Abstract
Inner ear hair cells have been suggested as attractors for growing afferent fibers, possibly through the release of the neurotrophin brain-derived neurotrophic factor (BDNF). Atoh1 null mice never fully differentiate hair cells and supporting cells and, therefore, may show aberrations in the growth and/or retention of their innervation. We investigated the distribution of cells positive for Atoh1- or Bdnf-mediated β-galactosidase expression in Atoh1 null and Atoh1 heterozygotic mice and correlated the distribution of these cells with their innervation. Embryonic day (E) 18.5 Atoh1 null and heterozygotic littermates show Atoh1- and BDNF-β-galactosidase–positive cells in comparable distributions in the canal cristae and the cochlea apex. Atoh1-β-galactosidase–positive but only occasional Bdnf-β-galactosidase–positive cells are found in the utricle, saccule, and cochlea base of Atoh1 null mutant mice. Absence of Bdnf-β-galactosidase expression in the utricle and saccule of Atoh1 null mice is first noted at E12.5, a time when Atoh1-β-galactosidase expression is also first detected in these epithelia. These data suggest that expression of Bdnf is dependent on ATOH1 protein in some but does not require ATOH1 protein in other inner ear cells. Overall, the undifferentiated Atoh1- and Bdnf-β-galactosidase–positive cells show a distribution reminiscent of that in the six sensory epithelia in control mice, suggesting that ear patterning processes can form discrete patches of Atoh1 and Bdnf expression in the absence of ATOH1 protein. The almost normal growth of afferent and efferent fibers in younger embryos suggests that neither fully differentiated hair cells nor BDNF are necessary for the initial targeted growth of fibers. E18.5 Atoh1 null mice have many afferent fibers to the apex of the cochlea, the anterior and the posterior crista, all areas with numerous Bdnf-β-galactosidase–positive cells. Few fibers remain to the saccule, utricle, and the base of the cochlea, all areas with few or no Bdnf-β-galactosidase–positive cells. Thus, retention of fibers is possible with BDNF, even in the absence of differentiated hair cells.
Keywords: hair cells, sensory neurons, inner ear, neurotrophins, guidance cues, cell death
INTRODUCTION
Hair cells of the inner ear have long been hypothesized to attract growing inner ear afferents by secreting neurotrophins (Cajal, 1919; Lorente de No, 1926; Van de Water, 1983). Recent experimental work has demonstrated that neurotrophins can attract afferent fiber growth in vitro (Ming et al., 2002) and in vivo (Tucker et al., 2001) in other sensory systems. In the inner ear, only two neurotrophins are expressed: Bdnf and neurotrophin 3 (Ntf3; Pirvola et al., 1992; Farinas et al., 2001). Mice lacking one of these neurotrophins have some initial fiber growth toward the sensory epithelia. But the afferents eventually die off in a neurotrophin-specific pattern soon after they reach the sensory epithelia (Fritzsch et al., 2004). All inner ear afferents are lost in double mutations of the two inner ear-expressed neurotrophins: Bdnf and Ntf3 (Ernfors et al., 1995; Fritzsch et al., 2004). It has been shown that all hair cells express Bdnf, whereas embryonic supporting cells express Ntf3 (Pirvola et al., 1992; Farinas et al., 2001). Misexpression of Bdnf in the ear can redirect afferent growth, suggesting that BDNF acts in late embryonic ears as a tropic factor for inner ear afferents (Tessarollo et al., 2004). Nevertheless, some initial fiber growth to sensory epithelia occurs even in the absence of BDNF, provided the death of sensory neurons is prevented through elimination of the cell death promoting factor Bax (Hellard et al., 2004). Some in vitro data suggest that afferent growth to sensory epithelia happens even in the absence of all hair cells (Sobkowicz, 1992), whereas other data show that hair cells can attract afferent fibers but that this action is not mediated by neurotrophins (Bianchi and Cohan, 1993). These in vitro data are somewhat compromised, as neurotrophins in the serum could provide survival factors and the approach allows only testing of two-dimensional growth. In the vestibular system, recent in vivo data suggest that directed growth of afferents can occur even in the complete absence of entire epithelia (Pauley et al., 2003), suggesting a function of the no-sensory epithelium in guiding fibers. Such a role has been attributed recently to semaphorins, as afferent fibers are found to bypass otherwise normal sensory epithelia in semaphorin 3a mice (Gu et al., 2003). Overall, these data support the prominent role of BDNF in attracting afferent fibers in late embryos, but do not fully address the issue of BDNF-independent hair cell attraction. Residual Bdnf expression in developing hair cells (Xiang et al., 2003) or the possible expression of Ntf3 in the area of nondifferentiating sensory epithelia (Farinas et al., 2001; Pauley et al., 2003) compromises the interpretation gained by these approaches. Specifically, none of the above-cited studies has analyzed the residual attraction of sensory epithelia primordia in the absence of both hair cells and BDNF. Such absence might unmask additional attraction mediated by the forming sensory epithelia and cues provided by the nonsensory part of the ear the fibers grow along. In addition, afferents are rapidly lost in the complete absence of a sensory epithelium (Pauley et al., 2003), but long-term retention of some afferents in mice with a late embryonic loss of hair cells has been reported (Xiang et al., 2003).
The recent finding that Atoh1 (formerly Math1) is an essential basic helix–loop– helix (bHLH) gene for hair cell development (Bermingham et al., 1999; Chen et al., 2002) allows investigating the effect of a complete absence of all hair cells in all six inner ear sensory epithelia on both the specific growth and the differential retention of auditory and vestibular sensory neuron afferents. Specifically, Atoh1 null mice show limited expression of Atoh1-β-galactosidase reaction product in otherwise uncharacterized cells (Bermingham et al., 1999; Ben-Arie et al., 2000). However, no recognizable hair cells ever form in the Atoh1 null mice. Furthermore, Atoh1 expression alone is able to convert undifferentiated cells or supporting cells into hair cells (Gao, 2003; Kawamoto et al., 2003). Together these findings support the notion that Atoh1 is both necessary and sufficient to initiate hair cell differentiation. We should be able to analyze, therefore, outgrowth and retention of afferent fibers in the absence of hair cells by studying Atoh1 null mice.
In addition, because the neurotrophin BDNF is primarily expressed in hair cells in embryos older than E16.5 days (Pirvola et al., 1992; Farinas et al., 2001), we expect to be able to analyze fiber growth in the absence of BDNF as well. Supporting cell and hair cell differentiation are closely linked by means of the delta-notch system (Zine et al., 2001; Daudet and Lewis, 2005). Therefore, absence of differentiated hair cells could also affect supporting cell differentiation and their ability to synthesize the second neurotrophin known to be expressed in the ear, Ntf3 (Pirvola et al., 1992; Farinas et al., 2001). Indeed, this idea recently gained support from in vitro data, which showed that no specific supporting cell markers develop in Atoh1 null mice (Woods et al., 2004). Moreover, this study suggested that ATOH1 is not only essential for hair cell formation but that it also regulates formation of sensory epithelia. Therefore, Atoh1 null mice might not only lack hair cells but also fail to express either of the two known neurotrophins that mediate embryonic inner ear afferent survival (Fritzsch et al., 2004). Atoh1 null mice, therefore, could permit studying initial fiber growth and survival in the absence of any neurotrophin release from the sensory epithelia, including any other hair cell or sensory epithelium mediated attraction, an issue that has not been studied in mice devoid of both Bdnf and Ntf3. The possibly complete absence of all neurotrophins and hair cell attraction could allow investigating the role of nonsensory guidance cues, such as Sema3a (Gu et al., 2003) in directed afferent fiber growth.
Contrary to our expectation, we report in Atoh1 null mice a cellular expression of both Bdnf-β-galactosidase and Atoh1-β-galactosidase in discrete patches identical to the sensory epithelium distribution in Atoh1 heterozygotic littermates. This finding demonstrates that patterning processes in the ear up-regulate these genes in the absence of ATOH1 protein. We also show that the initial afferent fiber growth is almost normal in Atoh1 null mice but is then rapidly lost in a unique pattern that does not follow those described in single neurotrophin null mice. Together these data demonstrate that neither differentiated hair cells nor BDNF are essential for growth of afferent fibers in the ear but are necessary for fiber retention.
RESULTS
Atoh1-LacZ Expression in Older Embryos
β-Galactosidase staining due to the expression of the LacZ reporter gene in the Atoh1-LacZ allele reproduces faithfully the endogenous Atoh1 expression pattern (Bermingham et al., 1999). Mice homozygous for Atoh1-LacZ allele lack the ATOH1 protein and die at birth due to respiratory failure (Ben-Arie et al., 1997), thus limiting our analysis to embryonic stages. The distribution of these β-galactosidase–stained cells in Atoh1-LacZ null mice followed very closely that of Atoh1-β-galactosidase–stained hair cells in Atoh1-LacZ heterozygotic littermates (Fig. 1). We could identify six discrete patches, each situated in a topgraphically comparable position to the six known sensory epithelia of the mouse ear (three cristae, utricle, saccule, organ of Corti) inside the various recesses or ampullae of the inner ear (Fig. 1). β-galactosidase–stained areas in the vestibular part of the ear were dramatically smaller in the Atoh1 null embryos compared with the five vestibular sensory epithelia of heterozygote littermates (Fig. 1) Only a very small area of Atoh1-β-galactosidase–stained cells was found in the horizontal crista of such mice (Fig. 1B,D).
Fig. 1.
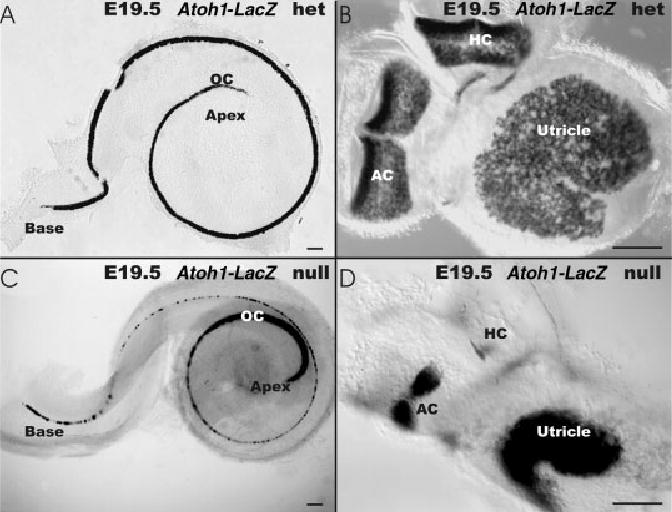
A–E: The distribution of β-galactosidase reaction product is shown in an Atoh1-LacZ heterozygotic embryonic day (E) 19.5 littermate (A,B) and an Atoh1-LacZ null mice (C,D). A,B: In the Atoh1-LacZ heterozygote, β-galactosidase stain is present in all hair cells of the organ of Corti of the cochlea (OC in A) and vestibular (B) epithelia. A: In the cochlea, the apex displays the weakest staining. B,D: In the Atoh1-LacZ null mice, the vestibular sensory epithelia are reduced in size, but the β-galactosidase staining (D) shows striking similarities in distribution and shape of expression compared with the Atoh1-LacZ heterozygotic littermates (B,D). For example, the two hemicristae of the anterior crista (AC) with the hair cell free cruciate eminence are obvious in both the mouse (D) and the heterozygotic littermate (B). D: No cruciate eminence exists in the horizontal crista (HC) and less β-galactosidase staining exists in this epithelium in the Atoh1-LacZ null mice. B,D: The utricle (U) shows the same kidney shape but is much smaller in the Atoh1-LacZ null mice (B, D). C: In the cochlea of the Atoh1-LacZ null mice, β-galactosidase staining is present along the entire length of the organ of Corti, with the most prominent staining being in the apical turn and at the basal tip. A: Comparison with the heterozygotic Atoh1-LacZ cochlea indicates that expression in the apical turn is actually more pronounced in the Atoh1-LacZ null littermate. Scale bars = 100 μm in A–D.
In the cochlea, all hair cells of the organ of Corti were Atoh1-β-galactosidase–stained (OC; Fig. 1A). In Atoh1-LacZ null mice, an area topographically equivalent to the organ of Corti showed Atoh1-β-galactosidase–stained undifferentiated cells (OC; Fig. 1C). Although overall similar, the detailed distribution of stained cells showed differences between the Atoh1-LacZ heterozygote and homozygote animals. In the heterozygote littermates, the hair cells near the apex showed little β-galactosidase staining, suggesting a delayed up-regulation of Atoh1-LacZ in this region (Fig. 1A). In contrast, the Atoh1-LacZ null mice showed the strongest and widest β-galactosidase staining in this area (Fig. 1C). This prominent staining pattern diminished to a single, interrupted line of Atoh1-β-galactosidase–positive cells in the upper middle turn of Atoh1-LacZ null mice. In the basal tip of the hook region, a continuous single row of cells showed strong β-galactosidase staining in Atoh1-LacZ null mice (Figs. 1C, 2B), whereas Atoh1-LacZ heterozygotic mice showed a single row of inner and three rows of out hair cells (Figs. 1A, 2A). We found a comparable cellular distribution in ears of E16.5, E17.5, and E18.5 old embryos, suggesting that this pattern is stable over this time interval. These data suggest that maintenance of Atoh1-LacZ expression occurs in a topographically and cellular restricted manner comparable to the wild-type organ of Corti and vestibular sensory epithelia in the absence of ATOH1 protein, suggesting that Atoh1 protein is not required to specify sensory epithelia or the location of Atoh1-β-galactosidase–stained cells.
Fig. 2.
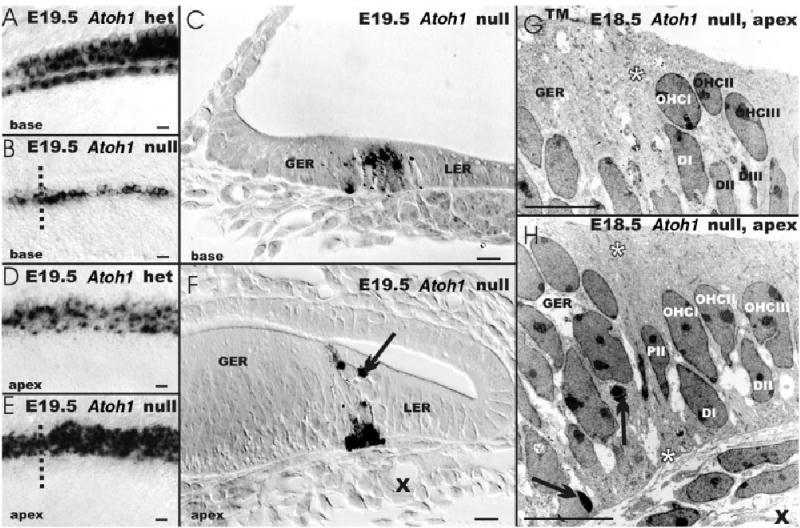
A–H: The distribution of β-galactosidase staining is shown in an Atoh1-LacZ heterozygotic animal (A,D) and its embryonic day (E) 19.5 Atoh1-LacZ null littermate (B,C,E–H). A,B: These whole-mounts show expression in four rows of hair cells in the base of an Atoh1-LacZ heterozygote (A) but only in one to three rows of cells in the Atoh1-LacZ null mouse (B). In the apex, there is no clear distinction between the Atoh1-LacZ heterozygotic and null mouse with respect to density and distribution of β-galactosidase–positive cells. D,E: If anything, the Atoh1-LacZ null mouse shows more rows of positive cells with a denser β-galactosidase staining. C,F: Sections (indicated by the dotted line in B,E) show that β-galactosidase–positive cells (C,F) are located at the intersection of the greater and lesser epithelial ridge (GER, LER). In the more differentiated basal turn, the cochlear epithelium is reduced to a single layer of columnar epithelium with Atoh1-β-galactosidase–positive cells extending through the entire epithelium. F: In contrast, the apex shows a layer of β-galactosidase–positive cells on the basilar membrane as well as β-galactosidase–positive cells near the lumen; these cells are modiolar to the spiral vessel (X). This position of these cells in Atoh1 null mice is identical to hair cells in wild-type or heterozygotic littermates. The transmission electron microscopy images show that the GER is multistratified. G,H: It is separated by two cells that extend through the entire epithelium (PII and asterisks) from a bistratified part of the epithelium. Typically three luminal cells are present in this bistratified area in radial sections (named OHCI, OHCII, OHCIII), which separated from the basilar membrane by three cells (named DI, DII, DIII). F–H: Arrows indicate apoptotic cells; Xs indicate the spiral capillary in F,H. Scale bars = 10 μm in A–H.
We next investigated whether any indication of the differentiation of hair cells could be found in the Atoh1-LacZ null mice. Epoxy resin sections of Atoh1-LacZ null mice showed that the β-galactosidase–stained cells were in the apex of the cochlea on the basilar membrane and near the lumen; the latter is a position typically occupied by hair cells (Fig. 2F). In the basal turn, the β-galactosidase–stained cells appeared to be concentrated near the basilar membrane (Fig. 2C) and formed a column at the boundary of the greater epithelial ridge (GER) and lesser epithelial ridge (LER; Fig. 2F). Apoptotic β-galactosidase–positive cells were found occasionally. The topology (near the spiral artery, between the GER and the single-cell layer of the LER) and distribution (near the lumen) of β-galactosidase–stained cells in the apex of the cochlea were consistent with those cells representing an undifferentiated organ of Corti (Fig. 2A–F).
We next analyzed the cellular appearance using transmission electron microscopy (TEM). For this analysis, we focused on the upper middle turn. Our TEM data showed some apoptotic cells near the basilar membrane and others being phagocytosed by cells of the GER (arrow; Fig. 2H). In addition, near the leading edge of the tectorial membrane at the transition from the multi-stratified GER to the single row of columnar cells of the LER, we found a small area that had a bistratified cellular distribution. Of interest, the bistratified cells were separated by a pair of cells that extended the full height of the epithelium in a manner reminiscent of pillar cells (asterisks in Fig. 2G,H). Toward the LER, we occasionally found cells that resembled in shape and distribution outer hair cells (Fig. 2G,H). Occasional nerve fibers were found passing through the habenula perforate, but no specializations at or near cells were detected. The vestibular epithelia showed Atoh1-β-galactosidase–stained cells in the more luminal layers, which are suggestive of hair cell primordia. Overall, the distribution of Atoh1-β-galactosidase–stained cells in whole-mounts, thick plastic sections, and TEM in Atoh1-LacZ null and heterozygote littermates suggests that these stained cells are topographically distributed like hair cells and form patches of undifferentiated Atoh-1– expressing cells in areas equivalent to the five vestibular sensory epithelia (utricle, saccule, and three cristae) and the organ of Corti of wild-type and heterozygotic littermates (Figs. 1, 2).
Bdnf-β-Galactosidase Expression in Older Embryos
Previous work had shown that none of the known hair cell markers ever develop in Atoh1 null mice (Bermingham et al., 1999; Chen et al., 2002). More recent work suggested that absence of Atoh1 “results in complete disruption of formation of the sensory epithelium of the cochlea, including the development of both hair cells and associated supporting cells” (Woods et al., 2004). In the inner ear, the neurotrophin Bdnf is expressed predominantly in hair cells in embryos of E16.5 or older, but also in other cells of the cruciate eminence of the cristae or the apical tip of the organ of Corti (Farinas et al., 2001; Fritzsch et al., 2002). We, therefore, next studied the expression of Bdnf-LacZ in Atoh1 null and control mice, using the Atoh1-Hprt/Bdnf-LacZ compound mice.
At E18.5, all hair cells of Atoh1-Hprt/Bdnf-LacZ double heterozygotes are Bdnf-β-galactosidase–stained in a pattern reminiscent of Atoh1-β-galactosidase staining in Atoh1-LacZ heterozygotic animals (Figs. 1A,B, 3A,B). In the Atoh1-Hprt null/Bdnf-LacZ heterozygotic mice, there was a distinct cellular expression of Bdnf-β-galactosidase in areas identifiable as undifferentiated sensory epithelia of the inner ear (Fig. 3C,D). The overall distribution of Bdnf-β-galactosidase in Atoh1-Hprt null mice and of Atoh1 β-galactosidase–positive cells in Atoh1-LacZ null mice was similar in several epithelia (Figs. 1C,D, 3C,D).
Fig. 3.
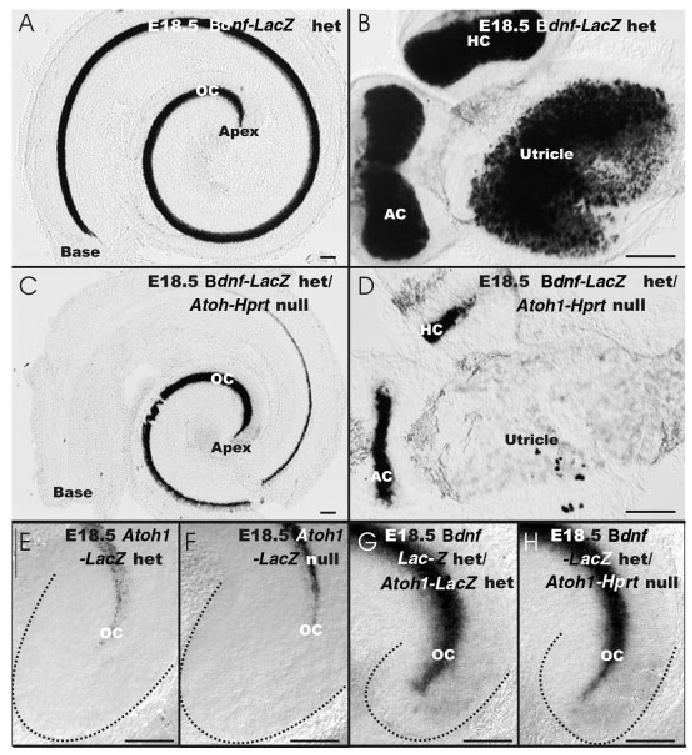
A–D,G,H: The distribution of brain-derived neurotrophic factor (Bdnf) -β-galactosidase staining is shown in a embryonic day (E) 18.5 wild-type littermate (A,B), Atoh1-Hprt null mouse (C,D,H) and Atoh1-LacZ/Bdnf-LacZ double heterozygote (G). E,F: In addition, the distribution of Atoh1-β-galactosidase staining is shown in the apex of Atoh1-LacZ heterozygote and wild-type littermate. A,B: In the wild-type animal, Bdnf-β-galactosidase staining is present in all hair cells of the organ of Corti (OC) of the cochlea (A) and vestibular epithelia (B). D: The presence of Bdnf-β-galactosidase staining in Atoh1-Hprt null mice is restricted to the cristae epithelia which are much reduced in size. B,D: The utricle of the Atoh1-Hprt null mouse shows only a few scattered Bdnf-β-galactosidase–positive cells, indicating a severe reduction of Bdnf-LacZ expression compared with the Atoh1 wild-type. C: In the organ of Corti, Bdnf-β-galactosidase staining is most prominent in the apex, falls off toward the middle turn, and is absent in the base. E,F: Comparisons of Atoh1-β-galactosidase staining in Atoh1-LacZ null and heterozygotes shows comparable extension of β-galactosidase–positive cells toward the tip of the cochlea (dotted lines). E–H: Comparison of β-galactosidase staining in compound Bdnf-LacZ/Atoh1-Lacz and Bdnf-LacZ het/Atoh1-Hprt null mice show a more apical staining than Atoh1-LacZ expression. G,H: No additional staining is found that could be related to Atoh1-LacZ in the Bdnf-LacZ double heterozygotic case, suggesting that all Atoh1-LacZ– expressing cells are also Bdnf-β-galactosi-dase–positive. AC, anterior crista; HC, horizontal crista. Scale bars = 100 μm in A–H.
This similarity was particularly striking in the apex of the cochlea, as noted by a strong Bdnf-β-galactosidase staining in cells. However, while Atoh1-β-galactosidase showed a reduced staining in the basal turn (Figs. 1C,D, 2B), no Bdnf-β-galactosidase staining was found in the basal turn of the Atoh1-Hprt null mice (Fig. 3C). In the vestibular system of Atoh1-Hprt null mice, the level of Bdnf-β-galactosidase stain was most pronounced in the anterior and posterior cristae (Fig. 3D). Bdnf-β-galactosidase staining was more extensive than the Atoh1-β-galactosidase staining observed in Atoh1-LacZ null mice (Figs. 1D, 3D). No Bdnf-β-galactosidase staining was found in the saccule of the Atoh1-Hprt null mice, while the utricle showed only a few scattered Bdnf-β-galactosidase–stained cells (Fig. 3D). We also studied the expression of Bdnf-β-galactosidase in Atoh1-LacZ heterozygotic and null mice. In these animals,the distribution of β-galactosidase–positive cells was indistinguishable from that of Atoh1-β-galactosidase stain alone, except for the cells of the cruciate eminence and the apical tip of the organ of Corti, which stained positive for Bdnf-β-galactosidase on any tested genetic background (Fig. 3E–H).
These E18.5 expression data suggest that, in the apex of the cochlea and the three cristae, Bdnf-LacZ expression does not require ATOH1 protein. However, in the saccule, utricle, and basal turn of the cochlea, Bdnf expression is apparently dependent on ATOH1 protein, either for initiation or maintenance. To differentiate between these two possibilities, we compared the distribution of Atoh1-β-galactosidase and Bdnf-β-galactosidase in younger embryos.
Atoh1-LacZ and Bdnf-LacZ Expression in Younger Embryos
At E12.5, the sensory epithelia of all cristae were Bdnf-β-galactosidase–stained in wild-type and Atoh1-Hprt null mice (Fig. 4A,B). Bdnf-β-galactosidase–stained cells were also visible in the utricle and saccule of Atoh1-Hprt wild-type animals, apparently in the future striolar region (Fig. 4A). No Bdnf-β-galactosidase–stained cells were found in the saccule, and only very few scattered and faintly labeled cells were observed in the utricle of Atoh1-Hprt null embryos (Fig. 4B). However, Bdnf-β-galactosidase–stained cells in the null mice were found only in the canal epithelia and the apex of the cochlea. In addition, cells delaminating from the utricle, saccule, and cochlea were Bdnf-β-galactosidase–positive in wild-type mice (Fig. 4). Because hair cells do not delaminate from the ear, we interpret these cells as vestibular and cochlear sensory neurons of the ear (Vgl; Fig. 4), in agreement with previous suggestions (Farinas et al., 2001; Fritzsch et al., 2002).
Fig. 4.
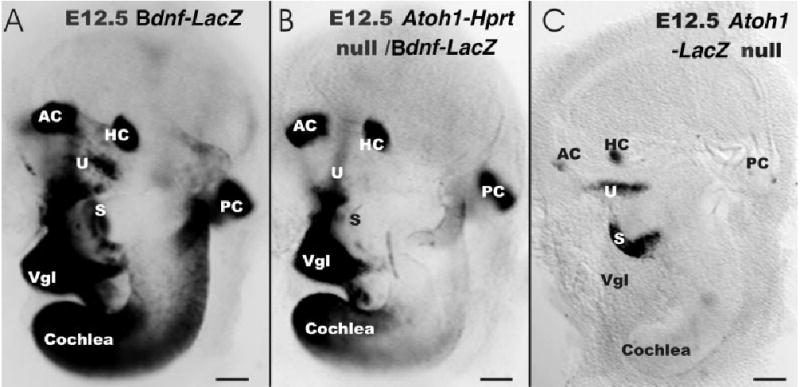
A–C: The expression of brain-derived neurotrophic factor (Bdnf; A,B) and Atoh1 (C) is shown in embryonic day (E) 12.5 wild-type (A) and Atoh1 null (B,C) mice, using the β-galactosidase histochemistry. A: Bdnf-β-galactosidase is present in all sensory epithelia in cells near the lumen and in delaminating neurons (Vgl, vestibular ganglion). B,C: In contrast, no Bdnf-β-galactosidase positive cells are found in the saccule (S) of Atoh1-Hprt null mice and only a few scattered, faintly labeled cells are observed in the utricle (U). All canal cristae and the apex of the cochlea show similar Bdnf-β-galactosidase staining, albeit reduced in overall intensity in the Atoh1-Hprt null mice. C: Atoh1-LacZ appears to be expressed at or before E12.5 in the saccule, utricle, and canal cristae. Absence of Atoh1-LacZ expression in the cochlea suggests that Bdnf-β-galactosidase staining is independent of Atoh1. B: The continued presence of Bdnf in canal cristae of Atoh1-Hprt null mice suggests that this Bdnf expression does not depend on ATOH1 protein. AC, anterior crista; HC, horizontal crista; PC, posterior crista. Scale bars = 100 μm in A–C.
We next studied the distribution of Atoh1-LacZ expression using the β-galactosidase staining at E12.5. These data showed expression of Atoh1-LacZ in the canal cristae, the utricle, and the saccule in a pattern comparable to the expression of Bdnf-LacZ in wild-type and Atoh1-Hprt null littermates (Fig. 4A–C). Combined with the data from E18.5 embryos (Fig. 3), these results suggest that Bdnf-LacZ expression in the canal cristae is independent of ATOH1 protein. In contrast, the expression of Bdnf-LacZ in the utricle, saccule, and basal turn of the cochlea depends on ATOH1 protein, as no Bdnf-β-galactosidase staining is ever found in these epithelia in Atoh1 null mice (Figs. 3D, 4B).
Tract Tracing With Lipophilic Dyes
The limited expression of Bdnf-LacZ in hair cell and sensory neuron precursors of E12.5 Atoh1 null embryos (Fig. 4B) could possibly provide some guidance cues as well as neurotrophic support for afferent nerve fiber growth in early embryos. Consistent with this assumption, our tract tracing results showed no differences in fiber growth at E11.5 (data not shown). At E12.5, fiber growth to the vestibular sensory epithelia was somewhat diminished (Fig. 5A,B). This was particularly obvious for the fiber growth to the posterior canal crista. Instead of radiating into the sensory epithelia (Fig. 5C), afferent fibers formed short branches off from the nerve to the posterior crista before it reached the posterior canal sensory epithelium (Fig. 5D). Only a few nerve fibers actually entered into the sensory epithelium (Fig. 5D). The effects of Atoh1 null mutation, therefore, emerge around E12.5, the earliest time point Atoh1 expression can be visualized using the LacZ marker (Fig. 4C).
Fig. 5.
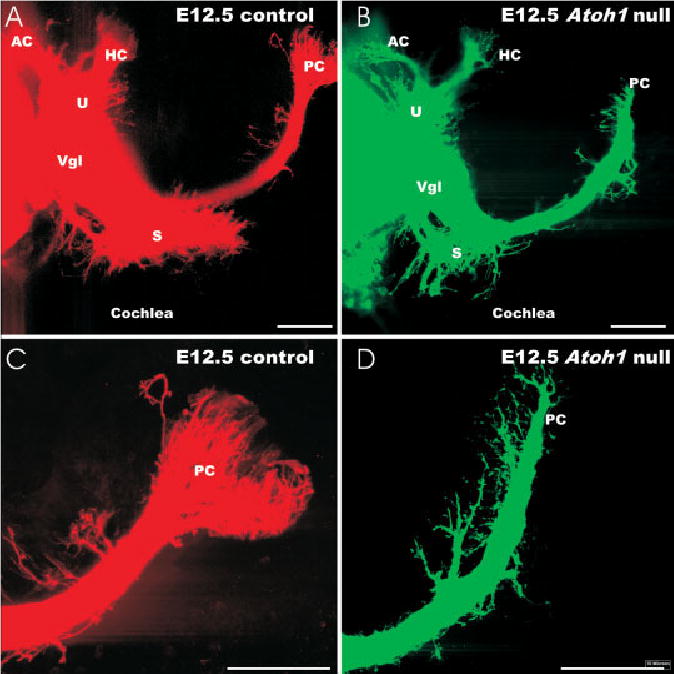
The projection of afferents in embryonic day (E) 12.5 Atoh1 wild-type and Atoh1-Hprt null mice is shown in these confocal images. A,B: Atoh1 null mice show fewer fibers to all vestibular epithelia. C,D: Higher magnifications of the posterior crista (PC) innervation shows that fewer fibers actually reach into the crista epithelium. D: Instead, in the Atoh1 null mice there are more fibers that branch off the main posterior crista nerve. AC, anterior crista; HC, horizontal crista; S, saccule; Vgl, vestibular ganglion; U, utricle. Scale bars = 100 μm in A–D.
In contrast to these early stages, older Atoh1 null embryos could be identified easily by their reduced ear innervation (Fig. 6A,B). Only a few afferents were present in the vestibular sensory epithelia at embryonic day 16.5 or 18.5 (Fig. 6D), the oldest stage studied here. Of interest, all vestibular sensory epithelia did receive some afferent innervation. The innervation density appeared highest in the posterior and anterior canal crista and the utricle and was reduced to individual fibers in the saccule and horizontal crista (Figs. 6D, 7G). The cochlea showed labeled spiral sensory neurons and afferent fibers extending radially toward the undifferentiated sensory epithelium (Figs. 6B, 7C). Compared with wild-type littermates, Atoh1 null animals exhibited a severe reduction of radial fibers in the basal turn (Fig. 6B). The very basal tip displayed a comparatively dense innervation pattern that was reduced to only an occasional radial fiber in the upper middle turn (Fig. 6B). In contrast, the apex showed an increasingly denser spacing of radial fibers and a dense packing of spiral sensory neurons (Fig. 7D). Higher magnifications revealed that afferent fibers did reach the undifferentiated area topographically equivalent to the organ of Corti (Fig. 7C) or the vestibular sensory epithelia (Fig. 7G). In addition, as fibers passed through the habenula perforate, they tended to branch, forming short terminals at or near cells of the organ of Corti (Fig. 7C). As pointed out earlier in this study, these few fibers were only rarely detected in the TEM analysis and nothing equivalent to a terminal was found.
Fig. 6.
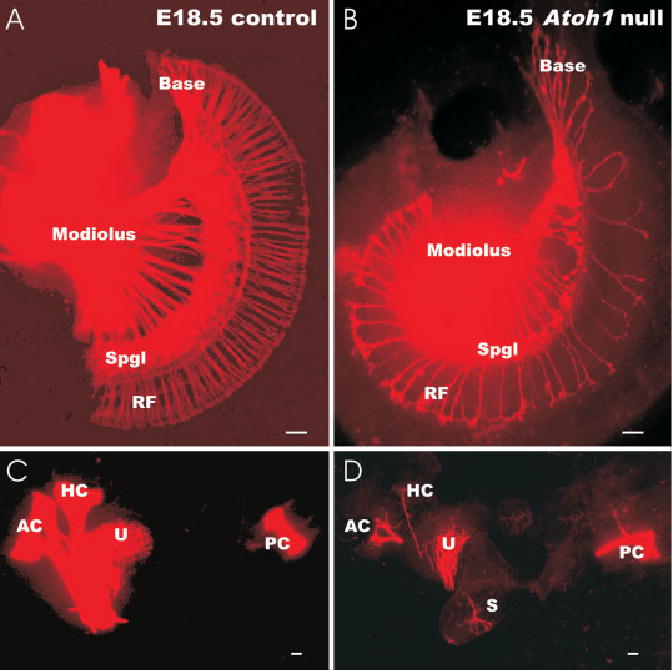
A–D: At embryonic day (E) 18.5, substantial differences exist between control litter-mates (A,C) and Atoh1-Hptr null mice (B,D) for afferent fiber innervation of epithelia. A,B: In the Atoh1 null mouse cochlea, only few spiral ganglion neurons (Spgl) are found in the basal turn. Likewise, many fewer radial fibers (RF) are present in the basal turn of the null mouse. Radial fibers extend in both Atoh1 null mice and control animals to the organ of Corti. A: However, only in the wild-type cochlea do these fibers form a continuous layer of terminals around the inner hair cells. B: In the mouse, there are large gaps between the radial fibers. C,D: In the vestibular system, there is a severe reduction of fibers to the horizontal crista (HC) and saccule (S) in the Atoh1-Hprt null mouse (D). Less severe is the reduction of fibers to the posterior crista (PC), utricle (U), and anterior crista (AC). Scale bars = 100 μm in A–D.
Fig. 7.
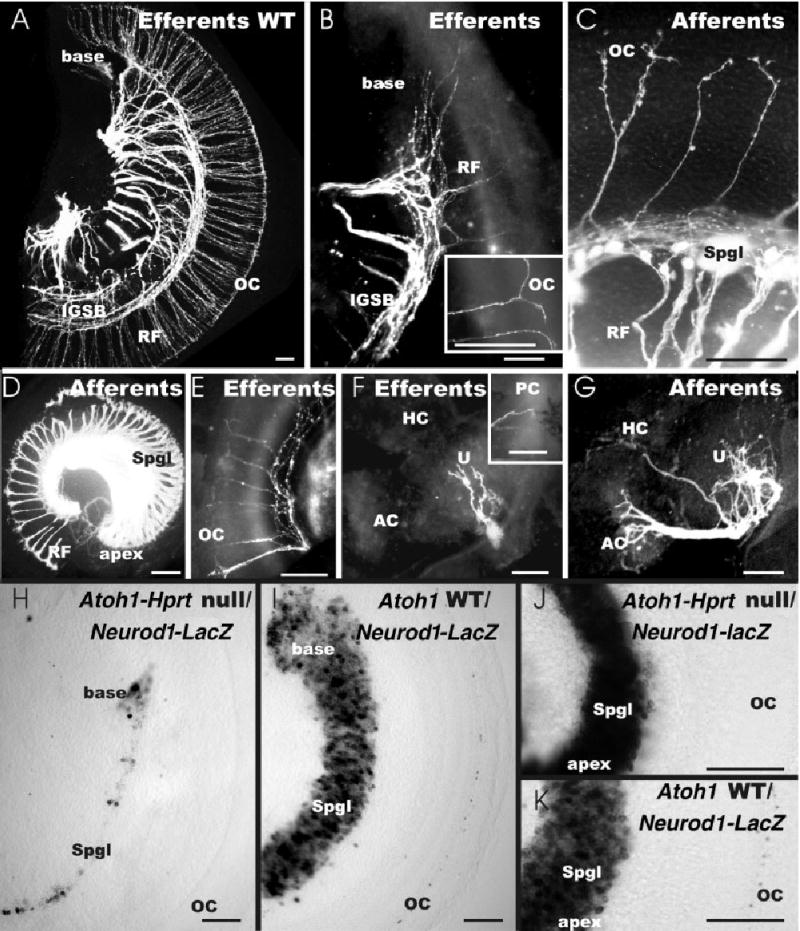
A–K: Details of afferent (C,D,G) and efferent (A,B,E,F) innervation of embryonic day (E) 18.5 Atoh1-LacZ null mice (B–G) and wild-type littermate (A) as well as the distribution of Neurod1-LacZ–positive cells in E18.5 Atoh1-Hprt backgrounds (H–K) is shown. C,D,H,J: Note the retention of many more spiral sensory neurons (Spgl) in the apex (D,J) compared with the basal turn (C,H) in the Atoh1 null mice. Correlated with the reduction of sensory neurons is the decrease in radial fiber (RF) density (D). C: Afferents extend as radial fibers toward the cochlea where they end with small enlargements on several terminal branches. Some of these branches extend past the spiral vessels into the undifferentiated area of the organ of Corti (OC in C). A,B: Efferent fibers form an intraganglionic spiral bundle (IGSB) from which radial fibers extend toward the cochlea; as with afferents, the spacing density of radial fibers is decreased in Atoh1 null mice. D,E: However, whereas afferents show a profound increase of radial fibers toward the apex (D), efferents show a decrease in Atoh1 null mice (E). B: At the end of a radial fiber (RF), efferent fibers bifurcate and run longitudinally toward the apex and base in an area equivalent to the position of the organ of Corti (insert in B). G,F: In contrast to afferents (G), which are prominent to the anterior crista (AC) and the utricle (U), efferent fibers extend sparingly to the utricle, but only occasionally reach a crista (insert in F). H,I: Neurod1-β-galactosidase staining shows a drastic reduction of spiral sensory neurons in the base of Atoh1-Hprt null mice compared with Atoh1 wild-type littermates. J,K: In contrast, many more spiral neurons are present in the apex of Atoh1-Hprt null mice (J) almost reaching the level of wild-type mice (K). H–K: Note that only wild-type mice have Neurod1-β-galactosidase staining in some hair cells of the organ of Corti, whereas no Neurod1-β-galactosidase–stained cells are found in the topographical equivalent of the organ of Corti of Atoh1-Hprt null mice. HC, horizontal crista. Scale bars = 100 μm in A–K.
Our previous work suggested that afferents provide highways for efferent fibers to grow along (Fritzsch et al., 1998; Karis et al., 2001). We, therefore, also investigated the projection of efferent fibers using PTIR271 injection into the medial vestibular nucleus to obtain ipsilateral and contralateral labeling of efferent fibers in the olivocochlear bundle. We found a greatly diminished distribution of efferent fibers to the vestibular system. Only occasional efferent fibers could be traced to the utricle (Fig. 7F). Despite robust labeling of afferents (Fig. 7G), cristae epithelia were devoid of efferent innervation, except for a single posterior crista in one of four ears (Fig. 7F, insert). The reduction in efferent fibers in the basal turn of the cochlea closely followed the pattern of reduction of afferent fibers (Figs. 6B, 7A–C). An intraganglionic spiral bundle was formed, despite the reduced number of spiral sensory neurons (Fig. 7B,E). Radial fibers could be traced occasionally to the cochlea, where they bifurcated and extended along the spiral artery toward the apical and basal tips (Fig. 7B). The apex showed a much reduced density of efferent fibers compared with afferents (Fig. 7D,E). This unusual pattern of afferent and efferent fiber distribution could not be investigated further in older stages owing to the neonatal lethality of the Atoh1 null embryos.
Neurod1 expression confirmed the dramatic reduction of spiral sensory neurons in the basal turn of Atoh1 null mice compared with Atoh1 wild-type littermates (Fig. 7H,I). As already noted for the labeling density of radial fibers (Fig. 7D), many more spiral neurons were present near the apex (Fig. 7J). As noted previously (Kim et al., 2001), some hair cells of the organ of Corti express Neurod1-LacZ in older wild-type embryos (Fig. 7I,K). However, although an area identified as the topographical equivalent of the organ of Corti was present in Atoh1 null mice, we could not detect any Neurod1-β-galactosidase–stained cells (Fig. 7G,I). Overall, these data confirm our findings obtained through tract tracing and establish that loss of sensory neurons is particularly profound in the base, whereas the apex is much less affected.
We also tested the correlation between Atoh1-LacZ–stained cells and fiber distribution directly using tubulin immunocytochemistry on β-galactosidase–reacted ears of Atoh1 heterozygote and null animals (Fig. 8E–J). These data confirmed and extended the findings revealed with dye tracing. Specifically, we found that fibers to the utricle and saccule meandered at the margin of the LacZ-positive patch of cells and only occasionally extended to the patch’s center. Neither dye tracing nor tubulin immunocytochemistry revealed more than a single fiber extending toward the horizontal crista, which exhibited the weakest Atoh1-β-galactosidase staining of all sensory epithelia. In the cochlea, tubulin immunocytochemistry identified only few fibers in the basal tip of the hook region compared with many more fibers at the apex (Fig. 8G,J). As in heterozygous Atoh1-LacZ littermates, fibers could be traced to the modiolar row of β-galactosidase–stained cells (Fig. 8G,J) indicating similar progression and targeting in this undifferentiated part of the organ of Corti.
Fig. 8.
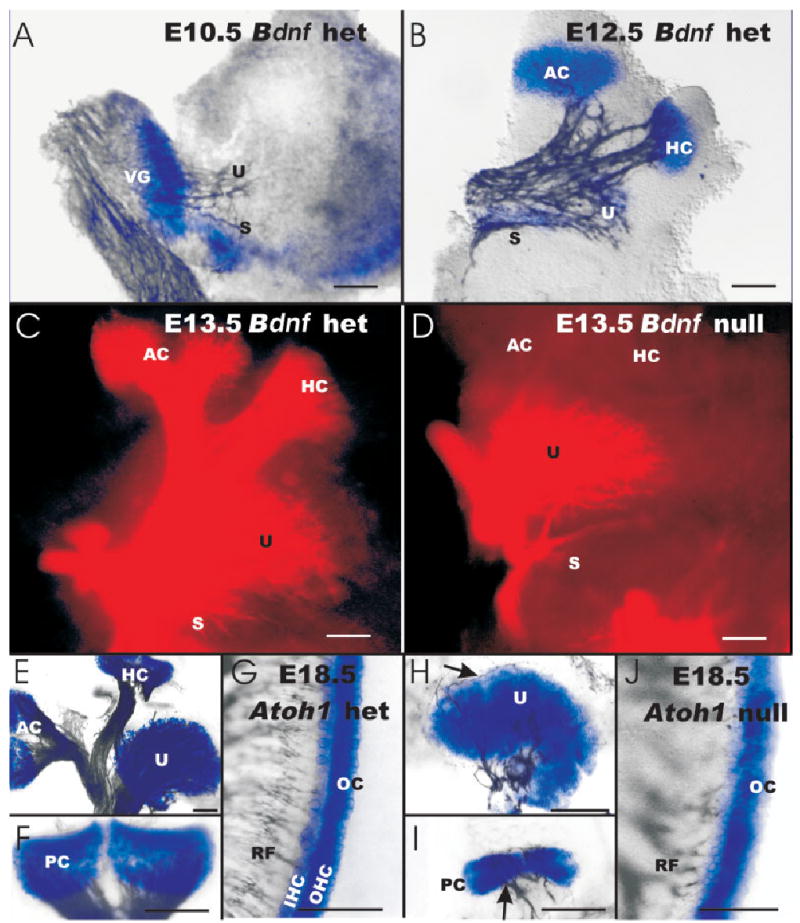
A–J: The expression of brain-derived neurotrophic factor (Bdnf) -LacZ in the ear and the effect of Bdnf null mutation on the pattern and density of afferent fiber innervation is shown (A–D) and compared with the Atoh1-LacZ expression and retention of fibers (E–J). A: The earliest expression of Bdnf at embryonic day (E) 10.5 shows no obvious correlation with the initial fiber outgrowth (revealed with acetylated β-tubulin immunoreactivity) toward the otocyst. B: By E12.5, afferent fibers extend toward the canal cristae, which show Bdnf-β-galactosidase staining. In the utricle (U) and saccule (S), the Bdnf-β-galactosidase staining is limited to the striola region but nerve fibers spread beyond this region. C,D: Loss of Bdnf expression results in complete loss of all afferent fibers to the canal cristae by E13.5. E–J: Acetylated β-tubulin immunoreactivity shows fibers to all Atoh1-β-galactosidase–stained epithelia in Atoh1-LacZ heterozygous (E–G) and null mice (H–J) mice. D,E,H: Density of fibers to the utricle is much reduced in Atoh1-LacZ null mice (H) compared with BDNF null mice (D) and Atoh1-LacZ heterozygous littermate (E). I: Canal innervation is also reduced in Atoh1-LacZ null mice compared with Atoh1-LacZ heterozygous littermates, but some fibers remain. D: In contrast, no fibers remain to canal cristae in Bdnf null mice. G,J: The organ of Corti (OC) of Atoh1-LacZ null mice and heterozygous littermates show comparable density of innervation in the upper middle turn but only the Atoh1-LacZ heterozygous mice show a clear separation of the β-galactosidase–stained cells into inner and outer hair cells (IHC, OHC) in the organ of Corti. AC, anterior crista; HC, horizontal crista; PC, posterior crista; RF, radial fibers; Vgl, vestibular ganglion. Scale bars = 100 μm in A–J.
Fiber Growth in Bdnf Null Mice
We next aimed to directly compare the loss of vestibular afferents in BDNF null mice with that in theAtoh1 null mice. Terminal mitosis of sensory neurons occurs over a prolonged period approximately 1 to 2 days before that of hair cells (Ruben, 1967). Our data showed an initial growth of afferents to the otocyst around the time of the earliest terminal mitosis of vestibular hair cells (Fig. 8A). Moreover, the initial fiber growth at E10.5 was not directed toward the areas of Bdnf-β-galactosidase staining. However, the areas of Bdnf-β-galactosidase staining changed rapidly between E10.5 and E12.5. At E10.5, Bdnf-LacZ was expressed widely around the caudoventral aspect of the otocyst. By E12.5, Bdnf-β-galactosidase staining in the vestibular system correlated with afferent distribution in the canal cristae. This was not the case for the utricle, where only the striolar region showed Bdnf expression (Fig. 8B). Together, these results indicate that initial fiber growth to the ear is not exclusively directed to areas of Bdnf expression but is targeted to these areas as early as E12.5. Loss of Bdnf expression leads to a rapid loss of all canal cristae innervation at E13.5 (Fig. 8B,C).
The retention of afferent fibers to the cristae in Atoh1 null mice (Figs. 6C, 7G, 8) indicates that the expression of Bdnf in the undifferentiated Atoh-LacZ–positive cells of these epithelia (Fig. 3D) is retaining the afferents. In contrast, Atoh1 null mice show no expression of Bdnf-LacZ in the utricle and saccule but show a more profound loss of afferents than simple Bdnf null mice (Fig. 8D,H). This finding suggests that the expression of Ntf3 that rescues those fibers (Fritzsch et al., 1995; Bianchi et al., 1996; Hellard et al., 2004) is possibly also compromised in Atoh1 null mice.
DISCUSSION
Atoh1 Null Mice Retain Some Cells That Express the Neurotrophin Bdnf
Our data confirm previous findings (Bermingham et al., 1999; Ben-Arie et al., 2000) that some Atoh1-β-galactosidase–stained cells are present in Atoh1-LacZ null mice. However, we have now extended this finding and show that the pattern of distribution of these cells closely resembles that of hair cells. Moreover, we show that some of these undifferentiated cells also express the neurotrophin Bdnf, a marker for hair cells in embryos of E16.5 or older (Farinas et al., 2001). Using whole-mounted, Atoh1-LacZ null ears, we can identify Atoh1-β-galactosidase–stained cells in six patches of cells in Atoh1-LacZ null mouse ears that are comparable in shape and topology in the ear to the five vestibular sensory epithelia (utricle, saccule, three cristae) and the organ of Corti. We can also identify four discrete patches of Bdnf-β-galactosidase–stained cells in the ears of compound Atoh1-Hprt null Bdnf-LacZ heterozygotic mice. These patches are similar in shape and topology to the three canal cristae and the apical turn of the organ of Corti of control littermates, albeit smaller in size (Figs. 1– 4). In addition, our sections reveal what appear to be more Atoh1-β-galactosidase–stained cells in the apical turn of the cochlea of Atoh1-LacZ null mice than are found in Atoh1-LacZ heterozygotic animals (Fig. 2). Moreover, cellular distribution at the electron microscope level suggests that some hair cell-like cells form in the area of outer hair cells (Fig. 2G,H). We confirm the previously reported apoptosis in Atoh1 null mice (Chen et al., 2002) and show that cells of the GER are phagocytosing this cellular debris (Fig. 2G).
Surprisingly, our findings suggest that Bdnf regulation is partially or completely independent of ATOH1 protein formation in several epithelia. Indeed, Bdnf-LacZ expression in canal cristae and the cochlear apex is present before the expression of Atoh1 in these epithelia (Fig. 4). In contrast, Bdnf-LacZ is expressed shortly after Atoh1-LacZ in other epithelia, notably the base of the cochlea, the utricle, and the saccule (Fig. 4). Thus, Bdnf-LacZ expression appears to depend on ATOH1 protein in the utricle, saccule, and basal turn of the organ of Corti, because Bdnf-β-galactosidase staining is always absent in these areas in Atoh1-Hprt null mice (Figs. 3, 4). In contrast, Bdnf-β-galactosidase staining is independent of ATOH1 protein in the basal turn of the organ of Corti and the three crista sensory epithelia. Potential candidates that could mediate Bdnf regulation in these epithelia include Neurog1, which affects hair cell formation (Ma et al., 2000) and may mediate the Bdnf expression in the delaminating sensory neurons (Fig. 4). Another candidate is the Pou domain factor Brn3c (Clough et al., 2004), which may show some limited expression even without ATOH1 protein formation (Rivolta et al., 2002). This complex pattern of regulation of Bdnf is consistent with the complex promoter structure of Bdnf (Timmusk et al., 1994; Shieh et al., 1998; Martinowich et al., 2003).
The formation of Bdnf-β-galactosidase– and Atoh1-β-galactosidase–staining cells in what appear to be the six sensory epithelia of the ear (utricle, saccule, three crista, organ of Corti) is consistent with suggestions that Atoh1 is a differentiation factor that does not determine hair cell progenitors (Chen et al., 2002). The lack of true proneural determination capacity of mammalian Atoh1 has also been noted in the nervous system, and it has been suggested that mammalian Atoh1 has lost the proneural capacity its orthologue, atonal, has in Drosophila (Bertrand et al., 2002). An as yet unidentified gene upstream of Atoh1 could be responsible for the Bdnf and Atoh1 up-regulation in these undifferentiated cells of Atoh1 null mice in a pattern comparable to wild-type sensory epithelia.
Our data do not support the recently proposed role of Atoh1 in regulation of development of entire sensory epithelia (Woods et al., 2004) but rather support the notion that topologically specific up-regulation of Bdnf-LacZ and Atoh1-LacZ occurs in cells of sensory epithelia independent of the presence of ATOH1 protein in older embryos. However, whether or not Atoh1 is initially up-regulated in both hair cell and supporting cell primordia in E12- to E14-day-old embryos, as recently claimed (Woods et al., 2004), cannot be resolved with our analysis, as Bdnf is not exclusively expressed in hair cells during that time. This unexpected residual expression of Atoh1-LacZ in the undifferentiated cells that form the six sensory epithelia and the coextensive expression of Bdnf in these same cells preclude a clear answer to our original question whether afferent fiber growth in vivo is completely independent of any signals from hair cells. This follows from the possibility that the undifferentiated cells revealed in this study might be capable of generating enough BDNF protein to avoid the Bdnf null phenotype of absence of any innervation to canal cristae (Figs. 7, 8). It is also conceivable that other as yet unknown factor(s) can be produced by these cells. Together, Bdnf and such factor(s) could provide enough attraction for guidance and some survival in virtually all epithelia. Nevertheless, the investigations of fiber growth and retention in these mice provided some interesting results that could not be obtained otherwise, as discussed below.
Initial Fiber Growth Is Almost Normal in the Absence of Differentiated Hair Cells
What attracts afferent fibers to grow to sensory epithelia is unknown and likely depends on a multitude of factors (Rubel and Fritzsch, 2002). Factors released from hair cells have been postulated to attract afferents (Bianchi and Cohan, 1993), and the neurotrophic factor BDNF has been shown to guide afferents (Tessarollo et al., 2004), possibly in combination with other guiding molecules (Gu et al., 2003). The sensory epithelia of Atoh1 null mice have been assumed to produce little, if any, such guidance factors in their undifferentiated epithelia precursors, because immunocytochemistry failed to detect any protein characteristic of differentiated hair cells or supporting cells (Woods et al., 2004). One would expect, therefore, that targeting errors might occur in Atoh1 null mice as fiber growth would depend predominantly on guidance cues other than attractions to sensory epithelia. In contrast to this expectation, all sensory epithelia of Atoh1 null mice receive some innervation, predominantly to areas expressing Bdnf-LacZ. The growing neuronal fibers exhibit only limited targeting errors (Fig. 5D) by E12.5, mostly near the sensory epithelia. Previous work has shown that this embryonic stage (E12.5) is before sensory neuron death mediated by neurotrophins (Fritzsch et al., 2004), and the misrouting, therefore, may be due to other unknown factors not released in proper amounts by the undifferentiated Atoh1-LacZ– expressing cells present in these epithelia. Importantly, the nerve fibers extend to and are maintained to all six Atoh1-β-galactosidase–stained sensory epithelia (Fig. 8E–J) in a pattern virtually identical to that in Atoh1 wild-type mice but different from that in simple neurotrophin null mice (Fig. 8D). These data show that growth of afferent nerve fibers to the sensory epithelia as well as branching within these sensory epithelia does not require differentiated hair cells or the neurotrophin BDNF (Figs. 3C, 7C, 8H–J).
Survival of Afferents Is Partially Independent of Differentiated Hair Cells in the Cochlea
The long-term retention of innervation correlates with the level of Bdnf-LacZ expression in many but not all sensory epithelia of Atoh1 null mice. For example, the strongest expression of Bdnf-LacZ is in the apex of the cochlea (Fig. 3C) and more Neurod1-β-galactosidase–stained sensory neurons remain in the apex than in the base (Fig. 7H,J). Likewise, most Atoh1-β-galactosidase–stained cells are in the apical turn of the organ of Corti (Fig. 1C), which receives a substantial innervation by spiral sensory neurons (Fig. 7D). In contrast, whereas there is no Bdnf-LacZ expression in the base (Fig. 3C), it still receives some innervation (Figs. 6B, 7C). Past research has shown that only the two neurotrophins Bdnf and Ntf3 are necessary to maintain all inner ear innervation, and in the absence of both or of their high affinity receptors, all innervation to the ear sensory epithelia is lost in embryos by E16.5 (Ernfors et al., 1995; Fritzsch et al., 2004). Ntf3 is produced in supporting cells during development (Pirvola et al., 1992; Wheeler et al., 1994; Farinas et al., 2001) and loss of Ntf3 causes loss of all radial fibers to the basal turn of the cochlea (Fritzsch et al., 1997). We therefore suggest that the retention of innervation to the base of the cochlea is likely related to the limited presence of the second neurotrophin of the ear, Ntf3.
There is no Bdnf expression in the utricle and saccule of E18.5 Atoh1 null mice, and there is a more severe reduction of afferent and efferent innervation to the Atoh1-β-galactosidase–stained cells than in Bndf null mice (Fig. 8D,H). It is likely that this more-severe reduction of afferent fibers in Atoh1 null mice relates to the reduced presence of the second neurotrophin, Ntf3, because it is expressed and mediates survival of afferents in these epithelia in the absence of Bdnf (Pirvola et al., 1992; Ernfors et al., 1995; Farinas et al., 2001). In addition, occasional fibers are found that do not enter the sensory epithelia of Atoh1 null mice but rather stay at the perimeter (Fig. 8H). This finding is particularly striking in the utricle (Fig. 8H) and saccule and suggests that the limited expression of Bdnf in a few cells only (Fig. 3D) may suffice for afferent survival but not for proper targeting. This misrouting of fibers is reminiscent of data recently described in Bdnf null mice in which cell death is prevented in the absence of Bax (Hellard et al., 2004). It may, therefore, again relate to the retained expression of Ntf3 (Farinas et al., 2001; Fritzsch et al., 2004).
Most interesting is the case of the canal epithelia. These sensory epithelia express Bdnf early and almost exclusively; thus, their afferent innervation is lost in Bdnf and ntrk2 null mice (Fritzsch et al., 1995; Bianchi et al., 1996). Atoh1 null mice do not resemble Bdnf null mice (Fig. 8D,I), as they retain innervation to the posterior and anterior crista, but only occasional fibers are found to the horizontal crista (Figs. 6D, 7G). Both Atoh1-β-galactosidase and Bdnf-β-galactosidase show strong staining in the anterior and posterior cristae in Atoh1 null mice, but there is a reduced expression of both reporters in the horizontal crista. Thus, in the anterior and posterior canal cristae, fiber loss conforms closely to sites of Bdnf expression. In the horizontal crista, there is a reduction in both reporter genes, suggesting a possible threshold effect of Bdnf expression on fiber retention in the horizontal crista.
In this context, it is interesting that the phenotype of efferent fiber loss is more severe than that of afferent fiber loss (Fig. 7B,E,F). This difference is particularly obvious in the apex of the organ of Corti (Fig. 7D,E) and the cristae (Fig. 7F,G). This finding suggests that the remaining afferents may be unable to provide guidance cues for the efferents, as previously proposed (Fritzsch et al., 1998). Alternatively, an unknown factor(s) may be released from differentiated hair cells and is in insufficient supply in the Atoh1 null mice owing to the undifferentiated nature of the cells in the sensory epithelia. This might limit growth and/or retention of efferent fibers to epithelia that still receive substantial afferent innervation, such as the anterior or posterior crista and the apex of the organ of Corti (Fig. 7).
The presence of sizable afferent fiber innervation to the apex of the cochlea as well as to most vestibular sensory epithelia even in the absence of overt hair cell differentiation raises hopes for clinical cases with congenital, embryological loss of hair cells. Combined with our previous demonstration that afferent fibers can be retained for the long-term even in cases with neonatal hair cell loss (Xiang et al., 2003), the current data indicate that possibly significant survival of afferents might occur in the apex of the cochlea of any child born lacking hair cells. Clearly, appropriate cochlear implants that can extend into the apex of the cochlear duct could provide stimulation to the surviving primary neurons. Possibly even in the most severe cases of congenital hair cell absence, as is found in the Atoh1 null mice of this study, cochlear implants could restore hearing in cases that never develop hair cells.
EXPERIMENTAL PROCEDURES
Two Atoh1 null alleles were used in this study. One allele carries a Hprt cassette (Atoh1-Hprt) and another one carries the LacZ reporter gene (Atoh1-LacZ) instead of the coding sequence (Ben-Arie et al., 1997, 2000). A mouse line carrying Bdnf-LacZ null allele was also used and maintained as described (Farinas et al., 2001). All Atoh1 null mice were bred from heterozygotes as previously described (Bermingham et al., 1999). Atoh1-LacZ–positive embryos were collected at embryonic days 11.5 (E11.5), E12.5, E14.5, E16.5, E17.5, E18.5, and E19.5. Embryos were perfusion- or immersion-fixed with 4% paraformaldehyde (PFA) for 30 min. Ears were rapidly dissected in 0.1 M phosphate buffer saline and subsequently reacted for LacZ (β-galactosidase) histochemistry overnight at room temperature (Farinas et al., 2001). Some ears of heterozygotic and homozygotic Atoh1 mice were also reacted for nerve fiber stain using acetylated tubulin antibodies as previously described (Fritzsch et al., 1997).
In addition, Atoh1 null mice carrying the Atoh1-Hprt allele were mated to generate null embryos without the LacZ gene (Ben-Arie et al., 1997). Timed-pregnant females were killed at E11.5, E12.5, E16.5, and E18.5. Tails of embryos were processed for genotyping, and all embryos were transcardially perfused with 4% PFA. Embryos were subsequently stored in 4% PFA in the refrigerator. Genotyped animals were coded and PTIR271 or PTIR278 (Tessarollo et al., 2004) injections in the brainstem were done blinded to the genotype. Afferent and efferent fibers were labeled in separate animals or combined by placing separate injections in both the differentiated alar and basal plate (Fritzsch and Nichols, 1993). Efferent fibers to the cochlea and efferents and afferents to the vestibular organs were labeled with an injection to the ipsilateral (efferents and afferents) or contralateral (efferents only) medial vestibular nucleus near the decussation of the olivocochlear bundle (Karis et al., 2001). Cochlear and vestibular afferents were labeled with injections into the descending vestibular nucleus and dorsal cochlear nucleus. In some cases we injected tracer into ventral cochlear nucleus, the vestibular nuclei, and olivocochlear bundle to label all afferent and efferent fibers to the ear. After sufficient diffusion time as determined previously (Maklad and Fritzsch, 2003), ears were dissected, mounted flat on slides, cover-slipped, and viewed with a confocal microscope (Bio-Rad Radiance 2000; LSM 510 META confocal scanning system). Data were documented, and phenotypic results were then compared against the genotype. Analysis of older embryos could not be fully blinded to the genotype, as dissected ears showed the diminished size of the sensory epithelia as previously described (Bermingham et al., 1999).
The neurotrophin Bdnf is expressed predominantly in differentiated hair cells in embryos of E16.5 or older (Farinas et al., 2001). We studied the Bdnf-LacZ expression in control and Atoh1 null littermates. To achieve this, we crossed Atoh1-Hprt heterozygote with Bdnf-LacZ heterozygote animals. Among the offspring, we selected Atoh1-Hprt/Bdnf-LacZ compound heterozygote males and bred these animals with Atoh1-Hprt heterozygote females. The resulting offspring produced Atoh1-Hprt null mice heterozygotic for Bdnf-LacZ at the expected Mendelian frequency of 1 in 8 embryos. We also generated compound mice that carried both the Atoh1-LacZ and the Bdnf-LacZ. In these animals, we tested whether cells other than the ones found in Atoh1-LacZ mice were positive for β-galactosidase.
The bHLH gene Neurod1 (formerly NeuroD) is expressed in all sensory neurons and in addition in some differentiated hair cells in older embryos (Kim et al., 2001). We studied the Neurod1-LacZ expression in control and Atoh1-Hprt null mouse littermates. For this analysis, we crossed Atoh1-Hprt heterozygote with Neurod1-LacZ heterozygote animals. In the offspring, we selected Atoh1-Hprt/Neurod1-LacZ compound heterozygote males and bred these animals with Atoh1-Hprt heterozygote females. We bred two litters of 15 E18.5 embryos in which we had 3 offspring that were Atoh1-Hprt null mice that were also heterozygous for Neurod1-LacZ. Ears of these E18.5 embryos were perfusion-fixed and reacted for β-galactosidase histochemistry as described above.
After β-galactosidase reaction, ears were dissected and mounted flat for analysis of the sensory epithelial area. Some ears were osmicated, embedded in epoxy resin, sectioned at 5–20 μm thickness and analyzed using a compound light microscope (Nikon Eclipse 800). Images were captured using a CCD camera and processed using ImagePro or Metamorph software. Thin sections were cut on a Reichert ultra-microtome, collected on 200-mesh copper grids, counterstained with uranyl acetate and lead citrate, and viewed in a transmission electron microscope (TEM; Phillips). Images were digitized and combined into plates by using CorelDraw software.
Acknowledgments
We thank Dr. H. Zoghbi for encouragement and suggestions throughout this project and two referees for critical comments on an earlier version of the manuscript. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number 1 C06 RR17417-01 from the National Center for Research Resources, National Institutes of Health. We acknowledge the use of the confocal microscope facility of the NCCB, supported by EP-SCoR EPS-0346476 (CFD 47.076). B.F. and K.W.B. were funded by grants from NIH, V.Y.W. is supported by a predoctoral NRSA fellowship and the McNair Scholarship.
References
- Ben-Arie N, Bellen HJ, Armstrong DL, Mc-Call AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169 –172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ, Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039 –1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Cohan CS. Effects of the neurotrophins and CNTF on developing statoacoustic neurons: comparison with an otocyst-derived factor. Dev Biol. 1993;159:353–365. doi: 10.1006/dbio.1993.1247. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. 1996. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF nullmutantmice.Development122:1965–1973. [DOI] [PubMed]
- Cajal SR. Accion neurotropica de los epitelios. Trab del Lab de Invest Biol. 1919;17:1–153. [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Clough RL, Sud R, Davis-Silberman N, Hertzano R, Avraham KB, Holley M, Dawson SJ. Brn-3c (POU4F3) regulates BDNF and NT-3 promoter activity. Biochem Biophys Res Commun. 2004;324:372–381. doi: 10.1016/j.bbrc.2004.09.074. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170 –6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Hear Res. 1993;65:51–60. doi: 10.1016/0378-5955(93)90200-k. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Smeyne R, Fagan AM, Barbacid M. Reduction and loss of inner ear innervation in trkB and trkC receptor knockout mice: a whole-mount DiI and scanning electron microscopic analysis. Audit Neurosci. 1995;1:401–417. [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Barbacid M, Silos-Santiago I. The combined effects of trkB and trkC mutations on the innervation of the inner ear. Int J Dev Neurosci. 1998;16:493–505. doi: 10.1016/s0736-5748(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Gao WQ. Hair cell development in higher vertebrates. Curr Top Dev Biol. 2003;57:293–319. doi: 10.1016/s0070-2153(03)57010-7. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 Conveys Semaphorin and VEGF Signaling during Neural and Cardiovascular Development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellard D, Brosenitsch T, Fritzsch B, Katz DM. Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice. Dev Biol. 2004;275:34 –43. doi: 10.1016/j.ydbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R. Etudes sur l’anatomie et la physiologie du labyrinthe de l’oreille et du VIII nerf. II. Quelques donnees au sujet de l’anatomie des organes sensoriels du labyrinthe. Trav Lab Rech Biol Univ Madrid. 1926;24:53–153. [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain Res Dev Brain Res. 2003;140:223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890 –893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC. Transcript profiling of functionally related groups of genes during conditional differentiation of a Mammalian cochlear hair cell line. Genome Res. 2002;12:1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM. 1992. The development of innervation in the organ of Corti. In: Romand R, editor. Development of auditory and vestibular systems 2. Amsterdam: Elsevier. p 59 –100.
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Belluardo N, Persson H, Metsis M. Developmental regulation of brain-derived neurotrophic factor messenger RNAs transcribed from different promoters in the rat brain. Neuroscience. 1994;60:287–291. doi: 10.1016/0306-4522(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29 –37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Van de Water TR. 1983. Embryogenesis of the inner ear: “in vitro studies”. In: Romand R, editor. Development of auditory and vestibular systems. New York: Academic Press. p 337–374.
- Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73:46 –56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310 –1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


