process, epidermal cells overlying the bud differentiate into nipple skin. Once the mammary sprout has reached the fat pad it begins a process of ductal branching morphogenesis that gives rise to the rudimentary ductal tree, consisting of a primary duct and 15–20 secondary branches, which is present at birth (Fig. 1d,1e).
Figure 1.
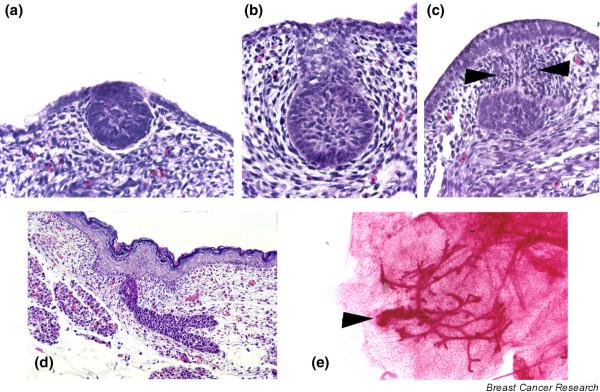
Embryonic mammary development. (a) Embryonic day (E)12.5. The epithelial cells have invaginated to form the initial bud, but the dense mammary mesenchyme has not yet formed. (b) Female bud at E14.5. The bud is fully formed. The epithelial cells are arrayed in a ball-on-stalk, or inverted bulb shape. The mesenchymal cells are arranged in four to five layers in a radial fashion around the epithelial cells. (c) Male bud at E14.5. Under the influence of testosterone, the mesenchymal cells condense around the stalk of the bud (arrowheads), constricting it until the connection with the surface epidermis is severed. After this occurs mammary mesenchyme cells and many epithelial cells undergo apoptosis. (d) Mammary sprout at E18.5. The epithelial bud has grown out from the mammary mesenchyme into the lower dermis, where it will enter the mammary fat pad and begin a period of active ductal branching morphogenesis. (e) A whole mount of the initial primary duct system from a 2-day-old mouse, the end-result of embryonic mammary morphogenesis. The arrowhead denotes the connection of the primary duct to the skin.
Specification of the milk line
Although the presence of the mammary line in mice had previously been questioned, recent studies have provided morphologic and molecular evidence of this structure [3-6]. Specification of the mammary line is dependant on canonical Wnt signaling [4]. One of the earliest described markers of the mammary line is the expression of a Wnt responsive β-galactosidase (TOPGAL) transgene in cells between the limb buds of E10.5 TOPGAL transgenic embryos. Following this, several Wnt genes become expressed within the mammary line between E11.25 and E11.5 (40–42 somite stage), including Wnt10b, Wnt10a, and Wnt6 [4-6]. Disruption of Wnt signaling within the developing epidermis through transgenic expression of the secreted Wnt inhibitor DKK1 has been shown to extinguish TOPGAL transgene expression and all evidence of the mammary line, including the expression of the Wnt genes mentioned above [4]. These findings suggest that specification of the mammary line requires an early Wnt signaling event that is then responsible for inducing a cascade of further Wnt gene expression and Wnt signaling within the milk line and placodes [4-6]. At this time it is not known which Wnt genes, receptors, or T cell factor family members are involved in this earliest specification of Wnt signaling. Chu and coworkers [4] demonstrated that several Wnts, including Wnt3, Wnt10b and Wnt6, are expressed at low levels throughout the epidermis at E10.5, qualifying them as candidates to mediate this function.
The fibroblast growth factor (FGF) signaling pathway also may contribute to mammary line specification. Knockout of the FGF10 and FGFR2b genes in mice has been shown to disrupt the formation of four out of the five mammary placodes (numbers 1, 2, 3, and 5) [7]. Between E10.5 and E11.5, Fgf10 is expressed in the most ventral-lateral reaches of the dermatomyotome of the somites adjacent to the developing mammary line [7]. FGFR2b is expressed within the mammary epithelial placodes, although it has not been identified specifically within the mammary line [7]. Eblaghie and colleagues [5] showed that another FGF receptor (FGFR1b) and four potential ligands, namely Fgf4, Fgf8, Fgf9, and Fgf17, are expressed within the mammary placodes. Unfortunately, those investigators did not report on the pattern of expression of these molecules at earlier time points during the formation of the mammary line. They did demonstrate that a chemical inhibitor of FGFR1 signaling inhibited the expression of the TBX3 gene (see below) in the mammary line and placodes in cultured embryos, although at the doses used in this study the inhibitor may not have been completely specific for this receptor. It also has been shown that inhibition of Wnt signaling does not alter expression of Fgf10 or FGFR1 signaling [4,5]. These data all suggest that FGF signaling is important to the earliest stages of mammary development and acts in parallel to Wnt signaling, rather than downstream of it.
Mammary gland aplasia or hypoplasia is a prominent feature of the mammary-ulnar syndrome, caused by mutations in the TBX3 gene, which encodes a T-box transcription factor [8,9]. TBX3-/- mice exhibit no morphologic evidence of mammary placodes and do not show evidence of Wnt10b or lymphoid enhancing factor (Lef)1 expression, two molecular markers of mammary placodes [9]. So, it is evident that TBX3 is important for placode formation. However, Eblaghie and coworkers [5] recently showed that TBX3 is expressed in the mammary line beginning at E10.25, raising the question of whether TBX3 might participate in the specification of the line itself. Consistent with this idea, TBX3 expression was induced by both FGF and Wnt signaling within the mammary line of cultured mouse embryos [5].
A working model that integrates these findings is shown in Fig. 2. Specification of the mammary line would be the result of FGF signals from the somite acting in concert with canonical Wnt signaling initiated by generally expressed Wnts in the ectoderm. This dual signal would activate TBX3 expression, which would in turn activate or amplify the expression of other Wnt and FGF pathway genes necessary for full mammary line development and the transition to placode formation. In this manner TBX3 would be both downstream and upstream of Wnt and FGF signaling, which is a known paradigm for T-box transcription factors.
Figure 2.
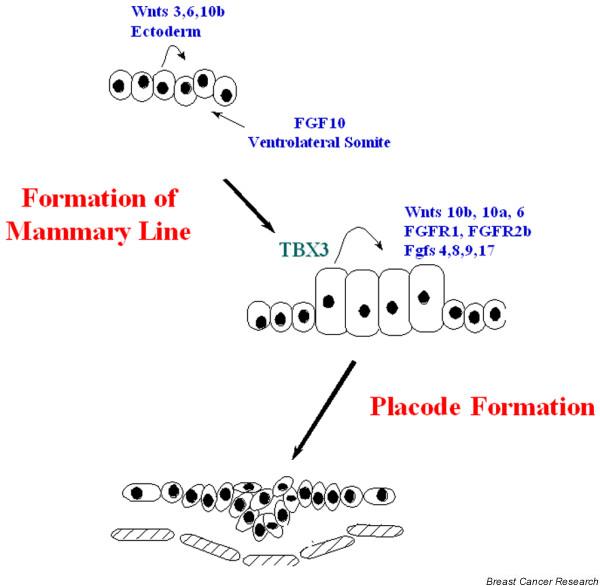
A model of mammary line specification and placode formation. Fibroblast growth factor (Fgf)10 from the ventrolateral portion of the somites acts in concert with Wnts expressed within the epidermis to specify the mammary line. This induces TBX3 expression, which in turn induces the expression of specific Wnts and Fgfs within the mammary line. These molecules act in an autocrine fashion and cooperate with other signaling pathways to form 10 pairs of placodes at specific locations along the original mammary line.
Placode formation
The same signaling pathways that have been implicated in the specification of the mammary line are also important for the development of the mammary placodes. TOPGAL transgene expression and Wnt10b expression have been reported to become discontinuous within the mammary line and localize to the forming placodes [4-6]. The placodes are thought to form from cell movements within the mammary line, and in TOPGAL embryos individual 'wandering' β-galactosidase positive cells can be seen clustering around the developing placodes [4]. In cultured embryos, activation of Wnt signaling using lithium chloride or Wnt3a results in the accelerated formation of enlarged mammary placodes [4]. Finally, Lef1-/- embryos form smaller placodes that then degenerate [3,10]. Wnt signaling is known to modulate cell adhesion and promote cell migration in other settings, and so it is attractive to speculate that Wnts might be involved in promoting the cell migration and invagination necessary for the formation of placodes.
As noted in the section above, the Fgf receptor FGFR2b is expressed within the developing mammary placodes, and disruption of this gene in mice inhibits the development of four pairs of placodes [7]. In addition, Fgf4, Fgf8, Fgf9, and Fgf17 are all expressed within the developing placodes, as is another Fgf receptor, FGFR1 [5]. In cultured embryos, beads soaked with Fgf8 have been shown to induce the ectopic expression of placodal markers when placed along the mammary line, and an FGFR1 inhibitor has been shown to inhibit the development of placodes from the mammary line [5]. Thus, it is likely that FGF signaling participates in regulating this process.
TBX3 and the related T-box family member TBX2 are both expressed at E11.5 in developing placodes [9]. As noted in the section above, mice lacking TBX3 fail to develop mammary placodes 1, 3, 4, and 5, and fail to express the placodal markers Wnt10b and Lef-1 [9]. Very little is known about the function of TBX2 in the mammary gland, but the phenotype of the TBX3 knockout mice suggests that TBX2 and TBX3 have nonoverlapping functions. Interestingly, TBX2 has been shown to regulate adhesion molecules such as cadherins and integrins [11], and so it is attractive to speculate that it may contribute to the migration and invagination of the mammary epithelial cells during placode formation.
Ectodysplasin (Eda) is a member of the tumor necrosis factor ligand superfamily [12]. Mice deficient in Eda (Tabby mice) or in its receptor (Edar) have defects in several epidermal appendages, including the mammary gland [13]. Eda is expressed in the underlying mesenchyme, whereas Edar is located in the epithelial cells of the mammary placode [12]. Transgenic mice overexpressing Eda-A in embryonic skin form enlarged and supernumerary mammary placodes along the mammary line [12]. These results suggest that Edar signaling promotes placode formation and/or directs the positioning of the placode along the mammary line. However, ectopic placodes only form along the mammary line, suggesting that the actions of Eda/Edar are downstream of the specification of this structure.
Bud formation
A growing number of signaling molecules have been described as being expressed within either the epithelial or mesenchymal cells of the mammary bud (Table 1). However, functional information exists for only a few of these signaling pathways. As with the previous stages, Wnt signaling appears to participate in the formation of the mammary buds. Wnt reporter (TOPGAL) gene expression remains induced in the epithelial cells of the mammary bud through to E15 [4]. In addition, many Wnt pathway genes are expressed within the mammary buds at E12.5 and E15 [4]. Lef1 is expressed in the mammary placode and bud at E11/12, and later, at around E14.5, it is expressed in the condensed mammary mesenchyme [14]. As noted in the section above, Lef1-/- mice form small placodes, which degenerate instead of going on to form mammary buds [3,10]. Whether this represents a failure of placode development or a block in the transition from placode to bud is not clear, because a detailed study of molecular markers of mammary development has not been done on these embryos. Furthermore, it is not clear whether the mammary defects in Lef1-/- mice are the result of the loss of Lef1 from epithelium or mesenchyme, or both.
Table 1.
Signaling molecules localized to mammary buds
| Epithelium | Mesenchyme |
| PTHrP | PTH1R |
| Wnts 10a, 10b, 6 | Eda-A1 |
| β-Catenin | Wnt11, Wnt5a |
| Axin2 | Lef1 |
| Lef1 | β-Catenin |
| Kremen 2 | Fgf7 |
| FGFR2b, FGFR1b | MSX2 |
| Fgfs 4,8,9,17 | TBX2 |
| Sonic Hedgehog, Indian Hedgehog | Hoxa9, Hoxb9, Hoxc9 |
| Gli2, Gli3, Ptc1 | BMP4 |
| BMP2 | ER-α, ER-β |
| Edar | Androgen receptor |
| MSX1, MSX2 | Pdgfr |
| Irx2 | Tenascin C |
| Lmx1b | |
| TBX3 | |
| PdgfA |
Edar, ectodysplasin receptor; FGF, fibroblast growth factor; Lef, lymphoid enhancing factor; Pdgfr, platelet-derived growth factor receptor; PTH1R, PTHrP receptor 1; PTHrP, parathyroid hormone related protein
The homeodomain-containing transcription factors MSX1 and MSX2 are both expressed in the mammary buds, and MSX2 is also expressed in the underlying mesenchyme [15,16]. Knockout of either MSX1 or MSX2 alone has no effects on mammary bud formation, although knockout of MSX2 does affect the next phase of mammary development. However, when both genes are disrupted placodes form but do not develop into mammary buds [16]. Thus, MSX1 and MSX2 appear to have necessary but redundant functions during the formation of the buds.
One of the molecules expressed by the mammary epithelial bud as it starts to invaginate into the mesenchyme is parathyroid hormone-related protein (PTHrP). Its receptor, PTH1R, is expressed in the mesenchyme underlying the developing bud [14,17]. If either PTHrP or the PTH1R is disrupted in mice, then morphologically normal mammary buds form but they degenerate and never grow out to form ductal trees [14,17]. This is because PTHrP is necessary for the mesenchyme to acquire a specialized mammary fate. When this does not occur, the mammary epithelial cells take on an epidermal fate, undergo squamous differentiation and morphogenesis fails. Another consequence is the loss of sexual dimorphism, because PTHrP is the epithelial factor that induces androgen receptor expression within the mammary mesenchyme [18]. PTHrP signaling is also necessary for the mammary mesenchyme to induce the overlying epidermis to form the nipple. Thus, in PTHrP and PTH1R knockout mice no nipples are formed, and when PTHrP is overexpressed in the epidermis the entire ventral surface of the embryo is transformed into nipple skin [14,17,18].
Rudimentary ductal tree
Although we know about several hormones and paracrine factors that regulate postnatal mammary ductal growth [19,20], we know very little about the mechanisms that are involved in the formation of the rudimentary ductal tree from the mammary buds. The initial branching morphogenesis of the embryonic mammary gland is hormone independent because mice that are deficient in either estrogen receptor (α or β), the prolactin receptor, the growth hormone receptor, or the progesterone receptor have no obvious embryonic mammary phenotype [19,20]. Likewise, the initial outgrowth of the bud occurs in the absence of growth factor receptors such as the insulin-like growth factor-1 receptor and the epidermal growth factor receptor, which are though to be important to the regulation of hormone dependant branching morphogenesis during puberty [20]. Four genetic models develop mammary buds but subsequently have defects in ductal outgrowth. These are PTHrP-/-, PTH1R-/-, MSX2-/- and RhoGAP p190B-/- mice [16,17,21]. In the case of PTHrP and its receptor, the failure of bud outgrowth is the result of defects in the mammary mesenchyme [14,17,18]. A similar mesenchymal defect might also hold for the MSX2-/- mice, because expression of this transcription factor is limited to the mesenchyme at this stage [15,16]. The mechanisms underlying the failure of transplanted RhoGAPp190B-/- buds to grow are currently under investigation [21]. At this point, we have very little idea as to what regulates the initial phase of ductal growth. This is an area ripe for investigation and one wonders whether the mechanisms at play here might shed light on the acquisition of hormone independent growth by breast cancers.
Conclusion
Embryonic mammary gland development requires the coordination of many signaling pathways to direct the cell shape changes, cell movements, and cell–cell interactions necessary for proper morphogenesis. Many of the processes that are necessary for development are recapitulated in breast cancer, especially in the metastatic cascade [22]. Although much progress has been made in the past several years, we remain in the early stages of our understanding of the specific molecular pathways that mediate the development of the embryonic gland. Our hope is that a better understanding of development will inform efforts to understand and eradicate metastatic breast cancer.
Abbreviations
E = embryonic day; Eda = ectodysplasin; FGF = fibroblast growth factor; Lef = lymphoid enhancing factor; PTHrP = parathyroid hormone-related protein.
Competing interests
The author(s) declare that they have no competing interests.
Note
This article is part of a review series on Key stages in mammary gland development, edited by Charles Streuli.
Other articles in the series can be found online at http://breast-cancer-research.com/articles/review-series.asp?series=bcr_keystages
Contributor Information
Julie R Hens, Email: julie.hens@yale.edu.
John J Wysolmerski, Email: john.wysolmerski@yale.edu.
References
- Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4:9–19. doi: 10.1023/A:1018748418447. [DOI] [PubMed] [Google Scholar]
- Sakakura T. Mammary embryogenesis. In: Neville MC, Daniel CW, editor. The Mammary Gland: Development, Regulation and Function. New York: Plenum; 1987. pp. 37–66. [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–29. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Song SJ, Kim JY, Akita K, Tickle C, Jung HS. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J Anat. 2004;205:1–13. doi: 10.1111/j.0021-8782.2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn. 2004;229:349–56. doi: 10.1002/dvdy.10441. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, Franceschini P, Lala R, Holmes LB, Gebuhr TC, Bruneau BG, Schinzel A, Seidman JG, Seidman CE, Jorde LB. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–5. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–73. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhong Q, Wang J, Cameron RS, Borke JL, Isales CM, Bollag RJ. Microarray analysis of Tbx2-directed gene expression: a possible role in osteogenesis. Mol Cell Endocrinol. 2001;177:43–54. doi: 10.1016/S0303-7207(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Mustonen T, Ilmonen M, Pummila M, Kangas AT, Laurikkala J, Jaatinen R, Pispa J, Gaide O, Schneider P, Thesleff I, Mikkola ML. Ectodysplasin A1 promotes placodal cell fate during early morphogenesis of ectodermal appendages. Development. 2004;131:4907–19. doi: 10.1242/dev.01377. [DOI] [PubMed] [Google Scholar]
- Sofaer JA. Aspects of the tabby-crinkled-downless syndrome. I. The development of tabby teeth. J Embryol Exp Morphol. 1969;22:181–205. [PubMed] [Google Scholar]
- Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, Dunbar M, Philbrick W, Wysolmerski J. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001;128:513–25. doi: 10.1242/dev.128.4.513. [DOI] [PubMed] [Google Scholar]
- Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, Woo I, Roberts-Clark D, Francis-West PH, Liu YH, Maxson R, Hill RE, Dale TC. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–37. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ, Philbrick WM, Dunbar ME, Lanske B, Kronenberg H, Broadus AE. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development. 1998;125:1285–94. doi: 10.1242/dev.125.7.1285. [DOI] [PubMed] [Google Scholar]
- Dunbar ME, Dann PR, Robinson GW, Hennighausen L, Zhang JP, Wysolmerski JJ. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126:3485–93. doi: 10.1242/dev.126.16.3485. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–75. doi: 10.1016/S1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/A:1015766322258. [DOI] [PubMed] [Google Scholar]
- Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen JM. p190-B RhoGAP regulates mammary ductal morphogenesis. Mol Endocrinol. 2003;17:1054–1065. doi: 10.1210/me.2002-0428. [DOI] [PubMed] [Google Scholar]
- Colletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Muller-Tidow C, Golub TR, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]


