ABSTRACT
Trematodes of the genus Diplostomum (Diplostomidae) are widely distributed and significant fish pathogens known for causing a range of negative effects. Any mechanism that protects the host from the parasite thus represents an evolutionary advantage. In this study, we observed an unusual localisation of Diplostomum metacercariae, which were displaced into the sac protruding from the lens, in North‐American bullhead catfish species of the genus Ameiurus. The occurrence of the sac was found in all sampling sites (Czech Republic) where A. nebulosus and A. melas were infected with Diplostomum, regardless of the parasite species, as both D. pseudospathaceum and D. spathaceum induced sac formation. The probability of sac formation increased with the intensity of Diplostomum infection. Experimental infection of juvenile A. melas showed that the first signs of sac formation appeared shortly after the infection. While visual observation under the light microscope suggested the first signs at 7 days post‐infection, histological examination documented the start of epithelial proliferation by the third day post‐infection. Our study suggests that the process of sac formation, along with parasite displacement, might present a host reaction to mitigate the negative effects of infection and the impaired vision caused by the parasite.
Keywords: Diplostomum, eye fluke, histology, Ictaluridae, lens protrusion, sac formation
1. Introduction
Trematodes of the genus Diplostomum von Nordmann, 1832 (Diplostomidae) are common parasites of freshwater fish worldwide (Niewiadomska and Laskowski 2002). Species that infect fish eyes are widely distributed and significant fish pathogens (Karvonen and Marcogliese 2020). Many of the negative effects observed in Diplostomum‐infected fish, such as increased mortality (Larsen et al. 2005; Michálková and Ondračková 2014), altered physiology (e.g., oxygen consumption [Voutilainen et al. 2008] or metabolic rate [Seppänen et al. 2009]), feeding activity (Crowden and Broom 1980; Voutilainen et al. 2008) and retarded growth (Voutilainen et al. 2008), are directly or indirectly associated with impaired vision.
Fish rely on vision as a dominant sensory system for foraging, predator avoidance and mate selection (Frey et al. 2022). The visual acuity of fish depends on the size of their spherical lens and the density of retinal cones (Sandström 1999). Because fish lack an air‐cornea interface, the refractive index of their cornea is similar to that of the surrounding water, depriving them of nearly 80% of their optical power and making it more difficult for them to focus on objects compared to terrestrial vertebrates. Since the refractive power of a fish's eye relies solely on the lens, the health of the lens is crucial for the quality of their vision (Collin 2009; Sandström 1999).
In severe infections by lens‐infecting species, damage caused by individual parasites results in opacity of the lens and leads to cataract formation, with the number of parasites and the size of the fish being the main factors influencing the severity of cataracts (Karvonen et al. 2004). In cases of extremely high infections, the lens capsule may rupture, or the lens may become dislocated, causing the fish host to lose its eyesight (Karvonen and Marcogliese 2020). Impaired vision in fish is caused also by metacercariae of species that primarily inhabit other parts of the eye, such as the vitreous humour and retina (Padrós et al. 2018; Schwelm et al. 2021; Ubels et al. 2018). Metacercariae of Diplostomum located in the choroidal layer behind the retina cause severe damage to the choroid, pigment epithelium and retina and reduce the electrophysiological function of the retina in yellow perch Perca flavescens (Mitchill, 1814) (Ubels et al. 2018). In Arctic charr Salvelinus alpinus (Linnaeus, 1758), Diplostomum metacercariae located in the eye retina alter the fish's visual acuity, potentially leading to severe or total visual impairment due to repair mechanisms (Padrós et al. 2018). In three‐spined stickleback Gasterosteus aculeatus Linnaeus, 1758, Diplostomum invading the subretinal space caused retinal detachments and damage to the retina, possibly by consuming the tissue (Frey et al. 2022).
Given the aforementioned negative effects caused by eye‐infecting Diplostomum species, any means of protecting the host from the parasite would represent an evolutionary advantage. Host traits that enable avoidance or clearance of an infection thus reduce the probability of an individual becoming infected (Lopes et al. 2022) or decrease its prevalence (Wedekind and Little 2004). As avoidance strategy seems too costly (Klemme and Karvonen 2016), investment in resistance and/or tolerance becomes essential (Klemme and Karvonen 2017). Most Diplostomum species infecting the eyes are located in the lens, a location that provides the metacercariae with a rich source of protein while also protecting them from the fish immune system (Ubels et al. 2018). It has generally been accepted that the classical adaptive response plays no role in resistance to primary parasite infection (Rauch et al. 2006) and that only the innate immunity of fish is activated against D. pseudospathaceum (Scharsack and Kalbe 2014). Nevertheless, recent studies have shown that, despite the fact that metacercariae of eye‐infecting Diplostomum species are situated in an immune‐privileged organ, the parasite may provoke the host's immune response. This response may also be partially related to the migration route, which begins with the penetration of the skin and ends with localisation in the eye (e.g., Haase et al. 2016; Fuad et al. 2024).
During an investigation of invasive North‐American invasive black bullhead Ameiurus melas (Rafinesque, 1820) and brown bullhead Ameiurus nebulosus Lesueur, 1819 (Siluriformes: Ictaluridae) in lentic water bodies in the Czech Republic (Ondračková et al. 2025), we repeatedly observed Diplostomum metacercariae clustered in a sac that protruded from the lens. Only a few studies reported similar observations. Some of these were directly related to sac formation on the lens of Ameiurus (La Rue et al. 1926; Larson 1965; Marcogliese and Compagna 1999), while others noted a capsule or sac‐like structure containing flukes in other parts of the fish eye, such as the internal surface of the cornea of pike Esox lucius L. (Steenstrup 1842) or the retina of Arctic charr (Padrós et al. 2018). Although findings on sac formation on the lenses of parasitised A. nebulosus and A. melas were published almost a hundred years ago (La Rue et al. 1926), no further attention has been paid to this unusual phenomenon in these lens‐infecting eye flukes.
As a basis for further detailed study, we first aimed to map the distribution of Diplostomum infection and evaluate the frequency of sac formation in naturally infected populations of A. melas and A. nebulosus in the study area. In the experimental part of this study, we describe the formation of the sac through visual observation and histological analysis in experimentally infected A. melas . As a first step in determining whether the process of sac formation is specific to a particular group of fish species (i.e., fish of the genus Ameiurus) or whether it is more widespread, we also present results from visual observations of the lens in experimentally infected common wels Silurus glanis Linnaeus, 1758 (Siluriformes: Siluridae), the most phylogenetically related native fish species in the study area.
2. Material and Methods
2.1. Fish and Parasite Collection
Bullhead catfishes A. melas and A. nebulosus were sampled using electrofishing gear or beach seines, depending on habitat type, at eight fishing grounds (ponds, pool) in the Czech Republic (Europe) between 2018 and 2023 (Table 1). For details on fish sampling sites, the number of fish examined, and their size, see Ondračková et al. (2025). Live fish were transported to the laboratory, where they were dissected to check for the presence of Diplostomum eye flukes. Parasites were isolated from the eye lens or from the sac protruding from the lens and counted. A subsample of parasites was preserved in 90% ethanol, separately from the lens and/or from the sac. Prevalence (i.e., the percentage of infected fish in a sample), mean intensity of infection (the mean number of metacercariae in infected fish) and total abundance (the number of parasites in all hosts in the sample) were calculated according to Bush et al. (1997) for Diplostomum spp. metacercariae in each parasite location (lens and/or sac). The proportion of metacercariae located in a sac was related to parasite abundance using arcsine‐transformed data and Spearman rank correlation test. The effect of the intensity of infection on sac formation probability was tested using a generalised linear model (GLM, Bernoulli distribution).
TABLE 1.
Occurrence of Diplostomum metacercariae in Ameiurus melas and Ameiurus nebulosus in water bodies in the Czech Republic, with prevalence (P), total abundance (A) and abundance in lens and lentic sac.
| N fish | P (%) | A | Lens | Sac | N sequenced | |
|---|---|---|---|---|---|---|
| Ameiurus melas | ||||||
| Mlazice pool | 16 | 38 | 228 | 67 (Dp, Ds) | 161 (Dp, Ds) | 32/38 |
| Stepnicky pond | 16 | 50 | 9 | 6 (Dp, Ds) | 3 (Dp) | 5/5 |
| Ameiurus nebulosus | ||||||
| Barbora pond | 20 | 100 | 231 | 21 (Dp) | 210 (Dp) | 11/11 |
| Za pilou pond | 20 | 30 | 23 | 1 | 22 (Dp, Ds) | 8/8 |
| Růžový pond | 20 | 65 | 67 | 22 (Dp) | 43 (Dp) | 5/5 |
| Starý pond | 22 | 91 | 399 | 102 (Dp, Ds) | 297 (Dp, Ds) | 15/16 |
| Hulík pond | 20 | 30 | 7 | 4 (Dp) | 3 (Dp) | 2/4 |
| Škřečoň pond | 20 | 25 | 6 | 4 | 2 | 0/3 |
Abbreviations: Dp, Diplostomum pseudospathaceum; Ds, Diplostomum spathaceum; N sequenced, number of successfully sequenced/out of the number of analysed metacercariae.
2.2. Diplostomum Species Determination
Molecular genetic methods were applied for parasite species determination. To exclude a possible parasite‐specific effect, we used molecular tools to identify the parasite species found. Diplostomum metacercariae exhibit considerable morphometric variability (Niewiadomska and Laskowski 2002), making morphology an insufficient method for species delimitation. The molecular approach, developed over the past few decades, has resulted in the delineation of several new species or species‐level lineages (Faltýnková et al. 2022; Georgieva et al. 2013; Schwelm et al. 2021) and is currently the most appropriate method for species identification of Diplostomum metacercariae (Faltýnková et al. 2022). Accordingly, sequencing of the ribosomal gene cluster ITS1‐5.8S‐ITS2 was used as one of the most commonly employed markers, with a number of corresponding sequences available in the GenBank database for the majority of Diplostomum species. DNA was extracted from a subsample of parasites from all sites. A total of 90 individual parasites were used, including specimens located either in the eye lens or sac from the same eye, to check potential parasite species‐specific localisation. DNA was extracted using the innuPREP Forensic Kit (Analytik Jena, Germany) and was used for sequencing in the barcode region of the internal transcribed spacer cluster (ITS1‐5.8S‐ITS2) of the rRNA gene, using the internal transcribed spacer 1 (ITS1)‐specific primers BD1 (forward: 5′‐GTCGTAACAAGGTTTCCGTA‐3′) and 4S (reverse: 5′‐TCTAGATGCGTTCGAARTGTCGATG‐3′) (Bowles et al. 1993; Luton et al. 1992) under the conditions described in Dudliv et al. (2024). Sanger sequencing of PCR products was performed commercially at Eurofins Genomics Germany GmbH (Germany), and the sequences were edited and aligned using Geneious v. 9.0.5 software (Kearse et al. 2012).
2.3. Experimental Infection
Young‐of‐the‐year (0+ juvenile) A. melas were collected by dip‐netting in a small pool near the town of Melnik in July 2021. Young‐of‐the‐year S. glanis were obtained from the Fisheries in Hodonin (https://www.rybarstvi‐hodonin.cz/). Ten fish per species were pre‐screened for eye‐fluke infection to confirm they were parasite‐free. The fish were then kept in outdoor fibreglass tanks (1.35 × 1.35 × 0.9 m) at the facilities of the Institute of Vertebrate Biology ASCR in Brno, Czech Republic and allowed to acclimatise for 2 weeks prior to the experimental infection.
As a source of infection, we used cercariae of D. pseudospathaceum released from its first intermediate host, the freshwater snail Lymnaea stagnalis (Linnaeus, 1758). Snails were collected from Vlkov pond in late July 2021, transported to the laboratory and checked for Diplostomum infection. Cercariae of Diplostomum type were preserved in 90% ethanol and parasite species identity was confirmed by sequencing the barcode region of the internal transcribed spacer cluster (ITS1‐5.8S‐ITS2) of the rRNA gene, as described above. Infected snails with confirmed parasite species were then used for experimental infection. Prior to infection, 10 infected snails were placed into a one‐litre container with standing tap water for 2 h to release cercariae at room temperature. Cercarial density in the suspension used for fish infection was estimated from ten 0.1 mL sub‐samples, from which the number of cercariae was counted with a binocular microscope (see Michálková and Ondračková 2014). The fish were individually placed into plastic boxes with 150 mL of water (22°C) and exposed to 50 cercariae for 1 h. After parasite exposure, the fish were moved to aquaria, where they were kept until dissection.
Prior to dissection, all fish were anaesthetised by overdosing with clove oil, in accordance with Law No. 246/1992 of the Czech Republic. For A. melas , a sample of fish was anaesthetised for each pre‐set time point (1, 3, 5, 7, 9, 12, 16 and 24 days post‐infection [DPI]) over 24 days. Six fish were checked for successful infection at 1 DPI with a binocular microscope. For the following samples (3–16 DPI), six fish were preserved in AFA (Alcohol–Formalin–Acetic Acid) fixative for further histological examination to capture the sac formation, while a subsample of non‐infected fish was used as a control. Another six fish per sample were measured, dissected and the number of metacercariae in each lens/lentic sac was counted. The presence of sac formation and the location of the metacercariae in the eye (i.e., lens or sac) were recorded (82 fish in total). Due to protozoan infection (Ichthyophthirius multifiliis Fouquet, 1876), the experiment had to be terminated at 24 DPI, when the last four fish were euthanised. Fish total length ranged from 30 to 51 mm, with mean ± SD (39 ± 6 mm) in dissected fish and 27 to 54 mm, with mean ± SD (40 ± 7 mm) for fish processed histologically. The area of the lenses and sacs was measured using an Olympus BX53 light microscope (Olympus Optical Co., Tokyo, Japan) fitted with the Olympus cellSens Standard v.3.2 digital image analysis package (Olympus Optical Co., Hamburg, Germany). For S. glanis , four fish were dissected on 1, 5, 7, 12, 16, 24, 30 and 40 DPI (36 fish in total) and checked for number and location of metacercariae in eyes, as well as any changes on the lens associated with the infection (e.g., cataract, sac formation, etc.). Fish total length ranged from 60 to 89 mm, with mean ± SD (72 ± 8 mm).
2.4. Histology
The whole fish ( A. melas ) was fixed in AFA (with several changes of fresh fixative) for at least 1 week. Prior to the histological procedure, the total length of the fish was measured (see above), and the heads were then separated for further processing. Using a magnetic stirrer, the fish heads were dehydrated through a graded alcohol series (70%, 80%, 90%, 96%) and acetone (≥ 99.5%, histological grade) and cleared in xylene. Subsequently, the heads were infiltrated in a graded series of xylene/BaWax (3:1, 1:1, 1:3) and finally embedded in BaWax (Bamed, Czech Republic). Serial sections (transverse and coronal) 6–8 μm thick were stained with haematoxylin‐eosin (HE) or Masson's trichrome (fast green method; MT) (Humason 1967). Mounted preparations were examined and photodocumented with light microscopy (LM) using an Olympus BX61 microscope equipped with an Olympus DP 71 digital camera and Stream Motion 1.9.3. image analysis software.
3. Results
3.1. Natural Diplostomum Infection and Frequency of Lentic Sac Occurrence
Both bullhead species, A. melas and A. nebulosus , were found to be infected with metacercariae of Diplostomum spp. (Table 1; Figure 1). In all sampling sites, metacercariae were located in the eye lens and/or the sac protruding from the lens. The sac was typically composed of a single chamber; less frequently, it was divided into several chambers (Figure S1). The proportion of metacercariae in the lens and sac varied between sites and reflected the overall parasite abundance at each locality (Table 1). The occurrence of metacercariae in the sac was more frequent in localities with higher parasite abundance (western part of the country), while in localities with lower prevalence and abundance (eastern part), more metacercariae were found in the lens (Figure 1). There was no case of sac occurrence without a Diplostomum infection. The probability of sac formation increased with the intensity of parasite infection (GLM, N = 77, p < 0.001), with the probability approaching nearly 100% at infection intensities around ten metacercariae (Figure 2). Accordingly, the proportion of metacercariae located in the sac of individual fish significantly increased with overall parasite abundance (r s = 0.64, p < 0.0001).
FIGURE 1.

Water bodies in the Czech Republic, where bullheads Ameiurus melas (AM) and A. nebulosus (AN) were infected with Diplostomum metacercariae. Location of Diplostomum in lens and sac is distinguished by blue and yellow colours, respectively.
FIGURE 2.
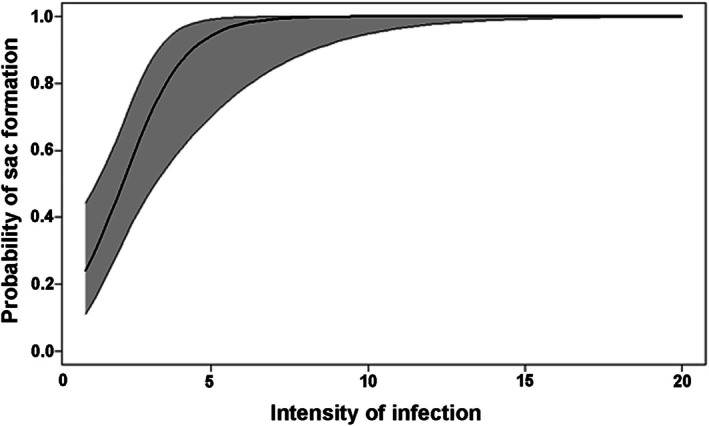
Probability of sac formation with increasing intensity of infection by Diplostomum spp. metacercariae in the lens of naturally infected bullhead catfish (generalised linear model, Bernoulli distribution). Shaded area corresponds to confidence intervals. For greater clarity, the x‐axis has been shortened from the maximum infection intensity of 68 to 20.
The occurrence of two species, D. pseudospathaceum and D. spathaceum (Rudolphi, 1819) was confirmed by molecular methods in both A. melas and A. nebulosus. Sequences from 76 parasites were obtained, resulting in an 84.4% success rate. The obtained sequences corresponded by 99.6%–100% to D. pseudospathaceum from Larus ridibundus Linnaeus, 1766 (Acc. No. KR269766) and D. spathaceum from L. cachinnans Pallas, 1811 (JX986844). Differences were mainly due to unreadable bases or gaps. While D. pseudospathaceum occurred in all water bodies where Diplostomum was successfully sequenced (i.e., except for Škřečoň pond), D. spathaceum was detected less frequently in four localities (Za Pilou, Mlazice pool, Štěpnický pond and Starý pond). Parasite location in the lens or sac was unrelated to Diplostomum species; both D. pseudospathaceum and D. spathaceum were found in the lens and sac (Table 1). Mixed infection, when both species were found in a single eye, was rare, occurring in three cases in the lens and one case in a sac (note that only a subsample of parasites was subjected to molecular analysis).
3.2. Light Microscopic Observations of Whole Lenses After Experimental Infection
Experimental infection with D. pseudospathaceum was performed on juvenile A. melas and S. glanis . All fish were successfully infected. In A. melas , the mean intensity of infection was 26.3, with a range of 5–44 metacercariae per individual fish. In S. glanis , the mean intensity of infection was 19.0, with a range of 6–35 metacercariae per individual fish. The intensity of infection was significantly higher in A. melas compared to S. glanis (t‐test; t = 3.21, p = 0.002). While metacercariae in S. glanis remained in the lens throughout the entire experiment without any obvious changes to the lens (40 days), the formation of a sac protruding from the lens and the dislocation of metacercariae into this sac were observed in A. melas . Upon analysing whole lenses of A. melas with a light microscope, no signs of sac formation were apparent up to 7 DPI, although slight undulation of the lens surface was noted (Figure 3A). The first indication of sac formation, in the form of a slight protrusion on the lens surface, was noted at 9 DPI in one eye (Figure 3B). In fish dissected at 12 DPI, three lenses with different stages of apparent sac formation were observed (Figure 3C,D). All fish, except for one, exhibited a sac on the surface of at least one lens by 24 DPI (Figure 3E). The formation of the sac resulted in a 12.3% average decrease in the lens area compared to the other lens without a sac, which was nearly three times higher than the average difference observed within the lens‐lens variability of the same fish (4.5%). Furthermore, in naturally infected fish with fully developed metacercariae, the area of the sac occasionally exceeded the area of the lens itself (Figure 3F).
FIGURE 3.
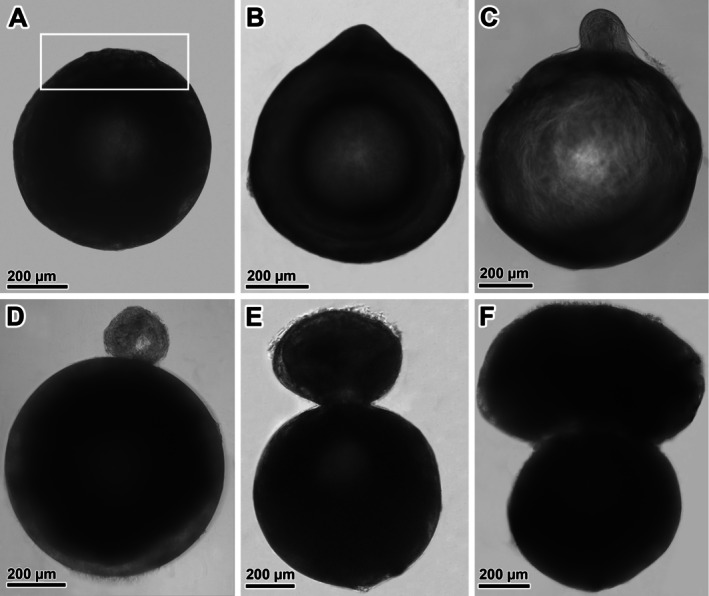
Light microphotographs of lenses with differently developed sac with Diplostomum metacercariae following experimental infection of young‐of‐the‐year Ameiurus melas . (A) No obvious signs of sac formation up to 7 DPI, with presence of undulated surface possibly indicating parasite‐induced proliferation of the lens epithelium (white frame). (B) First sign of sac formation at 9 DPI. (C, D) Different stages of sac formation observed at 12 DPI. (E) Fully developed sac observed at 16–24 DPI. (F) Fully developed sac with mature metacercariae in naturally infected fish.
3.3. Histopathological Examination of Control and Parasitised Eyes
The control (i.e., uninfected) eyes of A. melas showed typical teleost anatomy. The eye was divided into an anterior aqueous chamber bounded by the cornea and iris, and a posterior vitreous chamber bounded by the iris and sclera (Figure S2A). A round lens was suspended between these chambers by the retractor lentis muscle and ligaments. The posterior chamber further included retina vascularised by a capillary network, choroid, sclera, optic nerve (with up to five optic papilla) and the vitreous compartment was filled with vitreous humour. Single or branched capillaries, visible on some sections, indicated vascularisation of the retinal surface by a capillary network. The lens was composed of three layers: the outer acellular sheath (the capsule), the underlying cuboidal epithelial cell layer, and the innermost fibrous layer consisting of peripheral cortex and central nucleus. Infected eyes (Figure S2B) could be easily distinguished from uninfected control eyes (Figure S2A) by the presence of parasites in the lens and vitreous opacities with the presence of low to moderate numbers of inflammatory cells. Additionally, the substance of parasitised lenses crumbled during histological sectioning.
Damage to the surface of infected lenses, in the form of visible rupture of the capsule and subcapsular epithelium (presumed site of parasite invasion), was rarely detected in histological sections. At 3 DPI, a few proliferated epithelial cells were observed on the outside of the capsule in the wound site (Figure S3A). Some lenses showed partial sealing of the wound by proliferating epithelium growing outward (Figure 4A, Figure S3B). In some sections, migrating epithelial cells were visible in this area (Figure 4A). Tunnels left by the motile parasites, as well as the developing parasites themselves, were typically observed near the wound, under the epithelium in the superficial layers of the lens substance (Figure 4A, Figure S3A). Small lumps composed of proliferating epithelial cells began to form on the damaged lens surface (Figure 4B), which gradually enlarged by further cell proliferation at 5 DPI (Figure 4C,D, Figure S3C). Multiple epithelial lumps of varying sizes often appeared on a single lens, indicating asynchronous development of individual sacs (Figure S3D). The migrating parasites in the lens cortex created cavities around themselves, forming free spaces that separated them from the surrounding host tissue. Moreover, eosinophilic structures resembling bead strings could be observed passing perpendicularly from the surface of the parasite to the cavity wall (Figures 4B and 5A).
FIGURE 4.

Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 3 and 5 DPI. (A, B) Different stages of epithelial proliferation on lens outer surface at 3 DPI in longitudinally sectioned eyes. (C, D) Continued proliferation of the lens epithelium on the surface of its capsule at 5 DPI in longitudinally sectioned eyes. Note the migrating cell in the area of lens capsule disruption just below the epithelial outgrowth. LM, paraffin sections stained with HE (A, B, D) or MT (C). Black asterisk, eosinophilic structures; black arrow, proliferating epithelial cells; black arrowhead, migrating cell; ca, lens capsule; el, lens epithelial layer; lc, lens cortex; p, parasite; tu, tunnel excavated by the moving parasite.
FIGURE 5.

Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 5 and 7 DPI. (A) Cross sectioned parasite located within cavity in lens cortex at 5 DPI. Note the eosinophilic structures resembling strings of beads extending perpendicularly from the parasite surface to the cavity wall. (B) General view of a cross sectioned eye with parasitised lens at 7 DPI, revealing the tunnel burrowed by migrating parasite. (C) One stage of a longitudinally sectioned parasite during metamorphosis into metacercaria at 7 DPI. LM, paraffin sections stained with HE (B, C) or MT (A). ac, aqueous chamber; black asterisk, eosinophilic structures; ca, lens capsule; co, cornea; el, lens epithelial layer; ir, iris; lc, lens cortex; le, lens; p, parasite; re, retina; tu, tunnel excavated by the moving parasite; vc, vitreous chamber.
The cavities surrounding the individual parasites gradually enlarged, and the tunnels burrowed by migrating parasites through the lens substance were visibly longer at 7 DPI (Figure 5B,C). The epithelial cells forming the lump continued to proliferate, leading to the formation of a now clearly visible protuberance bulging on the outer surface of the lens (Figure 6A). These protuberances were formed by a mass of proliferating epithelial cells, which proximally (towards the lens) transitioned into a region with degenerating cells (Figure S4A). At 9 DPI, the growth of epithelial protuberances showed a significant progression as they began to transform into clearly demarcated sacs with a constricted base separating them from the lens (Figure 6B, Figure S4B). Each sac was covered by a thick layer of epithelial cells that gradually transitioned into a deeper layer consisting of eosinophilic fibres with only a few nuclei. At this stage, the epithelium gradually began to be covered by an acellular layer corresponding to the lens capsule. It was first visible at the interface with the lens and gradually extended into the more distal part of the sac (Figure 6B, Figure S4B). The parasites themselves or, at least, their tunnels, were usually present beneath the forming sacs.
FIGURE 6.

Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 7 and 9 DPI. (A) Continued proliferation of lens epithelium and sac formation at 7 DPI in a cross sectioned eye. Note the interconnection of the sac with retinal surface by the means of cellular network and vascularisation. (B) Ongoing formation of the sac at 9 DPI in cross sectioned eye. Note the thick layer of epithelial cells already covered by an acellular layer (resembling the lens capsule) and the underlying layer of eosinophilic fibres with only a few nuclei. LM, paraffin sections stained with HE. Black arrow, proliferating epithelial cells; ca, lens/sac capsule; el, lens epithelial layer; lc, lens cortex; n, cell nucleus; p, parasite; re, retina; s, sac; se, sac epithelium; tu, tunnel excavated by the moving parasite; v, vascularisation.
At 12 DPI, some sections revealed parasites attempting to migrate from the lens cortex into the interior of the growing sac (Figure 7A–C). Irregularly organised epithelial cells, covering the growing sac at earlier stages (Figure 6A, Figure S4A,B), gradually formed a multilayered epithelium (Figure 7B,C, Figure S4C). The sacs were mostly located laterally or dorsolaterally in the region of the lens equator, just behind the iris (e.g., Figure 7A, Figure S4D) and were clearly interacting with the retinal surface and the inner lining of the iris via a cellular network (Figures 6A and 7B,C, Figure S4A–C). This network consisted mainly of fibroblast‐like cells present on the surface of the sac, scattered epithelial cells, few macrophages and lymphocytes. Some sections of this interaction zone even showed traces of vascularisation, likely connecting the sac surface to the adjacent retina (Figures 6A and 7B,C, Figure S4A,C). While lymphocytes and a few macrophages could also be seen in the cloudy vitreous or near the retinal surface, inflammation was not significant. At this stage of experimental infection, the tunnels excavated beneath the surface of the lens, as well as the cavities around the growing parasites, became more spacious and were filled with eosinophilic bead string‐like structures (Figure 7C, Figure S4D). Compared to the lens of control fish, the lens fibres in parasitised parts were visibly disrupted, appearing liquefied.
FIGURE 7.

Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 12 DPI. (A) General view of the cross sectioned eye at 12 DPI, showing a clearly demarcated sac with constricted base separating it from the lens. (B) Detailed view of the sac at 12 DPI in a cross sectioned eye. Note the cellular network and vascularisation interconnecting its surface with adjacent retina. (C) Significant progression in sac formation at 12 DPI in cross sectioned eye. Note the parasites migrating from the lens cortex into the sac. LM, paraffin sections stained with HE. ac, aqueous chamber; ca, lens/sac capsule; co, cornea; el, lens epithelial layer; f, fibroblast‐like cell; ir, iris; l, lymphocyte; le, lens; lc, lens cortex; p, parasite; re, retina; s, sac; se, sac epithelium; tu, tunnel excavated by the moving parasite; v, vascularisation; vc, vitreous chamber.
The formation and growth of the sac was significantly advanced at 16 DPI (Figure 8A–C). The epithelial layer covering the sac became organised in the same manner as the subcapsular epithelium of the lens (Figure 8C). At this stage, the enlarged sac was completely pressed against the surface of the adjacent retina and often against the inner lining of the iris, leaving no space between the sac and the adjacent eye structure. Between 12 and 16 DPI, the number of parasites detected in the histological section increased, with many migrating from the lens to the inner part of the sac (Figures 7C and 8B). Parasites remaining in the lens were localised in its cortex, but never in the lens nucleus. The vitreous opacities became more pronounced (Figure 8A).
FIGURE 8.

Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 16 DPI. (A) General view of a cross sectioned eye with a fully formed sac at 16 DPI. Note the significant opacification of vitreous humour. (B, C) Detailed view of the sac organisation and its close interaction with the iris and retinal surface at 16 DPI in cross sectioned eyes. LM, paraffin sections stained with HE. ac, aqueous chamber; ca, lens/sac capsule; co, cornea; el, lens epithelial layer; ir, iris; lc, lens cortex; le, lens; ln, lens nucleus; on, optic nerve; p, parasite; re, retina; s, sac; se, sac epithelium; sf, sac fibres; tu, tunnel excavated by the moving parasite; vo, vitreous opacities.
In rare instances, the sac did not develop on the parasitised lens up to 16 DPI. In these cases, deformation and damage to the lens, as well as to the entire eye, accompanied by vitreous inflammation, were observed (Figure S2D). Additionally, metacercariae in these damaged eyes had escaped from the lens and invaded other parts of the eye. Conversely, there were also cases where, despite the presence of numerous parasites, a lens sac did not form, and the eye anatomy still appeared almost normal, except for a severely perforated lens cortex and mild vitreous opacities (Figure S2B). In two parasitised lenses (7 and 12 DPI), where the sac did not form, the capsule and subcapsular epithelium ruptured, and the lens contents began to extrude into the vitreous space (Figure S2C).
In later stages of infection, (DPI unidentified, naturally infected fish), the sac was often as large as the lens itself (Figure 9A). The capsule covering the large sac was relatively thick at the base of the sac but thinned significantly towards its distal end (Figure 9C) to the point of being often almost indistinguishable (Figure 9D). This was especially true for the area of intimate contact of the sac with the retina, where the extremely thin capsule was covered by a layer of fibroblast‐like cells (Figure 9D,E). Apart from marked vitreous opacities, the parasitised eye showed no obvious pathology (Figure 9A). In contrast, one fish with both lenses containing the metacercariae but not forming sacs exhibited significant pathology of both eyes, including cataract, lens irregularity, lens adherence to the inner parts of the eye and the overall eye deformation, with parasites invading other parts of the eye, including the anterior chamber (Figure S2E), vitreous and retina (Figure S2F). In this case, a more pronounced inflammatory reaction was present.
FIGURE 9.

Histopathological changes of Ameiurus melas lens naturally parasitised by Diplostomum pseudospathaceum. (A) General view of a cross sectioned eye with a well‐developed sac containing a mature metacercaria, accompanied by a vitreous opacification. (B) Detailed view of a sac showing its intimate interaction with the retinal surface in a cross sectioned eye. (C–E) High magnification showing the organisation of the sac wall in a cross sectioned eye. LM, paraffin sections stained with HE (A–D) or MT (E). ac, aqueous chamber; ca, lens/sac capsule; co, cornea; el, lens epithelial layer; f, fibroblast‐like cell; ir, iris; l, lymphocyte; lc, lens cortex; le, lens; p, parasite; re, retina; s, sac; se, sac epithelium; tu, tunnel excavated by the moving parasite; vo, vitreous opacities.
Whole‐head sectioning performed in this study revealed that lens infection was asymmetric in most infected fish, with one lens either not being parasitised at all at 16 DPI or showing a much lower intensity of infection. Moreover, it allowed us to confirm the restriction of the parasite to the eyes of infected fish and to exclude the presence of cercariae and metacercariae of D. pseudospathaceum in other parts of their heads. None of the eyes of control fish showed signs of Diplostomum parasitisation or the above pathology (Figure S2A).
4. Discussion
4.1. Occurrence of Sac Formation in Lenses of Infected Fish
The presence of a sac protruding from the lens of infected fish, containing Diplostomum metacercariae, was commonly observed in both A. nebulosus and A. melas , at all sampling sites, confirming that the formation of the sac in the lens of bullheads is not an exceptional phenomenon. On the contrary, it is more or less regular, especially at higher intensities of Diplostomum infection. Similar results were found by Larson (1965), who observed sac formation (referred to as ‘hernia’ in his study) in the majority of A. melas infected with Diplostomum at all sampling sites in North Dakota. It is, therefore, surprising that only a few notes regarding this unusual location of metacercariae have been reported to date, all involving the same fish species as in our study. In addition to the above‐mentioned study by Larson (1965), La Rue et al. (1926) examined A. nebulosus from Douglas Lake in Michigan, describing the location of Diplostomum metacercariae as occurring in a small knob or a projection from the lens. Marcogliese and Compagna (1999) noted that most Diplostomum metacercariae in 0+ A. nebulosus collected in the St. Lawrence River were present in a small sac protruding from the lens.
Apart from A. nebulosus and A. melas in Europe (this study) and North America (La Rue et al. 1926; Larson 1965; Marcogliese and Compagna 1999), we also observed sac formation in A. natalis collected in the Mississippi River basin in the Illinois and Wisconsin states. In the two examined fish infected with undetermined Diplostomum metacercariae, three and seven parasites, respectively, were all present in a sac protruding from the lens (Ondračková, personal observation). The absence of any other reports of this phenomenon in different fish species in the literature, combined with our experimental observations in S. glanis , suggests that it may be specific to the genus Ameiurus. In experimentally infected S. glanis , we did not observe any externally visible signs of damage to the lens even after 40 days post‐infection, indicating that this host species tolerates the eye‐fluke infection well. Moreover, published reports of diplostomid metacercariae in the related Ictalurus punctatus (another member of the family Ictaluridae) describe the parasite's niche as being restricted to the lens or vitreous humour (e.g., Baker and Crites 1976; Rosser et al. 2016). Compared to Ameiurus spp., I. punctatus have generally larger eyes with more distinctly differentiated retinal elements (Herrick 1941). Therefore, the infection of the lens in Ictalurus is not as limiting to the passage of light as in Ameiurus, in which the displacement of metacercariae from the area of the lens into the sac may allow a better passage of light.
It is possible that sac formation may occur in other fish species parasitised by eye flukes, such as rainbow trout, in which a structure labelled as a lens herniation (Figure 2 in Shariff et al. 1980) strikingly resembles the superficial or oblique sections of the early sacs in our study. Nevertheless, this phenomenon seems to be rather random and rare in rainbow trout, since the study by Shariff et al. (1980) reported pathologies such as subcapsular cataract, lens dislocation, as well as capsule rupture and duplication in acutely and chronically infected individuals.
4.2. External Factors Affecting the Sac Formation
The intensity of infection appears to be an important factor in the initiation of sac formation in Diplostomum‐infected lenses. Our data show that the probability of sac formation increases significantly with the intensity of infection, becoming nearly certain at an intensity of ten metacercariae per lens. However, the sac could also be observed in many lenses with much lower intensities; specifically, we found three fish with a single metacercaria forming the sac. Larson (1965) observed the sac formation in all infected fish at sites with a mean parasite abundance of 17 metacercariae or more. Extremely high infection intensities of Diplostomum metacercariae in the lens have been associated with rupture of the lens capsule (Karvonen and Marcogliese 2020) or ‘tumour‐like growth’ of the lens (Erasmus 1958). However, both of these cases differ from our observations in that they occur rarely and only in very severe infections. This suggests that sac formation and the displacement of metacercariae from the lens to the sac may represent a specific type of parasite tolerance in Ameiurus bullheads, potentially reducing the negative impact of the established infection on the host's fitness without directly harming the parasites, thereby reducing the severity of the infection (Klemme and Karvonen 2017). On the other hand, sac formation led to a significant reduction in lens size (an average of 12.3%). This reduction could also affect the fish's vision, as their ability to focus depends on the lens radius, which may therefore be sensitive to changes in lens size (Karvonen and Seppälä 2008).
In contrast, the species of the parasite does not seem to play a role in sac formation in the infected lens. In Europe, two common eye‐fluke species, D. pseudospathaceum and D. spathaceum , induced sac formation in the lenses of infected fish, without detecting any trend regarding a higher proportion of parasites located in the lens or sac in relation to parasite species. The relatively higher frequency of sac formation in D. pseudospathaceum is likely due to its generally wider distribution in our study area. Despite the parasite responsible for sac formation in the fish host's native range not being identified to the species level (although Larson [1965] suggested the species to be similar to D. flexicaudum ), it is highly probable that the fish were infected with Diplostomum species other than those detected in our study, reflecting their natural geographical distribution in the Palaearctic region (Locke et al. 2015).
4.3. Site of Parasite Entry Into the Lens and Initiation of Sac Formation
Visual examination of the entire lens, particularly histological examination, revealed that the process of sac formation begins very early after infection. The first signs of cell proliferation were evident 3 days post‐infection (Figure 4) and by 12 DPI, the sac was clearly visible with a binocular microscope (Figure 3). Unfortunately, although we documented the onset of epithelial proliferation at the site of apparent capsule disruption in one eye, we cannot definitively determine whether sac formation is restricted to the site of parasite entry. However, the lens capsule was always disrupted or at least thinner at the site of proliferating epithelium, and cells were observed at this site, which we assume migrated from the epithelium to the lens surface (Figure 4). If parasite entry indeed triggers sac formation at the wound site, then most of the parasites must invade the lens in the area just behind the iris. This would be consistent with the hypothesis of Shariff et al. (1980), who suggested that the migration speed of diplostomulae indicates their passage from the site of invasion on the fish surface to the eye is at least partly mediated by blood or lymph flow. Interestingly, asynchronous development of sacs as well as the occurrence of parasites of slightly different sizes in the same eye were observed in experimentally infected fish, suggesting that the parasites reach their target at different rates, over a period of up to several days.
The parasitised lens in our study could also be easily recognised microscopically (e.g., Figure 9A) due to increased vitreous opacification containing low numbers of lymphocytes and macrophages, most likely as a direct consequence of intraocular inflammation induced by the flukes invading the lens. Moreover, experimentally infected fish in this study clearly showed increased vitreous vascularisation extending from the retinal surface to the sac on the surface of the parasitised lens, in accordance with observations by Larson (1965). In our study, this vascularisation apparently interacted with the retinal surface or the inner lining of the iris, most often at the point where the retina ends just behind the iris. Therefore, we assume that the most likely portal of entry into the eye was either the iris or the choroid.
4.4. Histoarchitecture of the Sac
Larson (1965) described the pathology of sac formation as a herniation of a heavily parasitised lens near the attachment point of the dorsal suspensory ligament (i.e., the site of least resistance), caused by increasing pressure. This could be associated with a preference of metacercariae of some strigeid flukes to concentrate in the dorsal portion of the lens adjacent to the retina, as reported from three‐spined stickleback (Erasmus 1958). In contrast, our detailed observations of pathology development in experimentally infected fish, kept under controlled conditions, show that the occurrence of the sac is not restricted to the dorsal surface of the lens at the site of ligament attachment. Furthermore, the formation of the sac itself is a more complex process than simply herniation of the parasitised lens and may occur even at low infection intensities. The process of sac formation observed in our study indicates an increased proliferation of the subcapsular epithelium, extending outward from the lens above the capsule. Notably, the proliferating epithelial mass that appeared on the lens surface during the early stages of sac formation resembled the early cellular differentiation seen during embryonic lens development in zebrafish (Dahm et al. 2007). Similarly to the lens of adult zebrafish, where a monolayer of epithelial cells covers the anterior surface (i.e., the area of lens separation from the surface ectoderm) and expands towards the posterior pole, the epithelial layer in the fully developed sac is most prominent at the base (i.e., the site of epithelial proliferation initiation on the lens surface) and decreases distally. Consequently, the thin capsule, likely produced by the proliferating epithelial cells during sac growth, was often not discernible in its distal part. This could be due to the rapid growth of the sac, with the production of the capsule lagging behind. Interestingly, the capsule in the distal part of the large sacs was covered with a layer of strongly flattened cells resembling fibroblasts. Fibroblast‐like cells were also observed in the cellular network interconnecting the distal part of the sac to the iris lining and retinal surface. The presence of fibroblasts is not surprising, as they have been observed around the lens at the site of capsule rupture, alongside proliferating epithelial cells. It is even possible that these fibroblasts originate from metaplasia of the proliferating lens epithelium (Wilcock and Peiffer Jr. 1987; Li et al. 2021).
It should be noted that although fully developed sacs resembled lenses in their histological organisation, manipulation with fresh lenses revealed that the sacs were much softer and their internal substance was liquid. The soft nature of the sacs may have been due to the absence of a fibre nucleus, which is present in the lens and is harder than the fibre cortex, as noted in a previous study (Larson 1965). The liquefaction of the fibrous substance filling the sac occurred as a result of the parasites' activity. In the lens, the presence of parasites confined to the cortex is also associated with liquefaction of cortical fibres, which occurs during the parasite's movement, feeding and/or secretion/excretion of (Erasmus 1958; Graczyk 1988). Consequently, the parasites in both the lens and the sac were surrounded by a cavity that conformed to the shape of their bodies, as also observed in three‐spined stickleback and rainbow trout (Erasmus 1958; Shariff et al. 1980).
4.5. Sac Formation: Host Defence Response?
Despite considerable scientific interest in the metacercariae of Diplostomum eye flukes, there remains a paucity of information regarding the host's evasive mechanisms for mitigating the detrimental effects of ocular infection in fish. By lodging in the eyes and inducing cataract formation, parasites infecting the fish lens prevent light from reaching the retina (Seppälä et al. 2005), which is critical for vision. On the other hand, Ameiurus bullheads, possessing relatively small eyes (Herrick 1941), prefer benthic zones with limited light penetration (Ulikowski et al. 2022). Furthermore, infected A. melas that failed to develop lentic sac exhibited notable eye pathology, including cataracts and overall eye deformation, likely reducing their vision (Figure S2C–F). Sac formation in parasitised individuals may thus serve as a specific defence mechanism, allowing the fish to ‘clear’ the space within the infected lens, thereby protecting them from vision impairment caused by metacercariae or from the development of cataracts, which further hinder light transmission (Karvonen and Lindström 2018). This process likely utilises the multipotential morphogenetic capacities of the lens epithelium (Von Sallmann et al. 1966) and its high cellular activity following injury, a characteristic response across all animal species (Hirayama et al. 2003). The lens's ability to repair itself can further compensate for damage after parasites move into the sac, as indicated by the relatively normal structure of lenses with developed sacs (Larson 1965). The sac typically forms in the posterior segment, typically on the dorsolateral or lateral side of the lens, in the equatorial region just behind the iris, where it does not interfere with the fish's field of vision, further supporting our suggestion of the host's strategy to mitigate the limited light penetration due to the parasite infection.
Our study confirms that sac formation in Diplostomum‐infected lenses of Ameiurus bullheads is a common process, despite the generally limited awareness in the scientific literature. It is characteristic of the host species in both their native North American and non‐native European ranges and is associated with the infection by various species of Diplostomum trematodes. The displacement of metacercariae from the lens, an organ critical for the fish's vision, to the sac protruding into the posterior segment appears to mitigate the negative effects of infection and impaired vision caused by the parasite. Although our data indicate that sac formation is triggered by disruption of the lens capsule caused by parasite penetration, further studies are needed to verify this mechanism and fully understand its adaptive significance.
Author Contributions
Markéta Ondračková: conceptualization, investigation, funding acquisition, writing – original draft, writing – review and editing, formal analysis. Andrea Valigurová: investigation, writing – original draft, writing – review and editing, formal analysis. Iveta Hodová: writing – review and editing, formal analysis. Veronika Bartáková: writing – review and editing, formal analysis. Maria Yu. Tkachenko: investigation, writing – review and editing. Michal Janáč: formal analysis, writing – review and editing. Lukáš Vetešník: investigation, writing – review and editing, data curation.
Ethics Statement
This research was undertaken in line with the ethical requirements of the Czech Republic and has been approved by the appropriate ethics committee (permit No. MO‐2017‐02). The method of fish sampling, experimental infection and killing complied with the legal requirements of the Czech Republic (§ 7 law No. 114/1992 on the Protection of Nature and Landscape and § 6, 7, 9 and 10 regulation No. 419/2012 on the Care, Breeding and Use of Experimental Animals).
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. Lens with protruding sacs in naturally infected Ameiurus nebulosus with Diplostomum sp., photodocumented under stereomicroscope. (A) Sac with a single chamber. (B) Sac divided into several chambers.
Figure S2. Anatomy of the control eye of Ameiurus melas and histopathological changes of its lens during natural and experimental infections with Diplostomum pseudospathaceum. (A) General view of a cross sectioned control eye. (B) General view of a cross sectioned eye at 16 DPI, where no sac developed on the surface of the lens parasitised by several metacercariae. (C) General view of longitudinally sectioned eye showing the parasitised lens at 7 DPI, where the sac has not formed and the lens capsule ruptured, with part of the lens contents leaking into the vitreous space (encircled). (D) General view of a cross sectioned eye showing significant damage at 16 DPI, in which the sac has not developed on the lens surface. Note the parasite present in the vitreous with marked inflammation. (E, F) Longitudinal section through a damaged eye with naturally parasitised lens that failed to form the sac. Note metacercariae invading different parts of the eye such as anterior chamber (E) or retina (F). LM, paraffin sections stained with HE (B, C, D, E) or MT (A, F). ac, aqueous chamber; co, cornea; ir, iris; le, lens; on, optic nerve; p, parasite; re, retina; vc, vitreous chamber; vo, vitreous opacities.
Figure S3. Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 3 and 5 DPI. (A) Putative site of infection and proliferation of the lens epithelium at 3 DPI in a longitudinally sectioned eye. (B) Proliferation of the lens epithelium on its outer surface at 3 DPI in cross sectioned eye. (C) Continued proliferation of the lens epithelium on the capsule surface at 5 DPI in longitudinally sectioned eye. (D) View of different degrees of epithelial proliferation at two locations on the same lens at 5 DPI in a longitudinally sectioned eye. LM, paraffin sections stained with HE (A, D) or MT (B, C). Black arrow, proliferating epithelial cells; ca, lens capsule; el, lens epithelial layer; lc, lens cortex; tu, tunnel excavated by the moving parasite.
Figure S4. Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 7, 9 and 12 DPI. (A) Continued proliferation of lens epithelium and sac formation at 7 DPI in a cross sectioned eye. Note the interconnection of the sac with retinal surface by the means of cellular network and vascularisation. (B) Ongoing formation of the sac at 9 DPI in longitudinal sectioned eye. (C) Detailed view of the sac at 12 DPI in a cross sectioned eye. Note the cellular network and vascularisation interconnecting its surface with adjacent retina and iris. (D) Significant progression in sac formation at 12 DPI in cross sectioned eye. Note another sac growing in the posterior segment of the lens (encircled). LM, paraffin sections stained with HE (C, D) or MT (A, B). Black arrow, proliferating epithelial cells; ca, lens/sac capsule; co, cornea; dc, degenerating cells; f, fibroblast‐like cell; ir, iris; l, lymphocyte; le, lens; m, macrophage; p, parasite; re, retina; s, sac; se, sac epithelium; sf, sac fibres; tu, tunnel excavated by the moving parasite; v, vascularisation; vc, vitreous chamber.
Acknowledgements
We thank our colleagues from the Czech Academy of Sciences (Institute of Vertebrate Biology) for their support during fish sampling and the representatives of the Czech and Moravian Angling Associations for the opportunity to sample fish in their waters. We are greatly indebted to Gabriela Vágnerová (Faculty of Science, Masaryk University) for her help with the processing of samples for histological analysis. Open access publishing facilitated by Institute of Vertebrate Biology of the Czech Academy of Sciences, as part of the Wiley ‐ CzechELib agreement.
Funding: This study was financially supported by the Czech Science Foundation, project No. 20‐29111S.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Baker, J. C. , and Crites J. L. C.. 1976. “Parasites of Channel Catfish. Ictalurus punctatus Rafinesque, From the Island Region of Western Lake Erie.” Proceedings of the Helminthological Society of Washington 43: 37–39. [Google Scholar]
- Bowles, J. , Hope M., Tiu W. U., Liu X. S., and McManus D. P.. 1993. “Nuclear and Mitochondrial Genetic Markers Highly Conserved Between Chinese and Philippine Schistosoma japonicum .” Acta Tropica 55: 217–229. 10.1016/0001-706X(93)90079-Q. [DOI] [PubMed] [Google Scholar]
- Bush, A. O. , Lafferty K. D., Lotz J. M., and Shostak A. W.. 1997. “Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited.” Journal of Parasitology 83: 575–583. 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- Collin, S. P. 2009. “Evolution of the Visual System in Fishes.” In Encyclopedia of Neuroscience, edited by Binder M. D., Hirokawa N., and Windhorst U.. Springer. 10.1007/978-3-540-29678-2_3178. [DOI] [Google Scholar]
- Crowden, A. E. , and Broom D. M.. 1980. “Effects of the Eyefluke, Diplostomum spathaceum , on the Behaviour of Dace ( Leuciscus leuciscus ).” Animal Behaviour 28, no. 1: 287–294. 10.1016/S0003-3472(80)80031-5. [DOI] [Google Scholar]
- Dahm, R. , Schonthaler H. B., Soehn A. S., van Marle J., and Vrensen G. F.. 2007. “Development and Adult Morphology of the Eye Lens in the Zebrafish.” Experimental Eye Research 85, no. 1: 74–89. 10.1016/j.exer.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Dudliv, I. , Kvach Y., Tkachenko M. Y., et al. 2024. “Comparative Analysis of Parasite Load on Recently Established Invasive Pumpkinseed Lepomis gibbosus (Actinopterygii: Centrarchidae) in Europe.” Acta Parasitologica 69: 819–830. 10.1007/s11686-024-00794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus, D. A. 1958. “Studies on the Morphology, Biology and Development of a Strigeid Cercaria (Cercaria × Baylis 1930).” Parasitology 48, no. 3–4: 312–335. 10.1017/S0031182000021284. [DOI] [PubMed] [Google Scholar]
- Faltýnková, A. , Kudlai O., Pantoja C., Yakovleva G., and Lebedeva D.. 2022. “Another Plea for ‘Best Practice’ in Molecular Approaches to Trematode Systematics: Diplostomum sp. Clade Q Identified as Diplostomum baeri Dubois, 1937 in Europe.” Parasitology 149: 503–518. 10.1017/S0031182021002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, R. A. , Barrett L. M., Parkin L., et al. 2022. “Eye Flukes (Diplostomum spp) Damage Retinal Tissue and May Cause a Regenerative Response in Wild Threespine Stickleback Fish.” Experimental Eye Research 225: 109298. 10.1016/j.exer.2022.109298. [DOI] [PubMed] [Google Scholar]
- Fuad, M. M. H. , Tichopád T., Ondračková M., et al. 2024. “Trematode Diplostomum pseudospathaceum Inducing Differential Immune Gene Expression in Sexual and Gynogenetic Gibel Carp ( Carassius gibelio ): Parasites Facilitating the Coexistence of Two Reproductive Forms of the Invasive Species.” Frontiers in Immunology 15: 1392569. 10.3389/fimmu.2024.1392569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva, S. , Soldánová M., Pérez‐del‐Olmo A., et al. 2013. “Molecular Prospecting for European Diplostomum (Digenea: Diplostomidae) Reveals Cryptic Diversity.” International Journal for Parasitology 43: 57–72. 10.1016/j.ijpara.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Graczyk, T. 1988. “The Metacercaria of Diplostomum pseudospathaceum Niewiadomska, 1984 and Diplostomum spathaceum (Rudolphi, 1819) in the Ocular Lens of Fish and Reactions of the Lens to the Presence of the Parasites.” Wiadomości Parazytologiczne 34, no. 1: 29–36. [PubMed] [Google Scholar]
- Haase, D. , Rieger J. K., Witten A., et al. 2016. “Immunity Comes First: The Effect of Parasite Genotypes on Adaptive Immunity and Immunization in Three‐Spined Sticklebacks.” Developmental and Comparative Immunology 54: 137–144. 10.1016/j.dci.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Herrick, C. J. 1941. “The Eyes and Optic Paths of the Catfish, Ameiurus .” Journal of Comparative Neurology 75: 255–286. [Google Scholar]
- Hirayama, S. , Wakasugi A., Morita T., et al. 2003. “Repair and Reconstruction of the Mouse Lens After Perforating Injury.” Japanese Journal of Ophthalmology 47: 338–346. 10.1016/s0021-5155(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Humason, G. L. 1967. Animal Tissue Techniques. 2nd ed. W.H. Freeman and Company. [Google Scholar]
- Karvonen, A. , and Lindström K.. 2018. “Spatiotemporal and Gender‐Specific Parasitism in Two Species of Gobiid Fish.” Ecology and Evolution 8: 6114–6123. 10.1002/ece3.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen, A. , and Marcogliese D.. 2020. “Diplostomiasis (Diplostomum spathaceum and Related Species).” In Climate Change and Infectious Fish Diseases, edited by Woo P. T. K., Leong J.‐A., and Buchmann K., 434–456. CABI. 10.1079/9781789243277.0434. [DOI] [Google Scholar]
- Karvonen, A. , and Seppälä O.. 2008. “Eye Fluke Infection and Lens Size Reduction in Fish: A Quantitative Analysis.” Diseases of Aquatic Organisms 80: 21–26. 10.3354/dao01918. [DOI] [PubMed] [Google Scholar]
- Karvonen, A. , Seppälä O., and Valtonen E. T.. 2004. “Eye Fluke‐Induced Cataract Formation in Fish: Quantitative Analysis Using an Ophthalmological Microscope.” Parasitology 129: 473–478. 10.1017/s0031182004006006. [DOI] [PubMed] [Google Scholar]
- Kearse, M. , Moir R., Wilson A., et al. 2012. “Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data.” Bioinformatics 28: 1647–1649. 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemme, I. , and Karvonen A.. 2016. “Learned Parasite Avoidance Is Driven by Host Personality and Resistance to Infection in a Fish–Trematode Interaction.” Proceedings of the Royal Society B: Biological Sciences 283: 20161148. 10.1098/rspb.2016.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemme, I. , and Karvonen A.. 2017. “Vertebrate Defense Against Parasites: Interactions Between Avoidance, Resistance, and Tolerance.” Ecology and Evolution 7, no. 2: 561–571. 10.1002/ece3.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue, G. R. , Butler E. P., and Berkhout P. G.. 1926. “Studies on the Trematode Family Strigeidae (Holostomidae): No. IV. The Eye of Fishes, an Important Habitat for Larval Strigeidae.” Transactions of the American Microscopical Society 45, no. 4: 282–288. 10.2307/3221788. [DOI] [Google Scholar]
- Larson, O. R. 1965. “ Diplostomulum (Trematoda:Strigeoidea) Associated With Herniations of Bullhead Lenses.” Journal of Parasitology 51, no. 2: 224–229. 10.2307/3276087. [DOI] [PubMed] [Google Scholar]
- Larsen, A. H. , Bresciani J., and Buchmann K.. 2005. “Pathogenicity of Diplostomum Cercariae in Rainbow Trout, and Alternative Measures to Prevent Diplostomosis in Fish Farms.” Bulletin of the European Association of Fish Pathologists 25: 332–339. [Google Scholar]
- Li, Y. , Li Z., Quan Y., et al. 2021. “Macrophage Recruitment in Immune‐Privileged Lens During Capsule Repair, Necrotic Fiber Removal, and Fibrosis.” iScience 24, no. 6: 102533. 10.1016/j.isci.2021.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, S. A. , Al‐Nasiri F. S., Caffara M., et al. 2015. “Diversity, Specificity and Speciation in Larval Diplostomidae (Platyhelminthes: Digenea) in the Eyes of Freshwater Fish, as Revealed by DNA Barcodes.” International Journal of Parasitology 45: 841–855. 10.1016/j.ijpara.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Lopes, P. , Fench S., Woodhams D., and Binning S.. 2022. “Infection Avoidance Behaviors Across Vertebrate Taxa: Patterns, Processes, and Future Directions.” In Animal Behavior and Parasitism, 237. Oxford University Press. [Google Scholar]
- Luton, K. , Walker D., and Blair D.. 1992. “Comparison of Ribosomal Internal Transcribed Spacers From Two Congeneric Species of Flukes (Platyhelminthes: Trematoda: Digenea).” Molecular and Biochemical Parasitology 56: 323–328. 10.1016/0166-6851(92)90181-I. [DOI] [PubMed] [Google Scholar]
- Marcogliese, D. J. , and Compagna S.. 1999. “Diplostomatid Eye Flukes in Young‐Of‐The‐Year and Forage Fishes in the St. Lawrence River.” Quebec. Journal of Aquatic Animal Health 11: 275–282. . [DOI] [Google Scholar]
- Michálková, V. , and Ondračková M.. 2014. “Experimental Evidence for Parasite‐Induced Over‐Winter Mortality in Juvenile Rhodeus amarus .” Journal of Fish Biology 84, no. 5: 1377–1388. 10.1111/jfb.12363. [DOI] [PubMed] [Google Scholar]
- Niewiadomska, K. , and Laskowski Z.. 2002. “Systematic Relationships Among Six Species of Diplostomum Nordmann, 1832 (Digenea) Based on Morphological and Molecular Data.” Acta Parasitologica 47: 1230–1237. [Google Scholar]
- Ondračková, M. , Kvach Y., Tkachenko M., et al. 2025. “The Role of North American Bullhead Catfish as Parasite Reservoirs in Central European Fishing Grounds.” Aquaculture 599: 742100. 10.1016/j.aquaculture.2024.742100. [DOI] [Google Scholar]
- Padrós, F. , Knudsen R., and Blasco‐Costa I.. 2018. “Histopathological Characterisation of Retinal Lesions Associated to Diplostomum Species (Platyhelminthes: Trematoda) Infection in Polymorphic Arctic Charr Salvelinus alpinus .” International Journal for Parasitology—Parasites and Wildlife 7: 68–74. 10.1016/j.ijppaw.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, G. , Kalbe M., and Reusch T. B. H.. 2006. “One Day Is Enough: Rapid and Specific Host–Parasite Interactions in a Stickleback‐Trematode System.” Biology Letters 2: 382–384. 10.1098/rsbl.2006.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser, T. G. , Baumgartner W. A., Alberson N. R., et al. 2016. “ Austrodiplostomum sp., Bolbophorus sp. (Digenea: Diplostomidae), and Clinostomum marginatum (Digenea: Clinostomidae) Metacercariae in Inland Silverside Menidia beryllina From Catfish Aquaculture Ponds, With Notes on the Infectivity of Austrodiplostomum sp. Cercariae in Channel Catfish Ictalurus punctatus .” Parasitology Research 115: 4365–4378. 10.1007/s00436-016-5222-z. [DOI] [PubMed] [Google Scholar]
- Sandström, A. 1999. “Visual Ecology of Fish—A Review With Special Reference to Percids.” Fiskeriverket Rapport 2: 45–80. [Google Scholar]
- Scharsack, J. P. , and Kalbe M.. 2014. “Differences in Susceptibility and Immune Responses of Three‐Spined Sticklebacks (Gasterosteus aculeatus) From Lake and River Ecotypes to Sequential Infections With the Eye Fluke Diplostomum pseudospathaceum .” Parasites & Vectors 7: 109. 10.1186/1756-3305-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwelm, J. , Georgieva S., Grabner D., Kostadinova A., and Sures B.. 2021. “Molecular and Morphological Characterisation of Diplostomum phoxini (Faust, 1918) With a Revised Classification and an Updated Nomenclature of the Species‐Level Lineages of Diplostomum (Digenea: Diplostomidae) Sequenced Worldwide.” Parasitology 148: 1648–1664. 10.1017/S0031182021001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppänen, E. , Kuukka H., Voutilainen A., et al. 2009. “Metabolic Depression and Spleen and Liver Enlargement in Juvenile Arctic Charr Salvelinus alpinus Exposed to Chronic Parasite Infection.” Journal of Fish Biology 74: 553–561. [DOI] [PubMed] [Google Scholar]
- Seppälä, O. , Karvonen A., and Valtonen E. T.. 2005. “Impaired Crypsis of Fish Infected With a Trophically Transmitted Parasite.” Animal Behaviour 70: 895–900. 10.1016/j.anbehav.2005.01.021. [DOI] [Google Scholar]
- Shariff, M. , Richards R. H., and Sommerville C.. 1980. “The Histopathology of Acute and Chronic Infections of Rainbow Trout Salmo Gairdneri Richardson With Eye Flukes, Diplostomum spp.” Journal of Fish Diseases 3: 455–465. 10.1111/j.1365-2761.1980.tb00432.x. [DOI] [Google Scholar]
- Steenstrup, J. J. S. 1842. “Über den Generationswechsel, (Ubers. Lorenzen).” XVII + 140pp. 3 pls. 80 Kopenhagen.
- Ubels, J. L. , DeJong R. J., Hoolsema B., et al. 2018. “Impairment of Retinal Function in Yellow Perch (Perca flavescens) by Diplostomum baeri Metacercariae.” Internationa Journal for Parasitology—Parasites and Wildlife 7: 171–179. 10.1016/j.ijppaw.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulikowski, D. , Traczuk P., and Kalinowska K.. 2022. “Abundance and Size Structure of Invasive Brown Bullhead, Ameiurus nebulosus (Lesueur, 1819), in a Mesotrophic Lake (North‐Eastern Poland).” BioInvasions Records 11: 267–277. 10.3391/bir.2022.11.1.27. [DOI] [Google Scholar]
- Von Sallmann, L. , Halver J. E., Collins E., and Grimes P.. 1966. “Thioacetamide‐Induced Cataract With Invasive Proliferation of the Lens Epithelium in Rainbow Trout.” Cancer Research 26, no. 8: 1819–1825. [PubMed] [Google Scholar]
- Voutilainen, A. , Figueiredo K., and Huuskonen H.. 2008. “Effects of the Eye Fluke Diplostomum spathaceum on the Energetics and Feeding of Arctic Charr Salvelinus alpinus .” Journal of Fish Biology 73: 2228–2237. 10.1111/j.1095-8649.2008.02050.x. [DOI] [Google Scholar]
- Wedekind, C. , and Little T. J.. 2004. “The Clearance of Hidden Cestode Infection Triggered by an Independent Activation of Host Defense in a Teleost Fish.” Journal of Parasitology 90: 1329–1331. 10.1645/GE-225R. [DOI] [PubMed] [Google Scholar]
- Wilcock, B. P. , and Peiffer R. L. Jr. 1987. “The Pathology of Lens‐Induced Uveitis in Dogs.” Veterinary Pathology 24, no. 6: 549–553. 10.1177/030098588702400613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Lens with protruding sacs in naturally infected Ameiurus nebulosus with Diplostomum sp., photodocumented under stereomicroscope. (A) Sac with a single chamber. (B) Sac divided into several chambers.
Figure S2. Anatomy of the control eye of Ameiurus melas and histopathological changes of its lens during natural and experimental infections with Diplostomum pseudospathaceum. (A) General view of a cross sectioned control eye. (B) General view of a cross sectioned eye at 16 DPI, where no sac developed on the surface of the lens parasitised by several metacercariae. (C) General view of longitudinally sectioned eye showing the parasitised lens at 7 DPI, where the sac has not formed and the lens capsule ruptured, with part of the lens contents leaking into the vitreous space (encircled). (D) General view of a cross sectioned eye showing significant damage at 16 DPI, in which the sac has not developed on the lens surface. Note the parasite present in the vitreous with marked inflammation. (E, F) Longitudinal section through a damaged eye with naturally parasitised lens that failed to form the sac. Note metacercariae invading different parts of the eye such as anterior chamber (E) or retina (F). LM, paraffin sections stained with HE (B, C, D, E) or MT (A, F). ac, aqueous chamber; co, cornea; ir, iris; le, lens; on, optic nerve; p, parasite; re, retina; vc, vitreous chamber; vo, vitreous opacities.
Figure S3. Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 3 and 5 DPI. (A) Putative site of infection and proliferation of the lens epithelium at 3 DPI in a longitudinally sectioned eye. (B) Proliferation of the lens epithelium on its outer surface at 3 DPI in cross sectioned eye. (C) Continued proliferation of the lens epithelium on the capsule surface at 5 DPI in longitudinally sectioned eye. (D) View of different degrees of epithelial proliferation at two locations on the same lens at 5 DPI in a longitudinally sectioned eye. LM, paraffin sections stained with HE (A, D) or MT (B, C). Black arrow, proliferating epithelial cells; ca, lens capsule; el, lens epithelial layer; lc, lens cortex; tu, tunnel excavated by the moving parasite.
Figure S4. Histopathological changes of Ameiurus melas lens parasitised by Diplostomum pseudospathaceum at 7, 9 and 12 DPI. (A) Continued proliferation of lens epithelium and sac formation at 7 DPI in a cross sectioned eye. Note the interconnection of the sac with retinal surface by the means of cellular network and vascularisation. (B) Ongoing formation of the sac at 9 DPI in longitudinal sectioned eye. (C) Detailed view of the sac at 12 DPI in a cross sectioned eye. Note the cellular network and vascularisation interconnecting its surface with adjacent retina and iris. (D) Significant progression in sac formation at 12 DPI in cross sectioned eye. Note another sac growing in the posterior segment of the lens (encircled). LM, paraffin sections stained with HE (C, D) or MT (A, B). Black arrow, proliferating epithelial cells; ca, lens/sac capsule; co, cornea; dc, degenerating cells; f, fibroblast‐like cell; ir, iris; l, lymphocyte; le, lens; m, macrophage; p, parasite; re, retina; s, sac; se, sac epithelium; sf, sac fibres; tu, tunnel excavated by the moving parasite; v, vascularisation; vc, vitreous chamber.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


